Abstract
In the marine environment, macroalgae face changing environmental conditions and some species are known for their high capacity to adapt to the new factors of their ecological niche. Some macroalgal metabolites play diverse ecological functions and belong to the adaptive traits of such species. Because algal metabolites are involved in many processes that shape marine biodiversity, understanding their sources of variation and regulation is therefore of utmost relevance. This work aims at exploring the possible sources of metabolic variations with time and space of four common algal species from the genus Lobophora (Dictyotales, Phaeophyceae) in the New Caledonian lagoon using a UHPLC-HRMS metabolomic fingerprinting approach. While inter-specific differences dominated, a high variability of the metabolome was noticed for each species when changing their natural habitats and types of substrates. Fatty acids derivatives and polyolefins were identified as chemomarkers of these changing conditions. The four seaweeds metabolome also displayed monthly variations over the 13-months survey and a significant correlation was made with sea surface temperature and salinity. This study highlights a relative plasticity for the metabolome of Lobophora species.
Introduction
Together with marine sponges, macroalgae represent a high source of chemical diversity, also called specialized metabolites. Today, over 3,000 specialized metabolites were identified from red (Rhodophyta), green (Chlorophyta) and brown (Ochrophyta) algae1. Tropical macroalgal taxa have been shown to produce a higher diversity of metabolites than their temperate counterparts, with a majority of halogenated metabolites, phenolic, terpenoids, or acetogenins2. These small molecules (<1500 Da) are mainly regulated by genetic, developmental and environmental factors3. They can be seen as adaptive traits that have evolved under natural selection4. They are involved in chemical communication and play diverse ecological functions in macroalgae. Even if the best known and studied ecological role of these metabolites is the deterrence against competitors and herbivores5,6, they can also act as defense against pathogens7,8 (e.g. bacteria, fungus, virus), epibionts9, UV protector10 or sexual pheromones11. These chemicals might also be involved in the competition for space with other benthic organisms12,13.
Specialized metabolite concentrations may vary between and within species, temporally and spatially14,15 and their concentration can be affected by environmental factors (biotic and abiotic)3,16. Nevertheless, most studies on algal chemical variability used bioassays on the crude extract as a proxy of metabolites production or they focused on specific families of compounds therefore overlooking many metabolites likely to play important ecological functions. Because marine algae face changes in the surrounding physico-chemical and biotic parameters17, it is highly relevant to study their global metabolic response when exposed to changing environmental conditions.
The advent of metabolomics allows the study of a large set of metabolites (metabolome), through metabolomic fingerprinting approaches. The variation of macroalgal metabolomic fingerprints will bring useful information to the response of the seaweed to environmental changes. Several studies explored the metabolomic response of marine organisms to different biotic and abiotic factors. For example, the impacts of salinity and UV stress on the metabolome were explored in the brown macroalgae Sargassum cymosum18. The exo-metabolome was studied in the green algae Ulva, revealing differences according to growth stage and interaction with bacteria19. Defense mechanisms against herbivorous were assessed based on the metabolic profile of Gracilaria vermiculophylla20. Metabolomic changes related to chemical mediation are also studied for biotic interactions, notably in coral-algal competition21. However these interactions are still scarcely investigated at the global metabolomic level. Changes in Asparagopsis taxiformis metabolomic fingerprint were observed after contact with the coral Astroides calycularis22 and different coral-algal assemblages can alter the coral metabolome23. These above-mentioned studies indicate that the metabolome of macroalgae is influenced by abiotic and biotic factors, supporting its involvement in biological processes and adaptation to the environment. However, metabolomic studies specifically focused on spatio-temporal variations are rare for macroalgae (but see the study on the red alga A. taxiformis from temperate versus tropical regions24). Understanding how the metabolome varies at these scales and how they respond to different factors is relevant to understand adaptive phenomena. It can also help to understand the biochemical pathways involved in macroalgal/microbial cells in response to different conditions16,25.
Here, we studied the metabolomic variations in time and space of four common species of the brown algal genus Lobophora (Dictyotales, Phaeophyceae) in the New Caledonian lagoon using an untargeted UHPLC-QToF metabolomic fingerprinting approach. Lobophora is a key macroalgal component of tropical coral reefs, especially in New Caledonia where they are commonly found. Recent studies have unveiled their high species richness26,27. Importantly, some species are closely associated with corals and are strongly involved in coral-algal interactions28, leading in some cases to negative impacts on corals29. Only few rather non-polar metabolites have been characterized so far and they are likely to be derived from long chain fatty acids30,31. In this study, we chose four species of different morphologies living in various natural habitats across the lagoon of New Caledonia, i.e. L. monticola, L. obscura, L. rosacea and L. sonderii26. Lobophora rosacea usually grows attached to the bedrock by a basal mound of hairs niched within Acropora spp. branches or grows epiphyticly over L. sonderii. Lobophora monticola is also associated with branching corals (e.g. Acropora, Montipora) in turbid waters, and its blades can grow partially or completely in contact with them. Lobophora sonderii forms dense erected blades among Sargassum and Turbinaria beds. Finally, L. obscura grows on dead coral, coral rubbles or rock firmly attached to the substratum by ventral rhizoids.
First, we investigated the temporal variability of the metabolic fingerprints of the four Lobophora species during a 13-months survey. Physico-chemical parameters were assessed during the survey to highlight some factors likely to affect the metabolome composition. We then studied the spatial metabolomic variation of three species, either in their natural habitat by looking at different sites across the lagoon (five sites), or after short-term in situ cross-transplantations between different habitats (two species, three habitats).
Results
Temporal variation
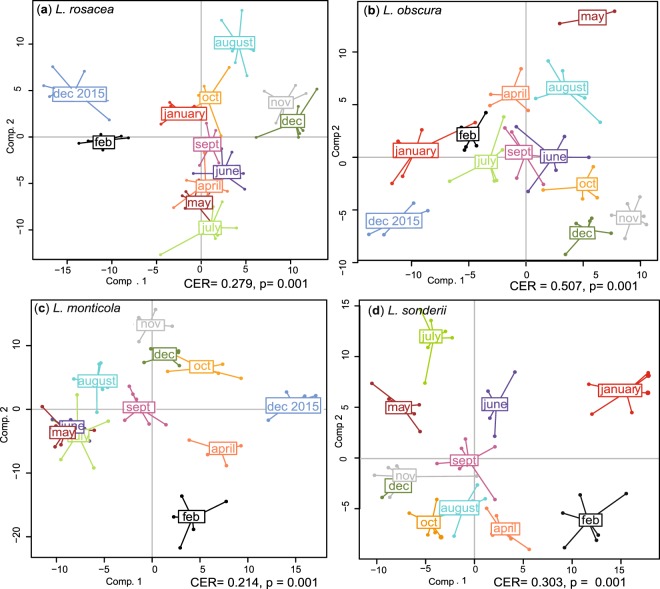
Temporal variation of the metabolome of the four Lobophora species was studied monthly over a 13-months period. After filtration, a total of 326 features were detected in L. rosacea (LR), 310 in L. sonderii (LS), 404 in L. monticola (LM), and 436 in L. obscura (LO). Supervised analyses PPLS-DA (Fig. 1) conducted for each species supported a significant effect of time on the metabolomic fingerprinting (CERLR = 0.279, CERLS = 0.303, CERLM = 0.214, CERLO = 0.507, p = 0.001; CER = Mean classification error rate with p-value after double cross model validation).
Figure 1.
Powered Partial Least-Squares-Discriminant Analysis (PPLS-DA) score plots of the metabolome profiles observed in the four Lobophora species over a 13-months period. (a) Lobophora rosacea, (b) Lobophora obscura, (c) Lobophora monticola and (d) Lobophora sonderii (CER = Mean classification error rate with p-value after double cross model validation).
The metabolome of L. rosacea, L. monticola and L. sonderii were more variable between months (post-hoc tests Tables S1–S3) compared to L. obscura (highest CER, post-hoc tests Table S4) even if no clear seasonal pattern was observed. December 2015 and December 2016 presented significant distinct metabotypes (metabolic phenotypes) for each species and the metabolomic variation does not appear to be yearly cyclic. The metabolome of December 2015 was more distinct on PPLS-DA loading plots for L. rosacea and L. monticola as was January for L. sonderii. These summer months exhibited high mean values of photoperiod, global radiation, Photosynthetically Active Radiation (PAR, one measure per month), sea surface temperature (SST) and salinity.
The correlation between environmental factors (monthly average) and the temporal metabolomic variability was then investigated for each species by PERMANOVA (9999 permutations): SST, photoperiod, global radiation, rainfall, salinity and PAR (Table 1). Salinity and SST were the main factors correlated with metabolomic variations in L. rosacea (pseudo-Fsalinity = 3.51, pseudo-Fsst = 3.35, p < 0.005), L. sonderii (pseudo-Fsalinity = 5.91, pseudo-Fsst = 3.68, p < 0.0001), L. monticola (pseudo-Fsalinity = 12.65, pseudo-Fsst = 10.24, p < 0.001) and L. obscura (pseudo-Fsalinity = 4.37, pseudo-Fsst = 4.42, p < 0.001). But other factors were also significantly correlated with L. sonderii metabolomic fingerprinting: photoperiod (pseudo-F = 4.89, p = 0.0001), PAR (pseudo-F = 3.5, p = 0.0005) and global radiation (pseudo-F = 2.55, p = 0.0063). All environmental factors significantly affected L. monticola metabolomic variability (Table 1, p < 0.001).
Table 1.
Results of Permanova tests (9999 permutations) on environmental factors explaining the temporal metabolomic variability in the four Lobophora species (LR: Lobophora rosacea, LO: Lobophora obscura, LM: Lobophora monticola and LS: Lobophora sonderii).
| Species | Response variable | F | Pr(>F) |
|---|---|---|---|
| LR | photoperiod | 1.80 | 0.0597 |
| PAR | 2.31 | 0.0120* | |
| global_radiation | 1.12 | 0.3017 | |
| rainfall | 1.54 | 0.1103 | |
| salinity | 3.51 | 0.0013** | |
| SST | 3.35 | 0.0020** | |
| LO | photoperiod | 2.61 | 0.0133* |
| PAR | 1.50 | 0.1131 | |
| global_radiation | 1.70 | 0.0803 | |
| rainfall | 1.32 | 0.1871 | |
| salinity | 4.37 | 0.0004*** | |
| SST | 4.42 | 0.0003*** | |
| LM | photoperiod | 5.24 | 0.0001*** |
| PAR | 4.44 | 0.0011** | |
| global_radiation | 5.8 | 0.0002*** | |
| rainfall | 4.9 | 0.0003*** | |
| salinity | 12.65 | 0.0001*** | |
| SST | 10.24 | 0.0001*** | |
| LS | photoperiod | 4.89 | 0.0001*** |
| PAR | 3.50 | 0.0005*** | |
| global_radiation | 2.55 | 0.0063** | |
| rainfall | 1.26 | 0.2234 | |
| salinity | 5.91 | 0.0001*** | |
| SST | 3.68 | 0.0003*** |
No clear chemomarkers driving differences between metabotypes of each month could be identified. Differentiation appeared to rely on several minor ions and no compound could be identified with the molecular network obtained from GNPS.
Spatial variation
Significant differences were observed between the metabolomes of the MeOH extracts of three species (L. rosacea, L. sonderii and L. obscura) and five sites studied (Ricaudy, Crouy, Canard islet, Larégnère and Banc Nord). The species explained most of the metabolic variability observed (PERMANOVA, pseudo-F = 19.34, p = 1e-04) compared to sites (pseudo-F = 11.28, p = 1e-04). Metabolites features were used for Hierarchical Clustering Analysis (HCA). The resulting dendrogram (Fig. S1) separated three clusters corresponding to each species: (A) L. rosacea, (B) L. sonderii and (C) L. obscura, supporting a specificity of the metabolome for each species over the influence of the site they come from. No specific chemomarker could be annotated but the inter-specific metabolomic variability of these Lobophora species has been previously investigated by Gaubert et al. (submitted).
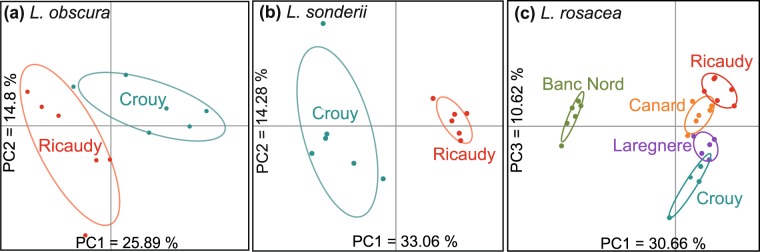
In a second step, metabolomic fingerprintings were analyzed for each species according to sites. For L. obscura, metabotypes from Ricaudy were compared to Crouy. The variance explained by the two first components on the principal component analysis (PCA, Fig. 2a) was 40.69% and two significant distinct clusters were visible (PPLS-DA, CER = 0.054, p = 0.007). Similarly, L. sonderii metabotype from Ricaudy was compared to Crouy. The variance was 47.34% (Fig. 2b) and the metabolome was significantly different between specimens from Ricaudy and Crouy (PPLS-DA, CER = 0, p = 0.004). Metabotypes of L. rosacea from Ricaudy, Crouy, Larégnère, Canard Islet and Banc Nord showed more evident separation along the 1–3 axes, with 41.29% of variance (Fig. 2c). Lobophora rosacea presented significant different metabotypes at each site (PPLS-DA, CER = 0.056, p = 0.001, post-hoc p < 0.05 for each pair, Table S5).
Figure 2.
Principal component analysis (PCA) of metabolomic fingerprints of (a) Lobophora obscura, (b) Lobophora sonderii and (c) Lobophora rosacea from different sites: Crouy, Ricaudy, Larégnère, Canard islet and Banc Nord. Only two sites were sampled for Lobophora sonderii and Lobophora obscura because these species were not found at Larégnère, Banc Nord and Canard islet.
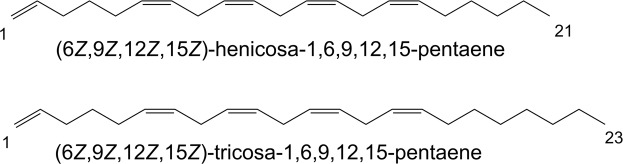
Correlation with the habitat (Table S6) did not show any clear pattern between species metabotypes and sites. The chemomarkers that explain the differences between sites where highlighted and appeared to be mainly minor intensity ions. However, we were able to annotate 9 chemomarkers after molecular network with GNPS, the use of Sirius and previous in-house chemical work on Lobophora. We putatively found one saturated C17 and three C20-C22 polyunsaturated and oxygenated fatty acids derivatives, two C14-C16 unsaturated alcohols and three polyolefins with 16, 21 and 23 carbon atoms respectively (Table 2). The last two polyolefins (C21H34 and C23H38) contain five unsaturations and are major compounds (see Fig. S2). Based on an analogy with already isolated lobophorenols and NMR data on a fraction containing these compounds (see Fig. S3 for example), we propose the structures below (Fig. 3). They could reasonably be assigned to (6Z,9Z,12Z,15Z)-henicosa-1,6,9,12,15-pentaene, as seen in Fucus vesiculosus32 and (6Z,9Z,12Z,15Z)-tricocosa-1,6,9,12,15-pentaene.
Table 2.
Chemomarkers responsible for the difference according to sites in Lobophora rosacea, Lobophora sonderii and Lobophora obscura (ion [M + NH4]+).
| Ion | Ion m/z | RT (s) | Molecular formula | Diff. ppm | Score MFG | Species |
|---|---|---|---|---|---|---|
| M230T256 | 230.2473 | 256 | C14H28O | 3.26 | 93.86 | L. rosacea & L. sonderii |
| M242T309 | 242.2841 | 309 | C16H32 | −0.15 | 89.47 | L. rosacea |
| M258T310 | 258.2797 | 310 | C16H32O | −1.72 | 96.4 | L. rosacea & L. sonderii |
| M288T251 | 288.2904 | 251 | C17H34O2 | −0.7 | 85.64 | L. rosacea |
| M304T338 | 304.3026 | 338 | C21H34 | −2.31 | 93.08 | L. rosacea & L. obscura |
| M332T377 | 332.3332 | 377 | C23H38 | −5.52 | 86.36 | L. rosacea & L. obscura |
| M344T346 | 344.3126 | 346 | C20H38O3 | −3.73 | 89.04 | L. rosacea |
| M368T338 | 368.3165 | 338 | C22H38O3 | −2.23 | 79.91 | L. rosacea & L. sonderii |
| M370T362 | 370.3319 | 362 | C22H40O3 | 1.71 | 95.11 | L. rosacea & L. sonderii |
The score MFG (molecular formulas generation) is the MFG overall match score (0–100%) combining the MS and MS/MS scores. For each ion M = molecular weight, T = retention time.
Figure 3.
Proposed chemical structures of the two polyolefins identified among chemomarkers explaining the spatial metabolomic variability.
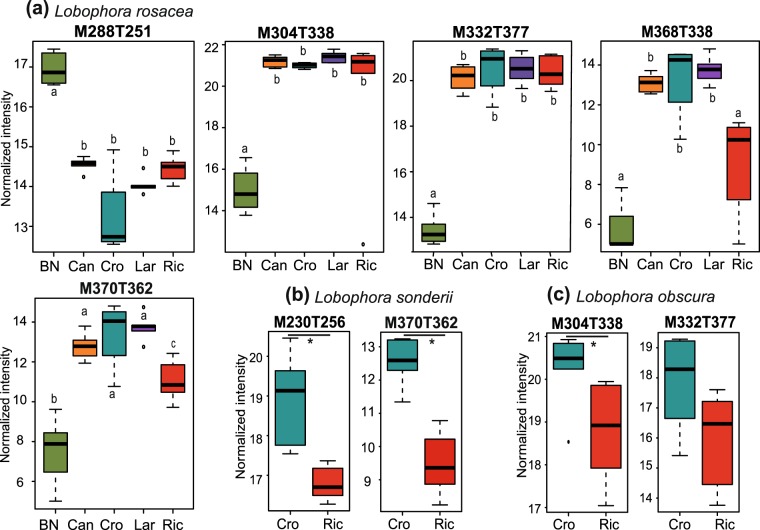
They were markers of Crouy in L. obscura and significantly under-expressed in Banc Nord compared to other sites in L. rosacea (see Figs 4 and S4). Four compounds are common between L. sonderii and L. rosacea (C14H28O, C16H32O, C22H38O3 and C22H40O3) and in higher amount at Crouy in comparison to Ricaudy in L. sonderii and depressed in Banc Nord for L. rosacea (Figs 4 and S4). Venn diagrams showed that no compound was specific to a site for L. obscura and L. rosacea while one compound was only detected at Ricaudy for L. sonderii: M888T571.
Figure 4.
Box plots of the chemomarkers annotated in Lobophora species responsible for metabolomic differences according to sites. Ion intensities of chemomarkers are expressed as mean normalized intensities ± SD (log-transformed data). For (a) Lobophora rosacea: n = 6 for Banc Nord (BN), Canard (Can) and Ricaudy (Ric), n = 5 for Larégnère (Lar) and n = 4 for Crouy (Cro). Statistical analyses were performed using Kruskal-Wallis (KW) followed by post-hoc Conover’s test. Letters represent distinct groups based on post-hoc pairwise comparisons between sites for each chemomarker (p < 0.05). Compounds M230T256, M242T309, M258T310 and M344T346 are shown in Fig. S4 and present the same trend as M332T377. For (b) Lobophora sonderii and (c) Lobophora obscura: n = 6 and differences between ion intensities at Crouy vs Ricaudy were tested with Mann-Whitney tests (*p < 0.01). Compounds M258T310 and M368T338 of Lobophora sonderii are shown in Fig. S4 and present the same trend as M370T362.
Transplantation experiments
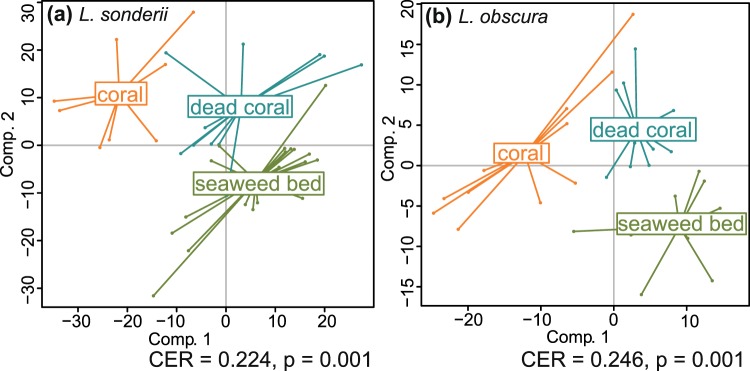
While no clear pattern between species metabotypes and habitat could be highlighted in the spatial study, we decided to further investigate the effect of the habitat on the metabolome of L. sonderii and L. obscura via cross-transplantations from their natural habitat to new ones (sites at a distance of <300 m). Control samples (in natural habitat) were collected at t0, t7 and t14 and transplants (in a new habitat) were analyzed after seven (t7) and 14 days (t14) of transplantation, allowing an assessment of both the impact of the habitat and the time of transplantation on algal metabolome. The habitat influenced significantly the metabolome of each Lobophora species (PERMANOVA, pseudo-FLO = 3.03, pseudo-FLS = 4.09, p < 0.05) and the time of transplantation also influenced L. sonderii metabolome (pseudo-F = 2.53, p = 0.005) with different metabolic fingerprints observed at each sampling time (PPLS-DA, CER = 0.108, p = 0.01, post-hoc p < 0.05 for each pair, Table S7). However, time is not correlated with metabolomic changes in L. obscura (p = 0.0627). For both species, different metabotypes were observed for each habitat (CERLS = 0.224, CERLO = 0.246, p = 0.001, post-hoc p < 0.05, Table S8, Fig. 5).
Figure 5.
Powered Partial Least-Squares-Discriminant Analysis (PPLS-DA) score plots of the metabolome profiles observed in (a) Lobophora sonderii and (b) Lobophora obscura according to the habitat (living coral, dead coral or seaweed bed) during the 14 days cross-transplantations (All time points (t0, t7 and t14) are included. CER = Mean classification error rate with p-value after double cross model validation).
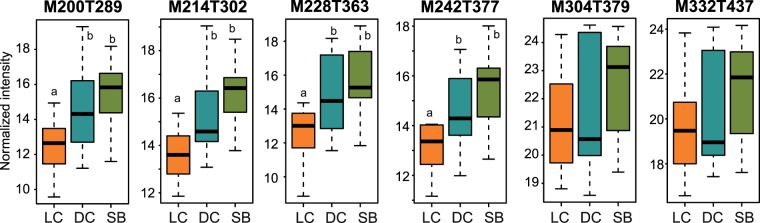
Two compounds were specifically detected in L. sonderii in its natural habitat (seaweed bed) while only quantitative changes occurred for both L. rosacea and L. sonderii in all the other habitats tested (Venn diagram test). No clear chemomarker linked to the transplantation conditions could be identified for L. obscura. In L. sonderii, four chemomarkers could be assigned to small alkenes (M200T289, M214T302, M228T363 and M242T377) and we also found the two previously highlighted polyolefins (C21H34 and C23H38; M304T379 and M332T437 respectively; see Table S9 for metabolites details). All these chemomarkers were under-expressed when algae were in contact with living corals (Fig. 6). The other chemomarkers did not match any known compounds.
Figure 6.
Box plots of the chemomarkers annotated in Lobophora sonderii responsible for metabolomic differences according to the substrate of transplantation (LC: living coral, DC: dead coral and SB: seaweed bed. All time points (t0, t7 and t14) are included). Ion intensities of chemomarkers are expressed as mean normalized intensities ± SD (log-transformed data, nLC = 8, nDC = 11, nSB = 23). Statistical analyses were performed using Kruskal-Wallis (KW) followed by post-hoc Conover’s test. Letters indicate distinct groupings based on post-hoc pairwise comparisons among transplantation conditions for each chemomarker (p < 0.05).
Discussion
Specialized metabolites play diverse ecological functions in macroalgae and are implied in the chemical communication with other marine organisms. However, their sources of variation and regulation are still poorly understood. Studying metabolomic variations at inter- and intraspecific scales, including with space and time, are important for understanding species ecology, community structure and ecosystems functioning33–35. In our study, we highlighted spatio-temporal metabolomic variations in the common macroalgae Lobophora.
A monthly variation of the metabolic fingerprinting was noticed in the four Lobophora species inhabiting a tropical lagoon, with a higher variability during summer (December 2015-February) but no clear seasonal pattern neither annual cycle was evidenced. Physical parameters tend to be more variable during the wet season (austral summer) in the New Caledonian lagoon, with episodes of heavy rains and cyclones36. Moreover, end of 2015-beginning of 2016 was marked by El Niño phenomenon, that brings unusually warm water to the equatorial Pacific37. Summer months (December 2015-February) exhibited high values of photoperiod, global radiation, PAR, SST and salinity, a set of abiotic factors that can affect metabolites biosynthesis. While seasonal patterns of secondary metabolism was reported for several temperate macroalgae like Fucus vesiculosus and Gracilaria vermiculophylla38,39 or other benthic organisms40, this trend is not clear and little is known for tropical species. No clear pattern of seasonal variation was seen in the red alga A. taxiformis studied in the Mediterranean sea24 while no temporal metabolomic variation was highlighted in the tropical macroalga Portieria hornemannii41. The metabolome of L. obscura exhibited less variability than the other species. Lobophora sonderii, commonly found in Sargassum beds, seems more exposed to biotic and abiotic factors. It presented a strong metabolomic variability over time, also supported by the transplantation experiments where metabolic changes appeared after seven days. The two other species, L. rosacea and L. monticola are closely associated with corals, and also showed a high metabolomic variability during the 13-months study, as supported by the permutational pairwise tests between months. Environmental factors explained some of the metabolomic variance observed, the SST and salinity having the highest explanatory power. The importance of temperature on the production of defense compounds was highlighted in marine organisms like sponges42 and macroalgae14. While we could not annotate any chemomarker and further investigate the metabolic pathways involved with changing environmental conditions, recent works showed the importance of lipids metabolism in case of salt stress in two microalgae species43,44. Other abiotic factors also influence the metabolome of Lobophora, like the global radiation and PAR as shown in other algae43, some factors linked to the light necessary for photosynthesis. A weak light irradiance could lead to a decrease in photosynthetic efficiency while a higher irradiance can cause oxidative damage. Global radiation and photoperiod being high during December 2015 and January, synthesis of UV-protectors may occur (e.g. polyamines, carotenoids or fatty acids44,45). Some additional factors may also influence the metabolome in Lobophora. For example, the nutrient availability can impact the metabolites production. But biotic factors, like herbivory pressure, the physiology or life-history stage46 are also known to affect metabolites production in macroalgae. Because life stages are not easily dissociable through Lobophora life-cycle, their influence on the metabolome cannot be assessed. However, it would be interesting to investigate more in depth the ontogeny and phenology of these species through the year, notably to see potential change in summer months which could be related to metabolomic changes. Little is known about Lobophora life cycle in New Caledonia but this genus is supposed to be reproductive along the full year. No data are recorded regarding herbivory pressure at Ricaudy and Sainte Marie but both sites are not protected areas.
Despite a higher interspecific variability, significant spatial variations around the lagoon were also evidenced in L. rosacea, L. sonderii and L. obscura. At a broader geographical scale, a significant variation of the metabolome was also highlighted for the red alga Asparagopsis taxiformis from temperate versus tropical regions24. Previous works on spatial variations mainly targeted specific metabolites or their families, like phenolic compounds47,48. In our metabolomic study, we spotted different metabotypes in sites distant from 2 to 11 km. In previous studies, metabolite variations were noticed in Laminaria groenlandica among sites as close as few meters in the northeastern Pacific49. While the environment influences the metabolome, the consideration of the type of habitat did not allow us to define a clear link between metabotypes and natural habitats. Except for L. sonderii, all sites were characterized by Acropora-dominated coral assemblage. Lobophora sonderii was collected in a Sargassum-dominated seaweed bed at Ricaudy (fringing reef) and Crouy (intermediate reef), which both present similar characteristics. Metabolic variations may arise from different nutrient concentrations and herbivory pressure between sites. Nevertheless, Canard islet and Larégnère are marine protected areas so herbivory pressure must be higher compared to other sites, potentially leading to an increase in algal chemical defense as suggested in other works6. To further investigate this hypothesis, it would be interesting to test the bioactivity of MeOH fractions among sites. Micro-environments may also explain some metabolic differences between sites. While the spatial metabolomic variation observed may arise from a complex set of abiotic and biotic factors, it could also be explained by local adaptation or genotypic selection across habitats50. Indeed, the clusters resulting from the metabolomic fingerprinting analyses possibly mirror genetic differences between populations. As mentioned earlier, some of these populations also present distinct ecological habits. Dispersal limitations may limit gene flows between these populations, shaping genetic, metabolomic and ecological differenciation. We recommend a population genetics study to test this idea.
As shown by PCAs’ variance, it also appeared that metabolomic changes induced by sites are of the same order than those induced over time (Figs 2 and S8).
Among the annotated chemomarkers responsible for the spatial discrimination, we putatively annotated some C20-C22 polyunsaturated and oxygenated fatty acid derivatives. Fatty acid derivatives present numerous essential roles in membrane structure fluidity, cell maintenance and signaling but are also involve in adaptation to diverse biotic and abiotic stresses17,51. We also found some polyolefins, notably a C21:5 as previously found in Fucus vesiculosus and other brown algae32,52 and a C23:5 homologue. These polyolefins could derived from the decarboxylation of the corresponding C22 and C24 unsaturated fatty acids. While we do not know their function here, they are among the major compounds found in our studied fractions and their structures are in agreement with our knowledge of the chemistry of these Lobophora species and closely related to the recently described lobophorenols, nonadecaketides and linear methyl ketones30,31.
In transplant experiments, different metabotypes were observed when L. sonderii and L. obscura were placed in a new habitat, suggesting an effect of the nearby environment (including the substrate) on the algal metabolome. These metabolic responses seem specific as metabotypes were significantly different in all tested conditions, notably after contact with living or dead corals. This observation supposed additional biotic sources of metabolic variation (e.g. different coral associated microbiome) beyond the scope of this study, which was a first investigation of the sources of chemical variation in Lobophora. We could not separate the physico-chemical from the biotic components in this experiment. Among chemomarkers linked to transplant conditions, we found putative small olefins and the two polyolefins early mentioned, under-expressed when L. sonderii was transplanted on a living coral compared to its natural habitat. It would be interesting to further assess the bioactivity of algal metabolomes which may increase after contact with corals, as seen in A. taxiformis22. Corals metabolome may also be altered by the contact with macroalgae23. Temporal metabolomic variation can even be noticed at a smaller time-scale (from 7 days), as shown in the cross-transplantations. However, we did not see any resilience to the pre-transplant metabolome so we cannot conclude if the pre-transplant metabolome could revert to the initial conditions after a longer time of transplantations or if the metabolome has just being adapted to new conditions. Moreover, despite any physical visible damage, we cannot exclude the transplantation may had caused a stress response in the transplanted algae, a point that would benefit from supplementary experiments.
This study revealed a high specific variability of Lobophora metabolome at the temporal and geographical scales across the New-Caledonian lagoon, in relation with physical factors and the nearby environment. It also suggests the involvement of other abiotic and biotic parameters to explain this variation. Multi-sources of metabolomic fluctuations had also been observed in several benthic organisms notably sponges and corals34 and all the factors implied are often difficult to unveil. The algal metabolome had adapted and evolved to adjust to a dynamic environment. Indeed, macroalgae face threats from a diverse range of organisms (e.g. pathogenic bacteria, epiphytes, herbivores) and are exposed to various stresses and their metabolome must then constantly adapt to new conditions. However, studies conducted in natural habitats do not allow control of all these parameters. Furthermore, biologically active compounds found in macroalgae may also be synthetized by their associated microbiome, as previously suggested for the lobophorolide in L. variegata7 and demonstrated in sponges and bryozoans53. Disentangling the metabolome from the host and its associated microbiome is challenging. The lack of specific metabolomic databases for marine organisms including macroalgae raises another issue17: metabolites annotation and identification remain the biggest challenge in global metabolomics54, notably for non-model species. This difficulty has been illustrated through the present work where only few chemomarkers could be identified, despite the discrimination of metabotypes according to time and space.
It also appeared that discrimination between groups is mainly driven by minor intensity ions, a problem previously mentioned in other works on macroalgae24. A total of 23 bioactive pure compounds were described in Lobophora55, included seven recently identified nonadecaketides31 and three lobophorenols (L. rosacea30). These major compounds were not detected as chemomarkers in our LC-MS conditions. Related C21 and C23 polyolefins have however been identified as major components. Interestingly no terpene derivatives were identified in the metabolome of the four studied species of Lobophora. This result came as a surprise considering that Lobophora is a genus belonging to the Dictyotales, known usually as a producer of this family of natural products. This observation could lead to interesting chemotaxonomic considerations for this particular group of brown macroalgae and the search for terpene synthases in a large set of Dictyotales.
Metabolomics helped us to gain insight into the impact of the environment on the metabolomic fingerprints of marine organisms, and more experimental data are needed to better understand this intrinsic relationship. A global understanding of the main sources of metabolome variations is important in the context of climate change faced by marine ecosystems. Sea surface temperature is predicted to increase by 0.3 °C–4.8 °C by the end of the 21st century and the pH to decrease by 0.06–0.32 units (RCP models56). Understanding the natural parameters influencing the metabolome in macroalgae will help in a predictive assessment of ecological success of some species fate in a changing ocean. Due to their ecological relevance, changes in the production of defensive metabolites in macroalgae will indeed have profound impacts on biological interactions with marine organisms and thus on the global ecosystems.
Methods
Sampling
Lobophora species were collected by SCUBA in ziplock plastic bags, immediately soaked into ice and frozen at −20 °C until chemical extraction. For the temporal study, six specimens (replicates) of L. rosacea, L. sonderii, L. obscura were collected monthly from December 2015 to December 2016 at Ricaudy (22°18.956′S; 166°27.405′E, Nouméa, New Caledonia) and at Sainte-Marie (22°18.269′S; 166°28.791′E, Nouméa, New Caledonia) for L. monticola. A total of 300 samples were collected for this temporal study (Table S10).
For the spatial study, a total of 51 samples of L. rosacea, L. sonderii and L. obscura were collected in austral summer 2015–2016 (December 2015, January and March 2016) at different locations into the lagoon: Ricaudy (22°18.956′S; 166°27.405′E), Canard islet (22°18.904′S; 166°26.147′E), Crouy (22°21.600′S; 166°20.402′E), Larégnère (22°19.3264′S; 166°19.1056′E) or Banc Nord (22°23.12.78′S; 166°31.369′E) (Fig. S6, Table S10). Lobophora monticola was not included because it is only found at Sainte Marie in the South-West lagoon of Nouméa. Habitats characterization was done for each site according to57.
Transplantations
To explore the influence of the environment on the metabolome, cross-transplantations from the natural habitat to new habitats were realized at Ricaudy. Experiments were performed on L. sonderii and L. obscura in summer 2016 (February-March) as presented in Table 3.
Table 3.
Experimental framework of Lobophora transplantation experiments.
| Species | Natural habitat | Substrate | Transplantations (x2) | |
|---|---|---|---|---|
| L. sonderii | seaweeds bed | rock slab | living coral | dead coral |
| L. obscura | dead coral, coral rubbles, rocks | dead coral, rocks | living coral | seaweed bed |
Eighteen fronds of each species were collected by SCUBA and directly fixed to their transplantation support with tulle strips. For the “dead coral” and “seaweed bed” transplants, supports were created with PVC slabs (297 × 420 mm), holding up 18 dead coral fragments fixed with Epoxy resin (Fig. S7). Two slabs were fixed with concrete reinforcing bars on the seaweed bed sandy floor (22°18.956′S; 166°27.405′E) or near the coral reef flat (22°18.945′S; 166°27.403′E) at Ricaudy. Algal fronds were then hooked up to the dead coral fragments. Some leaving coral colonies were used as support for the “leaving coral” transplant at Ricaudy (Table 3). Six replicates (when possible) of each transplantation condition were picked up after seven (t7) and 14 days (t14) of transplantation, and controls in natural habitat at the beginning of the experiment (t0) and after seven (t7) and 14 days (t14) of experiment. They were placed in ziplock plastic bags, immediately soaked into ice and frozen at −20 °C until chemical extraction (80 samples, Table S10).
Sample preparation
Prior to extraction, the 399 samples were freeze-dried and ground with liquid nitrogen. A mass of 250 mg was extracted 3 times with 5 mL of MeOH/CH2Cl2 (1:1) in an ultrasonic bath (5 min). The filtrates (paper filter, 4–12 µm, Macherey-Nagel) were concentrated under vacuum after adsorption to C18 silica powder (Polygoprep® Macherey-Nagel). The extracts were then fractioned by Solid Phase Extraction (Strata C18-E, 500 mg/6 mL, Phenomenex®) after cartridges cleaning (6 mL MeOH/CH2Cl2 1:1) and conditioning (6 mL H2O), via three successive elutions: 6 mL of H2O, 6 mL of MeOH and 6 mL of CH2Cl2. A volume of 1 mL of the MeOH fraction was then filtered (PTFE, 0.20 μm, Phenomenex®), dried and later used for UHPLC-HRMS (QToF) analyses.
Metabolomic analyses
UHPLC-HRMS (QToF)
LC-MS analyses were performed on a UHPLC-QToF (6540 UHD Accurate-Mass Quadrupole Time-of-Flight, Agilent Technologies) in Dual Agilent Jet Stream Electrospray Ionization mode. Mass spectra were acquired in positive mode, on an Acquity UPLC® BEH® Phenyl column (1.7 µm, 2.1 × 100 mm, Waters®) for the spatio-temporal samples and an Acquity UPLC® HSS T3 column (1.8 µm, 2.1 mm × 30 mm, Waters®) for the transplants samples. The mobile phase was: H2O + 0.1% formic acid + 10 mM ammonium formate (A) and acetonitrile/H2O (95:5) + 0.1% formic acid + 10 mM ammonium formate (B). Injection volume was set to 3 μL, elution rate to 0.4 mL.min−1 (5 µL and 0.5 mL.min−1 for the transplants samples), and column temperature maintained at 40 °C. Elution gradient was programed as follows: 40% B during 2 min, linear increased of B up to 100% from 2 to 8 min, 100% B during 4 min, return to initial condition from 12 to 14 min, and 3 min of post-run for column equilibration, with a total runtime of 17 min.
MS parameters were: nebulizer gas N2 at 30 psig, gas temperature: 300 °C, drying gas N2 at 7 mL.min−1, TOF spectra acquisition from m/z 100 to 1600, capillary voltage: 3500 V. MS² were acquired in the same conditions (frag = 175.0 V). For each study (spatial, temporal or transplantations), a quality control (QC) sample was prepared by mixing 25 µL of each sample, without any internal standard. QC samples allow checking for MS shift over time and ensure data normalization. Each study started with blanks injections, followed by 10 QC injections, then the samples and a QC between every five samples injected randomly along the run.
Data treatment and statistical analyses
LC–MS raw data files were converted to mzXML files with MSconvert using Python (version 2.7.11). mzXML files were then processed using the package XCMS for R software (R version 3.3.2, XCMS version 1.50.1). Optimized parameters for XCMS were used as follows: peak picking (method = “centwave”, peakwidth = c(2,20), ppm = 15, mzdiff = 0.05, prefilter = c(0,0)), retention time correction (method = “obiwarp”), matching peaks across samples (bw = 30, mzwid = 0.015, minfrac = 0.3) and filling in missing peaks. A matrix of compounds with peak intensity, m/z value and retention time was generated. The latter was filtered according to blanks and QC to remove technical variability using in-house R scripts (1-Filtering the matrix according to peaks present in blanks relative to pools (signal/noise ratio > 10), 2-filtering the matrix according to peaks coefficient of variation (CV) calculated on pool (CV < 20%) and 3-filtering the matrix according to autocorrelation between peaks). Data were normalized by log-transformation prior statistical analyses. To identify which significant factors were linked to the metabolites diversity, we used Permutational Multivariate Analysis of Variance using distance matrices (PERMANOVA, 9999 permutations, vegan package for R). Principal component analysis (PCA) was used to visualize the metabolome variation according to sites (ade4 package for R). Powered Partial Least-Squares-Discriminant Analysis (PPLS-DA) were used to find the maximum covariance between our data set and their class membership. Permutational tests based on cross model validation (MVA.test and pairwise.MVA.test) were applied to test differences between groups (RVAideMemoire package). In a second time, correlation circles were drown to identify discriminating compounds (RVAideMemoire package). Venn diagrams were constructed with the Vennerable package for R. Hierarchical Cluster Analysis (HCA) was performed with MetaboAnalyst 3.0 (distance measure: Euclidean, clustering algorithm: Ward). Molecular network based on MS2 spectra were constructed with GNPS58 and visualized under Cytoscape 3.5.059. Metlin (https://metlin.scripps.edu/), SIRIUS 4.060 and in-house work were used for putative annotation.
Physico-chemical parameters
Some parameters were recorded during the temporal sampling to correlate the chemical variation to environmental factors. Photoperiod was calculated for Nouméa thanks to the day length calendar from December 2015 to December 2016. Monthly means sea surface temperature (SST) and salinity were obtained from the GOPS observatory at Canard islet (22°18.439′N, 166°26.198′E) and Maitre islet (22°20.299′N, 166°24.109′E) stations respectively (measures each 1 min and 15 min respectively) (http://www.observatoire-gops.org). Photosynthetically Active Radiation (PAR) was obtained from CTD profiles at Moise station (22°14.600′N; 166°18.569′E, Nouméa, New Caledonia, one measure per month). Monthly means global radiation and rainfall were acquired at Meteo France Nouvelle-Calédonie at Nouméa station 98818001 (Table S11).
Supplementary information
Acknowledgements
The PhD of J. Gaubert is supported through a scholarship from Sorbonne University, Paris, France. Part of this project is carried out with the support of the Marine Institute (Grant-Aid Agreement No. PBA/MB/16/01) and is funded under the Marine Research Programme by the Irish Government. S. Greff (Aix-Marseille Université) is acknowledged for his help in data analysis and his precious comments. Thanks to the GOPS for salinity and SST data. We are grateful to G. Culioli (Université de Toulon) and M. Zubia (University of French Polynesia) for their fruitful comments.
Author Contributions
J.G., O.T. and C.P. designed the experiments. J.G. performed algal collections under the supervision of C.P. and carried out extractions and fractionations. J.G. and H.S. analyzed metabolomic fingerprints under the supervision of O.T. and J.G. performed statistical analyses. J.G. drafted the manuscript with input from C.V., C.P. and O.T.
Data Availability
Metabolomics data have been deposited to the EMBL-EBI MetaboLights database (10.1093/nar/gks1004. PubMed PMID: 23109552) with the identifier MTBLS707. The complete dataset can be accessed here https://www.ebi.ac.uk/metabolights/MTBLS707.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38177-z.
References
- 1.Pereira, R. C., da Gama, B. A. P. & Sudatti, D. B. In Mar. Macrophytes as Found. Species (Olafsson, E.) 26–42 (CRC Press 2016).
- 2.Amsler, C. D. Algal chemical ecology. Algal Chem. Ecol. (Springer-Verlag Berlin Heidelberg), 10.1007/978-3-540-74181-7 (2008).
- 3.Kooke R, Keurentjes JJB. Multi-dimensional regulation of metabolic networks shaping plant development and performance. J. Exp. Bot. 2011;63:3353–3365. doi: 10.1093/jxb/err373. [DOI] [PubMed] [Google Scholar]
- 4.Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry. 2003;64:3–19. doi: 10.1016/S0031-9422(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 5.Hay ME, Fenical W. Marine Plan-Herbivore Interactions: The Ecology of Chemical Defense. Annu. Rev. Ecol. Syst. 1988;19:111–145. doi: 10.1146/annurev.es.19.110188.000551. [DOI] [Google Scholar]
- 6.Dell C, Hay ME. Induced defence to grazing by vertebrate herbivores: Uncommon or under-investigated? Mar. Ecol. Prog. Ser. 2016;561:137–145. doi: 10.3354/meps11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubanek J, et al. Seaweed resistance to microbial attack: a targeted chemical defense against marine fungi. Proc. Natl. Acad. Sci. USA. 2003;100:6916–21. doi: 10.1073/pnas.1131855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremb S, et al. Aqueous extracts of the marine brown alga Lobophora variegata inhibit HIV-1 infection at the level of virus entry into cells. PLoS One. 2014;9:e103895. doi: 10.1371/journal.pone.0103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Gama BAP, Plouguerné E, Pereira RC. The antifouling defence mechanisms of marine macroalgae. Adv. Bot. Res. 2014;71:413–440. doi: 10.1016/B978-0-12-408062-1.00014-7. [DOI] [Google Scholar]
- 10.Pessoa MF. Algae and aquatic macrophytes responses to cope to ultraviolet radiation - a Review. Emirates J. Food Agric. 2012;24:527–545. doi: 10.9755/ejfa.v24i6.527545. [DOI] [Google Scholar]
- 11.Pohnert G, Boland W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002;19:108–122. doi: 10.1039/a806888g. [DOI] [PubMed] [Google Scholar]
- 12.Box S, Mumby P. Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Prog. Ser. 2007;342:139–149. doi: 10.3354/meps342139. [DOI] [Google Scholar]
- 13.Slattery M, Lesser MP. Allelopathy in the tropical alga Lobophora variegata (phaeophyceae): Mechanistic basis for a phase shift on mesophotic coral reefs? J. Phycol. 2014;505:493–505. doi: 10.1111/jpy.12160. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira AS, Sudatti DB, Fujii MT, Rodrigues SV, Pereira RC. Inter- and intrapopulation variation in the defensive chemistry of the red seaweed Laurencia dendroidea (Ceramiales, Rhodophyta) Phycologia. 2013;52:130–136. doi: 10.2216/12-058.1. [DOI] [Google Scholar]
- 15.Wright JT, De Nys R, Steinberg PD. Geographic variation in halogenated furanones from the red alga Delisea pulchra and associated herbivores and epiphytes. Mar. Ecol. Prog. Ser. 2000;207:227–241. doi: 10.3354/meps207227. [DOI] [Google Scholar]
- 16.Viant MR. Introducing genomics, proteomics and metabolomics in marine ecology Introduction. Mar. Ecol. Prog. Ser. 2007;332:247–248. doi: 10.3354/meps332301. [DOI] [Google Scholar]
- 17.Kumar M, Kuzhiumparambil U, Pernice M, Jiang Z, Ralph PJ. Metabolomics: an emerging frontier of systems biology in marine macrophytes. Algal Res. 2016;16:76–92. doi: 10.1016/j.algal.2016.02.033. [DOI] [Google Scholar]
- 18.Polo LK, et al. Metabolic profile of the brown macroalga Sargassum cymosum (Phaeophyceae, Fucales) under laboratory UV radiation and salinity conditions. J. Appl. Phycol. 2015;27:887–899. doi: 10.1007/s10811-014-0381-8. [DOI] [Google Scholar]
- 19.Alsufyani, T., Weiss, A. & Wichard, T. Time course exo-metabolomic profiling in the green marine macroalga Ulva (Chlorophyta) for identification of growth phase-dependent biomarkers. Mar. Drugs15 (2017). [DOI] [PMC free article] [PubMed]
- 20.Nylund, G. M., Weinberger, F., Rempt, M. & Pohnert, G. Metabolomic assessment of induced and activated chemical defence in the invasive red alga gracilaria vermiculophylla. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 21.Rasher DB, Hay ME. Competition induces allelopathy but suppresses growth and anti-herbivore defence in a chemically rich seaweed. Proc. R. Soc. B Biol. Sci. 2014;281:20132615–20132615. doi: 10.1098/rspb.2013.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greff S, et al. The interaction between the proliferating macroalga Asparagopsis taxiformis and the coral Astroides calycularis induces changes in microbiome and metabolomic fingerprints. Sci. Rep. 2017;7:42625. doi: 10.1038/srep42625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn RA, et al. Metabolomics of reef benthic interactions reveals a bioactive lipid involved in coral defence. Proc. Biol. Sci. 2016;283:20160469. doi: 10.1098/rspb.2016.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greff S, Zubia M, Payri C, Thomas OP, Perez T. Chemogeography of the red macroalgae Asparagopsis: metabolomics, bioactivity, and relation to invasiveness. Metabolomics. 2017;13:0. doi: 10.1007/s11306-017-1169-z. [DOI] [Google Scholar]
- 25.Hay ME. Marine chemical ecology: what’ s known and what’ s next? J. Exp. Mar. Bio. Ecol. 1996;200:103–134. doi: 10.1016/S0022-0981(96)02659-7. [DOI] [Google Scholar]
- 26.Vieira C, D’hondt S, De Clerck O, Payri CE. Toward an inordinate fondness for stars, beetles and Lobophora? Species diversity of the genus Lobophora (Dictyotales, Phaeophyceae) in New Caledonia. J. Phycol. 2014;50:1101–1119. doi: 10.1111/jpy.12243. [DOI] [PubMed] [Google Scholar]
- 27.Vieira C, et al. Historical biogeography of the highly diverse brown seaweed Lobophora (Dictyotales, Phaeophyceae) Mol. Phylogenet. Evol. 2017;110:81–92. doi: 10.1016/j.ympev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Rasher DB, Hay ME. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl. Acad. Sci. USA. 2010;107:9683–8. doi: 10.1073/pnas.0912095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira C, Payri C, De Clerck O. Overgrowth and killing of corals by the brown alga Lobophora hederacea (Dictyotales, Phaeophyceae) on healthy reefs in New Caledonia: A new case of the epizoism syndrome. Phycol. Res. 2015;63:152–153. doi: 10.1111/pre.12082. [DOI] [Google Scholar]
- 30.Vieira C, et al. Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci. Rep. 2016;6:18637. doi: 10.1038/srep18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutiérrez-Cepeda A, et al. Acetate-Derived Metabolites from the Brown Alga Lobophora variegata. J. Nat. Prod. 2015;78:1716–22. doi: 10.1021/acs.jnatprod.5b00415. [DOI] [PubMed] [Google Scholar]
- 32.Halsall TG, Hills I. Isolation of Heneicosa- 1,6,9,12,15,18-hexaene and -1,6,9,12,15-pentaene from the Alga Fucus vesiculosus. Chem. Commun. 1972;5:533–539. [Google Scholar]
- 33.Paul VJ, Arthur KE, Ritson-Williams R, Ross C, Sharp K. Chemical defenses: From compounds to communities. Biol. Bull. 2007;213:226–251. doi: 10.2307/25066642. [DOI] [PubMed] [Google Scholar]
- 34.Rohde S, et al. Spatial Variability in Secondary Metabolites of the Indo-Pacific Sponge Stylissa massa. J. Chem. Ecol. 2012;38:463–475. doi: 10.1007/s10886-012-0124-8. [DOI] [PubMed] [Google Scholar]
- 35.Reverter M, Tribalat MA, Pérez T, Thomas OP. Metabolome variability for two Mediterranean sponge species of the genus Haliclona: specificity, time, and space. Metabolomics. 2018;14:0. doi: 10.1007/s11306-018-1401-5. [DOI] [PubMed] [Google Scholar]
- 36.Le Borgne R, Douillet P, Fichez R, Torréton JP. Hydrography and plankton temporal variabilities at different time scales in the southwest lagoon of New Caledonia: A review. Mar. Pollut. Bull. 2010;61:297–308. doi: 10.1016/j.marpolbul.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Normile D. El Niño’s warmth devastating reefs worldwide. Science (80-.). 2016;352:15–16. doi: 10.1126/science.352.6281.15. [DOI] [PubMed] [Google Scholar]
- 38.Rickert E, Wahl M, Link H, Richter H, Pohnert G. Seasonal Variations in Surface Metabolite Composition of Fucus vesiculosus and Fucus serratus from the Baltic Sea. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0168196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surget G, et al. Seasonal phenology and metabolomics of the introduced red macroalga Gracilaria vermiculophylla, monitored in the Bay of Brest (France) J. Appl. Phycol. 2017;29:2651–2666. doi: 10.1007/s10811-017-1060-3. [DOI] [Google Scholar]
- 40.Ivanisevic J, et al. Biochemical trade-offs: Evidence for ecologically linked secondary metabolism of the sponge oscarella balibaloi. PLoS One. 2011;6:e28059. doi: 10.1371/journal.pone.0028059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Payo, D. A., Colo, J., Calumpong, H. & De Clerck, O. Variability of non-polar secondary metabolites in the red alga Portieria. Mar. Drugs9 (2011). [DOI] [PMC free article] [PubMed]
- 42.Reverter M, Perez T, Ereskovsky AV, Banaigs B. Secondary Metabolome Variability and Inducible Chemical Defenses in the Mediterranean Sponge Aplysina cavernicola. J. Chem. Ecol. 2016;42:60–70. doi: 10.1007/s10886-015-0664-9. [DOI] [PubMed] [Google Scholar]
- 43.Lu N, Wei D, Jiang XL, Chen F, Yang ST. Fatty Acids Profiling and Biomarker Identification in Snow Alga Chlamydomonas Nivalis by NaCl Stress Using GC/MS and Multivariate Statistical Analysis. Anal. Lett. 2012;45:1172–1183. doi: 10.1080/00032719.2012.673094. [DOI] [Google Scholar]
- 44.Takagi M, Karseno, Yoshida T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006;101:223–226. doi: 10.1263/jbb.101.223. [DOI] [PubMed] [Google Scholar]
- 45.Cronin G, Hay ME. Effects of light and nutrient availability on the growth, secondary chemistry, and resistance to herbivory of two brown seaweeds. Oikos. 1996;77:93–106. doi: 10.2307/3545589. [DOI] [Google Scholar]
- 46.Vergés A, Paul NA, Steinberg PD. Sex and life-history stage alter herbivore responses to a chemically defended red alga. Ecology. 2008;89:1334–1343. doi: 10.1890/07-0248.1. [DOI] [PubMed] [Google Scholar]
- 47.Le Lann K, Connan S, Stiger-Pouvreau V. Phenology, TPC and size-fractioning phenolics variability in temperate Sargassaceae (Phaeophyceae, Fucales) from Western Brittany: Native versus introduced species. Mar. Environ. Res. 2012;80:1–11. doi: 10.1016/j.marenvres.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Pereira RC, Soares AR, Teixeira VL, Villaça R, Da Gama BAP. Variation in chemical defenses against herbivory in southwestern Atlantic Stypopodium zonale (Phaeophyta) Bot. Mar. 2004;47:202–208. doi: 10.1515/BOT.2004.020. [DOI] [Google Scholar]
- 49.Van Alstyne KL, McCarthy JJ, Hustead CL, Duggins DO. Geographic variation in polyphenolic levels of northeastern Pacific kelps and rockweeds. Mar. Biol. 1999;133:371–379. doi: 10.1007/s002270050476. [DOI] [Google Scholar]
- 50.Moore B, Andrew R, Külheim C, Foley W. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014;201:733–750. doi: 10.1111/nph.12526. [DOI] [PubMed] [Google Scholar]
- 51.Dunn SR, Thomas MC, Nette GW, Dove SG. A Lipidomic Approach to Understanding Free Fatty Acid Lipogenesis Derived from Dissolved Inorganic Carbon within Cnidarian-Dinoflagellate Symbiosis. PLoS One. 2012;7:e46801. doi: 10.1371/journal.pone.0046801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Youngblood WW, Blumer M. Alkanes and alkenes in marine benthic algae. Mar. Biol. 1973;21:163–172. doi: 10.1007/BF00355246. [DOI] [Google Scholar]
- 53.Mouchka ME, Hewson I, Harvell CD. Coral-associated bacterial assemblages: Current knowledge and the potential for climate-driven impacts. Integr. Comp. Biol. 2010;50:662–674. doi: 10.1093/icb/icq061. [DOI] [PubMed] [Google Scholar]
- 54.Allard PM, Genta-Jouve G, Wolfender JL. Deep metabolome annotation in natural products research: towards a virtuous cycle in metabolite identification. Curr. Opin. Chem. Biol. 2017;36:40–49. doi: 10.1016/j.cbpa.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Vieira, C. et al. Biological activities associated to the chemodiversity of the brown algae belonging to genus Lobophora (Dictyotales, Phaeophyceae). Phytochem. Rev., 10.1007/s11101-015-9445-x (2015).
- 56.Contribution of Working Groups I, I. and I. to the F. A. R. of the I. P. on C. C. Climate Change 2013 - The Physical Science Basis, 10.1017/CBO9781107415324 (2014).
- 57.Andrefouet, S. In Fiches d’identification des habitats récifolagonaires de Nouvelle-Calédonie. Sci. la Mer. Biol. Mar. Notes Tech. 12 (2014).
- 58.Wang M, et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Böcker S, Dührkop K. Fragmentation trees reloaded. J. Cheminform. 2016;8:1–26. doi: 10.1186/s13321-016-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metabolomics data have been deposited to the EMBL-EBI MetaboLights database (10.1093/nar/gks1004. PubMed PMID: 23109552) with the identifier MTBLS707. The complete dataset can be accessed here https://www.ebi.ac.uk/metabolights/MTBLS707.