Abstract
Background
Pulmonary hypertension (PH) comprises a group of complex and heterogenous conditions, characterised by elevated pulmonary artery pressure, and which left untreated leads to right‐heart failure and death. PH includes World Health Organisation (WHO) Group 1 pulmonary arterial hypertension (PAH); Group 2 consists of PH due to left‐heart disease (PH‐LHD); Group 3 comprises PH as a result of lung diseases or hypoxia, or both; Group 4 includes PH due to chronic thromboembolic occlusion of pulmonary vasculature (CTEPH), and Group 5 consists of cases of PH due to unclear and/or multifactorial mechanisms including haematological, systemic, or metabolic disorders. Phosphodiesterase type 5 (PDE5) inhibitors increase vasodilation and inhibit proliferation.
Objectives
To determine the efficacy of PDE5 inhibitors for pulmonary hypertension in adults and children.
Search methods
We performed searches of CENTRAL, MEDLINE, Embase, CINAHL, and Web of Science up to 26 September 2018. We handsearched review articles, clinical trial registries, and reference lists of retrieved articles.
Selection criteria
We included randomised controlled trials that compared any PDE5 inhibitor versus placebo, or any other PAH disease‐specific therapies, for at least 12 weeks. We include separate analyses for each PH group.
Data collection and analysis
We imported studies identified by the search into a reference manager database. We retrieved the full‐text versions of relevant studies, and two review authors independently extracted data. Primary outcomes were: change in WHO functional class, six‐minute walk distance (6MWD), and mortality. Secondary outcomes were haemodynamic parameters, quality of life/health status, dyspnoea, clinical worsening (hospitalisation/intervention), and adverse events. When appropriate, we performed meta‐analyses and subgroup analyses by severity of lung function, connective tissue disease diagnosis, and radiological pattern of fibrosis. We assessed the evidence using the GRADE approach and created 'Summary of findings' tables.
Main results
We included 36 studies with 2999 participants (with pulmonary hypertension from all causes) in the final review. Trials were conducted for 14 weeks on average, with some as long as 12 months. Two trials specifically included children.
Nineteen trials included group 1 PAH participants. PAH participants treated with PDE5 inhibitors were more likely to improve their WHO functional class (odds ratio (OR) 8.59, 95% confidence interval (CI) 3.95 to 18.72; 4 trials, 282 participants), to walk 48 metres further in 6MWD (95% CI 40 to 56; 8 trials, 880 participants), and were 22% less likely to die over a mean duration of 14 weeks (95% CI 0.07 to 0.68; 8 trials, 1119 participants) compared to placebo (high‐certainty evidence). The number needed to treat to prevent one additional death was 32 participants. There was an increased risk of adverse events with PDE5 inhibitors, especially headache (OR 1.97, 95% CI 1.33 to 2.92; 5 trials, 848 participants), gastrointestinal upset (OR 1.63, 95% CI 1.07 to 2.48; 5 trials, 848 participants), flushing (OR 4.12, 95% CI 1.83 to 9.26; 3 trials, 748 participants), and muscle aches and joint pains (OR 2.52, 95% CI 1.59 to 3.99; 4 trials, 792 participants).
Data comparing PDE5 inhibitors to placebo whilst on other PAH‐specific therapy were limited by the small number of included trials. Those PAH participants on PDE5 inhibitors plus combination therapy walked 19.66 metres further in six minutes (95% CI 9 to 30; 4 trials, 509 participants) compared to placebo (moderate‐certainty evidence). There were limited trials comparing PDE5 inhibitors directly with other PAH‐specific therapy (endothelin receptor antagonists (ERAs)). Those on PDE5 inhibitors walked 49 metres further than on ERAs (95% CI 4 to 95; 2 trials, 36 participants) (low‐certainty evidence). There was no evidence of a difference in WHO functional class or mortality across both treatments.
Five trials compared PDE5 inhibitors to placebo in PH secondary to left‐heart disease (PH‐LHD). The quality of data were low due to imprecision and inconsistency across trials. In those with PH‐LHD there were reduced odds of an improvement in WHO functional class using PDE5 inhibitors compared to placebo (OR 0.53, 95% CI 0.32 to 0.87; 3 trials, 285 participants), and those using PDE5 inhibitors walked 34 metres further compared to placebo (95% CI 23 to 46; 3 trials, 284 participants). There was no evidence of a difference in mortality. Five trials compared PDE5 inhibitors to placebo in PH secondary to lung disease/hypoxia, mostly in COPD. Data were of low quality due to imprecision of effect and inconsistency across trials. There was a small improvement of 27 metres in 6MWD using PDE5 inhibitors compared to placebo in those with PH due to lung disease. There was no evidence of worsening hypoxia using PDE5 inhibitors, although data were limited. Three studies compared PDE5 inhibitors to placebo or other PAH‐specific therapy in chronic thromboembolic disease. There was no significant difference in any outcomes. Data quality was low due to imprecision of effect and heterogeneity across trials.
Authors' conclusions
PDE5 inhibitors appear to have clear beneficial effects in group 1 PAH. Sildenafil, tadalafil and vardenafil are all efficacious in this clinical setting, and clinicians should consider the side‐effect profile for each individual when choosing which PDE5 inhibitor to prescribe.
While there appears to be some benefit for the use of PDE5 inhibitors in PH‐left‐heart disease, it is not clear based on the mostly small, short‐term studies, which type of left‐heart disease stands to benefit. These data suggest possible harm in valvular heart disease. There is no clear benefit for PDE5 inhibitors in pulmonary hypertension secondary to lung disease or chronic thromboembolic disease. Further research is required into the mechanisms of pulmonary hypertension secondary to left‐heart disease, and cautious consideration of which subset of these patients may benefit from PDE5 inhibitors. Future trials in PH‐LHD should be sufficiently powered, with long‐term follow‐up, and should include invasive haemodynamic data, WHO functional class, six‐minute walk distance, and clinical worsening.
Plain language summary
PDE5 inhibitors for pulmonary hypertension
Review question:
We wanted to review whether a group of drugs called PDE5 inhibitors (which may work to open up the vessels in the lung) can help people with pulmonary hypertension (increased pressures in the blood vessels of the lungs). Cochrane researchers collected and analysed all relevant studies to answer this question, and found 36 studies.
Why the review is important:
Approximately three people in every 1000 have pulmonary hypertension, due to different causes. This can lead to reduced exercise capacity, reduced quality of life, increased hospitalisations, and early death. A group of drugs called PDE5 inhibitors may improve blood circulation in the right heart and lungs. We wanted to make sure that if these drugs are being used, there is evidence of benefit and little or no harm.
Main findings:
We included 36 studies with 2999 people. Trials were conducted for 14 weeks on average, with some as long as 12 months. Most trials involved adults, and two trials specifically included children.
Nineteen trials included those with group 1 pulmonary arterial hypertension (inherited, unknown, due to connective tissue diseases). People who were given PDE5 inhibitors were compared with those not given PDE5 inhibitors. This review shows that when given PDE5 inhibitors, on average people walked 48 meters further in six minutes (8 trials, 880 people). They also improved their functional class (reducing the physical limitations associated with PH), and were less likely to die (high‐certainty evidence). They were also more likely to have side effects, including headache, flushing and muscle aches.
Five trials included people with pulmonary hypertension due to left‐heart disease. This review shows that when given PDE5 inhibitors, these people were on average able to walk 34 metres further in six minutes (3 trials, 284 people; low‐certainty evidence). However, there was no difference in survival, compared to those who were not given PDE5 inhibitors. Five trials included people with pulmonary hypertension due to lung disease (mostly chronic obstructive pulmonary disease and some idiopathic pulmonary fibrosis). When given PDE5 inhibitors, they were able to walk 27 meters further in six minutes (low‐certainty evidence), but with no difference in survival, compared to those who were not given PDE5 inhibitors. Three trials included people with pulmonary hypertension due to blood clots; there was no significant difference in outcomes for those who used PDE5 inhibitors compared to those who did not.
Limitations:
There was good‐quality evidence for those with pulmonary arterial hypertension, giving us some confidence that the results are correct. The evidence for those with pulmonary hypertension due to heart disease was less certain. The quality of evidence in this group was low because there were few trials, small numbers of people taking part, and the trials were quite different from each other, making it difficult to draw firm conclusions.
Summary of findings
Background
Description of the condition
Pulmonary hypertension (PH) (defined as a mean pulmonary artery pressure ≥ 25 mmHg at rest on right‐heart catheterisation) comprises a complex group of conditions (see Table 8), characterised by increased right ventricular afterload, which ultimately leads to right‐heart failure (McLaughlin 2009). The increased afterload may be due to passive transmission of high left‐sided pressures (post‐capillary pulmonary hypertension), obstruction of the pulmonary arterial bed (pre‐capillary pulmonary hypertension) or a combination of both. Pulmonary arterial hypertension (PAH‐WHO Group 1) is a group of diseases where pulmonary hypertension occurs in the setting of increased pulmonary vascular resistance. The more recent availability of medications and therapies targeting the pulmonary arterial bed has led to the prospect of an improvement in what had previously been a very poor prognosis. Although PAH is rare, PH secondary to left‐heart disease and lung disease is much more common. The availability of drugs with efficacy in PAH has led to great interest in the use of these drugs in other forms of pulmonary hypertension.
1. World Health Organisation/World Symposium classification of pulmonary hypertension.
| WHO group | Classification |
| Group 1 | Pulmonary arterial hypertension
Pulmonary veno‐occlusive disease/pulmonary capillary haemangiomatosis Persistent pulmonary hypertension of the newborn |
| Group 2 | Pumonary hypertension due to left heart disease
Valvular heart disease Congenital post‐capillary obstructive lesions |
| Group 3 | Pulmonary hypertension due to chronic lung disease or chronic hypoxaemia, or both
|
| Group 4 | Pulmonary hypertension due to pulmonary artery obstruction
|
| Group 5 | Pulmonary hypertension with unclear mechanisms
|
PAH: pulmonary arterial hypertension
In PAH, increased pulmonary vascular resistance is caused by vascular remodelling and thickening in the small‐ and medium‐sized arterioles, fibrinoid necrosis, the formation of eccentric, concentric, or plexiform lesions, and the loss of vascular tone. This process of cellular hypertrophy and hyperplasia is mediated by intracellular calcium and protein kinase C, inflammatory cytokines, and altered energy metabolism. Remodelling and vasoconstriction lead to hypoxia, causing further vasoconstriction and further hypoxia (Guignabert 2013; Sim 2010).
Pulmonary hypertension is classified into five groups of multiple clinical conditions, grouped according to similar clinical presentations and pathophysiological and haemodynamic characteristics, with distinct treatment strategies for each group. Group 1 pulmonary arterial hypertension (PAH) includes idiopathic and heritable PAH and PAH due to pathology of the small pulmonary arterioles resulting from connective tissue disorders, drugs or toxins, portal pulmonary hypertension, and others (see Table 8). Pulmonary arterial hypertension is caused by increased pulmonary vascular resistance due to occlusive vasculopathy of the small pulmonary arteries and arterioles. Pulmonary arterial hypertension is a rare disease, with an estimated prevalence of 10 to 52 cases per million (Ling 2012; Peacock 2007). However, screening for pulmonary hypertension for all causes demonstrates a prevalence of 320 cases per 100,000 (Strange 2012).
Group 2 consists of pulmonary hypertension due to left‐heart disease, caused by increased flow through the pulmonary vasculature, or increased pulmonary pressures (e.g. mitral valve disease, left ventricular disease, and constrictive myopathies). Group 3 comprises pulmonary hypertension as a result of lung diseases or hypoxia, or both, caused by a decrease in the area of the pulmonary vascular bed (e.g. interstitial lung disease), or conditions that induce hypoxic vasoconstriction. Group 4 refers to cases of pulmonary hypertension due to chronic thromboembolic occlusion of pulmonary vasculature, and Group 5 consists of cases of pulmonary hypertension due to unclear or multifactorial mechanisms or both, including haematological, systemic, or metabolic disorders (see Table 8) (McLaughlin 2009). The extent to which WHO Groups 2 to 5 have pulmonary arterial bed changes analogous to WHO Group 1 (and thus respond similarly to the medications) remains unclear. Furthermore, potential detrimental effects of selective arterial vasodilatation (pulmonary oedema in WHO Group 2 and hypoxia in WHO group 3) will probably present a quite different risk/benefit profile to the same medications used in PAH.
People with PAH often present with symptoms of dyspnoea, fatigue, syncope, and right‐heart failure (Galiè 2016b). Right‐heart catheterisation remains the gold standard of diagnosis to confirm pulmonary hypertension and to further investigate potential causes and treatment targets. Pulmonary arterial hypertension is defined as a mean pulmonary artery pressure equal to or greater than 25 mmHg; a pulmonary artery wedge pressure, left atrial pressure, or left ventricular end‐diastolic pressure less than or equal to 15 mmHg; and a pulmonary vascular resistance greater than three Wood units (Galiè 2016b). Elevation of the pulmonary artery wedge pressure suggests pulmonary hypertension secondary to left‐heart disease. People with confirmed PAH should undergo acute vasodilator testing to assess for pulmonary vasoreactivity, as a small proportion may respond very favourably to long‐term high‐dose calcium channel blocker therapy (McLaughlin 2009).
Following history, examination, electrocardiogram, echocardiogram, and chest X‐ray, other investigations for people with pulmonary hypertension should include pulmonary function tests and high‐resolution computed tomography chest to assess for underlying lung disease, a ventilation/perfusion scan to assess for chronic thromboembolic pulmonary hypertension, thyroid function tests, autoimmune serology, HIV and hepatitis screening to assess for underlying aetiologies, and a six‐minute walk test or exercise testing, biomarkers to monitor response to treatment and for prognostication (Galiè 2016b).
The natural history and prognosis of pulmonary hypertension varies amongst the WHO Groups. However the presence of pulmonary hypertension irrespective of WHO group generally reflects the presence of a progressive and often fatal condition. Known independent predictors of poor prognosis include advanced WHO functional class, poor performance in six‐minute walk test, high right atrial pressure, significant right ventricular dysfunction, evidence of right ventricular failure, elevated pro‐B‐type natriuretic peptide (BNP), and low cardiac index (Thenappan 2007).
Description of the intervention
Recent years have seen the introduction of evolving therapies for PAH, with an improvement in the one‐year survival rate to 84% from 68% in the 1980s (Archer 2009). The goals of therapy are to achieve a state associated with good quality of life and exercise tolerance with low mortality risk and to maintain right ventricular function, using supplemental oxygen and treatment of the underlying cause. The underlying pulmonary artery endothelial dysfunction in Group 1 PAH enables the use of PAH‐specific targeted treatments promoting vasorelaxation and suppression of cellular proliferation within the pulmonary artery wall, including nitric oxide and phosphodiesterase type 5 inhibitors (PDE5i, prostanoids, endothelin receptor antagonists, and calcium channel blockers) (McLaughlin 2009).
How the intervention might work
Nitric oxide performs as a pulmonary vasodilator by activating soluble guanylate cyclase, stimulating the production of cyclic guanosine monophosphate (cGMP), which in turn activates myosin light chain phosphatase, which reduces phosphorylation of myosin to reduce pulmonary vascular tone. Increased intracellular cGMP also inhibits calcium entry, thereby reducing intracellular calcium leading to less hypertrophy and hyperplasia, as well as antiproliferative and pro‐apoptotic effects that may reverse pulmonary artery remodelling. Nitric oxide also inhibits platelet recruitment, adhesion, and aggregation (Sim 2010).
However, nitric oxide administration is not without risk. High levels of inhaled nitric oxide may lead to oxidative stress and cause tissue damage, reperfusion injury, and a pulmonary inflammatory reaction. Inhaled nitric oxide is rapidly absorbed into the blood stream, where it is converted to methaemoglobin, leading to impaired rather than improved oxygen delivery (Sim 2010).
Phosphodiesterase type 5 (PDE5) specifically reduces cGMP‐degrading enzyme activity, thereby increasing cGMP production. Phosphodiesterase type 5 inhibitors are not thought to induce the same levels of oxidation as inhaled nitric oxide (Ghofrani 2004d). Phosphodiesterase type 5 inhibitors that have been investigated for use in Group 1 PAH include sildenafil, tadalafil, and vardenafil. These agents have been shown in clinical trials to improve six‐minute walk distance and haemodynamics (Archer 2009; Galiè 2016b; McLaughlin 2009).
The data are less clear in WHO Group 2 to 5 patients, in whom this class of drug may be potentially harmful. There are different pathobiological and pathophysiological factors at play, leading to the development of pulmonary hypertension in these people. The nature of the pulmonary arteriopathy may be quite different (e.g. hypoxic vasoconstriction and pulmonary vascular bed obstruction in Group 3; thrombotic obstruction in Group 4). The consequences of 'selective' pulmonary arterial vasodilation may also be detrimental (pulmonary oedema in WHO group 2 and hypoxia in Group 3). Thus, as with other selective pulmonary vasodilators, a reduction in pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP) by PDE5 inhibitors may not improve the overall well‐being of patients, especially in the non‐PAH Groups (2 to 5) (Guazzi 2012).
Phosphodiesterase type 5 inhibitors may theoretically improve function in Group 2 patients with left‐heart disease. Previous studies in heart‐failure patients have demonstrated that nitric oxide is responsible for regulation of vascular tone, and infusion of NG‐monomethyl‐L‐arginine, an inhibitor of nitric oxide synthase, caused less vasoconstriction in heart‐failure patients compared to those with a normal pulmonary vascular resistance (Cooper 1996). Trials using sildenafil in Group 2 pulmonary hypertension patients have shown some evidence of improvement in exercise capacity, ventilation efficiency, and quality of life (Lewis 2007). However, other studies have demonstrated unbalanced pulmonary dilatation as a consequence of nitric oxide and analogues may lead to increased preload due to a poorly compliant left ventricle, and therefore a significant increase in pulmonary artery wedge pressure, which may even precipitate acute pulmonary oedema (Bocchi 1994).
Furthermore, trials using other PAH‐specific therapies including epoprostenol and endothelin receptor antagonists in people with Group 2 pulmonary hypertension demonstrated an increased risk of hospitalisations, disease progression, and hypoxaemia. People with left ventricular dysfunction may not be able to tolerate the increased flow across a newly‐dilated pulmonary vascular bed (Guazzi 2012).
People with Group 3 chronic lung diseases may experience worsening ventilation perfusion mismatch and increased hypoxaemia. A study in people with pulmonary hypertension associated with chronic obstructive pulmonary disease demonstrated an improvement in pulmonary artery pressures, but at the cost of worsening arterial oxygenation (Blanco 2010).
Why it is important to do this review
Given recent advances in the understanding of the pathophysiological mechanisms and treatments for pulmonary hypertension with significant contributions in the area in the last decade, we planned to summarise the current evidence relating to the use of PDE5 inhibitors in pulmonary hypertension.
This review aimed to quantify any potential benefit for PDE5 inhibitors in people with PAH in terms of haemodynamic measurements and patient‐centred outcomes, and to balance this against any potential treatment harms, in order to guide patient preference, clinician treatment choices, and guidelines for policymakers.
This review also examines the available evidence to determine whether there is any potential benefit or harm in using PDE5 inhibitors in people with Group 2 to 5 pulmonary hypertension.
This review builds on a previous review (Kanthapillai 2004), since which further concepts about pathophysiology have been developed, and a number of more recent randomised controlled trials using PDE5 inhibitors have been published.
Objectives
To determine the efficacy of PDE5 inhibitors for pulmonary hypertension in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We include single‐ or double‐blinded randomised controlled trials (RCTs) in which PDE5 inhibitors are compared to placebo or to any other treatment. We defined 'randomised' as studies described by the author as 'randomised' anywhere in the study report. All trials defined as such, published or unpublished, in any language, were eligible for inclusion.
Types of participants
We include any individual with a diagnosis of pulmonary hypertension from any cause who required medical treatment for their condition. We define pulmonary hypertension according to accepted criteria (Galiè 2016b; McLaughlin 2009).
Comparison 1 specifically assesses the effects of PDE5 inhibitors compared to placebo on Group 1 PAH, confirmed as a mean pulmonary artery pressure ≥ 25 mmHg by right‐heart catheterisation.
Comparison 2 compares PDE5 inhibitors with placebo in PAH participants on combination therapy.
Comparison 3 compares PDE5 inhibitors to ERAs in PAH participants.
Comparison 4 includes group 2 pulmonary hypertension participants with a diagnosis of pulmonary hypertension and left‐heart disease, as defined by the authors.
Comparison 5 includes group 3 pulmonary hypertension participants with a diagnosis of pulmonary hypertension and lung disease, as defined by the authors.
Comparison 6 includes group 4 pulmonary hypertension participants with a diagnosis of pulmonary hypertension and chronic thromboembolic disease (CTEPH), as defined by the authors.
Comparison 7 includes mixed group 2 to 5 pulmonary hypertension participants with a diagnosis of pulmonary hypertension as defined by the authors.
We planned to specify subgroups of adults (older than 18 years) and a paediatric population younger than 18 years.
Types of interventions
We include studies comparing any type of PDE5 inhibitors by any route of administration with placebo or any other treatment used for pulmonary hypertension. We include studies with co‐interventions provided they are not part of the randomised treatment. We aimed to perform subgroup analyses depending on the co‐interventions used. Where studies were too heterogenous for meta‐analyses, we describe them in narrative form.
Types of outcome measures
Primary outcomes
Change in WHO functional class
Six‐minute walk distance (6MWD)
Mortality
Secondary outcomes
Haemodynamic parameters, including change in mean pulmonary artery pressure, change in cardiac output, cardiac index
Exercise capacity other than six‐minute walk distance
Quality of life/health status, by any validated scale
Dyspnoea score, including visual analogue scale or Borg scale
Hospitalisation/intervention
Adverse events
Reporting one or more of the outcomes listed here in the study was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials from searches of the following databases up to 26 September 2018:
The Cochrane Airways Group Register of Trials;
Cochrane Central Register of Controlled Trials (CENTRAL) through the Cochrane Register of Studies Online (crso.cochrane.org);
MEDLINE (Ovid) 1950 to 26 September 2018;
Embase (Ovid) 1974 to 26 September 2018;
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
We provide the database search strategies in Appendix 1. We searched all databases from their inception to the present, with no restriction on language of publication. We searched for handsearched conference abstracts and grey literature through the CENTRAL database.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We handsearched reference lists of included studies, relevant chapters, and review articles. We used Google to search for grey literature and conference abstracts. We translated any relevant article into English for potential inclusion. Where data were missing, we attempted to contact the trial investigators.
Data collection and analysis
Selection of studies
Two review authors (HB and ZB) independently screened all abstracts to determine if they met the inclusion criteria for the review. We sought full‐text publications for those papers that possibly or definitely met the inclusion criteria. Two review authors independently reviewed all full‐text articles to determine eligibility, recording reasons for ineligibility of those that did not. We resolved any disagreements through discussion, or by seeking consensus from a third review author (AB). We included a PRISMA study flow diagram in the full review to document the screening process, and included a Characteristics of included studies table (Moher 2009).
Data extraction and management
Two review authors (HB and ZB) independently extracted data from included studies, and where appropriate, pooled data in the Cochrane statistical software Review Manager 5 for further analysis (RevMan 2014). We used a data collection form that we piloted on one study for inclusion in the review, containing the following data.
Methods: study design, duration, study setting, date of study.
Participants: number, mean age and age range, gender, inclusion and exclusion criteria.
Intervention: type of PDE5 inhibitor, dose, mode of administration, control drug, co‐interventions, and exclusions.
Outcomes: primary and secondary outcomes as specified, type of scale used, time points collected.
Risk of bias summary.
Other: funding for trial, any conflicts of interest for trial authors.
Assessment of risk of bias in included studies
Two review authors (HB and ZB) independently assessed the included studies for risks of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment
Incomplete outcome data.
Selective outcome reporting.
Other potential sources of bias.
We judged each potential source of bias as low risk, unclear risk (insufficient information to form a judgement), or high risk, and provided justification with evidence from each trial in the ‘Risk of bias’ table. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the previously published protocol, and justified any deviations from it in the Differences between protocol and review section of the review.
Measures of treatment effect
Where possible, we pooled and presented results from dichotomous data as odds ratios (ORs). Where possible, we presented results from continuous variables and calculated the mean difference (MD) or standardised mean difference (SMD) where scales are combined, with the 95% confidence interval (95% CI). Where we combined data from rating scales in a meta‐analysis, we ensured that they were entered with a consistent direction of effect (e.g. lower scores always indicate improvement). If both change from baseline and endpoint scores were available for continuous data, we used change from baseline scores. Where outcomes were reported at multiple time points, we consistently extracted and included the latest reported time point, but also considered outcomes reported at other time points. We only combined data reported at different time points if this was clinically appropriate.
We described skewed data narratively (e.g. as medians and interquartile ranges for each group).
We used intention‐to‐treat or 'full analysis set' analyses where they were reported (i.e. those where data have been imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants rather than events as the unit of analysis (i.e. number of children admitted to hospital, rather than number of admissions per child). Where rate ratios were reported in a study, we analysed them on that basis. We planned to only meta‐analyse data from cluster‐randomised controlled trials if the available data have been adjusted (or could be adjusted) to account for the clustering, however there were no cluster‐randomised controlled trials identified for inclusion.
Dealing with missing data
We contacted investigators in order to verify key study characteristics including methods of randomisation, and obtained missing numerical outcome data where possible (e.g. when we identified a study as an abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes. We planned to only meta‐analyse data from cluster‐RCTs if the available data had been adjusted (or could be adjusted), to account for the clustering.
Assessment of heterogeneity
For pooled analyses we quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across trials due to heterogeneity rather than to sampling error. We considered significant statistical heterogeneity to be present if the I2 is greater than 50%. Where we identified significant heterogeneity, we attempted to explore possible causes using prespecified subgroup analyses.
Assessment of reporting biases
Where sufficient studies were present, we planned to create and examine a funnel plot to explore small‐study and publication biases; however, there are currently insufficient studies.
Data synthesis
We used a fixed‐effects model and performed a sensitivity analysis comparing the fixed‐ and random‐effects model. Where possible we pooled dichotomous outcome variables using a Mantel‐Haenszel OR with 95% CIs. For continuous outcomes, we analysed data as MDs or standardised mean difference (SMD) where scales are combined, with the 95% confidence interval (95% CI). We calculated the number needed to treat for an additional beneficial outcome (NNTB) from the pooled OR and assumed control risk using the formula described in Section 12.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
'Summary of findings' table
We created seven 'Summary of findings' tables that include WHO functional class status, quality of life, mortality, change in haemodynamics, and six‐minute walk distance. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data for the prespecified outcomes. We used the methods and recommendations described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schüneman 2017), employing GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses:
Paediatric population up to 18 years and an adult population aged 18 years or over.
Dosage of PDE5 inhibitor.
Mode of administration.
With the following outcomes in subgroup analyses:
WHO functional class.
Mortality.
Six‐minute walk distance.
Haemodynamic criteria.
However, there were insufficient studies per comparison to perform meta‐analyses for these subgroups, so we present narrative results (described in‐text where applicable).
Sensitivity analysis
We planned to carry out the following sensitivity analyses:
Exclusion of trials identified as being at high risk of selection bias (described in‐text where applicable).
Fixed‐effect model compared with random‐effects model (presented in table form).
Results
Description of studies
Results of the search
We identified 4840 citations in the initial search, and after screening abstracts, selected 136 studies for full‐text review. We included 36 trials with 2999 participants in the final review, plus earlier published abstracts, further post hoc analyses and analyses of secondary outcomes (see Figure 1). We also found five ongoing studies (see Characteristics of ongoing studies).
1.
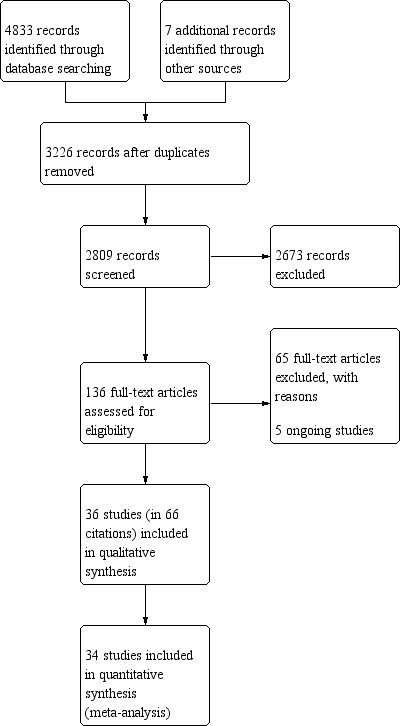
Study flow diagram.
Included studies
Group 1: Pulmonary arterial hypertension
We included all but two of the 36 studies in the final meta‐analysis; Jalalian 2015 included participants with pulmonary hypertension secondary to sickle cell disease, and Machado 2009 included participants with pulmonary hypertension secondary to beta‐thalassemia, and was stopped early due to increased adverse events in the treatment arm. All studies were randomised; 30 were parallel‐controlled and six were cross‐over trials.
Nineteen trials included group 1 PAH participants (Albini 2017; Bharani 2003; Bharani 2007; Boonstra 2005; Galiè 2005a; Galiè 2009; Galiè 2015; Iversen 2009 (specifically Eisenmenger's syndrome); Jing 2011; Mazzanti 2013; Mukhopadhyay 2011; (Barst 2012; Palii 2014 ‐ specifically children with PAH); Sastry 2004; Simonneau 2008; Singh 2006; Vizza 2017a; Wilkins 2005; Zhuang 2014). Most trials recruited participants with WHO functional class II and III (see Characteristics of included studies).
Eleven trials in PAH participants compared a PDE5 inhibitor to placebo: seven trials compared sildenafil to placebo (Barst 2012; Bharani 2003; Boonstra 2005; Galiè 2005a; Palii 2014; Sastry 2004; Singh 2006), three compared tadalafil to placebo (Bharani 2007; Galiè 2009; Mukhopadhyay 2011), and one compared vardenafil to placebo (Jing 2011).
Four studies compared PDE5 inhibitors to placebo, whilst on additional combination therapy (all as add‐on therapy) (Iversen 2009; Simonneau 2008; Vizza 2017a; Zhuang 2014), and four studies (Albini 2017; Galiè 2015; Mazzanti 2013; Wilkins 2005) compared PDE5 inhibitors to endothelin receptor antagonists.
In the PAH trials, sildenafil was prescribed in eight hourly divided doses, with dosages ranging from 20 to 100 mg three times daily. Where multiple doses were compared, we used results from the highest dose where appropriate (e.g. haemodynamics), and aggregated doses where appropriate (e.g. mortality). Tadalafil was dosed 2.5 to 40 mg daily and vardenafil 5 mg twice daily. The duration of trials ranged from two weeks to 12 months, with a mean treatment duration of 14 weeks.
In PAH participants, the primary outcomes of six‐minute walk distance (6MWD) was reported in 15 trials (Albini 2017; Bharani 2003; Bharani 2007; Boonstra 2005; Galiè 2005a; Galiè 2009; Iversen 2009; Jing 2011; Mukhopadhyay 2011; Palii 2014; Sastry 2004; Simonneau 2008; Singh 2006; Vizza 2017a; Zhuang 2014), WHO functional class improvement in seven of 19 trials (Bharani 2007; Galiè 2005a; Galiè 2015; Jing 2011; Mukhopadhyay 2011; Vizza 2017a; Zhuang 2014), and mortality in 13 of 19 trials (Barst 2012; Bharani 2003; Bharani 2007; Galiè 2005a; Galiè 2009; Galiè 2015; Jing 2011; Palii 2014; Sastry 2004; Simonneau 2008; Wilkins 2005; Vizza 2017a; Zhuang 2014). Haemodynamic data were reported in 12 of 19 trials (Albini 2017; Barst 2012; Bharani 2003; Bharani 2007; Galiè 2005a; Iversen 2009; Jing 2011; Mukhopadhyay 2011; Sastry 2004; Simonneau 2008; Singh 2006; Zhuang 2014), For quality of life, two trials used the SF‐36 form (scores 1 to 100, higher scores indicate better quality of life) (Galiè 2005a; Simonneau 2008); minimum clinical improvement difference (MCID) for physical functioning domain for PAH = 13, vitality = 15 (Gilbert 2009). One used a heart failure questionnaire (Sastry 2004), where the higher the score the better the quality of life, and one (Wilkins 2005) used the Kansas City Cardiomyopathy Questionnaire, where higher scores indicated better quality of life. Six trials measured dyspnoea using the Borg dyspnoea scale (MCID for PAH = 1 (Khair 2016)) (Bharani 2003; Bharani 2007; Galiè 2005a; Jing 2011; Sastry 2004; Vizza 2017a). Eleven studies reported adverse events (Albini 2017; Galiè 2005a; Galiè 2009; Galiè 2015; Iversen 2009; Jing 2011; Mukhopadhyay 2011; Sastry 2004; Simonneau 2008; Vizza 2017a; Zhuang 2014).
Group 2: Pulmonary hypertension due to left‐heart disease
Five trials included PH secondary to left‐heart disease (Bermejo 2017 (valvular heart disease); Guazzi 2011a (HFpEF); Hoendermis 2015 (HFpEF); Lewis 2007 (systolic heart failure); Ovchinnov 2015 (diastolic heart failure)). Heart failure was reported as optimally treated according to accepted guidelines at the time of enrolment. All five trials used sildenafil 25 to 75 mg three times daily compared to placebo. Three trials had a 12‐week duration, one trial was for six months (Bermejo 2017), and one trial (Guazzi 2011a) had a 12‐month duration. In terms of primary outcomes, three of the five reported improvement in WHO functional class (Bermejo 2017; Hoendermis 2015; Lewis 2007), three of the five reported 6MWD (Bermejo 2017; Lewis 2007; Ovchinnov 2015), and three of the five reported mortality (Bermejo 2017; Hoendermis 2015; Lewis 2007). Haemodynamic data were assessed using right‐heart catheterisation in three of the five trials (Guazzi 2011a; Hoendermis 2015; Lewis 2007), and pulmonary artery systolic pressure (PASP) using transthoracic ultrasound in one trial (Bermejo 2017). Quality of life was reported in one trial (Hoendermis 2015) using the Kansas City Cardiomyopathy Questionnaire (23 items, a higher score indicating better quality of life), and one trial (Guazzi 2011a) reported dyspnoea. Two trials reported adverse events, including exacerbation of heart failure (Bermejo 2017; Guazzi 2011a).
Group 3: Pulmonary hypertension due to lung disease
Five trials included PH secondary to lung disease, predominantly that of COPD (Blanco 2013; Goudie 2014; Rao 2011; Vitulo 2016), and one with IPF (Han 2013). The duration was 12 to 16 weeks (median 12 weeks). Four compared sildenafil to placebo (Blanco 2013; Han 2013; Rao 2011; Vitulo 2016) and one (Goudie 2014) compared tadalafil to placebo. The primary outcomes of improvement in WHO functional class were reported in only one trial (Rao 2011); 6MWD in all five trials, and no trials reported mortality. Only two trials (Rao 2011; Vitulo 2016) reported haemodynamic data. Blanco 2013 reported quality of life using SFGRQ (although this was reported using median and IQR) and SF‐36. Goudie 2014 reported SF‐36 and Minnesota Questionnaire. Vitulo 2016 reported SF‐36 and BODE index. No trials reported dyspnoea. Three trials reported adverse events, although insufficient data were provided to produce a meta‐analysis.
Group 4: Chronic thromboembolic pulmonary hypertension
Three trials included CTEPH participants (Galiè 2016a; Palazzini 2010; Suntharalingham 2008). All trials used sildenafil, with two (Palazzini 2010; Suntharalingham 2008) comparing to placebo, and Galiè 2016a comparing to bosentan. Trial duration was a mean of 3.5 months. Improvement in functional class was reported in one study, 6MWD in all three studies, mortality in all three studies, and haemodynamic data in all three studies. One study (Suntharalingham 2008) measured quality of life using the CAMPHOR scale (higher scores indicate worse quality of life), and also reported dyspnoea using the Borg scale. Only one study reported adverse events.
Mixed group 2 to 4 pulmonary hypertension
Two trials enrolled participants with PH across WHO Groups 2 and 3 (Dwivedi 2015; Salem 2013), including participants with secondary pulmonary hypertension due to COPD, heart failure, valvular heart disease, and cardiomyopathy. Sildenafil 20 to 50 mg three times daily was compared to placebo. Only improvement in functional class, 6MWD, and PASP were reported.
Group 5: Haematological disorders
Two trials included participants with PH secondary to haematological disorders (Machado 2009 (sickle cell disease); Jalalian 2015 (beta‐thalassemia)). We analysed these trials descriptively and separately.
Excluded studies
We excluded 65 studies for the following reasons: ineligible participant population (6); ineligible intervention (13); ineligible study design (22); extension of a previous study (9); post‐hoc analysis (15) (see Characteristics of excluded studies).
Risk of bias in included studies
We assessed the risks of bias in the included studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2017), and included the domains of allocation, blinding, incomplete outcome data, selective reporting, and other potential sources of bias. Please see Figure 2 for a summary of the 'Risk of bias' findings.
2.
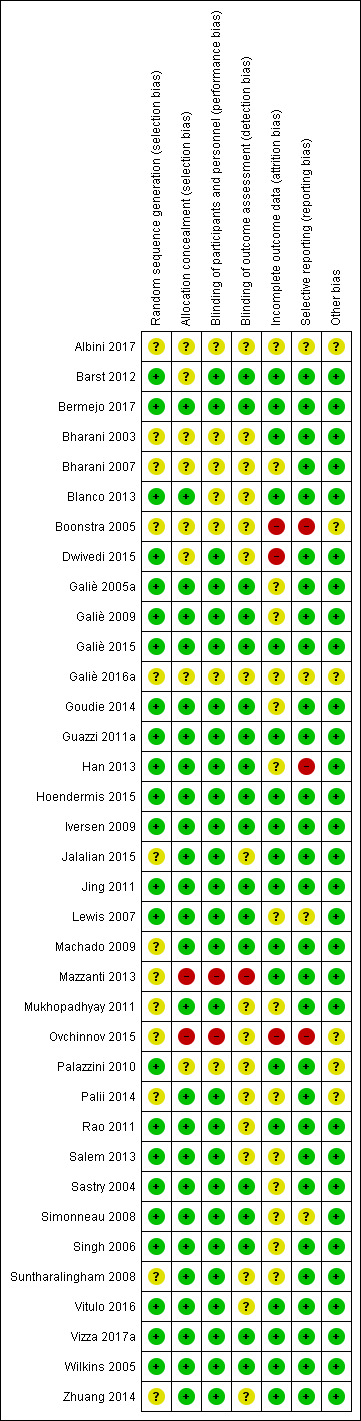
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed random sequence generation as low risk for 23 studies. Albini 2017; Bharani 2003; Bharani 2007; Boonstra 2005; Galiè 2016a; Jalalian 2015; Machado 2009; Mazzanti 2013; Mukhopadhyay 2011; Ovchinnov 2015; Palii 2014; Suntharalingham 2008; Zhuang 2014 were at unclear risk, as they did not report methods of random sequence generation.
We assessed allocation concealment as low risk for 26 studies and unclear risk for Albini 2017; Barst 2012; Bharani 2003; Bharani 2007; Boonstra 2005; Dwivedi 2015; Galiè 2016a; Palazzini 2010, as allocation concealment was not reported. We assessed Mazzanti 2013 and Ovchinnov 2015 as high risk, as each study was reported as an open‐label study.
Blinding
We assessed Bharani 2003; Bharani 2007; Blanco 2013; Boonstra 2005; Galiè 2016a; Palazzini 2010; Palii 2014; Rao 2011; Salem 2013; Suntharalingham 2008; Vitulo 2016 as being at unclear risk, as blinding of participants and personnel was not clearly reported. Ovchinnov 2015 and Mazzanti 2013 were assessed as being at high risk as the trials were reported as open‐label studies, so it was likely that participants knew which intervention they were receiving.
We assessed Albini 2017; Bharani 2003; Bharani 2007; Blanco 2013; Boonstra 2005; Dwivedi 2015; Galiè 2016a; Jalalian 2015; Mukhopadhyay 2011; Ovchinnov 2015; Palazzini 2010; Palii 2014; Rao 2011; Salem 2013; Suntharalingham 2008; Vitulo 2016; Zhuang 2014 as being at unclear risk of detection bias, as methods for blinding of outcomes were not clearly reported. We assessed Mazzanti 2013 as being at high risk, as this trial was reported as open‐label and there were no specified methods to keep outcomes blinded from assessors.
Incomplete outcome data
We assessed attrition bias as unclear risk in 15 studies: Albini 2017; Bharani 2007; Galiè 2005a; Galiè 2009; Galiè 2016a; Goudie 2014; Han 2013; Lewis 2007; Mukhopadhyay 2011; Palii 2014; Salem 2013; Sastry 2004; Simonneau 2008; Singh 2006; Suntharalingham 2008, given that only a proportion of participants who were enrolled completed the study, for reasons which were not given; however, this included both intervention and comparator arms in approximately equal proportions.
For Boonstra 2005 and Dwivedi 2015, about one‐third of enrolled participants did not complete the study, for unknown reasons, so we assigned this a high risk of bias. For Ovchinnov 2015, the number of enrolled participants and the number of completers were not reported, so we rated this at high risk.
Selective reporting
We found reporting bias to be high risk in Boonstra 2005, as only one outcome was reported. Han 2013 was a substudy of the STEP‐IPF trial, and it is not clear if this analysis was planned a priori so we assessed it as high risk. Ovchinnov 2015 only reported quantitative data from the intervention group and not from the comparator group, and no between‐group effect sizes were reported, so we rated it at high risk. Simonneau 2008 reported that they only assessed secondary outcomes if the primary outcomes were significant, although it is unclear if this affected the overall reporting of the trial outcomes, as a number of secondary outcomes were reported; we assessed this as high risk of bias.
Other potential sources of bias
We assessed Albini 2017; Boonstra 2005; Galiè 2016a; Ovchinnov 2015; Palazzini 2010; and Palii 2014 as unclear risk of other bias, as these were abstracts from conference proceedings, and were not recorded in any clinical trials registry, with limited information about methods used and limited data presented to determine if other significant risk was present.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. Group 1 Pulmonary arterial hypertension ‐ PDE5i compared to placebo.
| Group 1 Pulmonary arterial hypertension ‐ PDE5i compared to placebo | ||||||
| Patient or population: people with pulmonary arterial hypertension Setting: outpatients Intervention: PDE5 inhibitors Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with PDE5i | |||||
| Improvement in WHO functional class | 61 per 1000 | 358 per 1000 (204 to 549) | OR 8.59 (3.95 to 18.72) | 282 (4 RCTs) | ⊕⊕⊕⊕ HIGH | ‐ |
| Six‐minute walk distance | Ranges from 170 ‐ 319 ma | MD 48 metres higher (40 higher to 56 higher) | ‐ | 880 (8 RCTs) | ⊕⊕⊕⊝b MODERATE | 6MWD in PAH MCID is 41 metres |
| Mortality | 41 per 1000 | 9 per 1000 (3 to 28) | OR 0.22 (0.07 to 0.68) | 1119 (8 RCTs) | ⊕⊕⊕⊕ HIGH | ‐ |
| Quality of life SF‐36: (scores 1 to 100, higher scores indicate better QoL) EQ‐5D questionnaire: (higher scores indicate worse QoL) CHFQ: (lower scores indicate worse QoL) |
Galiè 2005a found a statistically significant improvement in all SF‐36 domains for sildenafil‐treated participants, and when compared to placebo in physical functioning (P < 0.001), general health (P < 0.001), and vitality (P < 0.05). There was also a statistically significant improvement in placebo‐treated participants in the physical functioning domain. Galiè 2005a found statistically significant improvements for the EQ‐5D current health status (P < 0.01) and utility index (P < 0.01). Sastry 2004 found a statistically significant difference for the CHFQ fatigue domain (sildenafil post‐treatment score 22.33, SD 4.82 compared to placebo post‐treatment score 20.67, SD 5.19; P = 0.04), and a non‐statistically significant difference in the emotional function domain (sildenafil post‐treatment score 37.33, SD 9.3, compared to placebo post‐treatment score 34.71, SD 10.91; P = 0.06), favouring sildenafil compared with placebo. |
163 (2 RCTs) |
‐ | Data considered too heterogeneous to meta‐analyse | ||
| PAP | ‐ | MD 6.43 mmHg lower (8.13 lower to 4.74 lower) | ‐ | 453 (6 RCTs) | ⊕⊕⊕⊝b MODERATE | The higher the mean PAP, the worse the PH |
| RAP | ‐ | MD 1.35 mmHg lower (2.34 lower to 0.36 lower) | ‐ | 341 (3 RCTs) | ⊕⊕⊕⊕ HIGH | The higher the RAP, the worse the PH |
| Cardiac index | ‐ | MD 0.28L/min/m2 higher (0.16 higher to 0.4 higher) | ‐ | 239 (4 RCTs) | ⊕⊕⊕⊝b MODERATE | The lower the cardiac index, the worse the PH |
| PVR | ‐ | MD 4.74 WU lower (6.13 lower to 3.35 lower) | ‐ | 266 (3 RCTs)) | ⊕⊕⊕⊕ HIGH | The higher the PVR. the worse the PH |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walk distance; CI: Confidence interval; EQ‐5D: EuroQoL 5D; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; PAP: pulmonary arterial pressure; PDE‐5i: phosphodiesterase‐5 inhibitor; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; RAP: right atrial pressure; RCT: randomised controlled trials; SD: standard deviation; SF‐36: Medical Outcomes Study 36‐item short form; WU: woods units; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aPost‐treatment values for participants in the placebo group were presented in two studies only; the remaining included studies presented a mean difference only. bDowngraded due to imprecision owing to significantly high heterogeneity, although the direction of effect is consistent.
Summary of findings 2. Group 1 Pulmonary arterial hypertension ‐ PDE5i compared to placebo, on combination therapy.
| Group 1 Pulmonary arterial hypertension ‐ PDE5i compared to placebo, on combination therapy | ||||||
| Patient or population: people with pulmonary arterial hypertension Setting: outpatients Intervention: PDE5 inhibitors plus other disease‐modifying therapies Comparison: placebo plus other disease‐modifying therapies | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo, on combination therapy | Risk with PDE5i | |||||
| Improvement in WHO functional class | 263 per 1000 | 300 per 1000 (191 to 437) | OR 1.20 (0.66 to 2.17) | 227 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ |
| Six‐minute walk distance | Ranges from 341 ‐ 377 mb | MD 20 metres higher (9 higher to 30 higher) | ‐ | 509 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | 6MWD in PAH MCID is 41 metres |
| Mortality | 32 per 1000 | 9 per 1000 (2 to 34) | OR 0.26 (0.07 to 1.06) | 492 (3 RCTs) | ⊕⊕⊕⊝ MODERATEc | ‐ |
| Quality of life physical functioning on SF‐36 (higher scores indicate better quality of life) |
0.3 (4.7 higher to 4.1 higher) | 7.8 (3.6 higher to 12.1 higher) | ‐ | 267 (1 RCT) |
⊕⊕⊕⊝ MODERATEd | ‐ |
| PAP | ‐ | MD 4.58 mmHg lower (6.14 lower to 3.01 lower) | ‐ | 387 (2 RCTs) | ⊕⊕⊕⊕ HIGH | The higher the PAP, the worse the pulmonary hypertension |
| Cardiac output | ‐ | MD 0.87 L/min higher (0.53 higher to 1.21 higher) | ‐ | 310 (3 RCTs) | ⊕⊕⊕⊕ HIGH | The lower the cardiac output, the worse the pulmonary hypertension |
| PVR | ‐ | SMD 0.48 lower (0.72 lower to 0.25 lower) | ‐ | 303 (3 RCTs) | ⊕⊕⊕⊕ HIGH | The higher the PVR, the worse the pulmonary hypertension |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walk distance; CI: Confidence interval; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; PAP: pulmonary arterial pressure; PDE‐5i: phosphodiesterase‐5 inhibitor; PVR: pulmonary vascular resistance; RCT: randomised controlled trials; SMD: standardised mean difference; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded due to imprecision owing to small participant numbers and inconsistent direction of effect. bRange of baseline values, as studies only presented mean difference values for analysis. cDowngraded due to imprecision as the confidence interval crosses the line of no difference. dDowngraded due to imprecision owing to small participant numbers in one trial.
Summary of findings 3. Group 1 Pulmonary arterial hypertension ‐ PDE5i compared to ERA.
| Group 1 Pulmonary Arterial Hypertension ‐ PDE5i compared to ERA | ||||||
| Patient or population: people with pulmonary arterial hypertension Setting: outpatients Intervention: PDE5 inhibitors Comparison: endothelin receptor antagonists(ERA) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ERA | Risk with PDE5i | |||||
| Improvement in WHO functional class | 339 per 1000 | 325 per 1000 (220 to 450) | OR 0.94 (0.55 to 1.60) | 244 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ‐ |
| Six‐minute walk distance | Ranges from 290 ‐ 354 mb | MD 49 higher (4 higher to 95 higher) | ‐ | 36 (2 RCTs) | ⊕⊕⊝⊝ LOWc | 6MWD in PAH MCID is 41 metres |
| Mortality | 14 per 1000 | 45 per 1000 (11 to 167) | OR 3.19 (0.74 to 13.64) | 272 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ |
| Quality of life Kansas City Cardiomyopathy Quality‐of‐Life questionnaire (higher scores indicate better quality of life) |
‐ | MD 22 higher (9 higher to 35 higher) | ‐ | 25 (1 RCT) | ⊕⊕⊝⊝ LOWc | ‐ |
| PAP | ‐ | MD 7.00 mmHg lower (4.82 lower to 18.82 higher) | ‐ | 11 (1 RCT) |
⊕⊕⊝⊝ LOWd | The higher the mean PAP, the worse the PH |
| RAP | ‐ | MD 2 mmHg higher (2.14 lower to 6.14 higher) | ‐ | 11 (1 RCT) |
⊕⊕⊝⊝ LOWd | The higher the RAP, the worse the PH |
| Cardiac index | ‐ | MD 0 L/min/m2 higher (0.49 lower to 0.49 higher) | ‐ | 11 (1 RCT) |
⊕⊕⊝⊝ LOWd | The lower the cardiac index, the worse the PH |
| PVR | ‐ | MD 0 WU lower (1.93 lower to 1.93 higher) | ‐ | 11 (1 RCT) |
⊕⊕⊝⊝ LOWd | The higher the PVR. the worse the PH |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walk distance; CI: Confidence interval; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; PDE‐5i: phosphodiesterase‐5 inhibitor; RCT: randomised controlled trials; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded once due to imprecision. bRange of baseline values, as studies only presented mean difference values for analysis. cDowngraded twice due to imprecision and small participant numbers. dDowngraded twice due to very small participant numbers and high risk of bias.
Summary of findings 4. Group 2 Pulmonary hypertension due to left‐heart disease ‐ PDE5i compared to placebo.
| Group 2 Pulmonary hypertension due to left‐heart disease ‐ PDE5i compared to placebo | ||||||
| Patient or population: people with pulmonary hypertension due to left‐heart disease Setting: outpatients Intervention: PDE5 inhibitors Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with PDE5i | |||||
| Improvement in WHO functional class | 403 per 1000 | 263 per 1000 (178 to 370) | OR 0.53 (0.32 to 0.87) | 285 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ |
| Six‐minute walk distance | No data reported | MD 34 metres higher (23 higher to 46 higher) | ‐ | 284 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ |
| Mortality | 22 per 1000 | 27 per 1000 (6 to 114) | OR 1.27 (0.28 to 5.80) | 286 (3 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ |
| Quality of life | 19.83 points higher (8.23 higher to 31.44 higher) | 12.05 points higher (1.14 higher to 22.96 higher) | ‐ | 52 (1 RCT) |
⊕⊕⊝⊝ LOWc | Kansas City Cardiomyopathy Questionnaire (higher scores reflect better health status) |
| Mean PAP | ‐ | MD 10.17 mmHg lower (11.99 lower to 8.35 lower) | ‐ | 130 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | The higher the mean PAP, the worse the pulmonary hypertension |
| Cardiac index | ‐ | MD 0.07 L/min/m2 higher (0.17 lower to 0.3 higher) | ‐ | 96 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | The lower the cardiac index, the worse the pulmonary hypertension |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walk distance; CI: Confidence interval; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; PAP: pulmonary arterial pressure; PDE‐5i: phosphodiesterase‐5 inhibitor; RCT: randomised controlled trials; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded due to high heterogeneity and inconsistent direction of effect. bDowngraded due to imprecision as the confidence interval crosses the line of no difference. cDowngraded twice due to imprecision owing to few participant numbers in one trial.
Summary of findings 5. Group 3 Pulmonary hypertension due to lung disease ‐ PDE5i compared to placebo.
| Group 3 Pulmonary hypertension due to lung disease ‐ PDE5i compared to placebo | ||||||
| Patient or population: people with pulmonary hypertension due to lung disease Setting: outpatients Intervention: PDE5 inhibitors Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with PDE5i | |||||
| Improvement in WHO functional class | 50 per 1000 | 700 per 1000 (201 to 956) | OR 44.33 (4.78 to 410.94) | 40 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ |
| Six‐minute walk distance | Ranges from 237 ‐ 297 metres | MD 27 metres higher (2 higher to 51 higher) | ‐ | 350 (5 studies) | ⊕⊕⊕⊕ HIGH | ‐ |
| Mortality | ‐ | ‐ | ‐ | No studies | ‐ | ‐ |
| Quality of life | ‐ | MD 0.19 higher (0.07 lower to 0.44 higher) | ‐ | 238 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | SF‐36 overall quality of life (higher scores indicate better quality of life) |
| Mean PAP | ‐ | MD 0.14 mmHg lower (6.65 lower to 6.37 higher) | ‐ | 61 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | The higher the mean PAP, the worse the pulmonary hypertension |
| Cardiac index | ‐ | MD 0.3 L/min/m2 higher (0.14 lower to 0.74 higher) | ‐ | 28 (1 RCT) | ⊕⊕⊝⊝ LOWa | The lower the cardiac index, the worse the pulmonary hypertension |
| PVR | ‐ | MD 1.31 WU lower (3.67 lower to 1.05 higher) | ‐ | 28 (1 RCT) | ⊕⊕⊝⊝ LOWa | The higher the PVR, the worse the pulmonary hypertension |
| RAP | ‐ | MD 0.36 mmHg higher (2.76 lower to 3.48 higher) | ‐ | 28 (1 RCT) | ⊕⊕⊝⊝ LOWa | The higher the RAP, the worse the pulmonary hypertension |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; PAP: pulmonary arterial pressure; PDE‐5i: phosphodiesterase‐5 inhibitor; PVR: pulmonary vascular resistance; RAP: right atrial pressure; RCT: randomised controlled trials; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded twice due to imprecision owing to small participant numbers. bDowngraded once due to imprecision.
Summary of findings 6. Group 4 Pulmonary hypertension due to CTEPH ‐ PDE5i compared to placebo.
| Group 4 Pulmonary hypertension due to CTEPH ‐ PDE5i compared to placebo | ||||||
| Patient or population: people with pulmonary hypertension due to CTEPH Setting: outpatients Intervention: PDE5 inhibitors Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with comparison (placebo/sildenafil) | Risk with PDE5i | |||||
| Improvement in WHO functional class | 0 per 1000 | 444 per 1000 | OR 17.18 (0.78 to 380.84) | 19 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ |
| Six‐minute walk distance ‐ sildenafil compared to placebo | Baseline 331 metres | MD 18 metres higher (24 lower to 59 higher) | ‐ | 19 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ |
| Six‐minute walk distance ‐ sildenafil compared to bosentan | Ranges from 422 ‐ 455 metres | MD 20 metres higher (28 lower to 69 higher) | ‐ | 227 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ |
| Mortality ‐ sildenafil versus placebo | No deaths reported | not estimable | 20 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ | |
| Mortality ‐ sildenafil versus bosentan | 40 per 1000 | 54 per 1000 (9 to 261) | OR 1.36 (0.22 to 8.48) | 106 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ |
| Quality of life | ‐ | MD 0.26 lower (1.17 lower to 0.64 higher) | ‐ | 34 (1 RCT) | ⊕⊕⊝⊝ LOWa | CamPHOR scale; higher scores indicate worse quality of life |
| Mean PAP ‐ sildenafil versus placebo | ‐ | MD 6.2 mmHg lower (12.4 lower to 0 higher) | ‐ | 19 (1 RCT) | ⊕⊕⊝⊝ LOWa | The higher the PAP, the worse the pulmonary hypertension |
| Mean PAP ‐ sildenafil versus bosentan | ‐ | MD 0.76 mmHg higher (3.96 lower to 5.48 higher) | ‐ | 227 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | The higher the PAP, the worse the pulmonary hypertension |
| Cardiac index ‐ sildenafil versus placebo | ‐ | MD 0 L/min/m2 higher (0.4 lower to 0.4 higher) | ‐ | 19 (1 RCT) | ⊕⊕⊝⊝ LOWa | The lower the cardiac index, the worse the pulmonary hypertension |
| Cardiac index ‐ sildenafil versus bosentan | ‐ | MD 0.04 L/min/m2 higher (0.22 lower to 0.31 higher) | ‐ | 227 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | The lower the cardiac index, the worse the pulmonary hypertension |
| PVR ‐ sildenafil versus placebo | ‐ | MD 0.89 WU lower (1.85 lower to 0.06 higher) | ‐ | 19 (1 RCT) | ⊕⊕⊝⊝ LOWa | The higher the PVR, the worse the pulmonary hypertension |
| PVR ‐ sildenafil versus bosentan | ‐ | MD 0.01 WU lower (0.27 lower to 0.25 higher) | ‐ | 227 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb | The higher the PVR, the worse the pulmonary hypertension |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; CTEPH: chronic thromboembolic pulmonary hypertension; MD: mean difference; OR: odds ratio; PAP: pulmonary arterial pressure; PDE‐5i: phosphodiesterase‐5 inhibitor; PVR: pulmonary vascular resistance; RCT: randomised controlled trials; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded twice due to imprecision owing to small participant numbers in only one trial. bDowngraded once due to imprecision.
Summary of findings 7. Mixed Pulmonary hypertension group 2 ‐ 4 ‐ PDE5i compared to placebo.
| Mixed pulmonary hypertension group 2 ‐ 4 ‐ PDE5i compared to placebo | ||||||
| Patient or population: people with pulmonary hypertension group 2 ‐ 4 Setting: outpatients Intervention: PDE5 inhibitors Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with PDE5i | |||||
| Improvement in WHO functional class | Study population | OR 11.31 (4.90 to 26.14) | 146 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | ‐ | |
| 137 per 1000 | 642 per 1000 (438 to 806) | |||||
| Six‐minute walk distance | No data provided | MD 51 metres higher (7 higher to 95 higher) | ‐ | 106 (1 RCT) | ⊕⊕⊕⊝ MODERATEa,b | ‐ |
| Mortality | ‐ | ‐ | no studies | ‐ | ‐ | ‐ |
| Quality of life | ‐ | ‐ | no studies | ‐ | ‐ | ‐ |
| PASP | ‐ | MD 10 mmHg lower (11.92 lower to 8.08 lower) | ‐ | 146 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | The higher the PASP, the worse the pulmonary hypertension |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; OR: odds ratio; PASP: pulmonary artery systolic pressure; PDE‐5i: phosphodiesterase‐5 inhibitor; RCT: randomised controlled trials; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded due to imprecision owing to small participant numbers. bThe information is from studies at low or unclear risk of bias.
Group 1: Pulmonary arterial hypertension
PDE5 inhibitors compared to placebo
Change in WHO functional class
Four studies assessed change in WHO functional class (Comparison 1). There was a significant improvement in WHO functional class favouring PDE5 inhibitors when compared to placebo, (odds ratio (OR) 8.59, 95% CI 3.95 to 18.72; P < 0.001; 4 trials, 282 participants; I2 = 0%; Analysis 1.1; see Figure 3).
1.1. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 1 Improvement in WHO functional class.
3.

Forest plot of comparison: 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, outcome: 1.1 Improvement in WHO functional class.
Six‐minute walk distance
Eight studies assessed 6MWD. There was a significant improvement in 6MWD using PDE5 inhibitors compared to placebo (mean difference between PDE5i and placebo (MD) 48 metres, 95% CI 40 to 56; P <0.001; 8 trials, 880 participants; Analysis 1.2). The effect was clinically significant, with a mean difference of 48 metres (MCID in PAH is 41 metres (Gilbert 2009)). The effect on 6MWD was comparable between drugs (sildenafil MD 57 metres, 95% CI 44 to 69; P < 0.001; 4 trials, 339 participants; tadalafil MD 38 metres, 95% CI 28 to 49; P < 0.001; 3 trials, 477 participants; vardenafil MD 69 metres, 95% CI 41 to 97; P < 0.001; 1 trial, 64 participants). There was significant heterogeneity between trials (I2 = 71; P = 0.03), which is not fully explained by the different drugs used in each trial (subgroup difference I2 = 51%; P = 0.03).
1.2. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 2 Six‐minute walk distance.
Mortality
There was a significant effect on mortality using PDE5 inhibitors compared to placebo (OR 0.22, 95% CI 0.07 to 0.68, P = 0.009; 8 trials, 1119 participants; Analysis 1.3; see Figure 4). In the placebo group four people out of 100 died, compared to one (95% CI 0 to 3) out of 100 for the PDE5 inhibitor group; see Figure 5. The number needed to treat to prevent one additional death was 32 (95% CI 27 to 78).
1.3. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 3 Mortality.
4.
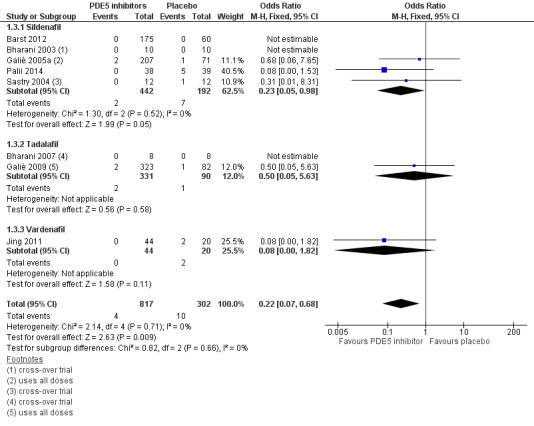
Forest plot of comparison: 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, outcome: 1.3 Mortality.
5.

Cates Plot for mortality with PDE5 inhibitor treatment in Group 1 PAH
Haemodynamics
There was a significant reduction in the mean PAP (MD −6.43 mmHg, 95% CI −8.13 to −4.74; P < 0.001; 6 trials, 453 participants; Analysis 1.4), in the right arterial pressure (RAP) (MD −1.35 mmHg, 95% CI −2.34 to −0.36; P = 0.008; 3 trials, 341 participants; Analysis 1.5), in cardiac index (MD 0.28 L/min/m2, 95% CI 0.16 to 0.40; P < 0.001; 4 trials, 239 participants; Analysis 1.6), in PVR (MD −4.74 WU, 95% CI −6.13 to −3.35; P < 0.0001; 3 trials, 266 participants; Analysis 1.7), and in PASP (MD −11.62 mmHg, 95% CI −25.18 to 1.94; P = 0.09; 2 trials, 48 participants; Analysis 1.8). There was significant heterogeneity between trials for mean PAP (I2 = 76%; P = 0.01). However, the test for subgroup difference was not significant (P = 0.23; I2 = 31%), suggesting that factors other than different drugs across trials may account for this heterogeneity. There was also significant heterogeneity for cardiac index (I2 = 77%; P = 0.005). The test for subgroup difference (P = 0.03; I2 = 82%) indicated that the difference between different drugs used may account for this heterogeneity.
1.4. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 4 PAP.
1.5. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 5 RAP.
1.6. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 6 CI.
1.7. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 7 PVR.
1.8. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 8 PASP.
Exercise capacity other than six‐minute walk distance
Two studies assessed exercise capacity. Sastry 2004 examined time on treadmill (using the Naughton protocol) and found a statistically significant difference favouring sildenafil with post‐treatment sildenafil 686.82 metres (SD 224.02) compared to placebo 475.05 metres (SD 168.02; P < 0.001). Singh 2006 assessed Mets achieved using a modified Bruce exercise protocol. There was a statistically significant difference favouring sildenafil post‐treatment 687 seconds (SD 243.9 sec) compared to placebo 452.1 seconds (SD 165.6 sec) (P < 0.001).
Quality of life
Only two studies assessed quality of life, and data were considered too heterogenous to combine in a meta‐analysis. Galiè 2005a assessed quality of life using two measures: the Medical Outcomes Study 36‐item short form (SF‐36), divided into physical functioning, general health, and vitality domains (scores 1 to 100, higher scores indicate better quality of life), and found a statistically significant improvement in all SF‐36 domains for sildenafil‐treated participants, and when compared to placebo in physical functioning (P < 0.001), general health (P < 0.001), and vitality (P < 0.05). There was also a statistically significant improvement in placebo‐treated participants in the physical functioning domain. They also used the EuroQol 5D (EQ‐5D) questionnaires at baseline and after 12 and 24 weeks of treatment (comprise five dimensions: mobility, self‐care, usual activities, pain/discomfort and anxiety/depression; higher scores indicate worse quality of life). Statistically significant improvements were also observed for the EQ‐5D current health status (P < 0.01) and utility index (P < 0.01).
Sastry 2004 assessed quality of life using a chronic heart failure questionnaire, includes the following domains: dyspnoea, fatigue, and emotional function of daily living; lower scores indicate worse quality of life. There was a statistically significant difference in the fatigue domain (sildenafil post‐treatment score 22.33, SD 4.82 compared to placebo post‐treatment score 20.67, SD 5.19; P = 0.04), and a statistically non‐significant difference in the emotional function domain (sildenafil post‐treatment score 37.33, SD 9.3, compared to placebo post‐treatment score 34.71, SD 10.91; P = 0.06), favouring sildenafil compared with placebo.
Dyspnoea
Five trials assessed dyspnoea: four (Bharani 2003; Bharani 2007; Galiè 2005a; Jing 2011) used the Borg scale and one (Sastry 2004) used a chronic heart failure questionnaire. When we combined the studies using Borg scale, there was a statistically significant improvement in dyspnoea (MD −0.72, 95% CI −0.99 to −0.44; P < 0.001; 4 studies, 239 participants; Analysis 1.9), but the effect size did not meet the minimum clinical improvement difference (MCID = 1, Khair 2016). There was significant heterogeneity across trials (I2 = 64%; P = 0.04), but the test for subgroup differences (I2 = 37%; P = 0.20) suggests that factors other than different drugs may account for differences across the trials.
1.9. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 9 Dyspnoea.
Hospitalisation/intervention
Three trials (746 participants) reported clinical worsening requiring intervention, including hospitalisation and initiation or addition of new therapy. There was no statistically significant difference, although the effect estimate favoured PDE5 inhibitors compared to placebo (OR 0.58, 95% CI 0.27 to 1.23; P = 0.16; Analysis 1.10).
1.10. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 10 Clinical worsening requiring intervention.
Adverse events
There were more adverse events in the PDE5 inhibitor participants compared to placebo (Analysis 1.11).
1.11. Analysis.
Comparison 1 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, Outcome 11 Adverse events.
There was a statistically significant increase in headache (OR 1.97, 95% CI 1.33 to 2.92; P < 0.001; 5 studies, 848 participants), in gastrointestinal (GI) upset (OR 1.63, 95% CI 1.07 to 2.48; P = 0.02; 5 studies, 848 participants), in flushing (OR 4.12, 95% CI 1.83 to 9.26; P < 0.001; 3 studies, 748 participants), and in muscle aches and joint pains (OR 2.52, 95% CI 1.59 to 3.99; P < 0.001; 4 studies, 792 participants).
There was no statistically significant difference in epistaxis (OR 2.37, 95% CI 0.83 to 6.77; P = 0.11; 2 studies, 682 participants), in respiratory symptoms (OR 1.74, 95% CI 0.89 to 3.40; P = 0.10; 3 studies, 748 participants), or in visual disturbance (OR 2.04, 95% CI 0.58 to 7.14; P = 0.27; 3 studies, 748 participants).
Although different formulation of drugs were not compared head‐to‐head, when comparing the odds of developing an adverse event, tadalafil appeared to cause greater frequency of headaches (OR 2.86, 95% CI 1.51 to 5.42; P = 0.001) compared to sildenafil (OR 1.43, 95% CI 0.83 to 2.44; P = 0.20), greater GI upset (OR 1.93, 95% CI 1.07 to 3.50; P = 0.03) compared to sildenafil (OR 1.50, 95% CI 0.81 to 2.78; P = 0.19), and greater frequency of muscle and joint pains (OR 4.02, 95% CI 2.05 to 7.89; P < 0.001) compared to sildenafil (OR 1.48, 95% CI 0.93 to 2.99; P = 0.27).
Subgroup analyses
There were no individual patient data to compare outcomes for a paediatric population versus an adult population. Palii 2014 recruited only children with PAH due to congenital cardiac shunts and found benefit in improvement in functional class, mortality (5 of 39 died at 6 to 12 months in the placebo group compared to none of 38 in the sildenafil group), improved exercise tolerance, and improved haemodynamics. Barst 2012 recruited only children with pulmonary arterial hypertension, and found no significant difference in terms of exercise duration or cardiopulmonary haemodynamics.
Almost all trials included participants with WHO functional class II/III. Boonstra 2005 compared participants with WHO FC I/II and III/IV and found that the six‐minute walk distance improved in both groups (49 metres improvement in WHO FC I/II (n = 27) and 54 metres improvement in WHO FC III/IV (n = 42)).
PDE5 inhibitors compared to placebo on additional therapy
Four trials compared PDE5 inhibitors to placebo, whilst on other therapy for pulmonary hypertension (Comparison 2). Iversen 2009 and Vizza 2017a compared sildenafil to placebo as add‐on therapy to bosentan. Simonneau 2008 compared sildenafil to placebo as add‐on therapy to intravenous epoprostenol. Zhuang 2014 compared tadalafil to placebo as add‐on therapy to ambrisentan.
Change in WHO functional class
There was no significant difference in WHO functional class (OR 1.20, 95% CI 0.66 to 2.17; P = 0.55; 2 trials, 227 participants; Analysis 2.1).
2.1. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 1 Improvement in WHO functional Class.
Six‐minute walk distance
There was a statistically significant difference in 6MWD using PDE5 inhibitors compared to placebo as add‐on therapy (MD 20, 95% CI 9 to 30; P < 0.001; 4 trials, 509 participants; Analysis 2.2), but this did not meet the MCID threshold (41 metres; Gilbert 2009).
2.2. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 2 Six‐minute walk distance.
Mortality
There was no statistically significant difference in mortality using PDE5 inhibitors compared to placebo as add‐on therapy (OR 0.26, 95% CI 0.07 to 1.06; P = 0.06; 3 trials, 492 participants; Analysis 2.3).
2.3. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 3 Mortality.
Haemodynamics
There was a statistically significant reduction in mean PAP (MD −4.58 mmHg, 95% CI −6.14 to −3.01; P < 0.001; 2 trials, 387 participants; Analysis 2.4), and in PVR (SMD −0.48, 95% CI −0.72 to −0.25; P > 0.001; 3 trials, 303 participants; Analysis 2.6) and a statistically significant increase in cardiac output (MD 0.87 L/min, 95% CI 0.53 to 1.21; P < 0.001; 3 trials, 310 participants; Analysis 2.5) using sildenafil compared to placebo as add‐on therapy.
2.4. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 4 PAP.
2.6. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 6 PVR.
2.5. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 5 Cardiac Output.
Exercise capacity other than six‐minute walk distance
No studies reported additional exercise testing.
Quality of life
One trial (Simonneau 2008) assessed quality of life using SF‐36. There was a significant improvement in the sildenafil group in terms of physical functioning (MD 0.3, 95% CI 4.7 to 4.1 for placebo versus MD 7.8, 95% CI 3.6 to 12.1 for sildenafil; P = 0.003), general health (MD 1.4, 95% CI 4.8 to 2.1 versus MD 6.6, 95% CI 3.3 to 9.9; P = 0.001), vitality (MD 0.8, 95% CI 3.3 to 4.9 versus MD 10.2, 95% CI 6.2 to 14.2; P = 0.001), social functioning (MD 2.5, 95% CI 7.8 to 2.9 versus MD 4.0, 95% CI 1.1 to 9.2; P = 0.05), and mental health (MD 3.7, 95% CI 7.0 to 0.5 versus MD 3.0, 95% CI 0.1 to 6.2; P = 0.001), but not in physical role (MD 3.6, 95% CI 5.4 to 12.7 versus MD 10.7, 95% CI 2.0 to 19.4; P = 0.20), bodily pain (MD 0.6, 95% CI 4.3 to 5.5 versus MD 5.8, 95% CI 1.0 to 10.6; P = 0.09), or emotional role (MD 4.6, 95% CI 4.2 to 13.5 versus MD 1.8, 95% CI 6.8 to 10.3; P = 0.60).
Dyspnoea
No studies assessed the effect on dyspnoea.
Hospitalisation/intervention
Four studies assessed clinical worsening requiring hospitalisation or additional therapy. There was a statistically significant difference favouring PDE5 inhibitors compared to placebo as add‐on therapy (OR 0.38, 95% CI 0.21 to 0.68; P = 0.001; 4 trials, 717 participants; Analysis 2.7).
2.7. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 7 Clinical worsening.
Adverse events
There was a statistically significant increased risk of headache (OR 2.49, 95% CI 1.74 to 3.56; P < 0.001; 5 studies, 768 participants), GI upset (OR 1.95, 95% CI 1.30 to 2.93; P = 0.001; 4 studies, 726 participants), muscle and joint pain (OR 2.17, 95% CI 1.28 to 3.67; P = 0.004; 3 studies, 494 participants), and visual disturbance (OR 3.95, 95% CI 0.97 to 16.08; P = 0.06; 2 studies, 368 participants). There was no statistically significant difference in flushing (OR 1.11, 95% CI 0.63 to 1.96; P = 0.71; 2 studies, 368 participants) or epistaxis (OR 1.32, 95% CI 0.34 to 5.12; P = 0.69; 2 studies, 358 participants) Analysis 2.8.
2.8. Analysis.
Comparison 2 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy, Outcome 8 Adverse events.
PDE5 inhibitors compared to endothelin receptor antagonists (ERA)
Three trials compared PDE5 inhibitors head‐to‐head with an endothelin receptor antagonist (Comparison 3): Albini 2017 compared monotherapy (ambrisentan or tadalafil) with combination therapy; for the purpose of this meta‐analysis ambrisentan was compared to tadalafil. Galiè 2015 compared ambrisentan monotherapy with tadalafil monotherapy with combination therapy; for this meta‐analysis, ambrisentan 10 mg daily monotherapy was compared to tadalafil 40 mg daily monotherapy. Wilkins 2005 compared sildenafil (50 mg twice daily for 4 weeks, then 50 mg three times daily) with bosentan (62.5 mg twice daily for 4 weeks, then 125 mg twice daily).
Change in WHO functional class
There was no significant difference in WHO functional class (OR 0.94, 95% CI 0.55 to 1.60; P = 0.82; 1 trial, 244 participants; Analysis 3.1).
3.1. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 1 Improvement in WHO functional class.
Six‐minute walk distance
There was a statistically and clinically significant difference in 6MWD favouring sildenafil compared to bosentan (MD 49 metres, 95% CI 4 to 95; P = 0.03; 2 trials, 36 participants; Analysis 3.2). As Galiè 2015 reported 6MWD as median and IQR, it was not possible to include it in the meta‐analysis (Higgins 2011). The median change from baseline distance in the tadalafil group was 23 metres (IQR −8 to 66) compared to the ambrisentan group 27 metres (IQR −14 to 63).
3.2. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 2 Six‐minute walk distance.
Mortality
There was no significant difference in mortality comparing PDE5 inhibitors to ERA (OR 3.19, 95% CI 0.74 to 13.64; P = 0.12; 2 trials, 272 participants; Analysis 3.3).
3.3. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 3 Mortality.
Haemodynamics
There was no significant difference in haemodynamics in one small study (32 participants), including mean PAP (MD 7.00, 95% CI −4.82 to 18.82; P = 0.25), RAP (MD 2.00, 95% CI −2.14 to 6.14; P = 0.34), cardiac index (MD 0.00, 95% CI −0.49 to 0.49; P = 1.00), and PVR (MD 0.00, 95% CI −1.93 to 1.93; P = 1.00). Wilkins 2005 assessed participants using cardiac magnetic resonance imaging (MRI) and found no significant difference in cardiac index (MD 0 L/min/m2, 95% CI −0.2 to 0.2).
Exercise capacity other than six‐minute walk distance
No studies reported additional exercise testing.
Quality of life
One study (Wilkins 2005) found a significant difference in quality of life favouring sildenafil compared to bosentan using the Kansas City Cardiomyopathy Quality‐of‐Life questionnaire (higher scores indicate better quality of life) (MD 22.00, 95% CI 9.00 to 35.00; P = 0.009).
Dyspnoea
No studies assessed dyspnoea.
Hospitalisation/intervention
There was a statistically significant incidence of clinical worsening in the ERA group compared to the PDE5 inhibitor group (OR 0.52, 95% CI 0.30 to 0.89; P = 0.02; 2 trials, 275 participants; Analysis 3.9).
3.9. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 9 Clinical worsening requiring hospitalisation.
Adverse events
Only one trial assessed adverse events (Analysis 3.10). There was no significant difference in headache (OR 1.10, 95% CI 0.65 to 1.87; P = 0.72), GI upset (OR 0.79, 95% CI 0.42 to 1.45; P = 0.44), flushing (OR 0.60, 95% CI 0.27 to 1.33; P = 0.21), muscle or joint pain (OR 1.34, 95% CI 0.60 to 3.00; P = 0.47), or respiratory symptoms (OR 0.80, 95% CI 0.47 to 1.35; P = 0.40).
3.10. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 10 Adverse events.
Group 2: Pulmonary hypertension due to left‐heart disease
Five trials assessed participants with pulmonary hypertension secondary to left‐heart disease (Comparison 4). All trials compared sildenafil with placebo.
Change in WHO functional class
Significantly fewer people receiving sildenafil improved in WHO functional class compared to placebo (OR 0.53, 95% CI 0.32 to 0.87; P = 0.01; 3 trials, 285 participants; Analysis 4.1), although there was considerable heterogeneity between trials (I2 = 67%, P = 0.05).
4.1. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 1 Improvement in WHO functional class.
Six‐minute walk distance
Three studies assessed the 6MWD. There was a significant difference favouring sildenafil (MD 34 metres, 95% CI 23 to 46; P < 0.001; 3 studies, 284 participants; Analysis 4.2; see Figure 6).
4.2. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 2 Six‐minute walk distance.
6.
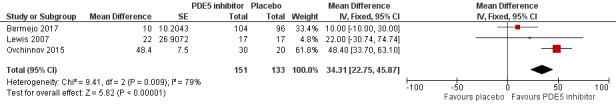
Forest plot of comparison: 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), outcome: 4.2 Six‐minute walk distance.
Mortality
Three studies assessed mortality. There was no significant difference between sildenafil and placebo (OR 1.27, 95% CI 0.28 to 5.80; P = 0.76; 3 trials, 286 participants; Analysis 4.3).
4.3. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 3 Mortality.
Haemodynamics
There was a significant reduction in mean PAP (MD −10.17 mmHg, 95% CI −11.99 to −8.35; P < 0.001, although with significant heterogeneity (I2 = 98%, P < 0.001); 3 trials, 130 participants; Analysis 4.4), transpulmonary pressure gradient (TPG) (MD −14.60 mmHg, 95% CI −15.63 to −13.57; P < 0.001; 1 trial, 44 participants; Analysis 4.6), and PASP (MD −27.60 mmHg, 95% CI −30.37 to −24.83; P < 0.001; 1 trial, 44 participants; Analysis 4.8) using sildenafil compared to placebo. There was no significant difference in cardiac index (MD 0.07 L/min/m2, 95% CI −0.17 to 0.30; P = 0.59; 2 trials, 96 participants; Analysis 4.5) or RAP (MD −1.00 mmHg, 95% CI −3.77 to 1.77; P = 0.48; 1 trial, 34 participants; Analysis 4.7). Bermejo 2017 reported results in median and IQR so we could not combine the data in the meta‐analysis, but there was no significant difference in haemodynamic parameters measured by Doppler echocardiography or magnetic resonance.
4.4. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 4 mean PAP.
4.6. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 6 TPG.
4.8. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 8 PASP.
4.5. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 5 Cardiac Index.
4.7. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 7 RAP.
Exercise capacity other than six‐minute walk distance
Two studies reported exercise capacity testing. Hoendermis 2015 reported no significant difference in VO2 peak (treatment effect sildenafil MD 0.2 mL/kg/min‐1 (95% CI ‐0.9 to 1.4) compared to placebo MD 0.70 mL/kg/min‐1 (95% CI −0.3 to 1.6); P = 0.51). Lewis 2007 reported a statistically significant difference in VO2 peak favouring sildenafil (post‐treatment sildenafil MD 1.8 ± 0.7 mL/kg/min‐1 compared to placebo MD 0.27 mL/kg/min‐1; P = 0.02).
Quality of life
One study (Hoendermis 2015) assessed quality of life using the Kansas City Cardiomyopathy Questionnaire (KCCQ: higher scores reflect better health status). The mean change in the KCCQ clinical summary score from baseline to 12 weeks was 12.05 points higher (95% CI 1.14 to 22.96) in the sildenafil group and 19.83 points higher (95% CI 8.23 to 31.44) in the placebo group, although the difference was not significant (P = 0.32).
Dyspnoea
One study (Guazzi 2011a) assessed dyspnoea using the 16‐item Chronic Heart Failure Questionnaire (CHQ) (Guyatt 1989). There was a significant improvement in dyspnoea using sildenafil compared to placebo (MD 1.15, 95% CI 0.51 to 1.79; P < 0.001; 1 trial, 44 participants; Analysis 4.9).
4.9. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 9 Dyspnoea.
Hospitalisation/intervention
There were mixed results in terms of clinical worsening requiring intervention including hospitalisation. Lewis 2007 (PH with systolic dysfunction) reported no significant difference in clinical worsening (OR 0.32, 95% CI 0.05 to 1.95; P = 0.22; 34 participants; Analysis 4.10), but Bermejo 2017 (valvular heart disease) reported an increased risk of hospitalisations, particularly relating to heart failure in those treated with sildenafil compared to placebo (OR 1.43; 95% CI 0.76 to 2.70; P = 0.05; 200 participants).
4.10. Analysis.
Comparison 4 Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo), Outcome 10 Clinical worsening.
Adverse events
Three studies reported adverse events. In Lewis 2007 the incidence of headache occurred more commonly in those treated with sildenafil compared to placebo (1 trial; P < 0.05) but there was no significant difference in terms of arrhythmias, hypotension, diarrhoea, flushing, or myalgia, and no incidence of visual disturbance. Hoendermis 2015 reported that adverse events occurred in 22 participants (85%) who received sildenafil and 21 participants (81%) who received placebo (P = 1.00). Prevalence of known adverse effects of sildenafil, such as headache, dyspepsia, (orthostatic) hypotension, increased erection and respiratory tract infection, were reported to be higher in the sildenafil group, but event data were not supplied. Bermejo 2017 reported an increased risk of gastrointestinal events (OR 2.82, 95% CI 0.91 to 10.41; P = 0.06), and infections (OR 0.22, 95% 0.02 to 1.13; P = 0.05).
Group 3: Pulmonary hypertension due to lung disease
Five trials included participants with pulmonary hypertension secondary to lung disease or hypoxaemia; in four trials this was secondary to COPD, and in one trial secondary to IPF (Comparison 5) (Han 2013) . Four trials compared sildenafil to placebo, and one trial (Goudie 2014) compared tadalafil to placebo.
Change in WHO functional class
One trial assessed improvement in WHO functional class, and found a statically significant improvement using sildenafil compared with placebo (OR 44.33, 95% CI 4.78 to 410.93; P < 0.001; 40 participants; Analysis 5.1).
5.1. Analysis.
Comparison 5 Group 3 Pulmonary Hypertension due to lung disease, Outcome 1 Improvement in WHO functional class.
Six‐minute walk distance
There was a significant improvement in the 6MWD with the use of sildenafil compared to placebo (MD 27 metres, 95% CI 2 to 51; P < 0.001; 5 trials, 350 participants; Analysis 5.2; see Figure 7).
5.2. Analysis.
Comparison 5 Group 3 Pulmonary Hypertension due to lung disease, Outcome 2 Six‐minute walk distance.
7.

Forest plot of comparison: 5 Group 3 Pulmonary Hypertension due to lung disease, outcome: 5.2 Six‐minute walk distance.
Mortality
No studies assessed mortality.
Haemodynamics
There was no significant difference in terms of mean PAP (MD −0.14 mmHg, 95% CI −6.65 to 6.37; P = 0.97; 2 trials, 61 participants; Analysis 5.4), cardiac index (MD 0.30 L/min/m2, 95% CI −0.14 to 0.74; P = 0.18; 1 trial, 28 participants; Analysis 5.5), RAP (MD 0.36 mmHg, 95% CI −2.76 to 3.48; P = 0.82; 1 trial, 28 participants; Analysis 5.7) and PVR (MD −1.31 mmHg, 95% CI −3.67 to 1.05; P = 0.28; 1 trial, 28 participants; Analysis 5.6).
5.4. Analysis.
Comparison 5 Group 3 Pulmonary Hypertension due to lung disease, Outcome 4 mean PAP.
5.5. Analysis.
Comparison 5 Group 3 Pulmonary Hypertension due to lung disease, Outcome 5 Cardiac Index.
5.7. Analysis.
Comparison 5 Group 3 Pulmonary Hypertension due to lung disease, Outcome 7 RAP.
5.6. Analysis.
Comparison 5 Group 3 Pulmonary Hypertension due to lung disease, Outcome 6 PVR.
Exercise capacity other than six‐minute walk distance
Blanco 2013 reported exercise capacity after three months pulmonary rehabilitation and treatment with sildenafil or placebo. There was no difference in the placebo‐corrected difference in the cycle endurance time: −7 seconds, 90% CI −540 to 244; P = 0.77, or maximal exercise tolerance 1 watt, 90% CI −2 to 5; P = 0.60.
Quality of life
Three studies assessed quality of life. Goudie 2014 used SF‐36 (lower scores indicate worse quality of life), and Vitulo 2016 assessed the BODE index and the Minnesota Questionnaire. Han 2013 used the St George's Respiratory Questionnaire (SGRQ), the EuroQOL, and the SF‐36. We pooled data for the SF‐36 physical function, and demonstrated no significant difference in overall quality of life (MD 0.19, 95% CI −0.07 to 0.44; P = 0.15; 2 trials, 238 participants; Analysis 5.3). Blanco 2013 reported outcomes using mean and IQR, so results could not be pooled in the meta‐analysis. There was no significant difference in SGRQ score (placebo‐corrected difference median 1.3, IQR −3.5 to 6.9, P = 0.53), or SF‐36 physical score (median −1.7, IQR −7.2 to 2.3, P = 0.38), or SF‐36 mental score (median 1.6, IQR −3.7 to 8.5 P = 0.64).
5.3. Analysis.
Comparison 5 Group 3 Pulmonary Hypertension due to lung disease, Outcome 3 QOL.
Dyspnoea
No studies assessed dyspnoea.
Hospitalisation/intervention
No studies assessed clinical worsening.
Adverse events
Three trials reported adverse events, but none reported event data that could be pooled in a meta‐analysis. Blanco 2013 reported that adverse events were similar across groups. Rao 2011 reported that headache was the most common side effect with sildenafil. Other adverse effects noted were epigastric pain or discomfort, headache, paraesthesias, and numbness. Vitulo 2016 reported that adverse events were observed in five participants in the sildenafil arm. The events were mild to moderate and included headache, diarrhoea, flushing, limb pain, dyspnoea, myalgia, and peripheral oedema. None interrupted the study treatment because of adverse events. No significant adverse effects were reported in the placebo arm. Two studies reported oxygenation parameters. Vitulo 2016 reported no difference in SpO2 or PaO2 in the sildenafil group from the start to the end of the study. Blanco 2013 reported no significant differences in arterial oxygen tension or saturation between the sildenafil and placebo groups.
Group 4: Chronic thromboembolic pulmonary hypertension (CTEPH)
Three trials included CTEPH participants (Galiè 2016a; Palazzini 2010; Suntharalingham 2008; Comparison 6). All trials used sildenafil, two (Palazzini 2010; Suntharalingham 2008) compared to placebo, and Galiè 2016a compared to bosentan.
Change in WHO functional class
Only one trial examined improvement in WHO functional class in CTEPH participants, and found no significant difference in the use of PDE5 inhibitors compared to placebo (OR 17.18, 95% CI 0.78 to 380.84; P = 0.07; 1 trial, 19 participants; Analysis 6.1).
6.1. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 1 Improvement in WHO functional class.
Six‐minute walk distance
There was no significant difference in 6MWD using PDE5 inhibitors when compared to placebo (MD 18 metres, 95% CI −24 to 59; P = 0.41; 1 trial, 19 participants; Analysis 6.2; see Figure 8) or when compared to bosentan (MD 20 metres, 95% CI −28 to 69; P = 0.41; 2 trials, 227 participants).
6.2. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 2 Six‐minute walk distance.
8.
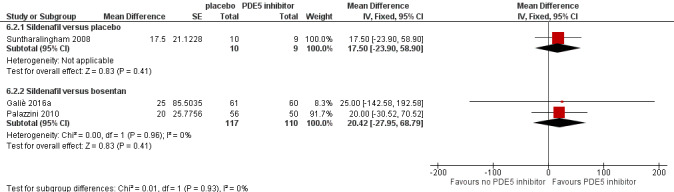
Forest plot of comparison: 6 Group 4 Pulmonary Hypertension due to CTEPH, outcome: 6.2 Six‐minute walk distance.
Mortality
There were no deaths in either arm in one trial comparing sildenafil with placebo (20 participants). There was no significant difference in mortality in one trial comparing PDE5 inhibitors to bosentan (OR 1.36, 95% CI 0.22 to 8.48; P = 0.74; 106 participants; Analysis 6.3).
6.3. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 3 Mortality.
Haemodynamics
There was no difference in haemodynamic parameters. There was no difference in mean PAP using PDE5 inhibitors when compared to placebo (MD −6.20 mmHg, 95% CI −12.40 to −0.00; P = 0.05; 1 trial, 19 participants; Analysis 6.4) or when compared to bosentan (MD 0.76 mmHg, 95% CI −3.96 to 5.48; P = 0.75; 2 trials, 227 participants). There was no difference in cardiac index using PDE5 inhibitors when compared to placebo (MD 0.00 L/min/m2, 95% CI −0.40 to 0.40; P = 1.0; 1 trial, 19 participants; Analysis 6.5) or when compared to bosentan (MD 0.04 L/min/m2, 95% CI −0.22 to 0.31; P = 0.76; 2 trials, 227 participants). There was no difference in PVR using PDE5 inhibitors when compared to placebo (MD −0.89, 95% CI −1.85 to 0.06; P = 0.07; 1 trial, 19 participants; Analysis 6.6) or when compared to bosentan (MD −0.01, 95% CI −0.27 to 0.25; P = 0.96; 2 trials, 227 participants). There was no difference in RAP using PDE5 inhibitors when compared to placebo (MD −0.90 mmHg, 95% CI −6.10 to 4.30; P = 0.73; 1 trial, 19 participants; Analysis 6.7) or when compared to bosentan (MD −1.00 mmHg, 95% CI −3.77 to 1.77; P = 0.48; 1 trial, 121 participants).
6.4. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 4 mean PAP.
6.5. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 5 Cardiac Index.
6.6. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 6 PVR.
6.7. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 7 RAP.
Exercise capacity other than six‐minute walk distance
No studies reported exercise capacity testing.
Quality of life
Only one trial examined quality of life, using the CamHOR scale (higher scores indicate worse quality of life). There was no significant difference (MD −0.26, 95% CI −1.17 to 0.64; P = 0.57; 34 participants; Analysis 6.8).
6.8. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 8 QOL.
Dyspnoea
No trials assessed dyspnoea.
Hospitaisation/intervention
No trials examined clinical worsening.
Adverse events
There were no significant differences in terms of headache (OR 2.57, 95% CI 0.19 to 34.47; P = 0.48; 19 participants; Analysis 6.9) or GI upset (OR 4.50, 95% CI 0.37 to 54.16; P = 0.24; 19 participants) between PDE5‐inhibitors and placebo in one trial.
6.9. Analysis.
Comparison 6 Group 4 Pulmonary Hypertension due to CTEPH, Outcome 9 Adverse events.
Mixed group 2 to 4 pulmonary hypertension
We combined two studies in this meta‐analysis: Salem 2013, which included participants with secondary pulmonary hypertension due to valvular heart disease, chronic thromboembolic disease, COPD, IPF, and idiopathic dilated cardiomyopathy, and Dwivedi 2015, which included participants with symptomatic secondary PAH including idiopathic dilated cardiomyopathy, heart failure with preserved ejection fraction, COPD, and other lung parenchymal disease, and valvular heart disease (Comparison 7).
Change in WHO functional class
There was a significant improvement in WHO functional class favouring PDE5 inhibitors (OR 11.31, 95% CI 4.90 to 26.14; P < 0.001; 2 trials, 146 participants; Analysis 7.1).
7.1. Analysis.
Comparison 7 Mixed Pulmonary Hypertension group 2‐4, Outcome 1 Improvement in WHO functional class.
Six‐minute walk distance
There was a significant difference in 6MWD in one trial favouring PDE5 inhibitors (MD 51 metres, 95% CI 7 to 95; P=0.02; 106 participants; Analysis 7.2).
7.2. Analysis.
Comparison 7 Mixed Pulmonary Hypertension group 2‐4, Outcome 2 6MWD.
Mortality
No trials assessed mortality.
Haemodynamics
There was a significant improvement in PASP (MD −10.00 mmHg, 95% CI −11.92 to −8.08; P < 0.001; 2 trials, 146 participants; Analysis 7.3). No other haemodynamic parameters were measured.
7.3. Analysis.
Comparison 7 Mixed Pulmonary Hypertension group 2‐4, Outcome 3 PASP.
Exercise capacity other than six‐minute walk distance
No studies reported exercise capacity testing.
Quality of life
No trials assessed quality of life.
Dyspnoea
No trials assessed dyspnoea.
Hospitalisation/intervention
No trials assessed clinical worsening.
Adverse events
No trials reported adverse events.
Sensitivity analyses
We planned to use the fixed‐effect model a priori to assess effect sizes. We present a comparison of fixed‐ versus random‐effects in Table 9. Where effect sizes differed, this was explained by high heterogeneity between trials.
2. Sensitivity analysis: fixed‐effect versus random‐effects.
| Meta‐analysis | Number of studies | Effect measure | Fixed‐effect size and CI | Random‐effect size and CI |
| Group 1 Pulmonary arterial hypertension ‐ PDE5i versus placebo | ||||
| Improvement in WHO functional class | 4 | OR | 8.59 (3.95 to 18.72) | 8.53 (3.90 to 18.67) |
| Six‐minute walk distance | 8 | MD | 48.17 (40.30 to 56.04) | 52.98 (40.74 to 65.23)1 |
| Mortality | 7 | OR | 0.22 (0.07 to 0.68) | 0.28 (0.08 to 0.95) |
| PAP | 5 | MD | −6.33 (−8.12 to −4.53) | −8.94 (−13.73 to −4.15) |
| RAP | 2 | MD | −1.52 (−2.79 to −0.24) | −1.52 (−2.79 to −0.24) |
| Cardiac index | 4 | MD | 0.28 (0.16 to 0.40) | 0.35 (0.08 to 0.61)a |
| PVR | 3 | MD | −4.74 (−6.13 to −3.35) | −5.02 (−7.02 to −3.02) |
| PASP | 2 | MD | −11.62 (−25.18 to 1.94) | −11.62 (−25.18 to 1.94) |
| Dypnoea | 4 | MD | −0.72 (−0.99 to −0.44) | −0.61 (−1.19 to −0.02) |
| Clinical worsening requiring intervention | 3 | OR | 0.58 (0.27 to 1.23) | 0.55 (0.25 to 1.23) |
| Group 1 Pulmonary arterial hypertension ‐ PDE5i versus placebo to on combination therapy | ||||
| Improvement in WHO functional Class | 2 | OR | 1.20 (0.66 to 2.17) | 1.09 (0.41 to 2.92) |
| Six‐minute walk distance | 4 | MD | 19.66 (9.22 to 30.10) | 18.94 (0.23 to 37.65) |
| Mortality | 3 | OR | 0.26 (0.07 to 1.06) | 0.38 (0.04 to 3.81) |
| PAP | 2 | MD | −4.58 (−6.14 to −3.01) | −5.12 (−8.21 to −2.03) |
| Cardiac output | 2 | MD | 0.87 (0.53 to 1.21) | 0.87 (0.53 to 1.21) |
| PVR | 3 | SMD | −0.48 (−0.72 to −0.25) | −0.36 (−0.84 to 0.12)a |
| Clinical worsening | 3 | OR | 0.34 (0.18 to 0.63) | 0.34 (0.18 to 0.63) |
| Group 1 Pulmonary arterial hypertension ‐ PDE5i versus ERA | ||||
| Improvement in WHO functional Class | 1 | OR | 0.94 (0.55 to 1.60) | 0.94 (0.55 to 1.60) |
| Six‐minute walk distance | 2 | MD | 49.38 (3.65 to 95.11) | 49.38 (3.65 to 95.11) |
| Mortality | 2 | OR | 3.19 (0.74 to 13.64) | 3.19 (0.74 to 13.64) |
| Quality of life | 1 | MD | 22.00 (9.00 to 35.00) | 22.00 (9.00 to 35.00) |
| PAP | 1 | MD | 7.00 (−4.82 to 18.82) | 7.00 (−4.82 to 18.82) |
| RAP | 1 | MD | 2.00 (−2.14 to 6.14) | 2.00 (−2.14 to 6.14) |
| Cardiac index | 1 | MD | 0.00 (−0.49 to 0.49) | 0.00 (−0.49 to 0.49) |
| PVR | 1 | MD | 0.00 (−1.93 to 1.93) | 0.00 (−1.93 to 1.93) |
| Clinical worsening | 2 | OR | 0.52 (0.30 to 0.89) | 0.52 (0.30 to 0.89) |
| Group 2 Pulmonary hypertension due to left‐heart disease | ||||
| Improvement in WHO functional Class | 3 | OR | 0.53 (0.32 to 0.87) | 0.70 (0.20 to 2.37) |
| Six‐minute walk distance | 3 | MD | 34.31 (22.75 to 45.87) | 28.44 (‐1.82 to 58.69)a |
| Mortality | 3 | OR | 0.01 (−0.03 to 0.04) | 0.01 (−0.03 to 0.04) |
| Mean PAP | 3 | MD | −10.17 (−11.99 to −8.35) | −6.58 (−22.28 to 9.12)a |
| Cardiac index | 2 | MD | 0.07 (−0.17 to 0.30) | 0.06 (−0.22 to 0.34) |
| TPG | 1 | MD | −14.60 (−15.63 to −13.57) | −14.60 (−15.63 to −13.57) |
| RAP | 1 | MD | −1.00 (−3.77 to 1.77) | −1.00 (−3.77 to 1.77) |
| PASP | 1 | MD | −27.60 (−30.37 to −24.83) | −27.60 (−30.37 to −24.83) |
| Dypnoea | 1 | MD | 1.15 (0.51 to 1.79) | 1.15 (0.51 to 1.79) |
| Clinical worsening requiring intervention | 2 | OR | 1.19 (0.66 to 2.14) | 0.87 (0.22 to 3.46) |
| Group 3 Pulmonary hypertension due to lung disease | ||||
| Improvement in WHO functional Class | 1 | OR | 44.33 (4.78 to 410.93) | 44.33 (4.78 to 410.93) |
| Six‐minute walk distance | 5 | MD | 14.04 (7.05 to 21.02) | 26.70 (2.00 to 51.39)1 |
| Mean PAP | 2 | MD | −0.14 (−6.65 to 6.37) | −0.14 (−6.65 to 6.37) |
| Cardiac index | 1 | MD | 0.30 (−0.14 to 0.74) | 0.30 (−0.14 to 0.74) |
| PVR | 1 | MD | −1.31 (−3.67 to 1.05) | −1.31 (−3.67 to 1.05) |
| RAP | 1 | MD | 0.36 (−2.76 to 3.48) | 0.36 (−2.76 to 3.48) |
| Quality of life | 3 | MD | 0.19 (−0.07 to 0.44) | 0.19 (−0.07 to 0.44) |
| Group 4 Pulmonary hypertension due to CTEPH | ||||
| Improvement in WHO functional Class | 1 | OR | 17.18 (0.78 to 380.85) | 17.18 (0.78 to 380.85) |
| Six‐minute walk distance | 3 | MD | 18.73 (−12.72 to 50.19) | 18.73 (−12.72 to 50.19) |
| Mortality | 2 | OR | 0.01 (−0.06 to 0.08) | 0.01 (−0.06 to 0.08) |
| Mean PAP | 2 | MD | −1.79 (−5.55 to 1.96) | −1.67 (−6.48 to 3.15) |
| Cardiac index | 3 | MD | 0.03 (−0.19 to 0.25) | 0.03 (−0.19 to 0.25) |
| PVR | 3 | MD | −0.07 (−0.32 to 0.18) | −0.12 (−0.50 to 0.25) |
| RAP | 2 | MD | −0.98 (−3.42 to 1.47) | −0.98 (−3.42 to 1.47) |
| Quality of life | 1 | MD | −0.26 (−1.17 to 0.64) | −0.26 (−1.17 to 0.64) |
| Mixed pulmonary hypertension group 2 to 4 | ||||
| Improvement in WHO functional Class | 2 | OR | 11.33 (4.91 to 26.15) | 11.33 (4.91 to 26.15) |
| Six‐minute walk distance | 1 | MD | 50.97 (44.88 to 57.06) | 50.97 (44.88 to 57.06) |
| PASP | 2 | MD | −10.00 (−11.92 to −8.08) | −10.00 (−11.92 to −8.08) |
astatistically high heterogeneity
CI ‐ confidence interval; CTEPH ‐ chronic thromboembolic pulmonary hypertension; OR ‐ odds ratio; MD ‐ mean difference; PAP ‐ pulmonary artery pressure; PASP ‐ pulmonary artery systemic pressure; PDE5i ‐ phosphodiesterase‐5 inhibitor; PVR ‐ pulmonary vascular resistance; RAP ‐ right atrial pressure; SMD ‐ standardised mean difference; TPG ‐ transpulmonary gradient; WHO ‐ World Health Organization
Group 5: Haematological disorders
Jalalian 2015 included 44 participants with beta‐thalassaemia intermedia and pulmonary hypertension on transthoracic echocardiogram, and were treated for six weeks with tadalafil (40 mg daily) or placebo. The authors reported a significant improvement in the PASP (post‐treatment tadalafil MD 34.26 mmHg; SE 1.15, compared to post‐treatment placebo MD 44.57 mmHg; SE 0.56; P < 0.001). In the tadalafil group two participants dropped out due to severe headache and one due to severe oedema. Three other participants experienced a mild headache with initiation of tadalafil, which resolved after one week. No deaths occurred after six weeks of this trial.
Machado 2009 included those with pulmonary hypertension secondary to sickle cell disease and tested sildenafil versus placebo. The study was prematurely stopped due to a statistically significant increase in serious adverse events requiring hospitalisation in the sildenafil arm.
Discussion
Summary of main results
This review demonstrates clear statistical and clinical benefit for the use of PDE5 inhibitors in group 1 PAH compared to placebo, in terms of mortality, improvement in WHO functional class, time to clinical worsening, haemodynamics, six‐minute walk distance (6MWD), and quality of life including dyspnoea. Clinicians and patients should be aware of side effects, especially headache, flushing, GI upset, and muscle and joint pain.
Three formulations of PDE5 inhibitors were used: sildenafil, tadalafil and vardenafil. Although none of these were compared head‐to‐head, a beneficial direction of effect appeared across all three drugs. Although the magnitude of effect appeared greater in sildenafil compared with tadalafil or vardenafil, this could be due to more studies and participant numbers in the sildenafil arm. In terms of adverse events, there appeared to be a higher chance of headache, GI upset and muscle aches and pains in the tadalafil arm compared to the sildenafil arm.
We demonstrate mixed evidence for the use of PDE5 inhibitors for left‐heart disease. The trials included were heterogenous in left‐heart disease secondary to different mechanisms. Overall, there was a statistically and clinically significant improvement across all trials in terms of WHO functional class and 6MWD, and an improvement in haemodynamics, quality of life, and dyspnoea, but this did not confer a clear benefit for mortality. It is unclear if this is due to underpowered studies. In addition, there was some evidence of heart failure exacerbations in one study which included participants with valvular heart disease, but despite this being a known potential side effect of these drugs, few studies reported any adverse events.
There is no clear benefit from the use of PDE5 inhibitors in pulmonary hypertension secondary to lung disease, according to this meta‐analysis. There was a statistically but not clinically significant difference in 6MWD, but no benefit for mortality, haemodynamics, quality of life, and clinical worsening. Most studies included COPD patients with pulmonary hypertension; only one included IPF patients. There are no data on PDE5 inhibitors in other lung disease patients.
There was no significant benefit for the use of PDE5 inhibitors in CTEPH, when compared to placebo or bosentan, for mortality, clinical worsening, 6MWD, and haemodynamics, but the numbers of participants were small.
There may be an improvement in cardiopulmonary haemodynamics with the use of PDE5 inhibitors in pulmonary hypertension due to beta‐thalassaemia intermedia. There may be evidence of harm from the use of PDE5 inhibitors in people with pulmonary hypertension secondary to sickle cell disease.
Overall completeness and applicability of evidence
There are sufficient trials of high quality in the use of PDE5 inhibitors compared to placebo for group 1 PAH. Most trials had a low risk of bias, and the direction of effect was consistent across studies. PDE5 inhibitors are one of five classes of drugs now available for group 1 PAH. Current European Society of Cardiology (ESC) guidelines stratify patients according to levels of risk, which correlate with expected survival (Boucly 2017; Hoeper 2017; Kylhammar 2018). The guidelines suggest the use of monotherapy with either a PDE5 inhibitor or an endothelin receptor antagonist (ERA) for people at low risk, and the use of combination therapy or intravenous prostacyclin for those at high risk. Two studies compared the use of PDE5 inhibitors with ERAs, and found a possible benefit for ERA in terms of 6MWD and quality of life, and a non‐statistically significant benefit for improvement in WHO functional class, and clinical worsening, favouring ERAs.
The use of step‐up or upfront combination therapy in people at intermediate risk has been suggested by several recent studies, including SERAPHIN 2013 (where macitentan was added to a PDE5 inhibitor or non‐parenteral prostacyclin, and demonstrated delay in clinical worsening), COMPASS‐2 2014 (where bosentan was added to sildenafil, and demonstrated a small but statistically significant improvement in 6MWD (22 m), but no change in morbidity or mortality), PACES (Simonneau 2008) (where sildenafil was added to epoprostenol and demonstrated a delay in clinical worsening), and GRIPHON 2015 (where the addition of selexipag to monotherapy demonstrated a delay in clinical worsening). The AMBITION 2015 study (included in this review) examined upfront combination therapy and demonstrated a reduction in clinical failure, and an improvement in 6MWD.
Pulmonary hypertension secondary to left‐heart disease (PH‐LHD) is by far the most common cause of pulmonary hypertension, and the presence of PH‐LHD leads to worse survival (Strange 2012). Studies including participants with PH‐LHD were mixed; when we pooled the studies there was a benefit for exercise parameters and quality of life, but an increased risk of heart failure exacerbations. Previous studies (Jiang 2014; Lindman 2012) have demonstrated acute haemodynamic benefit in these participants, but the long‐term effect remains controversial. Bermejo 2017 suggested an initial benefit in 6MWD at three months (although not statistically significant), but this effect disappeared at six months, with no difference in effect between the two arms. In contrast, the other long‐term study (Guazzi 2011a (12 months)) demonstrated a continued, persistent benefit in haemodynamics over time. This suggests that the controversy may lie in the heterogeneity of the cause of left‐heart disease leading to pulmonary hypertension.
The evidence for pulmonary hypertension secondary to lung disease was limited to people with COPD. Although there was one small study (an a priori planned substudy of a larger trial) in people with IPF, it is difficult to extrapolate the evidence presented in this meta‐analysis beyond the COPD population. Theoretically, worsening ventilation‐perfusion mismatch induced by pulmonary vasodilators may lead to more pronounced dyspnoea and clinical worsening (Smith 2013), but included studies did not assess dyspnoea, and limited data were provided on clinical worsening. Further trials should include these outcomes.
Appropriate and accurate outcome assessment did not occur in all studies. In trials with PH‐LHD, although most participants were initially diagnosed using right‐heart catheterisation, outcome assessment was performed with noninvasive measures such as PASP on transthoracic echocardiogram (TTE). Previous studies (Farber 2011) have demonstrated that these noninvasive techniques do not always correlate with invasive haemodynamics, especially in those with left‐heart disease. In addition, the presence of pulmonary hypertension in left‐heart disease has been shown to reduce exercise tolerance and increase hospitalisations for heart failure (Vachiéry 2013). However, despite this, only three of five studies reported exercise tolerance, and only two of five reported heart failure exacerbations. Any further studies in PH‐LHD should include 6MWD, invasive haemodynamic data, clinical worsening including heart failure exacerbations, and be adequately powered to detect a difference in these outcomes.
Quality of the evidence
For trials including group 1 PAH participants comparing PDE5 inhibitors to placebo, the quality of evidence was high across all outcomes except for six‐minute walk distance, and cardiac index, which were downgraded to moderate due to high heterogeneity, although the effect was consistent across trials. For PAH trials comparing PDE5 inhibitors to placebo whilst on additional therapy and trials comparing PDE5 inhibitors to ERAs, the quality of the evidence was reduced due to smaller participant numbers. Follow‐up was as long as 12 months, but the average follow‐up was only 14 weeks. Open‐label extension or real world registry data may be useful for longer‐term effects.
Although there were five studies examining PH‐LHD, the numbers in each study were small, and studies were significantly heterogeneous. Ovchinnov 2015 was a poor‐quality study, where randomisation and allocation concealment were not reported, dropouts and cross‐overs were not reported, and only quantitative data from the intervention arm was reported.
Potential biases in the review process
We conducted this review in accordance with established Cochrane standards. Two review authors independently screened search results and resolved discrepancies by discussion and consensus. We did not restrict the literature search by language. Publication bias is possible, whereby failure to identify unpublished negative trials could have led to an overestimation of effect.
Agreements and disagreements with other studies or reviews
Our findings are consistent with the current ESC/ERS guidelines (Galiè 2016b), which recommend the use of PDE5 inhibitors for monotherapy as level IA/B evidence for WHO functional class I ‐ III patients, and as level IIb evidence for WHO functional class IV patients (as there is stronger evidence of effect for combination therapy and intravenous prostacyclins). Similarly, the current ESC/ERS guidelines do not recommend the use of PDE5 inhibitors for left‐heart disease‐ or lung disease‐related pulmonary hypertension. Where PDE5 inhibitors are used as part of combination therapy, caution should be used in adding these to riociguat, due to potentially unfavourable side effects and lack of additional clinical benefit (Galiè 2015b).
No trials have compared individual PDE5 inhibitors head‐to‐head, and given the overall class benefit it is unlikely that these studies will be conducted. Our data suggest that there may be a better treatment effect with sildenafil compared to tadalafil, but this may be influenced by the greater number of studies conducted using sildenafil compared to tadalafil. The choice of agent is likely to be nuanced and influenced by the individual patient profile with respect to side effects. The SITAR study (Frantz 2014) rotated participants from sildenafil to tadalafil, and found an initial increase in side effects including headache and gastrointestinal upset, but this improved over time.
Our data demonstrating no clear benefit for the use of PDE5 inhibitors in those with lung disease‐associated pulmonary hypertension is consistent with the ESC guidelines (Galiè 2016b) and with other published data using PH‐specific therapy in people with interstitial lung disease (ILD). Han 2013 (pulmonary hypertension associated with lung disease) was a prespecified substudy of the STEP‐IPF trial (Zisman 2010), which included people with idiopathic pulmonary fibrosis (IPF), and found no benefit in terms of 6MWD, exacerbations or mortality.
Interestingly, there appeared to be no significant benefit for the use of PDE5 inhibitors in people with CTEPH. Current ESC/ERS guidelines (Galiè 2016b) recommend surgical pulmonary endarterectomy as first line treatment based on long term international registry data which demonstrates a mortality benefit (Delcroix 2016), and the use of riociguat medical therapy in those who are non‐operable candidates (Ghofrani 2013). Therefore, these treatments could be considered in preference to PDE5 inhibitors.
Authors' conclusions
Implications for practice.
Data from this review suggest a benefit for the use of PDE5 inhibitors in group 1 PAH, for improvement in WHO functional class, reduction in clinical worsening, and improvement in haemodynamics, six‐minute walk distance, quality of life, and mortality.
Sildenafil, tadalafil and vardenafil are all efficacious in this clinical setting.
Clinicians may wish consider the side‐effect profile for each drug when choosing which to prescribe to an individual patient.
This review suggests that a PDE5 inhibitor may be better than an ERA for six‐minute walk distance and quality of life, but that there appears to be no difference in WHO functional class or mortality. These conclusions are limited by the small number of trials.
While there appears to be some benefit for the use of PDE5 inhibitors in PH‐LHD, it is not clear based on the mostly small, short‐term studies which type of left‐heart disease stands to benefit. The use of PDE5 inhibitors does not appear to be beneficial in valvular heart disease.
This review suggests there is no clear benefit for PDE5 inhibitors in pulmonary hypertension secondary to lung disease or CTEPH.
There may be evidence of harm in the use of PDE5 inhibitors for pulmonary hypertension secondary to sickle cell disease.
Implications for research.
Further research is required into the mechanisms of pulmonary hypertension secondary to left‐heart disease.
Clinical trials in PH‐LHD should be sufficiently powered, with long‐term follow‐up, and should include invasive haemodynamic data, WHO functional class, six‐minute walk distance, and clinical worsening.
What's new
| Date | Event | Description |
|---|---|---|
| 28 February 2019 | Amended | Two minor amendments made to the "Agreements and disagreements with other studies or review" section of the discussion. Thank you to the Spanish translation team for highlighting these errors. 1. First paragraph: minor typographical error corrected 2. Final paragraph: Galiè 2016a reference corrected to Galiè 2016b and paragraph restructured to improve clarity |
Acknowledgements
The protocol was developed with the assistance of the Cochrane Airways protocol template, and comments were provided by the Cochrane Airways Group. The search strategy was developed with assistance from Liz Stovold, the Cochrane Airways Group’s Information Specialist. We thank the Cochrane Airways editors for their comments, including Rebecca Normansell. We acknowledge the authors Parthipan Kanthapilai and E. Haydn Walters for their previously published review.
Christopher Cates was the editor for this review and commented critically on the review.
The Backgroundand Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Database search strategies
CENTRAL & Cochrane Airways Register of Trials search strategy
#1 MESH DESCRIPTOR Hypertension, Pulmonary EXPLODE ALL TREES #2 MESH DESCRIPTOR Pulmonary Heart Disease #3 (pulmonary NEAR2 hypertensi*):TI,AB,KY #4 #1 OR #2 OR #3 #5 MESH DESCRIPTOR Phosphodiesterase 5 Inhibitors EXPLODE ALL TREES #6 (PDE5 or PDE5):TI,AB,KY #7 ("Phosphodiesterase 5" or Phosphodiesterase‐5):TI,AB,KY #8 (sildenafil or Viagra):TI,AB,KY #9 (tadalafil or Cialis):TI,AB,KY #10 (vardenafil or Levitra or Staxyn):TI,AB,KY #11 (avanafil or Stendra):TI,AB,KY #12 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 #4 AND #12
MEDLINE (Ovid) search strategy
1. exp Hypertension, Pulmonary/ 2. Pulmonary Heart Disease/ 3. (pulmonary adj2 hypertensi$).tw. 4. 1 or 2 or 3 5. exp Phosphodiesterase 5 Inhibitors/ 6. (PDE5 or PDE‐5).tw. 7. ("Phosphodiesterase 5" or Phosphodiesterase‐5).tw. 8. (sildenafil or viagra).tw. 9. (tadalafil or Cialis).tw. 10. (vardenafil or Levitra or Staxyn).tw. 11. (avanafil or Stendra).tw. 12. or/5‐11 13. 4 and 12 14. (controlled clinical trial or randomised controlled trial).pt. 15. (randomised or randomised).ab,ti. 16. placebo.ab,ti. 17. dt.fs. 18. randomly.ab,ti. 19. trial.ab,ti. 20. groups.ab,ti. 21. or/14‐20 22. Animals/ 23. Humans/ 24. 22 not (22 and 23) 25. 21 not 24 26. 13 and 25
Embase (Ovid) search strategy
1. exp pulmonary hypertension/ 2. (pulmonary adj2 hypertensi$).tw. 3. 1 or 2 4. exp phosphodiesterase V inhibitor/ 5. (PDE5 or PDE‐5).tw. 6. ("Phosphodiesterase 5" or Phosphodiesterase‐5).tw. 7. (sildenafil or viagra).tw. 8. (tadalafil or Cialis).tw. 9. (vardenafil or Levitra or Staxyn).tw. 10. (avanafil or Stendra).tw. 11. or/4‐10 12. 3 and 11 13. Randomized Controlled Trial/ 14. randomization/ 15. controlled clinical trial/ 16. Double Blind Procedure/ 17. Single Blind Procedure/ 18. Crossover Procedure/ 19. (clinica$ adj3 trial$).tw. 20. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 21. exp Placebo/ 22. placebo$.ti,ab. 23. random$.ti,ab. 24. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 25. (crossover$ or cross‐over$).ti,ab. 26. or/13‐25 27. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 28. human/ or normal human/ or human cell/ 29. 27 and 28 30. 27 not 29 31. 26 not 30 32. 12 and 31
Data and analyses
Comparison 1. Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional class | 4 | 282 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.59 [3.95, 18.72] |
| 1.1 Sildenafil | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.24 [3.69, 28.47] |
| 1.2 Tadalafil | 2 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.71 [1.88, 31.72] |
| 1.3 Vardenafil | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.66, 47.07] |
| 2 Six‐minute walk distance | 8 | 880 | Mean Difference (Fixed, 95% CI) | 48.17 [40.30, 56.04] |
| 2.1 Sildenafil | 4 | 339 | Mean Difference (Fixed, 95% CI) | 56.91 [44.40, 69.41] |
| 2.2 Tadalafil | 3 | 477 | Mean Difference (Fixed, 95% CI) | 38.46 [27.60, 49.31] |
| 2.3 Vardenafil | 1 | 64 | Mean Difference (Fixed, 95% CI) | 69.0 [41.00, 97.00] |
| 3 Mortality | 8 | 1119 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.07, 0.68] |
| 3.1 Sildenafil | 5 | 634 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 0.98] |
| 3.2 Tadalafil | 2 | 421 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.63] |
| 3.3 Vardenafil | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.82] |
| 4 PAP | 6 | 453 | Mean Difference (Fixed, 95% CI) | ‐6.43 [‐8.13, ‐4.74] |
| 4.1 Sildenafil | 4 | 333 | Mean Difference (Fixed, 95% CI) | ‐7.34 [‐9.35, ‐5.33] |
| 4.2 Tadalafil | 1 | 56 | Mean Difference (Fixed, 95% CI) | ‐3.67 [‐7.55, 0.21] |
| 4.3 Vardenafil | 1 | 64 | Mean Difference (Fixed, 95% CI) | ‐5.3 [‐10.60, ‐0.00] |
| 5 RAP | 3 | 341 | Mean Difference (Fixed, 95% CI) | ‐1.35 [‐2.34, ‐0.36] |
| 5.1 Sildenafil | 2 | 277 | Mean Difference (Fixed, 95% CI) | ‐1.20 [‐2.30, ‐0.11] |
| 5.2 Vardenafil | 1 | 64 | Mean Difference (Fixed, 95% CI) | ‐2.0 [‐4.30, 0.30] |
| 6 CI | 4 | 239 | Mean Difference (Fixed, 95% CI) | 0.28 [0.16, 0.40] |
| 6.1 Sildenafil | 2 | 152 | Mean Difference (Fixed, 95% CI) | 0.47 [0.28, 0.66] |
| 6.2 Tadalafil | 1 | 28 | Mean Difference (Fixed, 95% CI) | 0.02 [‐0.18, 0.22] |
| 6.3 Vardenafil | 1 | 59 | Mean Difference (Fixed, 95% CI) | 0.4 [0.10, 0.70] |
| 7 PVR | 3 | 266 | Mean Difference (Fixed, 95% CI) | ‐4.74 [‐6.13, ‐3.35] |
| 7.1 Sildenafil | 1 | 146 | Mean Difference (Fixed, 95% CI) | ‐3.87 [‐5.67, ‐2.08] |
| 7.2 Tadalafil | 1 | 56 | Mean Difference (Fixed, 95% CI) | ‐7.32 [‐10.42, ‐4.22] |
| 7.3 Vardenafil | 1 | 64 | Mean Difference (Fixed, 95% CI) | ‐4.7 [‐7.80, ‐1.60] |
| 8 PASP | 2 | 48 | Mean Difference (Fixed, 95% CI) | ‐11.62 [‐25.18, 1.94] |
| 8.1 Sildenafil | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐7.0 [‐23.64, 9.64] |
| 8.2 Tadalafil | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐20.75 [‐44.14, 2.64] |
| 9 Dyspnoea | 4 | 239 | Mean Difference (Fixed, 95% CI) | ‐0.72 [‐0.99, ‐0.44] |
| 9.1 Sildenafil | 2 | 155 | Mean Difference (Fixed, 95% CI) | ‐0.57 [‐0.90, ‐0.24] |
| 9.2 Tadalafil | 1 | 20 | Mean Difference (Fixed, 95% CI) | ‐0.64 [‐1.65, 0.37] |
| 9.3 Vardenafil | 1 | 64 | Mean Difference (Fixed, 95% CI) | ‐1.15 [‐1.70, ‐0.60] |
| 10 Clinical worsening requiring intervention | 3 | 746 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.27, 1.23] |
| 10.1 Sildenafil | 1 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.25] |
| 10.2 Tadalafil | 1 | 405 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.27, 5.95] |
| 10.3 Vardenafil | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.02, 2.46] |
| 11 Adverse events | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Headache | 5 | 848 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.33, 2.92] |
| 11.2 GI upset | 5 | 848 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.07, 2.48] |
| 11.3 Flushing | 3 | 748 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.12 [1.83, 9.26] |
| 11.4 Muscle and joint pain | 4 | 792 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.59, 3.99] |
| 11.5 Epistaxis | 2 | 682 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.83, 6.77] |
| 11.6 Respiratory symptoms | 3 | 748 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.89, 3.40] |
| 11.7 Visual disturbance | 3 | 748 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.58, 7.14] |
Comparison 2. Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus placebo, on combination therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional Class | 2 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.66, 2.17] |
| 2 Six‐minute walk distance | 4 | 509 | Mean Difference (Fixed, 95% CI) | 19.66 [9.22, 30.10] |
| 3 Mortality | 3 | 492 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 1.06] |
| 4 PAP | 2 | 387 | Mean Difference (Fixed, 95% CI) | ‐4.58 [‐6.14, ‐3.01] |
| 5 Cardiac Output | 3 | 310 | Mean Difference (Fixed, 95% CI) | 0.87 [0.53, 1.21] |
| 6 PVR | 3 | 303 | Std. Mean Difference (Fixed, 95% CI) | ‐0.48 [‐0.72, ‐0.25] |
| 7 Clinical worsening | 4 | 717 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.21, 0.68] |
| 8 Adverse events | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Headache | 5 | 768 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.74, 3.56] |
| 8.2 GI upset | 4 | 726 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.30, 2.93] |
| 8.3 Flushing | 2 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.63, 1.96] |
| 8.4 Muscle and joint pain | 3 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.28, 3.67] |
| 8.5 Epistaxis | 2 | 358 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.34, 5.12] |
| 8.6 Visual disturbance | 2 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.95 [0.97, 16.08] |
Comparison 3. Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional class | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Six‐minute walk distance | 2 | 36 | Mean Difference (Fixed, 95% CI) | 49.38 [3.65, 95.11] |
| 3 Mortality | 2 | 272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.74, 13.64] |
| 4 PAP | 1 | Mean Difference (Fixed, 95% CI) | 7.00 [‐4.82, 18.82] | |
| 5 RAP | 1 | Mean Difference (Fixed, 95% CI) | 2.0 [‐2.14, 6.14] | |
| 6 CI | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [‐0.49, 0.49] | |
| 7 PVR | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [‐1.93, 1.93] | |
| 8 Quality of life | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 9 Clinical worsening requiring hospitalisation | 2 | 275 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.89] |
| 10 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 GI upset | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Flushing | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Muscle or joint pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Respiratory symptoms | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.4. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 4 PAP.
3.5. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 5 RAP.
3.6. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 6 CI.
3.7. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 7 PVR.
3.8. Analysis.
Comparison 3 Group 1 Pulmonary Arterial Hypertension ‐ PDE5i versus ERA, Outcome 8 Quality of life.
Comparison 4. Group 2 Pulmonary hypertension due to left heart disease (sildenafil v placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional class | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.32, 0.87] |
| 2 Six‐minute walk distance | 3 | 284 | Mean Difference (Fixed, 95% CI) | 34.31 [22.75, 45.87] |
| 3 Mortality | 3 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.28, 5.80] |
| 4 mean PAP | 3 | 130 | Mean Difference (Fixed, 95% CI) | ‐10.17 [‐11.99, ‐8.35] |
| 5 Cardiac Index | 2 | 96 | Mean Difference (Fixed, 95% CI) | 0.07 [‐0.17, 0.30] |
| 6 TPG | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 RAP | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 PASP | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 Dyspnoea | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10 Clinical worsening | 2 | 234 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.22, 3.46] |
Comparison 5. Group 3 Pulmonary Hypertension due to lung disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional class | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Six‐minute walk distance | 5 | 350 | Mean Difference (Random, 95% CI) | 26.70 [2.00, 51.39] |
| 3 QOL | 2 | 238 | Mean Difference (Random, 95% CI) | 0.19 [‐0.07, 0.44] |
| 4 mean PAP | 2 | 61 | Mean Difference (Fixed, 95% CI) | ‐0.14 [‐6.65, 6.37] |
| 5 Cardiac Index | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 PVR | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 RAP | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 6. Group 4 Pulmonary Hypertension due to CTEPH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional class | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Six‐minute walk distance | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 Sildenafil versus placebo | 1 | 19 | Mean Difference (Fixed, 95% CI) | 17.5 [‐23.90, 58.90] |
| 2.2 Sildenafil versus bosentan | 2 | 227 | Mean Difference (Fixed, 95% CI) | 20.42 [‐27.95, 68.79] |
| 3 Mortality | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Sildenafil versus placebo | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Sildenafil versus bosentan | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.22, 8.48] |
| 4 mean PAP | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 Sildenafil versus placebo | 1 | 19 | Mean Difference (Fixed, 95% CI) | ‐6.2 [‐12.40, ‐0.00] |
| 4.2 Sildenafil versus bosentan | 2 | 227 | Mean Difference (Fixed, 95% CI) | 0.76 [‐3.96, 5.48] |
| 5 Cardiac Index | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 Sildenafil versus placebo | 1 | 19 | Mean Difference (Fixed, 95% CI) | 0.0 [‐0.40, 0.40] |
| 5.2 Sildenafil versus bosentan | 2 | 227 | Mean Difference (Fixed, 95% CI) | 0.04 [‐0.22, 0.31] |
| 6 PVR | 3 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 Sildenafil versus placebo | 1 | 19 | Mean Difference (Fixed, 95% CI) | ‐0.89 [‐1.85, 0.06] |
| 6.2 Sildenafil versus bosentan | 2 | 227 | Mean Difference (Fixed, 95% CI) | ‐0.01 [‐0.27, 0.25] |
| 7 RAP | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 Sildenafil versus placebo | 1 | 19 | Mean Difference (Fixed, 95% CI) | ‐0.9 [‐6.10, 4.30] |
| 7.2 Sildenafil versus bosentan | 1 | 121 | Mean Difference (Fixed, 95% CI) | ‐1.0 [‐3.77, 1.77] |
| 8 QOL | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 GI upset | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Mixed Pulmonary Hypertension group 2‐4.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional class | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.31 [4.90, 26.14] |
| 2 6MWD | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 PASP | 2 | 146 | Mean Difference (Fixed, 95% CI) | ‐10.0 [‐11.92, ‐8.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albini 2017.
| Methods | Randomised controlled trial 3 ‐ 6 months duration |
|
| Participants | Inclusion criteria:
WHO FC II/III n = 11 |
|
| Interventions | Tadalafil monotherapy (N=6) versus ambrisentan monotherapy (N=5) | |
| Outcomes | 6MWD, cardiopulmonary haemodynamics | |
| Notes | Compared tadalafil monotherapy to ambrisentan monotherapy No funding source stated Trial conducted at the University of Bologna, Bologna, Italy (dates not stated) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", although methods of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Unclear risk | Abstract from conference proceedings only, does not report a registration in a clinical trials database, limited information about methods used |
Barst 2012.
| Methods | Randomised, double‐blind, placebo‐controlled trial 16 weeks duration |
|
| Participants | Inclusion Criteria:
Exclusion criteria:
n = 234 WHO FC I/II |
|
| Interventions | Three sildenafil dose levels (low, medium, and high) (total N=174) were selected to achieve maximum plasma concentrations of 47, 140, and 373 ng/mL, respectively, at steady state after oral administration 3 times a day, versus placebo (N=60) | |
| Outcomes | Primary: percent change in PVO2 Secondary: change from baseline to week 16 in mPAP, PVRI, FC, cardiac index, right atrial pressure, physical and psychosocial scales of the Child Health Questionnaire–Parent Form 28 (for patients aged 5 years), and percent change from baseline in exercise duration Tertiary: Participant/parent and physician global assessments, changes in medications, and clinical worsening (defined as death, transplantation, hospitalisation due to PAH, or initiation of a prostacyclin analogue or bosentan for worsening PAH) |
|
| Notes |
NCT00159913 This study was sponsored by Pfizer Inc. Editorial support was provided by Tiffany Brake, PhD, and Janet E. Matsuura, PhD, from Compete Healthcare Communications, Inc, and was funded by Pfizer Inc Trial conducted in 16 countries (32 centers) in North, South, and Central America; Asia; and Europe between August 2003 and June 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The central computer‐generated pseudo‐random code used the method of random permuted blocks within stratum (block size 4). An automated interactive voice response system assigned randomisation numbers to eligible patients". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Drug supply consisted of a list of package numbers and corresponding treatment types. A unique package number identified each package of medication. The interactive voice response system assigned patients with a package number from the list corresponding to the treatment assigned." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
Bermejo 2017.
| Methods | Randomised placebo parallel controlled trial 6 months duration |
|
| Participants | Pulmonary hypertension following repaired valvular heart disease (WHO Group 2) Inclusion criteria:
Exclusion criteria:
n = 200 |
|
| Interventions | Sildenafil 40 mg 3 times a day (N=104) versus placebo (N=96) | |
| Outcomes | Primary outcome: clinical composite score combining all‐cause mortality, hospital admission for heart failure, WHO functional class, and the patient global assessment score 6MWD WHO functional class PASP Heart failure hospitalisations |
|
| Notes | Publicly funded The trial was conducted in 18 academic hospitals in Spain, enrolled from May 2009 to December 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation codes generated |
| Allocation concealment (selection bias) | Low risk | Quote: "The study drugs (i.e. sildenafil and placebo) were identical in appearance and were packaged in identical containers. The study drugs were prepared by an independent laboratory from commercially purchased sildenafil and specifically manufactured placebo powder". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators and participants were masked to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators and participants were masked to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Discontinuations and deviations were reported and equal among groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported; a primary composite outcome was reported with separate reporting of outcomes |
| Other bias | Low risk | Other bias is unlikely |
Bharani 2003.
| Methods | Randomised placebo‐controlled cross‐over trial 2 weeks duration |
|
| Participants | Inclusion criteria:
WHO FC II/III/IV n = 10 |
|
| Interventions | Sildenafil 25 mg 8‐hourly or matching placebo for 2 weeks
Washout at least 2 weeks before cross‐over Participants were receiving conventional therapy including: warfarin, nifedipine, diuretics, digoxin |
|
| Outcomes | Improvement in NYHA class 6MWD Borg dyspnoea index (scale 0 ‐ 10; the higher the score the worse the dyspnoea) PASP on TTE Side effects |
|
| Notes | Did not perform right‐heart catheterisation to confirm PAH Funding source not stated The trial was conducted through a large referral centre in central India (dates not stated) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was carried out at a large referral centre of central India as a prospective, randomised, double‐blind, crossover trial", but no further details are given Unclear method of randomisation/allocation/blinding of participants and personnel and measurement of outcomes |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation is not clearly stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not clear if participants and personnel were blinded, although authors state "placebo" was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the measurements were performed by those blinded to the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who were randomised were accounted for |
| Selective reporting (reporting bias) | Low risk | Most outcomes stated in the Methods are reported |
| Other bias | Low risk | None detected |
Bharani 2007.
| Methods | Randomised double‐blind placebo‐controlled cross‐over trial 4 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 11 |
|
| Interventions | 4 weeks of tadalafil 20 mg orally daily, or placebo orally daily
2‐week drug‐free interval
Cross‐over
4 weeks of tadalafil 20 mg orally daily or placebo orally daily Continued pre‐trial medications including: frusemide, spironolactone, warfarin, iron, digoxin |
|
| Outcomes | Improvement in NYHA class 6MWD Borg dyspnoea index (scale 0 ‐ 10; the higher the score the worse the dyspnoea) PASP on TTE Side effects |
|
| Notes | Did not perform right‐heart catheterisation to confirm PAH Funding source not stated Trial was conducted at MY Hospital, Indore, India, with enrolment from September 2005 to February 2006. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients received tadalafil 20 mg once a day or visually matching placebo for 4 weeks each with a drug free interval of at least 2 weeks between the two therapies in a randomised, doubled blind, cross over design, " but specific methods are not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation is not clearly stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not clear if participants and personnel were blinded, although authors state "placebo" was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the measurements were performed by those blinded to the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 8 of 11 participants completed the trial, for reasons which are unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the study design were reported, although some incompletely, in the text |
| Other bias | Low risk | None detected |
Blanco 2013.
| Methods | Randomised, double‐blind, placebo‐controlled trial 3 months duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 63 |
|
| Interventions | Sildenafil 20 mg orally 3 times daily and pulmonary rehabilitation programme, 3 times a week for 12 weeks, standardised across participating centres (N=29) Placebo, with pulmonary rehabilitation programme, 3 times a week for 12 weeks, standardised across participating centres (N=31) |
|
| Outcomes | Cycle endurance time 6MWD Quality of life including SGRQ and SF‐36 Lung function tests PAP on TTE Borg dyspnoea score |
|
| Notes | Publicly funded Trial conducted in Spain (dates not stated) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients meeting eligibility criteria were randomly assigned in a 1:1 ratio … via a four‐permuted‐block design, with no stratification." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation was performed by a pharmacologist not involved directly in the study." Central randomisation by a third party, as above, should be effective allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was a 3‐month, randomised, double‐blind, placebo‐controlled trial", "Asssessments before the study and at the end of the study were performed in the coordinating centre", but blinding is not made clear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear if the measurements were performed by those blinded to the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 289 patients were screened. 63 underwent randomisation, 60 made up the modified ITT population whose data were used in the primary effectiveness analysis Missing outcome data balanced across the groups Intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Low risk | Study protocol registered and adhered to, and reporting on planned outcomes appears complete |
| Other bias | Low risk | Other bias is unlikely |
Boonstra 2005.
| Methods | Randomised double‐blind, placebo‐controlled trial 12 weeks duration |
|
| Participants | Inclusion criteria:
WHO I/II and III/IV n = 136 |
|
| Interventions | sildenafil (20, 40, or 80 mg) 3 times daily (N=200) compared to placebo (N=66) |
|
| Outcomes | 6MWD | |
| Notes | Abstract only Funding source not stated Location of trial and dates of enrolment not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods for random sequence generation are not described within the abstract |
| Allocation concealment (selection bias) | Unclear risk | Methods for allocation concealment are not described within the abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel is not described within the abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment is not described within the abstract |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 200/278 participants are presented in the final outcome analysis. Reasons for attrition is not described within the abstract |
| Selective reporting (reporting bias) | High risk | Only 1 outcome is reported |
| Other bias | Unclear risk | Unlear if other risk is present ‐ abstract only from conference proceedings, and limited data are presented |
Dwivedi 2015.
| Methods | Randomised, parallel‐group, placebo‐controlled trial 6 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Total n = 106 |
|
| Interventions | Oral sildenafil 25 mg 3 times daily compared to placebo |
|
| Outcomes | 6MWD Improvement in NYHA functional class LVEF PASP RV function NT‐Pro BNP level safety and tolerability |
|
| Notes | Further information about the trial is taken from the Clinical Trials Registry ‐ India: CTRI/2017/01/007686 (Registered on: 12/01/2017) Publicly funded Study was conducted at King George Medical University, India, enrolled from December 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Authors report "centralised" though it is unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participant blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 106/122 participants completed the study, for reasons which are unclear |
| Selective reporting (reporting bias) | Low risk | Specified outcomes are reported |
| Other bias | Low risk | Other bias unlikely |
Galiè 2005a.
| Methods | Randomised, parallel, placebo‐controlled trial 12‐week study with an additional open‐label extension study |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 277 |
|
| Interventions | 20 mg Sildenafil n = 69
40 mg Sildenafil n = 67
80 mg Sildenafil n = 71 compared to placebo n = 70 People assigned to 80 mg sildenafil 3 times daily received 40 mg of sildenafil 3 times daily for the first 7 days before the dose was escalated to 80 mg; people randomised to the other 3 treatment groups underwent dummy dose escalation after 7 days Study medication added to their currently prescribed conventional therapy |
|
| Outcomes | 6MWD Haemodynamics Improvement in WHO functional class Borg dyspnoea score Mortality was reported, but the study was not powered to assess mortality Adverse events |
|
| Notes | Industry funded Trial was conducted in 53 centers in the United States, Mexico, South America, Europe, Asia, Australia, South Africa, and Israel between October 2002 and November 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation with respect to baseline walking distance and cause of pulmonary hypertension A stratified central‐randomisation scheme was used |
| Allocation concealment (selection bias) | Low risk | Placebo was used Participants randomly assigned to the other 3 treatment groups underwent dummy dose escalation after 7 days. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It is likely outcome assessment was blinded although it is not explicitly stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analyses were used
Very few (only 2) exclusions and balanced attrition between groups: 1 not treated 277 treated 4 died 8 withdrew ‐ 2 protocol violation, 2 withdrew consent, 4 side effects (reduced renal function, lower leg oedema, cardiac arrhythmias, headache) 265 completed 12 weeks 259 entered long‐term study 222 completed 1 year of treatment with monotherapy with sildenafil |
| Selective reporting (reporting bias) | Low risk | All outcomes proposed in Methods were described in the report, non‐significant outcomes reported |
| Other bias | Low risk | Other bias is unlikely |
Galiè 2009.
| Methods | Randomised, double‐blind, double‐dummy, placebo‐controlled, multicentre trial, followed by a blinded long‐term extension study 16 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 405 |
|
| Interventions | Participants were randomised to groups receiving tadalafil 2.5 mg (N=82), 10 mg (N=80), 20 mg (N=82), 40 mg (n=79), or placebo (n=82) once daily The randomisation was stratified for baseline walking distance (> 325 m or < 325 m), type of PAH (idiopathic/heritable and anorexigen use versus other types), and bosentan use |
|
| Outcomes | 6MWD Improvement in WHO functional class Clinical worsening Health‐related quality of life Borg dyspnoea score Adverse events |
|
| Notes | Industry funded Trial was conducted in 84 centers in Canada, the United States, Europe, and Japan between August 2005 and August 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permutated randomisation |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It is likely outcome assessment was blinded although it is not explicitly stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias is unlikely |
Galiè 2015.
| Methods | Multicentre, double‐blind, randomised, placebo‐controlled trial 24 weeks duration |
|
| Participants | Inclusion criteria:
WHO FC II/III n = 500 |
|
| Interventions | There were 3 groups: 253 were assigned to the combination‐therapy group, 126 to the ambrisentan‐monotherapy group, and 121 to the tadalafil‐monotherapy group Ambrisentan monotherapy was compared to tadalafil monotherapy for the purpose of this meta‐analysis |
|
| Outcomes | 6MWD Haemodynamics including PAP, cardiac index, PVR, RAP Improvement in WHO functional class ProBNP Adverse events |
|
| Notes | Industry funded Trial was conducted at 120 centers in 14 countries between October 18, 2010 (first visit), and July 31, 2014 (last study visit) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not stated, but likely low risk |
| Allocation concealment (selection bias) | Low risk | Placebo was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study design took into account that following each participant’s final assessment visit, investigators would need to make decisions regarding future treatment, and future treatment decisions would be informed by knowing what treatment participants had been randomised to. Therefore, investigators would need to be unblinded at a participant’s end‐of‐study visit so they could provide appropriate treatment for each participant upon study completion. To accommodate this, we included 2 database freezes. The first database freeze was planned after the last randomised participant had received ≥24 weeks of therapy and the 105th adjudicated first clinical failure event was projected to have occurred in the primary analysis set. At that time, all participants were notified to return for a final assessment visit or their Week 24 visit, whichever was later. The database was frozen for each participant’s visit‐based data through the final assessment visit. The primary analysis was performed on these data. Participants then returned to the clinic for their end‐of‐study visit, the investigator was unblinded and participants were treated per investigator discretion." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As described above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 610 participants were enrolled, but only 500 completed the final primary analysis; the reasons for withdrawals were documented in the supplementary index |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Galiè 2016a.
| Methods | Randomised double‐blind controlled trial Mean duration of follow‐up 4.7 months |
|
| Participants | Inclusion criteria:
n = 121 |
|
| Interventions | Sildenafil (20 mg 3 times ar day) (N=61) versus bosentan (125 mg bid) (N=60) | |
| Outcomes | 6MWD RAP Mean PAP CI PVR Adverse events |
|
| Notes | Abstract only States the abstract is funded by "none" Location of trial and dates of enrolment not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported, although information given was limited as this was an abstract only. We contacted the authors but there was no reply at the time of publication |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not clear if there were issues with attrition bias |
| Selective reporting (reporting bias) | Unclear risk | It is not clear if there was selective reporting bias |
| Other bias | Unclear risk | Unclear if other bias is present ‐ abstract only from conference proceedings |
Goudie 2014.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled trial 12 weeks duration |
|
| Participants | People with COPD confirmed on respiratory function tests, and associated pulmonary hypertension, confirmed on TTE (WHO Group 3) Inclusion criteria:
Exclusion criteria:
Baseline characteristics: Mean FEV1 1.06 ± 0.41 L (41% pred.), mPAP 30 ± 7 mmHg (PAT derived), RVSP 42 ± 10 mmHg. n = 120 |
|
| Interventions | Tadalafil 10 mg daily (N=60) compared to placebo (N=60) | |
| Outcomes | Primary endpoint: 6MWD Secondary endpoints: SGRQ; SF‐36 and Minnesota questionnaires (QOL); BNP DLCO and echo parameters. Inclusion: moderate‐severe COPD and PH (RVSP > 30 mmHg or a pulmonary acceleration time (PAT) < 120 ms, or both) | |
| Notes | Publicly funded Trial was conducted at three centres in Scotland, UK (Dundee, Perth, and Fife), between Sept 1, 2010, and Sept 1, 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned in a 1:1 ratio, via centralised randomisation with a computer‐generated sequence and block sizes of four, to receive daily tadalafil 10 mg or matched placebo, orally" |
| Allocation concealment (selection bias) | Low risk | Quote: "The intervention and placebo packs were identical in presentation (capsules and bottle) and double blinding was maintained throughout" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients, study investigators, outcome assessors, and those administering drugs were masked to group allocation. Unmasking was done only after the end of the trial once all data had been collected and analysed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, study investigators, outcome assessors, and those administering drugs were masked to group allocation. Unmasking was done only after the end of the trial once all data had been collected and analysed" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 120 patients were randomly assigned to receive tadalafil (n = 60) or placebo (n = 60), of whom 113 (94%) completed the study, reasons not given |
| Selective reporting (reporting bias) | Low risk | Unlikely selective reporting bias |
| Other bias | Low risk | Other risk unlikely |
Guazzi 2011a.
| Methods | Randomised placebo‐controlled trial 12 months duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
PH was confirmed on right‐heart catheterisation n = 44 |
|
| Interventions | Sildenafil (50 mg 3 times a day) (N=22) compared to placebo (N=22) | |
| Outcomes | Haemodynamics, including: mean RAP, PASP, pulmonary artery diastolic pressure, mean PAP, mean wedge pulmonary pressure, transpulmonary gradient, pulmonary arteriolar resistance, pulmonary arterial elastance, RV end‐diastolic pressure, RV mean systolic ejection rate, TAPSE, RV maximal short‐axis dimension, cardiac index, systemic vascular resistance, systolic arterial pressure, diastolic arterial pressure Quality of life, including breathlessness, fatigue, emotional function Lung function tests including FEV1, FVC, and DLCO |
|
| Notes | Publicly funded Trial conducted at San Paolo Hospital in Milan, Italy, and at Virginia Commonwealth University, Richmond, USA, enrolled between January 15, 2006, and December 18, 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1:1 ratio according to computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, with nursing staff checking compliance unaware of the study aims or assignment to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not explicitly stated but assumed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for: Quote: "No participants in the sildenafil group was lost to follow‐up; in the placebo group, 2 participants were withdrawn at months 7 and 9 because at repeated Holter monitoring they showed persistent atrial fibrillation". |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Han 2013.
| Methods | Double‐blind, placebo‐controlled trial 12 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
N = 119 |
|
| Interventions | Oral sildenafil (20 mg 3 times a day) (N=56) compared with placebo (N=63) | |
| Outcomes | 6MWD
SGRQ SF‐36 EuroQol visual analogue scores |
|
| Notes | This was a substudy of the STEP‐IPF study which examined all participants with IPF and compared sildenafil with placebo This substudy examined only those with pulmonary hypertension and IPF Industry‐funded Trial conducted at 14 centres in the USA, dates of enrolment not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants meeting eligibility criteria were randomly assigned in a 1:1 ratio to receive sildenafil or matched placebo with the use of a permuted‐block design, with stratification according to clinical centre |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All echocardiograms were performed prior to randomisation and scored independently and in a blinded fashion by two cardiologists with advanced expertise in echocardiography. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Sixty‐one echocardiograms could not be transferred to the study centre, thus ineligible for inclusion". |
| Selective reporting (reporting bias) | High risk | This is a substudy |
| Other bias | Low risk | Other bias is unlikely |
Hoendermis 2015.
| Methods | Single‐centre, randomised double‐blind, placebo‐controlled trial 12 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 52 |
|
| Interventions | Sildenafil, titrated to 60 mg 3 times a day (N=26), or placebo (N=26) | |
| Outcomes | The primary endpoint was change in mean PAP after 12 weeks Secondary endpoints were change in mean PAWP, cardiac output, and peak oxygen consumption (peak VO2) | |
| Notes | Publicly funded Trial conducted at University of Medical Center Groningen, Hanzeplein, The Netherlands, enrolled between October 2011 and September 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomly assigned in a 1:1 ratio according to a computer‐generated random sequence to 1 of the 2 treatment groups using a block size of 4 |
| Allocation concealment (selection bias) | Low risk | Sildenafil and matching placebo were administered orally in tablets and were provided by Pfizer Global Pharmaceuticals, were identical in appearance and were supplied to the study site in identical‐masked kits |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were recorded, and were even across the groups. Quote: "One participant in the sildenafil group withdrew due to heart failure, and four discontinued due to adverse events". |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were accounted for |
| Other bias | Low risk | Other bias is unlikely |
Iversen 2009.
| Methods | Randomised, placebo‐controlled, double‐blinded, cross‐over trial 3 months duration |
|
| Participants | Inclusion criteria:
WHO FC II/III n = 21 |
|
| Interventions | Participants were randomised to sildenafil 25 mg 3 times a day. for 2 weeks followed by 50 mg 3 times a day for 10 weeks or matching placebo as add‐on therapy to bosentan. After this period, evaluation identical to baseline examinations was performed again and a cross‐over was performed so that participants receiving sildenafil were now treated with placebo and vice versa All participants were treated open‐label with bosentan for the entire study period (9 months). During the first 2 weeks, the participants received bosentan 62.5 mg twice daily, and thereafter bosentan 125 mg twice daily for the rest of the trial |
|
| Outcomes | 6MWT Oxygen saturations N‐terminal pro‐brain natriuretic peptide Improvement in NYHA classification Cardiac catheterisation Magnetic resonance imaging | |
| Notes | Publicly funded Trial was conducted through Department of Cardiology at Copenhagen University Hospital Rigshospitalet in Copenhagen, Denmark between January 2006 and April 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by Copenhagen County Hospital Pharmacy |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not explicitly stated but assumed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for: Quote: "All the screened patients fulfilled the inclusion criteria and all were included in the study. No code breaks were done during the study. Two patients were excluded during the study (one due to side‐effects of the medication and one due to death)". |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias is unlikely |
Jalalian 2015.
| Methods | Randomised placebo‐controlled trial 6 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
(WHO Group 5) n = 22 |
|
| Interventions | Tadalafil (40 mg daily) (N=22) or placebo (N=22) | |
| Outcomes | PASP TRV and parameters related to systolic function of the right ventricle including changes in TAPSE, pulsed Doppler peak velocity at the tricuspid annulus (S') were measured by TTE | |
| Notes | Publicly funded Trial conducted at Babol, Northern Iran between December 2013 and April 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the participants were randomly divided" but methods are not stated |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for, and were even across both groups. Quote: "Three participants in the tadalafil group and one participant in the placebo group withdrew due to side effects" |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias is unlikely |
Jing 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial, 12 weeks duration, followed by an open‐label trial, of another 12 weeks duration | |
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 66 |
|
| Interventions | Vardenafil (5 mg once daily for 4 weeks then 5 mg twice daily; n = 44) or placebo (n = 22) | |
| Outcomes | The primary efficacy endpoint was the 6MWD, assessed at the end of the 12‐week randomised treatment period. Secondary measures of efficacy included cardiopulmonary haemodynamics (mean RAP, cardiac index, mean PAP, and PVR) measured by right‐heart catheterisation; the Borg dyspnoea index; WHO functional class (at baseline and Week 12); and occurrence of clinical worsening events defined as death (all causes) or hospitalisation for PAH progression | |
| Notes | Publicly funded Trial was conducted at nine centers in China and were enrolled in the trial between September 2008 and May 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated using the DMS System, with a block size of 6. The 2:1 randomisation ratio (vardenafil: placebo) was the same for each centre |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not explicitly stated but assumed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for: 2 participants randomised to placebo were lost to follow‐up without any follow‐up data being recorded. 4 participants in the placebo group and 1 participant in the vardenafil group withdrew because of clinical worsening. Thus, 59 participants completed the randomised phase |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Lewis 2007.
| Methods | Randomised, parallel‐group, double‐blinded, single‐centre, placebo‐controlled trial 12 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 34 |
|
| Interventions | Sildenafil (25 to 75 mg orally 3 times daily) (N=17) or placebo (N=17) | |
| Outcomes | 6MWD Cardiopulmonary exercise test Haemodynamics using right‐heart catheterisation, including RAP, PCWP, CI, stroke volume, PVR, SVR Quality of life questionnaire including the Minnesota Heart Failure Questionnaire (a 21‐question self‐administered instrument in which scores can range from 0 to 5 for each question and higher scores indicate a poorer quality of life) Clinical worsening Mortality Adverse events |
|
| Notes | Public and industry funded Trial conducted at multiple centres (location not stated) from May 2003 to March 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Massachusetts General Hospital Pharmacy was responsible for the blinding and randomisation procedure |
| Allocation concealment (selection bias) | Low risk | The Massachusetts General Hospital Pharmacy was responsible for the blinding and randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators adjudicated all primary and secondary endpoints and reviewed data on safety before unblinding of the study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear whether there were any withdrawals; does not explicitly state that all recruited completed the trial |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias is unlikely |
Machado 2009.
| Methods | Multicentre, placebo‐controlled, double‐blind trial 16 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 74, with a planned study size of 132 |
|
| Interventions | Oral sildenafil 20 mg 3 times daily for 6 weeks,followed by 40 mg 3 times daily for 4 weeks, followed by 80 mg 3 times daily for 6 weeks, compared to placebo in a similarly uptitrated fashion | |
| Outcomes | Primary outcome measures: Change in exercise capacity as assessed by 6MWD Secondary outcome measures: Change from baseline in PH at week 16 as assessed by tricuspid regurgitant jet velocity, Borg dyspnoea score, (severity is measured on a 10‐point scale with 0 = nothing at all and 10 = maximum severity of breathlessness), BNP levels. |
|
| Notes | The study was prematurely stopped due to a statistically significant increase in serious adverse events in the sildenafil arm Published as an abstract only, but also registered on the clinical trial registry: NCT00492531 Publicly funded Trial conducted at 10 United States and United Kingdom Centers, dates not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised, but methods not clearly stated |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals and reasons for trial termination clearly described |
| Selective reporting (reporting bias) | Low risk | Study was published despite early termination |
| Other bias | Low risk | Risk of other bias is unlikely. Although this is an abstract only, further methods were published on the clinical trials registry |
Mazzanti 2013.
| Methods | Randomised, open‐label trial Average duration of treatment: 4.1 ± 1.2 months |
|
| Participants | Inclusion criteria:
WHO FC not stated n = 205 |
|
| Interventions | Bosentan (125 mg twice daily) (N=100) compared with sildenafil (20 mg 3 times a day) (N=105) | |
| Outcomes | 6MWD Haemodynamics using right‐heart catheterisation Survival Adverse events |
|
| Notes | Abstract only Source of funding not stated Trial conducted at Department of Specialized, Diagnostic and Experimental Medicine, BOLOGNA, Italy, from November 2006 to January 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only ‐ reports as randomised but no further details given |
| Allocation concealment (selection bias) | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were reported and similar across both groups: 20 participants (20%) in the bosentan group and 13 (12%) in the sildenafil group did not complete the short‐term evaluation because of death, adverse events, protocol violations, lack of haemodynamic data or were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias is unlikely ‐ within the abstract clear and detailed methods and outcomes are presented |
Mukhopadhyay 2011.
| Methods | Randomised controlled cross‐over trial 6 weeks duration per treatment arm with a wash‐out of 2 weeks |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 28 |
|
| Interventions | Tadalafil 40 mg or matching placebo for 6 weeks, followed by cross‐over to the other drug after a wash‐out period of 2 weeks | |
| Outcomes | The primary endpoint was improvement in exercise tolerance by 6MWD The secondary end points were the effect of the drug on SO2, EPBF, PVR, SVR, and WHO functional class | |
| Notes | Drugs were supplied free of cost from industry. Other sources of funding were not stated Trial conducted at G.B. Pant Hospital, New Delhi, India (dates not given) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, but methods are not specified |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Any withdrawals and reasons were not stated. It is not clear whether all participants who were enrolled completed the study |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Ovchinnov 2015.
| Methods | Randomised parallel‐group placebo‐controlled trial 12 weeks duration |
|
| Participants | Inclusion criteria:
n = 50 |
|
| Interventions | Sildenafil (25 mg 3 times a day for 12 weeks followed by 50 mg 3e times a day for 12 weeks; n = 30) or to control group (no sildenafil, n = 20) | |
| Outcomes | 6MWD Cycle ergometer exercise duration Systolic pulmonary artery pressure RV function assessed by tricuspid annular systolic excursion Left‐sided diastolic function (E/é ratio) |
|
| Notes | Quantitative outcomes were only reported for the sildenafil group; the trial reported "no improvement in control group was observed" so this trial was unable to be included in the meta‐analysis We tried to contact all authors, but at the time of this review publication there has been no reply, or contact details were unavailable Funding source not stated Location and dates not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states participants were randomly assigned, but no methods were given |
| Allocation concealment (selection bias) | High risk | Compared sildenafil to "no sildenafil" ‐ unclear if placebo was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unclear if placebo was used or if participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of completers were not reported. Mortality or loss to follow‐up were not reported. Methods using intention‐to‐treat or per‐protocol analysis was not specified |
| Selective reporting (reporting bias) | High risk | Quantitative outcome data were only reported for the sildenafil group, not placebo group, and no P values between groups were reported |
| Other bias | Unclear risk | Not clear if other bias is possible ‐ abstract from conference proceedings with limited data presented |
Palazzini 2010.
| Methods | Randomised, open‐label trial Mean follow‐up 3.8 + 0.8 months |
|
| Participants | Inclusion criteria:
n = 128 |
|
| Interventions | Bosentan versus sildenafil (doses not provided) | |
| Outcomes | 6MWD Haemodynamics using right‐heart catheterisation including mean PAP, cardiac index, PVR Mortality |
|
| Notes | Abstract only Quote: "This abstract is funded by: none". Otherwise no other funding information is supplied Trial conducted at Orsola Malpighi Hospital, Bologna, Italy (dates not given) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation used |
| Allocation concealment (selection bias) | Unclear risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were reported: "6 withdrew from the bosentan group and 2 from sildenafil group due to side effects, 3 in bosentan and 1 in sildenafil group withdrew due to protocol violations, 2 did not undergo RHC, and 1 withdrew consent" |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Unclear risk | Unclear if other risk is present ‐ abstract only from conference proceedings with limited data presented |
Palii 2014.
| Methods | Monocentric, randomised placebo‐controlled trial 12 months duration |
|
| Participants | Inclusion criteria:
WHO FC III n = 77 |
|
| Interventions | Sildenafil – dose of 1 to 2 mg/kg/day every 8 hours (N=38), compared to placebo (N=39) | |
| Outcomes | NYHA functional class; 6MWT; O2 saturation; echocardiography PAPm, myocardial performance index (MPI/Tei index), right‐cardiac catheterisation – PVRI; questionnaire for adverse reactions | |
| Notes | Abstract only Funding source not stated Trial conducted at Institute of Mother and Child, Chisinau, Moldova (dates not given) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised, although information given was limited as this was an abstract only. We tried to contact authors but there was no reply at the time of publication |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were not specified |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Unclear risk | Unclear if other risk is present ‐ abstract only with limited data presented |
Rao 2011.
| Methods | Randomised, double‐blind placebo‐controlled trial 12 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 37 |
|
| Interventions | Oral sildenafil 20 mg 3 times a day (N=17) compared to identical placebo (N=20) | |
| Outcomes | Primary outcome: change in 6MWD. Improvement in PAP was taken as secondary outcome of the study | |
| Notes | Industry provided the drugs. No other funding source stated Trial conducted at SMS Medical College and Hospital, Jaipur (Rajasthan), India (dates of recruitment not stated) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sildenafil and placebo were dispensed according to random allocation of computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Drug was dispensed by an unblinded co‐ordinator, who dispensed the drug to blinded co‐ordinator, who gave it to the participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drug was dispensed by an unblinded co‐ordinator, who dispensed the drug to blinded co‐ordinator, who gave it to the participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for and similar across the 2 groups ‐ where in each group 1 was lost to follow‐up, 1e in the sildenafil group had an acute exacerbation, and 1 in the placebo group withdrew consent |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Salem 2013.
| Methods | Randomised, placebo‐controlled trial 6 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 40 |
|
| Interventions | Oral sildenafil (N=20) or matched placebo (N=20) was given in a titrated dose, starting with 25 mg 3 times daily for 1 week, then the dose was doubled to 50 mg 3 times daily according to side effects and tolerability until 6‐week period | |
| Outcomes | Improvement in NYHA functional class PASP LVEF |
|
| Notes | Funding source not stated Location of trial and dates of recruitment not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using simple randomisation (1:1) to either sildenafil therapy in a titrating dose or placebo |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was unclear if the outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not explicitly stated if all randomised participants completed the trial; no withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Sastry 2004.
| Methods | Randomised, double‐blind, cross‐over trial 6 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 22 |
|
| Interventions | Sildenafil compared to placebo Medication dosage was assigned on the basis of body weight, with participants weighing up to 25 kg receiving 25 mg 3 times daily, those weighing between 26 and 50 kg receiving 50 mg 3 times daily, and those weighing 51 kg receiving 100 mg 3 times daily Digoxin, diuretics, and oral anticoagulants were used at the clinician’s discretion. No other vasodilators were allowed, and participants were specifically advised not to take nitrate preparations in any form |
|
| Outcomes | Time spent on treadmill PASP Cardiac index as measured on doppler echocardiography Adverse events Quality of life (a chronic heart failure questionnaire with 16 questions, including 5 to assess dyspnoea, 4 to assess fatigue, and 7 to assess emotional function of daily living. The answers to each question may be scored from 1 (denoting worst function) to 7 (denoting best function). The maximum possible score of 108 would denote the best QOL, whereas a minimum score of 16 would denote worst QOL) |
|
| Notes | PAH diagnosed on echo, did not use right‐heart catheterisation Funding source not stated Trial was conducted at CARE Hospital, Hyderabad, India, between September 17, 2002, and December 13, 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed on the basis of computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participant, clinical investigator, echocardiographer, and the person supervising the exercise were blinded to the participant’s treatment regimen |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were reported and similar across groups: Quote: "One participant in the sildenafil first group opted out of the study one week after randomisation. This was not the result of any serious adverse effect of medication. Another participant in the placebo‐first group died one week after randomisation". |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Simonneau 2008.
| Methods | Multicentre, double‐blind, placebo‐controlled, parallel group trial 16 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 265 |
|
| Interventions | Placebo (N=133) or sildenafil (N=134), 20 mg 3 times daily, titrated to 40 mg and 80 mg 3 times daily, as tolerated, at 4‐week intervals | |
| Outcomes | Change from baseline in exercise capacity measured by 6MWD (primary endpoint)
Haemodynamic measurements, time to clinical worsening, and Borg dyspnoea score (secondary endpoints) Not clear whether right‐heart catheterisation was used |
|
| Notes | Also called the PACES study Industry funded Trial conducted at 46 centers in 11 countries (United States, 26; Canada, 6; France, 3; Netherlands, 2; Spain, 2; United Kingdom, 2; Belgium, 1; Czech Republic, 1; Denmark, 1; Israel, 1; and Italy, 1), recruited from 3 July 2003 to 27 January 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a central, computer‐generated pseudo‐random code (by using random, permuted blocks within strata) to assign patients to treatment groups across all centers. Eligible patients were randomly assigned to receive sildenafil or placebo in a 1:1 ratio. The randomisation was stratified by the baseline 6MWD (325 m or 325 m) and cause (idiopathic pulmonary arterial hypertension or pulmonary arterial hypertension due to other causes)". |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were accounted for and similar across groups: Quote: "Only 1 participant in the study (in the placebo group) did not adhere to treatment. No unplanned crossovers occurred during the trial" |
| Selective reporting (reporting bias) | Unclear risk | Quote: "We analysed treatment effects for the secondary end points sequentially only if a statistically significant effect was seen for the primary outcome" |
| Other bias | Low risk | Other bias is unlikely |
Singh 2006.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial 6 weeks duration with a wash‐out of 2 weeks |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 20 |
|
| Interventions | Oral sildenafil versus placebo Adult participants were given 25 mg sildenafil on the first day and repeated after 6 hours under observation. If there was no hypotension, they were given 100 mg 3 times a day. In children weighing < 30 kg, an initial dose of 3.125 mg of sildenafil was given and maintained at 25 mg 3 times daily. In older children weighing > 30 kg, initial dose of 6.25 mg was given and maintained at 50 mg 3 times a day. Participants were given second dose only if there was no significant fall in BP. Treatment was given for 6 weeks, which was followed by a 2‐week washout before crossing over Routine medication, as prescribed by the treating physician, including digitalis, diuretics, and oral anticoagulants, was continued throughout the study Compliance was assessed by the pill‐count method |
|
| Outcomes | The primary endpoint of efficacy was the improvement in distance covered in 6MWD. Secondary endpoints were reduction in PAP as measured by Doppler echocardiography after 6 weeks of treatment, improvement in clinical condition, NYHA class, and exercise duration and metabolic equivalents (Mets) achieved on modified Bruce exercise protocol | |
| Notes | Funding source not stated Location and dates of enrolment not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation, blinding, and drug/placebo administration were done by the Department of Pharmacology |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not explicitly stated if there were any withdrawals |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Suntharalingham 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial 12 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 19 |
|
| Interventions | Sildenafil at 40 mg 3 times a day (N=9) or placebo (N=10) at 2 tablets 3 times per day in a 1:1 ratio At the end of the study, all participants were transferred to open‐label sildenafil at 40 mg 3 times a day and offered repeat assessment at 12 months |
|
| Outcomes | Primary endpoint was change in 6MWD Secondary endpoints included changes in WHO class, cardiopulmonary haemodynamics, QOL scores, and NT‐proBNP | |
| Notes | Funding source not stated Trial conducted at the Pulmonary Vascular Diseases Unit, Papworth Hospital, Cambridgeshire, UK, enrolled between August 2004 and August 2007 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly stated if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were accounted for: Quote: "An extensive urticarial rash developed in one subject in the sildenafil arm, who was thus withdrawn from the study" |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Vitulo 2016.
| Methods | Randomised, placebo‐controlled double‐blind trial 16 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
n = 28 |
|
| Interventions | 20 mg sildenafil or placebo 3 times a day | |
| Outcomes | Primary endpoint was the reduction in pulmonary vascular resistance Secondary endpoints included BODE index, 6MWT, and quality of life questionnaire |
|
| Notes | There is a correction to the original study report: in Table 4 the difference in change for 6MWT should read +19.3, not −19.3, as has been communicated by the authors Dario Vizza with thanks. Industry funded Trial conducted at 7 Italian centers with expertise in the management of PH and COPD, from March 2012 to March 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A pharmacologist not involved directly in the study used an electronic system to manage randomisation |
| Allocation concealment (selection bias) | Low risk | A pharmacologist not involved directly in the study used an electronic system to manage randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A pharmacologist not involved directly in the study used an electronic system to manage randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for: Included in the intention‐to‐treat analysis were 3 participants who did not perform the end‐of‐study RHC for refusal or technical reasons |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
Vizza 2017a.
| Methods | Randomised, placebo‐controlled double‐blind trial 12 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 103 |
|
| Interventions | Sildenafil (20 mg 3 times daily) (N=53) or placebo (N=50) | |
| Outcomes | 6MWD WHO functional class Borg dyspnoea score Clinical worsening (death, lung transplantation, hospitalisation due to pulmonary hypertension, or clinical deterioration of PAH requiring additional therapy) Survival was assessed at week 64, including participants who had discontinued treatment |
|
| Notes | Industry funded Trial was conducted between September 2006 and August 2012 at 29 sites in 10 countries |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (via interactive voice response system incorporating a central randomisation and drug supply scheme) |
| Allocation concealment (selection bias) | Low risk | Participants and investigators were blinded to treatment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and investigators were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 104 randomised participants, 103 were treated in the double‐blind study and 91 continued in the extension |
| Selective reporting (reporting bias) | Low risk | Outcomes were all reported |
| Other bias | Low risk | Other bias is unlikely |
Wilkins 2005.
| Methods | Randomised double‐blind controlled trial 20 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC III n = 26 |
|
| Interventions | Sildenafil (50 mg twice daily for 4 weeks, then 50 mg 3 times daily) (N=14) or bosentan (62.5 mg twice daily for 4 weeks, then 125 mg twice daily) (N=12) | |
| Outcomes | Changes in right ventricular mass (using cardiovascular magnetic resonance), 6MWD, cardiac function, BNP, and Borg dyspnoea index | |
| Notes | Also called the SERAPH study Supported by public funding Trial conducted at Hammersmith Hospital, London, UK, during February 2002 to September 2003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The medication was blinded in identical‐looking gelatin capsules and randomised using a computer‐generated random list by the Hammersmith Hospital pharmacy |
| Allocation concealment (selection bias) | Low risk | The medication was blinded in identical‐looking gelatin capsules and randomised using a computer‐generated random list by the Hammersmith Hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The measurements from images were made by an experienced operator who was unaware of each participant’s clinical information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for: 1 participant died suddenly in the sildenafil arm |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk is unlikely |
Zhuang 2014.
| Methods | Randomised placebo‐controlled trial 16 weeks duration |
|
| Participants | Inclusion criteria:
Exclusion criteria:
WHO FC II/III n = 124 |
|
| Interventions | Tadalafil (40 mg a day) (N=60) or placebo to existing therapy with oral ambrisentan (10 mg a day) (n=64) | |
| Outcomes | 6MWD Improvement in WHO functional class Clinical worsening was defined as the occurrence of the following events: death, transplantation, arterial septostomy, hospitalisation due to worsening PAH, initiation of new therapy or worsening FC by week 16 Haemodynamic parameters were measured by right‐heart catheterisation |
|
| Notes | Funding source not stated Trial was conducted at Shanghai Tenth People’s Hospital of Tongji University, Shanghai, China, between September 2011 and March 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Type of randomisation is not clear The randomisation was stratified for baseline walking distance and type of PAH (idiopathic/familial and anorexigen use vs. other types) |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were accounted for: In the tadalafil group: 6 participants discontinued: 3 for toxicity, 1 for worsening PAH, 2 for non‐compliance In the placebo group: 5 participants discontinued: 4 for worsening PAH; 1 for irrelevant accidental death |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other risk of bias is unlikely |
6MWD: six‐minute walk distance; BNP: brain nutrietic peptide; BODE: Body mass index, airflow Obstruction, Dyspnoea and Exercise capacity; CHF: congenital heart failure; CI: cardiac index; COPD: chronic obstructive pulmonary disease; CTEPH: chronic thromboembolic pulmonary hypertension; DCMP: dilated cardiomyopathy; DLCO: diffusing capacity for carbon monoxide; EF: ejection fraction; EPBF: effective pulmonary blood flow; ERA: endothelin receptor antagonist; FEV1: forced expiratory volume in one second; HFpEF: heart failure with preserved ejection fraction; HIV: human immunodeficiency virus; IV: intravenous; LV: left ventricle; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; m: metres; n: number; NT‐Pro BNP: N‐terminal prohormone of brain natriuretic peptide; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PAP: pulmonary artery pressure; PASP: pulmonary artery systolic pressure; PAWP: pulmonary artery wedge pressure; PCWP: pulmonary capillary wedge pressure; PDA: patent ductus arteriosus; PDE5: phosphodiesterase 5; PEA: pulmonary endarterectomy; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; PVRI: pulmonary vascular resistance index; QOL: quality of life; RAP: right atrial pressure; RHC: right heart catheterisation; SF‐36: short form 36; SGRQ: St George's Respiratory Questionnaire; SO2: saturation of oxygen; SVR: systemic vascular resistance; TAPSE: tricuspid annular plane systolic excursion; TLC: total lung capacity; TTE: transthoracic echocardiogram; TRV: tricuspid regurgitation velocity; VO2: volume of oxygen; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aldashev 2005 | Ineligible patient population |
| Alkhayat 2016 | According to correspondence from the author, this study was not randomised |
| Arneson 2010 | Post hoc analysis |
| Bachetti 2015 | Ineligible intervention |
| Badesch 2005 | Post hoc analysis |
| Badesch 2007 | Post hoc analysis |
| Badesch 2011 | Post hoc analysis |
| Barst 2011 | Post hoc analysis |
| Bates 2011 | Ineligible patient population |
| Benza 2011 | Extension of a previous study |
| Benza 2018 | Point of randomisation was macitentan, not PDE5 inhibitor |
| Botha 2009 | Ineligible patient population |
| Butrous 2006 | Not randomised |
| Cappelleri 2010a | Post hoc analysis |
| Cappelleri 2010b | Ineligible intervention and study design |
| Cappelleri 2010c | Ineligible intervention and study design |
| Chin 2012 | Ineligible intervention |
| Corris 2005 | Post hoc analysis |
| Dandekar 2012 | Ineligible comparator |
| Farah 2013 | Ineligible intervention |
| Farrero 2009 | Not randomised |
| Feldman 2010 | Post hoc analysis |
| Fernandes 2014 | Ineligible study design |
| Fischler 2009 | Too short treatment duration |
| Galiè 2005b | Ineligible study design |
| Galiè 2011a | Post hoc analysis |
| Galiè 2011b | Extension of a previous study |
| Galiè 2013 | Extension of a previous study |
| Ghofrani 2002a | No comparator group, not randomised, acute term study |
| Ghofrani 2002b | Acute term study |
| Ghofrani 2002c | Acute term study |
| Ghofrani 2004a | Acute term study |
| Ghofrani 2004b | Acute term study (120 mins) |
| Ghofrani 2004c | Acute term study |
| Girgis 2009 | Open‐label extension of included study |
| Gotti 2014 | Post hoc analysis |
| Guazzi 2007 | Ineligible patient population |
| Guazzi 2011b | Ineligible patient population |
| Guazzi 2017 | Review |
| Jackson 2010 | Not randomised |
| Mclaughlin 2010 | Ineligible intervention |
| McLaughlin 2014 | Ineligible intervention |
| McLaughlin 2015 | Ineligible intervention |
| Mychaskiw 2010a | Ineligible intervention |
| Mychaskiw 2010b | Ineligible intervention |
| Nazeem 2014 | Ineligible intervention |
| Oudiz 2009a | Extension of a previous study |
| Oudiz 2009b | Extension of a previous study |
| Oudiz 2009c | Post hoc analysis |
| Oudiz 2009d | Post hoc analysis |
| Oudiz 2012 | Extension of a previous study |
| Peacock 2005 | Extension of a previous study |
| Roig 2014 | Ineligible study design |
| Rubin 2005 | Post hoc analysis |
| Rubin 2011 | Post hoc analysis |
| Santos‐Martinez 2015 | Ineligible patient population |
| Shamma 2002 | Not randomised |
| Sharma 2014 | Ineligible intervention |
| Shrestha 2017 | No placebo |
| Simonneau 2010 | Extension of a previous study |
| Sitbon 2005 | Post hoc analysis |
| Sun 2014 | No placebo or other intervention |
| Vizza 2011 | Ineligible comparator |
| Vizza 2017b | Ineligible comparator |
| Zeng 2012 | Ineligible study design |
Characteristics of ongoing studies [ordered by study ID]
Cooper 2013.
| Trial name or title | Sildenafil versus placebo in chronic heart failure (SilHF) |
| Methods | Double‐blind, parallel, randomised controlled trial, 2:1 |
| Participants |
|
| Interventions | Sildenafil 40 mg 3 times daily compared to placebo |
| Outcomes | 6MWD Quality of life (QoL) evaluation by EuroQol5D Kansas City Questionaire NYHA function class All measured at 24 weeks |
| Starting date | March 2013 |
| Contact information | Helse Stavanger HF |
| Notes | ClinicalTrials.gov Identifier: NCT01616381 Estimated study completion date: March 2018 |
Maron 2013.
| Trial name or title | Tadalafil for pulmonary hypertension due to chronic lung disease (TADA‐PHILD) |
| Methods | Randomised, double‐blind, parallel, placebo‐controlled trial |
| Participants |
|
| Interventions | Tadalafil 40 mg daily versus placebo |
| Outcomes |
Outcomes measured at 12 months |
| Starting date | October 1, 2013 |
| Contact information | VA Boston Healthcare System Jamaica Plain Campus, Jamaica Plain, MA |
| Notes | ClinicalTrials.gov Identifier: NCT01862536 Estimated study completion date: August 2019 |
NCT 02951429.
| Trial name or title | Efficacy, safety, and tolerability study of pirfenidone in combination with sildenafil in participants with advanced idiopathic pulmonary fibrosis (IPF) and risk of Group 3 pulmonary hypertension |
| Methods | Phase IIb, randomised parallel‐group controlled trial |
| Participants | Participants with advanced IPF and risk of Group 3 pulmonary hypertension (PH) |
| Interventions | Pirfenidone plus sildenafil versus pirfenidone plus placebo |
| Outcomes |
Measured at 52 weeks |
| Starting date | Currently recruiting |
| Contact information | global‐roche‐genentech‐trials@gene.com |
| Notes | ClinicalTrials.gov Identifier: NCT02951429 Currently recruiting |
NCT 03185364.
| Trial name or title | The safety and efficiency of sildenafil in the treatment of severe post‐capillary pulmonary hypertension caused by COPD |
| Methods | Randomised, double‐blind, parallel, placebo‐controlled trial |
| Participants | Aged 40 to 85 years with COPD and PH (mean PAP ≥ 35 mmHg, PAWP ≤ 15 mmHg) |
| Interventions | Sildenafil 20 mg t3 times daily |
| Outcomes |
Measured at 12 weeks |
| Starting date | June 14, 2017 |
| Contact information | Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Wuhan, Hubei, China, 430030 |
| Notes | ClinicalTrials.gov Identifier: NCT03185364 Currently recruiting |
REPLACE 2017.
| Trial name or title | REPLACE: A prospective, randomized trial of riociguat replacing phosphodiesterase 5 inhibitor therapy in patients with pulmonary arterial hypertension who are not at treatment goal |
| Methods | Prospective, randomised, multicentre, double‐arm, 24‐week, controlled, open‐label study |
| Participants | People with PAH not at treatment goal (WHO FC III, 6MWD 165 – 440 metres) despite receiving stable doses of PDE5i with or without ERA |
| Interventions | Participants will be randomised to continue PDE5i therapy or switch to riociguat (up to 2.5 mg 3 times daily) |
| Outcomes | The composite primary efficacy endpoint is satisfactory clinical response at Week 24, defined as fulfilment of at least 2 out of 3 efficacy parameters (≥ 10% or ≥ 30 metres increase from baseline in 6MWD, WHO FC I/II or ≥ 30% reduction from baseline in NT‐proBNP) in the absence of clinical worsening (independent central adjudication). Secondary outcomes include change from baseline in 6MWD and WHO FC (blinded assessment), NT‐proBNP and clinical worsening. Exploratory endpoints include quality of life (Living with Pulmonary Hypertension), REVEAL risk score and MRI parameters of right‐heart function. Safety will be assessed by adverse events and all‐cause mortality |
| Starting date | 8 September 2016 |
| Contact information | Bayer Clinical Trials Contact (+) 1‐888‐8422937 clinical‐trials‐contact@bayer.com |
| Notes | Recruiting ClinicalTrials.gov Identifier: NCT02891850 |
6MWD: six minute walk distance; COPD: chronic obstructive pulmonary disease; DLCO: diffusing capacity for carbon monoxide; ERA: endothelin receptor antagonist; FEV1: forced expiratory volume in one second; FVC: functional vital capacity; HF: heart failure; IPF: idiopathic pulmonary fibrosis; LVEF: left ventricular ejection fraction; NCT: National Clinical Trials portal; NT‐Pro BNP@ N‐terminal prohormone of brain natriuretic peptide; NYHA: New York Heart Association; PAP: pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PDE5: phosphodiesterase 5; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; QOL: quality of life; RV: right ventricle; SGRQ: St George's Respiratory Questionnaire; TAPSE: tricuspid annular plane systolic excursion; TTE: transthoracic echocardiogram; UCDS SOBQ ‐ The University of California, San Diego shortness of breath questionnaire; WHO: World Health Organization
Differences between protocol and review
In the protocol we intended to assess changes in New York Heart Association (NYHA) functional class, but the nomenclature for people with PAH is now WHO functional class. It should be noted, however, that the NHYA and WHO classification system both classify patients in the same way.
Contributions of authors
HB and ZB screened all abstracts, with TW providing third‐party consensus. HB and ZB extracted the data. HB drafted the review, and ZB and AB and TW provided comments and changes.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Other.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
HB: H Barnes received a travel scholarship that was determined by an independent abstract committee, funded by GSK. GSK had no involvement in the determination of recipients. The Cochrane funding arbiter was consulted and agreed it did not represent a conflict of interest as GSK had no role in the allocation of the travel scholarship.
ZB: none known
AB: none known
TW: Actelion Australia Scientific Advisory Board and Research/Education unrestricted grant; GSK Australia Scientific Advisory Board; Bayer Australia Scientific Advisory Board
Edited (no change to conclusions)
References
References to studies included in this review
Albini 2017 {published data only}
- Albini A, Dardi F, Rinaldi A, Monti E, Tanese N, Gotti E, et al. Hemodynamic and exercise effects of different types of initial oral combination therapy in pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2017;195:A3112. [Google Scholar]
Barst 2012 {published data only}
- Barst R, Ivy D, Gaitan G, Szatmari A, Rudzinski A, Garcia A, et al. A randomized, double‐blind, placebo‐controlled, dose‐ranging study of oral sildenafil citrate in treatment‐naive children with pulmonary arterial hypertension. Circulation 2012;125(2):324‐34. [DOI] [PubMed] [Google Scholar]
Bermejo 2017 {published data only}
- Bermejo J, Yotti R, Garcio‐Orta R, Sanchez‐Fernandez P, Castano M, Segovia‐Cubero J, et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double‐blind, randomized clinical trial. European Heart Journal 2018;39(15):1255‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bharani 2003 {published data only}
- Bharani A, Mathew V, Sahu A, Lunia B. The efficacy and tolerability of sildenafil in patients with moderate‐to‐severe pulmonary hypertension. Indian Heart Journal 2003;55(1):55‐9. [PubMed] [Google Scholar]
Bharani 2007 {published data only}
- Bharani A, Patel A, Saraf J, Jain A, Mehrotra S, Lunia B. Efficacy and safety of PDE‐5 inhibitor tadalafil in pulmonary arterial hypertension. Indian Heart Journal 2007;59(4):323‐8. [PubMed] [Google Scholar]
Blanco 2013 {published data only}
- Blanco I, Santos S, Gea J, Guell R, Torres F, Gimeno‐Santos E, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. European Respiratory Journal 2013;42(4):982‐92. [DOI] [PubMed] [Google Scholar]
- Blanco I, Santos S, Gea J, Guell R, Torres F, Gimeno‐Santos E, et al. Sildenafil treatment to improve the outcomes of pulmonary rehabilitation in COPD: A randomized, controlled trial. American Journal of Respiratory and Critical Care Medicine (Meeting Abstracts) 2013;187:A2266. [Google Scholar]
Boonstra 2005 {published data only}
- Boonstra A, Burgess G, Parpia T, Torbicki A. Sildenafil citrate benefits patients with pulmonary arterial hypertension (PAH) across functional classes. European Respiratory Journal 2005;26:3619. [Google Scholar]
Dwivedi 2015 {published data only}
- Dwivedi P, Narain VS, Saran RK, Dwivedi SK, Sethi R, Chandra S, et al. Assessment of short term effects of sildenafil therapy in patients with secondary pulmonary hypertension. Indian Heart Journal 2015;67:S128. [Google Scholar]
Galiè 2005a {published data only}
- Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. New England Journal of Medicine 2005;353(20):2148‐57. [DOI] [PubMed] [Google Scholar]
- Peacock A, Burgess G, Parpia T, Barst RJ. Hemodynamic effects of sildenafil in pulmonary arterial hypertension (PAH) patients. European Respiratory Society 15th Annual Congress; 2005 Sep 17‐20; Copenhagen, Denmark. 2005:Abstract No. 3127.
- Pepke‐Zaba J, Beardsworth A, Chan M, Angalakuditi M. Effects of tadalafil on health‐related quality of life in patients with pulmonary arterial hypertension. European Heart Journal 2009;30(suppl 1):442. [Google Scholar]
- Pepke‐Zaba J, Beardsworth A, Chan M, Angalakuditi M. Tadalafil therapy and health‐related quality of life in pulmonary arterial hypertension. Current Medical Research and Opinion 2009;25(10):2479‐85. [DOI] [PubMed] [Google Scholar]
- Pepke‐Zaba J, Brown M, Parpia T, Gilbert C, Burgess G. Sildenafil improves health‐related quality of life in pulmonary arterial hypertension (PAH) patients. European Respiratory Society Annual Congress. 2005 September 17‐20; Copenhagen, Denmark. 2005; Vol. 26:Abstract No. 848.
- Pepke‐Zaba J, Brown MCJ, Parpia T, Gilbert C, Burgess G. The impact of sildenafil citrate on health‐related quality of life in patients with pulmonary arterial hypertension (PAH) results of a multicenter, multinational, randomized double‐blind, placebo controlled trial. American Thoracic Society International Conference; 2005 May 20‐25; San Diego (CA). 2005:B16.
Galiè 2009 {published data only}
- Galiè N, Beardsworth A, Wrishko RE, Ghofrani HA. Tadalafil improves exercise capacity in patients with pulmonary arterial hypertension across the interdosing interval. American Thoracic Society International Conference; 2009 May 15‐20; San Diego (CA). 2009:A1045.
Galiè 2015 {published data only}
- Galiè N, Barbera JA, Frost A, Ghofrani HA, Hoeper M, McLaughlin V, et al. AMBITION: A randomised, multicenter study of first‐line ambrisentan and tadalafil combination therapy in subjects with pulmonary arterial hypertension (PAH). European Respiratory Journal 2014;44(Suppl 58):2916. [Google Scholar]
- Galiè N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. New England Journal of Medicine 2015;373(9):834‐44. [DOI] [PubMed] [Google Scholar]
- Hoeper M, Galiè N, Barbera J A, Frost A, Ghofrani A, McLaughlin V, et al. Initial combination therapy with ambrisentan (AMB) and tadalafil (TAD) in treatment naive patients with pulmonary arterial hypertension (PAH): Efficacy and safety in the AMBITION study intent to treat (ITT) population. European Respiratory Journal 2015;46(Suppl 59):OA4994. [Google Scholar]
- Rubin L, Barbera J, Frost A, Ghofrani HA, Hoeper M, McLaughlin V, et al. Upfront combination therapy with ambrisentan and tadalafil in treatment naive patients with pulmonary arterial hypertension (PAH): Subgroup analyses by functional class (FC), etiology, and region from the ambition study. Chest 2014;146(4 suppl 2):339A. [Google Scholar]
- Thierer J. In patients with pulmonary arterial hypertension treatment initiation with two drugs is associated with better outcome than with monotherapy: The AMBITION trial. Revista Argentina de Cardiologia 2015;83(5):487‐93. [Google Scholar]
- Vachiery JL, Galiè N, Barbera JA, Frost A, Ghofrani HA, Hoeper MM, et al. Initial combination therapy of ambrisentan and tadalafil reduced pulmonary arterial hypertension (PAH) related hospitalizations: Secondary analysis from the ambition trial. American Journal of Respiratory and Critical Care Medicine 2015;181:A2197. [Google Scholar]
- Vachiery JL, Galiè N, Barbera JA, Frost A, Ghofrani HA, Hoeper MM, et al. Initial combination therapy of ambrisentan and tadalafil reduced pulmonary arterial hypertension (PAH) related hospitalizations: Secondary analysis from the ambition trial. American Journal of Respiratory and Critical Care Medicine 2015;191:A2197. [Google Scholar]
Galiè 2016a {published data only}
- Galiè N, Gotti E, Palazzini M, Manes A, Bachetti C, Mazzanti G, et al. A randomized open label study comparing first‐line treatment with bosentan or sildenafil in chronic thromboembolic pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine 2016;193:A6468. [Google Scholar]
Goudie 2014 {published data only}
- Goudie A, Hopkinson P, Lipworth B, Struthers A. Do phosphodiesterase 5 inhibitors improve exercise capacity in patients with COPD associated pulmonary hypertension? (3P study). European Respiratory Society 23rd Annual Congress; 2013 September 7‐11; Barcelona, Spain. 2013.
- Goudie A, Lipworth B, Hopkinson P, Wei L, Struthers A. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double‐blind, parallel‐group, placebo‐controlled trial. Lancet Respiratory Medicine 2014;2(4):293–300. [DOI] [PubMed] [Google Scholar]
Guazzi 2011a {published data only}
- Guazzi M, Vicenzi M, Arena R, Guazzi M. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase‐5 inhibition in a 1‐year study. Circulation 2011;124(2):164‐74. [DOI] [PubMed] [Google Scholar]
Han 2013 {published data only}
- Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right‐sided ventricular dysfunction. Chest 2013;143(6):1699‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoendermis 2015 {published data only}
- Hoendermis ES, Liu LC, Hummel YM, Meer P, Boer RA, Berger RMF, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. European Heart Journal 2015;36(36):2565‐73. [DOI] [PubMed] [Google Scholar]
- Liu LC, Hummel YM, Meer P, Berger RM, Damman K, Veldhuisen DJ, et al. Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health‐related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. European Journal of Heart Failure 2017;19(1):116‐25. [DOI] [PubMed] [Google Scholar]
Iversen 2009 {published data only}
- Iversen K, Jensen AS, Jensen TV, Vejlstrup NG, Sondergaard L. Combination therapy with bosentan and sildenafil in Eisenmenger syndrome: A randomized, placebo‐controlled, double‐blinded trial. European Heart Journal 2010;31(9):1124‐31. [DOI] [PubMed] [Google Scholar]
Jalalian 2015 {published data only}
- Jalalian R, Moghadamnia AA, Tamaddoni A, Khafri S, Iranian M. Comparing the efficacy of tadalafil versus placebo on pulmonary artery systolic pressure and right ventricular function in patients with beta‐thalassaemia intermedia. Heart Lung and Circulation 2016;14(26):677–83. [DOI] [PubMed] [Google Scholar]
- Jalalian R, Tammadoni A, Iranian M, Saravi M, Mohgadamnia A, Khafi SE. Comparing the efficacy of tadalafil versus placebo on pulmonary artery systolic pressure and right ventricular function in patients with beta‐thalassemia intermedia. European Heart Journal 2015;36:502. [DOI] [PubMed] [Google Scholar]
Jing 2011 {published data only}
- Jing ZC, Yu ZX, Shen JY, Wu BX, Xu KF, Zhu XY, et al. Vardenafil therapy for pulmonary arterial hypertension: A randomised, double‐blind, placebo‐controlled, multicenter study. European Society of Cardiology, ESC Congress; 2010 Aug 28‐Sep 1; Stockholm, Sweden. 2010.
- Jing ZC, Yu ZX, Shen JY, Wu BX, Xu KF, Zhu XY, et al. Vardenafil in pulmonary arterial hypertension: a randomized, double‐blind, placebo‐controlled study. American Journal of Respiratory and Critical Care Medicine 2011;183(12):1723‐9. [DOI] [PubMed] [Google Scholar]
Lewis 2007 {published data only}
- Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 2007;115(1):59‐66. [DOI] [PubMed] [Google Scholar]
- Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007;116(14):1555‐62. [DOI] [PubMed] [Google Scholar]
Machado 2009 {published data only}
- Gladwin M, Machado R, Hassell K, Yovetich N, Gordeuk V, Gibbs S, et al. Treatment of pulmonary hypertension (PH) and sickle cell disease (scd) with sildenafil (sild) therapy (walk‐phasst): interim report. European Respiratory Society 19th Annual Congress; 2009 September 12‐15; Vienna, Austria. 2009; Vol. 171.
- Machado RF, Barst RJ, Yovetich NA, Hassell KL, Goldsmith JC, Woolson R, et al. Evaluation of sildenafil therapy for patients with sickle cell disease and increased tricuspid regurgitant velocity: preliminary results of the walk‐PHaSST trial. American Thoracic Society International Conference; 2010 May 14‐19; New Orleans (LA). 2010:A2514.
- Machado RF, Barst RJ, Yovetich NA, Hassell KL, Goldsmith JC, Woolson R, et al. Safety and efficacy of sildenafil therapy for doppler‐defined pulmonary hypertension in patients with sickle cell disease: preliminary results of the Walk‐PHaSST clinical trial. Blood 2009;114(22):Abstract no: 571. [Google Scholar]
- Yovetich NA, Hassell KL, Machado RF, Barst RJ, Gladwin MT. Treatment of pulmonary hypertension and sickle cell disease with sildenafil therapy (walk‐PHaSST): Background, rationale, study design, current enrollment and baseline data. American Journal of Hematology Conference: 3rd Annual Sickle Cell Disease Research and Educational Symposium and Grant Writing Institute and Annual National Sickle Cell Disease Scientific Meeting; 2009 Feb 15‐20; Fort Lauderdale (FL). 2009:E197‐8.
Mazzanti 2013 {published data only}
- Mazzanti G, Albini A, Palazzini M, Monti E, Bachetti C, Rinaldi A, et al. A randomized open label study comparing first‐line treatment with bosentan or sildenafil in pulmonary arterial hypertension (PAH). European Society of Cardiology Annual Congress; 2013 Aug 31‐Sep 4; Amsterdam, Netherlands. 2013:A3535.
- Mazzanti G, Palazzini M, Dardi F, Terzi F, D'Adamo A, Rinaldi A, et al. A randomized open label study comparing first‐line treatment with bosentan or sildenafil in pulmonary arterial hypertension. European Society of Congress; 2012 Aug 25‐29; Munchen, Germany. 2012:A4105.
- Mazzanti G, Palazzini M, Leci E, Dardi F, Rinaldi A, D'Adamo A, et al. A randomized open label study comparing first‐line treatment with bosentan or sildenafil in pulmonary arterial hypertension (PAH): Long‐term results. American Journal of Respiratory and Critical Care Medicine 2013;187:A3535. [Google Scholar]
Mukhopadhyay 2011 {published data only}
- Mukhopadhyay S, Nathani S, Yusuf J, Shrimal D, Tyagi S. Clinical efficacy of phosphodiesterase‐5 inhibitor tadalafil in Eisenmenger Syndrome‐A randomized, placebo‐controlled, double‐blind crossover study. Congenital Heart Disease 2011;6(5):424‐31. [DOI] [PubMed] [Google Scholar]
Ovchinnov 2015 {published data only}
- Artem Ovchinnikov AG, Potekhina AV, Gavryushina SV, Ageev FT. Sildenafil improves functional capacity and exercise hemodynamics in patients with HFpEF and predominantly combined pre‐and post‐capillary pulmonary hypertension. European Journal of Heart Failure Conference: Heart Failure 2018 and the 5th World Congress on Acute Heart Failure Austria. 2018; Vol. 20 (Supplement 1):567‐8.
- Ovchinnikov AG, Gavryushina SV, Masenko VP, Ageev FT. Phosphodiesterase‐5 inhibitor sildenafil decreases pulmonary and left ventricular filling pressures and improves functional capacity in patients with diastolic heart failure and reactive pulmonary hypertension. European Journal of Heart Failure 2015;17(Suppl. 1):5–441. [Google Scholar]
Palazzini 2010 {published data only}
- Palazzini M, Leci E, Bachetti C, Sgro F, Mazzanti G, Beciani E, et al. A randomized open label study comparing bosentan or sildenafil first‐line treatment in pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). European Society of Cardiology Congress; 2010 Aug 28‐Sep 1; Stockholm, Sweden. 2010.
Palii 2014 {published data only}
- Palii I, Vataman E, Maniuc L, Repin O, Vataman M, Cucu I. Clinical and haemodynamic effects of sildenafil in pulmonary hypertension secondary congenital systemic‐to‐pulmonary shunts and RV dysfunction. European Heart Journal 2011;32(Issue suppl_1, 113):P759. [Google Scholar]
- Palii I, Vataman E, Revenco N, Maniuc L, Vataman M, Repin. Outcome research in 77 patients with pulmonary arterial hypertension receiving sildenafil: A double‐blind, randomised controlled study. Archives of Disease in Childhood 2014;99(suppl 2):A31. [Google Scholar]
- Palii I, Vataman E, Stratulat P. Efficacy of sildenafil therapy in children with pulmonary hypertension secondary to congenital systemic‐to‐pulmonary shunts. European Heart Journal 2013;34:39. [Google Scholar]
Rao 2011 {published data only}
- Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. Sildenafil improves six‐minute walk distance in chronic obstructive pulmonary disease: a randomised, double‐blind, placebo‐controlled trial. Indian Journal of Chest Diseases and Allied Sciences 2011;53(2):81‐5. [PubMed] [Google Scholar]
- Rao RS, Singh S, Sharma BB, Singh V. Does sildenafil improve exercise capacity and pulmonary artery hypertension in chronic obstructive pulmonary disease? A controlled trial. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna, Austria. 2009:P3850.
Salem 2013 {published data only}
- Salem M, Diab A, Ateya A, Sanad O. Short term effects of sildenafil citrate therapy in secondary pulmonary hypertension. Egyptian Heart Journal 2014;66(1):49‐53. [Google Scholar]
Sastry 2004 {published data only}
- Sastry BK, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: A randomized, placebo‐controlled, double‐blind, crossover study. Journal of the American College of Cardiology 2004;43(7):1149‐53. [DOI] [PubMed] [Google Scholar]
Simonneau 2008 {published data only}
- Simonneau G, Burgess G, Collings L, Barst RJ, Galiè N, Rubin LJ, et al. Safety and efficacy of combination therapy with sildenafil and epoprostenol in patients with pulmonary arterial hypertension (PAH). American Thoracic Society International Conference; 2006 May 19‐21; San Diego. 2006:A58.
- Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost AE. Addition of sildenafil to long‐term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: A randomized trial. Annals of Internal Medicine 2008;149(8):521‐30. [DOI] [PubMed] [Google Scholar]
Singh 2006 {published data only}
- Singh T, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo‐controlled, double blind, cross‐over study to evaluate the efficacy and safety of oral sildenafil treatment in severe pulmonary artery hypertension. Indian Heart Journal 2005;57(5):Abstract no:533. [Google Scholar]
- Singh TP, Rohit M, Grover A, Malhotra S, Vijayvergiya R. A randomized, placebo‐controlled, double‐blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. American Heart Journal 2006;151(4):851.e1‐5. [DOI] [PubMed] [Google Scholar]
Suntharalingham 2008 {published data only}
- Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, et al. Long‐term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008;134(2):229‐36. [DOI] [PubMed] [Google Scholar]
Vitulo 2016 {published data only}
- Vitulo P, Callari A, Martino L, Stanziola A, Oggionni T, Meloni F, et al. Spheric‐1 (sildenafil and pulmonary hypertension in COPD): Intention‐to‐treat (ITT) analysis of safety and efficacy data. Journal of Heart and Lung Transplantation 2014;33(4):S148‐9. [Google Scholar]
- Vitulo P, Sofia M, Confalonieri M, Libertucci D Oggionni, Rottoli P, et al. COPD‐associated severe pulmonary hypertension (COPDPH): Results of a 16‐weeks prospective multicenter, double‐blind, placebo‐controlled randomized clinical trial (RCT) (SPHERIC‐1 (clinicaltrials.gov/ct2/show/NCT01441934)) investigating the effect of sildenafil citrate (Sld) on pulmonary vascular resistance (PVR) and PaO2. European Respiratory Journal 2013;42(Suppl 57):5151. [Google Scholar]
- Vitulo P, Stanziola A, Confalonieri M, Libertucci D, Oggionni T, Rottoli P, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: A randomized controlled multicenter clinical trial. Journal of Heart and Lung Transplantation 2016;36(2):166‐74. [DOI] [PubMed] [Google Scholar]
Vizza 2017a {published data only}
- Vizza C, Lansa P, Teal S, Dombi T, Zhou D. Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial. BMC Cardiovascular Disorders 2017;17(1):239‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkins 2005 {published data only}
- Wilkins MR, Paul GA, Strange JW, Tunariu N, Gin‐Sing W, Banya WA, et al. Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. American Journal of Respiratory and Critical Care Medicine 2005;171(11):1292‐7. [DOI] [PubMed] [Google Scholar]
Zhuang 2014 {published data only}
- Zhuang Y, Jiang B, Gao H, Zhao W. Randomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertension. Hypertension Research 2014;37(6):507‐12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aldashev 2005 {published data only}
- Aldashev AA, Kojonazarov BK, Amatov TA, Sooronbaev TM, Mirrakhimov MM, Morrell NW, et al. Phosphodiesterase type 5 and high altitude pulmonary hypertension. Thorax 2005;60(8):683‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alkhayat 2016 {published data only}
- Alkhayat K, Eid M. Sildenafil citrate therapy for secondary pulmonary arterial hypertension due to chronic obstructive lung disease. Egyptian Journal of Chest Diseases and Tuberculosis 2016;65(4):805‐9. [Google Scholar]
Arneson 2010 {published data only}
- Arneson C, McDevitt S, Klinger JR. Tadalafil in geriatric patients with pulmonary arterial hypertension. Chest 2010;138:367A. [Google Scholar]
Bachetti 2015 {published data only}
- Bachetti C, Manes A, Dardi F, Palazzini M, Mazzanti G, Rinaldi A, et al. Comparison between initial combination therapy and initial monotherapy in pulmonary arterial hypertension: a single centre blinded evaluation of patients enrolled in the AMBITION study. American Journal of Respiratory and Critical Care Medicine 2015;191(Meeting Abstracts):A4779. [Google Scholar]
- Bachetti C, Manes A, Dardi F, Palazzini M, Mazzanti G, Rinaldi A, et al. Hemodynamic effect of initial combination therapy as compared to initial monotherapy in pulmonary arterial hypertension: A single centre blinded evaluation of patients enrolled in the AMBITION study. European Heart Journal 2015;36:967. [Google Scholar]
Badesch 2005 {published data only}
- Badesch D, Burgess G, Parpia T, Torbicki A. Sildenafil citrate in patients with pulmonary arterial hypertension (PAH) results of a multicenter multinational randomized double‐blind placebo controlled trial by WHO functional class (FC). Thorax 2005;60(8):A57 [Poster: K39]. [Google Scholar]
Badesch 2007 {published data only}
- Badesch DB, Hill NS, Burgess G, Rubin LJ, Barst RJ, Galiè N, et al. Sildenafil for pulmonary arterial hypertension associated with connective tissue disease. Journal of Rheumatology 207;34(12):2417‐22. [PubMed] [Google Scholar]
Badesch 2011 {published data only}
- Badesch D, Hwang LJ, Teal S, Cwengros J, Watt S. Posthoc subgroup analysis: Sildenafil (SIL) added to long‐term epoprostenol therapy in patients with idiopathic and connective tissue disease (CTD)‐associated pulmonary arterial hypertension (PAH). European Society of Cardiology Congress; 2011 Aug 27‐31; Paris, France. 2011:173, 1179.
Barst 2011 {published data only}
- Barst R, Brundage B, Ghofrani A, Oudiz R, Simmoneau G, Beardsworth A, et al. [Tadalafil improves exercise capacity and delays time to clinical worsening in patients with pulmonary arterial hypertension (PAH)]. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin, Germany. 2008:3176.
- Barst RJ, Brundage BH, Ghofrani A, Oudiz RJ, Simonneau G, Beardsworth A, et al. Tadalafil improves exercise capacity, health related quality of life and delays time to clinical worsening in patients with symptomatic pulmonary arterial hypertension (PAH) [Abstract]. 2008; Vol. 134:39003s.
- Barst RJ, Oudiz RJ, Beardsworth A, Brundage BH, Simonneau G, Ghofrani HA, et al. Pulmonary arterial, hypertension; response to tadalafil study, group ‐ tadalafil monotherapy and as add‐on to background bosentan in patients with pulmonary arterial hypertension. Journal of Heart and Lung Transplantation 2011;30(6):632‐43. [DOI] [PubMed] [Google Scholar]
Bates 2011 {published data only}
- Bates MG, Thompson AA, Baillie JK, Sutherland AI, Irving JB, Hirani N, et al. Sildenafil citrate for the prevention of high altitude hypoxic pulmonary hypertension: double blind, randomized, placebo‐controlled trial. High Altitude Medicine and Biology 2011;12(3):207‐14. [DOI] [PubMed] [Google Scholar]
Benza 2011 {published data only}
- Benza RL, Seeger W, McLaughlin VV, Channick RN, Voswinckel R, Tapson VF, et al. Long‐term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open‐label extension. Journal of Heart and Lung Transplantation 2011;30(12):1327‐33. [DOI] [PubMed] [Google Scholar]
Benza 2018 {published data only}
- Benza RL, Raina A, Gupta H, Murali S, Burden A, Zastrow MS, et al. Bosentan‐based, treat‐to‐target therapy in patients with pulmonary arterial hypertension: results from the COMPASS‐3 study. Pulmonary Circulation 2018;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Botha 2009 {published data only}
- Botha P, Parry G, Dark JH, Macgowan GA. Acute hemodynamic effects of intravenous sildenafil citrate in congestive heart failure: comparison of phosphodiesterase type‐3 and ‐5 inhibition. Journal of Heart and Lung Transplantation 2009;28(7):676‐82. [DOI] [PubMed] [Google Scholar]
Butrous 2006 {published data only}
- Butrous G, Ghofrani HA, Weissmann N, Schermuly, Seeger W, Grimminger F. Oral sildenafil is a potent pulmonary vasodilator and improves gas exchange in patients with COPD with pulmonary hypertension. European Respiratory Society 16th Annual Congress; 2006 Sep 3‐6; Munich, Germany. 2006; Vol. 28:426s [P2494].
Cappelleri 2010a {published data only}
- Cappelleri JC, Mychaskiw MA, Hwang LJ, Mardekian J, Quazi K. Physical functioning, six‐minute walk distance, and pulmonary vascular resistance in adult patients with pulmonary arterial hypertension treated with sildenafil: examining the distributions of outcomes. Chest 2010;138(4):362A. [Google Scholar]
Cappelleri 2010b {published data only}
- Cappelleri JC, Hwang LJ, Mardekian J, Mychaskiw MA. PCV138 Measurement properties of peak VO2 in children with pulmonary arterial hypertension. Value in Health 2010;13(7):A367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cappelleri 2010c {published data only}
- Cappelleri JC, Hwang LJ, Mardekian J, Mychaskiw MA. PCV17 Response profiles of sildenafil citrate on exercise capacity, hemodynamic function, and health‐related quality of life in children with pulmonary arterial hypertension. Value in Health 2010;13(7):A343. [Google Scholar]
Chin 2012 {published data only}
- Chin K, D'Souza G, Jing ZC, Burgess G, Collings L, Teeter JG, et al. A multinational, randomized, double‐blind trial of sitaxentan monotherapy compared with sitaxentan in combination with sildenafil in patients with pulmonary arterial hypertension: The sitaxentan randomized prospective assessment adding sildenafil (SR‐PAAS). American Journal of Respiratory and Critical Care Medicine 2012;185:A4812. [Google Scholar]
Corris 2005 {published data only}
- Corris PA, Burgess G, Parpia T, Barst RJ. Sildenafil effects on 1‐year survival of patients with idiopathic pulmonary arterial hypertension (IPAH). European Respiratory Society 15th Annual Congress; 2005 Sep 17‐20; Copenhagen, Denmark. 2005; Vol. 26:Abstract No. 3128.
Dandekar 2012 {published data only}
- Dandekar PG, Verma Y. Efficacy and safety profile of phosphodiesterase 5 inhibitor sildenafil in pulmonary hypertension. International Congress of Cardiology; 2012 Feb 24‐26; Hong Kong. 2012:A7.
Farah 2013 {published data only}
- Farah P, Ahmad‐Ali A, Hanane G, Abbas E. Additive effect of phosphodiesterase inhibitors in control of pulmonary hypertension after congenital cardiac surgery in children. Iranian Journal of Pediatrics 2013;23(1):19‐26. [PMC free article] [PubMed] [Google Scholar]
Farrero 2009 {published data only}
- Farrero M, Perez‐Villa F, Arias A, Sionis A, Castel A, Roig E. Sildenafil or bosentan in patients considered inelegible for heart transplantation because of severe pulmonary hypertension. Journal of Heart and Lung Transplantation 2009;28(2):S265. [DOI] [PubMed] [Google Scholar]
Feldman 2010 {published data only}
- Feldman JP, Arneson C, Wade M. Does 16‐week response predict long‐term success? Results from a 52‐week analysis of patients receiving 40 mg once‐daily tadalafil. Chest 2010;138:364A. [Google Scholar]
Fernandes 2014 {published data only}
- Fernandes CJ, Martins BC, Jardim CV, Ciconelli RM, Morinaga LK, Breda AP, et al. Quality of life as a prognostic marker in pulmonary arterial hypertension. Health and Quality of Life Outcomes 2014;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischler 2009 {published data only}
- Fischler M, Maggiorini M, Dorschner L, Debrunner J, Bernheim A, Kiencke S, et al. Dexamethasone but not tadalafil improves exercise capacity in adults prone to high‐altitude pulmonary edema. American Journal of Respiratory and Critical Care Medicine 2009;180(4):346‐52. [DOI] [PubMed] [Google Scholar]
Galiè 2005b {published data only}
- Galiè N, Burgess G, Parpia T, Barst R. Effects of sildenafil on 1 year survival of patients with idiopathic pulmonary arterial hypertension (PAH). American Thoracic Society International Conference; 2005 May 20‐25; San Diego (CA). 2005:C88.
Galiè 2011a {published data only}
- Galiè N, Barst R, Brundage B, Ghofrani H, Oudiz R, Simonneau G. Long‐term outcomes of patients with pulmonary arterial hypertension associated with connective tissue disease as compared with idiopathic or heritable pulmonary arterial hypertension treated with tadalafil. Chest 2011;140:734A. [Google Scholar]
Galiè 2011b {published data only}
- Galiè N, Barst R, Brundage B, Ghofrani H A, Oudiz R, Simonneau G. Tadalafil in idiopathic or heritable pulmonary arterial hypertension compared to pulmonary arterial hypertension associated with connective tissue disease. European Respiratory Journal 2011;38:412s (P2299). [Google Scholar]
Galiè 2013 {published data only}
- Galiè N, Barst RJ, Oudiz RJ, Li B, Esler A, Simonneau G. Long‐term safety and survival outcomes of patients with pulmonary arterial hypertension enrolled in an open label continuation study with tadalafil 40mg daily. European Heart Journal 2013;34:49‐50. [Google Scholar]
Ghofrani 2002a {published data only}
- Ghofrani AH, Wiedermann R, Rose F, Schermuly T, Olschewski H, Weissmann N, et al. Sildenafil is a potent pulmonary vasodilator in pulmonary hypertension associated with interstitial lung disease: impact on gas exchange properties. European Respiratory Society 18th Annual Congress; 2008 October 3‐7; Berlin, Germany. 2008.
Ghofrani 2002b {published data only}
- Ghofrani HA, Wiedemann R, Rose F, Ischewski HO, Schermuly RT, Weissmann N, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Annals of Internal Medicine 2002;136(7):515‐22. [DOI] [PubMed] [Google Scholar]
Ghofrani 2002c {published data only}
- Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial (see comment). Lancet 2002;360(9337):895‐900. [DOI] [PubMed] [Google Scholar]
Ghofrani 2004a {published data only}
- Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek E, Seeger T, Schermuly RT, et al. Sildenafil reduces hypoxia‐induced pulmonary hypertension and increases exercise capacity during oxygen deprivation at a low altitude and at Mount Everest base camp: a randomized placebo controlled study. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando (FL). 2004:A56 Poster B3.
Ghofrani 2004b {published data only}
- Ghofrani HA, Voswinckel R, Reichenberger F, Olschewski H, Haredza P, Karadas B, et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase‐5 inhibitors in patients with pulmonary arterial hypertension ‐ a randomized prospective study. Journal of the American College of Cardiology 2004;44(7):1488–96. [DOI] [PubMed] [Google Scholar]
Ghofrani 2004c {published data only}
- Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek EH, Seeger T, Olschewski H, et al. Sildenafil increased exercise capacity during hypoxia at low altitudes and at Mount Everest base camp: a randomized, double‐blind, placebo‐controlled crossover trial. Annals of Internal Medicine 2004;141(3):169‐77. [DOI] [PubMed] [Google Scholar]
Girgis 2009 {published data only}
- Girgis R, Arneson C, Wade M. Tadalafil in patients with collagen vascular disease‐associated pulmonary arterial hypertension. Chest 2009;136(4):55S. [Google Scholar]
Gotti 2014 {published data only}
- Gotti E, Manes A, Palazzini M, Bernabe C, Bachetti C, Conficoni E, et al. A randomized open label study comparing first‐line treatment with bosentan or sildenafil in chronic thromboembolic pulmonary hypertension (CTEPH). European Heart Journal 2014;35:208. [Google Scholar]
Guazzi 2007 {published data only}
- Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long‐term use of sildenafil in the therapeutic management of heart failure. Journal of the American College of Cardiology 2007;50(22):2136–44. [DOI] [PubMed] [Google Scholar]
Guazzi 2011b {published data only}
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. A long term follow‐up study of patients with mild to moderate group 2 pulmonary hypertension treated with Pde5‐inhibition: A comparison between heart failure with reduced and preserved ejection fraction. Circulation 2011;124:A14722. [DOI] [PubMed] [Google Scholar]
Guazzi 2017 {published data only}
- Guazzi M. Is sildenafil neutral on cardiopulmonary performance in group 2 pulmonary hypertension? More details for interpretation. European Journal of Heart Failure 2017;19(5):691. [DOI] [PubMed] [Google Scholar]
Jackson 2010 {published data only}
- Jackson RM, Glassberg MK, Ramos CF, Bejarano PA, Butrous G, Orlando Gómez‐Marín O. Sildenafil therapy and exercise tolerance in idiopathic pulmonary fibrosis. Lung 2010;188(2):115‐23. [DOI] [PubMed] [Google Scholar]
Mclaughlin 2010 {published data only}
- McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. Journal of the American College of Cardiology 2010;55(18):1915‐22. [DOI] [PubMed] [Google Scholar]
McLaughlin 2014 {published data only}
- McLaughlin V, Channick R, Ghofrani HA, LeMarie JC, Naeije R, Packer M, et al. Effect of bosentan and sildenafil combination therapy on morbidity and mortality in pulmonary arterial hypertension (PAH): Results from the compass‐2 study. Chest. 2014; Vol. 146:860A.
McLaughlin 2015 {published data only}
- McLaughlin V, Channick RN, Ghofrani HA, Lemarie JC, Naeije R, Packer M, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. European Respiratory Journal 2015;46(2):405‐13. [DOI] [PubMed] [Google Scholar]
Mychaskiw 2010a {published data only}
- Mychaskiw MA, Hwang LJ, Mardekian J. Evaluation of sildenafil treatment on functional health status in patients with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2010;181:A3336. [Google Scholar]
Mychaskiw 2010b {published data only}
- Mychaskiw MA, Mardekian J, Teal SA, Hwang LJ, Quazi K. Relationship between pulmonary vascular resistance and physical functioning in two randomized controlled trials of adult patients with pulmonary arterial hypertension, including those with associated connective tissue disease. Chest 2010;138(4):363A. [Google Scholar]
Nazeem 2014 {published data only}
- Nazeem T, Thomas S, Kumar S. Severity based clinical efficacy and safety of oral sildenafil in the management of pulmonary arterial hypertension ‐ a pilot study. Nepal Journal of Epidemiology. 2014; Vol. 2.
Oudiz 2009a {published data only}
- Oudiz R, Ghofrani HA, Simonneau G, Barst R, Brundage B, Elion‐Mboussa A, et al. The effect of once‐daily tadalafil on exercise capacity and clinical deterioration with up to 68 weeks of treatment in patients with pulmonary arterial hypertension (PAH). European Society of Cardiology Congress 2009; 2009 August 28‐September 2; Barcelona, Spain. 2009:899.
Oudiz 2009b {published data only}
- Oudiz RJ, Arneson C, Wade M. Long‐term improvements in clinical worsening with tadalafil in patients with pulmonary arterial hypertension. Chest 2009;136(4):55S‐b. [Google Scholar]
Oudiz 2009c {published data only}
- Oudiz RJ, Beardsworth A, Chan MLS, Barst RJ. Blinded, long‐term safety and efficacy of tadalafil in treatment for pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2009;179:A1042 (Poster #715). [Google Scholar]
Oudiz 2009d {published data only}
- Oudiz RJ, Brundage B, Galie N, Simonneau G, Barst RB, Elion‐Mboussa A, et al. Effect of once‐daily tadalafil on exercise capacity and clinical deterioration with up to 68 weeks of treatment in patients with PAH. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna. 2009:[E1486].
Oudiz 2012 {published data only}
- Oudiz RJ, Brundage BH, Galiè N, Ghofrani HA, Simonneau G, Botros FT, et al. Tadalafil for the treatment of pulmonary arterial hypertension: a double‐blind 52‐week uncontrolled extension study. Journal of the American College of Cardiology 2012;60(8):768‐74. [DOI] [PubMed] [Google Scholar]
Peacock 2005 {published data only}
- Peacock A, Burgess G, Parpia T, Barst RJ. Hemodynamic effects of sildenafil in pulmonary arterial hypertension (PAH) patients. European Respiratory Society 15th Annual Congress; 2005 September 17‐20; Copenhagen, Denmark. 2005; Vol. 26:Abstract No. 3127.
Roig 2014 {published data only}
- Roig M, Torres L, Pacreu S, Londono O. Pulmonary arterial hypertension and sildenafil: A second stage control. European Heart Journal 2014;35:743. [Google Scholar]
Rubin 2005 {published data only}
- Rubin L, Burgess G, Parpia T, Simoneau G. Effects of sildenafil on 6 minute walk distance (6MWD) and WHO functional class (FC) after 1 year of treatment. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego (CA). 2005:B16.
Rubin 2011 {published data only}
- Rubin LJ, Badesch DB, Fleming TR, Galiè N, Simonneau G, Ghofrani HA, et al. Long‐term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER‐2 study. Chest 2011;140(5):1274‐83. [DOI] [PubMed] [Google Scholar]
Santos‐Martinez 2015 {published data only}
- Santos‐Martinez LE, Jimenez‐Santos M, Arenas‐Fonseca JG, Moreno‐Gonzalez A, Medina‐Concebida LE. Combined treatment of sildenafil and inhaled iloprost in pediatric patients with severe pulmonary arterial hypertension. Archivos de Cardiologia de Mexico 2015;85(1):80‐4. [DOI] [PubMed] [Google Scholar]
Shamma 2002 {published data only}
- Shamma V, Satish O, Mahesh G, Patnaik A, Srinivas B, Janardhana V, et al. Sildenafil citrate in patients with primary‐pulmonary hypertension. Indian Heart Journal 2002;54(5):143. [Google Scholar]
Sharma 2014 {published data only}
- Sharma SK, Ajmani S, Sharma A, Singh S, Sinha S, Vishnubhatla S. Comparison of efficacy of different treatment regimens in pulmonary hypertension secondary to lung disease and/or hypoxia. American Journal of Respiratory and Critical Care Medicine 2014;189:A1892. [Google Scholar]
Shrestha 2017 {published data only}
- Shrestha SK, Srivastava B, Karki M, Khatri DB, Pradhan RM. Effect of sildenafil citrate on pulmonary arterial systolic pressure and sub‐maximal exercise capacity in chronic obstructive pulmonary disease. Kathmandu University Medical Journal (KUMJ) 2017;15(60):271‐8. [PubMed] [Google Scholar]
Simonneau 2010 {published data only}
- Simonneau G, Rubin LJ, Galiè N, Fleming TR, Gillies H, Barst RJ. Long‐term oral sildenafil added to intravenous epoprostenol therapy improves outcomes in patients with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2010;181:A3332. [Google Scholar]
Sitbon 2005 {published data only}
- Sitbon O, Burgess G, Parpia T, Ghofrani HA. Effects of sildenafil on 6‐minute walk distance (6MWD) and WHO functional class (FC) in pulmonary arterial hypertension (PAH) patients after 1 year of treatment. European Respiratory Journal 2005;26(Suppl 49):3129. [Google Scholar]
Sun 2014 {published data only}
- Sun X, Wang K, Wang W, Li B. Clinical study on sildenafil in treatment of pregnant women with pulmonary arterial hypertension. Chung‐hua Fu Chan Ko Tsa Chih (Chinese Journal of Obstetrics & Gynaecology) 2014;49(6):414‐8. [PubMed] [Google Scholar]
Vizza 2011 {published data only}
- Vizza CD, Badagliacca R, Poscia R, Pasquini A, Cavalieri A, Pietro R, et al. Tadalafil in out‐of‐proportion pulmonary hypertension secondary to left ventricular dysfunction: Rationale and design of the study. Giornale Italiano di Cardiologia 2011;12:e44. [Google Scholar]
Vizza 2017b {published data only}
- Vizza CD, Sastry BK, Safdar Z, Harnisch L, Gao X, Zhang M, et al. Efficacy of 1, 5, and 20 mg oral sildenafil in the treatment of adults with pulmonary arterial hypertension: a randomized, double‐blind study with open‐label extension. BMC Pulmonary Medicine 2017;17(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2012 {published data only}
- Zeng WJ, Sun YJ, Gu Q, Xiong CM, Li JJ, He JG. Impact of sildenafil on survival of patients with idiopathic pulmonary arterial hypertension. Journal of Clinical Pharmacology 2012;52(9):1357‐64. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Cooper 2013 {published data only}
- Cooper TJ, Guazzi M, Al‐Mohammad A, Amir O, Bengal T, Cleland J, et al. Sildenafil in heart failure (SilHF). An investigator‐initiated multinational randomized controlled clinical trial: Rationale and design. European Journal of Heart Failure 2013;15(1):119‐22. [DOI] [PubMed] [Google Scholar]
Maron 2013 {published data only}
- Maron BA, Goldstein RH, Rounds SI, Shapiro S, Jankowich M, Garshick E, et al. Study design and rationale for investigating phosphodiesterase type 5 inhibition for the treatment of pulmonary hypertension due to chronic obstructive lung disease: the TADA‐PHiLD (TADAlafil for Pulmonary Hypertension associated with chronic obstructive Lung Disease) trial. Pulmonary Circulation 2013;3(4):889‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT 02951429 {unpublished data only}
- Behr J, Nathan SD, Harari S, Wuyts W, Kirchgaessler KU, Bengus M, et al. Sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: a Phase IIb, randomised, double‐blind, placebo‐controlled study ‐ Rationale and study design. Respiratory Medicine 2018;138:13‐20. [DOI] [PubMed] [Google Scholar]
- NCT02951429. Efficacy, safety, and tolerability study of pirfenidone in combination with sildenafil in participants with advanced idiopathic pulmonary fibrosis (IPF) and risk of group 3 pulmonary hypertension [A phase IIb, multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy, safety, and tolerability of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of group 3 pulmonary hypertension]. clinicaltrials.gov/ct2/show/NCT02951429 (first received 1 November 2016).
NCT 03185364 {unpublished data only}
- Jianguo He. The safety and efficiency of sildenafil in the treatment of severe post‐capillary pulmonary hypertension caused by COPD. NCT03185364 2017.
REPLACE 2017 {published data only}
- Hoeper MM, Ghofrani H, Benza RL, Corris PA, Gibbs J, Klinger JR, et al. REPLACE: a prospective, randomized trial of riociguat replacing phosphodiesterase 5 inhibitor therapy in patients with pulmonary arterial hypertension who are not at treatment goal. European Respiratory Journal 2017;50:PA2417. [Google Scholar]
- Hoeper MM, Ghofrani HA, Benza RL, Corris PA, Gibbs S, Klinger JR, et al. Rationale and design of the REPLACE trial: riociguat replacing phosphodiesterase 5 inhibitor (PDE5i) therapy evaluated against continued PDE5i therapy in patients with pulmonary arterial hypertension (PAH). American journal of respiratory and critical care medicine. 2017; Vol. 195:A2296.
Additional references
AMBITION 2015
- Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. New England Journal of Medicine 2015;373(9):834‐44. [DOI] [PubMed] [Google Scholar]
Archer 2009
- Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. New England Journal of Medicine 2009;361(19):1864‐71. [DOI] [PubMed] [Google Scholar]
Blanco 2010
- Blanco I, Gimeno E, Munoz P, Pizarro S, Gistau C, Rodriguez‐Roisin R, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine 2010;181(3):270–8. [DOI] [PubMed] [Google Scholar]
Bocchi 1994
- Bocchi EA, Bacal F, Auler Júnior JO, Carmone MJ, Bellotti G, Pileggi F. Inhaled nitric oxide leading to pulmonary edema in stable severe heart failure. American Journal of Cardiology 1994;74(1):70–2. [DOI] [PubMed] [Google Scholar]
Boucly 2017
- Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. European Respiratory Journal 2017;50(2):1700889. [DOI] [PubMed] [Google Scholar]
COMPASS‐2 2014
- McLaughlin V, Channick RN, Ghofrani HA, Lemarié JC, Naeije R, Packer M, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. European Respiratory Journal 2014;46(2):405‐13. [DOI] [PubMed] [Google Scholar]
Cooper 1996
- Cooper CJ, Landzberg MJ, Anderson TJ, Charbonneau F, Creager MA, Ganz P. Role of nitric oxide in the local regulation of pulmonary vascular resistance in humans. Circulation 1996;93(2):266–71. [DOI] [PubMed] [Google Scholar]
Delcroix 2016
- Delcroix M, Lang I, Pepke‐Zaba J, Jansa P, D'Armini A, Snijder R. Long‐term outcome of patients with chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2016;133(9):859–71. [DOI] [PubMed] [Google Scholar]
Farber 2011
- Farber HW, Foreman AJ, Miller DP, McGoon MD. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congestive Heart Failure 2011;17(2):56‐64. [DOI] [PubMed] [Google Scholar]
Frantz 2014
- Frantz R, Durst L, Burger C, Oudiz R, Bourge R, Franco V. Conversion From sildenafil to tadalafil: Results from the sildenafil to tadalafil in pulmonary arterial hypertension (SITAR) Study. Journal of Cardiovascular Pharmacology and Therapeutics 2014;19(6):550‐7. [DOI] [PubMed] [Google Scholar]
Galiè 2015b
- Galiè N, Müller K, Scalise AV, Grünig E. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. European Respiratory Journal 2015;45(5):1314‐22. [DOI] [PubMed] [Google Scholar]
Galiè 2016b
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Revista Espanola de Cardiologia 2016;69(2):177. [DOI] [PubMed] [Google Scholar]
Ghofrani 2004d
- Ghofrani HA, Pepke‐Zaba J, Barbera JA, Channick R, Keogh AM, Gomez‐Sanchez MA, et al. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. Journal of the American College of Cardiology 2004;43(12 Suppl S):68S‐72S. [DOI] [PubMed] [Google Scholar]
Ghofrani 2013
- Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH N. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. New England Journal of Medicine 2013;369(4):319‐29. [DOI] [PubMed] [Google Scholar]
Gilbert 2009
- Gilbert C, Brown MC, Cappelleri JC, Carlsson M, McKenna SP. Estimating a minimally important difference in pulmonary arterial hypertension following treatment with sildenafil. Chest 2009;135(1):137‐42. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 13 December 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
GRIPHON 2015
- Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, et al. Selexipag for the treatment of pulmonary arterial hypertension. New England Journal of Medicine 2015;373(26):2522‐33. [DOI] [PubMed] [Google Scholar]
Guazzi 2012
- Guazzi M, Borlaug B. Pulmonary hypertension due to left heart disease. Circulation 2012;126(8):975‐90. [DOI] [PubMed] [Google Scholar]
Guignabert 2013
- Guignabert C, Tu T, Hiress M, Ricard M, Sattler C, Seferian A. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. European Respiratory Review 2013;22(130):543‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 1989
- Guyatt GH, Veldhuyzen Van Zanten SJ, Feeny DH, Patrick DL. Measuring quality of life in clinical trials: a taxonomy and review. Canadian Medical Association Journal 1989;140(12):1441‐8. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altmam DG, Sterne JA (editors), on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hoeper 2017
- Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. European Respiratory Journal 2017;50(2):1700740. [DOI] [PubMed] [Google Scholar]
Jiang 2014
- Jiang G, Li B, Zhang G, Xu E, Liu Y, Xu Z. Effects of sildenafil on prognosis inpatients with pulmonary hypertension after left‐sided valvular surgery. Heart Lung Circulation 2014;23:680–5. [DOI] [PubMed] [Google Scholar]
Khair 2016
- Khair RM, Nwaneri C, Damico RL, Kolb T, Hassoun PM, Mathai SC. The minimal important difference in borg dyspnea score in pulmonary arterial hypertension. Annals of the American Thoracic Society 2016;13(6):842‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kylhammar 2018
Lindman 2012
- Lindman BR, Zajarias A, Madrazo JA, Shah J, Gage BF, Novak E, et al. Effects of phosphodiesterase type5 inhibition on systemic and pulmonary hemodynamics and ventricular function in patients with severe symptomatic aortic stenosis. Circulation 2012;125:2353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 2012
- Ling Y, Johnson MK, Kiely DG. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. American Journal of Respiratory and Critical Care Medicine 2012;186(8):790‐6. [DOI] [PubMed] [Google Scholar]
McLaughlin 2009
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on expert consensus documents. Journal of the American College of Cardiology 2009;53(17):1573–619. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peacock 2007
- Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. European Respiratory Journal 2007;30(1):104‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schüneman 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
SERAPHIN 2013
- Pulido T, Adzerikho I, Channick R, Delcroix M, Galiè N, Ghofrani H, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. New England Journal of Medicine 2013;369(9):809‐18. [DOI] [PubMed] [Google Scholar]
Sim 2010
- Sim J. Nitric oxide and pulmonary hypertension. Korean Journal of Anesthesiology 2010;58(1):4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith JS, Gorbett D, Mueller J, Perez R, Daniels CJ. Pulmonary hypertension and idiopathic pulmonary fibrosis: a dastardly duo. American Journal of Medical Science 2013;346(3):221‐5. [DOI] [PubMed] [Google Scholar]
Strange 2012
- Strange G, Playford D, Stewart S, Deague J, Nelson H, Gabbay E. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart 2012;98(4):1805‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thenappan 2007
- Thenappan T, Shah SJ, Rich S, Gomberg‐Maitland M. A USA‐based registry for pulmonary arterial hypertension: 1982–2006. European Respiratory Journal 2007;30(6):1103–10. [DOI] [PubMed] [Google Scholar]
Vachiéry 2013
- Vachiéry J‐L, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. Journal of the American College of Cardiology 2013;62(25 Suppl):D100‐8. [DOI] [PubMed] [Google Scholar]
Zisman 2010
- Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. New England Journal of Medicine 2010;363(7):620‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Barnes 2017
- Barnes H, Brown Z, Burns A, Williams T. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD012621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanthapillai 2001
- Kanthapillai P, Walters EH. Sildenafil for pulmonary hypertension. (Protocol). Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003562] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanthapillai 2004
- Kanthapillai P, Walters EH. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003562.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


