Abstract
Imidazo[1,2-a]pyridine-based compounds are clinically important to the treatments of heart and circulatory failures, while many are under development for pharmaceutical uses. In this study, a series of imidazo[1,2-a]pyridine-based derivatives 2(a–o) were synthesized by reacting a-haloketones with 2-aminopyridines in a basic media at ambient temperature. Single crystal X-ray diffraction studies suggest that with low degree-of-freedom, the introduction of bulky adamantyl or electron-rich biphenyl moiety into the imidazopyridine derivatives will not affect its structural occupancy. Imidazo[1,2-a]pyridine-based derivatives with biphenyl side chain are potential AChE inhibitors. Compound 2h which bears a biphenyl side chain and methyl substituent at the position R4 of the imidazo[1,2-a]pyridine ring showed the strongest AChE inhibition with an IC50 value of 79 µM. However, imidazo[1,2-a]pyridine derivatives with phenyl side chain exhibit better BChE inhibition effect among the series. Compound 2j with 3,4-dichlorophenyl side chain and unsubstituted imidazo[1,2-a]pyridine ring appears to be the strongest BChE inhibitor with an IC50 value of 65 µM and good selectivity. The inhibitory effects of active compounds were further confirmed by computational molecular docking studies. The results unveiled that peripheral anionic sites of AChE and acyl pocket of BChE were the predominated binding sites for the subjected inhibitors.
Introduction
Imidazo[1,2-a]pyridines are probably the most common nitrogen-bridgehead fused heterocycles used in pharmacology research, attributed to their activities spanning a diverse range of targets. These molecules exhibit antiviral1, antibacterial2, antiparasitic3, anti-inflammatory4 and antipyretic5 properties. Besides that, they also served as β-amyloid formation inhibitor, GABAA and benzodiazepine receptor agonists6 and cardiotonic agent7. Currently, four imidazo[1,2-a]pyridine-based derivatives are widely used in clinic, including hypnotic drug Zolpidem8, non-sedative anxiolytic drug Alpidem, antiulcer agent Zolmidine9 and phosphodiesterase III inhibitor Olprinone for the treatment of heart and circulatory failures10.
Aside from the progressive illness mentioned above, Alzheimer’s disease (AD) is a fatal brain degenerative disease that starts with dementia and loss of language, problem-solving and cognitive skills of a person. AD is lethal after a prolonged period of struggle and suffering11. Although the key nature of the pathophysiology of AD is still unclear, “cholinergic hypothesis” had been developed from the studies based on basal forebrain and rostral forebrain’s cholinergic pathway. Based on the cholinergic hypothesis, cholinesterase inhibitor is used to enhance cognitive function and slowdown AD progression by inhibiting the hydrolysis of cholinergic neurotransmitters12. There are two types of cholinesterases, which are acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) where these enzymes are responsible for the hydrolysis of the neurotransmitter acetylcholine into choline and acetic acid. As early as at the year of 1990s, a series of imidazo[1,2-a]pyridines derivatives (2-arylimidazo[1,2,-a]pyridinium salts) were subjected for the evaluation of electric eel acetylcholinesterase (AChE) inhibition. These compounds showed positive results with IC50 values ranging from 0.2 to 50.0 μM, meanwhile several of them showed protective effects against the organophosphorus AChE inhibitor soman in mice13. Besides, N-(benzylidene)imidazo[1,2-a]pyridine derivatives with halogen as substituent on the attached phenyl ring, showed potential AChE inhibition14. Recently, the cholinesterase inhibition effects of imidazole analogues designed to be multi-targeted, were confirmed through computational studies, pharmacological evaluation using animal model and mechanistic in-vitro cholinesterase inhibition study15.
In view of the potential cholinesterase inhibition activities associated with the imidazo[1,2-a]pyridine ring system, it led to our present study on the synthesis of fifteen new imidazo[1,2-a]pyridine-based derivatives, 2(a–o) and evaluation of their anti-cholinesterase activities. These new derivatives were characterized by spectroscopy methods and single crystal X-diffraction study. By using group comparison of single-crystal X-ray diffraction data, the structural conformation, structural occupancy and crystal packing similarity were studied. The AChE and BChE inhibitory activities of these derivatives were evaluated by Ellman’s colorimetric test16. In addition, we also investigated in-silico binding mode of the biologically active ligands into AChE and BChE enzymes in comparison with tacrine as reference and protocol validation. The molecular docking procedure was validated by using tacrine as the native ligand.
Results and Discussion
Spectroscopy analysis
Cyclization of 2(a-o) was confirmed by FTIR, as the N-H stretching bonds were in the range of 3500–3300 cm−1, which corresponded to the disappearance of primary amide. Furthermore, some peaks were observed at ~1370 and ~1200 cm−1, which corresponded to the C-N stretching of imidazole. The presence of unsaturated C-H (pyridine) stretching was observed near 3100 cm−1, and their v(C-C) and ω(C-H) were showed at ~1600 & 1450 cm−1 and ~750 cm−1, respectively. Imidazo[1,2-a]pyridines-based derivatives contain adamantyl, biphenyl or phenyl moiety as their side chains. The signature absorption bands of these moieties can be identified from their FTIR spectra. Adamantyl’s methylene and methine v(C-H) were observed as strong bands around 2900 and 2850 cm−1. Meanwhile, the v(C-H) of biphenyl and phenyl moieties were revealed at ~3000 cm−1. In the FTIR spectra of 2(a–o), the unexpected observations are the occurrences of O-H stretching in hydrated compounds 2b, 2c and 2e, as these compounds were co-crystallized with water molecules17,18.
In the 1H-NMR spectra of 2(a-o), the singlet corresponding to the methylene (-CH2-) group in 1 was replaced by methine (-CH-) protons at a higher chemical shift (δ ≈ 7.1–8.2 ppm) which associated with the other required aromatic peaks. For compounds 2(a-e), adamantyl moiety was presented as broad peaks in the lower region, centering around δ ≈ 2.1, 2.0 and 1.8 ppm with the integration values of 3:6:6. For compounds 2(f-i), the first phenyl ring of the biphenyl moiety was shown as doublet and two sets of triplet near δ ≈ 7.7 − 7.4 ppm with the integration values of 2:2:1. Two well-resolved sets of doublet were centered around δ ≈ 8.2 and 7.7 ppm with the integration values of 2:2, ascribing to the -CH- protons of second phenyl ring. Identical J-coupling values were used to distinguish different methine protons from imidazo[1,2-a]pyridine, biphenyl and phenyl ring systems. In addition, protons of -CH3 and -OCH3 groups were revealed in the up-field region near δ ≈ 2.5 and 3.9 ppm. The numbers of protons were in agreement with the proposed values based on the integration values.
The 13C NMR spectra of 2(a-o) showed four distinct sets of imidazole carbon, aromatic carbon, adamantyl carbon and saturated carbon signals. In the down-field region, both δ(C = N) and δ(C-N) imidazole carbon signals were centered around δ ≈ 146 and 145 ppm, respectively. The aromatic carbon signals of biphenyl and benzene groups were found in the range of δ ≈ 140 to 108 ppm. The adamantyl carbon signals were centered at δ ≈ 42, 37, 34 and 28 ppm, while the -CH3 and -OCH3 saturated carbon signals were observed in the up-field region, centering at δ ≈ 20 and 55 ppm, respectively18–20. All spectra are included in Supplementary Information.
X-ray crystal structure description
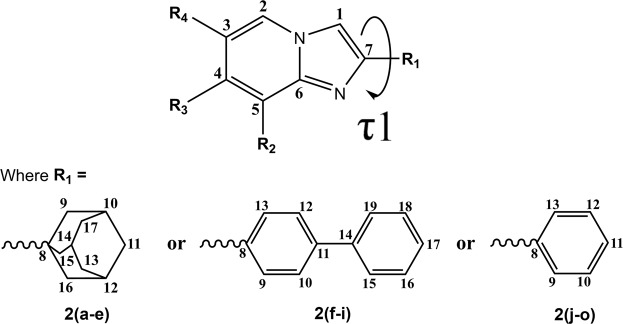
The asymmetric unit (Z’) of all studied compounds consist of a crystallographic independent molecule, except 2n and 2o, which consist of two molecules, while compound 2k consists of three molecules. Besides, the molecules of 2b and 2e lay on the mirror plane, therefore the asymmetric unit of 2b and 2e consist of half molecule. In addition, compounds 2b, 2c and 2e are crystallized with an extra water molecule as its solvate. Degree–of–freedom in the molecular conformations of the reported compounds can be characterized by the torsion angles between the imidazopyridine group with its attached adamantyl, biphenyl or phenyl groups C1–C7–C8–C9, τ1 (an extra torsion angle of C10–C11–C14–C15, τ2 for compounds containing biphenyl moiety, 2f-i).
For imidazo[1,2-a]pyridine derivatives with adamantyl side chain (2a–e), the torsion angles of C1–C7–C8–C9, τ1 are in the range of 0.0–7.1° (Fig. 1), while the imidazopyridine moiety is coplanar to the center of adamantyl (C9–C10–C13/14) with dihedral angle of 0.0–5.3°. Whereas, for compounds with biphenyl side chain (2f–i), the imidazopyridine and both phenyl rings are almost coplanar to each other with dihedral angles of 2.0–5.7° and 1.1–4.8°, respectively, as the torsion angle of τ1 and C10–C11–C14–C15, τ2 were nearly parallel with the range between 179.3–174.9° and 1.3–4.8°. As for imidazo[1,2-a]pyridine derivatives with phenyl side chain (2j-o), the phenyl ring had twisted away from the imidazopyridine ring, as the torsion angles of τ1 vary from 178.9° to 165.2°. Overall, the torsion angles in between imidazopyridine ring and its side chain (τ1) are always in periplanar conformation, although the dihedral angles have shown a discrepancy up to 25.7°. Selected torsional and dihedral angles for the reported compounds are tabulated in Table 1.
Figure 1.
General scheme of imidazo[1,2-a]pyridine derivatives with torsion angle τ1.
Table 1.
Selected torsional* and dihedral# angles (°) for compounds 2a–o.
| Compound | τ1 | τ2 | Dihedral 1 | Dihedral 2 |
|---|---|---|---|---|
| 2a | −3.4 | — | 3.6 | — |
| 2b | 0.0 | — | 0.0 | — |
| 2c | 7.1 | — | 5.3 | — |
| 2e | 0.0 | — | 0.0 | — |
| 2f | 179.3 | −4.7 | 3.5 | 4.00 |
| 2g | −174.9 | −4.8 | 5.7 | 2.7 |
| 2h | 178.4 | 2.6 | 2.0 | 1.09 |
| 2i | −176.1 | 1.3 | 3.6 | 4.83 |
| 2j | 176.2 | — | 5.4 | — |
| 2k | −175.1, 166.6, −165.2 | — | 5.7, 13.6, 11.9 | — |
| 2l | 167.6 | — | 12.44 | — |
| 2m | −169.0 | — | 10.0 | — |
| 2n | 175.4, 177.4 | — | 6.0, 25.7 | — |
| 2o | −175.6, 178.9 | — | 4.9, 3.9 | — |
*τ1 = Torsion angle of C1–C7–C8–C9; τ2 = Torsion angle of C10–C11–C14–C15.
#Dihedral 1 represents the dihedral angle between the mean plane of imidazopyridine and center of adamantyl (C9–C10–C13/14) or the first phenyl ring. Dihedral 2 represents the dihedral angle between the mean planes of the imidazole and the second phenyl ring.
Crystal packing similarity and structural occupancy
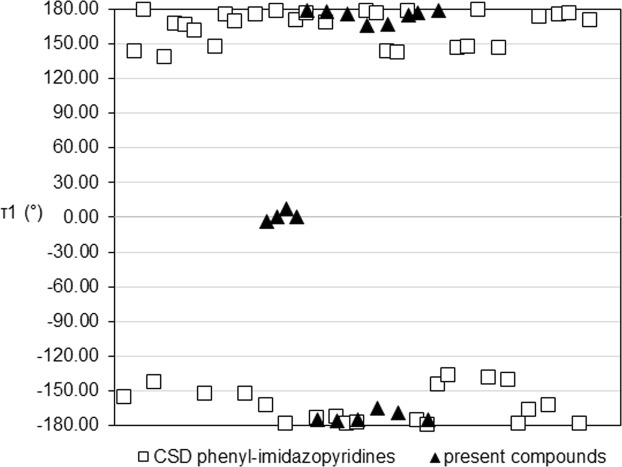
A Cambridge Structural Database (CSD, V5.38, last update Nov 201621) search using 2-phenylimidazo[1,2-a]pyridine as skeleton was performed to locate previously reported phenyl-imidazopyridine derivatives and 36 similar structures were found. In order to identify the effect of the replacement of phenyl ring with relatively bulky adamantyl or electron-rich biphenyl moiety on the molecular conformation and structure occupancy, fourteen present imidazo[1,2-a]pyridine derivatives were compared with 36 reported phenyl-imidazopyridine derivatives. In the aspect of molecular conformation, it is noteworthy to observe that no clinal conformation (τ1 between ± 30 °– ± 150°) is adopted by current studied compounds, unlike the variations displayed in phenyl-imidazopyrine derivatives (Fig. 2). All present compounds adopt periplanar conformation (τ1 between 0–30° or 150°–180°) with C1–C7–C8–C9 torsion angles falling in the range from 0–7.1° degree and 165.2–179.3°, which is comparable to those previously reported phenyl-imidazolepyridine derivatives that adopt the same conformation (152.9–179.8°).
Figure 2.
Graphical representation of the C1–C7–C8–C9 torsion angles, τ1, in present and previously reported compounds.
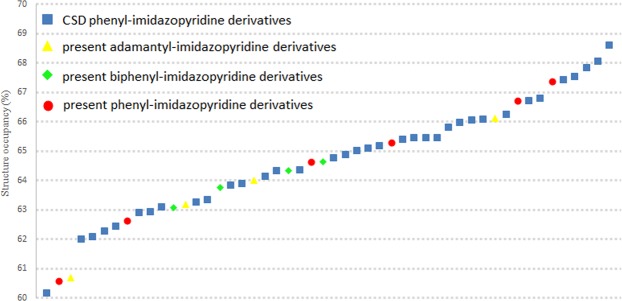
The comparison of crystal structure occupancy between the search results and the present compounds are listed in Table 2. In the previously reported phenacyl benzoate derivatives, the replacement of phenyl moiety with adamantyl moiety had reduced the occurrence of π···π interaction, hence reduced the crystal packing coefficient22. Whereas, the introduction of biphenyl moiety as a replacement for phenyl moiety in phenacyl benzoate derivative had encouraged the formation of weak intermolecular π···π and C-H···π interaction, thus increased their crystal packing coefficient23. In contrast to the previously reported phenacyl benzoate derivatives, replacement of phenyl moiety with the adamantyl or biphenyl moiety does not arise a direct impact on the crystal packing coefficient in the present imidazopyridine derivatives.
Table 2.
List of structural occupancy of the present and reported compounds.
| Compound | Packing coefficient (%) | Compound | Packing coefficient (%) | Compound | Packing coefficient (%) |
|---|---|---|---|---|---|
| 2a | 66.17 | DABTEI37 | 65.79 | OMIDEV38 | 62.42 |
| 2b | 63.25 | ECEGEA39 | 63.07 | QODZUG40 | 67.41 |
| 2c | 61.97 | FEMQOF41 | 66.70 | QODZUG0140 | 68.03 |
| 2e | 64.10 | HUPWIZ42 | 60.15 | QODZUG0240 | 66.78 |
| 2f | 64.32 | HUPWIZ0143 | 62.60 | QUQSEC.44 | 65.07 |
| 2g | 63.17 | HURZOL45 | 65.16 | RELQUW46 | 64.98 |
| 2h | 64.74 | KABMIM47 | 66.21 | RUJNEQ.48 | 64.61 |
| 2i | 63.81 | MIXZOJ49 | 65.44 | TIDVIN50 | 66.07 |
| 2j | 64.63 | MIXZUP51 | 67.52 | TUZYEU52 | 65.44 |
| 2k | 65.36 | MONREO53 | 66.12 | UTITEX54 | 62.07 |
| 2l | 67.34 | NAGGEH55 | 65.27 | VEGKAU56 | 64.34 |
| 2m | 60.69 | NAGGEH0157 | 64.84 | WUHKER58 | 62.24 |
| 2n | 62.87 | NOGRIM59 | 65.44 | YEDHIY60 | 63.06 |
| 2o | 66.69 | NONFOM61 | 64.30 | ZUNVOV62 | 68.57 |
| AHOMIV63 | 63.75 | NONFOM0161 | 65.95 | ZUPCOE62 | 66.04 |
| BEGTUE64 | 62.92 | NUBVUD65 | 63.87 | ZUSSAJ66 | 63.31 |
| CAJTIQ67 | 67.82 | NUBWAK65 | 64.00 |
As Fig. 3 shows the structure occupancy percentage, there is no obvious pattern as their structure occupancy were scattered within the range of the reported imidazopyridine derivatives. These might arise from the existence of imidazopyridine moiety and the low degree-of-freedom in the present compounds. As mentioned above, crystal packing coefficient were under direct influence of weak π···π and C-H···π interaction. While in the present compound, the existence of imidazopyridine ring system had play a vital role in those π···π and C-H···π interaction, as it is involves in 9 out of 14 crystal packing of those compound. In addition, low degree-of-freedom had limited the packing patterns of the present compound. Therefore, molecules in the present compound tend to pack in a similar pattern with average crystal packing coefficient, which indicated by the occurrence of isostructural with 3D and 2D packing similarities.
Figure 3.
Structural occupancy comparison of the present and reported compound.
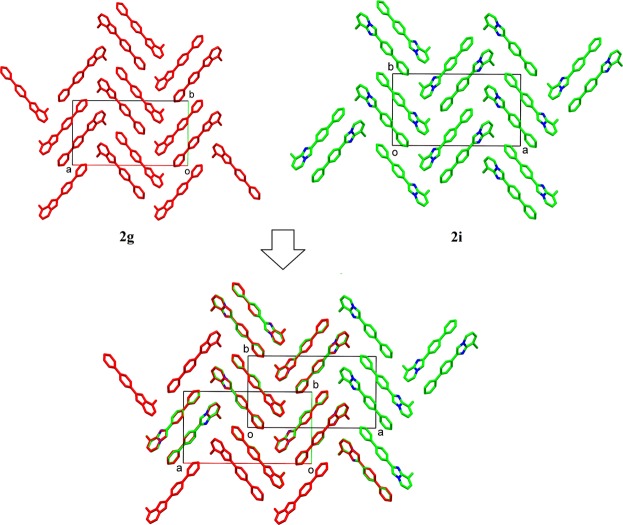
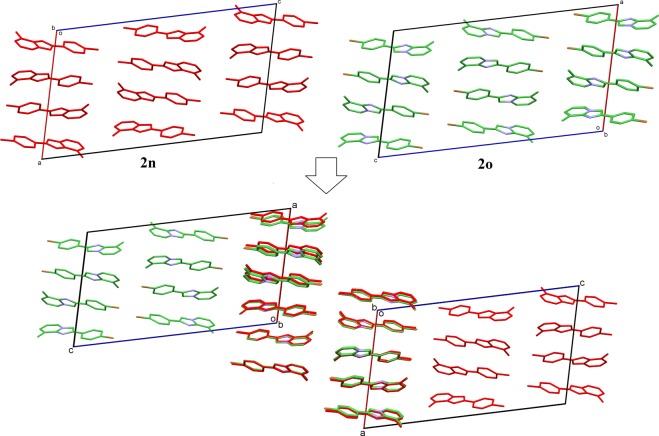
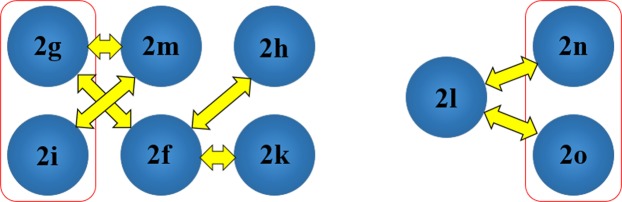
In the investigation of the crystal structural similarity among the present compounds, two pairs of compounds (2g/2i and 2n/2o) were found to be crystallized in the same space group with similar lattice constants, these are the two main designations for 3D structural similarity. In fact, their crystal structures overlaid diagrams show that those two pairs of compounds as isostructure with similar packing pattern (Figs 4 and 5). Besides, 2D similarities are observed in between 2l/2n and 2l/2o, while compound 2f, 2g, 2n, 2i, 2k and 2m shared also 2D similarity (Fig. 6).
Figure 4.
Crystal packing comparison between compounds 2g and 2i.
Figure 5.
Crystal packing comparison between compounds 2n and 2o.
Figure 6.
Crystal packing relationship in some studied compounds. Red boxes and yellow arrows indicate 3D and 2D similarities, respectively.
Cholinesterases Inhibitory Activities and Their Molecular Docking Studies
In the present study, all synthesized compounds were examined for their AChE and BChE inhibitory activities by Ellman’s assay16, and their IC50 values are tabulated in Table 3. The present imidazo[1,2-a]pyridine derivatives contain biologically active adamantyl, biphenyl or phenyl moiety as their side chains, which showed different levels of inhibitory activities towards AChE and BChE. Compounds 2a, 2f, 2j and 2l with unsubstituted imidazo[1,2-a]pyridine ring were synthesized as the reference compounds to accentuate the electronic effects of different substituents on the imidazo[1,2-a]pyridine ring, i.e. electron-donating (CH3) and electron-withdrawing (Cl) groups, upon their cholinesterase inhibitory activities.
Table 3.
IC50 values of the AChE and BChE inhibitory effects of 2(a–o).
| Compounds | Substituents | IC50 (µM) AChE | IC50 (µM) BChE | |||
|---|---|---|---|---|---|---|
| 2a | R1 = adamantan-1-yl | R2 = H | R3 = H | R4 = H | >2000 | >2000 |
| 2b | R1 = adamantan-1-yl | R2 = CH3 | R3 = H | R4 = H | 657 ± 60 | 706 ± 37 |
| 2c | R1 = adamantan-1-yl | R2 = H | R3 = CH3 | R4 = H | 270 ± 21 | >2000 |
| 2d | R1 = adamantan-1-yl | R2 = H | R3 = H | R4 = CH3 | >2000 | >2000 |
| 2e | R1 = adamantan-1-yl | R2 = H | R3 = Cl | R4 = H | >2000 | >2000 |
| 2f | R1 = 1,1′-biphenyl | R2 = H | R3 = H | R4 = H | 208 ± 17 | >2000 |
| 2g | R1 = 1,1′-biphenyl | R2 = CH3 | R3 = H | R4 = H | 288 ± 48 | 315 ± 6 |
| 2h | R1 =1,1′-biphenyl | R2 =H | R3 =H | R4 =CH3 | 79 ± 10 | 496 ± 27 |
| 2i | R1 = 1,1′-biphenyl | R2 = Cl | R3 = H | R4 = H | 253 ± 25 | >2000 |
| 2j | R1 = 3,4-dichlorophenyl | R2 = H | R3 = H | R4 = H | >2000 | 65 ± 13 |
| 2k | R1 = 3,4-dichlorophenyl | R2 = CH3 | R3 = H | R4 = H | >2000 | 191 ± 29 |
| 2l | R1 = 4-methoxyphenyl | R2 = H | R3 = H | R4 = H | >2000 | >2000 |
| 2m | R1 = 4-methoxyphenyl | R2 = CH3 | R3 = H | R4 = H | >2000 | >2000 |
| 2n | R1 = 4-chlorophenyl | R2 = CH3 | R3 = H | R4 = H | >2000 | 722 ± 48 |
| 2o | R1 = 4-bromophenyl | R2 = CH3 | R3 = H | R4 = H | >2000 | >2000 |
| Tacrine | 0.24 ± 0.04 | 0.03 ± 0.01 | ||||
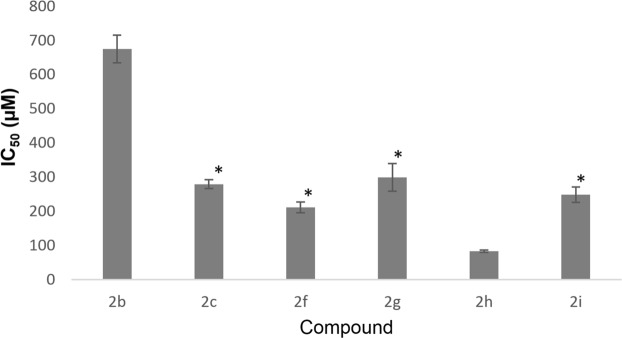
Compounds 2(f-i) with biphenyl side chain showed good AChE inhibition, while compounds 2(j-o) with phenyl side chain were inactive up to a concentration of 2000 μM, indicating the importance of biphenyl moiety in AChE inhibition. As compared to compound 2f (IC50 = 208 µM) with unsubstituted imidazo[1,2-a]pyridine ring, compounds 2g and 2i which have substituent group at the position R2 showed lower AChE inhibitory effect (IC50 = 288 and 253 µM, respectively), whereas, compound 2h with methyl substituent at the position R4 of imidazo[1,2-a]pyridine ring showed the highest AChE inhibition activity (IC50 = 79 µM). On the other hand, 2-(adamantan-1-yl)imidazo[1,2-a]pyridine derivatives 2(a-e) exhibited moderate to weak AChE inhibition activities. Compound 2c with methyl group substituted at the position R3 of imidazo[1,2-a]pyridine ring has an IC50 value of 270 µM, which is statistically comparable to compounds 2f, 2g and 2i (Fig. 7). Whereas, compound 2b with substituted methyl group at the position R2 of imidazo[1,2-a]pyridine ring showed even lower AChE inhibition activity (IC50 = 657 µM) as compared to 2c.
Figure 7.
The IC50 for AChE inhibition in mean ± SD (n = 3). * indicate p < 0.05.
In contrast to the inactive activity in AChE inhibition, derivatives with phenyl side chain gave better BChE inhibition as compared to the adamantyl or biphenyl side chain if their phenyl side chains are substituted with strong electron-withdrawing Cl group, i.e. compounds 2j, 2k and 2n. Compounds 2j and 2k bearing with 3,4-dichlorophenyl side chain are two strongest BChE inhibitors with IC50 values of 65 and 191 µM, respectively. There is a significant difference in the BChE inhibition activities of compounds 2n (IC50 = 722 µM) and 2o (IC50 > 2000 µM) although their phenyl side chains are substituted with electron-withdrawing groups, i.e. 4-chloro and 4-bromo groups, respectively, deducing that the oversized Br atom enfeebled the BChE inhibition of 2o. Meanwhile, compound 2m with its phenyl side chain substituted with electron-donating methoxy (OCH3) group is inactive towards BChE inhibition up to a concentration of 2000 µM, which emphasizes the importance of electron-withdrawing Cl substituent in BChE inhibition. Similar to AChE inhibitory activities of compounds 2f and 2g, the methyl substituent at the position R2 of imidazo[1,2-a]pyridine ring gave a negative impact towards BChE inhibition. As the BChE inhibition activity of 2k (IC50 = 191 µM) is two times lower than 2j (IC50 = 65 µM).
Molecular docking studies of the potent imidazo[1,2-a]pyridine-based derivatives were performed to provide putative binding modes within the cholinesterase enzymes. The validation of the docking accuracy was carried out by docking the native co-crystallized ligand (tacrine) back into the parent enzymes and the conformation of the best-scored pose was compared with the bound ligand in the native crystal. The overall structure of human’s BChE is similar to a human’s AChE with phenylalanines (Phe) in the acyl binding pocket of AChE being replaced by leucine (Leu) and valine (Val) in BChE24.
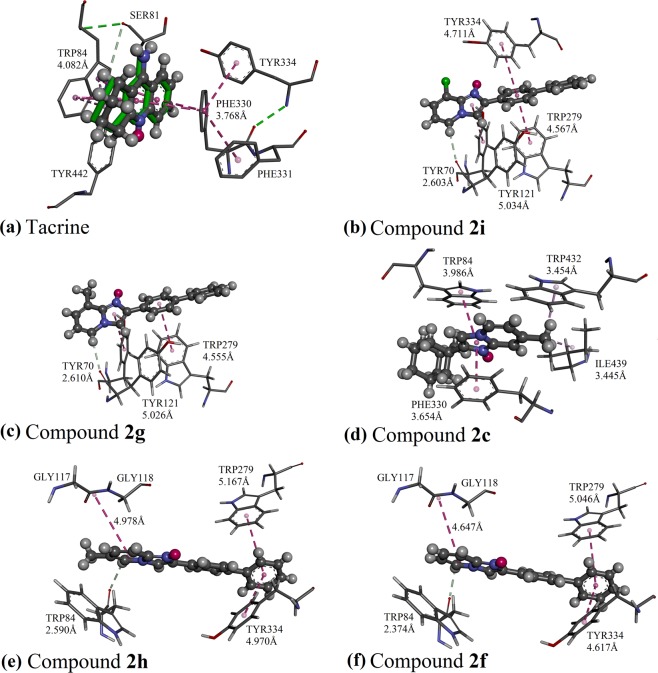
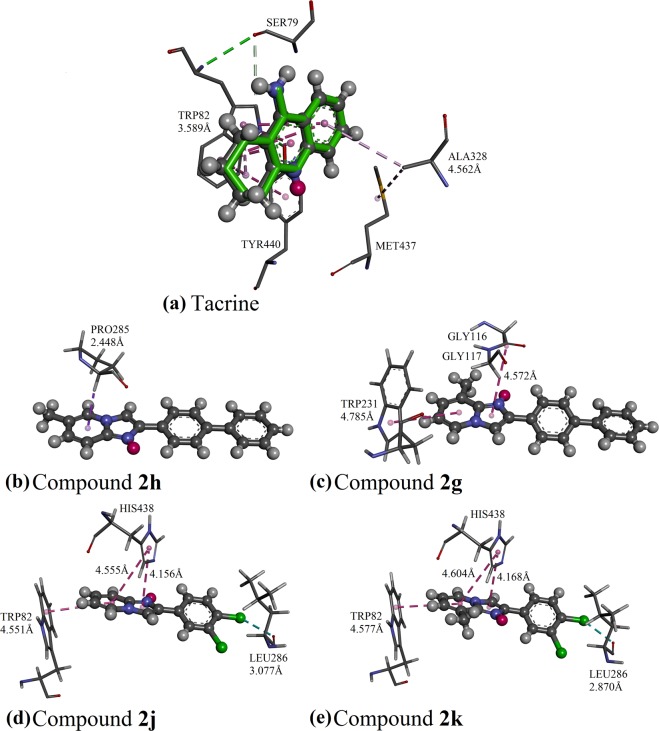
In the protein crystal structure of 1ACJ, the inhibitor tacrine was bound to the catalytic active site of the AChE protein via π···π interactions, involving residues Trp84 and Phe330 (Fig. 8a). The docking models of active compounds 2i, 2g, 2c, 2h and 2f are illustrated in Fig. 8b–f, respectively. The molecule of 2i and 2g, which bear substituted imidazo[1,2-a]pyridine ring at the position R2 were not penetrated to bound with any residue in the catalytic active site. Instead, the molecules of 2i and 2g only managed to bind only with the peripheral anionic site, which lies essentially on the surface of AChE25 by π···π and C-H···O interactions (involving residues Trp279, Tyr 334, Tyr112 and Tyr70 for 2i, and residues Trp279, Tyr121 and Tyr70 for 2g) (Fig. 8b,c) and resulted in weaker inhibitory effect. Similar to tacrine, the imidazole ring of molecule 2c was bound to the catalytic active site through π···π interactions with residues Trp84 and Phe330. Meanwhile, its methyl group substituted at the position R3 was favored to bind with the residues Trp437 and Ile439 of the hydrophobic pocket via C-H···π and C-H···alkyl interactions (Fig. 8d). However, molecule 2c failed to bind with any residues at the peripheral anionic site, which eventually leads to a moderate inhibition against AChE. As the most potent AChE inhibitors, the molecules of 2h and 2f were bound in the AChE enzyme with similar binding mode, involving both catalytic active site and peripheral anionic site. Their biphenyl side chains tend to form π···π interaction with residues Trp279 and Tyr334 at the peripheral anionic site26. The imidazo[1,2-a]pyridine rings of molecules 2h and 2f with unsubstituted position R2 were bound to the catalytic active site and oxyanion hole of the AChE enzyme through C-H···O and amide···π interactions, involving residues Trp84, Gly117 and Gly118 (Fig. 8e,f) and results in a stronger AChE inhibitory effect as compared to aforementioned compounds 2i, 2g and 2c.
Figure 8.
Differential validation in GOLD package by docking the native ligands of AChE into their binding sites (a). The native co-crystallized tacrine is represented as green sticks, while the docked ligands are shown in the form of balls and sticks, colored by elements. Putative binding modes of (b) compound 2i; (c) compound 2g; (d) compound 2c; (e) compound 2h; (f) compound 2f.
In the BChE molecular docking study with the protein crystal structure 4BDS, tacrine was bound to the catalytic active site of BChE with residues Trp82 and Ala328 through alkyl···π and π···π interactions (Fig. 9a). For compounds 2g and 2h with moderate BChE inhibitory activity, their molecules were only favored to bind at the acyl pocket via π···π interaction, involving residues Trp211 and Pro285 for 2g and 2h, respectively (Fig. 9b,c). Compound 2g (IC50 = 315 µM) which gives a better BChE inhibition than 2h (IC50 = 496 µM), could form an extra amide···π interaction at the oxyanion hole with residues Gly116 and Gly117. Similar to the most potent AChE inhibitors (2h and 2f), the imidazo[1,2-a]pyridine moieties of the most potent BChE inhibitors, 2j (IC50 = 65 µM) and 2k (IC50 = 191 µM) were able to bind at the catalytic active side through π···π interaction. The imidazo[1,2-a]pyridine rings of molecules 2j and 2k (Fig. 9d,e) were bound to two residues of the catalytic active site, i.e. Trp82 and His438. The dichloro-substituted phenyl side chains of 2j and 2k tend to bind tightly to the acyl pocket of the BChE enzyme, similar to a previously reported active compound, 2-(adamantan-1-yl)-2-oxoethyl 2,4-dichlorobenzoate27. The halogen···O interaction between the dichloro-substituted phenyl side chain and residue Leu286 of acyl pocket led to stronger BChE inhibitory effects of compounds 2j and 2k as compared to 2g and 2h.
Figure 9.
Differential validation in GOLD package by docking the native ligands of BChE into their binding sites (a). The native co-crystallized tacrine is represented as green sticks, while the docked ligands are shown in the form of balls and sticks, colored by elements. Putative binding modes of (b) compound 2h; (c) compound 2g; (d) compound 2j; (e) compound 2k.
As a summary, the molecular docking results showed that imidazo[1,2-a]pyridine ring was able to bind in the catalytic active site via π···π and C-H···O interactions (Trp84 in AChE; Trp82 and His438 in BChE). The finding reveals that AChE inhibitory effects are attributed to the biphenyl ring, which enable the imidazo[1,2-a]pyridine-based derivatives to bind in both catalytic active site and peripheral anionic site through π···π and C-H···O interactions. On the other hand, BChE inhibitory effects are mainly contributed by halogen···O interaction between the dichloro-substituted phenyl side chain and the residue of acyl pocket of BChE.
Material and Methods
The reagents and solvents were obtained commercially from Sigma Aldrich and Merck Corporation, and were used without additional purification. Melting points were determined on Stuart (UK) SMP10 apparatus. 1H and 13C nuclear magnetic resonance (NMR) spectra were determined in CDCl3 at 500 MHz and 125 MHz, respectively, using Bruker Avance III 500 spectrometer. Fourier transform infrared spectroscopy (FTIR) spectra were recorded on Perkin Elmer Frontier FTIR spectrometer equipped with attenuated total reflection (ATR). Mass spectra were recorded using Agilent 5975C TAD Gas Chromatograph/Mass Selective Detector System with HP-5MS GC column.
The X-ray analysis of all target compounds excluding 2d were performed using Bruker APEX II DUO CCD diffractometer employing Mo Kα radiation (λ = 0.71073 Å) with φ and ω scans. Data reduction and absorption correction were performed using SAINT and SADABS programs28. All structures were solved by direct methods and refined by full-matrix least-squares techniques on F2 using SHELXTL software package29. All non-hydrogen atoms were refined anisotropically (except for the minor disordered component of 2k with site-occupancy less than 0.2) and all C-bound H atoms were calculated geometrically with isotropic displacement parameters set to 1.2 (or 1.5 for methyl group) times the equivalent isotropic displacement parameters of the parent carbon atoms. Crystallographic data are collected in Supplementary Information.
Synthesis
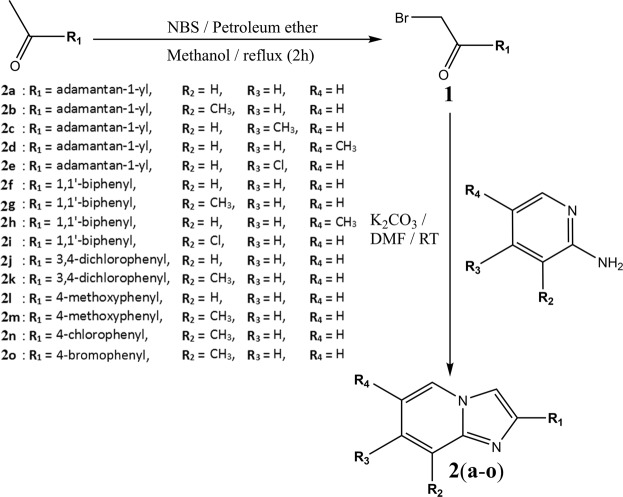
Target compounds were synthesized via a two-step reaction (Fig. 10). First, various substituted-ethanones were refluxed with N-bromosuccinimide (NBS) and petroleum ether in methanol at 333 K for two to five hours. Progress of the reactions were monitored by thin layer chromatography (TLC) plate using silica gel, with acetone:benzene (1:1) solvent system. The substituted-2-bromoethan-1-one (1) precipitate was filtered and recrystallized with ethanol. After that, 1 (0.002 mol) was reacted with 2-aminopyridines (0.003 mol) with the presence of potassium carbonate in DMF (8 ml) and stirred at room temperature for about three hours. The reaction progress was monitored by TLC method. After the reaction completed, the reaction mixture was poured into crushed ice. The precipitate formed, 2(a–o) were filtered out and recrystallized using acetone after dried30. All target compounds were synthesized in good yield and high purity, their chemical structures were characterized by using FTIR, NMR, mass spectroscopy and single-crystal X-ray diffraction analysis.
Figure 10.
Reaction scheme for the synthesis of imidazo[1,2-a]pyridine derivatives 2(a-o).
2-(adamantan-1-yl)imidazo[1,2-a]pyridine (2a)
Solvent for growing crystal: acetone; Yield 82%; M.P. 457–459 K; FT-IR (ATR (solid) cm−1): 3103 (Ar, C-H, v), 2901, 2845 (C-H, ν), 1634, 1449 (Ar, C-C, ν), 1352, 1274 (C-N, ν), 728 (Ar, C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.072–8.058 (d, 1 H, J = 7.0 Hz, 2CH), 7.680–7.591 (d, 1 H, J = 7.0 Hz, 5CH), 7.316 (s, 1 H, 1CH), 7.133–7.101 (t, 1 H, J = 7.0 Hz, 4CH), 6.735–6.708 (t, 1 H, J = 7.0 Hz, 3CH), 2.117 (bs, 3 H, 10CH, 12CH, 14CH), 2.070–2.064 (bd, 6 H, 9CH2, 15CH2, 16CH2), 1.836–1.824 (bt, 6 H, 11CH2, 13CH2, 17CH2); 13C NMR (125 MHz, CDCl3): δ ppm 157.34 (C6), 144.87 (C7), 125.46 (C2), 123.76 (C4), 117.25 (C3), 111.62 (C5), 106.59 (C1), 42.44 (C9, C15, C16), 36.93 (C11, C13, C17), 34.16 (C8), 28.64 (C10, C12, C14); GC-MS (EI) m/z: 252 (+M).
2-(adamantan-1-yl)-8-methylimidazo[1,2-a]pyridine hydrate (2b)
Solvent for growing crystal: acetone: methanol (1:1 v/v); Yield 78%; M.P. 363–365 K; FT-IR (ATR (solid) cm−1): 3326 (O-H, v), 3095 (Ar, C-H, v), 2899, 2847 (C-H, ν), 1632, 1492 (Ar, C-C, ν), 1355, 1271 (C-N, ν), 749 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 7.947-7.934 (d, 1 H, J = 6.8 Hz, 2CH), 7.306 (s, 1 H, 1CH), 6.922-6.908 (d, 1 H, J = 6.8 Hz, 4CH), 6.655-6.628 (t, 1 H, J = 6.8 Hz, 3CH), 2.643 (s, 3 H, 15CH3), 2.117 (bs, 3 H, 10CH, 12CH, 12ACH), 2.086-2.081 (bd, 6 H, 9CH2, 13CH2, 13ACH2), 1.863-1.804 (bq, 6 H, 11CH2, 11ACH2, 14CH2); 13C NMR (125 MHz, CDCl3): δ ppm 156.53 (C6), 144.17 (C7), 127.07 (C4), 123.34 (C2), 122.85 (C5), 111.70 (C5), 107.12 (C1), 42.40 (C9, C13, C13A), 36.92 (C11, C11A, C14), 34.18 (C8), 28.65 (C10, C12, C12A), 17.49 (C15); GC-MS (EI) m/z: 266 (+M).
2-(adamantan-1-yl)-7-methylimidazo[1,2-a]pyridine hydrate (2c)
Solvent for growing crystal: acetone: methanol (1:1 v/v); Yield 79%; M.P. 409-411 K; FT-IR (ATR (solid) cm−1): 3388 (O-H, v), 3143 (Ar, C-H, ν), 2899, 2845 (C-H, ν), 1645, 1449 (Ar, C-C, ν), 1357, 1234 (C-N, ν), 779 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 7.944-7.930 (d, 1 H, J = 6.9 Hz, 2CH), 7.353 (s, 1 H, 1CH), 7.223 (s, 1 H, 5CH), 6.566-6.552 (d, 1 H, J = 6.9 Hz, 3CH), 2.381 (s, 3 H, 18CH3), 2.108 (bs, 3 H, 10CH, 12CH, 14CH), 2.052-2.046 (bd, 6 H, 9CH2, 15CH2, 16CH2), 1.828-1.816 (bt, 6 H, 11CH2, 13CH2, 17CH2); 13C NMR (125 MHz, CDCl3): δ ppm 157.06 (C6), 145.37 (C7), 134.49 (C4), 124.70 (C2), 115.67 (C5), 114.21 (C3), 105.90 (C1), 42.44 (C9, C15, C16), 36.96 (C11, C13, C17), 34.09 (C8), 28.64 (C10, C12, C14), 21.30 (C18); GC-MS (EI) m/z: 266 (+M).
2-(adamantan-1-yl)-6-methylimidazo[1,2-a]pyridine (2d)
Yield 70%; M.P. 371-373 K; FT-IR (ATR (solid) cm−1): 3106 (Ar, C-H, v), 2896, 2847 (C-H, ν), 1632, 1446 (Ar, C-C, ν), 1355, 1274 (C-N, ν), 730 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 7.754 (s, 1 H, 2CH), 7.412-7.394 (d, 1 H, J = 9.2 Hz, 5CH), 7.134 (s, 1 H, 1CH), 6.886-6.867 (d, 1 H, J = 9.2 Hz, 4CH), 2.211 (s, 3 H, 18CH3), 2.116 (bs, 3 H, 10CH, 12CH, 14CH), 1.959-1.953 (bd, 6 H, 9CH2, 15CH2, 16CH2), 1.733-1.721 (bt, 6 H, 11CH2, 13CH2, 17CH2); 13C NMR (125 MHz, CDCl3): δ ppm 157.00 (C6), 143.89 (C7), 126.96 (C4), 123.24 (C2), 121.15 (C3), 116.50 (C5), 106.32 (C1), 42.44 (C9, C15, C16), 36.93 (C11, C13, C17), 34.11 (C8), 28.63 (C10, C12, C14), 18.06 (C18); GC-MS (EI) m/z: 266 (+M).
2-(adamantan-1-yl)-7-chloroimidazo[1,2-a]pyridine hydrate (2e)
Solvent for growing crystal: acetone; Yield 85%; M.P. 395-397 K; FT-IR (ATR (solid) cm−1): 3299 (O-H, v), 3149 (Ar, C-H, ν), 2901, 2850 (C-H, ν), 1626, 1451 (Ar, C-C, ν), 1352, 1234 (C-N, ν), 790 (C-Cl, v), 774 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 7.995-7.981 (d, 1 H, J = 7.2 Hz, 2CH), 7.603 (s, 1 H, 5CH), 7.293 (s, 1 H, 1CH), 6.743-6.729 (d, 1 H, J = 7.2 Hz, 3CH), 2.116 (bs, 3 H, 10CH, 12CH, 12ACH), 2.042-2.036 (bd, 6 H, 9CH2, 13CH2, 13ACH2), 1.831-1.818 (bt, 6 H, 11CH2, 11ACH2, 14CH2); 13C NMR (125 MHz, CDCl3): δ ppm 158.20 (C6), 142.25 (C7), 135.56 (C4), 125.59 (C2), 116.19 (C5), 113.35 (C3), 106.94 (C1), 42.29 (C9, C13, C13A), 36.88 (C11, C11A, C14), 34.32 (C8), 28.76 (C10, C12, C12A); GC-MS (EI) m/z: 286 (+M).
2-([1,1′-biphenyl]-4-yl)imidazo[1,2-a]pyridine (2f)
Solvent for growing crystal: acetone: methanol (1:1 v/v); Yield 77%; M.P. 472-474 K; FT-IR (ATR (solid) cm−1): 3129, 3030 (Ar, C-H, ν), 1634, 1476 (Ar, C-C, ν), 1373, 1252 (C-N, ν), 739 (Ar, C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.278-8.261 (d, 1 H, J = 8.6 Hz, 2CH), 8.163-8.146 (d, 2 H, J = 8.4 Hz, 9CH, 13CH), 8.037 (s, 1 H, 1CH), 7.774-7.757 (d, 1 H, J = 8.6 Hz, 5CH), 7.751-7.734 (d, 2 H, J = 8.4 Hz, 10CH, 12CH), 7.706-7.689 (d, 1 H, J = 8.0 Hz, 17CH), 7.649-7.633 (d, 2 H, J = 8.0 Hz, 15CH, 19CH), 7.513-7.483 (t, 2 H, J = 8.0 Hz, 16CH, 18CH), 7.434-7.405 (t, 1 H, J = 8.6, 4CH), 7.230-7.203 (t, 1 H, J = 8.6 Hz, 3CH); 13C NMR (125 MHz, CDCl3): δ ppm 145.90 (C6), 144.48 (C14), 140.65 (C7), 139.80 (C11), 138.03 (C8), 128.99 (C16, C18), 128.84 (C4), 127.98 (C9, C13), 127.88 (C17), 127.15 (C15, C19), 127.03 (C10, C12), 126.57 (C2), 123.03 (C5), 113.31 (C3), 108.51 (C1); GC-MS (EI) m/z: 270 (+M).
2-([1,1′-biphenyl]-4-yl)-8-methylimidazo[1,2-a]pyridine (2g)
Solvent for growing crystal: acetone; Yield 85%; M.P. 411-413 K; FT-IR (ATR (solid) cm−1): 3037 (Ar, C-H, v), 2928 (C-H, ν), 1629, 1478 (Ar, C-C, ν), 1372, 1258 (C-N, ν), 736 (Ar, C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.085-8.068 (d, 2 H, J = 8.5 Hz, 9CH, 13CH), 8.021-8.012 (d, 1 H, J = 6.8 Hz, 2CH), 7.898 (s, 1 H, 1CH), 7.714-7.697 (d, 2 H, J = 8.5 Hz, 10CH, 12CH), 7.691-7.667 (d, 2 H, J = 7.2 Hz, 15CH, 19CH), 7.498-7.467 (t, 2 H, J = 7.2 Hz, 16CH, 18CH), 7.396-7.367 (t, 1 H, J = 7.2 Hz, 17CH), 7.000-6.987 (d, 1 H, J = 6.8 Hz, 4CH), 6.731-7.714 (t, 1 H, J = 6.8 Hz, 3CH), 2.716 (s, 1 H, 20CH3); 13C NMR (125 MHz, CDCl3): δ ppm 146.20 (C6), 144.70 (C14), 140.86 (C7), 140.50 (C11), 132.95(C8), 128.78 (C16, C18), 127.75 (C5), 127.36 (C9, C13), 127.28 (C17), 126.99 (C15, C19), 126.58 (C10, C12), 123.52 (C2), 123.44 (C4), 112.50 (C3), 108.70 (C1) 17.16 (C20); GC-MS (EI) m/z: 284 (+M).
2-([1,1′-biphenyl]-4-yl)-6-methylimidazo[1,2-a]pyridine (2h)
Solvent for growing crystal: acetone; Yield 88%; M.P. 508-510 K; FT-IR (ATR (solid) cm−1): 3130, 3038 (Ar, C-H, ν), 2932 (C-H, ν), 1597, 1478 (Ar, C-C, ν), 1349, 1258 (C-N, ν), 733 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.105-8.080 (d, 2 H, J = 8.5 Hz, 9CH, 13CH), 8.068 (s, 1 H, 1CH), 8.010-7.992 (d, 1 H, J = 9.1 Hz, 5CH), 7.921 (s, 1 H, 2CH), 7.720-7.703 (d, 2 H, J = 8.5 Hz, 10CH, 12CH), 7.657-7.642 (d, 2 H, J = 7.2 Hz, 15CH, 19CH), 7.508-7.478 (t, 2 H, J = 7.2 Hz, 16CH, 18CH), 7.421-7.392 (d, 1 H, J = 7.2 Hz, 17CH), 7.332-7.314 (d, 1 H, J = 9.1 Hz, 4CH), 2.394 (s, 3 H, 20CH3); 13C NMR (125 MHz, CDCl3): δ ppm 145.49 (C6), 142.22 (C14), 140.10 (C7), 137.48 (C11), 136.99 (C8), 128.93 (C16, C18), 128.81 (C4), 127.80 (C3), 127.69 (C9, C13), 127.22 (C17), 126.98 (C15, C19), 126.83 (C10, C12), 126.04 (C2), 124.07 (C5), 108.14 (C1), 18.23 (C20); GC-MS (EI) m/z: 284 (+M).
2-([1,1′-biphenyl]-4-yl)-8-chloroimidazo[1,2-a]pyridine (2i)
Solvent for growing crystal: acetone: methanol: ethanol (1:1:1 v/v/v); Yield 70%; M.P. 491-493 K; FT-IR (ATR (solid) cm−1): 3135, 3063 (Ar, C-H, v), 1626, 1476 (Ar, C-C, ν), 1368, 1258 (C-N, ν), 784 (C-Cl, ν), 736 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.316-8.299 (d, 2 H, J = 8.4 Hz, 9CH, 13CH), 8.203-8.191 (d, 1 H, J = 7.0 Hz, 2CH), 8.178 (s, 1 H, 1CH), 7.787-7.770 (d, 2 H, J = 8.4 Hz, 10CH, 12CH), 7.761-7.744 (d, 1 H, J = 7.0 Hz, 4CH), 7.703-7.687 (d, 2 H, J = 8.0 Hz, 15CH, 19CH), 7.514-7.484 (t, 2 H, J = 8.0 Hz, 16CH, 18CH), 7.420-7.391 (t, 2 H, J = 8.0 Hz, 17CH), 7.061-7.032 (t, 1 H, J = 7.0 Hz, 3CH); 13C NMR (125 MHz, CDCl3): δ ppm 146.25 (C6), 144.82 (C14), 140.89 (C7), 140.50 (C11), 132.99(C8), 130.53 (C5), 128.79 (C16, C18), 127.54 (C4), 127.36 (C9, C13), 127.28 (C17), 126.98 (C15, C19), 126.58 (C10, C12), 123.52 (C3), 112.50 (C2), 108.70 (C1); GC-MS (EI) m/z: 304 (+M).
2-(3,4-dichlorophenyl)imidazo[1,2-a]pyridine (2j)
Solvent for growing crystal: acetone: methanol (1:1 v/v); Yield 81%; M.P. 439-441 K; FT-IR (ATR (solid) cm−1): 3122, 3033 (Ar, C-H, ν), 1637, 1451 (Ar, C-C, ν), 1371, 1252 (C-N, ν), 825 (C-Cl, ν), 744 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.145-8.135 (d, 1 H, J = 7.0 Hz, 2CH), 8.088 (s, 1 H, 9CH), 7.874 (s, 1 H, 1CH), 7.798-7.777 (d, 1 H, J = 9.0 Hz, 13CH), 7.655-7.637 (d, 1 H, J = 9.0 Hz, 12CH), 7.519-7.503 (d, 1 H, J = 7.0 Hz, 5CH), 7.245-7.213 (t, 1 H, J = 7.0 Hz, 4CH) 6.848-6.821 (t, 1 H, J = 7.0 Hz, 3CH); 13C NMR (125 MHz, CDCl3): δ ppm 145.79 (C6), 143.50 (C7), 133.91 (C10), 131.68 (C11), 130.68 (C12), 127.80 (C9), 125.70 (C13), 125.27 (C2), 125.14 (C4), 117.66 (C5), 112.87 (C3), 108.59 (C1); GC-MS (EI) m/z: 262 (+M).
2-(3,4-dichlorophenyl)-8-methylimidazo[1,2-a]pyridine (2k)
Solvent for growing crystal: acetone: methanol (1:1 v/v); Yield 80%; M.P. 410-412 K; FT-IR (ATR (solid) cm−1): 3114, 3076 (Ar, C-H, ν), 2923 (C-H, ν), 1632, 1457 (Ar, C-C, ν), 1372, 1136 (C-N, ν), 784 (C-Cl, ν), 741 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.105 (s, 1 H, 1CH), 8.020-8.007 (d, 1 H, J = 6.8 Hz, 2CH), 7.857 (s, 1 H, 13CH), 7.825-7.804 (d, 1 H, J = 8.4 Hz, 9CH), 7.516-7.499 (d, 1 H, J = 8.4 Hz, 10CH), 7.021-7.007 (d, 1 H, J = 6.8 Hz, 4CH), 6.758-6.730 (t, 1 H, J = 6.8 Hz, 3CH), 2.678 (s, 3 H, 14CH3); 13C NMR (125 MHz, CDCl3): δ ppm 146.28 (C6), 142.82 (C7), 134.15 (C8), 132.84 (C11), 131.48 (C12), 130.62 (C9, C13), 127.89 (C5), 125.28 (C10), 123.94 (C2), 123.51 (C4), 112.87 (C3), 109.07 (C1), 17.07 (C14); GC-MS (EI) m/z: 276 (+M).
2-(4-methoxyphenyl)imidazo[1,2-a]pyridine (2l)
Solvent for growing crystal: acetone; Yield 85%; M.P. 402-404 K; FT-IR (ATR (solid) cm−1): 3135, 3006 (Ar, C-H, ν), 2966 (C-H, ν), 1634, 1481 (Ar, C-C, ν), 1371, 1172 (C-N, ν), 1239, 1029 (C-O, ν), 744 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.133-8.119 (d, 1 H, J = 7.0 Hz, 2CH), 7.921-7.904 (d, 2 H, J = 8.9 Hz, 9CH, 13CH), 7.801 (s, 1 H, 1CH), 7.651-7.632 (d, 1 H, J = 7.0 Hz, 5CH), 7.195-7.163 (t, 1 H, J = 7.0 Hz, 4CH), 7.009-6.992 (d, 2 H, J = 8.9 Hz, 10CH, 12CH) 6.800-6.773 (t, 1 H, J = 7.0 Hz, 3CH), 3.879 (s, 3 H, 14CH3); 13C NMR (125 MHz, CDCl3): δ ppm 159.61 (C11), 145.73 (C6), 145.62 (C7), 127.32 (C9, C13), 126.44 (C8), 125.46 (C2), 124.50 (C4), 117.31 (C5), 114.16 (C10, C12), 112.30 (C3), 107.23 (C5), 55.33 (C14); GC-MS (EI) m/z: 224 (+M).
2-(4-methoxyphenyl)-8-methylimidazo[1,2-a]pyridine (2m)
Solvent for growing crystal: acetone; Yield 88%; M.P. 391-393 K; FT-IR (ATR (solid) cm−1): 3129, 3010 (Ar, C-H, ν), 2960, 2838 (C-H, ν), 1611, 1486 (Ar, C-C, ν), 1370, 1173 (C-N, ν), 1248, 1030 (C-O, ν), 776 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.013-7.999 (d, 1 H, J = 6.8 Hz, 2CH), 7.938-7.921 (d, 2 H, J = 8.9 Hz, 9CH, 13CH), 7.782 (s, 1 H, 1CH), 7.003-6.973 (m, 3 H, 4CH, 10CH, 12CH), 6.723-6.696 (t, 1 H, J = 6.8 Hz, 3CH), 3.877 (s, 3 H, 15CH3) 2.668 (s, 3 H, 14CH3); 13C NMR (125 MHz, CDCl3): δ ppm 159.54 (C11), 145.89 (C6), 144.89 (C7), 132.33 (C8), 127.51 (C9, C13), 126.47 (C5), 123.54 (C2), 123.36 (C4), 114.11 (C10, C12), 112.42 (C3), 107.77 (C1), 55.33 (C15) 17.18 (C14); GC-MS (EI) m/z: 238 (+M).
2-(4-chlorophenyl)-8-methylimidazo[1,2-a]pyridine (2n)
Solvent for growing crystal: acetone: methanol (1:1 v/v); Yield 82%; M.P. 417–419 K; FT-IR (ATR (solid) cm−1): 3034 (Ar, C-H, ν), 2923 (C-H, ν), 1629, 1473 (Ar, C-C, ν), 1367, 1256 (C-N, ν), 845 (C-Cl, ν), 736 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 8.002-7.988 (d, 1 H, J = 6.8 Hz, 2CH), 7.935-7.918 (d, 2 H, J = 8.6 Hz, 9CH, 13CH), 7.830 (s, 1 H, 1CH), 7.424-7.407 (d, 2 H, J = 8.6 Hz, 10CH, 12CH), 6.993-6.980 (d, 1 H, J = 6.8 Hz, 4CH), 6.727-6.699 (t, 1 H, J = 6.8 Hz, 3CH), 2.677 (s, 3 H, 14CH3); 13C NMR (125 MHz, CDCl3): δ ppm 146.24 (C6), 144.04 (C7), 133.48 (C8), 132.57 (C11), 128.84 (C9, C13), 127.61 (C5), 127.39 (C10, C12), 123.62 (C2), 123.45 (C4), 112.60 (C3), 108.67 (C1), 17.10 (C14); GC-MS (EI) m/z: 242 (+M).
2-(4-bromophenyl)-8-methylimidazo[1,2-a]pyridine (2o)
Solvent for growing crystal: acetone; Yield 85%; M.P. 405–407 K; FT-IR (ATR (solid) cm−1): 3135, 3037 (Ar, C-H, ν), 2920 (C-H, ν), 1629, 1468 (Ar, C-C, ν), 1370, 1253 (C-N, ν), 826 (C-Cl, ν), 733 (C-H, ω); 1H NMR (500 MHz, CDCl3): δ ppm 7.973-7.960 (d, 1 H, J = 6.8 Hz, 2CH), 7.861-7.844 (d, 2 H, J = 8.6 Hz, 9CH, 13CH), 7.810 (s, 1 H, 1CH), 7.566-7.549 (d, 2 H, J = 8.6 Hz, 10CH, 12CH), 6.980-6.966 (d, 1 H, J = 6.8 Hz, 4CH), 6.709-6.682 (t, 1 H, J = 6.8 Hz, 3CH), 2.668 (s, 3 H, 14CH3); 13C NMR (125 MHz, CDCl3): δ ppm 146.27 (C6), 144.04 (C7), 133.07 (C8), 131.76 (C9, C13), 127.67 (C10, C12), 127.61 (C11), 123.60 (C2), 123.45 (C4), 121.65 (C5), 112.58 (C3), 108.71 (C1), 17.10 (C14); GC-MS (EI) m/z: 286 (+M).
Cholinesterase inhibition assay
Ellman’s colorimetric method with minor modification was employed in the determination of AChE and BChE inhibitory activities16. Electric eel acetylcholinesterase (AChE, EC 3.1.1.7), equine serum butyrylcholinesterase (BChE, EC 3.1.1.8), 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB) and sodium phosphate dibasic (Na2HPO4) were purchased from Sigma-Aldrich (USA). S-butyrylthiocholine chloride (BTCC) from Sigma-Aldrich (Switzerland), acetylthiocholine iodide (ATCI) from Sigma-Aldrich (United Kingdom) and sodium phosphate monobasic (NaH2PO4) from Sigma-Aldrich (Germany) were purchased. Tacrine hydrochloride was purchased from Cayman Chemical Company and absolute ethanol from Merck Emsure® Germany was used as the solvent. Ethanol was used to replace the sample as a control in this assay31. Tacrine hydrochloride was used as the reference drug in the inhibition assays of AChE and BChE.
Statistical analysis
The results of cholinesterase inhibitory assay were expressed as mean ± standard deviation (SD) and were labeled if p < 0.05 by using ANOVA of IBM SPSS Statistics for Windows, Version 23.0 (IBM, New York).
Docking protocol
Molecular docking was performed for the most potent inhibitors using Genetic Optimization for Ligand Docking (GOLD) package 5.4.132–34. Crystal structures of AChE (PDB ID: 1ACJ35) and BChE (PDB ID: 4BDS36) isolated from Teronarce californica and Homo sapiens, respectively, with tacrine were obtained from Protein Data Bank. Ligand-protein binding space and the conformational flexibility of ligand inside the protein were explored by Genetic Algorithm (GA). A spherical binding site with a radius of 15 Å was used, it lays around the binding site of tacrine covering both catalytic anionic and peripheral anionic sites. 100 GA runs were carried out and the top 100 ranked docking poses were scored using the Piecewise Linear Potential (PLP) scoring function. Default values were used for all other parameters. The intermolecular interactions of the best scored pose of each ligand were analyzed and illustrated using Discovery Studio 4.5 software (Discovery studio v4.5.0.15071. Accelrys Inc 2015).
Conclusion
A series of imidazo[1,2-a]pyridine-based derivatives 2(a-o) were synthesized and characterized by FTIR, NMR and GCMS spectroscopy analyses. The 3D structures of these derivatives were further confirmed by single crystal X-ray diffraction studies and the result showed that they tend to adapt periplanar conformation with its substituted side chain at the position R1. With the presence of imidazo[1,2-a]pyridine ring and the low degree-of-freedom, the introduction of adamantyl or biphenyl does not affect their structural occupancies. Meanwhile, the present compounds tend to pack in a similar pattern with π···π and C-H···π interactions, involving the imidazo[1,2-a]pyridine ring, these had led to redundant 2D or 3D structural similarity in imidazo[1,2-a]pyridine-based derivatives. The AChE inhibitory effects of imidazo[1,2-a]pyridine-based derivatives increased in the order of phenyl <adamantyl < biphenyl side chain substituted at the position R1. Those derivatives with methyl substituent at the position R2 of the imidazo[1,2-a]pyridine ring exhibited weaker AChE and BChE inhibitory effects. The molecular docking studies of the imidazo[1,2-a]pyridine-based derivatives revealed that the π···π or halogen···O interaction between the active compounds and their surrounding residues in the peripheral anionic site or acyl pocket of the target enzymes are dominant as compared to the hydrophobic interaction in the catalytic active site.
Supplementary information
Acknowledgements
HCK thanks Malaysian Government for MyBrain15 (MyPhD) scholarship. The authors thank Malaysian Government and USM for Research University Individual Grant (1001/PFIZIK/8011080).
Author Contributions
Conceptualization: H.C.K. C.S.C.K. Data curation: H.C.K. Formal analysis: H.C.K. T.S.C. Funding acquisition: C.K.Q. Investigation: C.S.C.K. Y.L.M. T.S.C. Methodology: H.C.K. C.S.C.K. Project administration: C.K.Q. Resources: C.K.Q. S.H.M. G.K.L. Supervision: C.K.Q. Validation: H.C.K. T.S.C. S.C. Visualization: H.C.K. T.S.C. Writing – original draft: H.C.K. S.H.M. Writing – review & editing: H.C.K. All authors reviewed the manuscript.
Data Availability
The crystallographic data for 2(a, b, c and e-o) were deposited with the Cambridge Crystallographic Data Centre as supplementary publications with CCDC no. 1055984-1055988 and 1541130-1541138, respectively. Copies of available materials can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (Fax: + 44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huey Chong Kwong, Email: stonekg01@gmail.com.
Ching Kheng Quah, Email: ckquah@usm.my.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37486-7.
References
- 1.Mavel S, et al. Synthesis of imidazo[1,2-a]pyridine derivatives as antiviral agents. Arzneimittelforschung. 2001;51:304–309. doi: 10.1055/s-0031-1300042. [DOI] [PubMed] [Google Scholar]
- 2.Byth KF, Culshaw JD, Green S, Oakes SE, Thomas AP. Imidazo[1,2-a]pyridines. Part 2: SAR and optimisation of a potent and selective class of cyclin-dependent kinase inhibitors. Bioorganic & Medicinal Chemistry Letters. 2004;14:2245–2248. doi: 10.1016/j.bmcl.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Biftu T, et al. Synthesis and SAR studies of very potent imidazopyridine antiprotozoal agents. Bioorganic & Medicinal Chemistry Letters. 2006;16:2479–2483. doi: 10.1016/j.bmcl.2006.01.092. [DOI] [PubMed] [Google Scholar]
- 4.Lacerda RB, et al. Discovery of novel analgesic and anti-inflammatory 3-arylamine-imidazo[1,2-a]pyridine symbiotic prototypes. Bioorganic & Medicinal Chemistry. 2009;17:74–84. doi: 10.1016/j.bmc.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Almirante L, et al. Derivatives of Imidazole. I. Synthesis and Reactions of Imidazo[1,2-α]pyridines with Analgesic, Antiinflammatory, Antipyretic, and Anticonvulsant Activity. Journal of Medicinal Chemistry. 1965;8:305–312. doi: 10.1021/jm00327a007. [DOI] [PubMed] [Google Scholar]
- 6.Humphries AC, et al. 8-Fluoroimidazo[1,2-a]pyridine: Synthesis, physicochemical properties and evaluation as a bioisosteric replacement for imidazo[1,2-a]pyrimidine in an allosteric modulator ligand of the GABAA receptor. Bioorganic & Medicinal Chemistry Letters. 2006;16:1518–1522. doi: 10.1016/j.bmcl.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Davey D, et al. Cardiotonic agents. 1. Novel 8-aryl substituted imidazo[1,2-a]- and -[1,5-a]pyridines and imidazo[1,5-a]pyridinones as potential positive inotropic agents. Journal of Medicinal Chemistry. 1987;30:1337–1342. doi: 10.1021/jm00391a012. [DOI] [PubMed] [Google Scholar]
- 8.Harrison T, Keating G. Zolpidem. CNS Drugs. 2005;19:65–89. doi: 10.2165/00023210-200519010-00008. [DOI] [PubMed] [Google Scholar]
- 9.Ismail MA, et al. Novel Dicationic Imidazo[1,2-a]pyridines and 5,6,7,8-Tetrahydro-imidazo[1,2-a]pyridines as Antiprotozoal Agents. Journal of Medicinal Chemistry. 2004;47:3658–3664. doi: 10.1021/jm0400092. [DOI] [PubMed] [Google Scholar]
- 10.Mizushige K, Ueda T, Yukiiri K, Suzuki H. Olprinone: A Phosphodiesterase III Inhibitor with Positive Inotropic and Vasodilator Effects. Cardiovasc. Drug Rev. 2002;20:163–174. doi: 10.1111/j.1527-3466.2002.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 11.2015 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association11, 332–384, 10.1016/j.jalz.2015.02.003. [DOI] [PubMed]
- 12.Mirjana BC, Danijela ZK, Tamara DL-P, Aleksandra MB, Vesna MV. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundberg RJ, Dalvie D, Cordero J, Musallam HA. Carbamate derivatives of 2-arylimidazo[1,2-a]pyridinium salts as acetylcholinesterase inhibitors and protective agents against organophosphorus compounds. Chem. Res. Toxicol. 1993;6:506–510. doi: 10.1021/tx00034a018. [DOI] [PubMed] [Google Scholar]
- 14.Mohsen UA. Studies on Imidazopyridine Derivatives as Acetylcholinesterase Inhibitors. Asetilkolinesteraz inhibitörleri olarak imidazopiridin türevleri üzerine çalişmalar. 2012;2:119–123. [Google Scholar]
- 15.Gurjar AS, Darekar MN, Yeong KY, Ooi L. In silico studies, synthesis and pharmacological evaluation to explore multi-targeted approach for imidazole analogues as potential cholinesterase inhibitors with neuroprotective role for Alzheimer’s disease. Bioorganic & Medicinal Chemistry. 2018;26:1511–1522. doi: 10.1016/j.bmc.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Ellman GL, Courtney KD, Andres V, Jr., Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 17.Coates, J. Interpretation of Infrared Spectra, A Practical Approach.
- 18.Hamdouchi C, et al. 2-Amino-3-substituted-6-[(E)-1-phenyl-2-(N-methylcarbamoyl)vinyl]imidazo[1,2-a]pyridines as a Novel Class of Inhibitors of Human Rhinovirus: Stereospecific Synthesis and Antiviral Activity. Journal of Medicinal Chemistry. 1999;42:50–59. doi: 10.1021/jm9810405. [DOI] [PubMed] [Google Scholar]
- 19.Chezal J-M, et al. Synthesis and antiviral activity of an imidazo[1,2-a]pyrrolo[2,3-c]pyridine series against the bovine viral diarrhea virus. European Journal of Medicinal Chemistry. 2010;45:2044–2047. doi: 10.1016/j.ejmech.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Ulloora S, Shabaraya R, Aamir S, Adhikari AV. New imidazo[1,2-a]pyridines carrying active pharmacophores: Synthesis and anticonvulsant studies. Bioorganic & Medicinal Chemistry Letters. 2013;23:1502–1506. doi: 10.1016/j.bmcl.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Groom CR, Allen FH. The Cambridge Structural Database in Retrospect and Prospect. Angewandte Chemie International Edition. 2014;53:662–671. doi: 10.1002/anie.201306438. [DOI] [PubMed] [Google Scholar]
- 22.Kumar C, et al. Synthesis and Crystallographic Insight into the Structural Aspects of Some Novel Adamantane-Based Ester Derivatives. Molecules. 2015;20:18827. doi: 10.3390/molecules201018827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong HC, et al. Novel biphenyl ester derivatives as tyrosinase inhibitors: Synthesis, crystallographic, spectral analysis and molecular docking studies. PLOS ONE. 2017;12:e0170117. doi: 10.1371/journal.pone.0170117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J. Biol. Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 25.Sussman J, et al. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 26.Johnson G, Moore SW. The Peripheral Anionic Site of Acetylcholinesterase: Structure, Functions and Potential Role in Rational Drug Design. Curr. Pharm. Des. 2006;12:217–225. doi: 10.2174/138161206775193127. [DOI] [PubMed] [Google Scholar]
- 27.Kwong HC, et al. Cholinesterase Inhibitory Activities of Adamantyl-Based Derivatives and Their Molecular Docking Studies. Molecules. 2017;22:1005. doi: 10.3390/molecules22061005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc., Madison. Wisconsin, USA (2012).
- 29.Sheldrick GM. A short history of SHELX. A short history of SHELX. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 30.Kumar Chandraju Sadolalu, C. et al. In Zeitschrift für Kristallographie - Crystalline Materials. 229, 328–342 (2014).
- 31.Hülya Öztürk UKaCM. Antioxidant, Anticholinesterase and Antibacterial Activities of Jurinea consanguinea DC. Records of Natural Products. 2011;5:43–51. [Google Scholar]
- 32.Jones G, Willett P, Glen RC. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995;245:43–53. doi: 10.1016/S0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 33.Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking1. J. Mol. Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 34.Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. Improved protein–ligand docking using GOLD. Proteins: Structure, Function, and Bioinformatics. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- 35.Harel M, et al. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc Natl Acad Sci USA. 1993;90:9031–9035. doi: 10.1073/pnas.90.19.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachon F, et al. Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013;453:393–399. doi: 10.1042/bj20130013. [DOI] [PubMed] [Google Scholar]
- 37.Koudad M, Elaatiaoui A, Benchat N, Saadi M, El Ammari L. Crystal structure of 2-(4-methoxyphenyl)-6-nitroimidazo[1,2-a]pyridine-3-carbaldehyde. Acta Crystallographica Section E. 2015;71:o979–o980. doi: 10.1107/S2056989015021957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samanta S, et al. Switching the regioselectivity in the copper-catalyzed synthesis of iodoimidazo[1,2-a]pyridines. Organic & Biomolecular Chemistry. 2016;14:5073–5078. doi: 10.1039/C6OB00656F. [DOI] [PubMed] [Google Scholar]
- 39.Ma L, Wang X, Yu W, Han B. TBAI-catalyzed oxidative coupling of aminopyridines with [small beta]-keto esters and 1,3-diones-synthesis of imidazo[1,2-a]pyridines. Chemical Communications. 2011;47:11333–11335. doi: 10.1039/C1CC13568F. [DOI] [PubMed] [Google Scholar]
- 40.Mutai T, Shono H, Shigemitsu Y, Araki K. Three-color polymorph-dependent luminescence: crystallographic analysis and theoretical study on excited-state intramolecular proton transfer (ESIPT) luminescence of cyano-substituted imidazo[1,2-a]pyridine. CrystEngComm. 2014;16:3890–3895. doi: 10.1039/C3CE42627K. [DOI] [Google Scholar]
- 41.Kurteva VB, Lubenov LA, Nedeltcheva DV, Nikolova RP, Shivachev BL. Fast and efficient direct conversion of 2-aminopyridine into 2,3-disubstituted imidazo[1,2-a]pyridines. ARKIVOC. 2012;13:282–294. doi: 10.3998/ark.5550190.0013.824. [DOI] [Google Scholar]
- 42.Halasz I, Dinnebier RE. Structural and thermal characterization of zolpidem hemitartrate hemihydrate (form E) and its decomposition products by laboratory x-ray powder diffraction. J. Pharm. Sci. 2010;99:871–878. doi: 10.1002/jps.21883. [DOI] [PubMed] [Google Scholar]
- 43.Vega DR, Baggio R, Roca M, Tombari D. Aging-Driven Decomposition in Zolpidem Hemitartrate Hemihydrate and the Single-Crystal Structure of Its Decomposition Products. J. Pharm. Sci. 2011;100:1377–1386. doi: 10.1002/jps.22358. [DOI] [PubMed] [Google Scholar]
- 44.Ravi C, Chandra Mohan D, Adimurthy S. Dual role of p-tosylchloride: copper-catalyzed sulfenylation and metal free methylthiolation of imidazo[1,2-a]pyridines. Organic & Biomolecular Chemistry. 2016;14:2282–2290. doi: 10.1039/C5OB02475G. [DOI] [PubMed] [Google Scholar]
- 45.Yang D, et al. Catalyst-Free Regioselective C-3 Thiocyanation of Imidazopyridines. The Journal of Organic Chemistry. 2015;80:11073–11079. doi: 10.1021/acs.joc.5b01637. [DOI] [PubMed] [Google Scholar]
- 46.Yan R-L, et al. Cu(I)-Catalyzed Synthesis of Imidazo[1,2-a]pyridines from Aminopyridines and Nitroolefins Using Air as the Oxidant. The Journal of Organic Chemistry. 2012;77:2024–2028. doi: 10.1021/jo202447p. [DOI] [PubMed] [Google Scholar]
- 47.Yang D, et al. Catalyst-Free Regioselective C-3 Nitrosation of Imidazopyridines with tert-Butyl Nitrite under Neutral Conditions. Synthesis. 2016;48:122–130. doi: 10.1055/s-0035-1560508. [DOI] [Google Scholar]
- 48.Li Y-H, Liu W-Y, Gao Y, Wang Y-P. 2-(4-Chlorophenyl)imidazo[1,2-a]pyridine-3-carbaldehyde. Acta Crystallographica Section E. 2009;65:o3192. doi: 10.1107/S1600536809048302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anaflous A, et al. N-(2-Phenylimidazo[1,2-a]pyridin-3-yl)acetamide. Acta Crystallographica Section E. 2008;64:o926. doi: 10.1107/S1600536808011501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donohoe TJ, Kabeshov MA, Rathi AH, Smith IED. Direct preparation of thiazoles, imidazoles, imidazopyridines and thiazolidines from alkenes. Organic & Biomolecular Chemistry. 2012;10:1093–1101. doi: 10.1039/C1OB06587D. [DOI] [PubMed] [Google Scholar]
- 51.Anaflous A, et al. 2-Phenylimidazo[1,2-a]pyridine-3-carbaldehyde. Acta Crystallographica Section E. 2008;64:o927. doi: 10.1107/S1600536808011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Lu J, Zhang J-N, Zhang Z-H. Magnetic carbon nanotube supported Cu (CoFe2O4/CNT-Cu) catalyst: A sustainable catalyst for the synthesis of 3-nitro-2-arylimidazo[1,2-a]pyridines. Catal. Commun. 2016;78:26–32. doi: 10.1016/j.catcom.2016.02.004. [DOI] [Google Scholar]
- 53.Velázquez-Ponce M, et al. Intramolecular H-bonding interaction in angular 3-π-EWG substituted imidazo[1,2-a]pyridines contributes to conformational preference. Chem. Cent. J. 2013;7:20. doi: 10.1186/1752-153x-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chunavala KC, Joshi G, Suresh E, Adimurthy S. Thermal and Microwave-Assisted Rapid Syntheses of Substituted Imidazo[1,2-a]pyridines Under Solvent- and Catalyst-Free Conditions. Synthesis. 2011;2011:635–641. doi: 10.1055/s-0030-1258405. [DOI] [Google Scholar]
- 55.Tafeenko V. A., Paseshnichenko K. A. & Schenk, H. In Zeitschrift für Kristallographie - Crystalline Materials. 211, 457–463 (1996).
- 56.Duan G-Y, Tu C-B, Sun Y-W, Zhang D-T, Wang J-W. 2-(3-Bromo-4-methoxyphenyl)imidazo[1,2-a]pyridine. Acta Crystallographica Section E. 2006;62:o1141–o1142. doi: 10.1107/S1600536806005836. [DOI] [Google Scholar]
- 57.Dixon LI, et al. Unprecedented regiochemical control in the formation of aryl[1,2-a]imidazopyridines from alkynyliodonium salts: mechanistic insights. Organic & Biomolecular Chemistry. 2013;11:5877–5884. doi: 10.1039/C3OB41112E. [DOI] [PubMed] [Google Scholar]
- 58.Aydıner B, et al. Arylstyrylimidazo[1,2-a]pyridine-based donor–acceptor acidochromic fluorophores: Synthesis, photophysical, thermal and biological properties. J. Photochem. Photobiol. A: Chem. 2015;310:113–121. doi: 10.1016/j.jphotochem.2015.05.030. [DOI] [Google Scholar]
- 59.Marandi G, Saghatforoush L, Mendoza-Meroño R, García-Granda S. Catalyst-free synthesis of 3-(alkylamino)-2-arylimidazo[1,2-a]pyridine-8-carboxylic acids via a three-component condensation. Tetrahedron Letters. 2014;55:3052–3054. doi: 10.1016/j.tetlet.2014.03.121. [DOI] [Google Scholar]
- 60.Georges GJ, et al. Characterization of the physico-chemical properties of the imidazopyridine derivative Alpidem. Comparison with Zolpidem. European Journal of Medicinal Chemistry. 1993;28:323–335. doi: 10.1016/0223-5234(93)90149-9. [DOI] [Google Scholar]
- 61.Mutai T, Tomoda H, Ohkawa T, Yabe Y, Araki K. Switching of Polymorph-Dependent ESIPT Luminescence of an Imidazo[1,2-a]pyridine Derivative. Angewandte Chemie International Edition. 2008;47:9522–9524. doi: 10.1002/anie.200803975. [DOI] [PubMed] [Google Scholar]
- 62.Stasyuk AJ, Bultinck P, Gryko DT, Cyrański MK. The effect of hydrogen bond strength on emission properties in 2-(2′-hydroxyphenyl)imidazo[1,2-a]pyridines. J. Photochem. Photobiol. A: Chem. 2016;314:198–213. doi: 10.1016/j.jphotochem.2015.08.013. [DOI] [Google Scholar]
- 63.Liu S, et al. Organopromoted direct synthesis of 6-iodo-3-methylthioimidazo[1,2-a]pyridines via convergent integration of three self-sorting domino sequences. Organic & Biomolecular Chemistry. 2015;13:8807–8811. doi: 10.1039/C5OB01313E. [DOI] [PubMed] [Google Scholar]
- 64.Nair DK, Mobin SM, Namboothiri INN. Synthesis of Imidazopyridines from the Morita–Baylis–Hillman Acetates of Nitroalkenes and Convenient Access to Alpidem and Zolpidem. Organic Letters. 2012;14:4580–4583. doi: 10.1021/ol3020418. [DOI] [PubMed] [Google Scholar]
- 65.Seferoğlu Z, Ihmels H, Şahin E. Synthesis and photophysical properties of fluorescent arylstyrylimidazo[1,2-a]pyridine-based donor-acceptor chromophores. Dyes and Pigments. 2015;113:465–473. doi: 10.1016/j.dyepig.2014.09.016. [DOI] [Google Scholar]
- 66.Xiao X, et al. Transition-Metal-Free Tandem Chlorocyclization of Amines with Carboxylic Acids: Access to Chloroimidazo[1,2-α]pyridines. Organic Letters. 2015;17:3998–4001. doi: 10.1021/acs.orglett.5b01868. [DOI] [PubMed] [Google Scholar]
- 67.Aslanov LA, et al. Relationship between the structures of the molecules of indolizine and azaindolizines and the ability of these molecules to undergo rearrangement. J. Struct. Chem. 1983;24:427–434. doi: 10.1007/bf00747804. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallographic data for 2(a, b, c and e-o) were deposited with the Cambridge Crystallographic Data Centre as supplementary publications with CCDC no. 1055984-1055988 and 1541130-1541138, respectively. Copies of available materials can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK, (Fax: + 44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).