Abstract
Whether elevated serum uric acid levels (SUA) predict renal dysfunction remains controversial in the elderly. Therefore, we investigated the association between SUA and early renal function decline defined as an estimated glomerular filtration rate (eGFR) reduction ≥30% over 2 years. From 2001 to 2010, we conducted a longitudinal cohort study comprising 44,078 participants aged ≥65 years in the Taipei City Elderly Health Examination Database. Participants were classified by 1-mg/dL increment of SUA. We used multivariable logistic and Cox regression analyses to compare the risk of early renal function decline in different SUA groups. Compared to the reference SUA group of 5.0–5.9 mg/dL, hyperuricemic participants had increased risks of eGFR decline, starting at SUA ≥6.0 mg/dL (adjusted odds ratio [aOR] = 1.21, 95% confidence interval [CI] = 1.00–1.45). The risk progressively elevated as SUA increased, with the highest in the SUA ≥10.0 mg/dL group (aOR = 3.20, CI = 2.39–4.28). Multivariable Cox regression further confirmed that hyperuricemia was 1.12-fold (CI = 1.03–1.22, SUA ≥6.0 mg/dL) to 1.6-fold (CI = 1.37–1.86, SUA ≥10.0 mg/dL) more likely to develop early eGFR decline. Hyperuricemia-associated increased risks for early eGFR decline were consistent across subgroup and sensitivity analyses. Collectively, SUA ≥6.0 mg/dL independently predicted early renal dysfunction with eGFR decline ≥30% over 2 years in older people.
Introduction
Older adults constitute the fastest-growing population of kidney diseases. Among individuals aged ≥65 years, chronic kidney disease (CKD) is a rather common disease, which affects 35% to 44% of the older people1,2. Mounting evidence has indicated that the elderly are at higher risks for CKD occurrence and progression, and early progressive renal function decline contributes to multiple serious adverse events, including cognitive impairment, end-stage kidney failure, cardiovascular disease and death3,4. Therefore, timely recognition of those older people at-risk for early renal function decline provides opportunities for prevention and treatment of adverse outcomes5. Traditional approach using a doubling of serum creatinine concentration (corresponding to a reduction of estimated glomerular filtration rate [eGFR] of 57% or greater) to document renal progression is a late event which precludes timely intervention6. A lesser reduction (≥30%) of eGFR over 2 years is a novel validated definition for early renal function decline and has been positively acknowledged as a surrogate endpoint of end-stage renal disease in clinical research6–8. Identification of predictors for early renal function decline defined as the eGFR decline ≥30% will help further promptly detect and treat older people at risks for advanced renal impairment.
Uric acid, the final product of purine degradation in humans, has been proposed to play pathophysiologic roles in renal diseases through inducing endothelial dysfunction and activating renin-angiotensin-aldosterone system9. Experimental studies indicate that hyperuricemia causes primary renal arteriolopathy in normal rats and accelerates progression of renal disease in the rat remnant kidney model10–12. Epidemiological studies also highlight that an elevated serum uric acid (SUA) level is associated with the development and progression of CKD in the middle-aged adults13–17. Nonetheless, whether hyperuricemia predicts deterioration of renal function in elderly population remains controversial18–21. Earlier studies suggest that elevated SUA levels are independently associated with a decline in eGFR of ≥3 mL/min/1.73 m2 per year in community-based healthy elderly cohorts18,19. Altemtam et al. also reported that baseline hyperuricemia independently predicted a faster renal progression of eGFR decline >2 ml/min/1.73 m2 per year in 270 elderly patients with stage 3 or 4 diabetic CKD20. By contrast, Nacak et al. recently found that SUA levels were not associated with the decline in renal function in stage 3 to 5 CKD patients21. These contradictory results may be ascribed to the different definitions of renal progression and reference SUA levels, relatively small sample size, varying characteristics of study population, and unequal statistical adjustment for confounders18–21. Several important confounding factors including smoking21, alcohol consumption18–21, history of cardiovascular diseases18,19, and fasting glucose levels19–21 are not fully adjusted previously. Furthermore, the reference SUA levels among these studies are unequal and most studies categorize participants by quantiles,18,19 which markedly limit the comparability and clinical applicability. Till now, there is no study identifying a threshold SUA level beyond which the risk of renal progression increases in the elderly population.
Therefore, the present study aimed to explore whether hyperuricemia independently predicted early renal function decline by utilizing a well-validated novel endpoint of eGFR decline ≥30% in a large community-based elderly cohort. We also intended to determine the range of optimal SUA levels with least risks of deteriorating renal function among older people.
Methods
Data Source
The present study was based on a large, well-characterized Taiwanese longitudinal community-based cohort of older people from the Taipei City Elderly Health Examination Database22–24, which contained health examination data for citizens 65 years or older since 2001, when the Taipei City Government launched an annual, free-of-charge, comprehensive health examination program to promote the health of senior citizens25. The accuracy of the Taipei City Elderly Health Examination Database was audited by Department of Health, Taipei City Government and the cohort has been validated previously22,23. Detailed information regarding height, weight, blood pressure, and prior medical history were recorded at the examination. Demographic data like age, gender, marital status, education levels, smoking history, alcohol consumption, and medical history including hypertension, diabetes mellitus, coronary artery disease and cerebrovascular disease, were collected through a self-administered questionnaire. Overnight fasting blood was collected for the measurement of complete blood count, glucose, triglyceride, total cholesterol, high-density-lipoprotein cholesterol, albumin, blood urea nitrogen, creatinine, and SUA. The SUA level was assayed by the colorimetric uricase–peroxidase system26. A semiquantitative (negative, trace, 1+, 2+, 3+, 4+) urine dipstick test for albuminuria was also performed. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR, which more accurately estimates GFR and predicts the risks of end-stage renal disease and mortality than the Cockcroft-Gault and Modification of Diet in Renal Disease Study equations27,28. All participants provided informed consent authorizing the Taipei City Government to process the health examination data for research purposes. Detailed information on the health examination data is stored centrally in the Taipei City Elderly Health Examination Database and is de-identified before release to protect the privacy. The present study was approved by the institutional review board of the Taipei City Hospital (TCHIRB-1010323-E and TCHIRB-1030601-W).
Study Design and Participants
The study cohort comprised older adults participating in the Taipei City Elderly Health Examination Program with follow-up laboratory data from 2001 to 2010. Participants with repeated measurement of renal function were initially screened (n = 56,062). We excluded participants with missing measurements of SUA (n = 51), with the history of end-stage renal disease (n = 1,629), or with extremely low SUA levels (<2 mg/dL, n = 186) that could possibly be attributed to hereditary renal hypouricemia29. Those with follow-up periods less than 2 years (n = 2,854) or without regular follow-up (n = 7,264) were also excluded. Finally, 44,078 older people were enrolled in the present study. To identify the optimal range of SUA levels with the least risks for early renal function decline, eligible participants were further classified by 1-mg/dL increment of SUA and participants with the SUA of 5.0–5.9 mg/dL were set as the reference group, because the relation between SUA levels and new-onset kidney disease has been observed in those with SUA levels more than 6.0 mg/dL in the general population13.
Study Outcome and Follow-up
The study outcome was early renal function decline defined as the occurrence of ≥30% reduction in eGFR. Percent change in eGFR was calculated as (last eGFR at the follow-up period – baseline eGFR)/(baseline eGFR) × 100%6,8. All participants were followed until death or December 31, 2010, whichever occurred first.
Statistical Analysis
Categorical and continuous variables were expressed as percentages and median (interquartile range), respectively. Between-group comparisons were made by the Kruskal-Wallis test or Pearson χ2 test where appropriate. Multivariable logistic regression was used to assess the association between baseline SUA levels and the study outcome because the original definition of early renal progression proposed by Coresh et al. is calculated at the end of a 2-year follow-up6. Covariates including age, sex, body mass index, smoking, alcohol consumption, systolic and diastolic blood pressure, hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, cerebrovascular disease, baseline kidney function, plasma total cholesterol, triglyceride, high-density lipoprotein-cholesterol, hemoglobin, glucose, white blood cell count, and albumin were adjusted. Missing data of a certain baseline biochemical variable was replaced with the mean value of the same variable.
As SUA levels are inversely correlated with baseline kidney function, we also examined the predictive effect of SUA levels on eGFR decline in pre-specified stratified analyses according to baseline kidney function (eGFR >90, 60–90, 45–60 and <45 mL/min/1.73 m2). Consistency of the predictive effect of SUA levels on eGFR decline was further assessed in the subgroups based on age (<80 years and ≥80 years), gender, hypertension, diabetes, and coronary artery disease. Potential interactions between SUA levels and subgroups were explored by the likelihood ratio test. To further test the robustness of our findings, sensitivity analyses by assessing the association between SUA levels and the eGFR decline ≥30% over 1-year, 3-year, and 5-year follow-up periods, changing the threshold of eGFR decline to 40%, regrouping the participants into SUA deciles, and excluding participants who had albuminuria or baseline eGFR <60 mL/min/1.73 m2, participants who took urate-lowering agents, or participants who had missing value of other baseline biochemical covariates, were also performed. A cubic spline model was also constructed to describe the continuous association between SUA levels with early renal function decline, with three knots defined at SUA of 4.0 mg/dL, 6.0 mg/dL and 8.0 mg/dL. To further validate the association between hyperuricemia and early renal function decline, time-to-event analyses including Cox proportional hazards regression and competing-risk analysis by the Fine-Gray model, treating death as a competing risk event were also tested. In these time-to-event analyses, the study outcome was defined as an eGFR decline ≥30%. A two-tailed p value < 0.05 was considered significant. All analyses were conducted using the statistical software Stata (version 13.0; Stata Corp., College Station, TX).
Results
Baseline Characteristics
A total of 44,078 eligible participants (23,202 men and 20,876 women) were identified in the database from 2001 to 2010 (Supplemental Figure S1, Table 1). The median age of the study cohort was 71 (interquartile range, 9) years with 52.6% being male. Compared to the reference group (SUA level: 5.0–5.9 mg/dL), increasing SUA levels were associated with the higher age, body mass index, systolic and diastolic blood pressure, fasting plasma glucose and triglyceride levels, leukocyte count, and the percentage of male gender, smoking, alcohol use, hypertension, coronary artery disease, and cerebrovascular disease, but with lower baseline eGFR and high-density lipoprotein cholesterol levels (Table 1).
Table 1.
Demographic and clinical characteristics of study population by serum uric acid levels*.
| Characteristics | All | Serum Uric Acid (mg/dL) | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.0–2.9 | 3.0–3.9 | 4.0–4.9 | 5.0–5.9 | 6.0–6.9 | 7.0–7.9 | 8.0–8.9 | 8.0–9.9 | ≥10 | |||
| Number of participants | 44,078 | 522 | 2,656 | 7,708 | 11,076 | 10,047 | 6,435 | 3,387 | 1,344 | 903 | |
| Demographics | |||||||||||
| Male | 23,202 (52.6) | 165 (31.6) | 713 (26.8) | 2,577 (33.4) | 5,179 (46.8) | 6,070 (60.4) | 4,431 (68.9) | 2,451 (72.4) | 966 (71.9) | 650 (72.0) | <0.001 |
| Age, years | 71 (9) | 71 (9) | 69 (9) | 69 (9) | 70 (10) | 71 (9) | 72 (9) | 72 (9) | 73 (8) | 74 (8) | <0.001 |
| Smoking | 3,718 (8.4) | 22 (4.2) | 124 (4.7) | 440 (5.7) | 796 (7.2) | 940 (9.4) | 735 (11.4) | 382 (11.3) | 158 (11.8) | 121 (13.4) | <0.001 |
| Alcohol use | 6,200 (14.1) | 40 (7.7) | 227 (8.5) | 773 (10.0) | 1,501 (13.6) | 1,585 (15.8) | 1,156 (18.0) | 592 (17.5) | 195 (14.5) | 131 (14.5) | <0.001 |
| BMI, kg/m2 | 24.0 (4.2) | 22.6 (4.4) | 22.8 (4.3) | 23.2 (4.1) | 23.7 (4.1) | 24.3 (4.0) | 24.7 (4.1) | 25.0 (4.1) | 25.1 (4.4) | 25.2 (4.5) | <0.001 |
| Comorbidity | |||||||||||
| Hypertension | 23,352 (53.0) | 238 (45.6) | 1,220 (45.9) | 3,551 (46.1) | 5,614 (50.7) | 5,432 (54.1) | 3,727 (57.9) | 2,089 (61.7) | 859 (63.9) | 622 (68.9) | <0.001 |
| Diabetes | 4,560 (10.3) | 63 (12.1) | 311 (11.7) | 801 (10.4) | 1,133 (10.2) | 1,019 (10.1) | 648 (10.1) | 329 (9.7) | 162 (12.1) | 94 (10.4) | 0.086 |
| Dyslipidemia | 22,584 (51.2) | 269 (51.5) | 1,382 (52.0) | 4,136 (53.7) | 5,761 (52.0) | 5,050 (50.3) | 3,182 (49.4) | 1,673 (49.4) | 682 (50.7) | 449 (49.7) | <0.001 |
| CAD | 4,775 (10.8) | 52 (10.0) | 256 (9.6) | 751 (9.7) | 1,051 (9.5) | 1,105 (11.0) | 806 (12.5) | 449 (13.3) | 172 (12.8) | 133 (14.7) | <0.001 |
| CVD | 340 (0.8) | 5 (1.0) | 24 (0.9) | 48 (0.6) | 83 (0.7) | 77 (0.8) | 51 (0.8) | 27 (0.8) | 18 (1.3) | 7 (0.8) | 0.353 |
| Blood pressure, mmHg | |||||||||||
| Systolic | 133 (27) | 130 (24) | 130 (27) | 131 (25) | 133 (26) | 134 (27) | 135 (27) | 136 (26) | 137 (27) | 138 (26) | <0.001 |
| Diastolic | 76 (15) | 76 (13) | 75 (14) | 75 (15) | 76 (14) | 77 (14) | 77 (15) | 79 (16) | 78 (16) | 79 (16) | <0.001 |
| eGFR, mL/min/1.73 m2 | <0.001 | ||||||||||
| ≥90 | 4,920 (11.2) | 101 (19.3) | 645 (24.3) | 1,576 (20.4) | 1,456 (13.1) | 764 (7.6) | 262 (4.1) | 77 (2.3) | 28 (2.1) | 11 (1.2) | |
| 60–89 | 25,120 (57.0) | 323 (61.9) | 1,533 (57.7) | 4,687 (60.8) | 6,939 (62.6) | 6,034 (60.1) | 3,438 (53.4) | 1,448 (42.8) | 479 (35.6) | 239 (26.5) | |
| 45–59 | 10,466 (23.7) | 80 (15.3) | 401 (15.1) | 1,196 (15.5) | 2,167 (19.6) | 2,537 (25.3) | 2,016 (31.3) | 1,272 (37.6) | 466 (34.7) | 331 (36.7) | |
| 30–44 | 2,892 (6.6) | 12 (2.3) | 65 (2.4) | 206 (2.7) | 443 (4.0) | 590 (5.9) | 565 (8.8) | 476 (14.1) | 296 (22.0) | 239 (26.5) | |
| 15–29 | 526 (1.2) | 5 (1.0) | 5 (0.2) | 24 (0.3) | 46 (0.4) | 93 (0.9) | 124 (1.9) | 92 (2.7) | 65 (4.8) | 72 (8.0) | |
| <15 | 154 (0.3) | 1 (0.2) | 7 (0.3) | 19 (0.2) | 25 (0.2) | 29 (0.3) | 30 (0.5) | 22 (0.6) | 10 (0.7) | 11 (1.2) | |
| Total cholesterol, mg/dL | 198 (47) | 198 (45) | 199 (47) | 201 (47) | 199 (48) | 197 (46) | 195 (47) | 195 (49) | 196 (49) | 195 (52) | <0.001 |
| Triglyceride, mg/dL | 106 (72) | 84.5 (56) | 91 (56) | 96 (60) | 103 67) | 112 (76) | 119 (77) | 125 (83) | 133 (89) | 139 102) | <0.001 |
| HDL-cholesterol, mg/dL | 49 (18) | 55 (26) | 55.2 (25) | 54 (22) | 50 (18) | 47.3 (15) | 46.1 (13) | 46 (12) | 45 (10) | 44 (9) | <0.001 |
| WBC count, /mm3 | 5780 (1890) | 5460 (1900) | 5400 (1855) | 5500 (1800) | 5700 (1720) | 5800 (1820) | 5990 (1900) | 6110 (1920) | 6105 (2115) | 6450 (2160) | <0.001 |
| Albumin, g/dL | 4.3 (0.4) | 4.3 (0.4) | 4.3 (0.4) | 4.3 (0.4) | 4.3 (0.4) | 4.3 (0.4) | 4.3 (0.4) | 4.4 (0.4) | 4.4 (0.4) | 4.3 (0.4) | <0.001 |
| Hemoglobin, g/dL | 13.6 (1.7) | 13.1 (1.6) | 1.31 (1.4) | 13.3 (1.6) | 13.5 (1.6) | 13.7 (1.7) | 13.9 (1.8) | 13.9 (1.9) | 13.8 (2.1) | 13.6 (2.2) | <0.001 |
| Fasting glucose, mg/dL | 99 (18) | 97 (16) | 97 (17) | 98 (17) | 99 (17) | 99 (18) | 101 (19) | 101 (19) | 102 (21) | 102 (22) | <0.001 |
*Values for categorical variables are given as number (percentage); values for continuous variables are given as median (interquartile range).
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; SD, standard deviation; WBC, white blood cell
SI conversion factors: To convert uric acid value to μmol/L, multiply by 59.485; blood pressure value to pascal, multiply by 133.3; cholesterol (total or HDL-cholesterol) value to mmol/L, multiply by 0.0259; triglyceride value to mmol/L, multiply by 0.0113; WBC count to ×109/L, multiply by 0.001; albumin value to g/L, multiply by 10; hemoglobin value to g/L, multiply by 10; glucose value to mmol/L, multiply by 0.055.
Association between SUA levels and the risk of ≥30% eGFR decline in the elderly
There were 1190 participants who experienced early renal function decline with a ≥30% reduction in eGFR over 2 years. In multivariable analyses, hyperuricemic older people had increased risks for early renal function decline, starting at SUA ≥6.0 mg/dL (adjusted odds ratio [aOR] = 1.21, 95% confidence interval [CI] = 1.00–1.45), after adjustment for 20 demographic and clinical variables (Table 2). Notably, the risks for eGFR decline paralleled the increasing SUA levels with the highest risk in SUA group ≥ 10 mg/dL (aOR = 3.35, CI = 2.45–4.59). Remarkably, low SUA levels <3.0 mg/dL were also associated with a higher risk of eGFR decline (aOR = 1.69, CI = 1.08–2.63). After adjusting for proteinuria (dipstick trace or ≥1 + ), hyperuricemia still significantly predicted higher risks for early renal progression (Supplemental Table S1). In attempt to further decipher whether hyperuricemia induced renal dysfunction, the association between SUA levels and eGFR decline ≥30% over 2 years was tested in the participants free from CKD. Hyperuricemia remained significantly associated with early renal function decline in the group excluding participants with positive proteinuria or baseline eGFR <60 mL/min/1.73 m2 (Supplemental Table S1).
Table 2.
Incidence and risks of eGFR decline ≥30% over a 2-year follow-up period in older people.
| Serum uric acid (mg/dL) | Incidence | Logistic Regression Analysis | ||||
|---|---|---|---|---|---|---|
| No. of Events | No. of Participants | Crude Odds Ratio (95% CI) | P | Adjusted Odds Ratio (95% CI)a | P | |
| 2.0–2.9 | 23 (4.4%) | 522 | 2.07 (1.34–3.21) | 0.001 | 1.69 (1.08–2.63) | 0.021 |
| 3.0–3.9 | 64 (2.4%) | 2,656 | 1.11 (0.84–1.47) | 0.463 | 0.91 (0.68–1.20) | 0.491 |
| 4.0–4.9 | 205 (2.7%) | 7,708 | 1.23 (1.02–1.48) | 0.032 | 1.10 (0.91–1.33) | 0.319 |
| 5.0–5.9 | 241 (2.2%) | 11,076 | Reference | Reference | ||
| 6.0–6.9 | 233 (2.3%) | 10,047 | 1.07 (0.89–1.28) | 0.483 | 1.21 (1.00–1.45) | 0.048 |
| 7.0–7.9 | 220 (3.4%) | 6,435 | 1.59 (1.32–1.92) | <0.001 | 1.91 (1.58–2.32) | <0.001 |
| 8.0–8.9 | 103 (3.0%) | 3,387 | 1.41 (1.12–1.78) | 0.004 | 1.77 (1.38–2.26) | <0.001 |
| 9.0–9.9 | 41 (3.1%) | 1,344 | 1.41 (1.01–1.98) | 0.043 | 1.61 (1.13–2.29) | 0.008 |
| ≥10 | 60 (6.6%) | 903 | 3.20 (2.39–4.28) | <0.001 | 3.35 (2.45–4.59) | <0.001 |
aAdjusted for age, sex, body mass index, smoking, alcohol drinking, comorbidities and all biochemical data in Table 1.
Abbreviation: CI, confidence interval; eGFR, estimated glomerular filtration rate.
SI conversion factors: To convert uric acid value to μmol/L, multiply by 59.485.
Hyperuricemia and the risk of ≥30% eGFR decline according to baseline renal function
As SUA levels are inversely correlated with baseline kidney function, we also examined the effect-modifying role of baseline eGFR on hyperuricemia-associated early renal function decline. In the strata of eGFR >90 mL/min/1.73 m2 (aOR = 3.05, CI = 1.84–5.04), 60–90 mL/min/1.73 m2 (aOR = 1.77, CI = 1.37–2.28), and 45–60 mL/min/1.73 m2 (aOR = 2.47, C = 1.37–4.44), hyperuricemia was significantly associated with increased risks toward early eGFR decline once SUA levels exceeded 7.0 mg/dL. In the strata of baseline eGFR <45 mL/min/1.73 m2, risks of hyperuricemia-associated early renal function decline were significantly higher only when SUA levels surpassed 10.0 mg/dL (aOR = 1.96, CI = 1.02–3.79) (Fig. 1).
Figure 1.
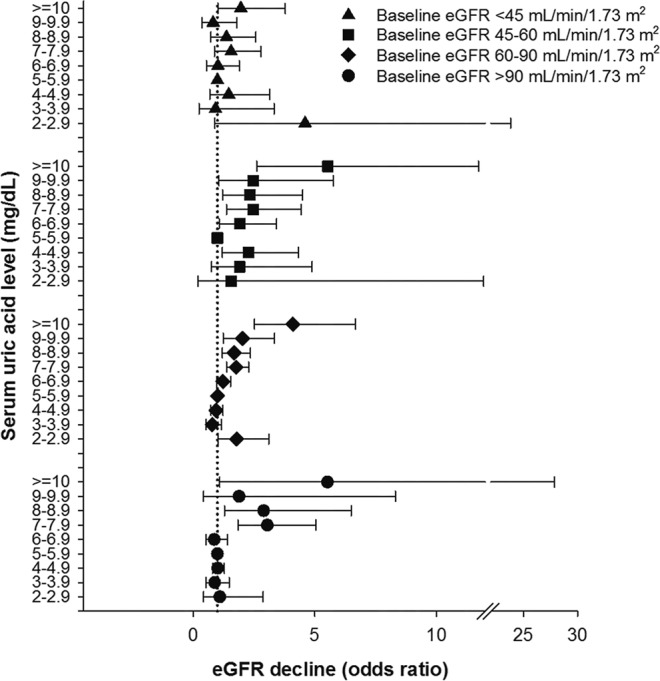
Association between serum uric acid levels and risks of early renal function decline stratified by baseline estimated glomerular filtration rate (eGFR). Odds ratios were calculated by multivariable logistic regression after adjusting for 20 demographic and clinical variables. Serum uric acid levels of 5.0–5.9 mg/dL served as the reference group. Bars denote 95% confidence intervals.
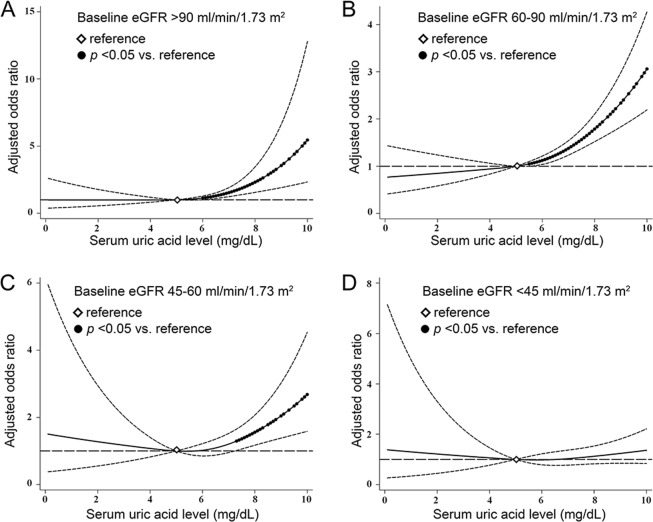
In order to offer more insight on the association between SUA levels and early renal function decline, SUA levels were treated as continuous variables and analyzed by cubic spline models with the reference at SUA of 5.0 mg/dL. Higher SUA levels were consistently associated with elevated risks of early renal function decline in the baseline eGFR >90, 60–90 and 45–60 ml/min/1.73 m2 strata. The risks of eGFR decline started to significantly increase once SUA levels went beyond 6.0 mg/dL in the baseline eGFR >90 ml/min/1.73 m2 strata, beyond 5.5 mg/dL in the baseline eGFR 60–90 ml/min/1.73 m2 strata, and beyond 7.3 mg/dL in the baseline eGFR 45–60 ml/min/1.73 m2 strata (Fig. 2).
Figure 2.
Cubic spline models for the association of serum uric acid levels with the risks of early renal function decline among strata of baseline eGFR (A) >90 ml/min/1.73 m2, (B) 60–90 ml/min/1.73 m2, (C) 45–60 ml/min/1.73 m2, and (D) <45 ml/min/1.73 m2. Models were adjusted for 20 demographic and clinical variables. Filled circles denote statistical significance (p < 0.05) compared to the reference (diamond) serum uric acid level of 5.0 mg/dL. Solid line (—) denotes adjusted odds ratio and dash line (—) denotes 95% confidence intervals.
Subgroup and sensitivity analysis of hyperuricemia-associated ≥30% eGFR Decline
In subgroups analyses, the association between hyperuricemia and early renal function decline remained consistent across all subgroups of interest. There was no significant interaction between SUA levels and subgroups on the risks of ≥ 30% eGFR decline over 2 years (Fig. 3).
Figure 3.
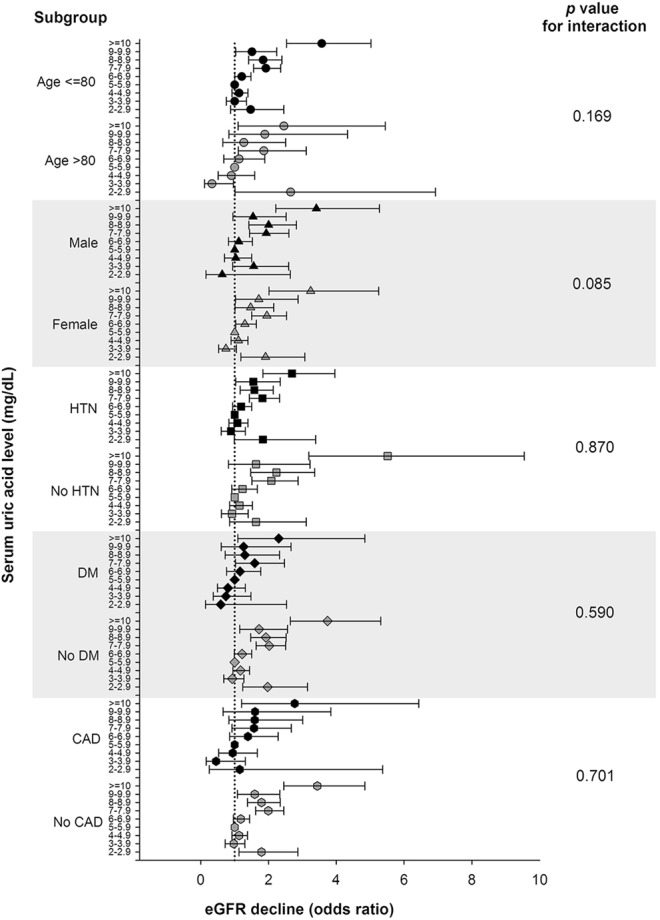
Subgroup analysis of the association between serum uric acid levels and risks of early renal function decline among older people. Odds ratios were calculated by multivariable logistic regression after adjustment for 20 demographic and clinical variables. Serum uric acid levels of 5.0–5.9 mg/dL served as the reference group. Bars denote 95% confidence intervals.
In the sensitivity analyses, where excluding participants taking urate-lowering agents or having missing baseline biochemical data, changing the threshold for eGFR to 40%, or re-grouping the participants into deciles, the associations between hyperuricemia and the risks of early renal function decline were consistently significant (Supplemental Tables S1–S3). In the time-to-event analyses, multivariable Cox proportional hazards regression analyses further confirmed that hyperuricemia was associated with 1.12-fold (95% CI = 1.03–1.22, for SUA 6.0–6.9 mg/dL) to 1.6-fold (95% CI = 1.37–1.86, SUA ≥ 10.0 mg/dL) higher hazards to develop early renal function decline. Competing-risk analyses also produced consistent results (Table 3).
Table 3.
Cox regression and competing-risk analyses for the risks of eGFR decline ≥0% over 2 years in older people.
| Serum uric acid (mg/dL) | Cox Regression Analysis | Competing Risk Analysis | ||
|---|---|---|---|---|
| Adjusted Hazard Ratio (95% CI)a | P | Adjusted Hazard Ratio (95% CI)a | P | |
| 2.0–2.9 | 1.12 (0.86–1.47) | 0.407 | 1.13 (0.87–1.47) | 0.374 |
| 3.0–3.9 | 1.01 (0.87–1.17) | 0.898 | 1.00 (0.87–1.16) | 0.955 |
| 4.0–4.9 | 1.01 (0.92–1.11) | 0.878 | 1.00 (0.91–1.10) | 0.965 |
| 5.0–5.9 | Reference | Reference | ||
| 6.0–6.9 | 1.12 (1.03–1.22) | 0.007 | 1.12 (1.03–1.22) | 0.006 |
| 7.0–7.9 | 1.19 (1.08–1.30) | <0.001 | 1.19 (1.08–1.31) | <0.001 |
| 8.0–8.9 | 1.29 (1.16–1.43) | <0.001 | 1.26 (1.14–1.41) | <0.001 |
| 9.0–9.9 | 1.36 (1.18–1.57) | <0.001 | 1.31 (1.13–1.51) | <0.001 |
| ≥10 | 1.60 (1.37–1.86) | <0.001 | 1.47 (1.26–1.73) | <0.001 |
aAdjusted for age, sex, body mass index, smoking, alcohol drinking, comorbidities and all biochemical data in Table 1.
Abbreviation: CI, confidence interval; eGFR, estimated glomerular filtration rate.
SI conversion factors: To convert uric acid value to μmol/L, multiply by 59.485.
The independent association between hyperuricemia and early renal function decline remained consistent either assessing the ≥ 30% eGFR reduction over 1, 3, or 5 years of follow-up periods. However, hypouricemia with SUA levels <3.0 mg/dL was no longer significantly associated with the higher risk of ≥30% eGFR decline over 3 years (aOR = 1.22, 95% CI = 0.72–2.06) and 5 years (aOR = 1.37, 95% CI = 0.75–2.53) as compared to the reference group (Supplemental Tables S4–S6).
Discussion
To the best our knowledge, the present study is the largest study to date to examine the association between hyperuricemia and early renal function decline among older people. By utilizing a large population-based elderly cohort including 44,078 participants, our study found that hyperuricemia with SUA levels ≥6.0 mg/dL carried a significantly higher risk for early renal function decline in terms of eGFR reduction ≥30% over 2 years. Notably, the risk of hyperuricemia for early renal function decline elevated in parallel with increasing SUA levels. This independent association between hyperuricemia and early renal function decline was consistent across subgroups and remained robust in the sensitivity analyses. Previous studies regarding the prognostic role of SUA on renal function decline yielded conflicting results, which were partly attributable for differences in sample size and the degree of adjustment for possible confounding factors18–21. Although most reports controlled age, sex, body weight, blood pressure, and baseline serum creatinine level, some investigators did not adjust for smoking21, alcohol consumption18–21, history of cardiovascular diseases18,19, and fasting glucose levels19–21. After fully adjustment for these confounding factors, our study confirmed that hyperuricemia independently predicted early renal function decline with a ≥30% eGFR decline over 2 years. In contrast to our findings, Nacak et al. did not find a significant association between renal function decline and the increase of SUA in stage III to IV CKD patients21. Our study indicated that hyperuricemia remained an independent predictor for early kidney function decline when SUA ≥7.0 mg/dL for those with baseline eGFR ≥45 mL/min/1.73 m2 and when SUA ≥10.0 mg/dL for those with baseline eGFR <45 mL/min/1.73 m2 even after extensive adjustment for possible confounding factors. This discrepancy may be explained by the relatively small patient number and absent adjustment of several confounders with kidney disease progression in Nacak’s study21. Furthermore, some other previous studies examined the association between quintiles of hyperuricemia and renal function decline18,19. This quantile classification approach is of less clinical applicability and also limited to unequal reference SUA levels18,19. By contrast, our large-scale cohort study can determine the threshold of SUA level ≥6.0 mg/dL above which the risk for eGFR decline is increased.
The present study also showed that hyperuricemia-associated higher risks for early renal function decline were consistently significant across different strata of baseline kidney function. SUA levels ≥7.0 mg/dL in older people with eGFR 45–60, 60–90 and >90 mL/min/1.73 m2 were associated with higher chances for early renal function decline even after adjusting other risks factors for renal disease progression. In those with eGFR <45 mL/min/1.73 m2, the risk of early renal function decline did not significantly increase until SUA levels exceeded 10.0 mg/dL. The higher SUA threshold for early renal function decline in the strata of eGFR <45 mL/min/1.73 m2 may be explained by that advanced renal dysfunction often accompanies with an increasing prevalence of non-traditional cardiovascular risk factors such as chronic inflammation, oxidative stress and calcium-phosphate imbalance30. These non-traditional factors could also result in renal deterioration and attenuate the contribution of hyperuricemia. In accordance to our findings, Liu et al. recently discovered that increased SUA had a causal effect in the Chinese older people with eGFR ≥80 mL/min/1.73 m2 by a Mendelian randomization approach31. Conversely, Hughes et al. found that increased SUA due to genetic variants of urate transporters was associated with improved renal function by utilizing a similar genetic statistical analysis32. Although Mendelian randomization is a valuable tool by using genetic variants as instrumental variables to test the causal relationship between SUA and eGFR change, this approach presumes that the genetic instruments should have strongly association with SUA levels, affect outcome solely through SUA, and have no association with known confounders33. The validity of genetic instruments was only partly verified in Hughes’ study and potentially influenced the association between SUA and renal function32. It should be also noted that the cohort analyzed by Hughes et al.32 were younger than ours. Clearly, further large-scale studies are still required to disentangle the causal mechanism between hyperuricemia and renal dysfunction. Taken together, our study provided important clinical insights that SUA levels ≥7.0 mg/dL in older people with eGFR ≥45 mL/min/1.73 m2 and SUA levels ≥10.0 mg/dL in those with eGFR <45 mL/min/1.73 m2 should prompt immediate surveillance of kidney function and management of eGFR decline.
Interestingly, in addition to hyperuricemia, low SUA levels <3.0 mg/dL were also associated with higher risks for early renal function decline in older people, suggesting a U-shaped association between SUA levels and eGFR decline. Increasing evidence has suggested that high eGFR is paradoxically associated with increased cardiovascular mortality, probably mediated by malnutrition34,35. High eGFR value either indicates a true high GFR or low serum creatinine level. Both SUA and creatinine are markers for nutritional status36,37. Malnourished people with both low SUA and creatinine levels can increase infection risks38, which probably accounts for higher risks of eGFR decline in our participants of low SUA levels and high eGFR.
The present study had several strengths. First, this is the first large-scale cohort study to examine the association of SUA levels with a novel validated renal endpoint in older people. Utilization of a 44,078-elderly cohort during a 10-year study period provided adequate statistical power. Second, by using a 1-mg/dL SUA increment to classify patients, our results were easily applied for daily practice to risk-stratify older patients. Third, we utilized the CKD-EPI equation, which provides more reliable estimation of renal function2,30. Fourth, the independent association between hyperuricemia and high risks for eGFR decline was ascertained in different statistical approaches including multivariable logistic and Cox regression, cubic spline model, sensitivity and subgroup analyses. Nonetheless, several potential limitations should be acknowledged. First, a single baseline SUA level was used to predict early renal progression. Second, albuminuria was determined by the semiquantitative dipstick analysis. However, positive (≥1 + ) and negative dipstick analyses have been reported to well correlate with a urine albumin-to-creatinine ratio ≥30 mg/g or <30 mg/g, respectively39,40. Third, the hospitalized or institutionalized older people were not included. Fourth, the medical history of participants was obtained by self-reported questionnaires and information bias cannot be excluded. Nonetheless, agreement between self-reported and hospital-acquired medical record is found to be substantial for diabetes, hypertension, myocardial infarction and stroke41. Finally, all study participants were Taiwanese and the conclusions may not be generalized to other ethnicities.
Conclusion
The present study found that an SUA level ≥6.0 mg/dL was independently associated with early renal function decline with ≥30% reduction in eGFR over 2 years in the elderly. Our data provided the epidemiological evidence that hyperuricemia was an independent risk factor for early decline of kidney function in the older population. Stringent surveillance regarding renal progression should be performed in the older people with hyperuricemia.
Supplementary information
Acknowledgements
This work was supported in part by the Ministry of Science and Technology [grant number MOST 102-2314-B-010-004-MY3, MOST 105-2314-B-010-016, MOST 106-2314-B-010-039-MY3, MOST 107-2314-B-075-064-MY3], the Taipei Veterans General Hospital [grant number V105C-013, V106C-147, V107C-127, V107B-037], the Department of Health, Taipei City Government [grant number 10101-62-083, 10501-62-081, 10601-62-036, 10701-62-054], Wan-Fang Hospital [grant number 106-swf-06], Foundation for Poison Control, and Ministry of Education’s Aim for the Top University Plan in the National Yang-Ming University, Taiwan. The funding sources had no role in the study design, conduct or reporting.
Author Contributions
Study conception and design: W.C. Tseng, Y.T. Chen; Acquisition of data. Y.T. Chen; Analysis and interpretation of the data: W.C. Tseng, Y.T. Chen, Y.P. Lin, D.C. Tarng; Drafting the article: W.C. Tseng; Critical revision of the article for important intellectual content: Y.T. Chen, Y.P. Lin, S.M. Ou, C.Y. Yang, C.H. Lin, D.C. Tarng; Final approval of the article: all authors.
Data Availability
The data and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because access to these data is contractually controlled by the Taipei City Hospital and the Department of Health, Taipei City Government. Only analytic methods are available on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
A comprehensive list of consortium members appears at the end of the paper.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei-Cheng Tseng and Yung-Tai Chen contributed equally.
Contributor Information
Der-Cherng Tarng, Email: dctarng@vghtpe.gov.tw.
The Taiwan Geriatric Kidney Disease (TGKD) Research Group:
Der-Cherng Tarng, Wei-Cheng Tseng, Ming-Tsun Tsai, Shuo-Ming Ou, Chih-Yu Yang, Yao-Ping Lin, Yu-Hsin Chen, Yi-Fang Chuang, Liang-Kung Chen, Kwua-Yun Wang, Chia-Jen Shih, Yung-Tai Chen, Yi-Sheng Lin, Szu-Chun Hung, Ko-Lin Kuo, Tung-Po Hung, Fen-Hsiang Hu, Nien-Jung Chen, Yu-Chi Chen, Chi-Hung Lin, Tung-Hu Tsai, Shie-Liang Hsieh, Yau-Huei Wei, Chih-Cheng Hsu, Jia-Sin Liu, Yu-Kang Chang, and Ming-Han Chiang
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37529-z.
References
- 1.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens LA, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP) Am. J. Kidney Dis. 2010;55:S23–33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol. Dial. Transplant. 2013;28:1810–1819. doi: 10.1093/ndt/gfs470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rifkin DE, et al. Rapid kidney function decline and mortality risk in older adults. Arch. Intern. Med. 2008;168:2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locatelli F, Vecchio LD, Pozzoni P. The importance of early detection of chronic kidney disease. Nephrol. Dial. Transplant. 2002;17(Suppl 11):2–7. doi: 10.1093/ndt/17.suppl_11.2. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang WX, et al. Predictors and the Subsequent Risk of End-Stage Renal Disease - Usefulness of 30% Decline in Estimated GFR over 2 Years. PLoS One. 2015;10:e0132927. doi: 10.1371/journal.pone.0132927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita K, et al. Risk of end-stage renal disease in Japanese patients with chronic kidney disease increases proportionately to decline in estimated glomerular filtration rate. Kidney Int. 2016;90:1109–1114. doi: 10.1016/j.kint.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzali M, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Renal Physiol. 2002;282:F991–997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Lozada LG, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, et al. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002;13:2888–2897. doi: 10.1097/01.ASN.0000034910.58454.FD. [DOI] [PubMed] [Google Scholar]
- 13.Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am. J. Kidney Dis. 2013;61:134–146. doi: 10.1053/j.ajkd.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch. Intern. Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iseki K, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am. J. Kidney Dis. 2004;44:642–650. doi: 10.1016/S0272-6386(04)00934-5. [DOI] [PubMed] [Google Scholar]
- 16.Obermayr RP, et al. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 2008;19:2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner DE, et al. Uric acid and incident kidney disease in the community. J. Am. Soc. Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chonchol M, et al. Relationship of uric acid with progression of kidney disease. Am. J. Kidney Dis. 2007;50:239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Yen CJ, et al. Hyperuricemia associated with rapid renal function decline in elderly Taiwanese subjects. J. Formos. Med. Assoc. 2009;108:921–928. doi: 10.1016/S0929-6646(10)60004-6. [DOI] [PubMed] [Google Scholar]
- 20.Altemtam N, Russell J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD) Nephrol. Dial. Transplant. 2012;27:1847–1854. doi: 10.1093/ndt/gfr561. [DOI] [PubMed] [Google Scholar]
- 21.Nacak H, et al. Uric acid is not associated with decline in renal function or time to renal replacement therapy initiation in a referred cohort of patients with Stage III, IV and V chronic kidney disease. Nephrol. Dial. Transplant. 2015;30:2039–2045. doi: 10.1093/ndt/gfv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YT, et al. Periodontal Disease and Risks of Kidney Function Decline and Mortality in Older People: A Community-Based Cohort Study. Am. J. Kidney Dis. 2015;66:223–230. doi: 10.1053/j.ajkd.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Shih CJ, et al. Observed Blood Pressure and Mortality Among People Aged 65 Years and Older: A Community-Based Cohort Study. J. Am. Med. Dir. Assoc. 2016;17:654–662. doi: 10.1016/j.jamda.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Ou SM, Chen YT, Shih CJ, Tarng DC. Impact of physical activity on the association between lipid profiles and mortality among older people. Sci. Rep. 2017;7:8399. doi: 10.1038/s41598-017-07857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health Examination for the Elderly. Public Health of Taipei, Annual Report 2009. page 55. http://www.health.gov.tw/Portals/0/Administrative/98ce/98.pdf Accessed September 1, 2018.
- 26.Gochman N, Schmitz JM. Automated determination of uric acid, with use of a uricase-peroxidase system. Clin. Chem. 1971;17:1154–1159. [PubMed] [Google Scholar]
- 27.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsushita K, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv. Chronic Kidney Dis. 2012;19:358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarnak MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, et al. Mendelian randomization analysis indicates serum urate has a causal effect on renal function in Chinese women. Int. Urol. Nephrol. 2017;49:2035–2042. doi: 10.1007/s11255-017-1686-8. [DOI] [PubMed] [Google Scholar]
- 32.Hughes K, Flynn T, de Zoysa J, Dalbeth N, Merriman TR. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int. 2014;85:344–351. doi: 10.1038/ki.2013.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376. doi: 10.1136/bmj.j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inrig JK, et al. Risk for cardiovascular outcomes among subjects with atherosclerotic cardiovascular disease and greater-than-normal estimated glomerular filtration rate. Clin. J. Am. Soc. Nephrol. 2007;2:1215–1222. doi: 10.2215/CJN.00930207. [DOI] [PubMed] [Google Scholar]
- 35.Ou SM, et al. Association of estimated glomerular filtration rate with all-cause and cardiovascular mortality: the role of malnutrition-inflammation-cachexia syndrome. J Cachexia Sarcopenia Muscle. 2016;7:144–151. doi: 10.1002/jcsm.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beberashvili I, et al. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition. 2015;31:138–147. doi: 10.1016/j.nut.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Shastri S, Sarnak MJ. Chronic kidney disease: High eGFR and mortality: high true GFR or a marker of frailty? Nat Rev Nephrol. 2011;7:680–682. doi: 10.1038/nrneph.2011.153. [DOI] [PubMed] [Google Scholar]
- 38.Hickson M. Malnutrition and ageing. Postgrad. Med. J. 2006;82:2–8. doi: 10.1136/pgmj.2005.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White SL, et al. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am. J. Kidney Dis. 2011;58:19–28. doi: 10.1053/j.ajkd.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Davidson MB, Smiley JF. Relationship between dipstick positive proteinuria and albumin:creatinine ratios. J. Diabetes Complications. 1999;13:52–55. doi: 10.1016/S1056-8727(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 41.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J. Clin. Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because access to these data is contractually controlled by the Taipei City Hospital and the Department of Health, Taipei City Government. Only analytic methods are available on reasonable request.