Abstract
Anti-inflammatory phytocompounds from Crateva adansonii DC leaf extracts were identified by GCMS analysis and its anti-inflammatory potential was evaluated by in silico molecular docking study against inflammatory molecular targets. Three different (Aqueous, Methanol and Petroleum ether) dried leaf extracts of Crateva adansonii were obtained from soxhlet extraction method. Preliminary phytoconstituents analysis of three different leaf extracts of C. adansonii confirmed the presence of various major classes of bioactive phytoconstituents such as polyphenols (tannins and flavonoids), steroids, alkaloid, coumarin, carbohydrate and terpenoids. Among three leaf extracts, methanolic leaf extract possess highest total phenolic content of 77 ± 1.65 µg gallic acid equivalent (GAE)/g of dry weight of leaf extract, subsequently methanolic leaf extract also shows maximal in vitro antioxidant activity (DPPH scavenging activity) of 71.22 ± 1.32% among three different leaf extracts. GC–MS analysis of petroleum ether leaf extract revealed the presence of nine phytocompounds representing 95.43% peak area percentage, among nine identified phytocompounds three phytocompounds of C. adansonii possess anti-inflammatory property namely phytol, 1-Hexyl-2-Nitrohexane and 2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate were chosen for in silico molecular docking study against four inflammatory receptor targets (COX-2, TNFα, IL-1β and IL-6) and they shows less binding energy with highest docking score ranging from −15.9500 to 5.0869. The present study substantially indicated and proven that anti-inflammatory potential of phytocompounds from C. adansonii leaf extracts which can be exploited for commercial designing of novel anti-inflammatory drug to treat various inflammatory disorders.
Keywords: Anti-inflammatory phytocompounds, Cyclooxygenase-2, Crateva adansonii, Inteeleukin-1β, Tumor necrosis factor α, Phytol
1. Introduction
Inflammation is a vital part of the human immune system. Inflammation is the body’s immediate primary physiologic defense mechanism that helps body to protect itself against infection, burn, toxic chemicals, allergens and other noxious stimuli [1]. The inflammatory reactions are protective and tightly regulated in the immune system. During the inflammation process, various inflammatory mediators including pro and anti-inflammatory mediators are synthesized and secreted from inflammatory cells and generate many cellular effects [2].
Inflammation is a major condition associated with various acute and chronic diseases. Drugs which are in use presently for the management of pain and inflammatory conditions are either steroidal or NSAIDs. All of these drugs present well known side effects and toxicity in long term uses. It is well documented that these nonsteroidal anti-inflammatory drugs (NSAIDs) produce intestinal tract ulcers with internal bleeding and erosions of the stomach lining for long-term users [3]. Even the new COX-2 selective inhibitor drugs only been reported to reduce intestinal tract damage by 50% and their toxicity to the liver and kidney is still under review [4]. Looking at the present scenario, medicinal compounds derived from plant sources could provide an excellent lead compounds to develop new anti-inflammatory agents, which could be served as a more efficacious, affordable, good therapeutic index and safer for patients.
Medicinal plants are known to play vital roles as sources of active anti-inflammatory agents, which can be used to treat various inflammatory disorders by targeting inflammatory receptors. It is obvious that numerous plants have been used in traditional medicine to treat diverse inflammatory disorders and have been thought to possess wound curing activities [5]. Natural compounds have been used extensively in the treatment of many inflammatory diseases conditions. Natural compounds from plant origin currently used in medicine exhibit a very wide chemical diversity and together with their analogues and several other natural products, they demonstrate the importance of compounds from natural sources in modern drug discovery efforts. Phytocompounds and their molecular mechanisms are highly important in the development of novel, clinically useful anti-inflammatory agents [6]. Interest in natural compounds has grown in recent years because of concerns about drug costs and safety in numerous inflammatory disease conditions.
Crateva adansonii DC belonging to the family Capparidaceae. Different parts of the plant are extensively used in folkloric medicine for the cure of many disease conditions. The leaf extracts of C. adansonii are applied externally to treat various inflammatory conditions associated with pain and it also used for treating ear infections. Powder of bark is used in rheumatism, itch, epilepsy, asthma, gastrointestinal and uterine infection [7], [8]. The aim of the present study is to identify bioactive anti-inflammatory phytocompounds of Crateva adansonii leaf extracts through phytoconstituents screening and in silico molecular docking approaches.
2. Materials and methods
2.1. Collection and processing of plant material
Crateva adansonii leaf was collected from Salem District, Tamil Nadu, India and authenticated by Botanical Survey of India (BSI), Coimbatore, Tami Nadu. The plant leaf was cleansed, shade dried and grounded into fine powder.
2.2. Preparation of plant extract
50 g of fine leaf powder of Crateva adansonii was packed with Whatman No 1 filter paper and placed in soxhlet apparatus along with 300 ml of methanol. Then the sample was boiled for ten soxhlet cycles to obtain methanolic leaf extract of C. adansonii and then evaporated under reduced pressure and dried using a rotary evaporator at 55 °C. The above mentioned procedure was repeated with aqueous and petroleum ether to obtain aqueous and petroleum ether leaf extracts of C.adansonii. Then the dried leaf extracts were labeled and stored in sterile screw capped bottles at 5 °C in the refrigerator for further use.
2.3. Preliminary phytoconstituents analysis
The freshly prepared three different leaf extracts of C. adansonii were subjected to qualitative chemical tests to identify various major classes of bioactive phytoconstituents present in the leaf extract as per the standard method of Trease and Evans [9].
2.4. Determination of total phenolic content
Total phenolic contents of the plant extract were estimated using Folin–Ciocalteu phenol reagent according to the method described by Kim et al. [10] with minor modifications. Briefly, 1 ml of standard solutions of gallic acid at different concentrations or diluted C. adansonii extracts was added to a 25 ml standard flask containing 9 ml of sterile distilled water. Later, 1 ml of Folin Ciocalteu phenol reagent was added to the flask, thoroughly mixed and incubated for another 5 minutes at room temperature. 10 ml of sodium carbonate solution (10% w/v) was added into the above mixture with constant stirring and immediately made up to 25 ml with sterile distilled water. The reaction mixture was incubated at 23 °C for 1 hour and the absorbance was measured at 750 nm. Total phenolic contents in Crateva adansonii plants were expressed as µg gallic acid equivalents (GAE) per gram dry weight of leaf extract [11], [12]. Sterile distilled water was used as the negative control for this experiment. All the determinations were carried out in triplicates.
2.5. In vitro antioxidant activity by DPPH radical scavenging assay
The scavenging activity for DPPH free radicals was measured according to the method described by Blios [13]. In brief, leaf extract of C. adansonii at various concentrations (10–160 µg/ml) was mixed with 5 ml of 0.1 mM methanolic DPPH solution and incubated for 20 minutes at 27 °C. After incubation, the absorbance of the reaction mixture was measured at 517 nm using UV-visible double beam spectrophotometer. A set of different concentrations (10–160 µg/ml) ascorbic acid reference standard were also taken and treated in the similar manner as sample. Sterile distilled water was used as the negative control for this experiment. Each assay was carried out in triplicate. The concentration of sample required to scavenge 50% of DPPH free radical (IC50) was determined from the curve of percent inhibitions plotted against the respective concentration.
2.6. Identification of phytocompounds by GCMS analysis
GC–MS analysis of petroleum ether extract was carried out using Perkin Elmer Clarus 680 gas chromatography mass spectrometer provided with fused silica column, packed with Elite-5MS (5% biphenyl 95% dimethyl polysiloxane, 30 m × 0.25 mm ID × 250 μm df) and the components were separated using Helium as carrier gas at a constant flow of 1 ml/min. The injector temperature was set at 260 °C during the chromatographic run. The 1μL of extract sample injected into the instrument the oven temperature was as follows: 60 °C (2 min); followed by 300 °C at 10 °C min−1; and 300 °C, where it was held for 6 min. The mass detector conditions were: transfer line temperature 240 °C; ion source temperature 240 °C; and ionization mode electron impact at 70 eV, a scan time 0.2 sec and scan interval of 0.1 sec and the fragments from 40 to 600 Da. The major peaks were analyzed by comparing its mass fragments pattern with standard spectra available in Perkin Elmer GCMS NIST library.
2.7. Molecular docking study
2.7.1. Receptor and its binding site
The three dimensional structures of four inflammatory receptor targets such as IL1β (PDBID: 5I1B), IL6 (PDBID: 1ALU), TNFα (PDBID: 2AZ5) and COX-2(PDBID: 4COX) retrieved from PDB database [14]. To determine the binding affinities between the ligand and receptor, the amino acids with the binding pockets was predicted at Q-site finder server [15].
2.7.2. Ligand generation
Among all identified phytocompounds of C. adansonii leaf extracts three anti-inflammatory phytocompounds such as Phytol, 1-Hexyl-2-Nitro cyclohexane and 2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate) were chosen for in silico molecular docking analysis with inflammatory molecular targets and their 2D structures were drawn in ACD-Chemsketch [16] and its SMILES notation was obtained. The SMILES notation was submitted to “Online SMILES convertor and Structure file generator‟ [17] and converted into 3D SDF format.
2.7.3. Flexible docking
The developed SDF structures were docked with the predicted binding site of all eight receptor binding site by using FlexX [18] with following parameters (i) default general docking information’s, (ii) base placement using triangle matching, (iii) scoring of full score contribution and threshold of 0.30 and No score contribution and threshold of 0.70. (iv) Chemical parameters of clash handling values for protein ligand clashes with maximum allowed overlap volume of 2.9 A03 and intra-ligand clashes with clash factor of 0.6 and considering the hydrogen in internal clash tests. (v) Default docking details values of 200 for both the maximum number of solutions per iteration and maximum number of solutions per fragmentation.
2.7.4. Prediction of ligand- receptor interactions
The interactions of two compounds with eight receptors in the docked complex were analyzed by the pose-view of LeadIT [19].
3. Results and discussion
3.1. Preliminary phytochemical analysis
Preliminary phytochemicals analysis was carried out on three different leaf extracts of Crateva adansonii and their results were presented in Table 1. The major secondary metabolites were present in all three extracts such as alkaloid, flavanoid, tannin, phlobatannin, terpenoid, coumarin, steroid and carbohydrate. On the other hand saponin is absent in aqueous and methaolic extract however it is present in petroleum ether extract likewise anthraquinone is absent in methanolic extract, cardiac giycosides and protein were absent in petroleum ether extract.
Table 1.
Preliminary phytochemical screening of the plant Crateva adansonii different leaves extracts.
| Plant Extract | Test | AE | ME | PE |
|---|---|---|---|---|
| Alkaloid | Mayer’s and Wagner’s test | ++ | + | + |
| Flavonoid | Ammonia test, Alkaline reagent test | + | + | + |
| Tannin | Ferric chloride test, Lead acetate test | ++ | + | + |
| Phlobatannin | Hydrochloric acid test | + | + | + |
| Saponin | Frothing test | − | − | + |
| Terpenoid | Salkowski test | + | + | + |
| Anthraquinone | Ammonia test | + | − | + |
| Cardiac Glycoside | Keller-Killiani test | ++ | + | − |
| Coumarin | Sodium Hydroxide test | + | + | ++ |
| Steroid | Liebermann’s test, Salkowski’s test | + | ++ | + |
| Carbohydrate | Molisch’s test | + | + | + |
| Protein | Ninhydrin test | ++ | + | − |
Legend: AE-Aqueous leaf Extract; ME- Methanolic leaf Extract; PE- Petroleum ether leaf Extract; − Negative; + Positive.
Plants contain numerous phytochemical constituents, many of which are known to be biologically active compounds and are responsible for exhibiting diverse pharmacological activities [20]. To explore the importance of any medicinal plant the initial step is to screen for its phytochemicals, as it gives a broad idea regarding the nature of compounds present in it. The results of preliminary phytochemical testing confirmed the presence of various classes of bioactive chemical constituents in three different extracts of Crateva adansonii leaves including polyphenols (tannins and flavonoids), steroids, alkaloid, coumarin, carbohydrate and terpenoids.
3.2. Determination of Total phenolic content
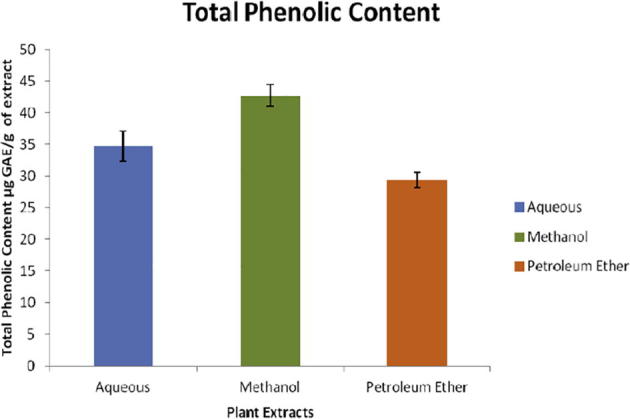
Total phenolic content of three different leaf extracts of Crateva adansonii was determined according to Folin-Ciocalteu method and the results were expressed as μg of gallic acid equivalent (GAE) per gram dry weight of leaf extract (Table 2 & Fig. 1). Total phenolic content was found to be affected by the solvents used for extraction. The total phenolic content in the three different extracts ranging from 29.38 to 42.77 μg of GAE/g. Methanol was found to be most effective significant solvent for extraction of phenolic compound than the other solvent used in the study. The total phenolic content of methanolic leaf extracts was found to be 42.77 μg of GAE/g than the aqueous and petroleum ether extracts which consisting of total phenolic content of 34.77 μg of GAE/g and 29.38 μg of GAE/g respectively. It has been reported that total phenolic content and antioxidant activity have significant and positive correlation [21]. In plants, phenolic antioxidants are produced primarily by secondary metabolism and their antioxidant properties mainly depends on redox properties and chemical structure i.e. number and position of hydroxyl group which play important role in scavenging free radical, chelating transitional metals and inhibiting lipoxygenase, a key enzyme involved in inflammatory immune responses [22].
Table 2.
Total phenolic content of the plant Crateva adansonii different leaves extracts.
| Plant extracts | Total phenolic content µg GAE/g of extract |
|---|---|
| Aqueous Extract | 34.77 |
| Methanol Extract | 42.77 |
| Petroleum Ether Extract | 29.38 |
Data are presented as the mean ± SD values of triplicate determinations.
Fig. 1.
Total phenolic content of the Crateva adansonii leaf extracts.
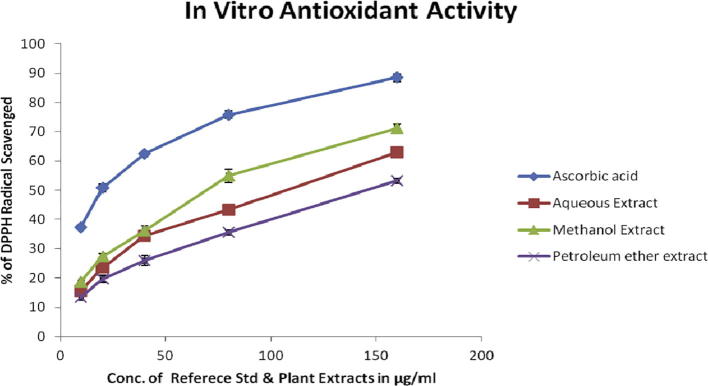
3.3. In vitro antioxidant activity by DPPH assay
Invitro Antioxidant activity of three different leaf extracts of Crateva adansonii as well as reference standards was investigated by DPPH radical scavenging activity assay. The scavenging effect of leaves extracts on the DPPH free radicals were expressed as% inhibition and they were compared with standard antioxidant ascorbic acid. All the three extracts showed a dose dependent scavenging activity of DPPH comparable to standard antioxidant. The IC50 value (in μg/ml) of the extracts was found in the order of methanol > aqueous > petroleum ether extract (Table 3 & Fig. 2). Maximum DPPH percentage of inhibition activity was measured at 160 μg/ml in all the extracts found to be aqueous (62.92 ± 1.25), methanol (71.22 ± 1.32), and petroleum ether (53.20 ± 0.87). The IC50 value for reference standards ascorbic acid 19.75 μg/ml as well as plant extracts aqueous, methanol and petroleum ether extracts was found to be 102.50, 72.50 & 144.25 μg/ml respectively. From the results it is known that Crateva adansonii leaf extracts possess scavenging free radicals. Furthermore, it was noticed that the leaf extract has least pronounced scavenging activity than that of the standard ascorbic acid. Similar previous antioxidant activity work was conducted on Crateva nurvala using DPPH radical scavenging assay and reported that leaf extracts has least scavenging activity than the standard [23].
Table 3.
DPPH radical scavenging activity assay of reference standards Ascorbic acid and different leaves extract of Crateva adasonii.
| Reference Standard & Plant Extracts | DPPH (% Inhibition) | IC50 | ||||
|---|---|---|---|---|---|---|
| Concentration (μg/ml) | ||||||
| 10 | 20 | 40 | 80 | 160 | ||
| Ascorbic acid | 37.42 ± 0.74c | 50.74 ± 1.30d | 62.37 ± 0.90e | 75.84 ± 1.39e | 88.44 ± 1.23e | 19.75 |
| Aqueous Extract | 15.64 ± 1.38a | 23.51 ± 0.60b | 34.52 ± 1.87c | 43.38 ± 0.95c | 62.92 ± 1.25e | 102.50 |
| Methanol Extract | 18.69 ± 0.49a | 27.39 ± 0.96b | 36.12 ± 1.78c | 54.88 ± 2.26d | 71.22 ± 1.32e | 72.50 |
| Petroleum ether Extract | 13.32 ± 0.98a | 19.59 ± 1.23b | 25.91 ± 1.73b | 35.53 ± 0.82c | 53.20 ± 0.87d | 144.25 |
Data are presented as the mean ± SD values of triplicate determinations. a–d Different superscript letters for a given value within a column are significantly different from each other (Tukeyis-HSD multiple range post hoc test, p < 0.05).
Fig. 2.
Invitro Antioxidant activity by DPPH radical scavenging activity assay of reference standard ascorbic acid and Crateva adansonii leaves extracts.
The antioxidant activity of phenolic compounds is mainly due to their reduced properties which allow them to act as metal chelators, absorb and neutralize free radicals [24]. During the DPPH free radical reaction, the degree of discoloration (decrease in absorbance) of the DPPH solution indicates the scavenging potentials of the sample antioxidant. The crude extracts of Crateva adansonii contain plant secondary metabolites such as alkaloids, tannins, saponins, glycosides, etc. All these bioactive compounds have the ability to discolor DPPH solution by their hydrogen donating ability [25].
3.4. Identification of phytocompounds by GCMS analysis
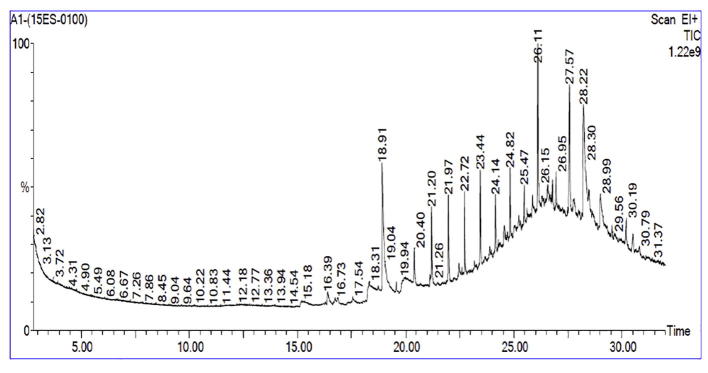
The phytocompounds of petroleum ether extract was analyzed by using GC–MS technique. The GC–MS analysis of petroleum ether extract had led to the identification and quantification of 9 different compounds representing 95.43% of the total extract (Table 4) and their GCMS chromatogram was shown in Fig. 3. These compounds mainly comprised of hydrocarbons, diterpene, alkane, alcohol and ketone. 2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate (25.29%) and Phytol (21.50%) was identified as a major chemical constituent followed by Nonacosane (11.26%), Tetratetracontane (11.12%), 1-Hexyl-2-Nitrohexane (10.18%), Tetratricontane (5.00%), Docosane 1,22- Dibromo (4.04%), 1-Hexyl-2-Nitro cyclohexane (3.35%), Heptacosane (3.33%). These compounds have previously been isolated from other medicinal plant species and were believed to play an important role in plant defense system [26].
Table 4.
Phytocomponents identified in the petroleum ether extract of the leaves of Crateva adansonii by GC -MS.
| S.NO | RT | Name of the Compound | Peak Area% | MW | MF |
|---|---|---|---|---|---|
| 1 | 18.915 | Phytol | 21.502 | 296 | C20H40O |
| 2 | 21.196 | Heptacosane | 3.332 | 380 | C27H56 |
| 3 | 23.442 | Tetratricontane | 5.008 | 478 | C34H70 |
| 4 | 26.113 | Tetratetracontane | 11.123 | 618 | C44H90 |
| 5 | 27.573 | Nonacosane | 11.262 | 408 | C29H60 |
| 6 | 27.793 | 1-Hexyl-2-Nitro cyclohexane | 3.354 | 213 | C12H23O2N |
| 7 | 28.219 | 2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate | 25.290 | 524 | C30H33O6Cl |
| 8 | 28.464 | Docosane 1,22- Dibromo | 4.404 | 466 | C22H44Br2 |
| 9 | 29.004 | 1-Hexyl-2-Nitrohexane | 10.186 | 213 | C12H23O2N |
Legend: RT- retention Time, MW- Molecular Weight, MF – Molecular Formula.
Fig. 3.
GCMS Chromatogram of Petroleum ether leaf extract of Crateva adansonii.
The identified bioactive phytocompounds of petroleum ether leaf extract possess many biological activities and its compound nature was presented in Table 5. The identified phytocompounds were reported by earlier studies found to have different biological activities such as Antioxidant, antimicrobial and anti-inflamattory and anti-diabetic activities etc., [27], [28], [29], [30], [31], [32], [33], [34], [35].
Table 5.
Biological activity and uses of phytoconstituents identified in Crateva adansonii petroleum ether leaf extract by GC–MS.
| S.NO | Name of the compound | Compound nature | Biological activity/Uses |
|---|---|---|---|
| 1 | Phytol | Diterpene alcohol | Precursor for manufacture of Vitamin E [27], antimicrobial, anticancer, anti-inflammatory, anti-diuretic, immuno stimulatory and anti-diabetic activity [28] |
| 2 | Heptacosane | Aliphatic hydrocarbon |
Antibacterial activity [29] |
| 3 | Tetratricontane | Alkane | Antifungal, antibacterial, antioxidant activity [30] |
| 4 | Tetratetracontane | Alkane | Promoted an effective action in bacterial reduction with the application of laser energy [31] |
| 5 | Nonacosane | Aliphatic hydrocarbon |
Antibacterial, Pheromone of female Anopheles stephensi mosquito [32] |
| 6 | 1-Hexyl-2-Nitro cyclohexane | Ketone | Neuroactive, anti-inflammatory, analgesic Property [33] |
| 7 | 2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate | Benzopyrone | Antioxidant, antimicrobial and anti-inflammatory activity [34] |
| 8 | Docosane 1,22- Dibromo | Alkane | Antibacterial activity [35] |
| 9 | 1-Hexyl-2-Nitrohexane | Ketone | No activity Reported |
Three phytocompounds with anti-inflammatory property such as phytol, 1-Hexyl-2-Nitro cyclohexane and 2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate were chosen to consider for in silico molecular docking study.
3.5. Molecular docking study
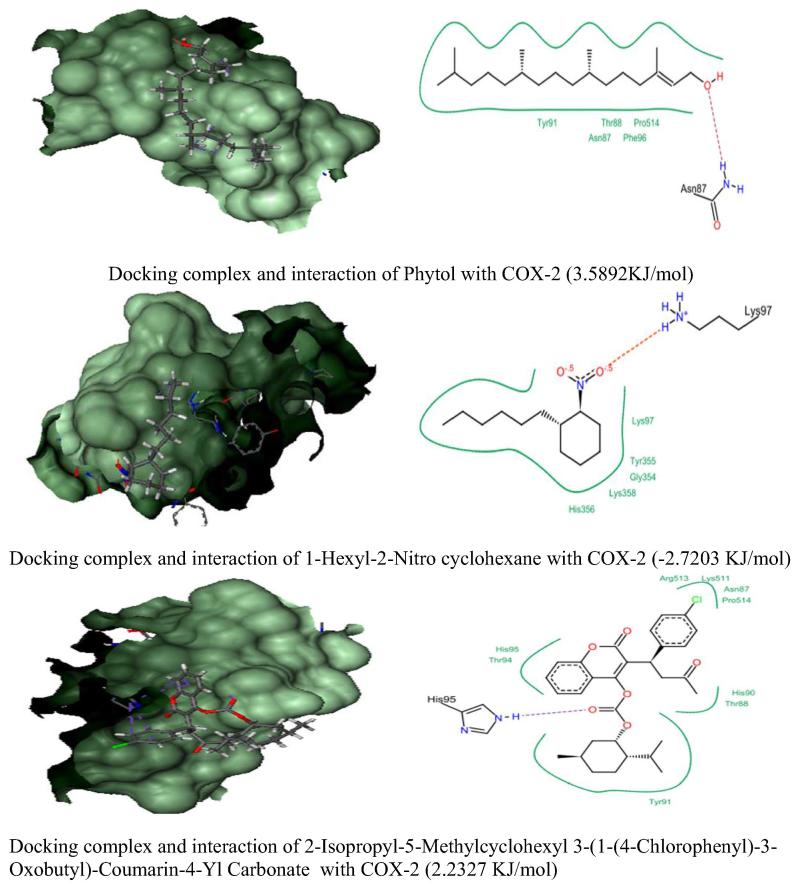
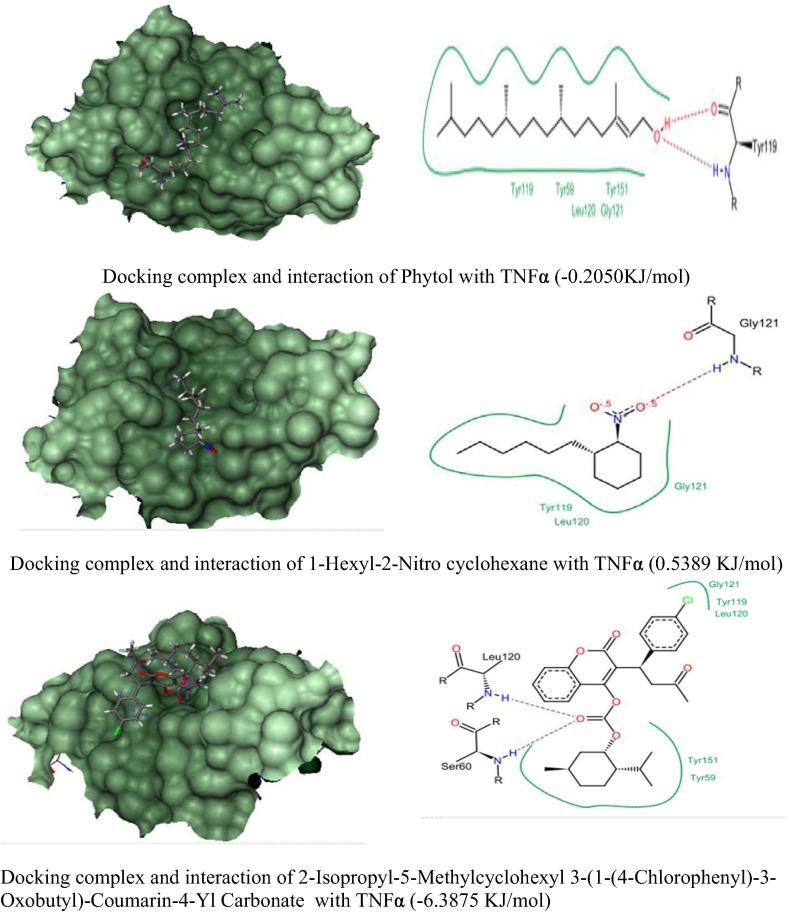
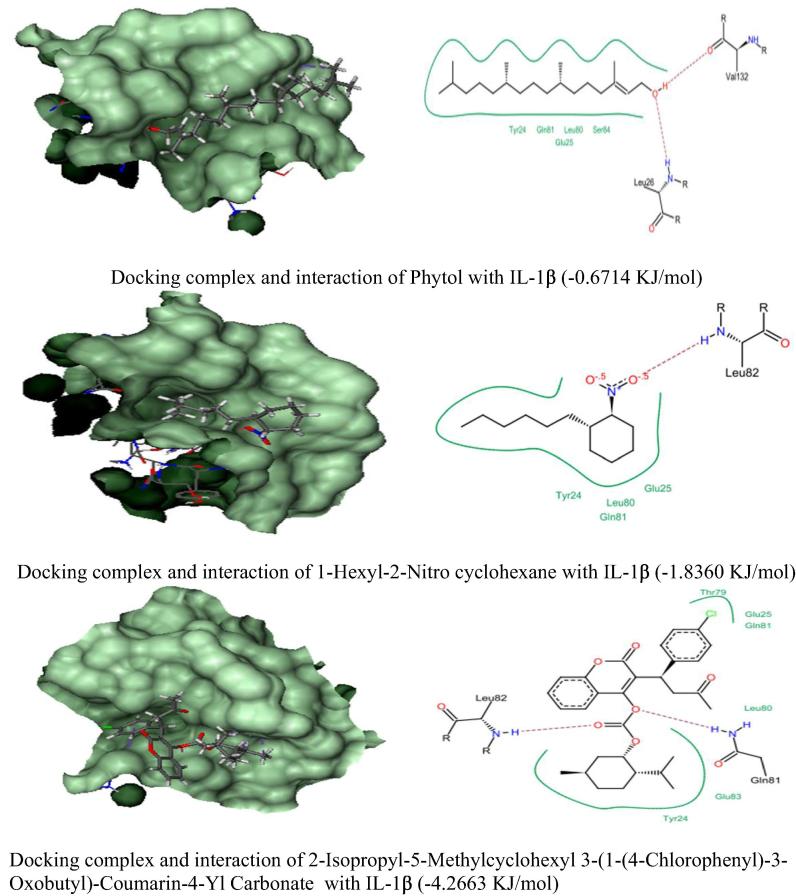
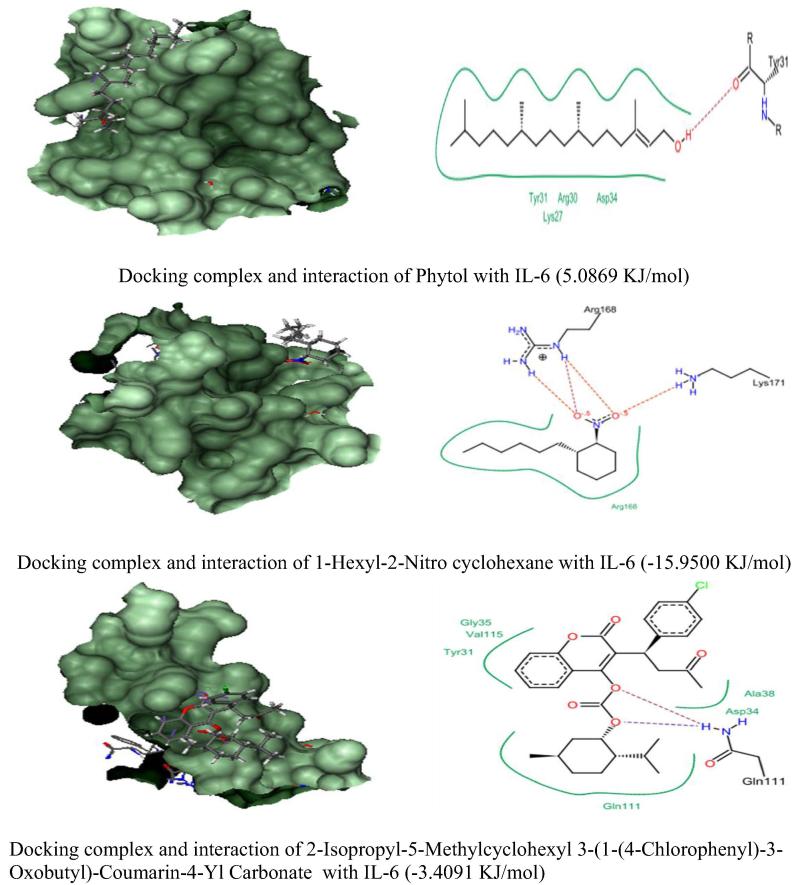
In silico molecular docking analysis of three anti-inflammatory phytocompounds from C. adansonii were screened against four inflammatory receptor targets and their results were presented in Fig. 4, Fig. 5, Fig. 6, Fig. 7 and Table 6. Among three phytocompounds coumarin derivative (2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate) exhibits highest docking score and lowest binding energy with TNF α (-6.3875 KJ/mol) and Il-1β (-4.2663 KJ/mol) whereas 1-Hexyl-2-Nitro cyclohexane exhibit highest docking score against COX-2 and IL-6 (-2.7203 KJ/mol & -15.9500 KJ/mol) respectively.
Fig. 4.
Docking Score and molecular interactions of anti-inflammatory phytocompounds of C. adansonii leaf extract against COX-2 inflammatory enzyme target.
Fig. 5.
Docking Score and molecular interactions of anti-inflammatory phytocompounds of C. adansonii leaf extract against TNFα inflammatory protein target.
Fig. 6.
Docking Score of anti-inflammatory phytocompounds of C. adansonii leaf extract against IL-1β inflammatory protein target.
Fig. 7.
Docking Score of anti-inflammatory phytocompounds of C. adansonii leaf extract against IL-6 inflammatory protein target.
Table 6.
Docking Score of Anti-inflammatory phytocompounds from petroleum ether leaf extract of C.adansonii with four inflammatory receptor targets.
| S.No | Phytocompounds | Binding Affinity KJ /mol |
|||
|---|---|---|---|---|---|
| COX-2 | TNF-α | IL-1β | IL-6 | ||
| 1. | Phytol | 3.5892 | −0.2050 | −0.6714 | 5.0869 |
| 2. | 1-Hexyl-2-Nitro cyclohexane | −2.7203 | 0.5389 | −1.8360 | −15.9500 |
| 3. | 2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate | 2.2327 | −6.3875 | −4.2663 | −3.4091 |
Molecular interaction results between ligand and four anti-inflammatory protein targets were presented in Table 7. Coumarin derivatives exhibits maximal molecular interaction with least docking score 5.0869 KJ/mol against COX-2 inflammatory target whereas reverse in the case with 1-Hexyl-2-Nitro cyclohexane against IL-6.Similar previous docking results was reported [36] in leaf extracts of C. adansonii phytocompounds have the ability to bind with different inflammatory molecular targets which will clearly proven the anti-inflammatory potential of phytocompounds from C. adansonii leaf extracts.
Table 7.
Molecular interactions of Anti-inflammatory phytocompounds from petroleum ether leaf extract of C.adansonii with four inflammatory receptor targets.
| S.No | Inflammatory Receptor Targets | Phytocompounds of C. adansonii |
||
|---|---|---|---|---|
| PHY | HEX | ISO | ||
| 1. | COX-2 | Asn87, Thr88, Tyr91, Phe96, Pro514 & #Asn87 | #Lys97*, Gly354, Tyr355, His356 & Lys358 | Thr88, Asn89, His90, Tyr91, Thr94, #His 95*, Lys511, Arg513 & Pro514 |
| 2. | TNF-α | Tyr 59, #Tyr 119*, Leu 120, Gly121 & Tyr 151 | Tyr119, Leu120 & #Gly121* | Tyr59, Ser60*, Tyr119, #Leu120*, Gly121 & Tyr151 |
| 3. | IL-1β | Tyr24, Glu25, Leu26*, Leu80, Gln81, Ser84 & Val132* | Tyr24, Glu25, Leu80, Gln81 & Leu82* | Tyr24, Glu25, Thr79, Leu80, #Gln81*, Leu82* & Glu83 |
| 4. | IL-6 | Lys27, Arg30, #Tyr31* & Asp34 | #Arg168* & Lys171* | Tyr31, Asp34, Gly35, Ala38, #Gln111* & Val115 |
Legend: *Hbond residues; #* Hbond & non bonded interaction; PHY- Phytol; HEX-1-Hexyl-2-Nitro cyclohexane; ISO -2-Isopropyl-5-Methylcyclohexyl 3-(1-(4-Chlorophenyl)-3-Oxobutyl)-Coumarin-4-Yl Carbonate.
4. Conclusion
The present study revealed that important bioactive phytocompounds of C. adansonii resolved by GC–MS analysis possess anti-inflammatory potential against inflammatory molecular targets. Thus this type of combinatorial analysis (In vitro Phytoconstituents analysis and In silico molecular docking study) helps to understand and reveal the bioactive principles of the medicinal plants, which will be useful for further fruitful logical research towards designing novel anti-inflammatory phytocompounds against multiple inflammatory molecular targets.
Footnotes
Peer review under responsibility of National Research Center, Egypt.
Contributor Information
Rathinavel Thirumalaisamy, Email: tmalaisamy@gmail.com.
Subramanian Ammashi, Email: asmanian68@gmail.com.
References
- 1.Huang M.T., Ghai G., Ho C.T. Comp Rev Food Sci Food Saf. 2004;3:127–139. doi: 10.1111/j.1541-4337.2004.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 2.Villarreal G., Zagorski J., Wahl S.M. Inflammation: Acute Ethno pharmaco. 2000;72:275. [Google Scholar]
- 3.Hayliyar J., Macpherson A., Bjarnason I. Drug Saf. 1991;7:86–105. doi: 10.2165/00002018-199207020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein F.E., Faich G., Goldstein J.L., Simon L.S., Pincus T., Whelton A. J Am Med Asso. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 5.Gacche R., Shaikh R., Pund M., Deshmukh R. J Pharmacog. 2011;3:57–64. [Google Scholar]
- 6.Strohl W.R. Drug Discov Today. 2000;5:9–41. doi: 10.1016/s1359-6446(99)01443-9. [DOI] [PubMed] [Google Scholar]
- 7.Sivarajan VV, Balachandran I. Oxford and IBH Publishing Company Pvt. Ltd., 1994, 234–456.
- 8.Gitte T.A., Kare M.A., Deshmukh A.M. Recent Res Sci Tech. 2012;4:08–10. [Google Scholar]
- 9.Trease GE, Evans WC. 14th ed. Bailliere Tindall Ltd, London, 2013, 832.
- 10.Kim D.O., Jeong S.W., Lee C.Y. Food Chem. 2003;81:321–326. [Google Scholar]
- 11.Bouayed J., Piri K., Rammal H., Dicko A., Desor F., Younos C., Souliman R. Food Chem. 2007;104:364–368. [Google Scholar]
- 12.Govarthanan Muthusamy, Rajinikanth Rathika, Kamala Kannan Seralathan, Selvankumar Thangasamy. J Gen Eng Biotech. 2015;13:25–29. doi: 10.1016/j.jgeb.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blios M.S. Nature. 1958;26:1199–1200. [Google Scholar]
- 14.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Shindyalov I.N., Bourne P.E. The protein data bank. Nucl Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurie A.T., Jackson R.M. Bioinformatics. 2005;21:1908–1916. doi: 10.1093/bioinformatics/bti315. [DOI] [PubMed] [Google Scholar]
- 16.ACD/ChemSketch Freeware, version 1. www.acdlabs.com, 2006.
- 17.Weininger D.J. Chem Inf Comput Sci. 1988;28:31–36. [Google Scholar]
- 18.Rarey M., Kramer B., Lengauer T., Klebe G. J Mol Biol. 1996;261:470–489. doi: 10.1006/jmbi.1996.0477. [DOI] [PubMed] [Google Scholar]
- 19.Stierand K., Maab P., Rarey M. Bioinformatics. 2006;22:1710–1716. doi: 10.1093/bioinformatics/btl150. [DOI] [PubMed] [Google Scholar]
- 20.Gu R., Wang Y., Long B., Kennelly E., Wu S., Liu B. Biol Pharm Bull. 2014;37:903–915. doi: 10.1248/bpb.b14-00084. [DOI] [PubMed] [Google Scholar]
- 21.Velioglu Y.S., Mazza G., Gao L., Oomah B.H. J Agric Food Chem. 1998;46:4113–4117. [Google Scholar]
- 22.Decker E.A. Nutr Rev. 1997;55:396–398. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 23.Tanwar Anshul, Bafna Anand, Bafna Pallavi. Inter J Phytother 2015;5: 37–43.
- 24.Mishra S.L., Sinhamahapatra P.K., Nayak A., Das R., Sannigrahi S. Indian J Pharm Sci. 2010;72(2):267–269. doi: 10.4103/0250-474X.65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waheed I., Ahmed M., Syed N.H., Ashraf R. Indian J. Pharm Sci. 2014;76:251–256. [PMC free article] [PubMed] [Google Scholar]
- 26.Kokate CK, Purohit AP, Gokhale SB. 42nd edition. Nirali Prakashan, 2008.
- 27.Yu F., Gapor A., Bender W. Cancer Detect Prev. 2005;29:383–388. doi: 10.1016/j.cdp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Sermakkani M., Thangapandian V. Asian J. Pharm. Clin Res. 2012;5:90–94. [Google Scholar]
- 29.Mihailovi V., Vukovi N., Niforovi N., Soluji S., Mladenovi M., Maškovi P. J Med Plant Res. 2011;5:1164–1174. [Google Scholar]
- 30.Uma B., Parvathavarthini R. Inter J Pharm Tech Res. 2010;2:1677–1680. [Google Scholar]
- 31.Ozdemir G., Horzum Z., Atakan S., Ulku K.Y. Turk J Chem. 2006;3:183–188. [Google Scholar]
- 32.Sun Y., Xing D., Shen L., Sun M., Fang M., Bi L., Sui Y., Zhang Z., Cao W. Appl Microbiol Biotechnol. 2013;97:5079–5087. doi: 10.1007/s00253-013-4903-0. [DOI] [PubMed] [Google Scholar]
- 33.Dinesh M.G. Indian J Res Pharm Biotech. 2014;2:1044–1057. [Google Scholar]
- 34.Swayam Sourav Sahoo Eur J Exp Biol. 2012;2:899–908. [Google Scholar]
- 35.Patel Rajesh M., Patel Natvar J. J Adv Pharm Edu Res. 2011;1:52–68. [Google Scholar]
- 36.Rathinavel Thirumalaisamy, Ammashi Subramanian, Shanmugam Gnanendra. Inter J Adv Interdis Res. 2017;4(1):6–14. [Google Scholar]