Abstract
Mass propagation of date palm through indirect somatic embryogenesis or organogenesis has attracted the interest of commercial producers. But, this technique still faces some problems that hindered the production of date palm plantlets in vitro. Tissue browning is one of the serious problems that reduce callus growth and shoot regeneration. So the objective of the present study is to investigate the effect of cold pretreatment on callus growth, shoot regeneration, and polyphenol oxidase (PPO) activity during the callus culture. Results showed that a high survival rate of callus cultures (100%) were obtained when cultures were incubated in low temperature (cold treatment) for 45 and 75 days. On the other hand, total amount on phenolic compounds was also reduced to 0.47 and 0.53 mg GAE/g after same period of incubation (45 and 75 days respectively) at low temperature. In additional, our results showed that the highest frequency of shoot formation (66.67 and 73.34, %) and the highest shoot numbers (7.8 and 8.6 shoots/100 mg) were obtained from callus treated with low temperature for 45 and 75 days, respectively.
Keywords: Cold pretreatment, Callus cultures, Phenolic compounds, Shoot regeneration, Polyphenol oxidase (PPO), SDS-PAGE
1. Introduction
Tissue blackening or browning is a very serious problem faces the date palm tissue. Phenolic compounds were produced when explants or tissue are excised from mother offshoot or during subcultures in vitro. However, phototoxic compounds produced as results of phenolic compound oxidation caused media and tissue blackening. The oxidized products bind to proteins thereby inhibiting enzyme activity and leading to lethal decline of explants [2]. In order to reduce the phenoloxidase activity and prevent tissue blackening, which are often widespread in plant tissue culture system. Research workers use different treatments like, appending the activated charcoal, polyvinyl pyroldine (PVP) or adding the chelating agent like ethylenediaminetetraacetic acid (EDTA) to the media. Store the cultures in the darkness for several days is also another treatment to minimize the phenoloxidase activity [3].
Low temperature is one of the important factors that affect the plant growth and morphogenesis in vitro and also it's help to decrease the tissue blackening and phenoloxidase activity and to increase explants survival rate [19]. In date palm, low temperature pretreatment for short period (3 days) with anti oxidant α-Tochopherol (Vitamin E) have been used to minimized the explant browning as well as gave rise to the highest callus production [22]. Also, Baiea [8] found that storing explants of date palm in refrigerator at 5 °C for 10 days before culturing succeeded in reducing necrosis and browning and increasing development. However, the induction of callus influenced by explants genotype, media composition, culture conditions, and cold pretreatment [14], [4], [5].
This investigation aims to determine the optimum cold pretreatment period for callus induction and shoot regeneration. Also, this study investigated the effect of low temperature treatment on phenolic compounds and antioxidant enzyme activity during callus incubation.
2. Materials and methods
The present study achieved in The Tissue Culture Laboratory of Date Palm Research Centre, Basra University, Basrah, Iraq, from the period 2014 to 2015.
2.1. Initiation stage
Five years old offshoots of superior female date palm (Phoenix dactylifera L.) cv. Barhee were selected and separated from healthy mother plants grown in Abul-kseeb Orchards. The shoot tips were excised from the offshoot heart and trimmed to the size about 0.8–1 cm in width and 1–2 cm in length and using them as starting material for present work. Explants surface sterilization were conducted by using 20% Clorox (2–5% sodium hypochlorite) mixed with two drops of tween 20 per100 ml for 20 min. The apical shoots were then divided into four equal segments and each segment was cultured individually on the initiation medium in the jar. There were sixteen replicates (quarters of terminal buds) at the beginning of the experiment. Initiation medium of [21] (MS) minerals salt supplemented with: myo-Inositol (100 mg l−1), glutamine (200 mg l−1), thiamine-hydrochloride (1 mg l−1), nicotinic acid (1 mg l−1), pyridoxine-hydrochloride (1 mg l−1), sucrose (30 g l−1), mg l−1 naphthalene acetic acid (NAA), 3 mg l−1 dimethyl amino-purine (2iP), activated charcoal (2.0 g l−1) and agar (7 g l−1).
After inoculation of the explants to the medium, cultures were incubated in growth chamber at 27 ± 2 °C under dark condition until callus was visible by the naked eyes. Callus was transferred to the modified MS medium enriched with the same components mentioned above but with different growth regulators combination (2 mg l−1 2iP, 6 mg l−1 NAA) suitable for callus growth. In addition, activated charcoal was reduced to 0.5 g l−1.
Medium pH was adjusted to 5.8 with 0.1 N NaOH or HCl, before the addition of agar. Then it was autoclaved at 121 °C and 1.04 kg/cm2 for 20 min.
2.2. First experiment
2.2.1. Effect of cold pretreatment on callus growth
In this experiment callus bloomed on growth media were divided into two groups, the first group was incubated at low temperature (5 °C) in a refrigerator under full darkness at different periods (45, 75 and 100 days). The second group was maintained in the dark under growth chamber conditions (27 ± 2 °C). Percentage of survival cultures were recorded for both groups. In this experiment, the degree of callus growth and browning in relation to treatments and incubation periods were scored according to Abul-Soad [1] as follows in Table 1:
Table 1.
The degree growth and browning percentage at callus that used in this study.
| Scores | Browning degree | Growth degree |
|---|---|---|
| − | Negative effect (No browning) | No growth |
| + | Poor effect | Poor growth |
| ++ | Moderate effect | Moderate growth |
| +++ | Sever effect | High growth |
2.3. Second experiment
2.3.1. Effect of cold pretreatment on indirect organogenesis
Callus cultures (callus grown in normal conditions in the growth chamber at 27 ± 2 °C) were transferred to modified MS medium fortified with the same composition mentioned above, but with different growth regulators combination (3 mg l−1 2iP + 1 mg l−1 NAA) suitable for shoot formation. Callus cultures were divided into two groups. First was kept under the dark condition at low temperature (5 °C) in a refrigerator. The second was incubated in a growth chamber at (27 ± 2 °C) and under the dark conditions. Both groups were maintained for different periods as follows: 45, 75 and 100 days. In order to study the effect of cold pretreatment on indirect shoot formation, callus cultures of the both groups were transferred at the end of incubation period mentioned before, to the controlled growth chamber at 27 ± 2 °C with a 16 h photoperiod under light intensity 30 µmol m−2 s−1. Data with regarding to the percentage of shoot regeneration and shoot number per jar were recorded after 12 weeks. About 100 mg callus was cultured on 50 ml medium in one jar. For each experiment a minimu4m of 15 replicates were used.
2.3.2. Determination of total phenolic content (TP)
Phenolics compound were extracted according to method described by Singleton and Rossi [30]. Gallic acid was used as a reference standard; Folin–Ciocalteu reagent was prepared (pre-diluted 10-fold with distilled water) and left at room temperature for 5 min, followed by addition of sodium bicarbonate (1.2 ml: 7.5%, w/v) to the mixture. After standing for 60 min at room temperature, absorbance was measured at 765 nm. The results recorded as gallic acid equivalent (mg GAE/g).
2.3.3. Assay of polyphenol oxidase (PPO) activity
Polyphenol oxidase (PPO) (EC 1.14.18.1) activity was measured according to Benjamin and Montgomery [7]. One unit of PPO activity was defined as the amount of enzyme that caused an increase in absorbance of 0.001 per min at 420 nm using spectrophotometer (UV–Vis spectrophotometer UV 9100B, LabTech). The enzyme activity was expressed as unit/mg protein.
2.3.4. Extraction of protein and gel electrophoresis
Proteins were extracted by homogenizing the 0.333 g of frozen dried callus to 1 ml of extraction buffer [0.2 M, tris hydroxymethyl aminomethane (Tris)+ 0.001 M ethylene diamine tetra acetic acid [(Na2 + EDTA) + 12% glycerol + 0.01 M dithiothreitol (DTT) + 0.05 mM phenyl methyl sulfonyl fluoride (PMSF)] by using the mortar and pestle. Samples then were centrifuged at 15,000g for 15 min, supernatant was used for determination of total protein content. Cracking puffer (0.125 M, Tris.HCl pH 6.8 + 4% SDS + 20%, glycerol + 10% β-mercaptoethanol + 0.01% bromophenol blue) was added to equal volume of protein sample. Protein samples were denaturized by boiling in the water bath at 90 °C for 3 min. Protein electrophoresis was performed in a discontinuous SDS polyacrylamide gel according to method described by Laemmli [20].
2.4. Statistical analysis
All the data were statistically analyzed by two-way analysis of variance (ANOVA). The least significant difference (LSD) method was used to test the difference between treatments and p ≤ 0.05 was considered statistically significant. A chi-square test was used to test the difference between treatments (%) in Table 1, Table 2. Statistical analysis was performed with SPSS packet software [6]. All treatments were replicated fifteen times.
Table 2.
Influence of various incubation temperatures on percentages of survival, browning percentage, browning degree and callus weight of date palm cv. Barhee. during different periods of callus incubation, means within each column followed by the same letter are not significantly different at p < 0.05. Browning intensity − = no browning response (white), + = slightly brown, ++ = moderate brown, +++ = high brown. Callus growth was scored as 0, no growth; +, slight growth; + +, moderate growth; and +++, maximum growth (n = 15).
| Incubation conditions | Incubation period | Callus Survival (%) | percent of callus browning (%) | Browning degree | Callus growth |
|---|---|---|---|---|---|
| Normal temperature (27 ± 2 °C) | 45 | 100 a | 0.0 d | − | + |
| 75 | 73.34 c | 26.67 b | + | ++ | |
| 100 | 53.34 d | 46.67 a | +++ | + | |
| Low temperature storage (5 °C) | 45 | 100 a | 0.0 d | − | ++ |
| 75 | 100 a | 0.0 d | − | +++ | |
| 100 | 86.67 b | 13.34 c | + | ++ | |
3. Results and discussion
3.1. Influence of cold pretreatment on explants culture
Results revealed that the high survival rate of cultures (100 and 86.67%), low browning intensity (− and +) and high callus growth degree (+++) were obtained when cultures were incubated at low temperature for 45, 75 and 100 days (Table 2). Whereas, incubation of cultures for 75 and 100 days under regular temperature at growth chamber increased the intensity of browning medium 26.67%, 46.67% respectively, and decreased the survival rate of explant cultures (73.34% and 53.34% respectively). On the other hand, callus growth recorded a moderate growth degree (++) and poor (+) when cultures incubated under the same conditions (27 ± 2 °C) for 75 and 100 days respectively (Fig. 1). These findings are in harmony with results of Mustafa et al. [22] who reported that pretreated of date palm shoot tip explants for 3 days at low temperature (5 °C) without antioxidant before culturing reduced the browning and increased callus production. However, findings also revealed that a prolonged incubation of callus (100 days) under cold conditions caused a poor response in callus growth (Table 2). This results in agreement with Rukmini et al. [25] who found that the prolonged cold pretreatment had an inhibitory effect.
Fig. 1.
Effect of various incubation temperatures 27 ± 2 °C (1) and 5 °C (2) on callus browning of date palm cv. 'Barhee after (a) 45 days (b) 75 days.
Anyway, incubation of the cultures at low temperature is one of techniques used for germplasm storage of different plant species. And under such condition, cells growth reduced by slow down many biochemical activities like carbohydrate translocation and protein synthesis. Moreover, cell division and elongation were retarded due to cell membrane thickening [12], [28].
3.2. Effect of cold pretreatment on callus development and shoot regeneration
Study also investigates the relation between callus cold pretreatment and shoot regeneration. Data (Table 3) showed that shoot induction was greatly affected by the cold pretreatment and the duration time of incubation. Where, the highest shoot induction percentage and numbers were obtained (73.34% and 66.67), (7.8 and 8.6 shoots/100 mg callus) when callus cultures incubated under cold conditions 5 °C for 45 and 75 days respectively (Table 3 and Fig. 2). In contrary less shoot percentage response and shoot numbers/100 mg were recorded in callus cultures incubated at normal temperatures for 100 days (20% and 3.2 shoots respectively) (Table 3). Some of the research workers support the view of using the cold pretreatment as promontory effect for cultures in vitro [24], [25], [22]. For date palm Mustafa et al. [22] found that cold pretreatment increase the rate of tissue swelling and callus induction, while Tang et al. [29] found that cold pretreatment remarkably improved embryos formation in callus of balsam pear (Momordica charantia L.). Moreover, in the earlier study done by Hiraoka and Kodama [16] was found that incubation of the callus culture of Bupleurum falcatum for one year under cold conditions retained their ability to organogenesis, while callus cultures grown under the normal temperature at 25 °C lost their ability to organogenesis during the first six months of subculture. However, the positive response obtained in this study reflex the influence of cold pretreatment on explant survival rate through preventing the phenols oxidation and quinine formation.
Table 3.
Effect of various incubation temperatures on a response percentage of callus for shoot formation and number of shoots/100 mg callus after 12 weeks from culturing for date palm cv. Barhee during different periods of incubation. Means of four replicates followed by standard error, ** - Means within each column followed by the same letter are not significantly different at p < 0.05. (n = 15).
| Incubation conditions | Incubation period | Response of callus (100 mg weight) for shoot regeneration % | Number of shoots/100 mg callus |
|---|---|---|---|
| Normal temperature (27 ± 2 °C) | 45 | 46.47 bc | 5.2 cd |
| 75 | 40.0 c | 4.8 d | |
| 100 | 20.0 d | 3.2 e | |
| Low temperature storage (5 °C) | 45 | 66.67 a | 7.8 b |
| 75 | 73.34 a | 8.6 a | |
| 100 | 53.34 b | 5.6 c | |
Fig. 2.
Shoot induction from callus cultured in MS medium supplemented with 3.0 mg l−1 2iP + 1.0 mg l−1 NAA after different periods from incubation of callus temperatures 27 ± 2 °C and 5 °C (a) 45 days, (b) 75 days, (c) 100 days respectively.
Present study also proved that the cold treatment used in all experimental investigations is the most effective for both callus induction as well as shoot regeneration, these results are in line with findings of Kaushal et al. [19] who stated that the cold treatment is very successful on all genotypes for callus formation and plant regeneration. But, a prolonged incubation of callus cultures (100 days) at low temperature reduced the ability of callus to organogenesis (Table 3).
This observations are supported by earlier report [9] who found that rice immature inflorescence lost their induction ability when incubated for long time under cold condition.
3.3. Effect of various incubation temperatures on the phenolic compounds
Fig. 3 shows that the incubation of callus cultures for 45 and 75 days under normal temperature caused accumulation of phenolic compounds (0.77 and 1 mg GAE/g) respectively, as compared with low accumulation of phenolic compounds when cultures were stored at 5 °C (0.47 and 0.53 mg GAE/g) for the same periods. Results also showed that regardless of temperature treatments (cold or normal) the accumulation of phenolic compounds was increased (0.81 mg and 1.43 GAE/g respectively) when callus cultures were incubated for a long period (100 days). Based on the observations presented previously (Table 2 and Fig. 3), data showed that the callus cultures of date palm cv. Barhee incubated under normal conditions at (27 °C) may face a deleterious effect due to oxidizing the phenolic compounds. In contrary, a cold pretreatment of callus cultures was helpful to delay the tissue browning and phenolic compound accumulation in tissues. These findings are in line with Mustafa et al. [22] who reported that the cold pretreatment for date palm tissues reduced the phenolic oxidation enzyme.
Fig. 3.
Total Phenol (mg GAE/g) content of callus of date palm cv. Barhee during different periods of callus incubation at 27 ± 2 °C and 5 °C.
Date palm tissues are very susceptible to browning and elimination or minimization of this process is an essential prerequisite to successful culture establishment. Mustafa et al. [22] and Shehata et al. [27] proved in their studies the correlation between the phenolic production and browning intensity in date palm cultured in vitro. So, in this study we were able to minimize date palm tissue browning by incubated the culture at 5 °C for 45 and 75 days.
3.4. Effect of various incubation temperatures and periods incubation on polyphenol oxidase activity (PPO)
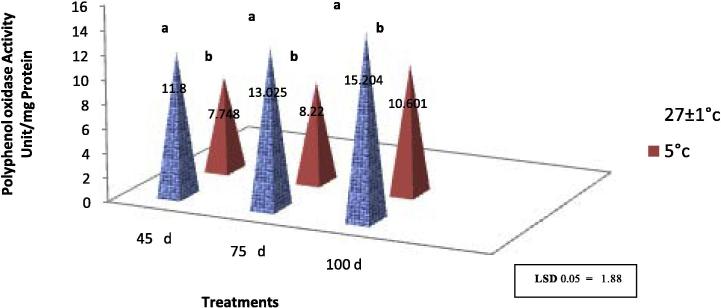
Results Fig. 4 shows that the higher PPO activity (11.8, 13.025 and 15.204 unit/mg) was obtained when callus was incubated under the normal temperature at various period of incubation (45, 75 and 100 days) respectively, which was significantly different compared with the other treatments. Whereas, lower PPO activity was obtained on callus tissues stored at 5 °C. These findings are in line with Dubravina et al. [11], Jin et al. [18] who reported that PPO activity was delayed when cultures were incubated under cold conditions. However, regardless of the incubation conditions (normal or cold temperature), the PPO activity was increased to 10.601 and 15.204 unit/mg when callus was incubated for a long period (100 days) (Fig. 4). PPO enzymes activity may be a main factor in the browning reaction. Due to the fact that phenolic compounds contribute to antioxidant capacity. The oxidation of phenolic compounds into quinones is mainly catalyzed by polyphenol oxidase. The brown colour that develops in callus cultures of diverse plant cultures is due to the formation of quinones which are inhibitory to plant cellular growth. Accumulation of quinones to a level which is detrimental for in vitro growth is common in some very important plants of economic importance [10], It is very important to reduce quinones in culture medium. Numerous studies suggest that PPO is responsible for enzymatic browning of plants [26]. Enzymatic browning in plants is mainly related to the oxidation of phenolic compounds to unstable o-quinines which are highly electrophilic molecules and their polymerization can lead to the appearance of brown, black pigmentation [17].
Fig. 4.
Effect of various incubation temperatures and incubation duration on polyphenol oxidase activity (PPO) in cv. Barhee callus in vitro.
3.5. SDS-PAGE protein pattern
The SDS-PAGE gel of micro propagated callus is shown in Fig. 5. Analysis of the gel showed that, incubation of date palm callus at 5 °C for 45 and 75 days shows three major bands stimulated due to the over-production of the polypeptides of 160, 93 and 47 KD, (Fig. 5 Lane 5 and 6). All the other polypeptides present in the control were still synthesized under 5 °C except that of 38 and 22 KD. The same results were obtained on callus incubated at 5 °C for 100 days (lane 7) where, a high intensity of high molecular weight polypeptides was appeared, while low molecular weight polypeptides disappeared. Robertson et al. [23] reported that low temperature treatment inhibited the synthesis of a 22 KD protein. But, Hassan [15] found that introducing the multiplied shoots to low temperature stimulated the synthesis of a new polypeptide of 47 KD. Eris et al. [13] showed that biochemical changes that have been associated with cold-acclimation include expression of specific proteins and the appearance of new isozyme.
Fig. 5.
SDS-PAGE of protein extracted from callus of date palm cv. Barhee incubated at various temperatures during different periods. (M = marker protein) Lane1 = (control), lanes (2 & 3) = callus incubated at normal temperatures conditions for 75 and 100 days respectively, lanes (4,5 & 6) callus incubated at 5 °C for 45,75 and 100 days respectively.
4. Conclusion
In view of the above, it can be concluded that cold pretreatment and duration of callus incubation for 45 and 75 days increased the ability of callus to growth, increased the survival rate of callus, shoot regeneration, also preventing tissues browning and blackening as compared with incubation under the normal temperature at various period of incubation (45, 75 and 100 days). Moreover, PPO activity had reduced in callus stored at 5 °C. Results also, suggested that the application of cold pretreatment at 5 °C for 45–75 days induced the synthesis of newly proteins bands with (160, 93 and 47 KD).
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Abul-Soad A.A. Influence of inflorescence explants age and 2, 4-D incubation period on somatic embryogenesis of date palm. Emir J Food Agric. 2012;24(5):434–443. [Google Scholar]
- 2.Alderson P.G. Micropropagation of woody plants. In: Alderson P., Dullforce W.M., editors. Micropropagation in horticulture, paractice and commercial problems. Univ Nottingham; 1986. pp. 37–52. [Google Scholar]
- 3.Al-Khalifah NS, Shanavaskhan AE. Micropropagation of date palms. Asia-Pacific Consortium on Agricultural Biotechnology (APCoAB) and Association of Agricultural Research Institutions in the Near East and North Africa (AARINENA); 2012. 54pp.
- 4.Al-Mayahi A.M. Effect of copper sulphate and cobalt chloride on growth of the in vitro culture tissues for date palm (phoenix dactylifera L.) cv. Ashgar Am J Agric Biol Sci. 2014;9(1):6–18. [Google Scholar]
- 5.Al-Mayah A.M.W. Effect of red and blue light emitting diodes ‘‘CRB-LED’’on in vitro organogenesis of date palm (Phoenix dactylifera L.) cv. Alshakr. World J Microbiol Biotechnol. 2016;32:160. doi: 10.1007/s11274-016-2120-6. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. SPSS. available at http://www.spss.com/. Chen L, Games DE, Jones J (2003). Isolation and identification of four flavonoid constituents from the seeds of Oroxylum indicum by high speed counter current chromatography. J Chrom. 2004;988(1):95–105. [DOI] [PubMed]
- 7.Benjamin N., Montgomery M.W. Polyphenol oxides of royal ann. cherries: purification and characterization. J Food Sci. 1973;38:799–806. [Google Scholar]
- 8.Baiea MH. Studies on propagation of some fruit species by using tissue culture techniques. M.Sc. Hort. Dept., Fac. of Agric. Moshtohor, Zagazig University, Egypt; 2002.
- 9.Croughan T.P., Chu Q.R. Rice (Oryza sativa L.): Establishment of callus cultures and the regeneration of plants. In: Bajaj Y.P.S., editor. Biotechnology in agriculture and forestry. Rice. Springer-Verlag; Berlin, Heidelberg, New York, Tokyo: 1991. pp. 20–37. [Google Scholar]
- 10.Daayf F., Bellajm E., Hassni E., Jaiti F., Hadrami E. Elicitation of soluble Phenolics in date palm callus by Fusarium oxysporum f.sp. albedinis culture medium. Environ Exp Bot. 2003;49:41–47. [Google Scholar]
- 11.Dubravina G.A., Zaytseva S.M., Zagoskina N.V. Changes in formation and localization of phenolic compounds in the tissues of european and canadian yew during differentiation In vitro. Russ J Plant Physiol. 2005;52:672–678. [Google Scholar]
- 12.Engelmann F. In vitro conservation methods. In: Ford-Lloyd B.V., Newburry J.H., Callow J.A., editors. Biotechnology and plant genetic resources: conservation and use. CABI; Wellingford: 1997. pp. 119–162. [Google Scholar]
- 13.Eris A., Gulen H., Barut E., Cansev A. Annual patterns of total soluble sugars and proteins related to cold-hardiness in olive (Olea europaea L. ‘Gemlik’) J Hort Sci Biotech. 2007;82:597–604. [Google Scholar]
- 14.Gioi T.D., Tuan V.D. Anther culture from crosses between IR64 and new plant type cultivar. Omonrice. 2004;12:27–32. [Google Scholar]
- 15.Hassan N.S. Effect of ABA and/or cold acclimation on protein profile and DNA fingerprints of micropropagated banana shoots. Egypt J Biotechnol. 2004;16:378–392. [Google Scholar]
- 16.Hiraoka N., Kodama T. Effects of non-frozen cold storage on the growth, organogenesis and secondary metabolism of callus cultures. Plant Cell Tiss Org Cult. 1984;3:349–357. [Google Scholar]
- 17.Holderbaum D.F., Kon T., Kudo T., Guerra M.P. Enzymatic browning, polyphenol oxidase activity, and polyphenols in four apple cultivars: dynamics during fruit development. Hort Sci. 2010;45(8):1150–1154. [Google Scholar]
- 18.Jin P., Wang S.W., Wanga C.W., Zeng Y. Effect of cultural system and storage temperature on antioxidant capacity and phenolic compounds in strawberries. Food Chem. 2011;124:262–270. [Google Scholar]
- 19.Kaushal L., Sharma R., Balachandran S.M., Ulaganathan K., Shenoy V. Effect of cold pretreatment on improving anther culture response of rice (Oryza sativa L.) J Exp Biol Agric Sci. 2014;2S:233–242. [Google Scholar]
- 20.Laemmli U.K. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Murashige T., Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 22.Mustafa N.S., Taha R.A., Hassan S.A.M., Zaid N.S.M., Mustafa E.A. Overcoming phenolic accumulation of date palm in vitro cultures using α- tochopherol and cold pre-treatment. Middle East J Sci Res. 2013;15(3):344–350. [Google Scholar]
- 23.Robertson A.J., Gusta L.V., Reaney M.J.T., Ishikawa M. Identification of protein correlated with increased freezing tolerance in bromegrass (Bromus inermis L. Cv. Manchar) cell cultures. Plant Physiol. 1988;86:344–347. doi: 10.1104/pp.86.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roininen I.T., Tanhuanpaa P., Immonen S. The effect of cold and heat treatments on the anther culture response of diverse rye genotypes. Euphytica. 2005;145(1–2):1–9. [Google Scholar]
- 25.Rukmini M., Rao G.J.N., Rao R.N. Effect of cold pretreatment and phytohormones on the anther culture efficiency of two indica rice (Oryza sativa L.) hybrids –Ajay and Rajalaxmi. J Exp Bio Agri Sci. 2013;1(2):69–76. [Google Scholar]
- 26.Ru Z., Lai Y., Xu C., Li L. Polyphenol oxidase (PPO) in early stage of browning of phalaenopsis. Leaf Explants. 2013;5(9):57–64. [Google Scholar]
- 27.Shehata W.F., Aldaej M.I., Alturki S.M., Ghazzawy H.S. Effect of ammonium nitrate on antioxidant production of date palm (Phoenix dactylifera L.) in vitro. Biotechnology. 2014;13(3):116–125. doi: 10.3923/pjbs.2014.1209.1218. [DOI] [PubMed] [Google Scholar]
- 28.Taiz L, Zeiger E. In: Plant physiology, 3rd ed. Sinauer Associates Pubilsher; 2002 [690pp].
- 29.Tang Y., Li Z., Li J., Ma C., Lai J., Li H. Effect of different pretreatment on callus formation from anther in balsam pear (Momordica charantia L.) J Med Plants Res. 2012;6(17):3393–3395. [Google Scholar]
- 30.Singleton V.L, Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungsic acid reagents. Amer.J Enol.Vitic. 1965;16:144–158. [Google Scholar]