Abstract
Keratinase are proteolytic enzymes which have gained much attention to convert keratinous wastes that cause huge environmental pollution problems. Ten microbial isolates were screened for their keratinase production. The most potent isolate produce 25.2 U/ml under static condition and was primarily identified by partial 16s rRNA gene sequence as Bacillus licheniformis ALW1. Optimization studies for the fermentation conditions increased the keratinase biosynthesis to 72.2 U/ml (2.9-fold). The crude extracellular keratinase was optimally active at pH 8.0 and temperature 65 °C with 0.7% soluble keratin as substrate. The produced B. licheniformis ALW1 keratinase exhibited a good stability over pH range from 7 to 9 and over a temperature range 50–60 °C for almost 90 min. The crude enzyme solution was able to degrade native feather up to 63% in redox free system.
Keywords: Bacillus licheniformisALW1, Keratinase, Feather waste, Optimization, Enzymatic feather degradation
1. Introduction
Keratin is an insoluble structural protein with high mechanical stability and resistant to microbial degradation, by common proteolytic enzymes, because of its extensive cross linkage by disulfide bonds, hydrogen bonding and hydrophobic interactions. Keratin mainly found in two forms either in a helix α-keratin (hair, nail, hooves, horns, etc.) or β-keratin sheets form (feather, scales, beaks, claws, etc.) [1], [2].
Feather waste was the most abundant keratinous material in the nature produced with high amounts in poultry slaughterhouses and contains 91% of β-keratin [3], [4]. It was recorded that, the daily accumulation of feather waste reached to five million tons around the world which are generally disposed of in dumps, land filled or burnt in incinerators that produced huge environmental pollution [5], [6]. Thus the need for processing of feather and usage of its value-added products gives us a new area of research.
Processing of feather by steam pressure, chemical treatment and feather milling are cost and labor intensive and may reduce the nutritional value of the products by destroying important amino acids. So the investigation of alternative technology seems justifiable. The application of biotechnology as environmentally friend technology by the use of microorganisms and their enzymes in feather processing considered conceptually appropriate [7], [8], [2].
Diverse groups of microorganisms, like fungi (Doratomyces microsporus, Alternaria radicina, Aspergillus sp., Rhizomucor sp., etc.), actinomycetes (Streptomyces pactum, S. thermoviolaceus, Thermoactinomyces candidus, etc.) and several bacterial species (Pseudomonas aeruginosa, Microbacterium sp., Bacillus licheniformis and B. pumilus) were reported to produce keratinase which is the specific class of proteolytic enzymes cleaving keratin containing substrates [9], [10], [11].
Okoroma et al. [8] estimated that growth substrates cost around 40% of the enzyme production cost. Thus usage of newly isolated bacterial cells under static condition for keratinase production from available low cost feather wastes is valued issue for studying.
2. Materials and methods
2.1. Chemicals
Turkey feathers were collected from a farm in Kafr Ghataty, Al-Haram, Giza, Egypt. Corn steep liquor (CSL) was obtained from the Egyptian Starch and Glucose Company, Mostorod, Qalubia, Egypt. All other chemicals were of analytical grade.
2.2. Microorganisms
In the present study 3 fungal, 3 actinomyces and 4 bacterial isolates were isolated from different keratinous wastes (feather, leather and wool) at the Department of Chemistry of Natural and Microbial Products in the National Research Center, Dokki, Giza, Egypt and were screened for keratinase production. Fungal isolates were maintained on potato dextrose agar slants at 4 °C after incubation at 30 °C for 7 days while actinomyces and bacterial isolates were maintained on tryptone soya agar (TSA) slants at 4 °C after incubation at 37 °C for 7 days.
2.3. Feather and soluble keratin preparation
Turkey feathers were prepared for the experiment according to Saber et al. [12]. Soluble keratin was prepared from turkey feathers according to the method of Mazotto et al. [1] and used as the substrate in keratinase assay.
2.4. Inoculum preparation
Ten milliliter of sterilized medium composed of (g/l): glucose 10.0; peptone 10.0; yeast extract 3.0; CaCl2·2H2O 2.0; pH 7.0 ± 0.2 [13] were added to slant and the surface was scratched with a sterile needle. The suspension was transferred to 250 ml Erlenmeyer flasks containing 40 ml of sterilized medium and incubated 72 h at 30 °C for fungi and 48 h at 37 °C for bacteria and actinomyces in shaking incubator (Thermo Scientific MaxQ481RHP) at 180 rpm.
2.5. Screening for keratinase production
To identify the most potent keratinase producing isolate, 250 ml Erlenmeyer flasks contain 2% (w/v) of native feather in 50 ml growth medium containing (g/l): NaCl 0.5; KH2PO4 0.7; K2HPO4 1.4; MgSO4.7H2O 0.1; pH 7.0 ± 0.2 [14] were inoculated with 5% of inoculum and incubated under static and shaking (180 rpm) conditions for different incubation periods. At the end of the fermentation period keratinase activity and the protein content were estimated after centrifugation of the fermented medium at 5000g for 15 min.
2.6. Keratinase assay
The keratinolytic activity of culture filtrate was assayed by using the prepared soluble keratin according to the method described by Cai et al. [15]. One unit of keratinolytic activity was defined as an increase of 0.01/min in absorbance at 280 nm against the blank, under the reaction condition.
2.7. Protein determination
Bovine serum albumin as standard was used to determine the protein content using the method of Lowry et al. [16].
2.8. Strain identification, 16s rDNA sequencing and phylogenetic analysis
The most potent isolated microorganism was identified by using Transmission Electron Microscopy (TEM, JEM-2100, JEOL USA) after growing on TSA plate for 3 days. Molecular identification was carried out were DNA extraction was done according to Gene Jet protocol of genomic DNA purification Kit (Thermo K0721). Forward primer AGA GTT TGA TCC TGG CTC AG, and reverse primer, GGT TAC CTT GTT ACG ACT T, PCR was made using Maxima Hot Start PCR Master Mix (Thermo K1051). Thermo-cycling process was done with initial denaturation and enzyme activation at 95 °C for 10 min and for 35 cycles (denaturation at 95 °C for 30 s, annealing at 65 °C for 1 min and extension at 72 °C for 90 s) and then the final extension at 72 °C for 10 min. The PCR product was purified using Gene JET™ PCR Purification Kit (Thermo K0701). Sequencing of the PCR product was done in GATC Company by use ABI 3730xl DNA sequencer using forward and reverse primers (Sigma Scientific Services Co).
2.9. Optimization of the most factors influencing keratinase production
The ability of the selected strain to utilize different keratinous substances to produce keratinase was tested, so feather in the fermentation medium was replaced by human hair, horn, nail and wool individually. Different temperature ranged from 28 to 47 °C was investigated for maximum production of keratinase. The basal medium was supplemented with six different carbon sources galactose, glucose, mannose, sucrose, dextrin and soluble starch at concentration 0.1%. Effect of different nitrogen sources was investigated by the addition of casein, urea, peptone, yeast extract, ammonium sulfate, CSL and baker’s yeast individually to the fermentation medium. The optimum concentration from the best keratinous substrate and nitrogen source were also identified. The inoculum size (0.75–5%) and other physical parameters of the culture medium as initial pH (5–8.5) were also optimized for maximum production of keratinase. The effect of the other medium constituent as K2HPO4 and NaCl was also examined in concentration range 0.8–1.8 g/l and 0.5–0.8 g/l respectively.
2.10. Properties of crude B. licheniformis ALW1 keratinase
2.10.1. Effect of temperature and pH
The optimum temperature of crude keratinase was investigated by incubating the reaction mixture at different temperature ranged from 40 °C to 80 °C for 15 min. under standard assay condition. The optimum pH was determined by carrying out the enzyme assay at different pH values using 0.1 N tris - HCl buffer in the range of 7.0–9.0 for 15 min. at optimum temperature.
2.10.2. Effect of substrate concentration
The enzyme activity with different concentrations of soluble keratin (0.3, 0.4, 0.5, 0.6, 0.7 and 0.8%) was estimated at the optimal temperature and pH of the tested enzyme.
2.10.3. Determination of thermal stability
Thermal stability of the crude keratinase was investigated by incubating the enzyme in 0.1 N tris - HCl buffer solution (without substrate) at different temperature (50–70 °C) for different time intervals (15, 30, 45, 60, 90, 120 min). The residual enzyme activity was measured under the optimum conditions.
2.10.4. Determination of pH stability
In the absence of substrate the crude enzyme solution was subjected to different pH values in the range of 7.0–9.0 (0.1 N tris - HCl buffer) for different time intervals (15, 30, 45, 60, 90, 120 min). The residual activity was measured under the optimum conditions.
2.11. Feather degradation by cell free crude keratinase
Conical flask (100 ml) containing 1.0 g feather with 15 ml 0.1 M phosphate buffer pH 8 was autoclaved for 15 min at 15 psi, 121 °C. The feather degradation process was started by the addition of 480 U of enzyme with final volume of 20 ml and the mixture incubated at 50 °C and 150 rpm. To study the effect of time on the degradation; the reaction was carried out for different period of 4–24 h. The impact of different pH on feather degradation was tested by using 0.1 M phosphate buffer at pH 6–8, 0.1 M tris- HCl buffer at pH 9 and 0.1 M carbonate – bicarbonate buffer at pH 10 instead of 0.1 M phosphate buffer at pH 8. The effect of temperature on degradation rate was evaluated by incubating the reaction mixture at different temperatures (30–60 °C). At the end of each experiment feather suspension was centrifuged at 5000g for 15 min and the precipitated dried at 80 °C to calculate the feather meal dry weight. The relative residual activity of keratinase and soluble protein (mg/g feather) were determined in the supernatant.
2.12. Statistical analysis of data
All experiments were performed in triplicates and the results were taken as the mean value ± SD. For optimization experiments ANOVA test was performed (using SPSS version 15) to calculate significant differences between means (represented as ∗∗) when compared to control of each experiment (∗) at a 95% confidence level.
3. Results and discussion
3.1. Screening for keratinase production
The time course production of keratinase under static and shaking conditions by 3 fungal, 3 actinomyces and 4 bacterial isolates indicated that the most significant amount of keratinase (25.2 U/ml) was secreted by one of the leather waste bacterial isolate under static condition after four days of incubation (unpublished data). The production of keratinase from different bacterial isolates had been reported in several studies [17], [18], [19], [2]. With exception of keratinase reported from some thermophilic bacteria, in general it was known that the production of keratinase achieved in submerged condition with high aeration [20], [10].
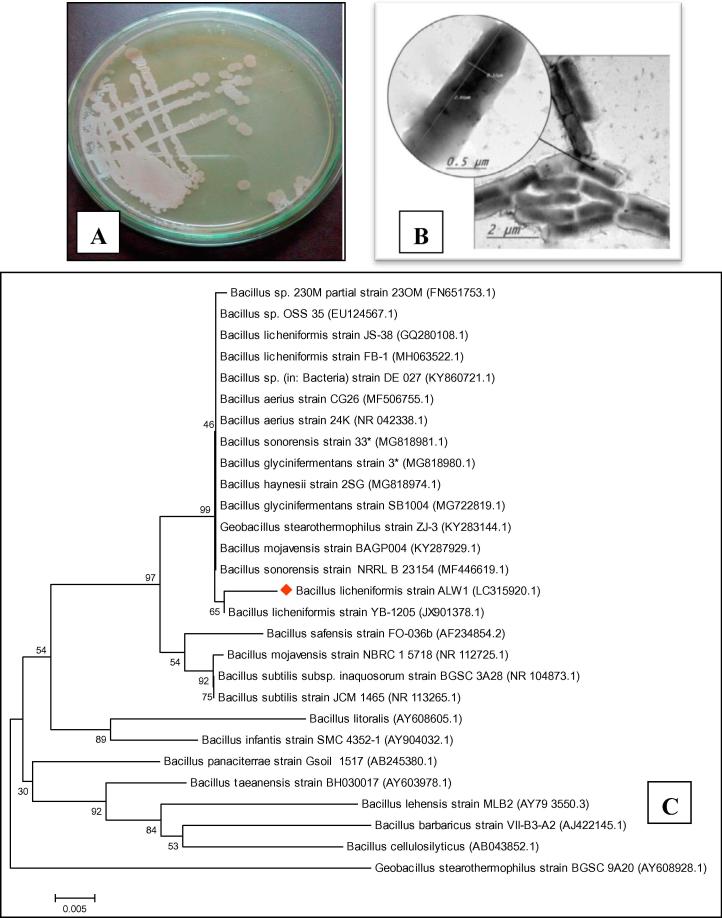
The growth of most potent bacterial isolate on TSA was represented in Fig. 1A. Transmission electron microscopy (Fig. 1B) and Gram stain examination of the most potent bacterial isolate showed rod shaped Gram positive cells with a 2 µm length and diameter of 0.5 µm. The 16S rDNA gene sequence analysis indicated that the isolate is closely related to B. licheniformis which was known as keratinase producer [21], [8]. The bacterial isolate has 99% homology with B. licheniformis YB-1205 as shown in the phylogenetic tree (Fig. 1C). The data of 16S rDNA partial sequence has been submitted to Gen Bank databases under the name of Bacillus licheniformis ALW1 with accession no. of LC315920.
Fig. 1.
The growth of most potent bacterial isolate on TSA (A), Transmission electron microscopy examination of the most effective bacterial isolate (B), Molecular phylogenetic analysis based on 16S rDNA partial sequence, showing the relation between isolate Bacillus licheniformis ALW1 and other species of Bacilli, The tree was constructed using MEGA6 programme by Maximum Likelihood method (C).
3.2. Optimization of B. licheniformis ALW1 keratinase production
It is well known that culture conditions as well as medium constituents were frequently influenced the produced metabolites by microorganisms. Therefore, in this part, variation of one factor at a time was used as a preliminary screening to investigate the most suitable nutritional and culture condition requirements for maximum production of keratinase by B. licheniformis ALW1.
3.2.1. The effect of different keratinous substances
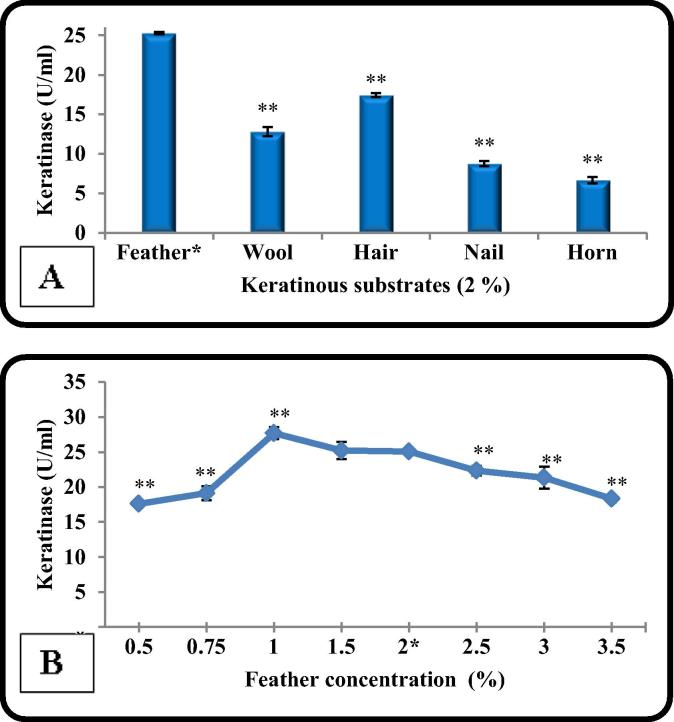
Among different keratinous materials tested at 2% level, feather was found to be the best substrate utilized by B. licheniformis ALW1 to induce keratinase (25.3 U/ml). A considerable amount of keratinase (17.4 U/ml) was also produced with hair which means that B. licheniformis ALW1 was able to utilize both α-keratin and β-keratin to produce keratinase. The lowest level of keratinase was recorded with horn (6.7 U/ml) as shown in Fig. 2A. Feather was recorded as the best source for keratinase production by other researchers [15], [10].
Fig. 2.
The effect of different keratinous substances on keratinase production by B. licheniformis ALW1 (A), The effect of different concentration of feather (%) on keratinase production (B).
Substrate concentration is very important factor for microbial growth and enzyme production. Applying feather at different concentrations in the culture medium indicated that (1%) was the most suitable concentration for keratinase production by B. licheniformis ALW1 (Fig. 2B). As the concentration of the feather increase the viscosity of the medium increase and showed substrate repression on keratinase production [22]. This result was in agreement with the results obtained by [8], [2].
3.2.2. The effect of incubation temperature on enzyme production
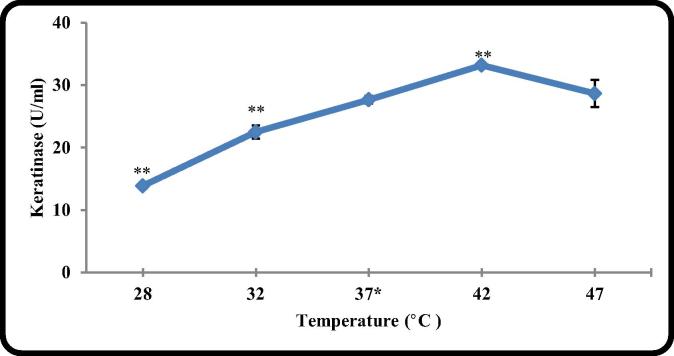
Temperature of the culture medium is an important factor for the growth of bacteria, the activity and the stability of the produced enzymes. The effect of incubation temperature on keratinase production by B. licheniformis ALW1 was illustrated in Fig. 3 and indicated that the highest level of the produced enzyme (33.2 U/ml) was obtained at an incubation temperature of 42 °C. It has been pointed out by other researchers that B. licheniformis is mesophilic, can grow well in a temperature range of 15–45 °C, and the best incubation temperature for keratinolytic enzyme production was between 40 and 45 °C [23], [24], [19].
Fig. 3.
The effect of different incubation temperature on keratinase production by B. licheniformis ALW1.
3.2.3. The effect of carbon sources addition
The effect of different carbon source additives on the keratinase production by B. licheniformis ALW1 was shown in Table 1. It was clear that there was no significant effect of carbon source on the keratinase production except in the case of the addition of glucose and galactose where the enzyme production increases by 5.5 and 10.3% respectively. Our results agree with what reported by Tiwary and Gupta [25] who reported an increase in the keratinase production from Bacillus licheniformis ER-15 by the addition of galactose and glucose. On the other hand glucose was reported to repress keratinase production for other bacterial strains [22], [10].
Table 1.
The effect of carbon source additives on keratinase production by B. licheniformis ALW1.
| Carbon source additives (0.1%) | Keratinase activity |
|
|---|---|---|
| (U/ml) | (%) | |
| None* | 33.22 ± 0.77 | 100.0 |
| Glucose | 35.06 ± 0.07 | 105.5** |
| Galactose | 36.63 ± 0.54 | 110.3** |
| Mannose | 34.82 ± 0.48 | 104.8 |
| Sucrose | 34.64 ± 0.39 | 104.3 |
| Dextrin | 33.76 ± 1.18 | 101.6 |
| Soluble starch | 34.83 ± 0.20 | 104.8 |
(**) mean that this value is significantly different when compared to control (*) at 95 % confidence level.
3.2.4. The effect of nitrogen sources addition
The results in Table 2 clearly indicated that the addition of CSL to the culture medium significantly increase the production of keratinase by 41% (51 U/ml) as CSL is rich in vitamins, minerals and proteins which may support the growth of the bacterium and enzyme production. This result was agreed with what reported by Ni et al. [26] for Bacillus licheniformis ZJUEL31410. On the other hand Tiwary and Gupta, [25] reported the decrease of the keratinase produced by Bacillus licheniformis ER-15 in the presence of corn steep.
Table 2.
The effect of nitrogen source additives on keratinase production by B. licheniformis ALW1.
| Nitrogen additives (0.0294 g N/100 ml) | Keratinase activity |
|
|---|---|---|
| (U/ml) | (%) | |
| None* | 36.05 ± 20.02 | 100.0 |
| (NH4)2SO4 | 32.01 ± 1.94 | 88.8 |
| Urea | 13.8 ± 0.82 | 38.3** |
| Baker’s yeast | 35.64 ± 3.6 | 98.9 |
| Casein | 32.69 ± 3.69 | 90.7 |
| CSL | 50.97 ± 0.29 | 141.4** |
| Peptone | 39.17 ± 4.18 | 108.7 |
| Yeast extract | 39.25 ± 1 | 108.9 |
(**) mean that this value is significantly different when compared to control (*) at 95 % confidence level.
The lowest level of the produced keratinase (13.8 U/ml) was observed with urea. This result agrees with Mabrouk et al. [27] and Park and Son, [24]. In the contrary Cai and Zheng [28] reported the increase in the production of keratinase with urea. All the other tested nitrogen sources almost had no significant effect on B. licheniformis ALW1 keratinase production.
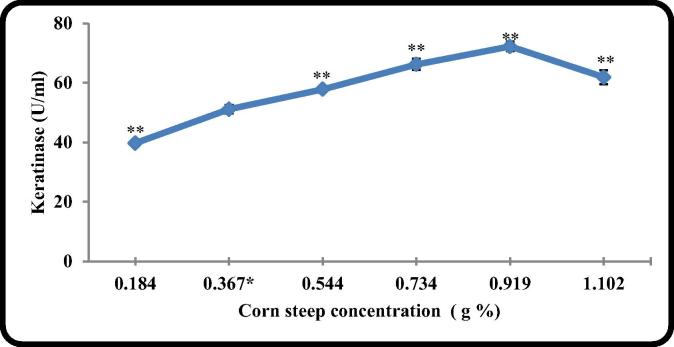
Applying CSL at different concentrations in the culture medium indicated that the highest level of keratinase (72.2 U/ml) produced at 0.92 g% (Fig. 4).
Fig. 4.
The effect of different CSL concentration on keratinase production by B. licheniformis ALW1.
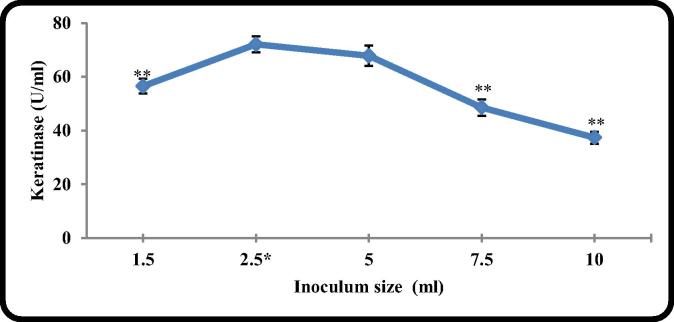
3.2.5. The effect of inoculum size
Inoculum size had a great influence on production of keratinase. The low density of the inoculum cells decreases the number of the grown cells in the enzyme production phase. On the other hand the high density of cells in the culture increases the viscosity and the compotation on the nutrients which also decrease the possibility of reaching the cells to the production phase and hence the enzyme production. The results in Fig. 5 indicated that the inoculation of the fermentation medium with 5% of 48 h old inoculum produced the highest value of keratinase (72.2 U/ml). This was in agreement with what was reported by other researchers [27], [29], [11].
Fig. 5.
The effect of inoculums size on keratinase production by B. licheniformis ALW1.
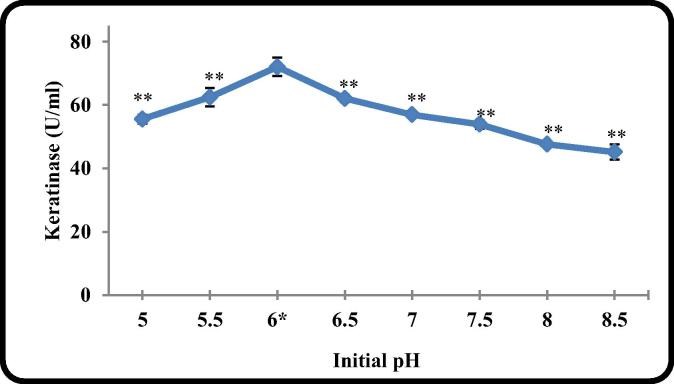
3.2.6. The effect of initial pH
pH of the culture medium has great impact on the growth of the bacteria and on the production, activity and stability of their produced metabolites. It was found that B. licheniformis ALW1 produce keratinase over a wide pH range with maximum production (72 U/ml) at pH 6.0 (Fig. 6). It was mentioned that the final pH of the entire initial tested pHs were in the range of 8.5–9 (unpublished data). Mao et al. [30] and Gajju et al. [31] recorded the production of proteases from Bacillus sp with slightly acidic initial pH fermented medium. Most of the researchers recorded neutral or alkaline pH as the suitable initial pH for production of keratinase from other Bacillus sp. [8], [19], [2].
Fig. 6.
The effect of different initial pH on keratinase production by B. licheniformis ALW1.
3.2.7. The effect of concentrations of K2HPO4 and NaCl
The effect of the concentration of K2HPO4 (0.8–1.8 g/l) on enzyme production was tested. It was found that 1.4 g/l was the most suitable concentration (72 U/ml). The results also indicated that the concentrations of K2HPO4 above 1.4 g/l in the tested range were not significantly affecting the production of keratinase. This result was nearly agree with that recorded by other researchers for keratinase production by Bacillus sp. [32], [26].
The results clarify that NaCl at 5% was the best concentration for production of keratinase by B. licheniformis ALW1 (72 U/ml). Increasing the concentration of NaCl up to 8% almost did not affect the production of keratinase. The ability of B. licheniformis ALW1 to hydrolyze feather in the optimized medium was shown in Fig. 7.
Fig. 7.
The ability of B. licheniformis ALW1 to hydrolyze feather in the optimized medium. A: represent feather after autoclaving; B: represent the hydrolysis of feather at the end of fermentation period.
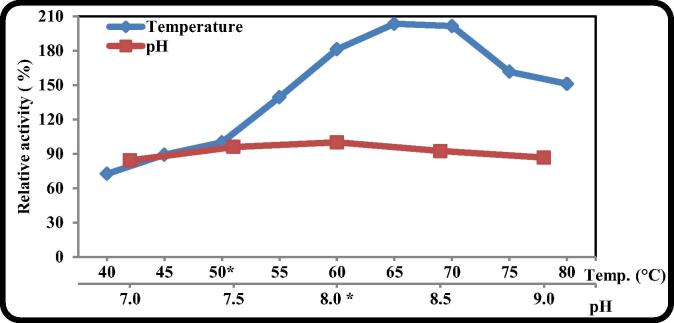
3.2.8. Effect of temperature and pH
The profile of extracellular B. licheniformis ALW1 keratinase activity at different temperatures and different pHs was shown in Fig. 8. The optimum temperature of B. licheniformis ALW1 keratinase was found to be 65 °C with 103% increase relative to control (50 °C). The B. licheniformis ALW1 keratinase exhibit a good activity over a wide range of pH (7–9) with optimum activity at pH 8 when the reaction was performed at 65 °C for 15 min. Optimal pH and temperature of bacterial keratinase was reported to be in the range of 7.5–10.0 and 30–80 °C respectively [33], [1], [9].
Fig. 8.
The effect of reaction temperature and pH on the activity of crude B. licheniformis ALW1 keratinase.
3.2.9. Effect of substrate concentration
The rate of substrate hydrolysis by B. licheniformis ALW1 keratinase increased as keratin concentration increased and reached maximum at 0.7%. Farther increase of substrate concentration up to 0.8% starts to decrease the rate of hydrolysis (8%) which may be due to substrate and/or products inhibition.
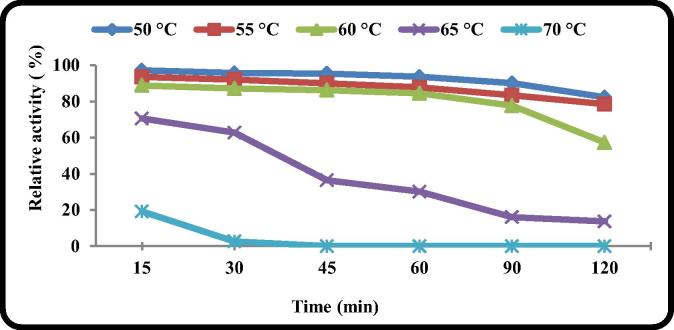
3.2.10. pH and thermal stability
pH and temperature stability of the enzymes are very important factors for their industrial application. The B. licheniformis ALW1 keratinase was thermostable over a temperature range 50–60 °C for almost 90 min (without substrate). The enzyme had a half life time over 2 h at 60 °C as shown in Fig. 9. Although the enzyme recorded excellent activity at 70 °C (Fig. 7) its residual activity after incubation for 15 min at 70 °C was only 20%. This agreed with the fact that the incubation of the enzymes at high temperature without their substrate decreases their observed stability [34]. The enzyme exhibited a good stability over neutral and alkaline pH range (7–9) as shown in Fig. 10. After 2 h of incubation the enzyme lost only 5% of its activity at pH 8 and 10% at pH 7 and 9.
Fig. 9.
Thermal stability of the crude B. licheniformis ALW1 keratinase.
Fig. 10.
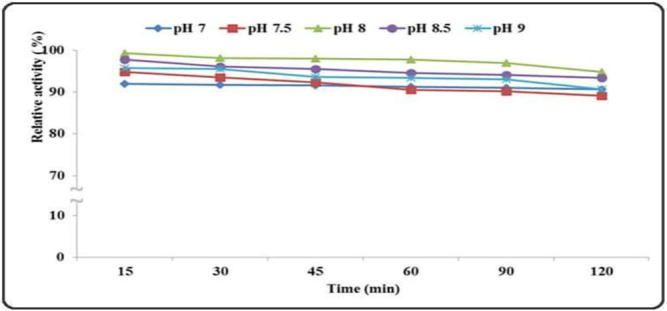
pH stability of the crude B. licheniformis ALW1 keratinase.
3.3. Enzymatic degradation of feather
The use of enzyme for feather degradation is highly efficient eco-friendly cheap method as it produce feather meal with high nutritional values and without any harmful on the produced amino acids which increase their industrial and biotechnological applications. So the optimization of the parameters necessary for efficient degradation of feather was carried out. Table 3 indicated that the feather degradation and total soluble protein increase by time up to 64% and 440 mg/g feather, respectively. The highest value of protein was released in the first 4 h, although the feather degradation was only 30%. The results also indicated that the residual activity of the enzyme decrease by the time. The effect of different pHs on feather degradation after 24 h clarify that as the pH increase the feather degradation increase except at pH 9 were the feather degradation (57%) was less than that at pH 8 (64%) this is may be attributed to the type of buffer. It was known that alkaline pH (pH 10) facilitate the feather degradation as it reduces the disulfide bond [35] but it reduce the value of the produced amino acids [36], so pH 8 was used in the next experiment. The impact of temperature on feather degradation at pH 8 and 24 h incubation was investigated. There was no increase in feather degradation at temperature higher than 50 °C although there was a little increase in soluble protein (11.7 mg/g feather). As high temperature has bad affect on the produced amino acids, so temperature 50 °C recommended to be used with the tested enzyme for feather degradation. As shown in Fig. 11 feather keratin was hydrolyzed by crude keratinase and dried to feather meal to be used in further application.
Table 3.
Effect of different parameters on feather degradation by crude B. licheniformis ALW1 keratinase.
| Parameter | Remaining activity (%) | Total protein (mg/g feather) | Feather degradation (%) |
|---|---|---|---|
| Time (h) | |||
| 0 | 100 | 77.2 | 0 |
| 4 | 81.2 | 297.1 | 29 |
| 8 | 61.1 | 307.1 | 40 |
| 12 | 57 | 332.6 | 52 |
| 16 | 48 | 357.7 | 57 |
| 20 | 44 | 400.7 | 62.3 |
| 24 | 42 | 440 | 64 |
| pH | |||
| 6.0 | 29.9 | 196.6 | 29 |
| 7.0 | 36 | 377.3 | 61 |
| 8.0 | 42 | 440 | 64 |
| 9.0 | 35.6 | 391.4 | 57 |
| 10 | 26.7 | 500.1 | 70 |
| Temp. | |||
| 30 °C | 53.1 | 267.09 | 43 |
| 40 °C | 45.65 | 375.56 | 59 |
| 50 °C | 42 | 440 | 63 |
| 60 °C | 39.38 | 451.69 | 63 |
Fig. 11.
Feather keratin completely hydrolyzed by crude keratinase (A), Dried feather meal (B).
4. Conclusion
Among different microbial isolates screened for keratinase production using turkey feather waste, a newly isolated B. licheniformis ALW1 with accession no. of LC315920 was found to be the most potent producer. This organism was able to utilize feather waste and CSL in medium supplemented with 0.1% glucose at initial pH 6 and 42 °C under static condition to produce keratinase. Optimization studies increase the produced keratinase by 2.9 fold (from 25.2 to 72 U/ml). The produced enzyme posses optimum activity at pH 8.0 and 65 °C and stable over a wide range of pH and temperature up to 2 h. B. licheniformis ALW1 keratinase was able to hydrolyze feather by 63% with soluble protein of 440 mg/g feather which can be used in many biological application.
Acknowledgments
Acknowledgment
Authors wish to thank National Research Centre, Cairo, Egypt for financial assistance of this paper as a part of PhD thesis.
Conflict of interest
The authors declare that the research was conducted in absence of any conflict of interest.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Mazotto A., de Melo A., Macrae A., Rosado A., Peixoto R., Cedrola S. World J Microbiol Biotechnol. 2011;27:1355–1365. doi: 10.1007/s11274-010-0586-1. [DOI] [PubMed] [Google Scholar]
- 2.Ire F.S., Onyenama A.C. J Adv Boil Biotechnol. 2017;13:1–13. [Google Scholar]
- 3.Mabrouk M.E. World J Microbiol Biotechnol. 2008;24:2331–2338. [Google Scholar]
- 4.Cedrola S., de Melo A., Mazotto A., Lins U., Zingali R., Rosado A. World J Microbiol Biotechnol. 2012;28:1259–1269. doi: 10.1007/s11274-011-0930-0. [DOI] [PubMed] [Google Scholar]
- 5.Vasileva-Tonkova E., Gousterova A., Neshev G. Int Biodeterior Biodegradation. 2009;63:1008–1012. [Google Scholar]
- 6.Mohamad N., Phang L., Abd-Aziz S. Biocatal Biotransform. 2017;35:41–50. [Google Scholar]
- 7.Wang X., Parson C.M. Poult Sci. 1997;76:491–496. doi: 10.1093/ps/76.3.491. [DOI] [PubMed] [Google Scholar]
- 8.Okoroma E.A., Garelick H., Abiola O.O., Purchase D. Int Biodeterior Biodegradation. 2012;74:54–60. [Google Scholar]
- 9.Han M., Luo W., Gu Q., Yu X. Afr J Microbiol Res. 2012;6:2211–2222. [Google Scholar]
- 10.Lakshmi P.J., Chitturi C.M., Lakshmi V.V. Int J Microbiol. 2013;2013:1–7. [Google Scholar]
- 11.Jani S., Malek S., Patel A., Pathak K., Baria K. Int J Curr Microbiol Appl Sci. 2017;6:1538–1552. [Google Scholar]
- 12.Saber W., El-Metwally M., El-Hersh M. Res J Microbiol. 2010;5:21–35. [Google Scholar]
- 13.Abdel-fattah A., Nashy E., Sabiel E., Hussien M., Attia A. Int J Appl Sci Biotechnol. 2015;3:609–618. [Google Scholar]
- 14.Wang J., Shih J. J Ind Microbiol Biotechnol. 1999;22:608–616. doi: 10.1038/sj.jim.2900667. [DOI] [PubMed] [Google Scholar]
- 15.Cai C., Lou B., Zheng X. J Zhejiang Univ Sci B. 2008;9:60–67. doi: 10.1631/jzus.B061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry O., Rosebrough N., Farr A., Randall R. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Laba W., Choinska A., Rodziewicz A., Piegza M. Br J Microbiol. 2015;46:691–700. doi: 10.1590/S1517-838246320140098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahni N., Sahota P.P., Phutela U.G. Int J Curr Microbiol Appl Sci. 2015;4:768–783. [Google Scholar]
- 19.Chaisemsaeng P., Sabu A., Ansanan S., Sirisan S. Int J Biotechnol Res. 2017;7:29–35. [Google Scholar]
- 20.Riessen S., Antranikian G. Extremophiles. 2001;5:399–408. doi: 10.1007/s007920100209. [DOI] [PubMed] [Google Scholar]
- 21.Manczinger L., Rozs M., Vagvolgyi C., Kevei F. World J Microbiol Biotechnol. 2003;19:35–39. [Google Scholar]
- 22.Kainoor P.S., Naik G.R. Indian J Biotechnol. 2010;9:384–390. [Google Scholar]
- 23.Kim M.J., Lim J.W., Suh J.H. Process Biochem. 2001;37:287–291. [Google Scholar]
- 24.Park G.T., Son H.J. Microbiol Res. 2009;164:478–485. doi: 10.1016/j.micres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Tiwary E., Gupta R. Bioresour Technol. 2010;101:6103–6110. doi: 10.1016/j.biortech.2010.02.090. [DOI] [PubMed] [Google Scholar]
- 26.Ni H., Chen Q., Chen F., Fu M., Dong Y., Cai H. Afr J Biotechnol. 2011;10:7236–7244. [Google Scholar]
- 27.Mabrouk S.S., Hashem A.M., El-Shayeb N.M., Ismail A.M., Abdel-Fattah A.F. Bioresour Technol. 1999;69:155–159. [Google Scholar]
- 28.Cai C., Zheng X. J Ind Microbiol Biotechnol. 2009;36:875–883. doi: 10.1007/s10295-009-0565-4. [DOI] [PubMed] [Google Scholar]
- 29.Jayasree D., Kumari T.D.S., Kishor P.B., Lakshmi M.V., Narasu M.L. Int J Sci Technol. 2009;1:79–82. [Google Scholar]
- 30.Mao W., Pan R., Freedman D. J Ind Microbiol. 1992;11:1–6. [Google Scholar]
- 31.Gajju H., Bhalla T.C., Agarwal H.O. Indian J Microbiol. 1996;36:153–155. [Google Scholar]
- 32.Ramnani P., Gupta R. Biotechnol Appl Biochem. 2004;40:491–496. doi: 10.1042/BA20030228. [DOI] [PubMed] [Google Scholar]
- 33.Gupta R., Ramnani P. Appl Microbiol Biotechnol. 2006;70:21–33. doi: 10.1007/s00253-005-0239-8. [DOI] [PubMed] [Google Scholar]
- 34.Sharpe D.J., Wong L.J. Biochimie. 1990;72:323–326. doi: 10.1016/0300-9084(90)90027-e. [DOI] [PubMed] [Google Scholar]
- 35.Riffel A., Lucas F., Heeb P., Brandelli A. Arch Microbiol. 2003;179:258–265. doi: 10.1007/s00203-003-0525-8. [DOI] [PubMed] [Google Scholar]
- 36.Tiwary E., Gupta R. J Bioprocess Biotechnol. 2012;2:1–5. [Google Scholar]