Abstract
Background
Systemic inflammation is pivotal in the pathogenesis of cardiovascular disease. As inflammation can directly cause cardiomyocyte injury, we hypothesised that established systemic inflammation, as reflected by elevated preoperative neutrophil-lymphocyte ratio (NLR) >4, predisposes patients to perioperative myocardial injury.
Methods
We prospectively recruited 1652 patients aged ≥45 yr who underwent non-cardiac surgery in two UK centres. Serum high sensitivity troponin T (hsTnT) concentrations were measured on the first three postoperative days. Clinicians and investigators were blinded to the troponin results. The primary outcome was perioperative myocardial injury, defined as hsTnT≥14 ng L−1 within 3 days after surgery. We assessed whether myocardial injury was associated with preoperative NLR>4, activated reactive oxygen species (ROS) generation in circulating monocytes, or both. Multivariable logistic regression analysis explored associations between age, sex, NLR, Revised Cardiac Risk Index, individual leukocyte subsets, and myocardial injury. Flow cytometric quantification of ROS was done in 21 patients. Data are presented as n (%) or odds ratio (OR) with 95% confidence intervals.
Results
Preoperative NLR>4 was present in 239/1652 (14.5%) patients. Myocardial injury occurred in 405/1652 (24.5%) patients and was more common in patients with preoperative NLR>4 [OR: 2.56 (1.92–3.41); P<0.0001]. Myocardial injury was independently associated with lower absolute preoperative lymphocyte count [OR 1.80 (1.50–2.17); P<0.0001] and higher absolute preoperative monocyte count [OR 1.93 (1.12–3.30); P=0.017]. Monocyte ROS generation correlated with NLR (r=0.47; P=0.03).
Conclusions
Preoperative NLR>4 is associated with perioperative myocardial injury, independent of conventional risk factors. Systemic inflammation may contribute to the development of perioperative myocardial injury.
Clinical trial registration
Keywords: inflammation, leukocyte count, cardiac risk, surgery, troponin
Editor's key points.
-
•
Pathophysiological mechanisms other than ischaemia or thrombosis may contribute to perioperative myocardial injury.
-
•
In this study, perioperative myocardial injury was associated with systemic inflammation.
-
•
Relative lymphopaenia and higher concentrations of circulating monocytes may play a mechanistic role.
Asymptomatic myocardial injury occurs in more than 30% of patients undergoing non-cardiac surgery.1, 2 Even apparently minor elevations in troponin are associated with prolonged hospitalisation and higher mortality. The extent and severity of coronary artery disease, however, does not correlate closely with the occurrence of perioperative myocardial injury.3 Furthermore, pharmacological interventions that are effective in acute coronary syndromes do not prevent myocardial infarction after surgery,4, 5, 6 although longer-term adverse cardiac outcomes may be improved by intensification of medical management for acute coronary syndrome.7, 8 These data suggest that pathophysiological mechanisms other than ischaemia, thrombosis, or both, may contribute to perioperative myocardial injury.
Systemic inflammation plays a major role in the development of cardiovascular disease.9, 10, 11 Inhibition of interleukin (IL)-1β, a cytokine that is pivotal to pro-inflammatory pathways, reduces cardiovascular events in patients with established cardiac disease.12 Mediators of systemic inflammation directly injure cardiomyocytes,13 modulate their response to damage,14, 15 or both. The systemic inflammatory response is an important contributor to myocardial injury, and to damage of other organs, after myocardial infarction and cardiac surgery. The extent to which systemic inflammation is involved in the pathogenesis of myocardial injury after non-cardiac surgery is not known.
The neutrophil-lymphocyte ratio (NLR) is a readily available and inexpensive marker of systemic inflammation driven by elevated concentrations of circulating cytokines which have been shown to modulate myocardial injury.16, 17 NLR enhances the Framingham-based risk prediction of cardiovascular mortality,18 with higher NLR associated with adverse outcomes after acute coronary syndromes19 and decompensated heart failure.20 NLR is also associated with worse perioperative outcomes,21 albeit for ill-defined, mechanistically unclear reasons. Other leukocyte subsets, including higher monocyte counts,22 and lower eosinophil counts,23 may also contribute to the development of cardiovascular morbidity.
Here, we hypothesised that the development of perioperative myocardial injury may be modified by mechanisms invoked by systemic inflammation. We assessed prospectively whether systemic inflammation, as reflected by elevated preoperative NLR, leukocyte subsets, or both, implicated in the pathogenesis of cardiomyocyte dysfunction, may promote perioperative myocardial injury. As a further test of this hypothesis, we assessed whether circulating monocytes, which are pivotal for the repair of myocardial injury, generated more injurious reactive oxygen species (ROS) when activated ex vivo in patients with higher NLR.
Methods
We report the results of a prospectively designed substudy from two UK centres which captured leukocyte data where investigators, patients, and healthcare providers were blinded to troponin results throughout the study period. The study was approved by UK National Research Ethics Committee London (MREC:10/WNo03/25). It was conducted in accordance with the principles of the declaration of Helsinki and institutional guidelines. Participants undergoing elective non-cardiac surgery provided written informed consent before surgery.
Participants
Participants were aged 45 yr or older and underwent non-cardiac surgery under general or regional anaesthesia at The Royal London Hospital (Barts Health NHS Trust) or at University College Hospital (University College London Hospitals NHS Foundation Trust) who required at least 1 night in hospital after surgery. Participants were excluded if they declined consent or if they had previously enrolled in the VISION study.1
Data collection
Researchers collected a detailed and standardised dataset from patients and their medical records, before and during the 30 days after surgery, including postoperative morbidity (defined by PostOperative Morbidity Survey and Clavien–Dindo grading). Full definitions of the variables included in this analysis are documented in the Supplementary material. Blood samples were obtained before, between 6 and 12 h after the end of surgery, and on postoperative Days 1, 2, and 3.
Exposures
Leukocyte subsets were measured before operation by observers masked to troponin results (Sysmex XE2100 analyser, Sysmex, Milton Keynes, UK). NLR>4 was defined prospectively as an established median threshold value associated with subclinical inflammation and adverse clinical outcomes derived from studies totalling 40 559 patients.24 Moreover, a population-based study reported mean NLR 1.76 [95% confidence intervals (CI): 0.83–3.92] in >8500 participants.25 We assessed the contributions of specific leukocyte subsets to perioperative myocardial injury using absolute counts and thresholds associated with cardiovascular risk in the general cardiovascular literature. Lymphopaenia was defined as <1×109 lymphocytes L−1.26 The following thresholds for absolute subset counts were used: neutrophil count ≥6×109 cells L−1,22 eosinophil count <0.05 cells 109 L−1,23 and monocyte count ≥0.7×109 cells L−1.22
Primary outcome
The primary outcome measure was perioperative myocardial injury defined as serum high sensitivity troponin T (hsTnT) concentration ≥14 ng L−1, measured by the hsTnT assay (Elecsys, Roche, Basel, Switzerland) within 3 days after surgery. This hsTnT assay enables the detection of cardiac troponin T at the 99th percentile of an apparently healthy reference population with <10% variability, with a 5 ng L−1 limit of detection.27 We did not seek to define ischaemic vs non-ischaemic causes of hsTnT elevation, as elevated troponin is linked to poorer clinical outcomes regardless of its aetiology.28 The secondary clinical outcome was length of hospital stay, stratified by NLR</>4, hsTnT≥14 ng L−1, or both.
Preoperative monocyte ROS generation
Preoperative monocyte ROS generation was measured by flow cytometry in a subset of 21 patients. Investigators undertaking flow cytometry were masked to NLR values. Monocytes were identified by forward/side scatter characteristics and CD14+ CD16− surface staining (Supplementary Fig. S1). Using fresh whole blood samples obtained before operation, loaded with dihydrorhodamine (DHR)-123 (10 μm), monocyte ROS were quantified after dimethyl sulfoxide (control) or phorbol 12-myristate 13-acetate (PMA) incubation for 10 min at 37°C in a CO2 incubator (Phagoburst, Orpegen, Heidelberg, Germany).29 The ROS-reactive dye DHR is converted to cationic green fluorescent rhodamine-123 upon oxidation by PMA, trapping it intracellularly. Median fluorescence intensity was quantified by flow cytometry (CyAn ADP flow cytometer, Beckman Coulter, Wycombe, UK). PMA-induced ROS was expressed as fold-change over each individual's unstimulated (time-matched control) sample.
Statistical analysis
The statistical analysis was prospectively planned and registered on a public database (Research Registry:3927). We used NCSS 11 (Kaysville, UT, USA) and STATA version 14 (StataCorp LP, College Station, TX, USA) to analyse the data. We ordered the sample according to integer values of NLR</>4 and stratified the baseline characteristics of the cohort according to this threshold. Binary data were expressed as percentages, normally distributed continuous data as mean with standard deviation, and non-normally distributed continuous data as median with inter-quartile range. We used multivariable logistic regression analysis to test for associations between leukocyte subsets (absolute counts) and perioperative myocardial injury, taking into account established conventional risk factors [age, sex, and the Revised Cardiac Risk Index (RCRI)].30, 31, 32, 33 The selection of covariates was based on prior evidence of association with the dependent variable or similar clinical outcomes, rather than using univariable analysis or P-value based approaches.34, 35 Covariates were treated as categorical variables. Missing data were handled by list-wise deletion. Fold-change in monocyte ROS (stimulation/basal ROS concentrations) was correlated with NLR using simple linear regression.
Sample size estimation
Our previous work in four UK centres showed that preoperative NLR>4 is present in approximately 20% of patients undergoing non-cardiac surgery.36 Observations from the main VISION study, suggest that ∼25% of patients develop hsTnT≥4 ng L−1 within 72 h after surgery.1 We therefore estimated that at least 1554 patients would be required to detect a 10% absolute difference in incidence of hsTnT≥14 ng L−1 within 72 h after surgery between patients with and without preoperative NLR>4 (α=0.05; 1-β=0.9; estimated dropout rate of ∼7%). For the monocyte ROS experiment, we estimated that a total sample size ≥19 subjects would be required, assuming a correlation coefficient r=0.6 (α=0.05; 1-β=0.8).
Results
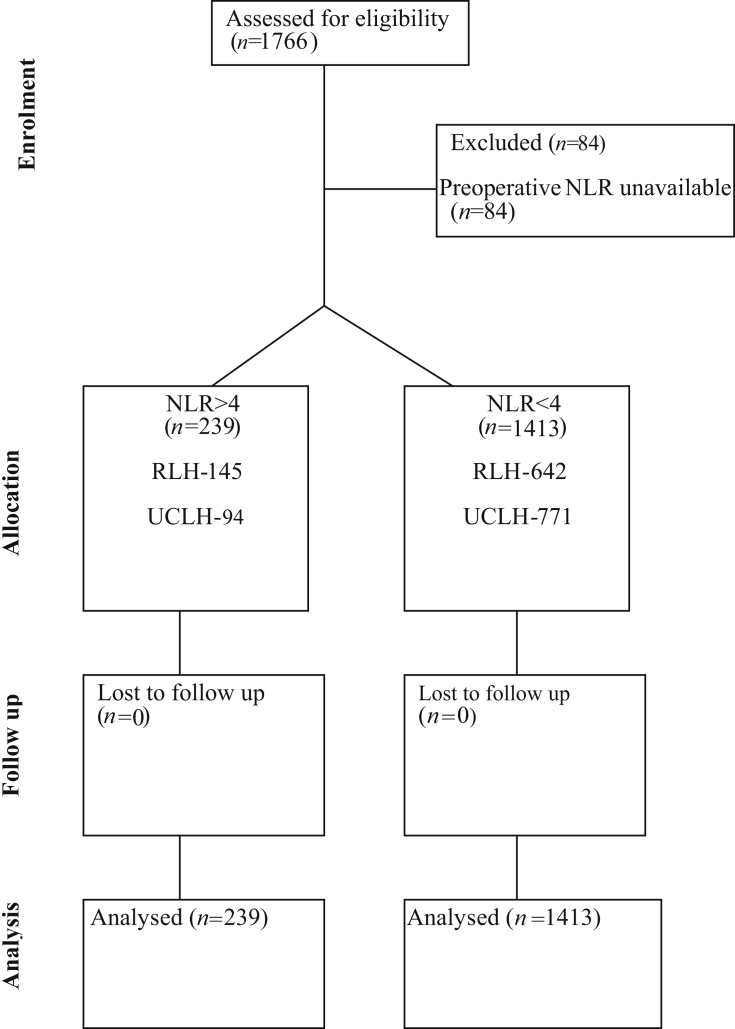
The study included 1682 patients, who were recruited between March 2011 and May 2014 (Figure 1). Preoperative NLR>4 was present in 239 (14.2%) patients (Supplementary Fig. S2). An RCRI score >2 was more common in 52/239 (21.8%) patients with NLR>4 [OR 2.36 (95% CI: 1.66–3.36); P<0.001], but patient age, sex, and other perioperative and preoperative cardiovascular parameters were similar (Table 1). Measures of frailty, cardiovascular medication, or surgical subspecialty were similar as stratified by NLR>4 (Supplementary Figs S3–5).
Fig 1.
Patient flow diagram showing cases included in the primary analysis. NLR, neutrophil-lymphocyte ratio; RLH, Royal London Hospital; UCLH, University College London Hospitals.
Table 1.
Patient characteristics. Descriptive data stratified by neutrophil-lymphocyte ratio (NLR) </>4, presented as numbers with percentages (%) or means with standard deviations (SD). Age is rounded to nearest whole number. eGFR, estimated glomerular filtration rate; RCRI, Revised Cardiac Risk Index. *Preoperative C-reactive protein available in 241 patients
| Characteristic | NLR>4 | NLR<4 | P-value |
|---|---|---|---|
| No. of patients | 239 | 1413 | |
| Age (yr) | 67 (58–76) | 66 (57–74) | 0.03 |
| Female sex, n (%) | 98 (41.0) | 715 (50.6) | 0.005 |
| BMI (SD) | 30 (7) | 28 (7) | 0.02 |
| Systolic BP (mm Hg) | 138 (21) | 135 (21) | 0.09 |
| Diastolic BP (mm Hg) | 75 (12) | 73 (13) | 0.004 |
| HR (beats min−1) | 76 (13) | 77 (13) | 0.36 |
| RCRI≥2, n (%) | 52 (21.8) | 185 (13.1) | <0.001 |
| Estimated GFR (ml min−1) | 81 (21) | 79 (33) | 0.23 |
| Haemoglobin (g L−1) | 123 (21) | 134 (16) | <0.001 |
| White cell count (cells 109 L−1) | 9.6 (4.3) | 7.1 (2.1) | <0.001 |
| Neutrophil count (cells 109 L−1) | 7.5 (3.9) | 4.2 (1.5) | <0.001 |
| Lymphocyte count (cells 109 L−1) | 1.2 (0.5) | 2.1 (0.8) | <0.001 |
| Monocyte count (cells 109 L−1) | 0.68 (0.34) | 0.56 (0.23) | <0.001 |
| Basophil count (cells 109 L−1) | 0.02 (0.03) | 0.04 (0.1) | <0.001 |
| Eosinophil count (cells 109 L−1) | 0.14 (0.15) | 0.2 (0.19) | <0.001 |
| Platelets (cells 109 L−1) | 275 (118) | 255 (75) | 0.004 |
| C-reactive protein* (mg L−1) | 16 (4–72) | 3 (1–14) | <0.001 |
| Albumin (g dl−1) | 41 (6) | 45 (4) | <0.001 |
| Moderate- or high-risk surgery, n (%) | 214 (89.5) | 1289 (91.2%) | |
| Hepatobiliary | 7 (2.9) | 75 (5.3) | |
| Gastrointestinal | 31 (13.0) | 241 (17.1%) | |
| Vascular | 22 (9.2) | 59 (4.2) | |
| Urology | 38 (15.9) | 246 (17.4%) | |
| Neurosurgery | 16 (6.7) | 85 (6.0) | |
| Gynaecological | 20 (8.4) | 129 (9.1%) | |
| Head/neck/ear, nose, throat | 12 (5.0) | 54 (3.8) | |
| Orthopaedic | 71 (29.7) | 440 (31.1) | |
| Duration of surgery (min) | 135 (96–204) | 130 (90–195) | 0.47 |
| Allogeneic blood products, n (%) | 14 (5.8) | 53 (3.8) | 0.15 |
| Postoperative critical care admission | |||
| Level 2, n (%) | 39 (16.3) | 239 (16.9) | 0.52 |
| Level 3, n (%) | 15 (6.2) | 57 (4.0) |
Primary outcome: myocardial injury and NLR
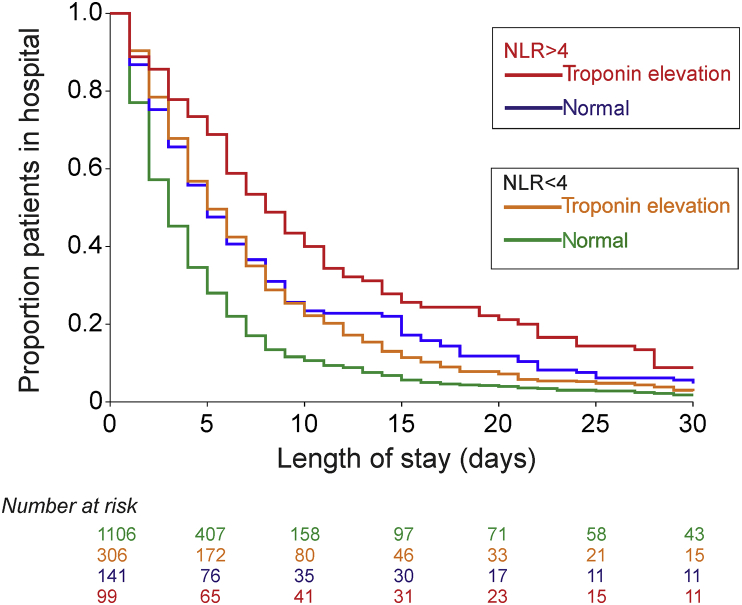
Perioperative myocardial injury was sustained in 405/1652 (24.5%) patients. Signs, symptoms, or both, of myocardial ischaemia were reported in 2/405 (0.49%) patients. Median troponin increase was 18 (14–26) ng L−1 in patients with troponin ≥14 ng L−1 within 72 h after surgery. No patients required coronary angiography during the same hospital stay. Perioperative myocardial injury was more frequent in 99/239 (41.4%) patients with preoperative NLR>4, compared with 306/1413 (21.7%) patients with NLR≤4 [OR 2.56 (95% CI: 1.92–3.41); P<0.0001]. Adjusting for RCRI, patient age, and sex, NLR was independently associated with perioperative myocardial injury either when dichotomised by NLR</>4 [OR 2.27 (95% CI: 1.67–3.09); P<0.001] or considered as a continuous variable [OR 1.06 (95% CI: 1.02–1.10); P<0.001] (Supplementary Tables S1–3). The combination of high preoperative NLR and myocardial injury was associated with prolonged length of stay [hazard ratio 1.45 (95% CI: 1.17–1.80); P<0.001; Figure 2]. This relationship was similar across surgical subspecialties (Supplementary Fig. S6).
Fig 2.
Length of hospital stay in relation to troponin events and neutrophil-lymphocyte ratio (NLR). Kaplan–Meier plot showing time to hospital discharge, stratified by patients' preoperative NLR, development of raised troponin, or both. Numbers at risk for each category are matched to coloured lines shown in graph panel.
Leukocyte subsets and myocardial injury
Using thresholds described in the wider cardiovascular literature, we found that myocardial injury was more frequent among 45/105 (42.6%) patients with preoperative lymphopaenia, compared with 345/1549 (22.3%) patients with a lymphocyte count >1×109 cells L−1 [OR 2.62 (95% CI: 1.75–3.92); P<0.0001]. Similarly, a higher proportion of patients with an absolute neutrophil count >6×109 cells L−1 (88/294; 29.9%) sustained myocardial injury [OR 1.5 (95% CI: 1.14–1.99); P<0.0001). For monocytes, myocardial injury was more frequent in 129/440 (29.3%) patients with a monocyte count ≥0.7×109 cells L−1,22 compared with 261/1213 (21.5%) patients with a monocyte count <0.7×109 cells L−1 [OR 1.51 (95% CI: 1.18–1.94); P<0.0001]. No relationship was found between previously described thresholds for eosinophil count (<0.05 cells 109 L−1) and myocardial injury [OR 0.99 (95% CI: 0.69–1.43]; P=0.55].
Multivariable logistic regression analysis of leukocyte subsets and myocardial injury
Adjusting for RCRI, age, and sex (Table 2), elevated postoperative troponin was independently associated with lower preoperative absolute lymphocyte count [OR 1.80 (95% CI: 1.50–2.17); P<0.0001] and higher preoperative absolute monocyte count [OR 1.93 (1.12–3.30); P=0.017].
Table 2.
Multivariable logistic regression model of leukocyte subsets associated with myocardial injury after non-cardiac surgery. Dependent variable is myocardial injury within the first 3 days of surgery. Lower absolute lymphocyte and higher absolute monocyte counts were independently associated with myocardial injury. Absolute leukocyte subset counts were treated as continuous variables. Results presented as odds ratios with 95% confidence intervals (CI). RCRI, Revised Cardiac Risk Index
| Odds ratio (95% CI) | P-value | |
|---|---|---|
| Age | 1.03 (1.02–1.04) | <0.0001 |
| Male sex | 1.08 (0.84–1.38) | 0.553 |
| RCRI (compared with zero score) | ||
| 1 | 1.64 (1.27–2.12) | <0.0001 |
| 2 | 3.55 (2.28–5.53) | <0.0001 |
| ≥3 | 6.29 (2.47–16.05) | <0.0001 |
| Neutrophils | 1.04 (0.98–1.10) | 0.202 |
| Lymphocytes | 0.56 (0.46–0.67) | <0.0001 |
| Monocytes | 1.93 (1.12–3.30) | 0.017 |
| Eosinophils | 1.58 (0.70–3.58) | 0.274 |
| Basophils | 3.81 (0.13–109.80) | 0.436 |
Monocyte ROS generation in relation to preoperative NLR
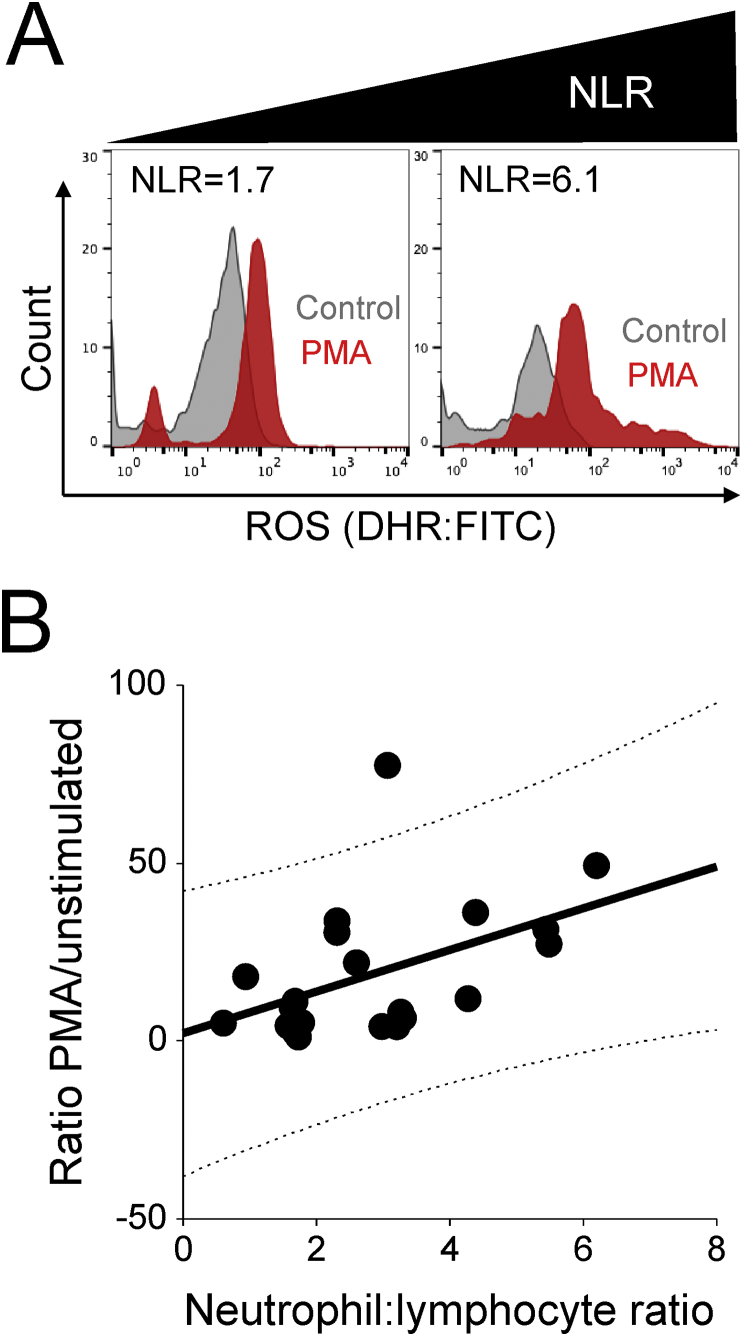
Clinical characteristics for these patients are shown in Supplementary Table S4. Higher preoperative NLR was significantly associated with more ROS release from activated monocytes (n=21 patients; r=0.47; P=0.03; Figure 3).
Fig 3.
Neutrophil-lymphocyte ratio and monocyte reactive oxygen species generation. (A) Histograms showing monocyte intracellular reactive oxygen species before (grey) and after (red) whole blood samples obtained before operation from 21 patients were stimulated with phorbol 12-myristate 13-acetate (PMA). Monocyte responses are shown from patients with high and low preoperative neutrophil-lymphocyte ratio (NLR). For clarity, unstained samples are not shown. (B) Correlation between preoperative NLR and fold-change (over basal values) in monocyte reactive oxygen species (ROS) after PMA stimulation. DHR, dihydrorhodamine; FITC, fluorescein isothiocyanate.
Discussion
The principal finding of this prospective observational cohort study involving more than 1600 elective patients undergoing non-cardiac surgery showed that elevated preoperative NLR, which reflects established systemic inflammation, was associated with increased risk of myocardial injury as measured by a high-sensitivity, cardiac-specific troponin assay. Our data implicate specific leukocyte subsets, suggesting that relative lymphopaenia and higher concentrations of circulating monocytes may play a mechanistic role in the development of perioperative myocardial injury. Monocytes, when obtained from patients with higher NLR, generate more injurious ROS when activated ex vivo. Taken together, these data suggest that a common, readily attainable leukocyte-based biomarker indicates that dysregulated inflammation may contribute to perioperative troponin increases.
These data reflect the findings of several studies exploring NLR in the cardiovascular literature, although these have primarily focussed on longer term outcomes rather than short-term risk of cardiac injury.37 Our data provide further mechanistic insight, as preoperative NLR appears to be associated with a potentially more injurious monocyte phenotype. Murine models of myocardial infarction show that monocytes produced in the bone marrow and spleen are recruited to the injured myocardium in two distinct phases.38 Within 24 h after myocardial infarction, angiotensin II mediates the release of monocytes from the spleen. The first phase involves B lymphocyte coordinated recruitment of monocytes; depletion of B-cells reduces monocyte infiltration and infarct size.39 At least in mice, this first phase is characterised by Ly-6chigh monocytes which give rise to inflammatory macrophages that clear damaged tissue by phagocytosis and secretion of proteolytic enzymes. This is followed by the infiltration of Ly-6clow macrophages that promote wound healing, angiogenesis and myofibroblast differentiation. Mobilisation of circulating monocytes in patients at higher risk of perioperative myocardial injury, that are capable of releasing more injurious ROS, may contribute to more extensive myocardial tissue damage.
In contrast, relative higher lymphocyte counts were associated with less myocardial injury. These findings are consistent with previous prospective work we have undertaken showing that lymphopaenia is associated with excess cardiovascular morbidity after elective orthopaedic surgery.26 Genetic deficiency, or depletion of CD4+ T-cells impairs wound healing in murine models of myocardial infarction.40 CD4+ T-cells modulate monocyte infiltration; their absence increases left ventricular dilatation and mortality after murine myocardial infarction.41 We have previously shown that lymphopaenia in perioperative patients is characterised by lower T- and B-cell populations,26 so the relationship between protective T-cell and deleterious B-cell phenotypes requires further elucidation. However, reduced NLR is also associated with poor cardiorespiratory reserve,36 which may also contribute to perioperative myocardial injury.42
A strength of our large, prospective, generalisable study is the blinded measurement of hsTnT, an objective biomarker linked to adverse outcome.1 The exploration of widely available leukocyte measurements adds further generalisability. As causality cannot be inferred from observational data, we are unable to discount that intraoperative management and drug therapy may play an important role in mitigating or exacerbating the influence of chronically elevated systemic inflammation. While the aim of this study was to identify a broadly defined inflammatory phenotype linked to myocardial injury, a further limitation is the lack of additional, integrative functional immune data. Although numbers of circulating leukocytes may correlate with function, they do not necessarily reflect their phenotype at sites of tissue injury. We cannot discount that frailty contributes to poorer outcome, given that in elderly cancer patients, frailty is positively correlated with higher NLR.43 Similarly, other unmeasured confounders may influence these data. Further investigations are required to establish how a number of inflammatory mechanisms reflected by higher NLR may be involved in perioperative myocardial injury. We have previously shown that non-classical monocyte subsets, which produce the highest concentrations of inflammatory cytokines in response to toll-like receptor ligands,44 are more prevalent in patients with higher perioperative risk.45 Upregulation of endothelial adhesion molecules, chemokines, cytokines, or all three, may promote arrhythmias.46 Excess inflammation may destabilise atherosclerotic plaques47 or exacerbate microvascular pathophysiology.48
In summary, these data provide support for an alternative hypothesis that may, in part, explain the high incidence of perioperative myocardial injury being related to chronically elevated systemic inflammation.
Authors' contributions
Designed the analysis plan: GLA.
Performed the data analysis independently: GLA, TEFA.
Drafted the manuscript: GLA, TEFA, RMP.
Revised the manuscript after critical review: all authors.
Declarations of interest
RP holds research grants, and has given lectures, performed consultancy work, or both, for Nestle Health Sciences, BBraun, Medtronic, GlaxoSmithKline, Intersurgical, and Edwards Lifesciences, and is a member of the associate editorial board of the British Journal of Anaesthesia. PD has received other funding from Roche Diagnostics and Abbott Diagnostics for investigator-initiated studies. GLA is a member of the editorial advisory board for Intensive Care Medicine Experimental, editor for British Journal of Anaesthesia, and has undertaken consultancy work for GlaxoSmithKline. There are no other relationships or activities that could appear to have influenced the submitted work.
Funding
Medical Research Council and British Journal of Anaesthesia clinical research training fellowship (grant reference MR/M017974/1) to TEFA; UK National Institute for Health Research Professorship to RP; British Journal of Anaesthesia/Royal College of Anaesthetists basic science Career Development award, British Oxygen Company research chair grant in anaesthesia from the Royal College of Anaesthetists, and British Heart Foundation Programme Grant (RG/14/4/30736) to GLA.
Editorial decision: 3 September 2018
Handling editor: P.S. Myles
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2018.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Writing Committee for the V.S.I., Devereaux P.J., Biccard B.M. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317:1642–1651. doi: 10.1001/jama.2017.4360. [DOI] [PubMed] [Google Scholar]
- 2.Puelacher C., Lurati Buse G., Seeberger D. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137:1221–1232. doi: 10.1161/CIRCULATIONAHA.117.030114. [DOI] [PubMed] [Google Scholar]
- 3.Sheth T., Chan M., Butler C. Prognostic capabilities of coronary computed tomographic angiography before non-cardiac surgery: prospective cohort study. BMJ. 2015;350:h1907. doi: 10.1136/bmj.h1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereaux P.J., Mrkobrada M., Sessler D.I. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 5.Devereaux P.J., Sessler D.I., Leslie K. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–1513. doi: 10.1056/NEJMoa1401106. [DOI] [PubMed] [Google Scholar]
- 6.Devereaux P.J., Yang H., Yusuf S. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 7.Foucrier A., Rodseth R., Aissaoui M. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014;119:1053–1063. doi: 10.1213/ANE.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 8.Devereaux P.J., Duceppe E., Guyatt G. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391:2325–2334. doi: 10.1016/S0140-6736(18)30832-8. [DOI] [PubMed] [Google Scholar]
- 9.Weber C., Zernecke A., Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 10.Hansson G.K., Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 11.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 12.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 13.Tavener S.A., Long E.M., Robbins S.M., McRae K.M., Van Remmen H., Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res. 2004;95:700–707. doi: 10.1161/01.RES.0000144175.70140.8c. [DOI] [PubMed] [Google Scholar]
- 14.Xiao H., Li H., Wang J.J. IL-18 cleavage triggers cardiac inflammation and fibrosis upon beta-adrenergic insult. Eur Heart J. 2018;39:60–69. doi: 10.1093/eurheartj/ehx261. [DOI] [PubMed] [Google Scholar]
- 15.Oyama J., Blais C., Jr., Liu X. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 16.Kantola T., Klintrup K., Vayrynen J.P. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729–1736. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z.Y., Raghav K., Lieu C.H. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112:1088–1097. doi: 10.1038/bjc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah N., Parikh V., Patel N. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: insights from the National Health and Nutrition Examination Survey-III. Int J Cardiol. 2014;171:390–397. doi: 10.1016/j.ijcard.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Tamhane U.U., Aneja S., Montgomery D., Rogers E.K., Eagle K.A., Gurm H.S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Uthamalingam S., Patvardhan E.A., Subramanian S. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–438. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Forget P, Machiels JP, Coulie PG Neutrophil:lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol. 2013;20(Suppl 3):S650–S660. doi: 10.1245/s10434-013-3136-x. [DOI] [PubMed] [Google Scholar]
- 22.Shah A.D., Denaxas S., Nicholas O., Hingorani A.D., Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: a CALIBER cohort study. J Am Coll Cardiol. 2017;69:1160–1169. doi: 10.1016/j.jacc.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah A.D., Denaxas S., Nicholas O., Hingorani A.D., Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart. 2016;3:e000477. doi: 10.1136/openhrt-2016-000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Templeton A.J., McNamara M.G., Seruga B. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 25.Fest J., Ruiter R., Ikram M.A., Voortman T., van Eijck C.H.J., Stricker B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8:10566. doi: 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards M.R., Sultan P., del Arroyo A.G. Metabolic dysfunction in lymphocytes promotes postoperative morbidity. Clin Sci (Lond) 2015;129:423–437. doi: 10.1042/CS20150024. [DOI] [PubMed] [Google Scholar]
- 27.Giannitsis E., Kurz K., Hallermayer K., Jarausch J., Jaffe A.S., Katus H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 28.Gualandro D.M., Puelacher C., Mueller C. High-sensitivity cardiac troponin in acute conditions. Curr Opin Crit Care. 2014;20:472–477. doi: 10.1097/MCC.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 29.Hirt W., Nebe T., Birr C. [Phagotest and Bursttest (Phagoburst), test kits for study of phagocyte functions] Wien Klin Wochenschr. 1994;106:250–252. [PubMed] [Google Scholar]
- 30.Vascular Events in Noncardiac Surgery Patients Cohort Evaluation Study Investigators, Devereaux P.J., Chan M.T. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 31.Botto F., Alonso-Coello P., Chan M.T. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–578. doi: 10.1097/ALN.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 32.Hawn M.T., Graham L.A., Richman J.S., Itani K.M., Henderson W.G., Maddox T.M. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–1472. doi: 10.1001/jama.2013.278787. [DOI] [PubMed] [Google Scholar]
- 33.Lee T.H., Marcantonio E.R., Mangione C.M. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 34.Abbott T.E.F., Pearse R.M., Archbold R.A. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION Study. Anesth Analg. 2018;126:1936–1945. doi: 10.1213/ANE.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott T.E.F., Pearse R.M., Archbold R.A. Association between preoperative pulse pressure and perioperative myocardial injury: an international observational cohort study of patients undergoing non-cardiac surgery. Br J Anaesth. 2017;119:78–86. doi: 10.1093/bja/aex165. [DOI] [PubMed] [Google Scholar]
- 36.Ackland G.L., Minto G., Clark M. Autonomic regulation of systemic inflammation in humans: a multi-center, blinded observational cohort study. Brain Behav Immun. 2018;67:47–53. doi: 10.1016/j.bbi.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Afari M.E., Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–577. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 38.Honold L., Nahrendorf M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res. 2018;122:113–127. doi: 10.1161/CIRCRESAHA.117.311071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zouggari Y., Ait-Oufella H., Bonnin P. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann U., Frantz S. Role of T-cells in myocardial infarction. Eur Heart J. 2016;37:873–879. doi: 10.1093/eurheartj/ehv639. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann U., Beyersdorf N., Weirather J. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 42.Abbott T.E.F., Minto G., Lee A.M. Elevated preoperative heart rate is associated with cardiopulmonary and autonomic impairment in high-risk surgical patients. Br J Anaesth. 2017;119:87–94. doi: 10.1093/bja/aex164. [DOI] [PubMed] [Google Scholar]
- 43.Nishijima T.F., Deal A.M., Williams G.R., Guerard E.J., Nyrop K.A., Muss H.B. Frailty and inflammatory markers in older adults with cancer. Aging (Albany NY) 2017;9:650–664. doi: 10.18632/aging.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong K.L., Tai J.J., Wong W.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 45.Sultan P., Edwards M.R., Del Arroyo A.G. Preoperative systemic inflammation and cardiopulmonary exercise capacity. Mediators Inflamm. 2014;2014:727451. doi: 10.1155/2014/727451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S., Frangogiannis N.G. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br J Pharmacol. 2018;175:1377–1400. doi: 10.1111/bph.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nilsson J. Atherosclerotic plaque vulnerability in the statin era. Eur Heart J. 2017;38:1638–1644. doi: 10.1093/eurheartj/ehx143. [DOI] [PubMed] [Google Scholar]
- 48.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.