Abstract
Macrophage is an important innate immune cell that not only initiates inflammatory responses, but also functions in tissue repair and anti-inflammatory responses. Regulating macrophage activity is thus critical to maintain immune homeostasis. Tyro3, Axl, and Mer are integral membrane proteins that constitute TAM family of receptor tyrosine kinases (RTKs). Growing evidence indicates that TAM family receptors play an important role in anti-inflammatory responses through modulating the function of macrophages. First, macrophages can recognize apoptotic bodies through interaction between TAM family receptors expressed on macrophages and their ligands attached to apoptotic bodies. Without TAM signaling, macrophages cannot clear up apoptotic cells, leading to broad inflammation due to over-activation of immune cells. Second, TAM signaling can prevent chronic activation of macrophages by attenuating inflammatory pathways through particular pattern recognition receptors and cytokine receptors. Third, TAM signaling can induce autophagy which is an important mechanism to inhibit NLRP3 inflammasome activation in macrophages. Fourth, TAM signaling can inhibit polarization of M1 macrophages. In this review, we will focus on mechanisms involved in how TAM family of RTKs can modulate function of macrophage associated with anti-inflammatory responses described above. We will also discuss several human diseases related to TAM signaling and potential therapeutic strategies of targeting TAM signaling.
Keywords: anti-inflammatory response, cell signaling, innate immunity, macrophage, TAM family of receptor tyrosine kinase
INTRODUCTION
Macrophage is a key component of innate immunity which orchestrates initial inflammation and immune homeostasis (Mosser and Edwards, 2008). Similar to the paradigm of helper T cells, macrophages are classified as M1 or M2 population depending on which stimulus they are first exposed to within a certain microenvironment (Mills et al., 2000). Specific cytokine or external antigen is a major factor that determines the fate of macrophages. Interferon (IFN)-γ and lipopolysaccharide (LPS) can induce M1 macrophage differentiation while interleukin (IL)-4, IL-10, IL-13, and chitin can induce M2 macrophage differentiation (Lawrence and Natoli, 2011).
M1 macrophages act on immediate defense against foreign antigens and are essential for initiating adaptive immune response (Mills and Ley, 2014). Proinflammatory cytokines secreted by M1 macrophages are major driven source of inflammatory responses (Mills and Ley, 2014). M2 macrophages can repair wounded tissues or help form extracellular matrix by secreting IL-10 or transforming growth factor (TGF)-β (Mills and Ley, 2014). Many human diseases are related to dominant M1 or M2 macrophage phenotype (Mills, 2012). Therefore, maintaining a balance between M1 and M2 macrophages is important for immune homeostasis.
Receptor tyrosine kinases (RTKs) are integral membrane proteins that transmit extracellular signal through phosphorylation of tyrosine residue within their cytoplasmic domains (Robinson et al., 2000). Among 20 subfamilies of RTKs, TAM receptor family consisting of Tyro3, Axl, and Mer is the main family that gives a pleiotropic anti-inflammatory response. Although TAM receptor signaling is essential for NK cell development (Caraux et al., 2006), it down-regulates activities of T cell, invariant NKT (i NKT) cell, NK cell, and dendritic cell (Behrens et al., 2003; Carrera et al., 2013; Paolino et al., 2014; Smiley et al., 1997). Supporting these observations, TAM deficient (Tyro3−/−Axl−/−Mer−/−) mice developed spontaneous autoimmune diseases due to chronic inflammatory responses (Lu and Lemke, 2001). Cancer metastasis in TAM deficient mice is also dampened due to the lack of inhibitory signal of NK cell activity (Paolino et al., 2014). Moreover, recent evidence has demonstrated that TAM receptor signaling gives anti-inflammatory responses through modulating macrophage activities (Rothlin et al., 2015).
In this review, we will focus on several mechanisms that describe how TAM receptor signaling inhibits inflammatory responses via manipulation of macrophage phenotype. We will also discuss clinical relevance of TAM receptor signaling in terms of developing new therapeutics against inflammatory diseases and cancer.
TAM FAMILY RECEPTORS AND THEIR LIGANDS
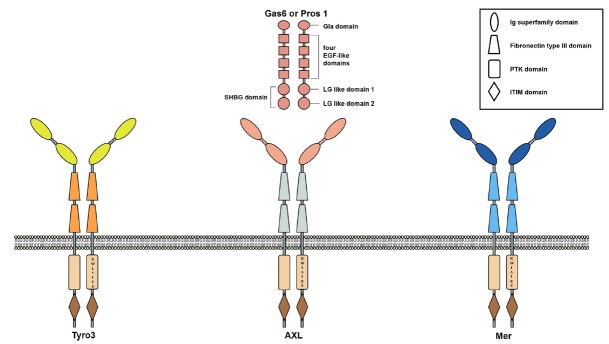
TAM family receptors share several unique signatures within their structures (Graham et al., 1994; Lai et al., 1994; O’Bryan et al., 1991)(Fig. 1). Two immunoglobulin (Ig) superfamily domains and two fibronectin type III domains are well conserved within extracellular domains of TAM family receptors (Graham et al., 1994; Lai et al., 1994; O’Bryan et al., 1991)(Fig. 1). In the cytoplasmic tail of each TAM family receptor, “KW(I/L)A(I/L)ES” signature sequences are well conserved (Graham et al., 1994; Lai et al., 1994; O’Bryan et al., 1991)(Fig. 1). Each TAM family receptor also contains intracellular region consisting of a conserved tyrosine kinase domain, autophosphorylation sites, and immunoreceptor tyrosine based inhibitory motif (ITIM) domain (Linger et al., 2011)(Fig. 1). Like other RTKs, TAM family receptor can form homodimer by autophosphorylation of tyrosine residues within the cytoplasmic tail and transfer signals after interacting with their ligands (Sasaki et al., 2006)(Fig. 1).
Fig. 1. Structures of TAM family receptors and their ligands.
Ig superfamily domains of each TAM receptor recognize their ligands. The extracellular domain of each TAM family receptor contains two Ig superfamily domains and two fibronectin type III domains. The cytoplasmic tail of each TAM family receptor contains well conserved “KW(I/L)A(I/L)ES” signature sequence. Also, the cytoplasmic tail of each TAM family receptor contains a conserved protein tyrosine kinase (PTK) domain and immunoreceptor tyrosine based inhibitory motif (ITIM) domain. Autophosphorylation sites of each TAM family receptor are located within PTK domain. Both Gas6 and Pros1 have γ-carboxyglutamate-rich domain (Gla domain) at their amino terminus. Also, both proteins contain four epidermal growth factor (EGF)-like domains and one sex hormone binding globulin (SHBG) domain which consists of two globular laminin G-like (LG) domain. Gas6 can bind to all three TAM family receptors with the highest affinity to Axl, whereas Pros1 can interact with Mer or Tyro3, but not Axl.
Growth arrest-specific gene 6 (Gas6) and Protein S (Pros1) are two well-known ligands for TAM receptors (Stitt et al., 1995). Although Gas6 and Pros1 share only 40% amino acid identities, they have several conserved domains (Fig. 1). For example, γ-carboxyglutamate-rich domain (Gla domain) is located at their amino terminus of both proteins (Nagata et al., 1996). Gas6 and Pros1 need vitamin-K dependent γ-carboxylation of glutamate for binding to phosphatidylserine on plasma membrane (Huang et al., 2003). Gas6 and Pros1 also contain four epidermal growth factor-like domains and one sex hormone binding globulin (SHBG) domain which consists of two globular laminin G-like (LG) domains (Hafixi and Dahlback, 2006)(Fig. 1). Based on Gas6 and Axl interaction model, each of two carboxyl terminus SHBG domains of TAM ligands can interact with each Ig superfamily domain of TAM receptor dimer (Sasaki et al., 2006)(Fig. 1).
Gas6 and Pros1 have different affinities to each member of TAM family receptors. Gas6 can deliver signal through all three TAM family receptors. It has the highest affinity to Axl. However, Pros1 can only transfer signal through Mer or Tyro3, not Axl (Nagata et al., 1996). Besides Gas6 and Pros1, Tubby, tubby-like protein 1 (Tulp-1), and Galectin-3 can act as ligands of TAM family receptors (Caberoy et al., 2010; 2012). The relevant physiological role of interactions between these ligands and TAM family receptors is not well characterized yet.
TAM family receptor is expressed on a broad range of cell types, including hematopoietic lineage cells such as macrophages, dendritic cells, NK cells, i NKT cells, and T cells, and non-hematopoietic cells such as endothelial cells, epithelial cells, neurons, and osteoclasts (Rothlin et al., 2015). However, TAM deficient (Tyro3−/−Axl−/−Mer−/−) mice are viable without severe developmental defects (Lu et al., 1999). The only obvious defect is spermatogenesis in TAM deficient mice (Lu et al., 1999). These results may reflect that the function of TAM family receptor is overlapped with other RTK proteins during development. Therefore, the function of TAM family receptor might not be essential for living, although it controls the homeostasis of particular biological events.
CLEARANCE OF APOPTOTIC BODIES BY TAM FAMILY RECEPTORS
Analysis of TAM deficient (Tyro3−/−Axl−/−Mer−/−) mice has given the first clue showing that TAM family receptors are involved in the clearance of apoptotic bodies because TAM deficient mice exhibited sperm abnormality due to inability to remove dead Sertoli cells in testis (Lu et al., 1999). Several other reports have also demonstrated that TAM family receptor-mediated phagocytosis is the major mechanism involved in the removal of apoptotic bodies. For example, Mer deficient (Mer−/−) macrophage cannot remove dead cells efficiently when Mer deficient (Mer−/−) thymocytes are challenged by apoptotic que (Scott et al., 2001). Mer is also involved in the clearance of retinal pigment epithelial cells and deletion of Mer in retina can lead to blindness (Prasad et al., 2006). A recent study has also suggested that microglia need TAM family receptors to regulate phagocytosis for adult neurogenesis or brain damage repair (Fourgeaud et al., 2016). Therefore, TAM family receptors contribute to the inhibition of inflammatory responses by initiating phagocytosis through the recognition of apoptotic cells (Lemke et al., 2010)(Fig. 2A). Otherwise, unremoved apoptotic cell debris often causes severe inflammation, similar to autoimmune disease-like symptoms (Lu and Lemke, 2001).
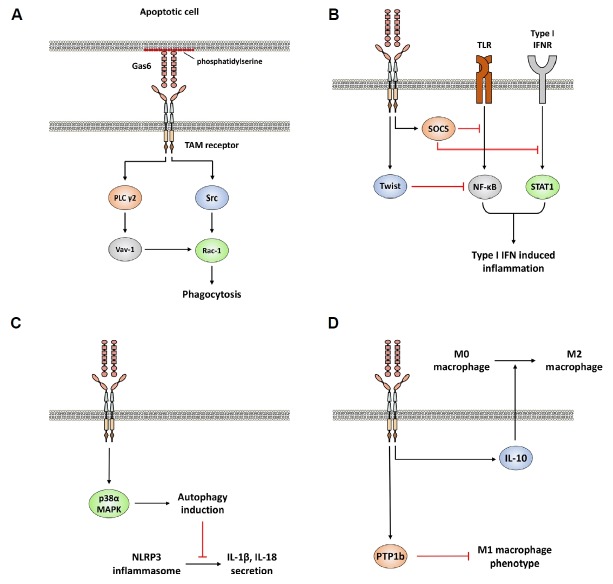
Fig. 2. Pleotropic inhibition of inflammatory responses by TAM family receptors expressed on macrophages.
(A) TAM receptor signaling enhances phagocytosis of apoptotic cells. (B) Attenuation of type I IFN-mediated inflammatory responses by TAM receptor signaling. (C) TAM receptor signaling suppresses NLRP3 inflammasome activation by autophage induction. (D) TAM receptor signaling inhibits M1 macrophage polarization and enhances M2 macrophage polarization by induction of IL-10.
TAM family receptor-mediated phagocytic activity in macrophages and dendric cells is only observed when they recognize apoptotic cells because the clearance of bacteria or synthetic particles by phagocytosis still occurs in macrophages and dendritic cells of TAM deficient mice (Scott et al., 2001). However, recent observation has demonstrated that TAM family receptor signaling can enhance the overall phagocytic activity indirectly by upregulating IL-10 (Segawa et al., 2014), a well-known inducer of phagocytosis (Jung et al., 2004; Lingnau et al., 2004).
Recently, the mechanism of how TAM family receptor recognizes apoptotic body has been identified. In the steady state, normal cells do not expose phosphatidylserine to the extracellular surface of plasma membrane (Segawa et al., 2014). However, Flippase which suppresses the exposure of phosphatidylserine to extracellular surface of plasma membrane becomes inactive in apoptotic cells (Segawa et al., 2014). Subsequently, exposed phosphatidylserine on the extracellular surface can bind to Gas6 or pros1 via Gla domain (Lew et al., 2014)(Fig. 2A). TAM family receptors expressed on macrophages or dendritic cells can then recognize their ligands and transduce signals to induce phagocytosis through activation of Rac1, one of GTP-binding proteins (Todt et al., 2004; Wu et al., 2005). Rac1 acts as a positive regulator of phagocytosis by inducing cytoskeletal rearrangement (Castellano et al., 2000). TAM family receptor signaling activates Rac1 through two different pathways: TAM-phospholipase C γ2 (PLC γ2)-Vav-1-Rac1 and TAM-Src family kinase-Rac1 axes (Todt et al., 2004; Wu et al., 2005) (Fig. 2A). The TAM-Src pathway also enhances αvβ5 integrin-mediated phagocytosis of apoptotic cells (Wu et al., 2005).
ATTENUATING INFLAMMATORY RESPONSES BY TAM FAMILY RECEPTORS
The first observation that demonstrated anti-inflammatory effects of TAM family receptors was reported in Mer deficient (Mer−/−) mice (Camenisch et al., 1999). Mer deficient mice are very susceptible to low dose of lipopolysaccharide injection (Camenisch et al., 1999). Supporting this observation, further study has revealed that Gas6/Mer signaling in macrophages can inhibit mRNA transcription of proinflammatory cytokines including IL-1β, IL-6, and TNF-α by blocking translocation of NFκB through phosphoinositide 3-kinase-protein kinase B (Akt) axis (Alciato et al., 2010).
Additionally, TAM family receptors on macrophages can attenuate severe inflammatory responses by inhibiting signal transduction of Toll-like receptor (TLR) and type I IFN (Rothlin et al., 2007). TLRs expressed on macrophages and dendritic cells can recognize various pattern associated molecular patterns (PAMPS) and initiate inflammatory response via TLR-mediated signals (Medzhitov et al., 2009). TLR-mediated mediated Type I IFN production is known to promote initial inflammatory responses because type I IFN signaling can initiate mRNA transcription of many genes involved in innate immunity, including proinflammatory cytokines (Takeuchi and Akira, 2010). TAM receptor signaling induce suppressor of cytokine signaling (SOCS) proteins in a Type I IFN receptor (INFAR) and signal transducer and activator of transcription 1 (STAT1)-dependent manner (Rothlin et al., 2007). These induced SOCS proteins can act as E3 ubiquitin ligases that target mediator proteins of TLR and type I IFN signaling (Rothlin et al., 2007). Ubiquitinated molecules then undergo degradation by proteasomal complex (Rothlin et al., 2007).
During type I IFN-mediated inflammation, TAM receptors expressed on macrophage can also suppress production of TNF-α, one of proinflammatory cytokines, by activating Twist transcriptional suppressors (Sharif et al., 2006). Twist proteins (Twist1 and Twist2) are transcriptional repressors of TNF-α because Twist proteins can bind to an E-box of TNF-α promoter and inhibit the activity of p65 subunit of NFκB (Sosic et al., 2003). Upon binding with its ligands, Axl signaling turns on mRNA transcription of Twist gene and induces Twist protein which inhibits TNF-α transcriptionally (Sharif et al., 2006). Combined together, these observations indicate that TAM receptor signaling is essential for anti-inflammatory role of type I IFNs and also inhibits type I IFN signaling (Rothlin et al., 2007; Sharif et al., 2006)(Fig. 2B). Interestingly, type I IFN signaling is required for the induction of TAM receptor and Gas6 expressions (Rothlin et al., 2007; Sharif et al., 2006). Type I IFN receptor deficient mice or STAT1 deficient mice did not induce mRNA transcripts of Axl in macrophages (Rothlin et al., 2007). Therefore, TAM receptor on macrophages seems to provide a negative feedback system which may prevent chronic inflammation status (Rothlin et al., 2007; Sharif et al., 2006).
INHIBITION OF NLRP3 INFLAMMASOME ACTIVATION VIA AUTOPHAGE INDUCTION BY TAM FAMILY RECEPTORS
A recent study has demonstrated that AXL signaling can inhibit inflammation through autophagy induction in macrophages (Han et al., 2016)(Fig. 2C). Autophagy is critical for regulating cell homeostasis that can enhance cell survival through degradation of damaged organelles or cytoplasmic components in eukaryotic cells (Klionsky and Emr, 2000). Two tyrosine residues in the cytoplasmic domain of Axl are autophosphorylated when Gas6 binds to Axl on macrophages (Han et al., 2016). This autophosphorylation activates p38α mitogen-activated protein kinase (MAPK) (MAPK14) pathway. Activated MAPK14 can induce autophagy formation through increasing expression of Atg5, Becn1, and Map1lc3b genes (Han et al., 2016). The induced autophagy then restrains NLRP3 inflammasome because Gas6/Axl induced autophagy can remove reactive oxygen species released from damaged mitochondria (Han et al., 2016). Previous observations have also demonstrated that reactive oxygen species released from damaged mitochondria can activate NLRP3 inflammasomes (Nakahira et al., 2011; Zhou et al., 2011). Inhibition of NLRP3 inflammasome activation is known to block the activity of caspase 1, a key enzyme for maturation and secretion of IL-1β and IL-18 (Han et al., 2016; Schroder and Tschopp, 2010; Stutz et al., 2009) (Fig. 2C). These results provide additional information on how TAM receptor expressed on macrophage inhibits inflammatory responses.
INHIBITION OF M1 MACROPHAGE POLARIZATION BY TAM FAMILY RECEPTORS
Cancer cells can change their microenvironment by recruiting immune cells to support their survival and metastasis (Solinas et al., 2009). Macrophages are dominant population of immune cells that infiltrate into the tumor microenvironment. Up to 50% of immune cells that migrate into tumor niche are macrophages (Solinas et al., 2009). In tumor microenvironment, macrophages can become tumor-associated macrophages to secret abundant IL-10 with phenotype similar to M2 macrophage (Grivennikv et al., 2010; Lewis and Pollard, 2006; Sica and Bronte, 2007). Therefore, new strategies need to be developed to remove tumor-associated macrophages from tumor niche for better anticancer therapy.
Recent evidence has indicated that cancer cells can utilize TAM receptor signaling to convert tumor-infiltrated monocytes into tumor-associated macrophages (Loges et al., 2010; Ubil et al., 2018). Some malignant tumors can secret Gas6 and Pros1 to bind to TAM receptor on macrophages (Loges et al., 2010; Ubil et al., 2018). After binding to Gas6, TAM receptor signaling on macrophages can produce more macrophage colony-stimulating factor and IL-10, leading to recruitment of more monocytes into tumor niche and differentiation of more monocytes into M2 macrophages or tumor-associated macrophages (Loges et al., 2010)(Fig. 2D). Pros1 secreted by malignant tumors also initiates Mer and Tyros3 signaling to activate protein tyrosine phosphatase 1b (PTP1b) on macrophages (Ubil et al., 2018). Activated PTP1b then suppresses the activity of M1 macrophages by inhibiting MAPK 14-mediated M1 macrophage gene expression (Ubil et al., 2018). Therefore, tumors can utilize TAM receptor on macrophages to enhance their survival against immune surveillance (Fig. 2D).
CONCLUSION
Currently, immunotherapy is a major tool to treat many human diseases including hypersensitivity, autoimmune disease, graft rejection, and cancer. Immunotherapy is designed based on immune modulatory effects of particular immune cell types. As an immune modulator, macrophage is a powerful governor that initiates or block inflammatory responses by manipulating activities of other immune cell populations. Therefore, finding new key molecule that can regulate the activity of macrophage may be a beneficial strategy to design novel immunotherapy.
As described above, numerous observations have suggested that TAM family receptor expressed on macrophage can act as a powerful inhibitor against a broad range of inflammatory responses (Fig. 2). Accordingly, enhancement of TAM receptor signaling may be one option to suppress inflammatory diseases such as graft rejection and autoimmune disease. In contrast, inhibition of TAM receptor signaling may be necessary for successful anti-cancer therapy or applied to new regimen to treat M2-prone allergic responses. Indeed, several examples have already shown the possibility of TAM receptor signaling as a new drug target. For example, TAM receptor signaling inhibits an allogenic type I IFN response in murine islet or heart transplant model (Zhang et al., 2018). Also, specific delivery of Gas6 targeting central nervous system can protect axon against damage in experimental autoimmune encephalomyelitis (Gruber et al., 2014). Furthermore, Gas6 weakens inflammatory cytokines in oral squamous cell carcinoma (Hirschi et al., 2018). Gas6 deficiency in mice enhances allergic airway disease (Shibata et al., 2014).
The possible drawback to using TAM family receptor as a therapeutic agent which modulates macrophage activity is an unexpected side effect because TAM receptor signaling exhibits a functional pleiotropy on the broad range of cells (Rothlin et al., 2015). For example, Axl inhibitor, R428 (BGB324), is currently under clinical trials in patients with several cancers including acute myeloid leukemia and non-small cell lung cancer (Sheridan, 2013; Wu et al., 2018). However, major effect of Axl inhibitor does not rely on modulating activity of macrophage, but blocks proliferation and migration of certain types of malignant tumors (Ben-Batalla et al., 2013; Gjerdrum et al., 2010; Linger et al., 2013). Therefore, more precise analyses with various clinical samples are need to be performed and relevant clinical trials should be conducted using Gas6 to solidify the concept of novel anti-inflammatory regimen using TAM receptor signaling.
ACKNOWLEDGMENTS
This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (2017M3A 9C8060392).
REFERENCES
- Alciato F., Sainaghi P.P., Sola D., Castello L., Avanzi G.C. TNF-alpha, IL-6, and IL-1 expression is inhibited by Gas6 in monocytes/macrophages. J Leukoc Biol. 2010;87:869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- Behrens E.M., Gadue P., Gong S.Y., Garrett S., Stein P.L., Cohen P.L. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. Eur J Immunol. 2003;33:2160–2167. doi: 10.1002/eji.200324076. [DOI] [PubMed] [Google Scholar]
- Ben-Batalla I., Schultze A., Wroblewski M., Erdmann R., Heuser M., Waizenegger J.S., Riecken K., Binder M., Schewe D., Sawall S., et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood. 2013;122:2443–2452. doi: 10.1182/blood-2013-03-491431. [DOI] [PubMed] [Google Scholar]
- Caberoy N.B., Alvarado G., Bigcas J.L., Li W. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol. 2012;227:401–407. doi: 10.1002/jcp.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy N.B., Zhou Y., Li W. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J. 2010;29:3898–3910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch T.D., Koller B.H., Earp H.S., Matsushima G.K. A novel receptor tyrosine kinase, Mer, inhibits TNF-α production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- Caraux A., Lu Q., Fernandez N., Riou S., Di Santo J.P., Raulet D.H., Roth C. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7:747–754. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- Carrera Silva E.A., Chan P.Y., Joannas L., Errasti A.E., Gagliani N., Bosurgi L., Jabbour M., Perry A., Smith-Chakmakova F., Mucida D., et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity. 2013;39:160–170. doi: 10.1016/j.immuni.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano F., Montcourrier P., Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J Cell Sci. 2000;113:2955–2961. doi: 10.1242/jcs.113.17.2955. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L., Través P.G., Tufail Y., Leal-Bailey H., Lew E.D., Burrola P.G., Callaway P., Zagórska A., Rothlin C.V., Nimmerjahn A., et al. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532:240–244. doi: 10.1038/nature17630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerdrum C., Tiron C., H⊘iby T, Stefansson I., Haugen H., Sandal T., Collett K., Li S., McCormack E., Gjertsen B.T., et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci USA. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.K., Dawson T.L., Mullaney D.L., Snodgrass H.R., Earp H.S. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994;5:647–657. [PubMed] [Google Scholar]
- Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R.C., Ray A.K., Johndrow C.T., Guzik H., Burek D., de Frutos P.G., Shafit-Zagardo B. Targeted GAS6 delivery to the CNS protects axons from damage during experimental autoimmune encephalomyelitis. J Neurosci. 2014;34:16320–16335. doi: 10.1523/JNEUROSCI.2449-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S., Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- Han J., Bae J., Choi C.Y., Choi S.P., Kang H.S., Jo E.K., Lee M.S. Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice. Autophagy. 2016;12:2326–2343. doi: 10.1080/15548627.2016.1235124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K.M., Chapman S., Hall P., Ostergar A., Winden D.R., Reynolds P.R., Arroyo J.A. Gas6 protein induces invasion and reduces inflammatory cytokines in oral squamous cell carcinoma. J Oral Pathol Med. 2018;47:748–754. doi: 10.1111/jop.12738. [DOI] [PubMed] [Google Scholar]
- Huang M., Rigby A.C., Morelli X., Grant M.A., Huang G., Furie B., Furie B.C. Structural basis of membrane binding by Gla domains of vitamin K–dependent proteins. Nat Struct Biol. 2003;10:751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- Jung M. Expression profiling of IL-10-regulated genes in human monocytes and peripheral blood mononuclear cells from psoriatic patients during IL-10 therapy. Eur J Immunol. 2004;34:481–493. doi: 10.1002/eji.200324323. [DOI] [PubMed] [Google Scholar]
- Klionsky D.J., Emr S.D. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C., Gore M., Lemke G. Structure, expression, and activity of Tyro 3, a neural adhesion-related receptor tyrosine kinase. Oncogene. 1994;9:2567–2578. [PubMed] [Google Scholar]
- Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Lemke G., Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Ann NY Acad Sci. 2010;1209:23–29. doi: 10.1111/j.1749-6632.2010.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew E.D., Oh J., Burrola P.G., Lax I., Zagórska A., Través P.G., Schlessinger J., Lemke G. Differential TAM receptor–ligand–phospholipid interactions delimit differential TAM bioactivities. Elife. 2014;3:e03385. doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C.E., Pollard J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Linger R.M., Cohen R.A., Cummings C.T., Sather S., Migdall-Wilson J., Middleton D.H., Lu X., Barón A.E., Franklin W.A., Merrick D.T., et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32:3420–3431. doi: 10.1038/onc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger R.M., Keating A.K., Earp H.S., Graham D.K. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnau M., Hoflich C., Volk H.D., Sabat R., Docke W.D. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Hum Immunol. 2007;68:730–738. doi: 10.1016/j.humimm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Loges S., Schmidt T., Tjwa M., Van Geyte K., Lievens D., Lutgens E., Luttun A. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115:2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- Lu Q., Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- Lu Q., Gore M., Zhang Q., Camenisch T., Boast S., Casagranda F., Earp H.S. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Mills C.D. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- Mills C.D., Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun. 2014;6:716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. [Google Scholar]
- Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Ohashi K., Nakano T., Arita H., Zong C., Hanafusa H., Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- Nakahira K., Haspel J.A., Rathinam V.A., Lee S.J., Dolinay T., Lam H.C., Englert J.A., Rabinovitch M., Cernadas M., Kim H.P. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryan J.P., Frye R.A., Cogswell P.C., Neubauer A., Kitch B., Prokop C., Espinosa R., Beau M.M.L., Earp H.S., Liu E.T. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino M., Choidas A., Wallner S., Pranjic B., Uribesalgo I., Loeser S., Jamieson A.M., Langdon W.Y., Ikeda F., Fededa J.P., et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507:508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D., Rothlin C.V., Burrola P., Burstyn-Cohen T., Lu Q., de Frutos P.G., Lemke G. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33:96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Robinson D.R., Wu Y.M., Lin S.F. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- Rothlin C.V., Carrera-Silva E.A., Bosurgi L., Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin C.V., Ghosh S., Zuniga E.I., Oldstone M.B., Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Sheridan C. First Axl inhibitor enters clinical trials. Nat Biotechnol. 2013;31:775–756. doi: 10.1038/nbt0913-775a. [DOI] [PubMed] [Google Scholar]
- Shibata T., Ismailoglu U.B., Kittan N.A., Moreira A.P., Coelho A.L., Chupp G.L., Kunkel S.L., Lukacs N.W., Hogaboam C.M. Role of growth arrest-specific gene 6 in the development of fungal allergic airway disease in mice. Am J Respir Cell Mol Biol. 2014;51:615–625. doi: 10.1165/rcmb.2014-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley S.T., Stitt T.N., Grusby M.J. Cross-linking of protein S bound to lymphocytes promotes aggregation and inhibits proliferation. Cell Immunol. 1997;181:120–126. doi: 10.1006/cimm.1997.1210. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Knyazev P.G., Clout N.J., Cheburkin Y., Gohring W., Ullrich A., Timpl R., Hohenester E. Structural basis for Gas6-Axl signalling. EMBO J. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Scott R.S. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Segawa K. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- Sharif M.N., Sosic D., Rothlin C.V., Kelly E., Lemke G., Olson E.N., Ivashkiv L.B. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A., Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- Sosic D., Richardson J.A., Yu K., Ornitz D.M., Olson E.N. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-κB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Stitt T.N. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Stutz A., Golenbock D.T., Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Todt J.C., Hu B., Curtis J.L. The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages. J Leukoc Biol. 2004;75:705–713. doi: 10.1189/jlb.0903439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubil E., Caskey L., Holtzhausen A., Hunter D., Story C., Earp H.S. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J Clin Invest. 2018;128:2356–2369. doi: 10.1172/JCI97354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Ma Z., Cheng Y., Hu W., Deng C., Jiang S., Li T., Chen F., Yang Y. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol Cancer. 2018;17:20. doi: 10.1186/s12943-018-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Singh S., Georgescu M.M., Birge R.B. A role for Mer tyrosine kinase in alphavbeta5 integrin mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- Zhang L., DeBerge M., Wang J., Dangi A., Zhang X., Schroth S., Zhang Z., Thorp E.B., Luo X. Receptor tyrosine kinase MerTK suppresses an allogenic type I IFN response to promote transplant tolerance. Am J Transplant. 2018 doi: 10.1111/ajt.15087. [In press] Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]