Abstract
Proteases are the hydrolytic enzymes which hydrolyzes peptide bond between proteins with paramount applications in pharmaceutical and industrial sector. Therefore production of proteases with efficient characteristics of biotechnological interest from novel strain is significant. Hence, in this study, an alkaline serine protease produced by Bacillus cereus strain S8 (MTCC NO 11901) was purified and characterized. The alkaline protease was purified by ammonium sulfate precipitation (50%), ion exchange (DEAE-Cellulose) and gel filtration (Sephadex G-100) chromatographic techniques. As a result of this purification, a protein with specific activity of 300U/mg protein was obtained with purification fold 17.04 and recovery percentage of 34.6%. The molecular weight of the purified protease was determined using SDS-PAGE under non-reducing (71 kDa) and reducing conditions (35 kDa and 22 kDa). Zymogram analysis revealed that proteolytic activity was only associated with 22 kDa. These results indicate that existence of the enzyme as dimer in its native state. The molecular weight of the protease (22 kDa) was also determined by gel filtration (Sephadex G-200) chromatography and it was calculated as 21.8 kDa. The optimum activity of the protease was observed at pH 10.0 and temperature 70 °C with great stability towards pH and temperature with casein as a specific substrate. The enzyme was completely inhibited by PMSF and TLCK indicating that it is a serine protease of trypsin type. The enzyme exhibits a great stability towards organic solvents, oxidizing and bleaching agents and it is negatively influenced by Li2+ and Co2+ metal ions. The purified protein was further characterized by Matrix Assisted Laser Desorption Ionization/Mass Spectroscopy (MALDI/MS) analysis which reveals that total number of amino acids is 208 with isoelectric point 9.52.
Keywords: Bacillus cereusstrain S8 (MTCC NO 11901), Alkaline protease, Purification, SDS-PAGE, Kinetic studies, FTIR, MALDI-TOFF
1. Introduction
Proteases are a complex group of ubiquitous enzymes in nature, belongs to a class of hydrolases with immense physiological and commercial significance [6]. Proteases form a large group of industrial enzymes with multitude of applications in various fields. Bacterial proteases are exploited widely than others because of their extracellular nature and high yield of production. With increasing industrial demand for proteases, it is expected that hyperactive strains will emerge and that the enzymes produced by new exotic microbial strains could be used as biocatalysts in the presently growing biotechnological era. Thermostability is a very important property of industrial enzymes and it is advantageous as that it contributes to the reduction of microbial contaminants and cooling costs. Hence it is imperative to search for alkaline proteases with desirable properties for commercial viability from bacterial sources [13].
Purification of enzyme (protease) is very important for developing a better understanding of the functioning of the enzyme. Different strategies adopted for purification of enzymes are on similar lines as that of proteins. Despite the diversity in the origin of enzymes they are purified using a generalized overall approach, which involves initial recovery of protein, concentration/primary purification and finally high end resolution chromatographic purification.
For most commercial purposes, crude protease preparations are generally employed while pure preparations are needed for pharmaceutical and other medical applications. The purified enzyme is also required for property studies and better understanding of structure function relationship. Protein separation techniques have traditionally been used to isolate and to purify specific proteins in order to facilitate studies of their enzymatic, physical, chemical and structural properties. These kinds of studies are necessary in order to elucidate the biological role of individual proteins in the cell and to understand the mechanism by which the activity of specific enzymes is controlled. A number of alkaline proteases from different sources have been purified & characterized. Factors affecting culture conditions, productivity and properties of alkaline protease was considered as significant in the purification and characterization of enzyme [14].
Once purified, most enzymes are subjected to a battery of characterization studies which include functional characteristics, evidence of purity, structural studies (molecular mass, amino acid composition, amino acid sequencing, analysis of secondary/tertiary/quaternary structure) etc., [11]. The purified protein was further characterized by Matrix Assisted Laser Desorption Ionization/Mass Spectroscopy (MALDI/MS) analysis. The combination of electrophoretic separation & mass spectrometric analysis are considered to be very powerful tool for protein analysis.
The properties of the enzyme identified during these studies help in determining the areas of its possible application. As the recovery costs of enzymes are nearly 70% of the total manufacturing costs [2] it is necessary to identify the characteristics of an enzyme to determine whether it has the potential for being adopted as a commercial enzyme.
The present chapter describes the purification, biochemical and molecular characterization and kinetic studies of extracellular alkaline protease from Bacillus cereus strain S8 with the aim of understanding the properties of the enzyme and assessing its worthiness as a commercial enzyme.
2. Materials and methods
2.1. Production of crude alkaline protease
Production of crude alkaline protease from Bacillus cereus strain S8 (MTCC NO 11901) was carried out by statistically optimized media supplemented with molasses, 1%(w/v); potassium nitrate, 0.75%(w/v); salt solution, 5%(v/v) – {MgSO4·7H2O, 0.5%(w/v); KH2PO4, 0.5%(w/v)}; FeSO4.7H2O, 0.01%(w/v) and CaCO3, 0.5% formulated by [3].
2.2. Purification of alkaline protease
The alkaline protease from Bacillus cereus strain S8 was purified by conventional purification procedures in three steps as follows.
Step 1: Ammonium sulfate precipitation
To the chilled crude enzyme, solid ammonium sulfate (10–100% saturation) was slowly added as per standard chart [9], [10] to precipitate the enzyme. Precipitation was done at 4 °C. The precipitate obtained was collected by centrifugation (8000g at 4 °C for 15 min) and dissolved in minimum quantity of Glycine-NaOH buffer (pH 10.0). This preparation was treated as partially purified enzyme.
Step 2: DEAE-Cellulose Ion exchange chromatography
DEAE-cellulose was purchased from Sigma and activated as per manufacturer’s instructions. The resin was packed into column. Care was taken to avoid trapping of air bubbles. All the buffers used were filtered and degassed before each run. The column was pre-equilibrated with Glycine-NaOH buffer (pH 10.0). The unbound proteins were eluted with the equilibration buffer. Fractions of 5 ml were collected at a flow rate of 60 ml/h and were assayed for protein by measuring their absorbance at 280 nm and enzyme by casein as substrate. The elution of the bound protein was done with a linear gradient of 0.1 to 1.0 M NaCl and fractions of 5 ml were collected at a flow rate of 60 ml/h. These fractions were assayed for protein by measuring their absorbance at 280 nm and enzyme assay by casein as substrate.
Step 3: Gel filtration on Sephadex G-100
The lyophilized material obtained after elution with NaCl gradient was dissolved in Glycine-NaOH buffer (pH 10.0) and then loaded on to equilibrated Sephadex G-100 column and fractions were collected with a flow rate of 12 ml/h by monitoring the absorbance of protein at 280 nm and activity of enzyme by assay method.
The purity of the sample at each step was checked by Polyacrylamide gel electrophoresis (PAGE) according to the method of [19] in 10% Polyacrylamide gel. Silver staining was performed according to the method of [4]. Glycoprotein staining [8] was performed to check the glycoprotein nature of the enzyme. Molecular weight of the purified protease was determined by SDS-PAGE and gel filtration chromatography on Sephadex G-200. Zymography was performed to check the activity of enzyme. Activity gel was prepared according to the method of [16] with some modifications.
2.3. Characterization of purified protease
Characterization of the purified enzyme was carried out for several parameters like substrate specificity (casein, gelatin, ovalbumin and bovine serum albumin), activity and stability at different pH (2.0–12.0) and temperatures (40–80 °C).
Effect of different metal ions (NiCl2, MnCl2, CuCl2, CaCl2, ZnCl2, MgCl2, FeCl3, HgCl2, MoCl2, AgNO3, LiSO4, BaCl2, CoCl2 and PbCl2), inhibitors (phenyl methyl sulphonyl fluoride (PMSF), Tosyl-L-lysyl-chloromethane ketone (TLCK), Tosyl phenylalanyl chloromethyl ketone (TPCK), iodoacetic acid (IAA), β-Mercaptoethanol, ethylene-diamine tetraacetic acid (EDTA), organic solvents (methanol, ethanol, isopropanol, acetonitrile, ethyl ether, hexane, benzene, acetone, toluene, xylene, butanol, dimethylsulphoxide (DMSO)), surfactants (Sodium dodecyl sulphate (SDS), Triton X-100, CTAB, Tween-20, Tween-80) and bleaching agents (H2O2, Sodium hypochlorite and Sodium perborate) were studied.
2.4. Analysis of casein hydrolysates
Casein was hydrolyzed by the purified protease enzyme at optimal conditions i.e. at 70 °C and pH 10.0 for 60 min. The hydrolysate was centrifuged (10,000 rpm, 10 min, 4 °C) and the supernatant was collected. The supernatant was freeze dried and used for FTIR analysis. Spectroscopic measurements were performed using approximately 25 mg freeze dried hydrolysate. The hydrolysate was mixed with 225 mg dried KBr (10% w/w) and used for analysis.
2.5. Determination of kinetic parameters
The kinetic parameters for purified alkaline protease was calculated by using various concentration of casein (0.25, 0.5, 0.75, 1.0 1.25, 1.5, 1.75, 2.0%) with standard assay conditions. The kinetic rate constants, KM and Vmax were determined by Michaelis-Menten equation.
2.6. Characterization of protease by MALDI-TOF MS
MALDI-TOF mass spectra were used for the analysis of peptide mass fingerprinting and MS/MS ion search. The 20 μl of trypsin digested purified protein solution was used for the MALDI-TOF MS analysis, recorded on an AB SCIEX Voyager DE Pro MALDI-TOF (Applied Biosystems, Foster City, CA) time-of-flight spectrometer, with a pulsed nitrogen laser (337 nm; 3-ns pulse width). The spectra were recorded in the linear, positive high-mass mode. A saturated solution of a-cyano-4-hydroxycinnamic acid in a 1:1 mixture of acetone and water along with 0.1% trifluoroacetic acid was used for obtaining the mass spectra.
3. Results
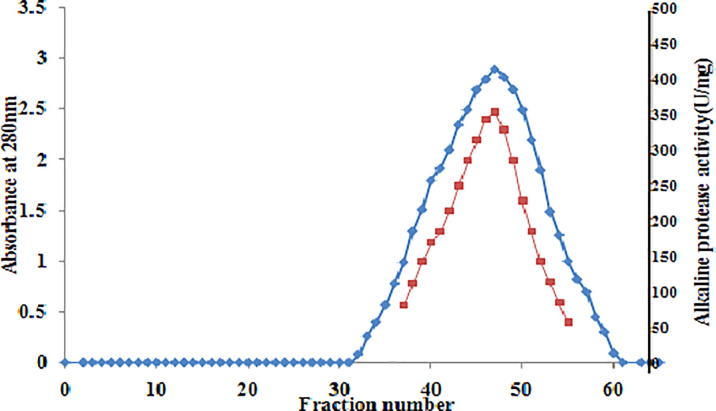
Purification of the enzyme is performed with the crude enzyme of the culture broth of Bacillus cereus strain S8. Protein with highest specific activity was achieved at 50% ammonium sulfate saturation. The dialyzed sample was loaded on DEAE-cellulose column previously equilibrated with Glycine-NaOH buffer, pH 10.0. Active fractions were obtained with 0.3 M NaCl gradient (0.1 M−1.0 M) elution (Fig. 1). The lyophilized sample obtained from previous step was loaded on Sephadex G-100 column and elution was carried out (Fig. 2). The extent of purification at each step was shown in purification table (Table 1). The homogeneity of the purified enzyme was checked by native PAGE (Fig. 3a) and silver staining (Fig. 3b). A single protein band was obtained for Sephadex G-100 fraction in 10% gel showed the extent of purification. Zymography of protease also revealed a clear zone of casein hydrolysis around protein band. Protease was negative towards periodic acid Schiff’s staining (Fig. 3a) suggesting that absence of carbohydrate moieties in the enzyme.
Fig. 1.
Ion exchange chromatography of ammonium sulfate fraction on DEAE-cellulose.
Fig. 2.
Gel filtration of alkaline protease on Sephadex G-100 of the DEAE-cellulose preparation.
Table 1.
Purification Table.
| Preparation | Volume (ml) | Total protein (mg) | Total activity units | Specific activity U/mg protein | Yield % | Fold purification |
|---|---|---|---|---|---|---|
| Crude | 200 | 2950 | 52,000 | 17.6 | 100 | 0 |
| 50% Ammonium sulphate | 100 | 950 | 28,000 | 29.47 | 53.8 | 1.67 |
| Ion exchange chromatography | 60 | 160 | 25,200 | 157.5 | 48.4 | 8.94 |
| Gel filtration chromatography | 40 | 60 | 18,000 | 300 | 34.6 | 17.04 |
Fig. 3a.

Native PAGE, Lane-1: Crude fraction. Lane-2: Ammonium sulfate fraction. Lane-3: Ion exchange fraction. Lane-4: Gel filtration fraction. Lane-5: PAS staining. Zymogram: Specific/activity staining.
Fig. 3b.
Silver staining, Lane-1: Crude extract of protease. Lane-2: Recombinant protein markers (kDa). Lane-3: Protease from ion exchange fraction. Lane-4: Protease from gel filtration fraction.
3.1. Determination of molecular weight
Under non-reducing conditions, SDS-PAGE of purified protein resulted in a fine single band and the molecular weight was found to be 71 kDa (Fig. 3c). But reducing SDS-PAGE of the same purified protein sample provides different result by exhibiting two protein bands of molecular weights 35 kDa and 22 kDa (instead of 71 kDa) respectively (Fig. 5a, Fig. 5b) with proteolytic activity confined to 22 kDa (Fig. 3c). It indicates that the existence of the enzyme as a dimer (linked by disulphide bridges) in its native state and further studies were carried out with protein of 22 kDa. Gel filtration of protease on Sephadex G-200 was carried out (Fig. 4a) and the molecular weight of the enzyme, as calculated from the plot (Ve/Vo versus log molecular weight) was 21.8 kDa (Fig. 4b).
Fig. 3c.

SDS-PAGE, Lane-1: Non-reducing SDS- PAGE (Single band. Lane-2: Reducing SDS- PAGE (Two protein bands). Lane-3: Recombinant protein markers.
Fig. 5a.
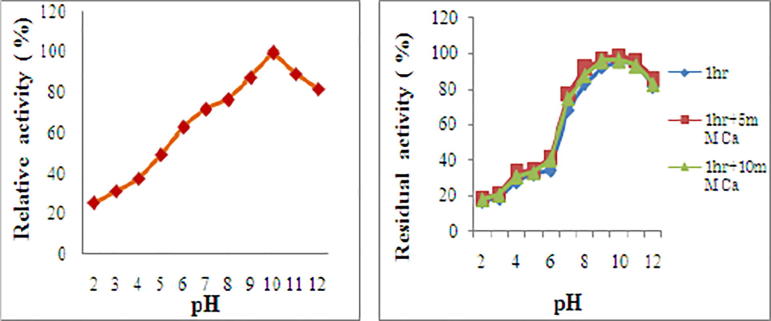
Effect of pH on the activity and stability of protease.
Fig. 5b.
Effect of temperature on the activity and stability of protease.
Fig. 4a.
Elution pattern of alkaline protease on Sephadex G-200 column.
Fig. 4b.
Molecular weight determination of alkaline protease by gel filtration Sephadex G-200 column.
3.2. Characterization of purified protease
Characterization studies of the purified enzyme from Bacillus cereus strain S8 showed that protease exhibits highest activity with casein (186 ± 0.18) than other substrates with optimum activity at pH 10.0 and great deal of stability in the pH range 7.0–12.0 (Fig. 5a). It is active at all the temperatures (30–80 °C) tested, with maximum activity at 70 °C, qualifying it to be designated as a significant thermoactive protease. It exhibits a great deal of stability in the temperature range 50–75 °C in presence of calcium (Fig. 5b). Activity of enzyme was positively influenced by Mo2+, Cu2+, Fe2+, Mg2+, Ni2+ and Ca2+ followed by Mn2+ and Ba2+, which slight decrease in the activity. However, Zn2+ ions marginally reduced activity. The activity of enzyme was negatively influenced by Li2+ and Co2+ ions while. Pb2+, Ag2+, and Hg2+ completely inhibited the enzyme activity (Fig. 6a). The alkaline protease was completely inhibited by PMSF, a serine protease inhibitor and TLCK, which is a trypsin inhibitor (Fig. 6b) suggesting that it is a serine protease of trypsin type.
Fig. 6a.
Effect of metals on the activity of protease (at 5 mM & 10 mM).
Fig. 6b.
Effect of inhibitors on the activity of protease (at 1 mM & 5 mM).
The protease activity was enhanced by the addition of xylene, ethyl ether, toluene, acetonitrile and DMSO, whereas there was decrease in enzyme activity observed with hexane, isopropanol, methanol, ethanol, butanol and benzene respectively (Fig. 6c). The enzyme retained significant activity with Tween 80, CTAB, SDS, Triton X-100 and Tween 20 at low concentrations but inhibited at highest concentrations (Fig. 6d). It was found to be stable at low concentrations of oxidizing agents (H2O2, Sodium hypochlorite and sodium perborate) (Fig. 6d).
Fig. 6c.
Effect of organic solvents on the activity of protease.
Fig. 6d.
Effect of surfactants and bleaching agents on the activity of protease.
3.3. Analysis of casein hydrolysates
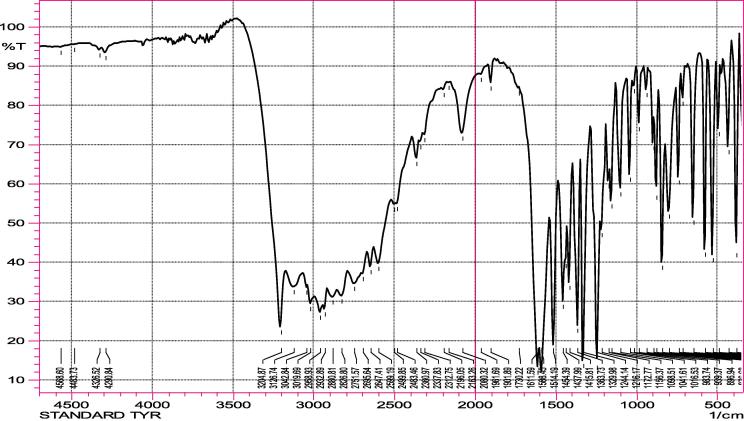
Casein was hydrolyzed by the purified protease enzyme at optimal conditions i.e. at 70 °C and pH 10.0 for 60 min. The hydrolysate was centrifuged (10,000 rpm, 10 min, 4 °C) and the supernatant was collected. The supernatant was freeze dried and used for FTIR analysis. Spectroscopic measurements were performed using approximately 25 mg freeze dried hydrolysate. The hydrolysate was mixed with 225 mg dried KBr (10% w/w). The FTIR spectra were recorded between 4000 and 500 cm−1 (Fig. 7a, Fig. 7b) on FTIR spectrometer (Schimadzu, model-IR Crestige 21). The spectra of hydrolysate and standard tyrosine are similar indicating that tyrosine is one of the major aminoacid released by the action of protease from casein.
Fig. 7a.
FTIR Spectra of standard Tyrosine.
Fig. 7b.
FTIR Spectra of Casein hydrolysate.
3.4. Kinetic studies of APBCS8
Effect of casein concentration on protease activity was determined by incubating the APBCS8 with 0.25% to 2.0% of casein at temperature 70 °C and pH 10.0. It was observed that the protease activity was increased with increase in substrate concentration and maximum protease activity was observed at 1.0% casein, beyond which there was slight inhibition in activity was recorded (Fig. 8a).
Fig. 8a.
Hyperbolic curve (substrate-velocity curve) of enzyme catalyzed reaction.
The hyperbolic curve did not give an accurate value of Vmax and hence KM. Therefore, a linear Lineweaver-Burk plot for protease activity was plotted (Fig. 8b) and the KM and Vmax values were determined to be 0.25 mg/ml and 310 U/ml respectively.
Fig. 8b.
Lineweaver-Burk plot for alkaline protease that shows Michaelis-Menten Kinetics.
3.5. Characterization of protease by MALDI-TOF MS
The purified protein was further characterized by MALDI/MS analysis. For the MALDI-TOF analysis of purified protease, the enzyme was subjected to tryptic digestion which showed major peaks corresponding to the molecular weight of 23.3 kDa (Fig. 9). The total number of amino acids 208 and the isoelectric point was found to be 9.52.
FTNSHYKKSNKQTNQTNQVKQENKRNHAFAKLEKEYNAKTVAYHADDRAVGALLRQNSIEALDERITYTRKAFASTSKDTGMTLKVRYSDSTAHNLIKSAFEKILREMGDTVTNSEREVNPGETHDTSTPKSFTLGTVLPSEKRNTTGDKLIRAGVPKSYGTRNDIAIIWPPNKPIVLSILSNHDKDDTLIADATKIVLETLKVTNK
Fig. 9.
MALDI spectra of resulted peptide fragments.
Compositional analysis of protease by protein information resource showed that it is rich in threonine, lysine, alanine, leucine, asparagine and serine. Moderate amounts of aspartic acid, glutamic acid, isoleucine, arginine, glycine, histidine and tyrosine were observed. Smaller amounts of the rest of the amino acids were observed. In its composition, the number of basic amino acids prevailed over the acidic amino acids (Fig. 10).
Fig. 10.
Composition of purified protease.
4. Discussion
Purification of protease is very important for developing a better understanding of the functioning of the enzyme. It also required for property studies and better understanding of structure function relationship. Protease can be purified by a combination of chromatographic procedures like affinity chromatography (AC), Ion exchange chromatography (IEC), hydrophobic interaction chromatography (HIC) and gel filtration chromatography. Alkaline protease from the Bacillus cereus strain S8 was purified to 17.04-fold of purification by employing ion exchange and gel filtration chromatographic techniques. Thirumala and Vishnuvardhan Reddy [15] reported the use of cellulose column chromatography for the purification of protein with increase in the purity of the protein by 20.74-fold from Bacillus caseinilyticus. Proteases with unique properties like thermostability, pH stability, stability towards solvents, oxidizing agents and surfactants are significant features in current pharmaceutical and industrial sector. As Serine Alkaline protease of trypsin type from the Bacillus cereus strain S8 fulfills this criterion, it is considered as important enzyme which meets current industrial sector. Annamalai et al. [17] reported that purified protease of B. firmus CAS 7 was active over wide range of pH between 5.0 and 12.0 and optimum being at pH 9.0. Shaghayegh et al. [7] reported an enzyme from Bacillus subtilis DR8806, with highest activity at 45 °C. Swati and Satyanarayana [12] reported positive effects with Co2+ and Ca2+ on protease rBLAP from Bacillus lehesis while Hg2+ completely inhibited rBLAP. Amina et al. [1] reported serine protease from Bacillus circulans strain DZ100. Nadia et al. [20] reported alkaline protease SAPB of Bacillus pumilus with organic solvent stability activities. Protease from Bacillus subtilis GACAS8 were reported by [18] with stability to oxidizing agents and surfactants.
Kinetic studies of alkaline protease from the Bacillus cereus strain S8 revealed that lower KM value of protease reflects a stronger binding affinity of this protease to substrate. Most of the previous studies have revealed that highest activity of alkaline proteases showed with casein as compared to other substrates. The Kinetic parameters (KM and Vmax) were determined for thermostable protease from Bacillus halodurans JB 99 by [5] at 70 °C and pH 11.0 for concentrations ranging between 0.5 and 7.5 mg/ml of casein. The KM and Vmax of purified protease was found to be 3.3 mg/ml and 15 U/mg protein respectively. The results for alkaline protease from Bacillus licheniformis Bl8 reported by [9] showed that the enzyme followed a typical Michaelis-Menten kinetics from a double reciprocal plot and the apparent KM of the enzyme for casein was found to be 3.2 mg/ml. Iqbal et al. [14] reported mutant purified protease with KM and Vmax values were 0.03064 µM and 69.76 U/mL using casein as substrate.
The result of the present investigation is fruitful and it reveals that there is a pronounced increase in specific activity of protease was obtained after the ammonium sulfate precipitation, ion exchange and gel filtration chromatography. The purified protease was further characterized by electrophoretic and matrix assisted laser desorption ionization/mass spectroscopy (MALDI/MS) analysis and found that molecular weight of the protease was 23.3 kDa.
Characterization studies of the purified protease from B. Cereus strain S8 reveals that important features exhibited by this protease like, high activity and stability at high pH and temperature, as well as stability in the presence of surfactants, oxidizing agents, organic solvents and metal ions are the attractive features from application point of view. There are no reports available until now with all these prominent features exhibited in a single enzymes and it proves the novelty of the work. There is a great industrial demand for the organic solvent tolerant proteases for application in the synthesis of useful products in the presence of organic solvents and thermostability might be an advantage for using this protease in industrial application such as laundry detergent formulations. Stability at its high pH is desirable for its applications in detergents and tanning processes. Inhibition studies with different inhibitors concluded that protease belongs to serine protease of trypsin type. Low KM particularly exhibited by this protease (incomparison with other proteases from literature) indicates that it is advantageous as it has greater affinity with the substrate and provides a scope for its use in different industrial sectors.
Acknowledgments
Acknowledgement
The authors are very thankful to the Department of Microbiology and Biochemistry, Andhra University for providing laboratory facilities to carry out this work.
Conflict of interest
The authors would like to declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
B.K.M Lakshmi, Email: kamalab42@gmail.com.
D. Muni Kumar, Email: dmunikumar.sch@cst.auvsp.edu.in.
K.P.J Hemalatha, Email: hemalathakpj@gmail.com.
References
- 1.Amina B., Zarai J.N., Abdelmalek B., Feriel R., Boulkour T.S., Hatem R. Biochemical and molecular characterization of a thermo and detergent-stable alkaline serine keratinolytic protease from Bacillus circulans strain DZ100 for detergent formulations and feather-biodegradation process. Int Biodet Biodeg. 2013;83:129–138. [Google Scholar]
- 2.Atkinson B, Mavituna. Biochemical engineering and biotechnology handbook, 2nd ed. London: Macmillan; 1991.
- 3.Lakshmi B.K.M., Hemalatha K.P.J. Production of alkaline protease from Bacillus licheniformis through statistical optimization of growth media by response surface methodology. Ferm Technol. 2016;5:2. [Google Scholar]
- 4.Blum H., Beier Hidburg, Gross Hans J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 5.Shrinivas D., Naik G.R. Characterization of alkaline thermostable keratinolytic protease from thermo alkalophilic Bacillus halodurans JB 99 exhibiting dehairing activity. Int Biodet Biodeg. 2011;65:29–35. [Google Scholar]
- 6.Sigma D.S., Mooser G. Chemical studies of enzyme active sites. Annu Rev Biochem. 1975;44:889–931. doi: 10.1146/annurev.bi.44.070175.004325. [DOI] [PubMed] [Google Scholar]
- 7.Shaghayegh F., Ahmad A., Milad L. Purification, biochemical characterization and structural modeling of a potential htrA-like serine protease from Bacillus subtilis DR8806. J Mol Cat B: Enzym. 2015;115:51–58. [Google Scholar]
- 8.Fairbanks G., Steck T.L., Wallach D.F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 9.Lakshmi G., Prasad N.N. Purification and characterization of alkaline protease from a mutant Bacillus licheniformis Bl8. Adv Biol Res. 2015;9(1):15–23. [Google Scholar]
- 10.Green, Hughes Methods Enzymol. 1955;1:67–90. [Google Scholar]
- 11.Walsh G. John Wiley & Sons; England: 2004. Protein purification and characterization. In proteins biochemistry and biotechnology; pp. 89–176. [Google Scholar]
- 12.Swati J., Satyanarayana T. Characteristics and applications of a recombinant alkaline serine protease from a novel bacterium Bacillus lehensis. Biores Tech. 2013;131:76–85. doi: 10.1016/j.biortech.2012.12.124. [DOI] [PubMed] [Google Scholar]
- 13.Saeki K., Ozaki K., Kobayashi T., Ito S. Detergent alkaline proteases: enzymatic properties, genes and crystal structures. J Biosci Bioeng. 2007;6:501–508. doi: 10.1263/jbb.103.501. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal Mohsin, Asgher Muhammad, Bashir Fareeha. Purification and kinetic characterization of alkaline protease for UV-90 mutant of bacillus subtilis. J Biochem Anal Stud. 2018;3(1) [Google Scholar]
- 15.Thirumala M., Vishnuvardhan Reddy S. Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacillus caseinilyticus 3. Biotechnology. 2016;6:53. doi: 10.1007/s13205-016-0377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M.G., Rollo E.E., Grodberg J., Oliver D.B. Nucleotide sequence of the secA gene and secA (Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988;170:3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annamalai N., Rajeswari M.V., Sunil Kumar S., Balasubramanian T. Purification and characterization of solvent stable, alkaline protease from Bacillus firmus CAS 7 by microbial conversion of marine wastes and molecular mechanism underlying solvent stability. Process Biochem. 2014;49:1012–1019. [Google Scholar]
- 18.Ramamoorthy S., Gnanakkan A., Jeganathan A. Production, purification and characterization of alkaline protease by ascidian associated Bacillus subtilis GACAS8 using agricultural wastes. Biocat Agric Biotech. 2015;4:214–220. [Google Scholar]
- 19.Laemmli U.K. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Nadia Z.J., Bassem J., Nushin A., Samir B. The over expression of the SAPB of Bacillus pumilus CBS and mutated sapB-L31I/T33S/N99Y alkaline proteases in Bacillus subtilis DB430: new attractive properties for the mutant enzyme. Bioresour Technol. 2012;105:142–151. doi: 10.1016/j.biortech.2011.11.115. [DOI] [PubMed] [Google Scholar]