Abstract
Necrotizing enterocolitis (NEC) is the leading cause of death among infants born at <30 weeks’ gestation, but donor human milk can reduce the incidence of NEC. Unfortunately, freezing or pasteurizing human milk deactivates beneficial bioactive components. We evaluated the feasibility, safety, and impact of feeding very preterm infants fresh (unprocessed) mother’s own milk within 4 hours of expression. In our multicentre prospective cohort analytic study, we fed 109 control and 98 intervention infants previously frozen donor or mother’s own milk; only the intervention group was fed fresh mother’s own milk once daily from enrollment until 32 weeks’ corrected age. Control group mothers could not commit to provide fresh milk daily and were less likely receive antenatal corticosteroids than mothers in the intervention group. In the intervention group, 87.5% (98/112) of mothers were able to provide at least one feed of fresh milk a day. No critical incidents or non-compliance with the protocol were reported. The duration of mechanical ventilation and total parenteral nutrition use were shorter in the intervention group than controls (P < 0.01) but the length of hospital stay was similar (P = 0.57). Although the study might be underpowered, the intervention group had lower unadjusted rates of the composite outcome NEC ≥ stage 2 or mortality (8% vs 20%, P = 0.04), sepsis (22% vs 38%, P = 0.02), retinopathy of prematurity (17% vs 39%, P < 0.01) and bronchopulmonary dysplasia (32% vs 47%, P < 0.01) than the control. These results indicated that feeding fresh mother’s own milk once daily was safe, feasible, and may reduce morbidity.
Introduction
Necrotizing enterocolitis (NEC) is a severe inflammatory disorder of the intestine that primarily affects very low birth weight (<1500 grams [g]) or very preterm infants (≤32 weeks’ gestation); it is also the leading cause of death in the neonatal intensive care unit (NICU)1,2. Currently there is no known effective treatment for NEC. Therefore, the best treatment for NEC is prevention, and mother’s own milk is the best practice for preventing NEC3. When mother’s own milk is not available, the options are donor human milk or formula. The use of formula in very low birth weight or preterm infants was reported to increase the risk of developing NEC when compared with donor human milk4.
Human milk contains not only nutritional components (such as proteins, amino acids, fats, carbohydrates, vitamins, and minerals), but also a plethora of components with profound bioactivity that are diminished during pasteurisation or freezing of fresh milk. Milk bioactives include both non-cellular (such as cytokines5, hormones, growth factors6, and oligosaccharides7) and cellular components (such as immune cells and stem cells)8, many of which exert protective antimicrobial and anti-inflammatory roles in the infant as well as potential developmental effects9–13. Recently, Hassiotou (now Kakulas) et al. showed that human milk is also a rich source of multilineage stem cells capable of self-renewal and differentiation into cells of all three germ layers14. In a mouse study, Kakulas et al. demonstrated that similar stem cells exist in mouse milk. Mouse milk stem cells survived in the gastrointestinal tract of the pups after nursing, entered their blood stream, and were incorporated into their major organ systems, where the stem cells were found alive and active (e.g. they contributed to pancreatic insulin production) even after the nursing period was completed8,15,16. Although the function and fate of milk stem cells are not yet well understood, it is possible that human milk stem cells benefit the infant through their ability to promote growth, development and regeneration12.
In addition, the current evidence strongly suggests that fresh human milk may have a protective effect against both infection and NEC17. However, current practices related to the handling and use of human milk in the NICU raise questions about whether we are deriving the maximum benefit from human milk and inversely whether the processing of human milk adversely affects infants. Currently, the standard NICU procedure is to freeze and store milk expressed by mothers of preterm infants in the milk bank. When the infant requires oral feeds, an order is placed with the milk bank and the oldest batch of milk is defrosted, fortifier is added as needed, and then the milk is sent to the NICU for use. The act of freezing human milk (either at −20 °C or −80 °C) along with the passage of time are known to decrease the energy content and reduce the amounts of several useful components of human milk18,19, including fat, carbohydrates, secretory immunoglobulin A, lactoperoxidase, lysozyme, antibacterial factors, and antioxidants18–23. In addition, the defrosted milk does not contain any live cells, and stem cells in milk have a half-life of 4 hours on average8,24.
The current NICU human milk feeding procedure exists as a means of ensuring that infants have consistent access to their mother’s milk even if the mother is not able to spend time in the NICU. The process also allows for strict quality and infection control, as well as computerised inventory and monitoring via electronic health records. However, the process deprives infants of the benefits of the cellular content of human milk, including both the immune cells and stem cells4. For the use of fresh human milk in China, there are several barriers to overcome: (1) many hospitals do not allow parents into the NICU during their infant’s stay; (2) mothers may produce less milk than is needed, either initially or throughout the breastfeeding period, so some feeds may need to be supplemented with donor milk or formula; (3) previous reports indicated that fresh human milk may be a source of bacterial and viral contamination, especially by cytomegalovirus (CMV)25; however, at the same time numerous more recent studies show that fresh human milk contains a unique microbiome that normally transfers numerous benefits to the developing gastrointestinal tract of the infant26.
We performed a multicentre prospective cohort analytic study to evaluate the feasibility and safety of providing very preterm infants born at <30 weeks’ gestation with fresh mother’s own milk within 4 hours of expression. While we acknowledge that the pilot study was not powered to detect a statistically significant difference, our secondary objective was to identify if fresh human milk had the potential to improve infant outcomes, particularly the occurrence of NEC and sepsis. We hypothesized that it is feasible for mothers to provide to their infant at least 1 feed per day of fresh, unprocessed human milk within 4 hours of expression, and that fresh mother’s own milk may decrease the prevalence of NEC and sepsis.
Materials and Methods
Study design
The study was a prospective cohort design including infants born at <30 weeks’ gestation at one of four tertiary NICUs in China (Children’s Hospital of Zhengzhou University, Nanjing Maternity and Child Health Hospital, Nanjing Children’s Hospital, and Xiangya Second Hospital). All NICUs were of similar size (≥45 beds) and had donor human milk available for infants born preterm. In the intervention (fresh human milk) group, mothers were asked to provide at least 1 feed of fresh milk within 4 hours of milk expression per day from the time of enrollment until the infants were 32 weeks’ corrected age. The control group included mothers who did not agree to provide fresh milk, but agreed for their infants to receive exclusively human milk (donor or frozen mother’s own milk) and allowed their information to be collected and analyzed.
Our design included several elements to ensure the safety of the mothers and infants. Every infant admitted to the NICU completed the TORCH screen for toxoplasma, rubella, CMV, and herpes simplex27,28. If we identified infants as CMV-IgG (+), we completed a human milk CMV-DNA test. We continued to feed the infant fresh human milk if the CMV-DNA level was below 1.0 × 104 copies/ml; however, if the CMV-DNA was above this level, we pasteurized the human milk. For an individual patient, the feeding protocol was stopped if there was an occurrence of NEC or any other medical condition that standard practice required stopping feeds. All cases of mortality, NEC, sepsis, and critical incidence reports (including mix-up of milk destined for individual infants, infection that was related to feeds, missed feeds arising from delays in milk preparation, and any other incident that concerned the clinicians) were critically reviewed by a Data Safety and Monitoring Committee. Stopping criteria were also decided by the Data Safety and Monitoring Committee. The Children’s Hospital of Zhengzhou University was the study coordinating centre, and the study was approved by the research ethics board of each participating hospital and conducted in accordance with their guidelines.
Study population
The study population included very preterm infants born at <30 weeks’ gestation between January 1, 2016 and December 31, 2017 and admitted to one of the four participating NICUs described above. Infants were eligible if they were born at <30 weeks’ gestation and had never received infant formula. Infants with major congenital anomalies, receiving palliative care, or where illness of the mother or infant prevented the administration of human milk feeds in the first week of the infant’s life were excluded from the study. Eligible mothers were approached at their first visit to the NICU following their infant’s admission and asked to provide informed consent to participate in the study. Only infants whose parents provided informed consent participated in the study. At the time of recruitment, mothers who committed to providing at least 1 feed of fresh human milk a day, 7 days a week, were included in the fresh human milk group, and mothers who allowed their data to be collected and their infants to receive exclusively human milk (donor or frozen mother’s own milk), but were unable to commit to providing at least 1 feed of fresh human milk a day, were included in the control group. If mothers were unable to produce milk for the duration of the study, the infant was fed donor human milk or formula and dropped from the fresh human milk group.
Feeding intervention
Following standard procedures, all infants in the study were initially provided nutrition intravenously (total parenteral nutrition [TPN]). If the infant was not suffering from any condition that affects the gastrointestinal system function, oral feeds were introduced as early as possible through a nasogastric tube using mother’s own milk or donor human milk. Once the infant was receiving all their nutrition from human milk (‘full enteral feeds’), fortifiers and other nutritional supplements, including probiotics, were added to each feed. Cow’s milk based human milk fortifier was added first, then seven days later vitamins and iron were added to each feed.
All infants were fed following their unit’s feeding procedures and received frozen human milk; however, the fresh human milk group received fresh mother’s own milk instead of frozen milk once a day. Neither the control group nor the fresh human milk group infants were fed formula. For the frozen human milk, all mothers who provided expressed milk followed the standard NICU protocols; nurses picked up the expressed human milk from the mothers, then it was frozen in the milk bank. Each evening the feeds for the following day were ordered, and the oldest batch of human milk was defrosted and prepared. Mothers in the fresh human milk group provided the nurses at least one fresh feed (within 4 hours of milk expression) seven days a week that was fed directly to their infant(s) without freezing or heating until the infants were 32 weeks’ corrected age. Infants were moved from enteral feeds to breastfeeding when they were clinically judged able to do so.
Data collection
Infant and maternal characteristics, delivery information, and outcomes data were collected on a data collection form and entered by a research assistant into a database for subsequent analysis. Study-specific variables were also collected, including rate of recruitment, rate of consent, rate of retention, compliance with the intervention protocol, the number and volume of fresh milk feeds per infant per day, and the response of each infant following the feed.
The primary outcome of the study was feasibility as measured by the percentage of mothers who provided at least one feed a day of fresh human milk from the time of enrollment until the infant was 32 weeks’ corrected age, the rate of consent to participate in the study, rate of recruitment to the fresh human milk group, and compliance with the intervention protocol. Secondary outcomes included infant growth, mortality, sepsis, NEC, retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), and intraventricular hemorrhage (IVH). A post-hoc analysis included the composite outcome NEC or mortality. Infant growth was measured by the weight z-score and change in weight z-score at 63 days of age29. Sepsis was defined on the basis of a positive blood culture and treatment with antibiotics for ≥5 days. NEC was defined as ≥stage 2 according to Bell’s criteria30. ROP was defined according to the International Committee for the Classification of Retinopathy of Prematurity31. BPD was defined as oxygen need at 36 weeks’ postmenstrual age32. IVH grade 3 or 4 and periventricular leukomalacia were defined as described by Papile et al.33.
Sample size
The mean number of infants born at <30 weeks’ gestation and admitted to participating NICUs in 2014 was over 500 infants per year. We aimed to include 100 infants in each group and expected that 25% of mothers approached would consent to be in the fresh human milk group. In 2013 the mean annual rate of NEC among participating hospitals was 9.3% in infants born at <29 weeks’ gestation, so we expected to see 9 cases of NEC in the control group. Although the study was not powered to detect a statistically significant difference in outcome rates, the occurrence of 5 or less cases of NEC in the fresh human milk group would be an indication that further study is warranted.
Statistical analyses
The primary outcomes, secondary outcomes, infant and maternal characteristics were summarized using descriptive methods and compared using the chi-square test for categorical variables and t-test for continuous variables. Multivariable logistic regression analyses were performed to assess the impact of the type of milk on NEC or mortality outcomes. For continuous variables, multiple linear regression analysis was used and adjusted for potential confounders. Crude relative risks (RR) with 95% confidence intervals (95% CIs) were estimated. The level of statistical significance was set at P < 0.05. The SPSS software version 21.0 (SPSS Chicago, Illinois, USA) was used for statistical analysis and data management.
Results
Feasibility and safety of the fresh human milk study
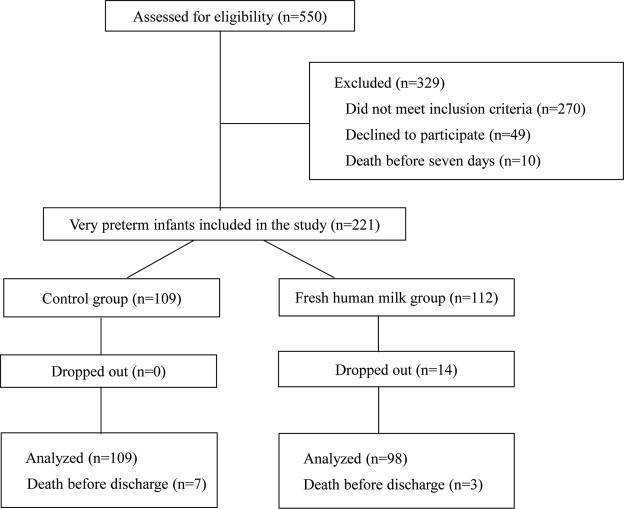
Between January 1, 2016 and December 31, 2017, there were 550 preterm infants born at <30 weeks’ gestation who were assessed for eligibility. Ten of the potentially eligible infants died before 7 days of age, and 270 infants were excluded because they did not meet the inclusion criteria (Fig. 1). Another 49 infants were excluded because their parents declined to participate resulting in a consent rate of 81.9% (221/270). Of the 221 very preterm infants included in this study, we were able to recruit 50.7% (112/220) of consenting mothers to the fresh human milk group and the remaining 109 infants were in the control group. In the fresh human milk group, 14 infants dropped out because their mothers could not produce enough fresh human milk, which means 87.5% (98/112) of mothers in the fresh human milk group were able to provide at least one feed of fresh milk a day. Therefore, we included 98 infants in the fresh human milk group and 109 in the control group in the analyses (Fig. 1).
Figure 1.
Consort Diagram Flow chart of included infants.
All NICUs were able to fully comply with the intervention protocol. No critical incidents, including CMV infection or feeding errors, were reported during the study. One mother’s milk contained higher than normal CMV-DNA levels, but the mother was from the control group and her milk was pasteurized. None of the infants in this study were infected with CMV. Furthermore, none of the following incidents were observed in either study group: mix-up of milk destined for individual infants, infection that was related to feeds, missed feeds arising from delays in milk preparation, or any other incident that concerned the clinicians.
Variation in maternal and infant characteristics
The majority of maternal characteristics were similar between the fresh human milk and control groups, except more mothers in the fresh human milk group had a college diploma or university degree, were treated with antenatal corticosteroids, and received magnesium sulphate during labor (Table 1). Several infant characteristics varied between the fresh human milk and control groups (Table 2). The average Apgar Scores were higher (P < 0.01) and the duration of mechanical ventilation (P < 0.01) and TPN use (P < 0.01) were shorter in the fresh human milk group than in the control group.
Table 1.
Comparison of maternal characteristics of fresh human milk and control groups.
| Maternal Characteristics | Fresh Human Milk N = 98 | Controla N = 109 | P value |
|---|---|---|---|
| Age of mothers (years), mean ± SD | 30.6 ± 5.5 | 29.8 ± 5.7 | 0.26 |
| Pregnancy weight (kg), mean ± SD | 58.2 ± 7.8 | 57.2 ± 5.8 | 0.31 |
| Marital status (married), n (%) | 97(99) | 107(98) | 0.50 |
| Education | <0.01 | ||
| Elementary school, n (%) | 7(7) | 7(6) | |
| High school, n (%) | 47(48) | 84(77) | |
| Trade certificate, n (%) | 5(5) | 10(9) | |
| Professional registration, n (%) | 0 | 1(1) | |
| College diploma, n (%) | 16(16) | 3(3) | |
| University degree, n (%) | 23(23) | 4(4) | |
| Conception by assisted reproductive technology, n (%) | 7(7) | 17(16) | 0.20 |
| Antenatal corticosteroid, n (%) | 47(50) | 13(12) | <0.01 |
| Gestational diabetes, n (%) | 11(11) | 4(4) | 0.11 |
| Hypertension or pre-eclampsia, n (%) | 18(18) | 11(10) | 0.31 |
| MgSO4 during labour, n (%) | 25(26) | 4(4) | <0.01 |
| Clinical chorioamnionitis, n (%) | 8 (8) | 7(6) | 0.80 |
| Antenatal bleeding, n (%) | 10(10) | 22(20) | 0.14 |
| Postpartum haemorrhage, n (%) | 2(2) | 8(7) | 0.37 |
| Length of ROM, n (%) | 0.04 | ||
| <24 hours | 68(69) | 91(83) | |
| 24 hours to 1 week | 17(17) | 10(9) | |
| >1 week | 6(6) | 1(1) | |
| Unknown | 7(7) | 7(6) | |
| Labor initiation (spontaneous), n (%) | 63(64) | 78(72) | 0.22 |
| Mode of delivery, n (%) | 0.69 | ||
| Vaginal | 64(65) | 78(69) | |
| Assisted vaginal | 1(1) | 2(2) | |
| Emergency caesarean | 26(27) | 22(20) | |
| Elective Caesarean | 7(7) | 7(6) | |
| Presentation (vertex), n (%) | 84(86) | 99(91) | 0.26 |
aInfants in the control group were fed frozen milk.
Abbreviations: N, number in category; n, number in group; SD, standard deviation; ROM, Rupture of membrane.
Table 2.
Comparison of infant characteristics and treatments in fresh human milk and control groups.
| Infant Characteristics | Fresh Human Milk N = 98 | Controla N = 109 | P value |
|---|---|---|---|
| Age of infant at recruitment (hours), mean ± SD | 10.3 ± 6.0 | 10.8 ± 7.0 | 0.58 |
| Male sex, n (%) | 60(61) | 59(54) | 0.32 |
| Gestational age at birth (weeks), mean ± SD | 28.3 ± 3.6 | 28.7 ± 1.2 | 0.20 |
| Apgar Scores | |||
| One minute, mean ± SD | 7.3 ± 2.3 | 5.9 ± 2.3 | 0.01 |
| Five minutes, mean ± SD | 8.2 ± 1.8 | 7.2 ± 2.1 | 0.01 |
| Ten minutes, mean ± SD | 8.8 ± 1.4 | 7.9 ± 1.6 | 0.01 |
| Number of births in pregnancy | |||
| Singleton, n (%) | 79(81) | 67(61) | 0.01 |
| Twins, n (%) | 17(17) | 39(36) | |
| Triplets, n (%) | 1(1) | 3(3) | |
| Respiratory Support | |||
| CPAP (days), mean ± SD | 8.1 ± 7.2 | 8.9 ± 9.4 | 0.49 |
| Mechanical Ventilation (days), mean ± SD | 2.9 ± 5.3 | 5.6 ± 6.9 | 0.01 |
| Oxygen (fraction of inspired oxygen) | |||
| 28 days, mean ± SD | 24.7 ± 5.0 | 29.4 ± 6.4 | 0.01 |
| 36 weeks, mean ± SD | 27. 2 ± 4.1 | 28.1 ± 2.7 | 0.60 |
| Surfactant administration, n (%) | 65(66) | 74(68) | 0.88 |
| Caffeine administration, n (%) | 85(87) | 86(79) | 0.15 |
| TPN(days), mean ± SD | 17.3 ± 8.0 | 21.8 ± 12.7 | 0.01 |
| Duration of hospitalization (days), mean ± SD | 47.0 ± 18.1 | 48.5 ± 19.6 | 0.57 |
aInfants in the control group were fed frozen human milk.
Abbreviations: N, number in category; n, number in group; SD, standard deviation CPAP, Continuous Positive Airway Pressure; TPN, Total Parenteral Nutrition.
Study outcomes
To determine if infant growth was affected by fresh human milk, we assessed infant weight gain and change in weight over the course of the study. The birth weight z-score of the fresh human milk and control groups were similar (P = 0.42), and 9 weeks after birth (63 days) the average weights remained similar for both groups (P = 0.25, Table 3). However, the infants in the fresh milk group gained weight faster than the control group as indicated by the significant difference between the two groups in the change of weight z-score at 63 days of age (P = 0.01, Table 3).
Table 3.
Comparison of the weight gain of preterm infants in the fresh human milk and control groups.
| Weight | Fresh Human Milk (N = 98) mean ± SD | Controla (N = 109) mean ± SD | P value |
|---|---|---|---|
| Birth weight z-score | 0.35 ± 0.90 | 0.25 ± 0.83 | 0.42 |
| Weight z-score at 7 days of age | −0.38 ± 0.77 | −0.11 ± 0.44 | 0.34 |
| Weight z-score at 14 days of age | −0.35 ± 2.1 | −0.71 ± 0.66 | 0.10 |
| Weight z-score at 21 days of age | −0.70 ± 0.74 | −0.64 ± 2.40 | 0.80 |
| Weight z-score at 28 days of age | −0.92 ± 0.75 | −1.08 ± 0.68 | 0.13 |
| Weight z-score at 35 days of age | −1.19 ± 0.74 | −1.28 ± 0.69 | 0.44 |
| Weight z-score at 42 days of age | −1.51 ± 0.72 | −1.58 ± 0.68 | 0.62 |
| Weight z-score at 49 days of age | −1.70 ± 0.77 | −1.74 ± 0.68 | 0.77 |
| Weight z-score at 56 days of age | −1.93 ± 0.71 | −1.97 ± 0.67 | 0.83 |
| Weight z-score at 63 days of age | −1.91 ± 0.78 | −2.17 ± 0.61 | 0.25 |
| Change in weight z-scoreb | −1.72 ± 0.32 | −2.10 ± 0.52 | 0.01 |
aInfants in the control group were fed frozen human milk.
bZ-score at 63 days of age minus z-score at birth.
Abbreviations: N, number in group; SD, standard deviation.
We used linear regression analyses to analyze weight gain velocity and other outcomes after adjusting for confounding factors including gestational age, birth weight, sepsis, and duration of mechanical ventilation. Weight gain velocity was significantly higher in the fresh human milk group than the control group, even after adjusting for confounding factors (Table 4). In addition, the fresh human milk group had a shorter duration of TPN than the control group (P = 0.02; Table 4). A trend was found in favour of the fresh human milk group for duration of hospitalization, but the results were not significant (Table 4).
Table 4.
Linear regression analysis of the impact of fresh human milk versus control milk on hospital stay, weight gain and total parenteral nutrition.
| Standardized Coefficients | 95% CI | ||
|---|---|---|---|
| Beta | Lower Limit | Upper Limit | |
| Duration of hospitalization, days | 0.02 | −3.97 | 5.10 |
| Weight gain, g/d | 1.90 | 0.12 | 3.67 |
| Change in weight z-scoreb | 0.27 | 0.04 | 0.51 |
| Total parenteral nutrition, days | 0.15 | 0.64 | 6.02 |
aThe fresh human milk group was compared to the control group and the model was adjusted for gestational age, birth weight, sepsis, and duration of mechanical ventilation.
bZ-score at 63 days of age minus z-score at birth.
Fresh human milk did not impact the mortality rates, which were similar between the fresh human milk and control groups (Table 5). The incidence of NEC was lower, although not significant, in the fresh human milk group than in the control group. However, the risk of the composite outcome NEC ≥ stage 2 or mortality was significantly lower in the fresh human milk group than in the controls. In addition, significantly fewer infants in the fresh human milk group had sepsis, ROP, or BPD than the control group (Table 5).
Table 5.
Comparison of the neonatal outcomes of preterm infants fed with and without fresh human milk.
| Outcomes | Fresh Human Milk N = 98 | Controla N = 109 | Relative Riskb (95%CI) | P value |
|---|---|---|---|---|
| NEC ≥ stage 2 or mortality, n (%) | 8(8) | 20(18) | 0.45(0.21–0.96) | 0.04 |
| Mortality, n (%) | 3(3) | 7(6) | 0.48(0.13–1.79) | 0.09 |
| NEC ≥ stage 2, n (%) | 6(6) | 15(14) | 0.46(0.19–1.10) | 0.11 |
| Sepsis, n (%) | 22(22) | 41(38) | 0.60(0.38–0.93) | 0.02 |
| ROP diagnosis, n (%) | 17(17) | 43(39) | 0.44(0.27–0.72) | <0.01 |
| ROP required treatment, n (%) | 9(9) | 15(14) | 0.64(0.30–1.40) | 0.29 |
| BPD, n (%) | 3(3) | 20(18) | 0.17(0.05–0.54) | <0.01 |
| IVH ≥ Grade 3, n (%) | 6(6) | 13(12) | 0.51(0.20–1.30) | 0.23 |
| PVL, n (%) | 5(5) | 12(11) | 0.46(0.17–1.27) | 0.14 |
| RDS, n (%) | 85(78) | 97(89) | 0.98(0.88–1.08) | 0.67 |
aInfants in the control group were fed frozen human milk.
bThe relative risk was calculated by comparing the probability of fresh human milk group to the outcomes of the control group.
Abbreviations: CI, confidence interval; RR, relative risk; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome; BPD, bronchopulmonary dysplasia.
Discussion
Our study indicated that feeding preterm infants (born at <30 weeks’ gestation) fresh human milk, within 4 hours post-expression, is a feasible and safe practice in the NICU. Of the 221included infants, 112 mothers agreed to participate in the fresh human milk intervention. While 14 mothers were unable to produce enough milk and dropped out, the remaining 98 mothers in the fresh human milk group all complied with the intervention protocol. Importantly, we did not have any critical incidents, including CMV infection, throughout the duration of the study. While we acknowledge that our pilot study was not powered to detect a statistically significant difference, our results suggest that only one fresh human milk feed per day has the potential to improve infant outcomes such as sepsis and NEC. Together, these results suggest that the use of fresh human milk in very preterm infants is feasible, safe, and could improve infant outcomes.
Human milk is a fresh, living fluid containing much more than nutrition. It is a cellular, as well as non-cellular, bioactive factory with live stem cells, immune cells, and numerous antioxidants, antibacterial, prebiotic, probiotic, and immune-boosting properties in addition to proteins, essential fats, enzymes, and hormones, all of which are uniquely human34. Whenever nursing or immediately expressed milk is unavailable35, properly stored human milk continues to provide safe and adequate nutrition, superior to formula feeding36, and is the gold standard for infant feeding. However, like any other living tissue or liquid, human milk is sensitive to the effects of temperature37, and some nutrients and bioactive properties are adversely affected by storage conditions. For example, pasteurized human milk stored at −20 °C has significantly less fat, lactose, and energy content than fresh human milk38. Furthermore, freezing human milk at −80 °C significantly decreases the energy content from both fat and carbohydrates lower than the levels observed in human milk stored at −20 °C19. Last, freezing depletes the live immune and stem cells in human milk, which are known to provide protective and developmental benefits to the infant11,12.
One potential problem with fresh human milk is the risk of CMV infection, which is of particular concern in the case of prematurity39; however, the topic is controversial and guidelines vary. Often, pasteurization of mother’s own milk is recommended for preterm infants because of the potential risk of CMV contamination. In our pilot study, we did not detect any CMV infection in the infants in the control or fresh human milk groups suggesting that it is safe to feed preterm infants fresh human milk.
Given previous reports that associated human milk with a decrease in NEC40,41, the NEC outcomes in our study were interesting. Keeping in mind the limitations in our study design, fresh human milk was protective against NEC when compared to the control, which is in agreement with a previous study showing that an early and rapid increase in enteral feeding with human milk was associated with a decreased risk of NEC41. Furthermore, a Belgian randomised controlled trial (RCT) pointed out that with fresh milk rather than pasteurised milk there was a lower frequency of NEC that required surgery40.
The potential of fresh human milk to reduce the incidence of BPD is also intriguing. Previous studies showed that oxidative stress is a major causative factor of BPD42, and fresh human milk has antioxidant properties43 that are altered by pasteurisation44. For example, fresh milk contains vitamins C and E and enzymes; including superoxide dismutase, catalase, and glutathione peroxidase; that are known to protect against the potentially-harmful effects of oxidative stress45. Our study supports further investigating the impact of fresh human milk on the incidence of BPD in a future RCT.
There are several limitations with our study. First, we observed differences in the baseline characteristics between the fresh human milk and control groups that we could not control for because our pilot study was under-powered. The educational level of mothers and the incidence of antenatal corticosteroid and magnesium sulphate use were higher in the fresh human milk group than the control group. It is possible that the better antenatal care and understanding of the benefits of fresh human milk by the mothers in the fresh human milk group impacted the infant outcomes in this group. Furthermore, the infants in the fresh human milk group had higher Apgar scores, were more likely to be singletons, spent fewer days on mechanical ventilation, and fewer days on TPN than the control group, all of which could have led to better outcomes for the fresh human milk group. Last, the definition of NEC is still heterogeneous and depends on the clinical judgement of the physician.
Despite the limitations, our study indicates that it is feasible and safe to feed preterm infants <30 weeks’ gestation fresh human milk. In China, there are no recent recommendations regarding the use of mother’s own fresh milk in NICUs. Practices differ widely among NICUs, but a high number of centres use fresh human milk to feed very preterm infants in recent years. The American Academy of Pediatrics recommends that all preterm infants are routinely fed fresh mother’s own milk13. To further evaluate the benefits of fresh human milk, an RCT is warranted.
The lessons learned from our study will provide important insights for our future RCT. For example, in China parents are not allowed in the NICU with their infants, which can make the logistics of providing fresh milk challenging. In our study, mothers consented to provide at least one feed of fresh human milk a day, and we allowed mothers into the NICU to pump to ensure human milk was delivered expediently. Future RCTs should also ensure mothers have a place in the NICU to pump human milk to enable compliance with the fresh human milk requirements. Furthermore, our estimate that 25% of mothers would consent to be in the fresh human milk group was low. We found that 81.9% (221/270) consented to participate in the study and 50.7% (112/220) of consenting mothers agreed to be in the fresh human milk group, which is the number that should be used for sample size calculations in the future. In a future multicenter randomized trial we will need to use multi-level models adjusting for potential confounders to analyze the data.
Conclusion
In summary, our multicentre prospective cohort study found that feeding preterm infants <30 weeks’ gestation fresh mother’s own milk once a day was safe and feasible in the NICU. Furthermore, our results suggested a potential reduced risk of NEC, sepsis, ROP, and BPD for these infants. Going forward, a large randomized multicentre study is needed to analyze the impact of fresh human milk on the outcomes of infants born preterm. If fresh human milk is shown to improve preterm infant outcomes, it will prompt new milk preparation and feeding guidelines for these vulnerable infants.
Acknowledgements
This study was supported by CHINA-CANADA Clinical Research Program and the Canadian Institutes of Health Research (CTP 87518). We thank the parents for allowing their very preterm infants to take part in this study; and the Research Centers of the Children’s Hospital of Zhengzhou University and Nanjing Maternity and Child Health Care Hospital, Children’s Hospital of Nanjing Medical University, the Third Xiangya Hospital of Central South University for their assistance. We thank Sarah Hutchinson, PhD, from the Maternal-Infant Care Research Centre at Mount Sinai Hospital, Toronto, Ontario, Canada for editing support. Funding was provided by the CHINA-CANADA Clinical Research Program and the Canadian Institutes of Health Research (CTP 87518). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Clinicaltrials.gov #NCT03258957 registered August 23, 2017.
Author Contributions
H.S., S.H., R.C., M.H. contributed equally to this work. Concept and study design: S.K.L., F.K. Data acquisition and analysis: H.S., S.H., R.C., M.H., S.K.L. Drafting the manuscript and the figures: H.S. Manuscript revisions: F.K.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med. 2011;16:145–150. doi: 10.1016/j.siny.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez KM, Moss RL. Necrotizing enterocolitis. Clin Perinatol. 2012;39:387–401. doi: 10.1016/j.clp.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Alshaikh B, Kostecky L, Blachly N, Yee W. Effect of a Quality Improvement Project to Use Exclusive Mother’s Own Milk on Rate of Necrotizing Enterocolitis in Preterm Infants. Breastfeed Med. 2015;10:355–361. doi: 10.1089/bfm.2015.0042. [DOI] [PubMed] [Google Scholar]
- 4.Quigley, M. & McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev, Cd002971, (2014). [DOI] [PubMed]
- 5.Li C, Solomons NW, Scott ME, Koski KG. Subclinical mastitis (SCM) and proinflammatory cytokines are associated with mineral and trace element concentrations in human breast milk. J Trace Elem Med Biol. 2018;46:55–61. doi: 10.1016/j.jtemb.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Khodabakhshi A, Mehrad-Majd H, Vahid F, Safarian M. Association of maternal breast milk and serum levels of macronutrients, hormones, and maternal body composition with infant’s body weight. Eur J Clin Nutr. 2018;72:394–400. doi: 10.1038/s41430-017-0022-9. [DOI] [PubMed] [Google Scholar]
- 7.Xiao L, et al. Human Milk Oligosaccharide 2’-Fucosyllactose Improves Innate and Adaptive Immunity in an Influenza-Specific Murine Vaccination Model. Front Immunol. 2018;9:452. doi: 10.3389/fimmu.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassiotou F, Geddes DT, Hartmann PE. Cells in human milk: state of the science. J Hum Lact. 2013;29:171–182. doi: 10.1177/0890334413477242. [DOI] [PubMed] [Google Scholar]
- 9.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Underwood MA. Human milk for the premature infant. Pediatr Clin North Am. 2013;60:189–207. doi: 10.1016/j.pcl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassiotou F, Geddes DT. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Adv Nutr. 2015;6:267–275. doi: 10.3945/an.114.007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassiotou F, Hartmann PE. At the dawn of a new discovery: the potential of breast milk stem cells. Adv Nutr. 2014;5:770–778. doi: 10.3945/an.114.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakulas F. Breast milk: a source of stem cells and protective cells for the infant. Infant. 2015;11:187–191. [Google Scholar]
- 14.Hassiotou F, et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30:2164–2174. doi: 10.1002/stem.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassiotou FHB, et al. Breastmilk stem cell transfer from mother to neonatal organs (216.4) The FASEB Journal. 2014;28:216.214. [Google Scholar]
- 16.Hassiotou FMA, Geddes D, Hartmann P, Wilkie TBreastmilk. Imparts the Mother’s Stem Cells to the Infant. The FASEB Journal. 2015;29:876.878. [Google Scholar]
- 17.Dicky O, et al. Policy of feeding very preterm infants with their mother’s own fresh expressed milk was associated with a reduced risk of bronchopulmonary dysplasia. Acta paediatr. 2017;106:755–762. doi: 10.1111/apa.13757. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Lara NR, et al. Effect of freezing time on macronutrients and energy content of breastmilk. Breastfeed Med. 2012;7:295–301. doi: 10.1089/bfm.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lev HM, et al. Major losses of fat, carbohydrates and energy content of preterm human milk frozen at −80 degrees C. J Perinatol. 2014;34:396–398. doi: 10.1038/jp.2014.8. [DOI] [PubMed] [Google Scholar]
- 20.Akinbi H, et al. Alterations in the host defense properties of human milk following prolonged storage or pasteurization. J Pediatr Gastroenterol Nutr. 2010;51:347–352. doi: 10.1097/MPG.0b013e3181e07f0a. [DOI] [PubMed] [Google Scholar]
- 21.Chang JC, et al. Influence of prolonged storage process, pasteurization, and heat treatment on biologically-active human milk proteins. Pediatr Neonatol. 2013;54:360–366. doi: 10.1016/j.pedneo.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Sari FN, et al. Antioxidant capacity of fresh and stored breast milk: is −80 degrees C optimal temperature for freeze storage? J Matern Fetal Neonatal Med. 2012;25:777–782. doi: 10.3109/14767058.2011.592230. [DOI] [PubMed] [Google Scholar]
- 23.Silvestre D, et al. Frozen breast milk at −20 degrees C and −80 degrees C: a longitudinal study of glutathione peroxidase activity and malondialdehyde concentration. J Hum Lact. 2010;26:35–41. doi: 10.1177/0890334409342987. [DOI] [PubMed] [Google Scholar]
- 24.Hassiotou F, Savigni D, Hartmann P, Geddes D. Optimization of Cell Isolation from Human Milk. The FASEB Journal. 2015;29:582.587. [Google Scholar]
- 25.Anne-Aurelie L, Souad B, Leila K. Clinical Findings and Autopsy of a Preterm Infant with Breast Milk-Acquired Cytomegalovirus Infection. AJP Rep. 2016;6:e198–202. doi: 10.1055/s-0035-1566249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez L, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69:1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 27.de Jong EP, Vossen AC, Walther FJ, Lopriore E. How to use… neonatal TORCH testing. Arch Dis Child Educ Pract Ed. 2013;98:93–98. doi: 10.1136/archdischild-2012-303327. [DOI] [PubMed] [Google Scholar]
- 28.Nahmias AJ, Walls KW, Stewart JA, Herrmann KL, Flynt WJ., Jr. The ToRCH complex-perinatal infections associated with toxoplasma and rubella, cytomegol-and herpes simplex viruses. Pediatr Res. 1971;5:405–406. doi: 10.1203/00006450-197108000-00144. [DOI] [Google Scholar]
- 29.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell MJ, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol123, 991–999 (2005). [DOI] [PubMed]
- 32.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 33.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 34.Bransburg-Zabary S, Virozub A, Mimouni FB. Human Milk Warming Temperatures Using a Simulation of Currently Available Storage and Warming Methods. PLoS One. 2015;10:e0128806. doi: 10.1371/journal.pone.0128806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arena Ansotegui J. Breastfeeding in the “global strategy for infant and young child feeding”. An Pediatr (Barc) 2003;58:208–210. doi: 10.1016/s1695-4033(03)78038-2. [DOI] [PubMed] [Google Scholar]
- 36.Boyd AE, Spatz DL. Breastfeeding and human lactation: education and curricular issues for pediatric nurse practitioners. J Pediatr Health Care. 2013;27:83–90. doi: 10.1016/j.pedhc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Handa D, et al. Do thawing and warming affect the integrity of human milk? J Perinatol. 2014;34:863–866. doi: 10.1038/jp.2014.113. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Lara NR, et al. Effect of Holder pasteurization and frozen storage on macronutrients and energy content of breast milk. J Pediatr Gastroenterol Nutr. 2013;57:377–382. doi: 10.1097/MPG.0b013e31829d4f82. [DOI] [PubMed] [Google Scholar]
- 39.Josephson CD, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168:1054–1062. doi: 10.1001/jamapediatrics.2014.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cossey V, et al. Pasteurization of mother’s own milk for preterm infants does not reduce the incidence of late-onset sepsis. Neonatology. 2013;103:170–176. doi: 10.1159/000345419. [DOI] [PubMed] [Google Scholar]
- 41.Nangia, S. et al. Early Total Enteral Feeding in Stable Very Low Birth Weight Infants: A Before and After Study. J Trop Pediatr. (2018). [DOI] [PubMed]
- 42.Sandal G, et al. Evaluation of treatment with hydrocortisone on oxidant/antioxidant system in preterm infants with BPD. Eur Rev Med Pharmacol Sci. 2013;17:2594–2597. [PubMed] [Google Scholar]
- 43.Mehta R, Petrova A. Is variation in total antioxidant capacity of human milk associated with levels of bio-active proteins? J Perinatol. 2014;34:220–222. doi: 10.1038/jp.2013.151. [DOI] [PubMed] [Google Scholar]
- 44.Silvestre D, et al. Antioxidant capacity of human milk: effect of thermal conditions for the pasteurization. Acta paediatr. 2008;97:1070–1074. doi: 10.1111/j.1651-2227.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 45.Tsopmo A, et al. Tryptophan released from mother’s milk has antioxidant properties. Pediatr Res. 2009;66:614–618. doi: 10.1203/PDR.0b013e3181be9e7e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.