Highlights
-
•
A high epilepsy prevalence in the Aketi health zone was observed despite 14 years of community-directed treatment with ivermectin.
-
•
The high prevalence of OV16 antibodies in children is indicative of high ongoing onchocerciasis transmission.
-
•
High onchocerciasis transmission is the consequence of high exposure to blackflies and low intake of ivermectin.
-
•
Head nodding seizures were observed in 13.8% of the persons with epilepsy.
-
•
Ivermectin coverage needs to be increased and bi-annual distribution should be considered.
Keywords: Onchocerciasis, Epilepsy, Ivermectin, Prevalence, Incidence, Case–control, Focus group discussion, Stigma
Abstract
Objectives
To investigate the reasons for the high prevalence of epilepsy (>6%) discovered in 2015 in the Aketi health zone in the north of the Democratic Republic of the Congo.
Methods
Persons with epilepsy (PWE) diagnosed in a door-to-door survey in 2015 were traced and re-examined in 2017 by a neurologist. Confirmed PWE were paired with matched controls. For onchocerciasis assessment, children 7–10 years old were tested for IgG4 Onchocerca volvulus (OV16) antibodies, a rapid epidemiological mapping of onchocerciasis (REMO) study was performed, and ivermectin coverage was investigated.
Results
Forty-three (61.4%) previously diagnosed PWE were traced; the neurologist confirmed the epilepsy diagnosis in all of them. The overall OV16 positivity rate was 64.5%. Poor ivermectin coverage (55.9%) and a high prevalence of onchocercal nodules (>70%) were observed. The prevalence of epilepsy was 5.7% in Aketi rural town, with nine PWE (13.8%) experiencing head nodding seizures. A case-control study showed that PWE had lower body weight and higher ivermectin coverage in 2017 than healthy controls.
Conclusions
The high prevalence of epilepsy in the Aketi health zone, despite 14 years of community-directed treatment with ivermectin (CDTI), was found to be associated with high onchocerciasis transmission and low ivermectin use. An awareness programme to increase ivermectin coverage and the introduction of a bi-annual CDTI programme should be considered.
Introduction
A high prevalence of epilepsy has been reported in many onchocerciasis endemic areas (Pion et al., 2009, Boussinesq et al., 2002, Kaiser et al., 1996, Ovuga et al., 1992, Prischich et al., 2008, Colebunders et al., 2016a, Gerrits, 1983, Rwiza et al., 1992), including the Democratic Republic of Congo (DRC) (Levick et al., 2017, Colebunders et al., 2016b, Colebunders et al., 2016c, Casis, 1938). Recent studies have suggested that the Onchocerca volvulus parasite is the trigger behind the seizures in a large percentage of persons with epilepsy in onchocerciasis endemic regions and that in these regions, treatment with ivermectin protects against epilepsy (Levick et al., 2017, Colebunders et al., 2016c, Colebunders et al., 2018a, Chesnais et al., 2018). However, the pathophysiological mechanism by which O. volvulus triggers epilepsy remains to be elucidated (Colebunders et al., 2018b, Colebunders and Titulaer, 2017, Johnson et al., 2017, Idro et al., 2016).
A house-to-house epilepsy prevalence survey was conducted in April 2015 in the Aketi health zone, an onchocerciasis endemic area in the province of Bas-Uélé in the north of the DRC. Results from that study revealed an epilepsy prevalence and incidence of 6.8% and 1.1%, respectively, in Wela village, and of 8.4% and 1.4%, respectively, in Makoko village (Levick et al., 2017). These prevalence and incidence rates are surprisingly high given the fact that a community-directed treatment with ivermectin (CDTI) programme had been implemented for 14 years in these villages, and that based on interviews of household members, ivermectin coverage in 2015 was calculated to be 65.1% in Wela and 78.1% in Makoko (Levick et al., 2017). Meanwhile in 1999, prior to CDTI introduction, these villages were known to be onchocerciasis hyperendemic, with 98% of adults presenting onchocercal nodules during a rapid epidemiological mapping of onchocerciasis (REMO) assessment (Levick et al., 2017).
To investigate the reasons for these very high epilepsy prevalence and incidence rates, the epilepsy and onchocerciasis situation in this health zone was re-investigated in April 2017. During the 2015 survey, epilepsy cases were confirmed by a non-specialist medical doctor. To exclude an overestimation of epilepsy prevalence due to inappropriate confirmation in 2015, it was necessary to have a neurologist re-confirm these cases. The high pre-CDTI onchocerciasis endemicity in Wela and Makoko (98% REMO findings), coupled with sub-optimal ivermectin coverage in 2015 led us to suspect that high O. volvulus transmission may have been the cause of the observed epilepsy prevalence and incidence. An assessment of the onchocerciasis situation in these villages was necessary to verify this hypothesis. Lastly, given that previous surveys focused only on rural villages, it was thought that data on onchocerciasis and epilepsy obtained in a sub-urban setting within the same health zone would be informative.
Materials and methods
Study sites
Studies were conducted in three different sites in the Aketi health zone (the villages of Wela and Makoko and the rural town of Aketi) located in the province of Bas-Uélé in the north of the DRC (Figure 1).
Figure 1.
The Aketi health zone (in red) located in the province of Bas-Uélé (in yellow) in the Democratic Republic of the Congo.
In the Aketi health zone, the morbidity pattern is dominated by poverty-related conditions, among which infectious diseases and epilepsy are the main reasons for consultation and admission to health care facilities. Agriculture, hunting, and fishing are the main economic activities of the health zone inhabitants. Five main rivers flow marginal to or across the health zone: the Itimbiri, Likati, Rubi, Telé, and Aketi rivers.
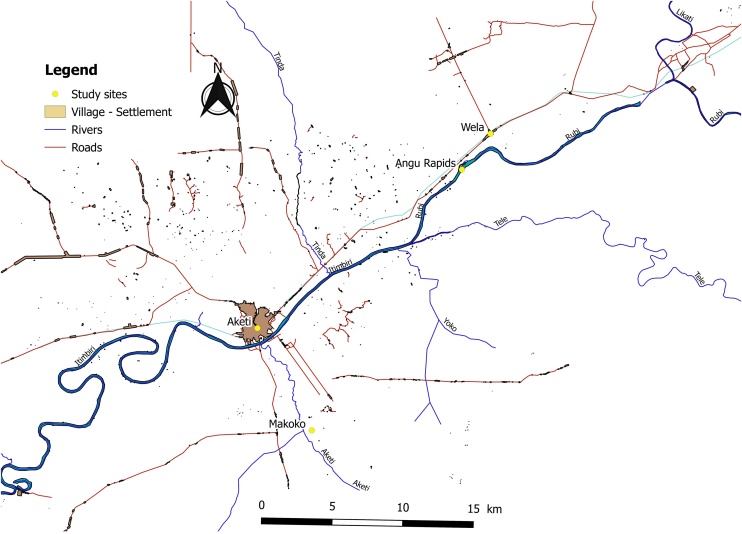
The village of Wela is situated on the road from Buta to Aketi, at 20 km distance from Aketi. The village is located 1 km from the Angu rapids of the Rubi River (Figure 2). Makoko is situated 1 km south-east of Aketi, on the left bank of the Itimbiri River and along the Aketi River. The total population of Wela and Makoko at the time of the study was 14 683. Aketi is the largest town of the three study sites, an estimated 41,913 residents in 2012 (Archive.today, 2013) and is situated on the right bank of the Itimbiri River. The Rubi and Itimbiri rivers are breeding sites for the vector Simulium damnosum (blackflies). A CDTI programme (Mectizan) for the control of onchocerciasis has been ongoing in the Aketi health zone since 2003.
Figure 2.
The villages of Aketi, Makoko, and Wela, and the Itimbiri and Rubi rivers located in the Aketi health zone.
Epilepsy definition
Epilepsy was defined as the occurrence of at least two unprovoked seizures separated by 24 h or more in the past 5 years (adapted from the operational definition of the International League Against Epilepsy (ILAE) guidelines of 2014 (Fisher et al., 2014)). A person with onchocerciasis-associated epilepsy (OAE) was defined as recently proposed (Colebunders et al., 2017): (1) a PWE according to the ILAE 2014 definition above; (2) age at onset of seizures between 3 and 18 years; (3) no obvious cause of epilepsy in the patient’s history or on physical examination; (4) residence in an onchocerciasis endemic area for at least 3 years; (5) no history of abnormal psychomotor development prior to epilepsy onset. Nodding seizures were defined as reported repetitive, involuntary drops of the head to the chest in a previously normal person, with onset between the ages of 3 and 18 years. ‘Nakalanga’ features (Foger et al., 2017) that were sought during the physical examination by the neurologist included growth retardation or short stature without an obvious cause, delayed or absence of external signs of sexual development, malformations, and mental retardation (Foger et al., 2017).
Study design
This study used a mixed design approach. In Wela and Makoko, the studies listed below were performed.
-
(1)
Confirmation of epilepsy diagnosis: Persons identified with epilepsy by the non-specialist physician during the 2015 survey were traced by community health workers and referred to the health centre, where they were interviewed and examined by a neurologist (DM) to ascertain the diagnosis of epilepsy.
-
(2)
Anti-O. volvulus IgG4 antibody serosurvey among children aged 7–10 years: In both villages, parents with eligible children were invited to participate in the study. Testing for O. volvulus antibodies was performed on the children using the rapid OV16 test (Standard Diagnostics, Inc., Gyeonggi-do, Republic of Korea).
-
(3)
REMO assessment study: In both villages, men >20 years old with a length of stay of at least 10 years in the village were examined for the presence of onchocercal nodules.
-
(4)
Ivermectin coverage survey: In both villages, an evaluation of ivermectin coverage in 2017 for the resident population (those who had been in the village for at least 6 months) was done using a standardized questionnaire, by inquiring about personal ivermectin intake by the household members during the last CDTI programme.
-
(5)
Focus group discussions (FGDs): Six focus group discussions were conducted, three in Wela and three in Makoko. In each village, we had one FGD with youths >18 years old, one with adult females, and one with adult males. Each group consisted of 10 purposively selected participants. Discussions were conducted in the local language on the basis of a pre-tested question guide, were recorded in digital format, and lasted at least 50 min but no more than 1 h. Discussions were geared particularly towards investigating (1) CDTI challenges, (2) epilepsy knowledge and practice, and (3) stigma faced by PWE and their next-of-kin.
Additionally, the studies outlined below were conducted in the rural town of Aketi.
-
(1)
Epilepsy prevalence survey: All households in a part of Aketi along the Itimbiri River were visited by community directed distributors of ivermectin (CDDs) trained to detect suspected cases of epilepsy. If household members of a selected house were not at home, the next house was visited. All household heads and parents of children present in the household were interviewed in their local language. The age and sex of every household member and their individual history of ivermectin intake were recorded. Screening for epilepsy was performed using the five-item questionnaire validated by Diagana et al. (2006). Anyone whose response to at least one of the five questions was positive was considered to be a suspected case of epilepsy and was referred to the health centre where he/she was seen by the neurologist (DM). The person and/or his family member was asked to describe the types of seizures (or to show what happens during a seizure), to report the precipitating circumstances, the duration of seizures, whether they were associated with uncontrollable tongue biting or passing of urine or stool, and whether there were episodes of absence (decreased consciousness of sudden onset and short duration) with or without nodding of the head. The neurologist examined suspected epilepsy cases to establish the final diagnosis of epilepsy. The autonomy of the participants was grossly assessed based on whether the daily life activities of the participant were affected by epilepsy or not. Confusion was defined as a disturbed orientation with regard to time, place, or person.
-
(2)
Epilepsy case–control study: Persons with confirmed epilepsy diagnosed by the neurologist during the Aketi rural town epilepsy survey were included as cases. Controls were persons without epilepsy from neighbouring households who were of similar age and of the same sex as the cases. Cases and controls were all interviewed and examined by the neurologist and tested for the presence of OV16 antibodies using the rapid OV16 test.
Study period
All studies were performed from April 5 to April 17, 2017, except for the ivermectin coverage study which was performed in November 2017, 4 months after the distribution of ivermectin.
Data analysis
In the quantitative studies, summary characteristics were obtained for both categorical (frequencies) and continuous variables (mean or median). Univariate models (logistic regression for categorical variables and linear regression for variables measured on a continuous scale) adjusted by age, sex, and village of origin were used to identify significant differences between the two groups. Matching was done to eliminate confounding effects due to age, sex, and village of origin. While considering the confounding variables (the cluster or strata effect), a multivariable generalized linear mixed model (GLMM) with logit link was used to determine the association between epilepsy and risk factors. The multivariable model was selected using the Akaike information criterion (AIC) (Agresti, 2003, Kutner et al., 2005) and all estimates were obtained with a significance level of 0.05. All p-values were two-sided.
In the qualitative study, FGD transcripts were imported into Atlas.ti 6. Codes were discussed and finalized by a team with expertise in qualitative research methods. The final report was made based on a descriptive model.
Results
Epilepsy in Wela and Makoko
Of the 70 PWE diagnosed in 2015, 43 (61.4%) could be found in 2017. The neurologist (DM) examined 23 (59.0%) of the 39 suspected epilepsy cases from Wela and 20 (64.5%) of the 31 suspected cases from Makoko who were identified during the 2015 epilepsy survey, confirming the diagnosis of epilepsy in all of them. The remaining suspected epilepsy cases identified in 2015 could not be examined because they had either died, relocated, or were unavailable due to farming activities at the time of the survey.
Onchocerciasis transmission in Wela and Makoko
The prevalence of OV16 antibodies in children aged 7–10 years was 80% in Wela and 46% in Makoko (p < 0.0001) (Table 1). The overall OV16 positivity rate was 64.5%.
Table 1.
OV16 test results for children aged 7–10 years in Wela and Makoko.
| Age (years) | Wela |
Makoko |
||
|---|---|---|---|---|
| Number examined | OV16-positive n (%) |
Number examined | OV16-positive n (%) |
|
| 7 | 60 | 46 (77%) | 43 | 19 (44%) |
| 8 | 43 | 33 (77%) | 35 | 18 (51%) |
| 9 | 21 | 18 (86%) | 17 | 6 (35%) |
| 10 | 28 | 25 (89%) | 35 | 17 (49%) |
| All ages | 152 | 122 (80%) | 130 | 60 (46%) |
Amongst the children tested for OV16 antibodies, ivermectin coverage was 80% in Wela compared to 96% in Makoko (p = 0.0001). When considering both villages, 152 (62%) of the 246 children who reported having taken ivermectin at least once were OV16-positive compared to 30 (83%) of the 36 who reported not having taken ivermectin (p = 0.014).
During REMO assessment, 43 (86%) of 50 adult men examined in Wela and 21 (70%) of 30 examined in Makoko presented with onchocercal nodules (p = 0.085). In Wela, 38 (76%) men had more than one nodule (range 2–11 nodules) and in Makoko, 12 (40%) men had more than one nodule (range 2–6 nodules).
Ivermectin coverage in Makoko and Wela
Ivermectin use in 2017 was reported by 64.5% of the inhabitants of Makoko and by 51.8% in Wela (p = 0.0002). Ivermectin coverage was significantly lower in the 5–10 years age group compared to the older age groups, and in Wela compared to Makoko (Table 2). However, in both villages combined, only three (10%) of 30 children aged 5 years and 21 (52.5%) of 41 children aged 6 years had taken ivermectin during the last distribution 4 months earlier.
Table 2.
Ivermectin intake during the 2017 CDTI by sex, age group, and village.
| Makoko | Wela |
p-Value (Makoko vs. Wela) |
Total (Makoko and Wela) |
p-Value | ||
|---|---|---|---|---|---|---|
| Sex-specific ivermectin coverage, n (%) | Male | 90/140 (64.3%) | 152/305 (49.8%) | 0.003 | 242/445 (54.3%) | Male vs. female, p = 0.18 |
| Female | 95/147 (64.6%) | 158/294 (53.7%) | 0.014 | 253/441 (57.4%) | ||
| Total | 185/287 (64.5%) | 310/599 (51.8%) | 0.0002 | 495/886 (55.9%) | ||
| Age-specific ivermectin coverage, n (%) | 5–10 years | 37/65 (56.9%) | 72/126 (57.1%) | 0.4880 | 109/191 (57.1%) | 0.0001a |
| 11–20 years | 46/55 (83.6%) | 75/102 (73.5%) | 0.0749 | 121/157 (77.1%) | ||
| 21–30 years | 29/33 (87.9%) | 48/77 (62.3%) | 0.0040 | 77/110 (70.0%) | ||
| 31–40 years | 28/32 (87.5%) | 51/75 (68.0%) | 0.0178 | 79/107 (73.8%) | ||
| >40 years | 45/53 (84.9%) | 64/100 (64.0%) | 0.0030 | 109/153 (71.2%) | ||
| Total | 185/238 (77.7%) | 310/480 (64.6%) | 0.0002 | 495/718 (68.9%) | ||
CDTI, community-directed treatment with ivermectin.
Chi-square test, corresponding p-value across age groups.
Of the 374 residents who did not receive ivermectin in 2017, the reasons were age below 5 years (51.6%), refusal to take the drug (27.0%), absence during the distribution (14.7%), pregnancy and breastfeeding (4.0%), and severe illness (2.7%). Epilepsy was not considered a contraindication to ivermectin use. Children between the ages of 5 and 10 years were less likely to have taken ivermectin than older children and adults.
Qualitative study in Makoko and Wela
During the FGDs, most respondents knew about ivermectin as well as its role in treating filarial diseases and in preventing blindness. One participant mentioned that it helps to reduce epilepsy. However, about two-thirds of participants reported either never taking ivermectin, having stopped using it, or taking it irregularly. The main reason for refusal to take ivermectin was fear of adverse effects preventing them from working on the farm. Certain participants complained about the number of tablets to be taken, which they considered to be too many and therefore could result in severe adverse effects and even death. Another reason was “being away from home, to work at the farm, during the distribution of ivermectin”.
Epilepsy was well known by the community; however, for most community members, epilepsy was caused by wizards and bad spirits, and therefore traditional healers were consulted. There was also a general belief that epilepsy was infectious. For example, it was mentioned that “dishes, beds, toilets and clothes should not be shared with a person with epilepsy” and that “children with epilepsy should not go to school because they could contaminate other children”. Concerning anti-epileptic treatment, they reported that phenobarbital was known to calm the seizures but had to be bought in the pharmacy, which most families could not afford. Many participants also complained of having been duped financially by traditional healers who had promised to cure them.
Aketi rural town epilepsy prevalence
During the household survey in Aketi rural town, 350 households were visited; 125 (5.7%) of the 2180 people screened were suspected to have epilepsy based on the five questions. In 20 (5.7%) of the 350 households there were at least two persons suspected to have epilepsy. Most suspected PWE were in the 20–29 years age group (Table 3).
Table 3.
Aketi age-specific prevalence rates of epilepsy.
| Age (years) | Population | Number of persons suspected to have epilepsy | Probable epilepsy prevalence (%) |
|---|---|---|---|
| >40 | 366 | 8 | 2.2 |
| 30–39 | 232 | 16 | 6.9 |
| 20–29 | 334 | 40 | 12.0 |
| 10–19 | 546 | 50 | 9.2 |
| <10 | 702 | 11 | 1.6 |
| All ages | 2180 | 125 | 5.7 |
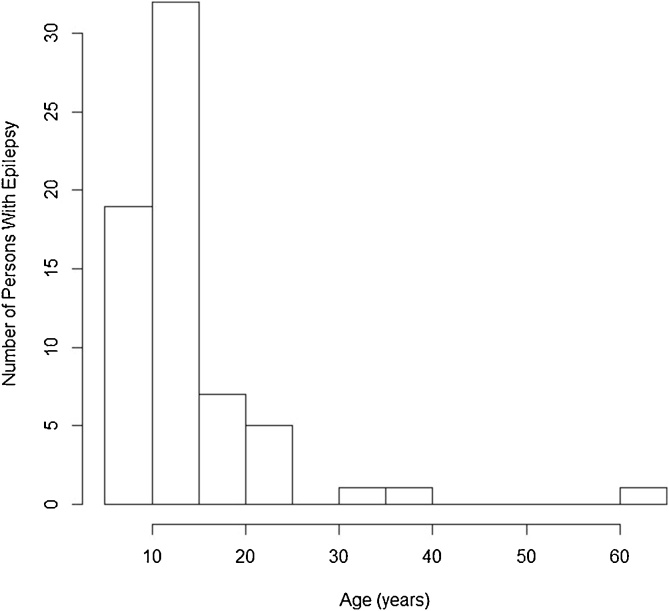
Of the 125 persons suspected to have epilepsy, only 65 (52%) were seen by the neurologist (DM). The diagnosis of epilepsy was confirmed in all of them. The median age of PWE was 19 years (range 14–27 years). The first seizures appeared most frequently in the 10–15 years age group (Figure 3).
Figure 3.
Numbers of persons with epilepsy with onset per age group.
In 52 (83.9%) of the 62 PWE for whom information was available, seizures had started between the ages of 3 and 18 years; 47/62 (75.8%) PWE met all the diagnostic criteria for OAE; five did not meet the criteria because they had lived in the village for less than 3 years.
Thirty-eight (58.5%) PWE experienced tonic-clonic seizures; nodding seizures were reported in nine (13.8%). Furthermore, 22 (33.8%) PWE presented with one or more features of Nakalanga: short stature (n = 17; 26.2%), delayed/absent secondary sexual characteristics (n = 10; 15.4%), and deformities of either the spine or thorax (n = 9; 13.8%). Eight (15.3%) PWE were confused. Autonomy during daily life activities was altered in 22 (33.8%) PWE.
Epilepsy treatment
Twenty-seven (41.5%) PWE had never taken any anti-epileptic treatment. Phenobarbital was the only anti-epileptic drug used. Only six (9.2%) PWE reported taking anti-epileptic drugs regularly, 26 (40%) took anti-epileptic drugs only for a short period following seizures, and six (9.2%) had stopped taking treatment. Thirty-three (50.8%) PWE used traditional medicine. Forty (61.5%) PWE reported at least one seizure daily.
Case–control study
The 65 confirmed PWE were matched for sex and age with one control each, thus there were 65 case–control pairs. The mean age was 19 years (standard deviation 10.8 years) and median age was 23.4 years, and 47.7% were female pairs. Cases had a lower body weight, reported more ivermectin intake, and more often presented O. volvulus antibodies compared to controls (Table 4).
Table 4.
Characteristics of cases and controls in the Aketi case–control study—univariate analysis.
| Cases n = 65 |
Controls n = 65 |
p-Value | |
|---|---|---|---|
| Female | 31 (47.7%) | 31 (47.7%) | 1 |
| Median age (years) | 23.5 | 23.5 | 1 |
| Median length of stay in Aketi (years) | 17 | 17 | 1 |
| Complicated delivery | 2 (3.1%) | 1 (1.5%) | 0.6 |
| Febrile convulsions | 2 (3.1%) | 0 | 0.15 |
| Serious illness during infancy | 6 (9.2%) | 10 (15.4%) | 0.3 |
| Mean length of stay in the village (years) | 18.12 | 17.62 | 0.8 |
| Mean body weight (kg) | 37.9 | 52.3 | <0.0001 |
| Confusion | 8 (12.3%) | 2 (3.1%) | 0.05 |
| Ivermectin intake last year | 49 (75.3%) | 37 (56.9%) | 0.03 |
| OV16 positivity | 48 (73.8%) | 37 (56.9%) | 0.04 |
Upon fitting the multivariate model, only weight and ivermectin intake differed significantly between the cases and controls (Table 5).
Table 5.
Parameter estimates from the generalized linear mixed model.
| Parameter | Estimate | Standard error | p-Value |
|---|---|---|---|
| Intercept | −6.6324 | 1.8352 | 0.0003 |
| Length of stay in Aketi | −0.0321 | 0.0187 | 0.0872 |
| Body weight | 0.1321 | 0.0233 | <0.0001 |
| Ivermectin intake last year | 1.1351 | 0.5341 | 0.0336 |
| Complicated delivery | 1.4827 | 1.4283 | 0.2992 |
| Serious illness during infancy | −0.8418 | 0.6039 | 0.1634 |
| OV16 positivity | 0.5749 | 0.4916 | 0.2423 |
| Strata effect | 0.0457 | 0.0187 | 0.0401 |
Discussion
A high prevalence of epilepsy in the villages of Wela (6.8%) and Makoko (8.4%) and the town of Aketi (5.7%) was observed despite 14 years of CDTI. This high prevalence is similar to previous findings in other onchocerciasis endemic areas (Pion et al., 2009, Boussinesq et al., 2002, Prischich et al., 2008, Gerrits, 1983, Levick et al., 2017) and higher than the median epilepsy prevalence of 1.42% observed in Sub-Saharan Africa (Ba-Diop et al., 2014). The fact that approximately three-quarters of the PWE met the criteria for OAE and that some of them presented nodding seizures and Nakalanga features, support the presence of OAE in the Aketi health zone.
An additional argument supporting OAE in the Aketi health zone is the high prevalence of OV16 antibodies in children below the age of 10 years, indicating a high ongoing O. volvulus transmission rate, which is likely to be caused by the location of the villages very close to blackfly breeding sites and a high blackfly biting rate.
The low ivermectin coverage observed is a clear indication that the CDTI programmes in Wela and Makoko have failed. The REMO results showing >70% of examined men with nodules in both villages further suggests very poor onchocerciasis control during the past 14 years. The poor coverage is partly explained by the lack of an education campaign about the benefits of the CDTI.
To reduce the onchocerciasis transmission rate in the Aketi health zone, bi-annual CDTI complemented with proper sensitization needs to be considered. Indeed, bi-annual CDTI has been shown to improve treatment uptake, and proper sensitization will curb the high rates of refusal of ivermectin due to the fear of side effects (Frempong et al., 2016). Better timing of distribution campaigns to coincide with periods of least farm activity would also contribute to increasing the therapeutic coverage of ivermectin, as revealed by the qualitative survey. Education programmes involving radio broadcasts and film projection in the local language could help increase the proportion of consenting villagers. The reason why children 5–10 years of age were less likely to have taken ivermectin than older children is explained by the low number of 5–6-year-old children who had taken ivermectin – 34.2% compared with 70.2% of the 7–10-year-olds. Five years is the age at which children are eligible to take ivermectin (Burnham, 1998). It is possible that some of the 5-year-olds at the time of the survey were not yet 5 years old, 4 months earlier, at the time of ivermectin distribution. Another explanation is that ivermectin distribution is not done based on age but on height, by measuring children with a stick to determine the number of tablets that need to be given. Therefore, it is possible that certain 5–6-year-old children who were too small did not receive the ivermectin. The ivermectin intake of children 7–10 years old who were tested for the presence of OV16 antibodies was very high (80%). However, a social desirability response bias needs to be considered.
The case–control study, using a multivariable model, revealed that the PWE had lower body weights and were more likely to have taken ivermectin than controls. We have noticed before that PWE in onchocerciasis endemic regions tend to take more ivermectin than controls, probably because ivermectin reduces onchocerciasis-associated itching, which is frequently present in persons with OAE (Levick et al., 2017, Colebunders et al., 2016b). On the other hand, we observed previously that before the development of epilepsy there was less ivermectin use, suggesting a protective effect of ivermectin in the development of OAE (Levick et al., 2017, Colebunders et al., 2016b).
Children who had taken ivermectin were less likely to have OV16 antibodies compared to those who were ivermectin-naïve (p = 0.014). This is perhaps because ivermectin use may protect against acquiring a sufficiently high microfilariae density to induce OV16 antibodies.
The management of epilepsy is far from optimal in the Aketi health zone, as only 9.2% of PWE had regular anti-epileptic drug treatment. Consequently, 61.5% of PWE reported daily seizures. The qualitative survey revealed that the main reason for poor epilepsy treatment was the lack of financial resources to purchase the drugs, similar to what was found in another onchocerciasis endemic area in Cameroon (Dongmo et al., 2004). Poverty and lack of knowledge result in the families going to consult traditional healers, thus increasing costs, delaying medical care, and increasing the risk of PWE being refractory to anti-epileptic drugs (Nzuzi et al., 2016). Proper sensitization of the population is needed to raise awareness of the benefits of early epilepsy treatment. Furthermore, a decentralized system of epilepsy care with continuous availability of cheap/free drugs would be recommended in such resource-limited settings.
This study has several limitations. First, during his short stay in Aketi, the neurologist was only able to see a limited number of persons suspected to have epilepsy to confirm the diagnosis. We do not have information about the persons identified during the 2015 survey who could not be traced. Some of them may have died, moved to another village, or were not interested in attending for a visit because they felt healthy. Therefore, the PWE seen by the neurologist cannot be considered representative of all PWE in the area. However, all suspected cases in the rural town of Aketi who were available to consult with the neurologist were indeed confirmed as PWE, suggesting a satisfactory positive predictive value for the five-question screening tool. Laboratory and radiological investigations were not performed to identify the exact cause of epilepsy. Although it was possible to exclude perinatal brain damage as an important cause of epilepsy by properly taking the PWE birth history, it was, however, difficult to determine the attributable fraction of, for instance, epilepsy caused by neurocysticercosis. The onset of epilepsy due to neurocysticercosis may also occur between the ages of 11 and 20 years, similar to OAE (Monteiro et al., 1995). However, the mean age at seizure onset in neurocysticercosis is usually after 25 years (Monteiro et al., 1995, Medina et al., 1990).
In conclusion, this study showed a very high prevalence of OAE in the Aketi health zone. An awareness programme about the importance of proper onchocerciasis control needs to be implemented to increase ivermectin coverage, together with a bi-annual CDTI programme. In addition, there is an urgent need for a decentralized system to treat all PWE in the health zone. This needs to include uninterrupted access to affordable anti-epileptic treatment and will require training of the local healthcare workers on how to treat PWE. Such a project is currently being planned by the Ministry of Health of Bas-Uélé in collaboration with a non-governmental organization, VZW Aketi.
Acknowledgements
We thank the Fons Peeters and the VZW Aketi for logistical assistance, and the populations of Wela, Makoko, and Aketi for their collaboration in the study. R. Colebunders received funding from the European Research Council (ERC) (grant number 671055, project title NSETHIO). The funder had no role in the study design, in the collection, analysis, and interpretation of the data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Acknowledgments
Data statement
The study data can be obtained from the corresponding author upon reasonable request.
Ethics statement
The study was approved by the Ethics Review Board of the School of Public Health of the University of Kinshasa and the Ethics Review Board of the University of Antwerp. Written informed consent was obtained from all adults and from the parents and guardians of children who participated in the quantitative and qualitative studies. In addition, assent was obtained from children above the age of 8 years. For illiterate individuals, consent was obtained by finger printing.
Conflict of interest
None of the authors has a conflict of interest.
Author contributions
RC and FT wrote the study protocol; DM, FT, IA, AR, and AL performed the field studies and collected the data; RC and DM wrote the first draft; JNC and AH contributed to the study design and writing of the paper; CNN, SC, BK and SM analysed the data; all co-authors critically reviewed the paper.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Contributor Information
Deby Mukendi, Email: debymukendi@yahoo.fr.
Floribert Tepage, Email: floritepage@yahoo.fr.
Innocent Akonda, Email: boseluc@gmail.com.
Joseph Nelson Fodjo Siewe, Email: JosephNelson.SieweFodjo@uantwerpen.be.
Anke Rotsaert, Email: anke_rotsaert@hotmail.com.
Carl Nwana Ndibmun, Email: carlnwana.ndibmun@student.uhasselt.be.
Anne Laudisoit, Email: laudisoit@ecohealthalliance.org.
Simon Couvreur, Email: simonjkcouvreur@gmail.com.
Blandine Kabutako, Email: drblandine.kabutako@gmail.com.
Sonia Menon, Email: sonia.menon@uantwerpen.be.
An Hotterbeekx, Email: an.hotterbeekx@uantwerpen.be.
Robert Colebunders, Email: robert.colebunders@uantwerpen.be.
References
- Agresti A. 2nd ed. John Wiley and Sons Ltd.; New Jersey: 2003. Categorical data analysis. p. 202. [Google Scholar]
- Ba-Diop A., Marin B., Druet-Cabanac M., Ngoungou E.B., Newton C.R., Preux P.M. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13:1029–1044. doi: 10.1016/S1474-4422(14)70114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussinesq M., Pion S.D., Demanga N., Kamgno J. Relationship between onchocerciasis and epilepsy: a matched case-control study in the Mbam Valley, Republic of Cameroon. Trans R Soc Trop Med Hyg. 2002;96:537–541. doi: 10.1016/s0035-9203(02)90433-5. [DOI] [PubMed] [Google Scholar]
- Burnham G. Onchocerciasis. Lancet. 1998;351:1341–1346. doi: 10.1016/S0140-6736(97)12450-3. [DOI] [PubMed] [Google Scholar]
- Casis S. El Sindrome Epileptico y sus reaciones con Onchocercosis. Boletin de Salubridad e Higiene. 1938:1. [Google Scholar]
- Chesnais C.B., Nana-Djeunga H.C., Njamnshi A.K. The temporal relationship between onchocerciasis and epilepsy: a population-based cohort study. Lancet Infect Dis. 2018 doi: 10.1016/S1473-3099(18)30425-0. pii: S1473-3099(18)30425-0. [DOI] [PubMed] [Google Scholar]
- Colebunders R., Titulaer M.J. Nodding syndrome: preventable and treatable. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aam8532. [DOI] [PubMed] [Google Scholar]
- Colebunders R., Hendy A., Mokili J.L. Nodding syndrome and epilepsy in onchocerciasis endemic regions: comparing preliminary observations from South Sudan and the Democratic Republic of the Congo with data from Uganda. BMC Res Notes. 2016;9:182. doi: 10.1186/s13104-016-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R., Mandro M., Mokili J.L. Risk factors for epilepsy in Bas-Uele Province, Democratic Republic of the Congo: a case-control study. Int J Infect Dis. 2016;49:1–8. doi: 10.1016/j.ijid.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R., Tepage F., Rood E. Prevalence of river epilepsy in the Orientale Province in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R., Nelson Siewe F.J., Hotterbeekx A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol. 2017 doi: 10.1016/j.pt.2017.11.009. pii: S1471-4922(17)30283-0. [DOI] [PubMed] [Google Scholar]
- Colebunders R., Nelson Siewe F.J., Hotterbeekx A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol. 2018;34:208–216. doi: 10.1016/j.pt.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Colebunders R., Mandro M., Njamnshi A.K. Report of the first international workshop on onchocerciasis-associated epilepsy. Infect Dis Poverty. 2018;7:23. doi: 10.1186/s40249-018-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Democratic Republic of Congo Population List 2014. Available from: https://archive.is/20130210151812/http://world-gazetteer.com/wg.php?x=&men=gcis&lng=en&des=gamelan&srt=npan&col=abcdefghinoq&msz=1500&geo=-46. [cited 2018 June 1]
- Diagana M., Preux P.M., Tuillas M., Ould Hamady A., Druet-Cabanac M. Depistage de l’epilepsie en zones tropicales: validation d’un questionnaire en Mauritanie. Bull Soc Pathol Exot. 2006;99:103–107. [PubMed] [Google Scholar]
- Dongmo L., Druet-Cabanac M., Moyou S.R. Cysticercosis and epilepsy: a case-control study in Mbam Valley, Cameroon. Bull Soc Pathol Exot. 2004;97:105–108. [PubMed] [Google Scholar]
- Fisher R.S., Acevedo C., Arzimanoglou A. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Foger K., Gora-Stahlberg G., Sejvar J. Nakalanga syndrome: clinical characteristics, potential causes, and its relationship with recently described nodding syndrome. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frempong K.K., Walker M., Cheke R.A. Does increasing treatment frequency address suboptimal responses to ivermectin for the control and elimination of river blindness. Clin Infect Dis. 2016;62:1338–1347. doi: 10.1093/cid/ciw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits C. A West African epilepsy focus. Lancet. 1983;1:358. doi: 10.1016/s0140-6736(83)91663-x. [DOI] [PubMed] [Google Scholar]
- Idro R., Opar B., Wamala J. Is nodding syndrome an Onchocerca volvulus-induced neuroinflammatory disorder? Uganda’s story of research in understanding the disease. Int J Infect Dis. 2016;45:112–117. doi: 10.1016/j.ijid.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Johnson T.P., Tyagi R., Lee P.R. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aaf6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C., Kipp W., Asaba G. The prevalence of epilepsy follows the distribution of onchocerciasis in a west Ugandan focus. Bull World Health Organ. 1996;74:361–367. [PMC free article] [PubMed] [Google Scholar]
- Kutner H.M., NJC, Neter J., Li W., editors. Applied Linear models. 5th ed. Mc Hraw. Hill International Edition; 2005. p. 2005. [Google Scholar]
- Levick B., Laudisoit A., Tepage F. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M.T., Rosas E., Rubio-Donnadieu F., Sotelo J. Neurocysticercosis as the main cause of late-onset epilepsy in Mexico. Arch Intern Med. 1990;150:325–327. [PubMed] [Google Scholar]
- Monteiro L., Nunes B., Mendonca D., Lopes J. Spectrum of epilepsy in neurocysticercosis: a long-term follow-up of 143 patients. Acta Neurol Scand. 1995;92:33–40. doi: 10.1111/j.1600-0404.1995.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Nzuzi T. Matonda Ma, Lelo G.M., Mpembi Nkosi M. Are the children with epilepsy treated traditionally a disadvantaged group? A pilot study. Pan Afr Med J. 2016:23. [Google Scholar]
- Ovuga E., Kipp W., Mungherera M., Kasoro S. Epilepsy and retarded growth in a hyperendemic focus of onchocerciasis in rural western Uganda. East Afr Med J. 1992;69:554–556. [PubMed] [Google Scholar]
- Pion S.D., Kaiser C., Boutros-Toni F. Epilepsy in onchocerciasis endemic areas: systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3:e461. doi: 10.1371/journal.pntd.0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prischich F., De Rinaldis M., Bruno F. High prevalence of epilepsy in a village in the Littoral Province of Cameroon. Epilepsy Res. 2008;82:200–210. doi: 10.1016/j.eplepsyres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Rwiza H.T., Kilonzo G.P., Haule J. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian district: a community-based study. Epilepsia. 1992;33:1051–1056. doi: 10.1111/j.1528-1157.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]