Abstract
Most autoimmune disease are driven by a dysfunction in T and B cells, but B cells are still an interesting area of research, perturbations in their development are implicated in autoimmune diseases. B cell differentiating factor (BCDF) plays a part in the differentiation of B cells. The aim was To assess the levels of BCDF, IgM and IgG in SLE patients and whether they have any peculiarity in the clinical context of SLE. Thirty six patients with SLE and 24 healthy volunteers as control were enrolled in the study. BCDF was measured using Sandwich ELISA, total human IgM and IgG were measured by calorimetric methods. The mean concentrations of BCDF and IgM were significantly higher in patients with SLE as compared with controls (P < 0.001 and P < 0.0001 respectively). No significant difference was observed as regard IgG. We observed positive correlation between BCDF and IgM (r = 0.281, P = 0.03), and between IgG and IgM, duration of the disease (r = 0.468, P = 0.004, r = 0.337, P = 0.008 respectively). Moreover we observed lower IgM level in patients with discoid lesion (P = 0.009) and lower IgG level in those with hematologic manifestations (P = 0.02). ROC analysis revealed area under curve (AUC) 0.861 for BCDF and 0.902 for IgM, they can delineate SLE from controls at a cut-off value of 98.5 pg/ml, and 18 mg/dl IgM respectively.
Conclusion
BCDF and IgM are increased in SLE patients and are promissing diagnostic markers for SLE.
Keywords: BCDF, IgM, IgG, SLE
1. Introduction
A high proportion of patients display autoimmune features [1]. Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by different clinical manifestations [2]. Molecules responsible for tissue damage and affected sites are antibodies that are directed against a large numbers of self-antigens. These pathogenic autoantibodies are produced from autoreactive B cells [3]. The disease is driven by a loss of immune tolerance and abnormal B- and T- cell function [4]. Jackson et al., stated that B cells have been recognized for their importance for lupus pathogenesis because of their production of pathogenic antinuclear antibodies (ANA) and that dysregulation in B cell signaling was implicated in the initiation of systemic autoimmunity [5]. B cell may proliferate without secreting immunoglobulin (Ig) [6] its differentiation depends on the presence of B cell differentiation factor [6], [7], [8]. The role of immunoglobulin isotypes has attracted attention in many human autoimmune diseases [9] where many isotypes of autoantibodies can be detected. The IgG anti-dsDNA isotype is largely studied in SLE patients [10] because of their pathogenic role but, the behavior of the IgM anti-dsDNA isotype has been a matter of polemic [11]. Human self-reactive natural IgM antibodies are common in health and disease and can play fundamental roles in tissue homeostasis and the maintenance of immune equilibrium. High levels of IgM and IgG autoantibodies was observed to be associated with many autoimmune diseases [12]. Recently, we found increase serum BCDF in patients with rheumatoid arthritis [13]. Because of the clinical heterogeneity of SLE and the lack of pathogenomonic tests we aim to assess the levels of BCDF, IgM and IgG in SLE patients and whether they have any peculiarity in the clinical context of SLE.
2. Subjects and methods
2.1. 1Patients
Thirty six patients with SLE who fulfilled the American Rheumatism Association (ARA) criteria for SLE [14] followed up at rheumatology outpatient clinic of the center of excellence at National Research Centre Cairo- Egypt, participated in the study.
They were 32 (88.9%) females, and 4 (11.1%) males, mean age was 32.2 ± 10.4 years with duration ranged from one to 20 years with mean: 8.92 ± 5.7 years. Twenty-four apparently healthy individuals, 21 (87.5%) females, and 3 (12.5%) males, mean age: 39.3 ± 1.6 years were used as controls. Patients and controls were subjected to detailed medical history and assessment of BCDF, IgM and IgG. We excluded any patients with autoimmune diseases other than SLE, concurrent infection, or malignancy. Lupus disease activity was assessed by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [15].
The study was approved by the ethical committee of National Research Centre according to the ethical standards established by the Declaration of Helsinki and an informed consent was taken from each participant in the study.
2.2. Laboratory tests
2.2.1. Blood sampling
Five millimeter of blood were withdrawn from all subjects and centrifuged at 3,000 rpm for 10 min. Sera were isolated and stored at −80 until the determination of laboratory investigations.
2.2.2. Biochemical assays
Serum levels of BCDF, was measured using a commercial enzyme linked immunosorbent assay ELISA kit, produced by Glory Science Co., Ltd. 2400 Veterans Blvd. Suite 16–101, Del Rio, TX 78840, USA. www.glorybioscience.com. Quantitative determination of total human IgM and IgG were measured by colorimetric kinetic method produced by Chronolab systems, S.L., C/Aragon, 271,6 planta,08007 Barcelona, Spain www. All these analysis were performed at NRC, medical physiology department.
2.3. Statistical analysis
The study analysis was carried out using statistical package for social science (spss) version 16 IBM, Chicago IL, U.S.A statistical software The statistical significance was set at P < 0.05 and the significant difference between the two groups was determined by using independent “t” test expressed as mean ± SE, Pearson correlation was used to analyze the correlation between different variables. For the evaluation of the diagnostic performance of BCDF, IgM and IgG, we used a receiver operating characteristic curve (ROC). The curve was constructed to show their sensitivity and specificity at different decision cut-off levels. In this type of curve, the x-axis represents the false-positive rate (1-specificity). The y-axis represents the true- positive rate (sensitivity). The best cut-off is the nearest point to the upper left corner. Area under the curve was constructed to determine the overall performance of the test.
3. Results
3.1. Demographic and clinical characteristics of SLE patients
Thirty-six SLE patients participated in the study their disease duration ranged from one to 20 years with mean: 8.92 ± 5.7 years, SLEDAI ranged from 4 to 41, mean: 20.58 ± 1.61.
Clinical data of the SLE patients are presented in Table 1.
Table 1.
Clinical data of SLE patients.
| Manifestations of SLE | n (%) |
|---|---|
| Oral ulcers | 19 (52.8%) |
| Malar rash | 21 (58.3%) |
| Photosensitivity | 17 (47.2%) |
| Discoid rash | 3 (8.8%) |
| Maculo-Papular rash | 9 (26.5%) |
| Alopecia | 21 (58.3%) |
| Arthritis | 28 (77.8%) |
| Vasculitis | 14 (38.9%) |
| Serositis | 8 (22.2%) |
| Renal (nephrotic nephritic) | 16 (44.4%) |
| Neurologic (psychosis, depression, seizures, headache) | 11 (30.6%) |
| Haematology (anemia, lymphopenia) | 7 (19.4%) |
| Total number of patients n = 36 | 36 (100%) |
Mean values of BCDF and IgM were significantly higher in SLE patients compared to controls P < 0.001 and P < 0.0001 respectively. However IgG did not show significant difference between SLE patients and controls (Table 2).
Table 2.
BCDF, IgM and IgG in SLE patients and controls.
| Parameters | SLE patients (n = 36) | Controls (n = 24) | P value |
|---|---|---|---|
| BCDF, pg/ml | 201.9 ± 25.8 | 105.1 ± 9.2 | p < 0.001* |
| IgM, mg/dl | 60.9 ± 6.6 | 19.8 ± 2.6 | p < 0.0001** |
| IgG, mg/dl | 135.5 ± 11.4 | 132.5 ± 4.9 | p = 0.5 |
p significant.
p highly significant, BCDF: B cell differentiating factor.
3.2. Relation of BCDF, IgM and IgG to clinical and laboratory data of SLE patients
Pearson correlation test was used to assess the correlations between BCDF, IgM IgG, age, duration of the disease and SLEDAI. There was significant positive correlation beween BCDF and IgM (r = 0.281, p = 0.03) (Fig. 1) and between IgG and duration of the disease, IgM (r = 0.468, p = 0.004; r = 0.337, p = 0.008, respectively) (Figs. 2, 3.). No significant correlation was found with SLEDAI (see Figs. 4, 5).
Fig. 1.
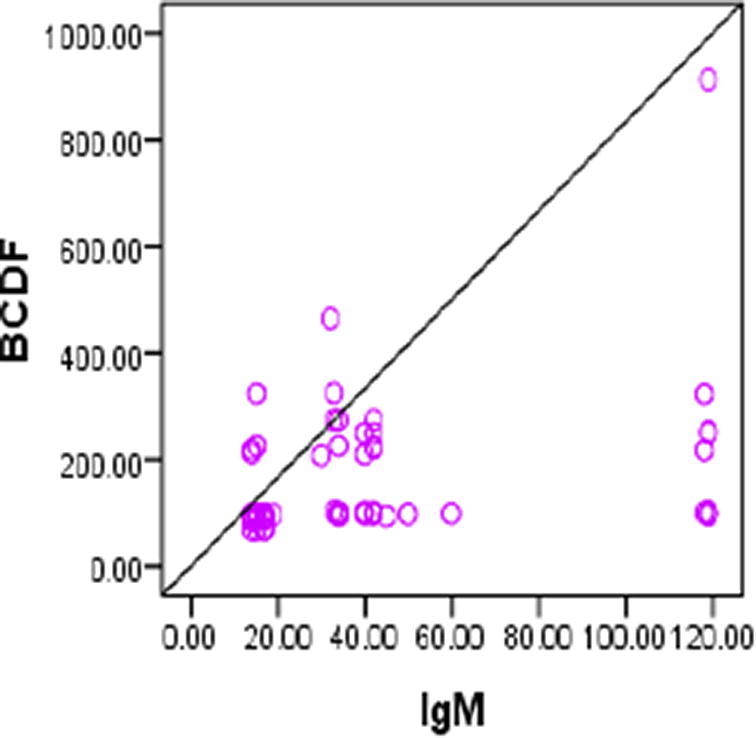
Positive correlation between. BCDF and IgM (r = 0.281,p = 0.03).
Fig. 2.
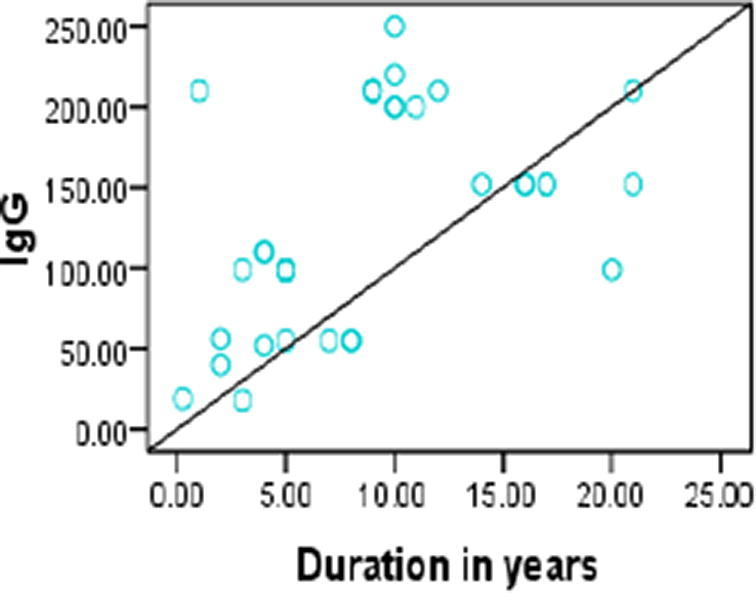
Positive Correlation between IgG and duration of disease (r = 0.468, p = 0.004).
Fig. 3.
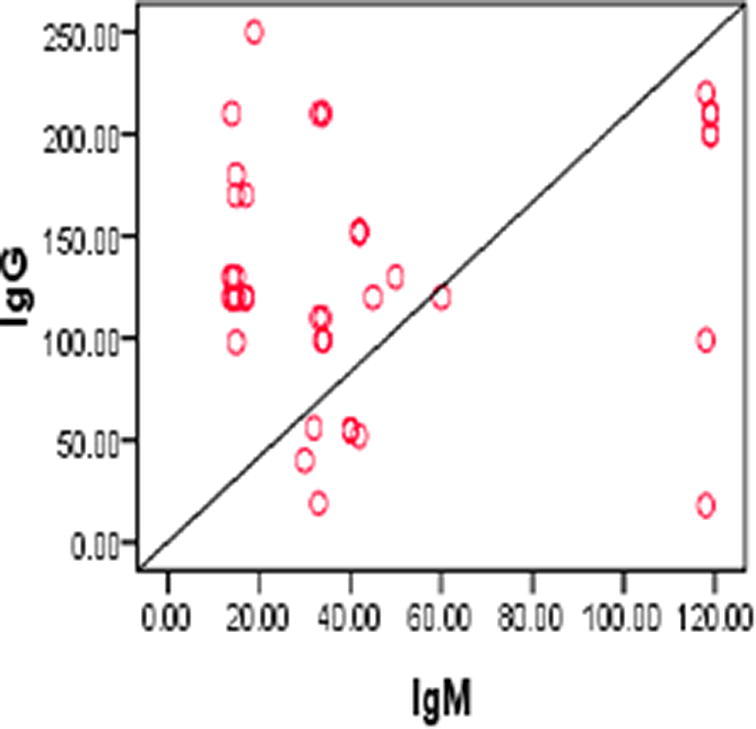
Positive Correlation correlation between IgG and IgM. (r = 0.337, p = 0.008).
Fig. 4.
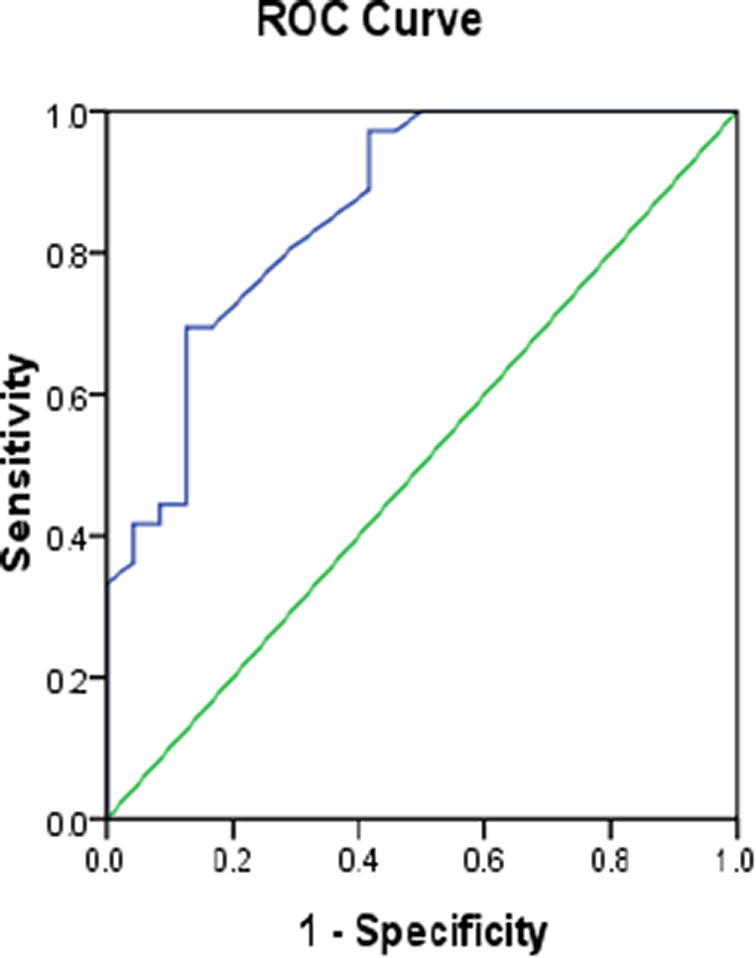
ROC curve of BCDF in SLE patients.
Fig. 5.
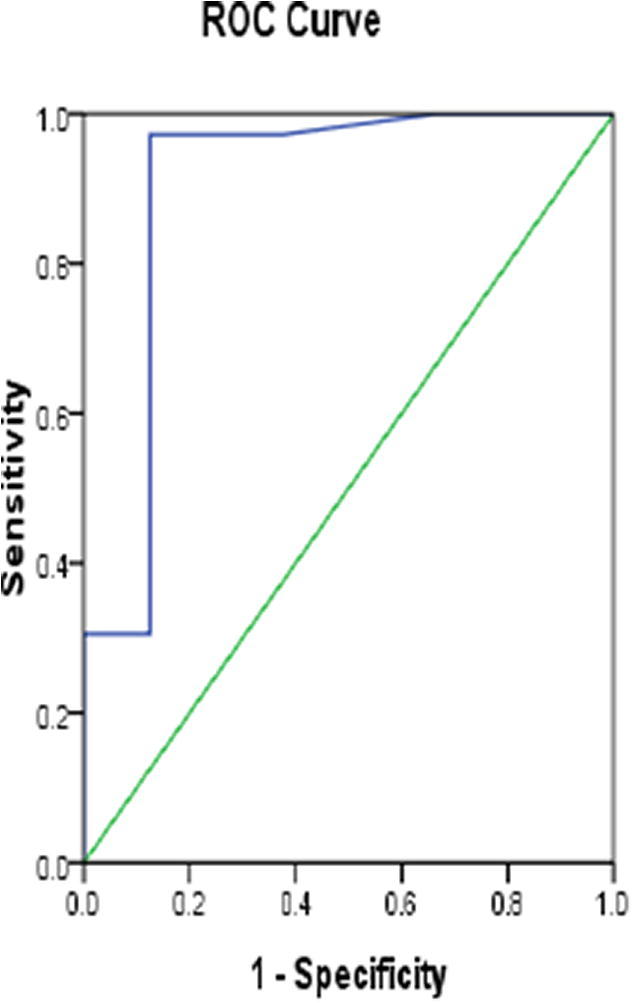
ROC curve of IgM in SLE patients.
We investigated the relation between BCDF, IgM, IgG and any of the clinical manifestation, we found that IgM was significantly lower in SLE patients with discoid lupus compared to SLE patients without discoid lesion. Moreover, IgG was significantly lower in SLE patients with hematological manifestations compared to those without hematological manifestations (Table 3, Table 4).
Table 3.
Mean BCDF level in different clinical manifestations of SLE patients.
| Manifestations of SLE | Mean ± SE of BCDF (pg/ml) |
P value | |
|---|---|---|---|
| SLE patients with manifestation | SLE patients without manifestation | ||
| Renal | 182.6 ± 22.7 | 217.4 ± 42.9 | 0.48 |
| Neurologic | 149.5 ± 20.7 | 225 ± 35.2 | 0.07 |
| Oral | 167 ± 19.8 | 240.3 ± 49 | 0.18 |
| Arthritis | 208.4 ± 31.9 | 179.2 ± 32.1 | 0.52 |
| Vasculitis | 162 ± 20.2 | 227 ± 39.6 | 0.15 |
| Malar rash | 167.0 ± 18.6 | 250.2 ± 54.8 | 0.17 |
| Photosens | 151.6 ± 17.6 | 241.6 ± 44.8 | 0.09 |
| Discoid rash | 191 ± 46.9 | 209.6 ± 29.3 | 0.75 |
| Maculopapular rash | 187.4 ± 29.7 | 215.4 ± 35.2 | 0.54 |
| Cutaneous | 171 ± 26.3 | 215 ± 35.2 | 0.32 |
| Alopecia | 162.3 ± 17.1 | 257 ± 54.9 | 0.11 |
| Serositis | 260 ± 98 | 185 ± 18.6 | 0.47 |
| Haematology | 155 ± 27 | 213 ± 31 | 0.18 |
SLE: systemic lupus erythematosus,
* p significant
Table 4.
Mean IgM and IgG levels in different clinical manifestations of SLE patients.
| Manifestations of SLE | Mean ± SE of IgM (mg/dl) |
P value | Mean ± SE of IgG (mg/dl) |
P value | ||
|---|---|---|---|---|---|---|
| SLE patients with manifestation | SLE patients without manifestation | SLE patients with manifestation | SLE patients without manifestation | |||
| Renal | 68.2 ± 10.1 | 55 ± 8.6 | 0.32 | 119.7 ± 18.4 | 148 ± 14 | 0.22 |
| Neurologic | 60.0 ± 11.3 | 61.0 ± 8.1 | 0.92 | 127.0 ± 18.9 | 138.0 ± 14.3 | 0.64 |
| Oral | 61.2 ± 9.3 | 60.5 ± 9.4 | 0.95 | 144.0 ± 15.0 | 125.0 ± 17.5 | 0.43 |
| Arthritis | 65.5 ± 7.7 | 44.8 ± 10.6 | 0.13 | 141 ± 13.2 | 115 ± 21.7 | 0.31 |
| Vasculitis | 55.6 ± 9.1 | 64.2 ± 9.0 | 0.50 | 114 ± 15.6 | 148 ± 15.3 | 0.13 |
| Malar rash | 63.0 ± 8.93 | 58.0 ± 9.84 | 0.70 | 142 ± 14.5 | 126 ± 18.5 | 0.50 |
| Photosens | 62.2 ± 9.1 | 59.7 ± 9.5 | 0.85 | 146.8 ± 15.3 | 125.4 ± 16.7 | 0.35 |
| Discoid rash | 38.6 ± 2.4 | 59.3 ± 7.02 | 0.009* | 102 ± 28 | 137.5 ± 12.6 | 0.33 |
| Mp rash | 44.2 ± 9.6 | 62.3 ± 7.98 | 0.16 | 117 ± 23.4 | 140 ± 13.7 | 0.41 |
| Cutaneous | 57.8 ± 11.93 | 62.3 ± 7.98 | 0.75 | 124 ± 20.8 | 140 ± 13.7 | 0.53 |
| Alopecia | 50.7 ± 7.53 | 75.1 ± 10.9 | 0.08 | 144 ± 13.4 | 123 ± 20 | 0.40 |
| Serositis | 66.6 ± 15.4 | 59.2 ± 7.30 | 0.67 | 157 ± 28.9 | 129.0 ± 12.1 | 0.39 |
| Haematology | 60 ± 15.1 | 61.1 ± 7.39 | 0.94 | 76.1 ± 24 | 149 ± 11.5 | 0.02* |
SLE: systemic lupus erythematosus.
P significant.
3.3. Value of BCDF, IgM as diagnostic markers in SLE patients
We performed ROC curve analysis to evaluate the diagnostic value of BCDF and IgM. We found that BCDF can discriminate SLE patients from healthy volunteers at cut-off value of 98.5 pg/ml with sensitivity 80.6%, specificity 70.8% and area under curve (AUC) 0.861 (p < 0.001, 95%CI 0.765–0.956), and IgM can discriminate SLE patients from healthy volunteers at cut-off value of 18 mg/dl with sensitivity 97.2%, specificity 87.5% and AUC of 0.902(p < 0.001, 95%CI 0.806–0.998).
4. Discussion
B cell hyperactivity have several roles and represents a central feature in the development of SLE [16] and its pathogenesis [17] via production of pathogenic immunoglobulin [18]. To the best of our knowledge this is the first study assessing the level of BCDF in SLE patients and its relation to IgM and IgG and clinical manifestations of SLE. Our findings revealed that levels of B cell differentiating factor (BCDF) were higher in SLE patients compared to healthy controls and no difference in IgG between patients and controls. This may suggest that BCDF differentiate to produce more IgM than IgG. A recent study released by Shaikh and colleagues., revealed that SLE is caused by a loss of immune tolerance and abnormal B and T cell function [4]. Hirano and colleagues proposed that BCDF induces the differentiation of B cells into Ig-secreting cells [6]. This was explained by Kikutani, who suggested that BCDF induces an increase in the level of mRNA specific for secretory heavy chains, and then induces the final maturation of B cells into immunoglobulin-secreting cells [8]. Later Huang and colleagues speculated that B cell may express receptors for BCDF, in addition they observed that BCDF stimulates a rapid rise in intracellular calcium which is an important event in the terminal differentiation of B cell mediated by BCDF [19]. This let us to deduce that the increase of BCDF explains why B cell represents a central feature of SLE.
SLE patients are characterized by the presence of high levels of circulating IgM and IgG autoantibodies [7], [20] this was supported by a recent study postulated that the serum of SLE patients induces the normal B cell to secrete autoantibodies [21]. In our study there was a significant increase of IgM in SLE patients in agreement with the findings of Jost and colleagues [22], moreover we found a direct correlation between BCDF and IgM which let us deduce that the increase of BCDF in SLE patients enhances the maturation of B cell and hence the secretion of immunoglobulin IgM. On the contrary to IgM, we did not find a significant difference in IgG levels between patients and controls, which could be attributed to the importance of IgM in the containment of disease, where IgM autoantibodies could decrease the IgG autoantibody production by autoreactive B- cells [22], [23], [24]. Another study suggested that natural IgM may have protective properties in autoimmunity [25], this was explained by Boes and colleagues [26] who suggested that secreted IgM may lessen the severity of autoimmune pathology associated with IgG autoantibodies, they observed that mice without the ability to produce IgM have a predisposition for development of pathogenic IgG autoantibodies specific for double-stranded DNA (dsDNA) and histones and suffered from more severe lupus-like autoimmunity and glomerulonephritis. Another study revealed that IgM can induce specific anti-inflammatory signaling pathway that depends on the phosphatase mitogen activated protein kinase (MAPK) in dendritic cells derived from bone marrow [27] and block the pro-inflammatory influences of lupus- associated RNA or DNA IgG immune complexes in SLE patients [12]. IgM is a potent activator of complement that promotes the removal of autoantigens and reduces the chance of autoreactive B cells to be activated [28]. Deficiency in secreted IgM has the same effect on IgG antibody responses to both foreign antigens and self-antigens as deficiency in complement components [29] indicating that secreted IgM may affect the development of IgG autoantibodies and autoimmune disease through the same pathways as complement components, these IgM antibody responses are highly inducible and their up-regulation can be a powerful means for the host to survive in a setting of chronic inflammation [25]. IgM protects against autoimmunity by inducing B cell tolerance when there is too much autoreactive IgM, autoantibody response may be dominant, such as in the advanced stage of SLE [30]. In addition there was a direct correlation between IgG and IgM because BCDF induced the maturation of B cell, and increased the immunoglobulin levels [19]. B cell may proliferate without secreting Ig but in the presence of BCDF biosynthesis of immunoglobulin will increase [19]. We found a direct correlation between IgG and the duration of disease supporting the early finding of Saiki [31], as IgG antibodies appear with disease progression [32] and the levels of IgG autoantibodies to dsDNA in serum are known to fluctuate with disease activity in SLE and they often increase prior to the flaring of disease activity [33]. Although, most of the SLE patients in our study have high disease activity the IgG was not elevated which may be due to the higher IgM that has negative effect on the production of IgG [24].
When we tested for the presence of any association between BCDF, IgM, IgG and any of the clinical manifestation of SLE, we found lower IgM in SLE patients with discoid lupus compared to other SLE patients and lower IgG in SLE patients with hematologic manifestation compared to other SLE patients
Discoid lupus erythematosus (DLE) occurs in 25% of SLE patients [34], it has been postulated that IgM autoantibodies could decrease IgG autoantibody production by autoreactive B cells, and act as competitive inhibitors with their IgG counterparts by binding to the same circulating nuclear antigens [23], [24] where these mechanisms may be important in preventing the systemic spread in DLE patients [22].
Hematologic manifestations are identified in 10–83% of SLE. In the present study, we found lower IgG in SLE patients with hematologic manifestation [35]. In contrast to our study, Liu and colleagues [36] found that IgG fractions in the sera of active SLE patients acquire a suppressive effect on haematopoietic progenitor cells. Thus, IgG autoantibodies to primitive haematopoietic progenitor cells are demonstrated to be present in the sera of a significant proportion of active SLE patients with anaemia and leukocytopenia and to suppress the progenitor cell growth this could be attributed to the low number of patients with hematologic manifestations which make the statistical comparison unfair.
We assessed the diagnostic performance of BCDF and IgM and we found that they are promising diagnostic markers in SLE as the ROC curve analysis of BCDFand IgM showed AUC 0.861 and 0.902 respectively. BCDF at cut-off 98.5 pg/ml is can discriminate SLE patients from healthy individuals with sensitivity 80.6%, specificity 70.8% and also IgM can discriminate SLE patients from healthy volunteers at cut-off value of 18 mg/dl with sensitivity 97.2%, specificity 87.5% .
5. Conclusion
We observed increased levels of BCDF, IgM in SLE patients in comparison to healthy controls. The increased levels of BCDF may be the cause of the expansion of autoreactive B cell clones leading to the increase of IgM. This might reopen a field of interest in BCDF and its clinical association with SLE. BCDF and total human IgM are promising diagnostic markers for SLE.
Funding
There was no funding.
Disclosure of potential conflicts of interest
Author one: Hala Zaki Raslan, Author two: Hiba Sibaii, Author three:Salwa Refat El-Zayat, Author four: Hagar Hassan, Author five: Mahitab El- Kassaby, declare that they have no conflict of interest.
Research involving human participants (Ethical approval)
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional (National Research Centre) committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgment
We would like to express our gratitude to the National Research Center for providing us the outpatient clinic as a facility and the laboratory equipments.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Yong P.F., Thaventhiran J.E., Grimbacher B. “A rose is a rose is a rose”, but CVID is Not CVID common variable immune deficiency (CVID), what do we know in 2011? Adv Immunol. 2011;111:47–107. doi: 10.1016/B978-0-12-385991-4.00002-7. [DOI] [PubMed] [Google Scholar]
- 2.Ng R., Bernatsky S., Rahme E. Disease characterization of systemic lupus erythematosus (SLE) patients in Quebec. Lupus. 2017;26:1005–1011. doi: 10.1177/0961203317692435. [DOI] [PubMed] [Google Scholar]
- 3.Choi S.C., Morel L. B cell contribution of the CD4+ T cell inflammatory phenotypes in systemic lupus erythematosus. Autoimmunity. 2017;37:41. doi: 10.1080/08916934.2017.1280028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaikh M.F., Jordan N., D'Cruz D.P. Systemic lupus erythematosus. Clin Med. 2017;17:78–83. doi: 10.7861/clinmedicine.17-1-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson S.W., Kolhatkar N.S., Rawlings D.J. B cells take the front seat: dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr Opin Immunol. 2015;33:70–77. doi: 10.1016/j.coi.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K, Nakajima K, Pyun KH, Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci USA;82 (1985): 5490–5494. [DOI] [PMC free article] [PubMed]
- 7.Yoshizaki K., Nakagawa T., Fukunaga K., Tseng L.T., Yamamura Y., Kishimoto T. Isolation and characterization of B cell differentiation factor (BCDF) secreted from a human B lymphoblastoid cell line. J Immunol. 1984;132:2948–2954. [PubMed] [Google Scholar]
- 8.Kikutani H., Taga T., Akira S., Kishi H., Miki Y., Saiki O., Yamamura Y., Kishimoto T. Effect of B cell differentiation factor (BCDF) on biosynthesis and secretion of immunoglobulin molecules in human B cell lines. J Immunol. 1985;134:990–995. [PubMed] [Google Scholar]
- 9.Rey D.C., Bogdanos D.P., Leung P.S., Anaya J.M. ME Gershwin IgM predominance in autoimmune disease: genetics and gender. Autoimmun Rev. 2012;11:A404–A412. doi: 10.1016/j.autrev.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Sinico RA, Bollin B, Sabadini E, DiToma L, Radice A. The use of laboratory tests in diagnosis and monitoring of systemic lupus erythematosus. J Nephrol;15 (2002):S20–S7. [PubMed]
- 11.Keiserman B., Ronchetti M.R., Monticielo O.A., Keiserman M.W., Staub H.L. Concomitance of IgM and IgG anti-dsDNA antibodies does not appear to associate to active lupus nephritis. Open Rheumatol J. 2013;7:101–104. doi: 10.2174/1874312901307010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vas J., Gronwall C., Marshak-Rothstein A., Silverman G.J. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64:3388–3398. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokhles M.A., Raslan H.Z., Sibaii H., El-Zayat S.R., El-Kassaby M., Hassan H. Studying of B-cell differentiation and macrophage colony-stimulating factors as new players in the diagnosis of rheumatoid arthritis. J Arab Soc Med Res. 2017;12:79–85. [Google Scholar]
- 14.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classi®cation of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 15.Bombardier C., Gladman D.D., Urowitz M.B., Caron D., Chang C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 16.Dörner T., Giesecke C., Lipsky P.E. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nashi E, Wang YH, and Diamond B. The Role Of B Cells in Lupus Pathogenesis Int J Biochem Cell Biol; 42 (2010):543–550. [DOI] [PMC free article] [PubMed]
- 18.Rowley M.J., Nandakumar K.S., Holmdahl R. The role of collagen antibodies in mediating arthritis. Mod Rheumatol. 2008;18:429–441. doi: 10.1007/s10165-008-0080-x. [DOI] [PubMed] [Google Scholar]
- 19.Huang R., Cioffi J., Kimberly R., Edberg J., Mayer L. B cell differentiation factor-induced human B cell maturation: stimulation of intracellular calcium release. Cell Immunol. 1995;164:227–233. doi: 10.1006/cimm.1995.1165. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen C.T., Østergaard O., Stener L., Iversen L.V., Truedsson L., Gullstrand B., Jacobsen S., Heegaard N.H.H. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012;64:1227–1236. doi: 10.1002/art.34381. [DOI] [PubMed] [Google Scholar]
- 21.Zheng LT, Zhao LD, Zhao C, Zhao Y, ZhAng X, Zheng FC, Zeng XF, Tang FL, You X, Zazhi ZN. Effect of serum interleukin 21 on B cell secretory capacity and apoptosis in patients with systemic lupus erythematosus. China Medical Abstracts (Internal Medicine); 56 (2017): 116–120. [DOI] [PubMed]
- 22.Jost SA, Tseng LC, Loderick AM, Rebecca V, Song Z, Yancey B Kim, and Chong Benjamin F. IgG, IgM, and IgA Antinuclear Antibodies in Discoid and Systemic Lupus Erythematosus Patients. The Scientific World Journal 2014: (2014) Article ID 171028, 7 pages. [DOI] [PMC free article] [PubMed]
- 23.Witte T. IgM antibodies against dsDNA in SLE. Clinical Reviews in Allergy and Immunology; 34(2008): 3, 345–347. [DOI] [PubMed]
- 24.Villalta D, Bizzaro N, Bassi N, et al. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease. PLoS ONE; 8(2013) Article ID e71458. [DOI] [PMC free article] [PubMed]
- 25.Grönwall C., Silverman G.J. Natural IgM: beneficial autoantibodies for the control of inflammatory and autoimmune disease. J Clin Immunol. 2014;34:S12–S21. doi: 10.1007/s10875-014-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak- Rothstein A, Chen J. Accelerated development of IgGautoanti- bodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA; 97(2000):1184–9. [DOI] [PMC free article] [PubMed]
- 27.Gronwall C., Akhter E., Oh C., Burlingame R.W., Petri M., Silverman G.J. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Son M., Santiago-Schwarz F., Al-Abed Y., Diamond B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc Natl Acad Sci USA. 2012;109:E3160–E3167. doi: 10.1073/pnas.1212753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 30.Vaishnaw A.K., McNally J.D., Elkon K.B. Apoptosis in the rheumatic diseases. Arthritis Rheum. 1997;40:1917–1927. doi: 10.1002/art.1780401102. [DOI] [PubMed] [Google Scholar]
- 31.Saiki O., Saeki Y., Tanaka T., Doi S., Hara H., Negoro T., Igarashi S., Kishimoto S. Development of selective igm deficiency in systemic lupus erythematosus patients with disease of long duration. Arthritis Rheum. 1987;30:1289–1292. doi: 10.1002/art.1780301112. [DOI] [PubMed] [Google Scholar]
- 32.Papp K., Végh P., Tchorbanov A., Vassilev T., Erdei A., Prechl J. Progression of lupus-like disease drives the appearance of complement-activating IgG antibodies in MRL/lpr mice. Rheumatology. 2010;49:2273–2280. doi: 10.1093/rheumatology/keq278. [DOI] [PubMed] [Google Scholar]
- 33.Kenderov A., Minkova V., Mihailova D., Giltiay N., Kyurkchiev S. Lupus-specific kidney deposits of HSP90 are associated with altered IgG idiotypic interactions of anti-HSP90 autoantibodies. Clin Exp Immunol. 2002;129:169–176. doi: 10.1046/j.1365-2249.2002.01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pistiner M., Wallace D.J., Nessim S. Lupus erythematosus in the 1980s: a survey of 570 patients. Semin Arthritis Rheum. 1980;21(1991):55–64. doi: 10.1016/0049-0172(91)90057-7. [DOI] [PubMed] [Google Scholar]
- 35.Fayyaz A., Igoe A., Kurien B.T. Haematological manifestations of lupus. Lupus. Science Med. 2015;2:e000078. doi: 10.1136/lupus-2014-000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H., Ozaki K., Matsuzaki Y., Abe M., Kosaka M., Saito S. Suppression of haematopoiesis by IgG autoantibodies from patients with systemic lupus erytbematosus (SLE) Clin Exp Immunol. 1995;100:480–485. doi: 10.1111/j.1365-2249.1995.tb03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


