Abstract
The ability to synthesize Indole-3-acetic acid (IAA) is widely associated with the plant growth promoting rhizobacteria (PGPR). The present work deals with isolation and characterization of such bacteria from the rhizosphere of medicinal plant Stevia rebaudiana and optimization of IAA production from its isolates. The optimization of IAA production was carried out at different pH and temperature with varied carbon and nitrogen sources of culture media. Out of different isolates obtained, three of them were screened as efficient PGPRs on the basis of different plant growth promoting attributes. Isolates CA1001 and CA2004 showed better production of IAA at pH 9 (91.7 µg ml−1) and at temperature 37 °C (81.7 µg ml−1). Dextrose (1%) was found to be the best carbon source for isolate CA1001 with 104 µg ml−1 IAA production. Isolate CA 2004 showed best production of IAA 36 µg ml−1 and 34 µg ml−1 at 1.5% and 1% Beef extract as nitrogen source respectively. Isolate CA 1001 showed 32 µg ml−1 IAA production at 0.5% nicotinic acid concentration. From the current study, CA1001 and CA2004 emerged as noble alternatives for IAA production further which also resulted in root and shoot biomass generation in crop plants, hence can be further used as bio-inoculants for plant growth promotion.
Keywords: Indole-3-acetic acid, Rhizobacteria, Optimization, Stevia rebaudiana
1. Introduction
Plant growth in soil depends upon a number of biotic and abiotic factors. The thin layer of soil immediately surrounding the root of a plant is an extremely important area for root activity and metabolism and is known as the rhizosphere. It is home to a number of bacteria, fungi, protozoa and algae whereby bacteria outnumber the else by a huge margin. Plants select those bacteria which are beneficial for their growth by release of particular organic compounds through root exudates [1] thus creating a very selective environment where only a limited species of bacteria can survive and hence diversity is low [2], [3]. This happens due to the corresponding ability of bacteria to utilize these compounds [4]. Thus, rhizosphere acts as a unique ecological niche for each plant and those beneficial bacteria associated with plants are referred to as plant growth promoting bacteria. A number of bacterial species belonging to Azospirillum, Alcaligenes, Arthrobacter, Acinetobacter, Bacillus, Burkholderia, Bradyrhizobium, Enterobacter, Erwinia, Flavobacterium, Pseudomonas, Rhizobium and Serratia have been found to be associated with rhizosphere and are able to exert beneficial effects on plant growth [5], [6].
Indole-3-acetic acid (IAA) is the main member of auxins family produced by plants as it plays an important role in a number of plant activities such as leaf formation, embryo development, root initiation and development, abscission (falling of leaves), phototropism, geotropism, fruit development, etc. IAA helps in the enhancement of root length with increase in number of root branches, root hairs and root laterals that aid in uptake of nutrients from surrounding [7]. Since IAA has been found to be very important for plant growth and development, extensive studies have been performed on IAA after its discovery as a plant hormone. It has been found out that different bacteria, fungi and algae are capable of producing physiologically active amounts of IAA. IAA is synthesized by plants and microbes via a number of interrelated pathways of which tryptophan dependent pathway is the best understood. [8]. As plants grow, they release many water-soluble compounds such as amino acids, sugars and organic acids. So the rhizospheric bacteria get abundant supply of substrates required for secondary metabolite production of which IAA is one.
In this study, the one factorial approach has been employed for optimization by varying one factor and keeping all the others fixed during the trial. The aim of this study was to isolate IAA producing bacteria and to increase IAA production by varying different physiological parameters such as pH, temperature, carbon and nitrogen sources, so that we get the intended conditions at which the IAA production is maximized. IAA does not function as a hormone in microbial cells (PGPRs) but their ability to produce the same might have evolved because of its importance in plant microbe relationships. The present study reports optimization of growth parameters for IAA production by PGPRs isolated from rhizosphere of Stevia rebaudiana.
2. Materials and methods
2.1. Isolation of bacteria from rhizospheric soil
Soil samples were collected from the BIT Medicinal plants garden and Institute of Forest Productivity, Garhkhatanga, Ranchi, Jharkhand. The intact plant with root was dug out carefully with 15 cm soil slab. The clumps of soil tightly bound to the roots were carefully stored in sterile polyethylene and used for isolation of PGPRs.
Standard tenfold serial dilution method was used for the bacterial isolation from the soil [9]. Soil was air dried to remove the excess moisture. 1 gm of soil was suspended in 10 ml autoclaved distilled water and 1 ml of soil solution from each tube was passed on to the next tube and subsequently a dilution range of 10−1 to 10−10 was prepared. 50 μl of soil solution was spread on sterile nutrient agar plates and incubated at 37 °C for 24 h. Several bacterial colonies appeared whereby the morphologically distinguishable colonies were picked and streaked on nutrient agar plates. Re-streaking was carried on until pure cultures were obtained. IAA-producing isolates were selected by growing them in IAA production medium as described below. The isolate producing maximum amount of IAA was further selected for the optimization of IAA production. Finally, selected isolates were named according to their collection sites and analyzed for various plant growth promoting activities. Pure cultures were maintained in nutrient agar slants at 4 °C in sterile conditions for further use.
2.2. Characterization of IAA production
IAA production was detected by the method described by Brick et al. [1991] with some modification [10]. 25 ml nutrient broth was inoculated with freshly grown cultures and kept at 37 °C for 36 h at 120 rpm in an incubator shaker. Cultures were centrifuged at 10,000 rpm for 15 min. at room temperature. Carefully 1 ml supernatant was pipetted out and 2 ml Salkowski reagent (2% 0.5 M FeCl3 in 35% perchloric acid) added. Further, two drops of orthophosphoric acid added to it and kept in dark for color formation. The optical density was recorded at 530 nm after 2 h. IAA concentrations were determined using the standard plot of IAA.
2.3. Standard assays
Standard IAA was purchased from HiMedia and different concentrations (25, 50, 100, 150, 200, 250 and 300 µg ml−1) were prepared in distilled water. For color formation, Salkowski reagent was added to different dilution of IAA (1:2) followed by the addition of two drops of orthophosphoric acid and kept in dark. Spectrometry measurements were made at 530 nm and standard curve was plotted.
2.4. IAA production and optimization of production parameters
For IAA production, the culture medium was inoculated with 24 h grown cultures (O.D 0.5) of isolates CA1001, CA2003 and CA2004. Four different parameters viz. Temperature, pH, carbon source and nitrogen source were taken for the study. The basic IAA production medium consisted of the peptone 10 g l−1 yeast extract 6 g l−1 and NaCl 5 g l−1. All cultures were incubated at 37 °C at 120 rpm for 36 h for observation. pH is one of the most important physicochemical parameters for IAA production. A range of pH 5–9 was examined for its effect on IAA production by different isolates. Temperature is also an important parameter for IAA production since the growth of bacteria affected by low or high temperature and IAA production is dependent upon the correct growth of microorganism. Thus, its production was tested at 25, 30, 35, 37, 40 and 45 °C at 120 rpm. Five different sugars viz. Dextrose, mannitol, sucrose, mannose and starch at different concentrations of 0.5%, 1.0% and 1.5% were tested. Different nitrogen sources viz. beef extract, soybean meal, urea, glycerine, nicotinic acid, ammonium nitrate and potassium nitrate at varying concentrations of 0.50%, 0.75%, 1%, 1.25% and 1.50% were used for the study. Quantification of IAA production was performed through the standard plot.
2.5. Effect of selected isolates in crop plants for plant growth activities
Seeds of three crop plants viz. mungbean (strain no-SML66), sorghum (strain no-CSH2255) and millet (strain no-A404) were procured from Department of plant breeding, Birsa Agricultural University, Kanke, Ranchi, Jharkhand and seeds of wheat were purchased from market.
Healthy seeds were sterilized by washing them with tap water to remove all the dirt and dust followed by washing twice with distilled water to remove any tap water. Then seeds were treated with a 0.2% solution of a fungicide Bavistin for 2–3 min followed by dipping them in 0.1% of HgCl2 for 2–3 min. Seeds were then washed with 70% ethanol 3 times followed by rinsing them with autoclaved distilled water 5 times. The selected PGPRs were grown in nutrient broth for 18 h at 37 °C in an incubator shaker at 120 rpm. The surface sterilized seeds were inoculated in broth culture of the three PGPRs for 5–6 h. MS medium was prepared and 100 ml was poured in 500 ml conical flask. 5 seeds for each crop plant were then washed in autoclaved distilled water to remove broth and placed at a equal distance in the flask using sterile forceps. Uninoculated seeds were treated with distilled water and worked as control. Flasks were plugged using sterile cotton plug and kept in the plant growth chamber at 25 °C for 30 days. After 30 days flasks were taken out and plants were carefully uprooted. The shoots and roots were separated and dried in an oven at 68 ± 2 °C for 48 h to calculate the dried root and shoot biomass.
3. Results and discussion
Isolates were screened for IAA production. Out of twenty isolates (not shown here) of plant growth promoting rhizobacteria (PGPRs), three were selected as efficient producers of IAA; hence they were used for optimization of IAA production. All three isolates produce IAA in the presence of tryptophan, but in this study medium was kept devoid of any tryptophan because peptone contains tryptophan and in earlier qualitative tests these isolates were able to produce IAA in the absence of tryptophan.
Lower pH limits the growth of plants, as concentration of metal ions could reach toxic levels in the soil at low pH [11]. A number of physiological and metabolic processes taking place in the rhizosphere can be affected by soil pH and metal cations present in the vicinity, therefore, impact of pH range of 5–9 was only checked for IAA production (Fig. 1). CA1001 was able to produce good amount of IAA throughout the pH range tested, which varied from 74.3 µg ml−1 at pH 5 to 91.7 µg ml−1 IAA produced at pH 9.
Fig. 1.
Effect of pH on IAA production by PGPR [bar = mean ± SD].
For isolate CA2003 at pH 5 it showed maximum production (73.71 µg ml−1) and started decreasing when moved through the pH range 6–9. Least production was 15.33 µg ml−1 observed at pH 9. CA2004 produced 21.16 µg ml−1 of IAA at pH 5 and produced maximum 53.06 µg ml−1 of IAA at pH 6 and then the production decreased with subsequent increase in pH. Bharucha et al. [2013] showed the maximum IAA production at pH 7.5 in Pseudomonas putida UB-1 [12] whereas Shanti et al. [2007] suggested that production varied between pH 6.4 and 7.8 [13] and maximum was observed at 7.2. pH range of 6–8 was also found optimum by Sachdev et al. [2009] with maximum production at pH 8 [14].
The effect of temperature was studied in the range 25–45 °C whereby maximum yield (84.3 µg ml−1) was observed at 37 °C by isolate CA 1001 (Fig. 2). Similar result was shown in other studies [14], [15] where 37 °C was the best temperature for IAA production for Rhizobium and Bacillus spp.
Fig. 2.
Effect of temperature on IAA production by PGPR [bar = mean ± SD].
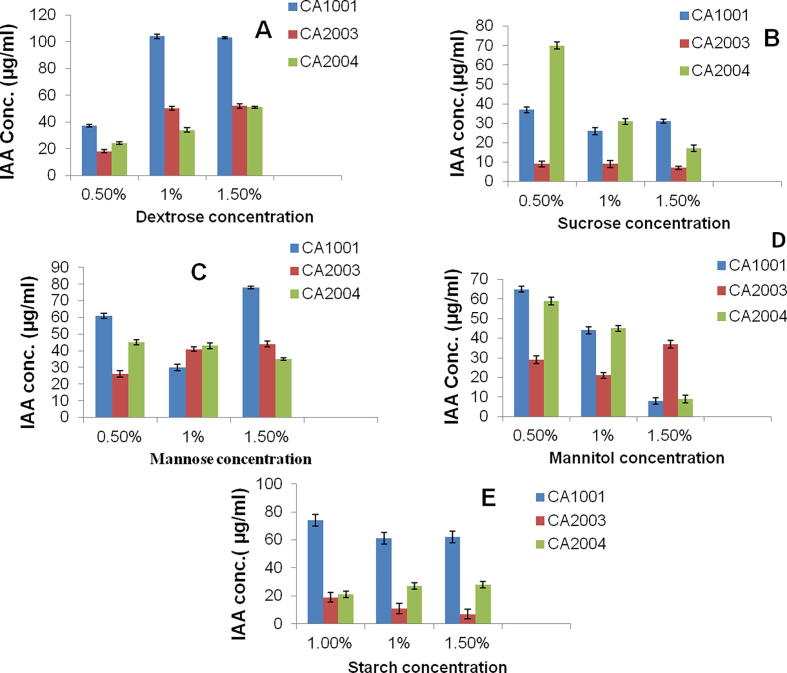
The carbon sources that are used in production of secondary metabolites have profound effect on the overall efficiency of biosynthesis. Five different sugars (dextrose, sucrose, mannose, mannitol and starch) were used in this study to check their effect on IAA production [Fig. 3A–E].
Fig. 3.
(A–E) – Effect of different sugar sources on IAA production by PGPR [bar = mean ± SD].
Dextrose was found as the best carbon source [Fig. 3(A)–(E)] producing 104 µg ml−1 IAA at 1% and 103 µg/ml at 1.5% concentration of Dextrose for isolate CA1001 followed by starch and mannose. For isolate CA2003 dextrose was the best sugar source producing 50 µg ml−1 followed by mannose and mannitol. IAA production declined to 7 µg ml−1 in case of starch at 1.5%. For isolate CA2004 best sugar was found to be dextrose followed by mannose and mannitol. Monosaccharides were better sources than disaccharides and polysaccharides. Therefore dextrose was found as the best sugar source for IAA production. Studies by Shanti et al. [2007] and Sridevi et al. [2008] on bacteria have suggested that individual carbon sources affect the IAA production [13], [16]. Studies on carbon sources suggested that effect of different concentrations of sugar sources in basal media was different due to variable utilization of sugars by bacteria during their growth.
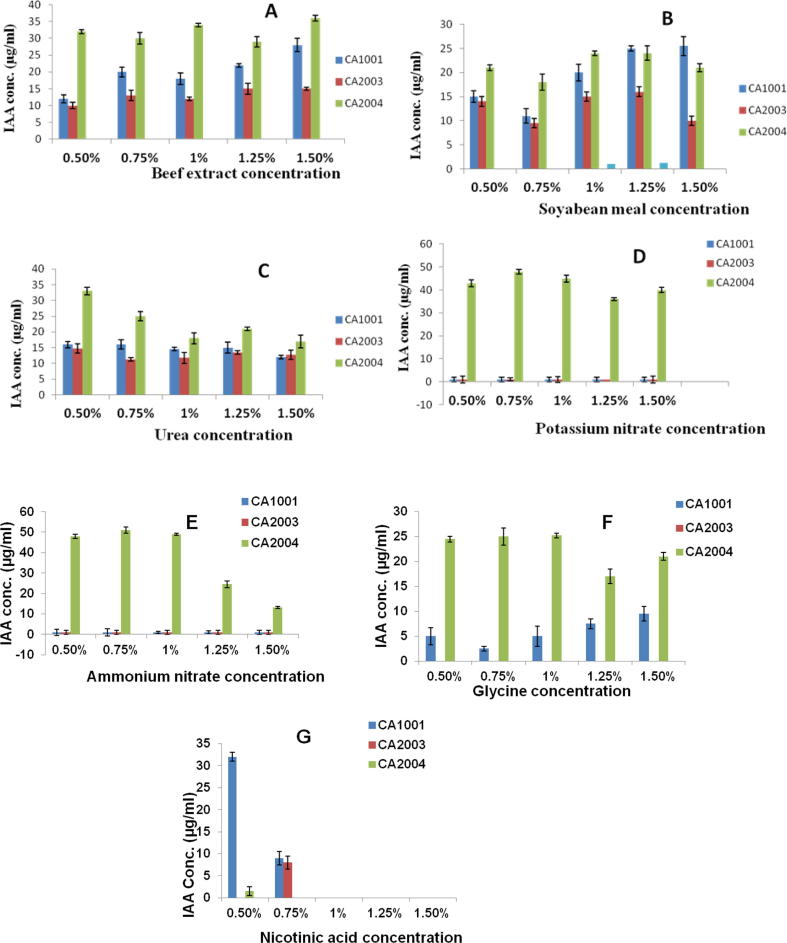
Utilization of varied nitrogen sources showed varied response for production of IAA. Source of inorganic nitrogen as ammonium nitrate by isolate CA 2004 showed production of 51 µg ml−1 at 0.75% and a very similar production 48 and 49 µg ml−1 at 0.5% and 1% respectively. Isolate CA 2004 showed production of 48 µg ml−1 IAA at 0.75% potassium nitrate. Among organic sources, Beef extract and nicotinic acid showed better production compared to soyabean meal and glycine as nitrogen sources. Isolate CA 2004 showed the best production of IAA 36 µg ml−1 and 34 µg ml−1 at 1.5% and 1% beef extract respectively. Isolate CA 1001 showed 32 µg ml−1 production of IAA at 0.5% nicotinic acid concentration.
Impact of nitrogen sources on IAA production was studied by addition various nitrogen sources such as beef extract, urea, soyabean meal, glycine, nicotinic acid, ammonium nitrate and potassium nitrate at the concentrations of 0.5%, 0.75%, 1%, 1.25% and 1.50% [Fig. 4(A)–(G)].
Fig. 4.
(A–G) Effect of nitrogen sources on IAA production by PGPR [bar = mean ± SD].
In case of Isolates CA1001 and CA2003, IAA production declined when nitrogen sources were used. The best nitrogen source was found to be the beef extract followed by soyabean meal and urea. When inorganic nitrogen sources were used, no growth was observed in case of isolates CA1001 and CA2003 whereas nicotinic acid acted as a growth inhibitor when concentration was increased from 1 to 1.5%. At low concentrations of 0.5%, isolate CA1001 was able to grow and IAA production was measured at 32 µg ml−1 whereas isolate CA2003 and CA2004 showed very little to no growth on nearly zero production of IAA. Isolate CA2004 gave best results in case of nitrogen sources. IAA production was about 45 µg ml−1 when ammonium nitrate and potassium nitrate were used whereas it was in range of 20–35 µg ml−1 consistently in case of beef extract, soyabean meal and urea. Studies for nitrogen sources showed that ammonium chloride to be the best nitrogen source for Acetobacter diazotrophicus and IAA production by Pseudomonas fluorescence to be 48 µg ml−1 [17]. Another study by Balaji [2012] showed yeast extract (210 µg ml−1) as the best nitrogen source for Pseudomonas species and IAA production to be 18.08 mg l−1 when soyabean meal was used as the nitrogen source [18]. Recent study [19] reported a 13.4-fold improvement in IAA production by Enterobacter sp. DMKU-RP206. Enterobacter sp. DMKU-RP206 produced a higher amount of IAA than previously reported for the genus Enterobacter. 0.85% of lactose as a carbon source, 1.3% of yeast extract as a nitrogen source, 1.1% of l-tryptophan as a precursor, 0.4% of NaCl, an initial pH of 5.8, an incubation temperature at 30 °C, and a shaking speed of 200 rpm were found to be the optimum conditions for IAA production. In a similar study [20] antagonistic Pseudomonas aeruginosa PS24 showed multiple plant growth promoting attributes such as phosphate solubilization activity, indole acetic acid (IAA), siderophore, and HCN production. Another recent study [21] showed the production of IAA by enteric bacteria Salmonella which is also colonizing plant tissues and help in interaction with plants and animals which also provides new incentives to gain insight into the function of this plant hormone in a larger biological context. The results of this work suggest that the Salmonella ipdC (indole-3-pyruvate decarboxylase) contributes to the production of IAA in laboratory cultures and this gene is expressed during root colonization, and that the product of the reaction involving IpdC activates plant auxin-responsive promoter.
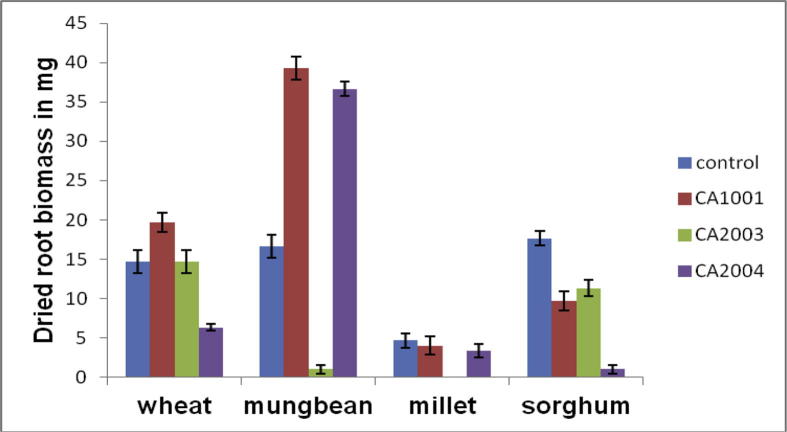
To study the effect of PGPR on plant growth, seeds of different crop plants were treated with isolates CA 1001, CA2003 and CA2004. Isolates CA1001 and CA 2004 showed better response on treating the seeds of mungbean with production of 39.33 mg of dried root biomass compared to control as 16.66 mg in mungbean plant, whereas in wheat it was 19.66 mg by isolate CA1001. Isolate CA2004 also showed correspondingly good result in mungbean plant with generation of 36.66 mg of dried root biomass. And there is higher root biomass produced as compared to wheat and sorghum. Isolate CA 2003 gave better result in wheat and Sorghum with generation of 14.66 mg and 11.33 mg of dried root biomass respectively. Similarly, dried shoot biomass of 43 mg and 41 mg were also obtained in mungbean plant when seeds were treated with isolates CA 1001 and CA 2004 respectively followed with 19.6 mg in wheat. In millet and sorghum no prospective results were obtained.
The property of IAA production by rhizobacterial isolates is considered as effective tool for screening beneficial microorganisms and they have profound effect on plant growth [22]. Previous studies also confirmed the involvement of rhizobacterial isolates in enhancing the plant growth by synthesizing IAA. Root elongation was found to occur in Sesbania aculeata by inoculation with Azotobacter spp. and Pseudomonas spp., in Brassica campestris by Bacillus spp [23], in Vigna radiate by Pseudomonas putida [24] and in Pennisetum americanum by Azospirillum brasilense [25]. Effect of IAA producing isolate was also observed in Solanum lycopersicum, [26] where it significantly increased the shoot and root biomass and chlorophyll (a and b) contents as compared to control plants. In another study [27] PGPR applications had desirable effects on plant growth. It revealed the effect of P. aeruginosa strain (AK20 and AK31), P. fluorescens strain (AK18 and AK45) and B. subtilis strain (AK38) in rice, caused significant variations between weight and length of shoot and root (see Fig. 5, Fig. 6).
Fig. 5.
Effect of varied isolates on root biomasss production.
Fig. 6.
Effect of varied isolates on shoot biomasss production.
4. Conclusion
Present study showed that maximum yield of IAA was obtained at pH 9 (91.7 µg ml−1) and at temperature 37 °C (81.7 µg ml−1) by isolate CA1001. Dextrose (1%) was found to be the best carbon source for isolate CA1001 with 104 µg ml−1 IAA production. Isolate CA 2004 showed maximum production of 51 µg ml−1 IAA at 0.75% ammonium nitrate. Isolate CA 2004 showed best production of IAA 36 µg ml−1 and 34 µg ml−1 at 1.5% and 1% Beef extract respectively. Isolate CA 1001 showed 32µg ml−1 production of IAA at 0.5% nicotinic acid concentration. From the current study, CA1001 and CA2004 emerged as noble alternative for IAA production further which also resulted in root and shoot biomass generation in crop plants, hence can be further used as bio-inoculants for plant growth promotion. Further studies are required to explore more on production of IAA by rhizosheric bacteria and study their effect on various crop plants in the field where plants are exposed to various abiotic and biotic factors.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
References
- 1.Jones D. John Wiley & Sons; Chichester, Sussex, UK: 1990. The Rhizosphere; p. 458. [Google Scholar]
- 2.Barriuso J., Solano B.R., Lucas J.A., Lobo A.P., García-Villaraco A., Mañero F.J.G. Wiley-VCH Verlag GmbH and Co. KGaA; Weinheim: 2008. Ecology, genetic diversity and screening strategies of plant growth promoting rhizobacteria (PGPR) pp. 1–17. [Google Scholar]
- 3.Marilley L., Aragno M. Phytogenetic diversity of bacterial communities differing in degree of proximity of Lolium perenne and Trifolium repens roots. Appl Soil Ecol. 1999;13:127–136. [Google Scholar]
- 4.Nye P. The Rhizosphere. In: Curl E.A., Truelove B., editors. (Advanced Series in Agricultural Sciences, Coordinating Editor B. Yaron) Springer–Verlag; Berlin–Heidelberg–New York–Tokyo: 1987. pp. 284–285. [Google Scholar]
- 5.Tilak K.V.B.R., Ranganayaki N., Pal K.K., De R., Saxena A.K., Nautiyal C.S. Diversity of plant growth and soil health supporting bacteria. Curr Sci. 2005;89(1):136–150. [Google Scholar]
- 6.Egamberdiyeva D. Plant-growth-promoting rhizobacteria isolated from a calcisol in a semi-arid region of Uzbekistan:biochemical characterization and effectiveness. J Plant Nutr Soil Sci. 2000;168(1):168. [Google Scholar]
- 7.Datta C., Basu P.S. Indole acetic acid production by a Rhizobium species from root nodules of a leguminous shrub, Cajanus cajan. Microbiol Res. 2000;155(2):123–127. doi: 10.1016/S0944-5013(00)80047-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribbons D.W. Academic Press; 2006. Extremophiles. Methods in microbiology; p. 543. [Google Scholar]
- 10.Brick J.M., Bostock R.M., Silverstones S.E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbial. 1991;57(2):535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohit B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr. 2013;13(3):638–649. [Google Scholar]
- 12.Bharucha U., Trivedi U.B., Patel K. Optimization of indole acetic acid production by Pseudomonas putida UB1 and its effect as plant growth-promoting rhizobacteria on Mustard (Brassica nigra) Agric Res. 2013;2(3):215–222. [Google Scholar]
- 13.Shanti M., Keshab C., Dey S. Optimization of cultural and nutritional conditions for indole acetic acid production by a Rhizobium sp. isolated from root nodules of Vigna mungo (L.) Hepper. Res J Microbiol. 2007;2:239–246. [Google Scholar]
- 14.Sachdev D.P., Chaudhari H.G., Kasture V.M., Dhavale D.D., Chopade B.A. Isolation and characterization of indole acetic acid (IAA) producing klebsiella pneumonia strains from rhizosphere of wheat (Triticum aestivum) and their effect on plant growth. Ind J Exp Boil. 2009;47:993–1000. [PubMed] [Google Scholar]
- 15.Sudha M., Shyamala G.R., Prbhavati P., Astapritya P., Yamuna Devi Y., Saranya A. Production and optimization of Indole acetic acid by indigenous microflora using agro waste as substrate. Pak J Biol Sci. 2012;15(1):39–43. doi: 10.3923/pjbs.2012.39.43. [DOI] [PubMed] [Google Scholar]
- 16.Sridevi M., Yadav N.C.S., Mallaiah K.V. Production of indole acetic acid by Rhizobium isolates from Crolataria species. Res J Microbiol. 2008;3(4):276–281. [Google Scholar]
- 17.Jeyanthi V., Ganesh P. Production, optimization and characterization of phytohormone Indole Acetic Acid by Pseudomonas fluorescence. Int J. Pharma Biol Arch. 2013;4(2):514–520. [Google Scholar]
- 18.Balaji N., Lavanya S.S., Muthamizhselvi S., Tamilarasan K. Optimization of fermentation condition for indole acetic acid production by Pseudomonas species. Int J Adv Biotechnol Res. 2012;3:797–803. [Google Scholar]
- 19.Nutarata P., Monprasit A., Srisuk N. High-yield production of indole-3-acetic acid by Enterobacter sp. DMKU-RP206, a rice phyllosphere bacterium that possesses plant growth-promoting traits. 3. Biotech. 2017;7:305. doi: 10.1007/s13205-017-0937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uzair B., Kausar R., Bano S.A., Fatima S., Badshah M., Habiba U. Isolation and molecular characterization of a model antagonistic Pseudomonas aeruginosa divulging in vitro plant growth promoting characteristics. Hind BioMed Res Int. 2018;2018:1–7. doi: 10.1155/2018/6147380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox C.E., Brandl M.T., de Moraes M.H., Gunasekera S., Teplitski M. Production of the plant hormone auxin by Salmonella and its role in the interactions with plants and animals. Front Microbiol. 2018;8:2668. doi: 10.3389/fmicb.2017.02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahyudi A.T., Astuti R.P., Widyawati A., Meryandini A., Nawangsih A.A. Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrobiol. 2011;3:34–40. [Google Scholar]
- 23.Ghosh S., Penterman J.N., Little R.D., Chavez R., Glick B.R. Three newly isolated plant growth-promoting bacilli facilitate the seeding growth of canola Brassica campestris. Plant Physol Biochem. 2003;41:277–281. [Google Scholar]
- 24.Pattern C.L., Glick B.R. Role of Pseudomanas putida indole acetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tien T.M., Gaskinsa M.H., Hubbell N.D.D.H. Plant growth substances produced by Azospirillurn brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol. 1979;37:1016–1024. doi: 10.1128/aem.37.5.1016-1024.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan A.L., Halo B.A., Elyassi A., Ali S., Al-Hosni K., Hussain J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Elect J Biotechnol. 2016;21:58–64. [Google Scholar]
- 27.Karnwal A. Isolation and identification of plant growth promoting rhizobacteria from maize (Zea mays L.) rhizosphere and their plant growth promoting effect on rice (Oryza sativa L.) J Plant Protect Res. 2017;57(2):144–151. [Google Scholar]