Abstract
The unique antimicrobial mechanism of antimicrobials make them a promising substitute for antibiotics for fighting drug-resistant bacteria. Both melittin and thanatin have antimicrobial bioactivity. However, thanatin does not inhibit the growth of Staphylococcus aureus. Melittin can inhibit S. aureus and has strong hemolytic activity. In the present study, the mutant fragments of melittin and thanatin were combined by flexible peptides to form a novel hybrid peptide, which was synthesized based on the secondary and tertiary structure prediction. The hybrid peptide inhibited S. aureus with a hemolytic concentration of above 45 μmol/L and inhibition rate in SMMC-7721 cells of 19.14%. The hybrid antimicrobial peptide, which was designed by the combination of α-helix and β-lamellar antimicrobial peptides, showed that both types of peptides did not interact with each either on spatial structure or biological activities, thereby providing a novel idea for the design of artificial antimicrobial peptides.
Keywords: Melittin, Thanatin, Hybrid peptide, Structure, Activity
Introduction
Generally, the antimicrobial functions of traditional antibiotics are achieved by destroying the bacterial cell wall or blocking the biosynthesis of substances required for the biological activity of bacteria (Goossens et al. 2005; Mangoni and Bhunia 2016). However, the development of antibiotic-resistant bacteria often affects the clinical use of antibiotics. The antimicrobial mechanism of antimicrobial peptides has not been fully understood. Known mechanisms of action include the destruction of cell membranes, interference with nucleic acid and protein synthesis, the inhibition of cell wall synthesis, and interference with cell division (Bolintineanu et al. 2012; Lee and Park 2014; Ursic-Bedoya et al. 2011; Lee et al. 2016; Fabbretti et al. 2012; Malmsten 2014; Cho et al. 2012; Xia et al. 2018). Antimicrobial peptides have unique antimicrobial properties without detectable resistance, making them a promising substitute for traditional antibiotics (Costa et al. 2012; Durrant and Amaro 2015). Aside from determining antimicrobial peptides through biological approaches, researchers often design peptides artificially. Artificial peptides are widely used because of increased antimicrobial spectrum, in vivo stability and biological activity, and reduced cytotoxicity (Wang et al. 2015; Walsh et al. 2011; Godballe et al. 2011).
Thanatin, with a primary structure of GSKKPVPIIYCNRRTGKCQRM, is a type of antimicrobial peptide consisting of 21 amino acid residues. It is found in the insect Podisus maculiventris. Despite its broad-spectrum antimicrobial properties, it does not inhibit S. aureus (Fehlbaum et al. 1996; Mandard et al. 1998).
Melittin was first obtained from bee venom by Habermann and Jentsch (1967). It is a peptide consisting of 26 amino acids with a primary structure of GIGAVLKVLTTGLPALISWIKRKRQQ. It shows antimicrobial, anti-inflammatory, anti-radiation, anti-arthritic, anti-tumor, anti-AIDS, and other biological activities; It inhibits S. aureus (Wachinger et al. 1998; Saini et al. 1999; Gajski and Garajvrhovac 2011). However, its clinical application is limited by its strong toxicity (mainly hemolytic activity), genotoxicity, and influence on gene expression (Gajski et al. 2016; Wu et al. 2015). To improve the biological activity of melittin and reduce its hemolytic activity, many researchers have studied the structure–activity relationship and structural modification of melittin (Blondelle and Houghten 1991; Asthana et al. 2004; Li et al. 2003; Sun et al. 2005).
To expand the antimicrobial spectrum of thanatin and avoid the toxic effects of melittin, a hybrid antimicrobial peptide was designed according to a previous study (Blondelle and Houghten 1991). Hemolysis was slightly affected by the removal of C-terminal amino acids of melittin, according to the previous study (Jahnsen et al. 2015). Four alkaline amino acids (KRKR) are located at the C-terminal amino acids of melittin. The hybrid antimicrobial peptide that includes the full-length of thanatin and the C-terminal of melittin may reveal a stronger and safer antibacterial effect (Leonardo et al. 2012). The hybrid antimicrobial peptide GLPLLISWIKRKRQQ-AGP-GSKKPVPIIYCNRRTGKCQRM was designed using melittin’s C-terminal 15-amino acid mutant GLPL*LISWIKRKRQQ (L*wild type was A) as the N-terminus and thanatin as the C-terminus and by ligating with AGP to create a hybrid peptide that could inhibit S. aureus without hemolytic activity. The fusion expression of the hybrid peptide was carried out in Escherichia coli by using genetic engineering techniques, and the acid hydrolysis site AP was added to the N-terminus of the hybrid peptide. After the engineered bacteria were fermented, separated, acid-hydrolyzed, and purified, another proline residue was found on the N-terminus of the resulting peptide, that is, PGLPLLISWIKRKRQQGSKKPVPIIYCNRRTGKCQRM. In vitro antimicrobial experiments showed that this hybrid peptide inhibited the growth of S. aureus. However, pure hybrid peptide was not obtained due to problems associated with acid hydrolysis. Therefore, the author designed an artificial hybrid peptide (GLPLLISWIKRKRQQGSKKPVPIIYCNRRTGKCQRM) to further explore its antimicrobial, hemolytic, and anti-cancer activities.
Materials and methods
Design, physicochemical properties, and structure prediction of polypeptides
The 15 modified amino acid C-terminus of melittin and the complete amino acid sequence of thanatin were ligated using the flexible peptide linker alanine–glycine–proline. Thus, the designed hybrid peptide sequence was GLPLLISWIKRKRQQAGPGSKKPVPIIYCNRRTGKCQRM. By utilizing online tools, the secondary and tertiary structure, molecular weight, and isoelectric point of the original peptides and the designed hybrid thanatin and melittin peptide were predicted. Secondary structure prediction was based on GOR (http://npsa-Pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page = npsa_gor4.html) and the HNN method (https://prabi.ibcp.fr/htm/site/web/home), and the advanced structure was predicted based onPhyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/html) and SWISS-MODEL (https://swissmodel.expasy.org/) programs.
Polypeptide biosynthesis
The peptide was synthesized from the C-terminus to N-terminus end by the solid-phase synthesis method (ChinaPeptides Co., Ltd.). The synthesized peptide chain was oxidized with dimethyl sulfoxide (DMSO) to form a ring, after which it was purified by high performance liquid chromatography, and freeze-dried into powder with a purity of 98.06%.
Bacteria
Escherichia coli JM109 strain was obtained from the School of Life Science, Huzhou Univesity. S. aureus (1.282), Bacillus subtilis (1.15792) and Salmonella typhimurium (1.1190) were purchased from China General Microbiological Culture Collection Center (CGMCC). The sterile defibrinated sheep blood was obtained from Pingrui Biotechnology (Beijing) Co., Ltd.
Antibacterial test
Escherichia coli JM109, S. aureus, B. subtilis, and S. typhimurium were inoculated into liquid PB medium (1% peptone and 0.9% sodium chloride) at 37 °C and 180 rpm for 24 h, and the PB medium was diluted to 300 bacteria/80 μL. The hybrid antimicrobial peptide was diluted with PB medium into 1.5, 3, 6, 12.5, 25, 50, 100, 200, 400, and 12 μmol/L. Twenty microliters of the hybrid antimicrobial peptide solution was added to a 96-well plate, and then 80 μL of the diluted bacterial solution was added for a total of 100 μL. A positive control (PB medium + ampicillin) and a negative control (PB medium only) were also included. The 96-well plates were incubated for 12 h at 25 °C with slow shaking (~ 100 rpm) (Taguchi et al. 2000).
Hemolysis test
Two milliliters of defibrinated sheep blood were obtained and centrifuged at 2000 rpm for 10 min, and the pellet was kept and washed with normal saline until no blood color remained. Next, saline was added and diluted to 2% of the red blood cell suspension. A volume of 2.5 mL of the red blood cell suspension was added into seven test tubes. Then, 2.5 mL of increasing concentrations of the antimicrobial peptide (final concentration: 5, 15, 30, 45, and 60) was also added to each tube, whereas 2.5 mL of normal saline and 2.5 mL of distilled water were used as a negative and positive controls, respectively. After homogenization, the tubes were incubated in a water bath at 37 °C and observed every 15 min for the first hour and once an hour for the next 3 h (Wei et al. 2010).
Anticancer tests
These tests took three experimental groups of the culture medium, control, and test group (the concentration of hybrid peptide was 100 µg/mL). Each group contains 3 double-wells and was repeated thrice. After the preserved SMMC-7721 cell line (BNCC338089, purchased from Bnbio company, Beijing, China) were reactivated by the RPMI-1640 medium and were cultured to the logarithmic phase, the cells were washed by phosphate buffer (pH 7.3) thrice. Then, the cells were digested with 0.25% of trypsin–EDTA-2Na for 2–3 min. Digestion was terminated by the cultured medium, and the cell suspension was transferred into the centrifuge tube. After 3 min of centrifugation (1000 rpm), the precipitated cells were resuspended by the RPMI-1640 medium and then diluted to 2.5 × 104 cells/mL. All cell plates were covered with board. The culture medium group was mixed with 200 µL of the RPMI-1640 medium, and the control and test group were mixed with 100 µL of cell suspension. Then, each group was incubated in 5% CO2 and saturation vapor incubator for 3–4 h at 37 °C. A total of 100 µL of the RPMI-1640 medium was added into the control group, and 100 µL of hybrid peptide was added into the test group after incubation. The culture was continued for 44 h. The medium was absorbed, and each plate was washed with phosphate buffer. After washing, 100 µL of phosphate buffer and 20 µL of MTT were added into each group. After 4 h, incubation was completed, and the media were consumed. A volume of 100 µL of DMSO was then added into each hole. After 10 min of low-speed shock, the absorbance (A490 mm) values were measured using the microplate reader.
Results
Physicochemical properties and structure prediction of polypeptides
Physicochemical properties of the hybrid peptide
The isoelectric point and molecular weight of the designed hybrid peptide are shown in Table 1 (thanatin data from the reference) (Fehlbaum et al. 1996).
Table 1.
Physicochemical properties of hybrid peptide
| Peptides | Isoelectric point (pI) | Molecular weight (Da) |
|---|---|---|
| Melittin Peptide | 12.02 | 1836.26 |
| Thanatin | 10.48 | 2436.20 |
| Hybrid Peptide | 11.50 | 4576.56 |
Secondary structure prediction by the GOR method
The secondary structure of the modified C-terminal amino acid sequence of melittin was predicted to be: ccccchhhhhcccee, where h represents alpha helix, c represents random coil, and e represents extended strand.
The predicted secondary structure of thanatin was: ccccceeeeeecccccccccc.
Therefore, as anticipated, the predicted secondary structure of the hybrid peptide was: ccccchhhhhhhhhccccccccceeeeeeccccccccee.
Secondary structure prediction by the HNN method
The secondary structure of the modified C-terminal amino acids of melittin based on the HNN method are as follows:
DSC ccchhhhhhhhhccc
MLRC ceeeeeeeeeeeeec
PHD ccceceehhhhhhhc
Sec.Cons. ccce?eehhhhh??c
The predicted secondary structure of thanatin is as follows:
DSC cccccceeeeecccccccccc
MLRC ccccceeeeeeeccccccccc
PHD ccccccceeeecccccccccc
Sec.Cons. cccccceeeeecccccccccc
The predicted secondary structure of the hybrid peptide is as follows:
DSC cccchhhhhhhhhhcccccccccceeeeecccccccccc
MLRC ccchhhhhhhhhhhcccccccccceeeeeeccccccccc
PHD cceeeeeehhhhhhhcccccccceeeeeeeccccccccc
Sec.Cons. ccc?hhhhhhhhhhcccccccccceeeeeeccccccccc
Advanced structural prediction
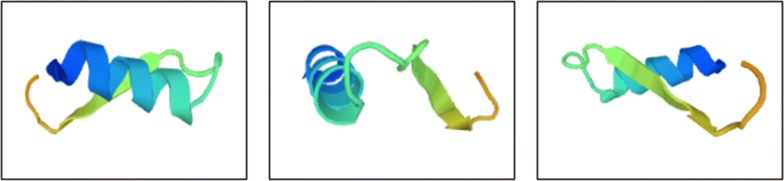
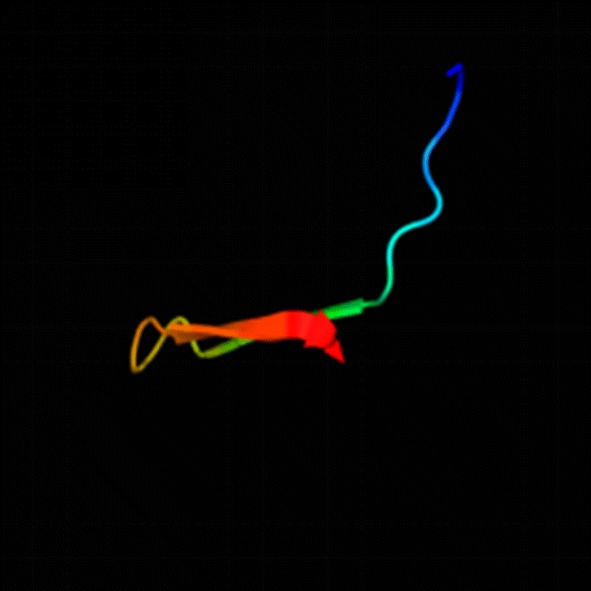
To further investigate the hybrid peptide structure, we determined the predicted tertiary structure by using two methods. First, Phyre2 was used in our research. The tertiary structure prediction based on Phyre2 is shown in Fig. 1. The second method utilized for predicting the tertiary structure of the hybrid peptide was SWISS-MODEL. Seven similar templates were predicted with a consistency of 31.03. The spatial simulation structure is shown in Fig. 2.
Fig. 1.
Phyre2 predicted tertiary structure of the hybrid peptide (arrow from N C)
Fig. 2.
SWISS-MODEL prediction tertiary structures
Antibacterial test
The minimum inhibitory concentration (MIC) of the hybrid antimicrobial peptide and melittin on E. coli, S. aureus, B. subtilis and S. typhimurium were detected and listed in Table 2.
Table 2.
Minimum inhibitory concentration (MIC) range of antimicrobial peptides
| Bacterial name | Inhibitory concentration range (hybrid) | Inhibitory concentration range (melittin) |
|---|---|---|
| Escherichia coli JM 109 | 1.2–2.5 μmol/L | 0.6–1.2 μmol/L |
| Staphylococcus aureus | 1.2–2.5 μmol/L | 0.9–1.5 μmol/L |
| Bacillus subtilis | 2.5–5 μmol/L | 0.6–1.2 μmol/L |
| Salmonella typhimurium | 1.2–2.5 μmol/L | 0.3–0.6 μmol/L |
MIC and hemolysis test
Functional evaluation of the hybrid peptide and melittin was determined by establishing the minimum inhibitory concentration (MIC) and by performing a hemolysis test. The MIC of the hybrid antimicrobial peptide against E. coli JM109, S. aureus, B. subtilis, and S. typhimurium is shown in Table 2.
Hemolysis test performed using the hybrid peptide showed that hemolysis did not occur at 45 μmol/L but rather at 60 μmol/L, indicating that the hemolytic concentration of the hybrid antimicrobial peptide was greater than 45 μmol/L.
Anticancer tests
The result of the mean difference analysis showed a significant difference between the control and test groups (P < 0.05), and the average inhibitory rate of the hybrid peptide in the SMMC-7721 cells was 19.14%.
Discussion
Protein structural modification and the antimicrobial peptides have always been a research hotspot, as well as the antimicrobial peptides. The modification of various antimicrobial peptide structures (Gao and Zhu 2012; Wang et al. 2015), the splicing of different peptides (Acuña et al. 2012; Kim et al. 2016; Memariani et al. 2016; Orrapin and Intorasoot 2014), antimicrobial peptides incorporating non-natural amino acids, or artificial and computer-aided design of antimicrobial peptides (Wang et al. 2016) are all common means of peptide modifications. Melittin’s hemolytic activity has limited its clinical application, prompting researchers to study the causes of hemolysis and modify it (Wu et al. 2016; Sun et al. 2005; Yan et al. 2003; Juvvadi et al. 2010).
In the present study, the hybrid peptide was designed by using melittin’s C-terminal 15-amino acid mutant (with weak hemolytic activity) (Li et al. 2003) as the N-terminus and thanatin as the C-terminus. Based on the secondary structure prediction by using the GRO method, the secondary structure of the hybrid peptide’s C-terminus was not changed compared with thanatin. However, the N-terminus showed an increased helicity compared with the mutant fragments of melittin. From the secondary structure prediction by using the HNN method, the secondary structure of the hybrid peptide’s C-terminus was not changed compared with thanatin. The N-terminal helicity was not reduced compared with the mutant fragments of melittin. The melittin fragment and thanatin contained less than 30 amino acid residues, thereby contributing to the uncertainty over the tertiary structure prediction. Hence, the spatial structure of the three peptides cannot be compared. From the tertiary structure of the hybrid peptide model generated by the Phyre2 and SWISS-MODEL, the N-terminal melittin fragment had an α-helix structure, whereas the C-terminal thanatin was composed of three independent structures, including random peptide fragments, β-lamella, and random peptide fragments. Considering the presence of independent α-helix and β-lamellar structures, the hybrid peptide retained the ability to inhibit S. aureus. The MIC of the hybrid peptide on S. aureus and B. subtilis was nearly consistent with that of the melittin fragment and thanatin, and its MIC on E. coli JM109 and S. typhi was also increased (Fehlbaum et al. 1996; Li et al. 2003; Taguchi et al. 2000). These results suggested that the melittin fragment and thanatin almost did not interact with each another on the spatial structure of the hybrid peptide, and each peptide fragment retained its original antimicrobial properties. The retention of the melittin fragment’s activity might contribute to the anti-cancer activity of the hybrid peptide. In this study, a hybrid antimicrobial peptide was designed by the combination of α-helix and β-lamellar antimicrobial peptides. Both types of peptide did not interact with each other either in terms of spatial structure or biological activities, thereby providing ideas for the design of artificial antimicrobial peptides.
Authors’ contributions
XFJ and KQ designed and conceptualized the project; GPL, LYS and XFJ designed the experiments; XFJ, GPL, GQZ and JFL performed the experiments; XFJ, KQ, GPL, XQF and ZBL analyzed the data. XFJ wrote the manuscript and KQ, LYS, XQF, HXG and ZBL contributed extensively in revising the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Xiaogang Xu to provide great help in data analysis.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Please contact author for data requests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All experimental procedures were approved by the Committee on Human Material Care and Use and the Committee on the Ethic of Human Material Experiments of Zhejiang Sci-Tech University (No. 20180024). And we confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Funding
This research was funded by Science and Technology Project of Huzhou (2016GY04) and the Start-up Project of Zhejiang Sci-Tech University (116129A4Y17186).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaofeng Jiang, Email: kizi24@126.com, Email: xfjiang@zstu.edu.cn.
Kun Qian, Email: qiankun@zjhu.edu.cn.
Guangping Liu, Email: liugp0093@aliyun.com.
Laiyu Sun, Email: sunly@zjhu.edu.cn.
Guoqing Zhou, Email: zgq@zjhu.edu.cn.
Jingfen Li, Email: ljfljf@zjhu.edu.cn.
Xinqiang Fang, Email: fxq@zjhu.edu.cn.
Haixia Ge, Email: gehaixia@zjhu.edu.cn.
Zhengbing Lv, Email: zhengbingl@126.com.
References
- Asthana N, Yadav SP, Ghosh JK. Dissection of antibacterial and toxic activity of melittin: a leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. J Biol Chem. 2004;279:55042–55050. doi: 10.1074/jbc.M408881200. [DOI] [PubMed] [Google Scholar]
- Blondelle SE, Houghten RA. Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry. 1991;30:4671–4678. doi: 10.1021/bi00233a006. [DOI] [PubMed] [Google Scholar]
- Bolintineanu DS, Vivcharuk V, Kaznessis YN. Multiscale models of the antimicrobial peptide protegrin-1 on gram-negative bacteria membranes. Int J Mol Sci. 2012;13:11000–11011. doi: 10.3390/ijms130911000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Hwang IS, Choi H, Hwang JH, Hwang JS, Lee DG. The novel biological action of antimicrobial peptides via apoptosis induction. J Microbiol Biotechnol. 2012;22:1457–1466. doi: 10.4014/jmb.1205.05041. [DOI] [PubMed] [Google Scholar]
- Costa CFD, Pinheiro AC, Almeida MVD, Lourenço MCS, Souza MVND. Synthesis and antitubercular activity of novel amino acid derivatives. Chem Biol Drug Des. 2012;79:216–222. doi: 10.1111/j.1747-0285.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Amaro RE. Machine-learning techniques applied to antibacterial drug discovery. Chem Biol Drug Des. 2015;85:14–21. doi: 10.1111/cbdd.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbretti A, Brandi L, Petrelli D, Pon CL, Castañedo NR, Medina R, Gualerzi CO. The antibiotic furvina® targets the p-site of 30 s ribosomal subunits and inhibits translation initiation displaying start codon bias. Nucleic Acids Res. 2012;40:10366. doi: 10.1093/nar/gks822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlbaum P, Bulet P, Chernysh S, Briand JP, Roussel JP, Letellier L, Hetru C, Hoffmann JA. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc Natl Acad Sci USA. 1996;93:1221–1225. doi: 10.1073/pnas.93.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajski G, Garajvrhovac V. Bee venom induced cytogenetic damage and decreased cell viability in human white blood cells after treatment in vitro: a multi-biomarker approach. Environ Toxicol Pharmacol. 2011;32:201–211. doi: 10.1016/j.etap.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Gajski G, Domijan AM, Žegura B, Štern A, Gerić M, Novak JI, Vrhovac I, Madunić J, Breljak D, Filipič M, Garaj-Vrhovac V. Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon. 2016;110:56–67. doi: 10.1016/j.toxicon.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Gao B, Zhu SY. Alteration of the mode of antibacterial action of a defensin by the amino-terminal loop substitution. Biochem Biophys Res Commun. 2012;426:630–635. doi: 10.1016/j.bbrc.2012.08.143. [DOI] [PubMed] [Google Scholar]
- Godballe T, Nilsson LL, Petersen PD, Jenssen H. Antimicrobial β-peptides and α-peptoids. Chem Biol Drug Des. 2011;77:107–116. doi: 10.1111/j.1747-0285.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- Goossens H, Ferech M, Vander SR, Elseviers M, Group EP Outpatient antibiotic use in europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- Habermann E, Jentsch J. Sequence analysis of melittin from tryptic and peptic degradation products. Hoppe Seylers Z Physiol Chem. 1967;348:37–50. doi: 10.1515/bchm2.1967.348.1.37. [DOI] [PubMed] [Google Scholar]
- Jahnsen RO, Sandbergschaal A, Frimodtmøller N, Nielsen HM, Franzyk H. End group modification: efficient tool for improving activity of antimicrobial peptide analogues towards gram-positive bacteria. Eur J Pharm Biopharm. 2015;95:40–46. doi: 10.1016/j.ejpb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Juvvadi P, Vunnam S, Merrifield EL, Boman HG, Merrifield RB. Hydrophobic effects on antibacterial and channel-forming properties of cecropin a–melittin hybrids. J Pept Sci. 2010;2:223–232. doi: 10.1002/psc.63. [DOI] [PubMed] [Google Scholar]
- Kim H, Jang JH, Kim SC, Cho JH. Enhancement of the antimicrobial activity and selectivity of GNU7 against Gram-negative bacteria by fusion with LPS-targeting peptide. Peptides. 2016;82:60–66. doi: 10.1016/j.peptides.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Lee JK, Park Y. Mechanism of action of antimicrobial peptides against bacterialn membrane. J Bacteriol Virol. 2014;44:140–151. doi: 10.4167/jbv.2014.44.2.140. [DOI] [Google Scholar]
- Lee TH, Hall KN, Aguilar MI. Antimicrobial peptide structure and mechanism of action: a focus on the role of membrane structure. Curr Top Med Chem. 2016;16:25–39. doi: 10.2174/1568026615666150703121700. [DOI] [PubMed] [Google Scholar]
- Leonardo A, Gianluca P, Fernando S, Morero RD, Augusto B. A new hybrid bacteriocin, ent35-mccv, displays antimicrobial activity against pathogenic gram-positive and gram-negative bacteria. Febs Open Bio. 2012;2:12–19. doi: 10.1016/j.fob.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Yan H, Liu G, He B, Jiang L. Studies on synthesis and biological activities of analogues of melittin. Chem Res Chin Univ. 2003;24:449–453. [Google Scholar]
- Malmsten M. Antimicrobial peptides. Upsala J Med Sci. 2014;119:199–204. doi: 10.3109/03009734.2014.899278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandard N, Sodano P, Labbe H, Bonmatin JM, Bulet P, Hetru C, Ptak M, Vovelle F. Solution structure of thanatin, a potent bactericidal and fungicidal insect peptide, determined from proton two-dimensional nuclear magnetic resonance data. FEBS J. 1998;256:404–410. doi: 10.1046/j.1432-1327.1998.2560404.x. [DOI] [PubMed] [Google Scholar]
- Mangoni M, Bhunia A. Editorial: antimicrobial peptides in medicinal chemistry: advances and applications. Curr Top Med Chem. 2016;16:2–3. doi: 10.2174/1568026615999150914160355. [DOI] [PubMed] [Google Scholar]
- Memariani H, Shahbazzadeh D, Sabatier JM, Memariani M, Karbalaeimahdi A, Bagheri KP. Mechanism of action and invitro, activity of short hybrid antimicrobial peptide pv3 against pseudomonas aeruginosa. Biochem Biophys Res Commun. 2016;479:103–108. doi: 10.1016/j.bbrc.2016.09.045. [DOI] [PubMed] [Google Scholar]
- Orrapin S, Intorasoot S. Recombinant expression of novel protegrin-1 dimer and LL-37-linker–histatin-5 hybrid peptide mediated biotin carboxyl carrier protein fusion partner. Protein Expr Purif. 2014;93:46–53. doi: 10.1016/j.pep.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Saini SS, Chopra AK, Peterson JW. Milittin activates endogenous phospholipase D during cytolysis of humanmonocytic leukemia cells. Toxicon. 1999;37:1605–1619. doi: 10.1016/S0041-0101(99)00110-5. [DOI] [PubMed] [Google Scholar]
- Sun X, Chen S, Li S, Yan H, Fan Y, Mi H. Deletion of two c-terminal Gln residues of 12-26-residue fragment of melittin improves its antimicrobial activity. Peptides. 2005;26:369–375. doi: 10.1016/j.peptides.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Taguchi S, Kuwasako K, Suenaga A, Okada M, Momose H. Functional mapping against Escherichia coli for the broad-spectrum antimicrobial peptide, thanatin, based on an in vivo monitoring assay system. J Biochem. 2000;128:745–754. doi: 10.1093/oxfordjournals.jbchem.a022811. [DOI] [PubMed] [Google Scholar]
- Ursic-Bedoya R, Buchhop J, Joy JB, Durvasula R, Lowenberger C. Prolixicin: a novel antimicrobial peptide isolated from Rhodnius prolixus with differential activity against bacteria and trypanosoma cruzi. Insect Mol Biol. 2011;20:775–786. doi: 10.1111/j.1365-2583.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, Holle R, Salmons B, Erfle V, Brack-Werner R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral geneexpression. J Gen Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- Walsh EG, Maher S, Devocelle M, O’Brien PJ, Baird AW, Brayden DJ. High content analysis to determine cytotoxicity of the antimicrobial peptide, melittin and selected structural analogs. Peptides. 2011;32:1764–1773. doi: 10.1016/j.peptides.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhong W, Lin D, Xia F, Wu W, Zhang H, Lv L, Liu S, He J. Antimicrobial peptides derived from fusion peptides of influenza a viruses, a promising approach to designing potent antimicrobial agents. Chem Biol Drug Des. 2015;86:487–495. doi: 10.1111/cbdd.12511. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang YJ, Chen YN, Zhao HY, Zhang S. Computer-aided design, structural dynamics analysis, and in vitro susceptibility test of antibacterial peptides incorporating unnatural amino acids against microbial infections. Comput Methods Programs Biomed. 2016;134:215–223. doi: 10.1016/j.cmpb.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Wei W, Wu XM, Li YJ. Experimental methodology of pharmacology. Shelton: People’s Medical Publishing House; 2010. pp. 503–504. [Google Scholar]
- Wu X, Zhao B, Cheng Y, Yang Y, Huang C, Meng X, Wu B, Zhang L, Lv X, Li J. Melittin induces ptch1 expression by down-regulating mecp2 in human hepatocellular carcinoma smmc-7721 cells. Toxicol Appl Pharmcol. 2015;288:74–83. doi: 10.1016/j.taap.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Wu X, Singh AK, Wu X, Lyu Y, Bhunia AK, Narsimhan G. Characterization of antimicrobial activity against listeria and cytotoxicity of native melittin and its mutant variants. Colloid Surf B. 2016;143:194–205. doi: 10.1016/j.colsurfb.2016.03.037. [DOI] [PubMed] [Google Scholar]
- Xia X, Cheng L, Zhang S, Wang L, Hu J. The role of natural antimicrobial peptides during infection and chronic inflammation. Anton Leeuw. 2018;111:5–26. doi: 10.1007/s10482-017-0929-0. [DOI] [PubMed] [Google Scholar]
- Yan H, Li SX, Mi H, He B. Individual substitution analogs of mel (12-26), melittin’s c-terminal 15-residue peptide: their antimicrobial and hemolytic actions. FEBS Lett. 2003;554:100–104. doi: 10.1016/S0014-5793(03)01113-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.