Abstract
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, which in turns accounts for the sixth most common cancer worldwide. Despite being the 6th most common cancer it is the second leading cause of cancer related deaths. HCC typically arises in the background of cirrhosis, however, about 20% of cases can develop in a non-cirrhotic liver. This particular subgroup of HCC generally presents at an advanced stage as surveillance is not performed in a non-cirrhotic liver. HCC in non-cirrhotic patients is clinically silent in its early stages because of lack of symptoms and surveillance imaging; and higher hepatic reserve in this population. Interestingly, F3 fibrosis in non-alcoholic fatty liver disease, hepatitis B virus and hepatitis C virus infections are associated with high risk of developing HCC. Even though considerable progress has been made in the management of this entity, there is a dire need for implementation of surveillance strategies in the patient population at risk, to decrease the disease burden at presentation and improve the prognosis of these patients. This comprehensive review details the epidemiology, risk factors, clinical features, diagnosis and management of HCC in non-cirrhotic patients and provides future directions for research.
Keywords: Hepatocellular carcinoma, Non-cirrhotic liver, Hepatitis B, Hepatitis C, Risk factors, Clinical features, Diagnostic modalities, Management strategies, Future directions
Core tip: Hepatocellular carcinoma (HCC) is the 2nd leading cause of cancer related deaths. Majority of HCC arise in a cirrhotic liver, however, 20% of cases can develop in non-cirrhotic liver. This comprehensive review focuses on risk factors, clinical features, diagnostic modalities, management strategies and future directions for HCC in non-cirrhotic liver.
INTRODUCTION
Primary liver cancer is the sixth most common cancer worldwide, 90% of which are hepatocellular carcinoma (HCC)[1,2]. HCC is the second leading cause of cancer-related deaths worldwide[3]. Although, most of the cases occur in developing countries, its incidence in developed countries has increased recently[4]. HCC typically arises in the setting of cirrhosis however; approximately 20% of HCC’s have been known to develop in a non-cirrhotic liver[5,6]. This sub-group of HCC often presents at advanced stages because surveillance is not performed in a non-cirrhotic liver. Fibrolamellar carcinoma (FLC), a rare variant of HCC also occurs without any background cirrhosis or hepatitis[6,7]. This review discusses the epidemiology, risk factors, clinical features, diagnosis and management of HCC in non-cirrhotic patients as well as provides future direction for research in this population.
LITERATURE SEARCH
A comprehensive literature search was performed and research papers regarding non-cirrhotic HCC and related literature was analyzed to prepare this review article. Special emphasis was placed on research related to the diagnosis and management of non-cirrhotic HCC in the last 5 yr.
EPIDEMIOLOGY
Worldwide, liver cancer is the fifth most common cancer in men and the ninth in women. It is the 2nd leading cause of cancer death in men and the sixth leading among women, with about 745500 deaths in 2012. In the United States, there is expected to be an estimated 42220 new cases and 30200 death cases of liver and intrahepatic bile duct carcinomas in 2018[8,9]. HCC is a little over 2 times more likely in men over women, the incidence being 5.5 per 100000 in male and 2 per 100000 in female in the United States[8]. Non-cirrhotic HCC has a bimodal age distribution, peaking during the 2nd and 7th decade of life[5]. The FLC variant comprises 1%-9% of all HCC and accounts for less than 1% of HCC in the United States[10,11]. Overall, there is a lack of significant data on HCC that arises in non-cirrhotic liver. Given that HCC is one of the fastest growing cause of cancer-related deaths and has a survival rate of less than 12% in the United States, there is a need for further research to explore the epidemiology of non-cirrhotic HCC[8].
RISK FACTORS
Table 1.
Incidence of different risk factors for hepatocellular carcinoma in cirrhotic and non-cirrhotic liver in various studies
| Parameter | Study | No (NC) | No (CL) | Percentage (NC) | Percentage (CL) | Mean Incidence (NC) | Mean Incidence (CL) |
| Alcohol | Schütte et al[16] | 14 | 285 | 15% | 50% | 21.77% | 30% |
| Nzeako et al[52] | 25 | 130 | 7% | 28% | |||
| Trevisani et al[4] | 15 | 105 | 16.50% | 30% | |||
| Techathuvanan et al[153] | 26 | 48 | 36% | 34% | |||
| Chang et al[154] | 98 | 99 | 44% | 44.50% | |||
| Grazi et al[155] | 29 | 67 | 21.50% | 23% | |||
| Shim et al[28] | 22 | 22 | 12.40% | 4.10% | |||
| HBV | Nzeako et al[52] | 17 | 43 | 5% | 9.30% | 30.60% | 41.65% |
| Trevisani et al[4] | 10 | 81 | 10% | 22.55 | |||
| Techathuvanan et al[153] | 5 | 26 | 10.60% | 32% | |||
| Chang et al[154] | 142 | 150 | 64.30% | 69% | |||
| Grazi et al[155] | 17 | 46 | 12.60% | 14.90% | |||
| Shim et al[28] | 105 | 443 | 59% | 83.30% | |||
| Kew et al[156] | NR | NR | 52.80% | 60.50% | |||
| HCV | Trevisani et al[4] | 48 | 76 | 15% | 76% | 14.36% | 44.18% |
| Chang et al[154] | 39 | 57 | 18.10% | 27.40% | |||
| Grazi et al[155] | 33 | 182 | 24.40% | 56.80% | |||
| Shim et al[28] | 13 | 27 | 7.30% | 5.10% | |||
| Albeldawi et al[157] | 6 | 107 | 7% | 55.70% | |||
| NASH | Schütte et al[16] | 6 | 37 | 6.45% | 6.48% | NA | NA |
| Cigarette smoking | Schütte et al[16] | 55 | 231 | 40% | 47.60% | 28.37% | 32.10% |
| Trevisani et al[4] | 16 | 70 | 20% | 28% | |||
| Techathuvanan et al[153] | 4 | 5 | 4.20% | 2.80% | |||
| Chang et al[154] | 110 | 111 | 49.30% | 50% | |||
| Diabetes Mellitus | Schütte et al[16] | 59 | 368 | 71.20% | 83.70% | 40.73% | 46.40% |
| Shim et al[28] | 27 | 84 | 15.20% | 15.80% | |||
| Albeldawi et al[157] | 29 | 113 | 35.80% | 39.70% | |||
| Family History | Techathuvanan et al[153] | 9 | 7 | 12.30% | 4.90% | 13.85% | 9.60% |
| Chang et al[154] | 32 | 30 | 15.40% | 14.30% | |||
| Cryptogenic | Schütte et al[16] | 53 | 65 | 57% | 11.38% | 39.15% | 9.44% |
| Shim et al[28] | 38 | 40 | 21.30% | 7.50% |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; NASH: Non-alcoholic steatohepatitis; NC: Non-cirrhotic liver; CL : Cirrhotic liver.
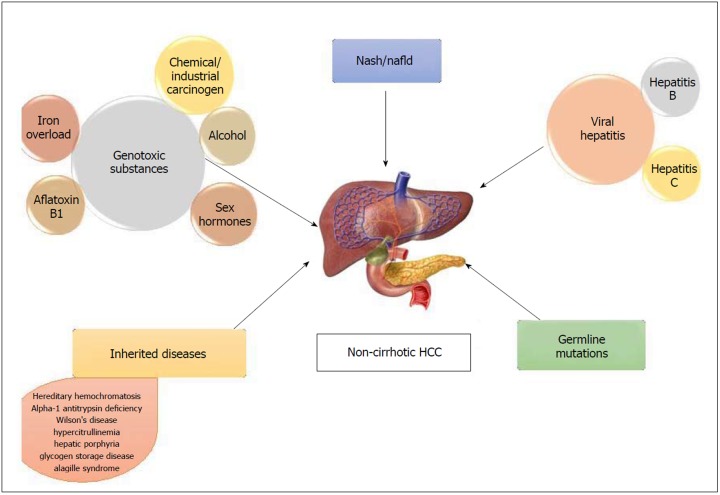
Figure 1.
Causes of non-cirrhotic hepatocellular carcinoma. HCC: Hepatocellular carcinoma.
Non-alcoholic fatty liver disease /Non-alcoholic steatohepatitis
Non-alcoholic fatty liver disease (NAFLD) comprises of a spectrum that includes isolated steatosis, non-alcoholic steatohepatitis (NASH, hepatic inflammation and cell death), fibrosis and cirrhosis (Table 1). With the growing obesity epidemic, NAFLD has become the most common liver disorder in the United States. A strong association has been reported between fatty liver disease and HCC in non-cirrhotic livers[12,13]. NAFLD-related HCC has been acknowledged as a growing burden in this country[14,15]. It has also been recognized as the most common etiology in new HCC cases, likely due to the recent advances in viral hepatitis, especially hepatitis C virus (HCV) treatment[15]. In a study performed by Schütte et al[16] the etiology of majority of HCC in non-cirrhotic liver was related to metabolic syndrome (MS). The causes of non-cirrhotic HCC is shown in Figure 1.
NAFLD, with or without NASH is the hepatic manifestation of MS and predisposes to HCC in non-cirrhotic patients[17]. Type 2 diabetes mellitus and obesity that is associated with NAFLD and MS leads to the release of multiple pro-inflammatory cytokines like TNF-alpha, IL-6, leptin and resistin and decreased amounts of adiponectin. These processes favor the development of hepatic steatosis and inflammation within the liver and subsequently precede the development of HCC[12]. Even though type 2 diabetes and obesity have both been implicated as independent risk factors for HCC, studies that establish a clear link to HCC in non-cirrhotic livers are scarce[18-20]. Not only MS and obesity but being overweight is also associated with higher risk of HCC. Overweight and obesity increase HCC prevalence in general population and especially in hepatitis B virus (HBV) and HCV patients[21]. Other features of MS like hypertension and dyslipidemia have not been extensively studied for the linkage. A recent Australian study comparing HCC characteristics between cirrhotic and non-cirrhotic NAFLD found that apart from the presence or absence of cirrhosis, the fibrosis stage was the only patient characteristic that conferred a worse prognosis as this was related to the size of the tumors (P = 0.03)[22].
Viral hepatitis
30% of HBV-related HCC occurs in non-cirrhotic patients[23] (Figure 2). HBV, a partially double stranded DNA virus is able to integrate into the host cell and acts as a mutagenic agent causing secondary chromosomal rearrangement and increasing genomic instability. In addition, transactivation of genes by the regulatory protein HBx is known to increase cell proliferation, deregulate cell cycle control and interfere with DNA repair and apoptosis[24]. Certain risk factors in chronic hepatitis B patients in turn impart a higher risk for non-cirrhotic HCC. BCP T1762/A1764 mutation and high viral loads have been reported to be strong viral factors and independent predictors of HCC in non-cirrhotic patients who are chronic HBV carriers[25,26]. Older patients with chronic Hepatitis B had a higher annual incidence of non-cirrhotic HCC compared to cirrhotic patients; 1.1% per year in men and 0.3%-0.4% per year in women greater than the age of 55[27]. African American and Asian race has also been associated with a higher incidence of HCC in non-cirrhotic chronic hepatitis B patients[27]. About half of the cryptogenic HCC cases have occult Hepatitis B infection defined by the presence of HBV DNA in the liver or blood without HBsAg[28].
Figure 2.
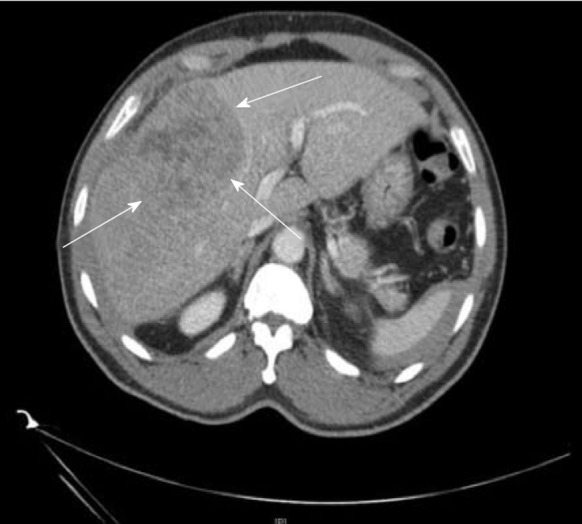
Computed tomography image of a 64-yr-old male with hepatitis B. No cirrhosis found to have large 9 cm mass in right lobe (arrows) on CT abdomen done for abdominal pain.
HCC in chronic Hepatitis C patients primarily occurs in the background of cirrhosis that is related to a necroinflammatory state with tissue damage, regeneration, repair and fibrosis[29,30]. HCV being a single stranded RNA virus cannot integrate with the host genome due to the absence of a DNA intermediate. However, it still possesses direct oncogenic potential although lower compared to HBV; with several of its gene products capable of contributing to carcinogenesis[4,31]. The core protein can alter cell regulation via enhanced telomerase activity[31]. Non-structural proteins like NS3, NS4B and NS5A can potentially induce carcinogenesis through interactions with cellular promoters and proteins[32]. The incidence of HCC in non-cirrhotic HCV patients ranges from 4.4%-10.6%[32]. Its role as a major risk factor is suggested by the development of HCC even after eradication of the virus[33]. In treatment naïve HCV patients, male gender, advanced age, persistently elevated aminotransferases, high gamma-glutamyl transferase levels, hepatic steatosis, diabetes and alcohol abuse have all been shown to increase the risk for non-cirrhotic HCC[34,35]. In patients with sustained virologic response after chronic HCV treatment, those who had diabetes mellitus and increased Fibrosis-4 index were at a higher risk. Studies have also shown increased risk of HCC in patients with hepatitis C and F3 fibrosis. Apart from hepatitis C, this increased risk is noted in even patients with hepatitis B and NAFLD who have F3 fibrosis[22,25,27,36]. The exact pathophysiology behind this still remains to be elucidated.
Genotoxic substances
Alcohol: Several studies have shown heavy alcohol intake in patients with non-cirrhotic HCC, however most of these were not statistically significant[4]. Alcohol may not be a major cause in non-cirrhotic patients, but it should still be treated as a serious risk factor for the development of HCC. This is related to its direct genotoxic effect in the development of HCC mediated through endotoxin production, oxidative stress and inflammation[37]. Heavy alcohol intake in the setting of other risk factors like chronic HCV and diabetes mellitus may potentiate the oxidative stress and free radical damage; leading to rapid progression to HCC[38-40].
Aflatoxin B1: Aflatoxin B1 (AFB1) is an extremely potent hepatocarcinogen that is a secondary metabolite produced by fungi, Aspergillus flavus and Aspergillus parasiticus. They are typically found in tropical and sub-tropical regions of the world in which grains such as rice stored in hot humid conditions promote growth of these toxin-producing fungi[41]. Most cases occur in sub-Saharan Africa, Southeast Asia and China where HBV is highly prevalent. However, its incidence in the United States is extremely low; 0.003 in HbsAg negative and 0.08 in HbsAg-positive patients[42]. In addition, its burden in non-cirrhotic individuals is unknown. AFB1 is metabolized by the P450 enzymes in the liver to generate an epoxide, which binds to DNA and induces mutation of the p53 tumor suppressor gene[24,43]. Like cirrhotic patients, non-cirrhotic patients with chronic HBV are also at a higher risk for aflatoxin-mediated HCC[44,45].
Iron overload: Secondary iron overload is seen in patients with hematological disease like myelodysplastic syndrome, chronic anemia and polytransfusion[46-48]. The notion that increased liver iron stores in the liver cause HCC in non-cirrhotic patient has been present for at least three decades and there are several case reports that have highlighted the role of excess iron as a potential carcinogen[48,49]. Iron-induced carcinogenesis could be related to the production of reactive oxygen species via Fenton reaction inducing oxidative stress. This in turn promotes protein and DNA modification, blunts immune response by impairing T cell proliferation against tumor transformed cells and induces of p53 mutations[50,51].
Miscellaneous: Chemical and industrial carcinogens like nitrosamines, azo dyes, aromatic amines, vinyl chloride, organic solvents, pesticides and arsenic have been known to induce carcinogenesis in non-cirrhotic liver[52,53]. Studies have shown KRAS mutations in vinyl chloride-induced HCC and HRAS mutations in methylene chloride-induced liver tumors[23]. Polycyclic aromatic hydrocarbons derived from red meat, cigarette smoking, and environmental pollutants also increase the risk of HCC by forming DNA adducts[53-55].
Sex hormones: Several case reports have established a link between chronic anabolic androgen steroid abuse and non-cirrhotic HCC in young professional bodybuilders[56-58]. “Adenoma-carcinoma sequence” or “de novo carcinoma development” are the two proposed mechanisms for carcinogenesis[56]. Oral contraceptive (OCs) is also a known risk factor for the development of non-cirrhotic HCC[59,60]. In a retrospective case series of 26 white women aged under 50 who developed HCC in a non-cirrhotic liver, women who used OCs for 8 or more years had a 4.4-fold increased risk (P < 0.01)[59]. Both anabolic steroids and estrogen undergo significant enterohepatic circulation and slow biliary excretion, which increases intrahepatic concentrations and causes direct toxicity[59].
Inherited diseases
Hereditary hemochromatosis and alpha-1 antitrypsin deficiency are inherited diseases in which HCC occurs frequently without cirrhosis[61-65]. Acute hepatic porphyrias which include 3 autosomal dominant disorders: Acute intermittent porphyria (AIP), variegate porphyria (VP) and hereditary coproporphyria (HCP) has also been considered as a cause of non-cirrhotic HCC[66]. Overproduction of aminolevulinic acid and/or porphobilinogen overproduction and excretion has been implicated as a cause of hepatic carcinogenesis in AIP, but the mechanism is poorly understood in VP and HCP[67].
Hypercitrullinemia, a hereditary urea cycle disorder is associated with hepatocarcinogenesis in non-cirrhotic liver likely via citrulline-mediated promotion effect on hepatocyte proliferation[68].
Wilson’s disease, an autosomal recessive disorder of copper metabolism is also implicated as cause of HCC in scattered case reports. The mechanism is related to copper mediated stimulation of fibroblast growth factor-2 and copper induced stabilization of hypoxia-inducible-factor-1 alpha, which causes expression of genes that promote angiogenesis[69,70]. Even though HCC prevalence in Wilson’s disease is lower than other inherited forms of liver disease, it remains a significant risk factor for development of HCC in non-cirrhotic liver as well.
Glycogen storage disorders (GSD) are a group of inherited metabolic disorders characterized by the accumulation of excessive abnormal glycogen in liver, muscle or both. HCC typically occurs without cirrhosis in glycogen storage disease type 1 (GSD-1) via adenoma-carcinoma sequence[62]. HCC in GSD type III is extremely rare and generally occurs in the background of cirrhosis, however Oterdoom et al[71] reported the first documented case in a non-cirrhotic.
Alagille syndrome, an autosomal dominant disease that causes significant neonatal jaundice and cholestasis in older children has been shown in case reports to cause non-cirrhotic HCC[72,73].
Hepatic vascular disease like Budd-Chiari syndrome and nodular regenerative hyperplasia have been associated with non-cirrhotic HCC in case reports, however the mechanism of hepatocarcinogenesis in the absence of cirrhosis is unknown[74,75].
Germline mutations
Studies establishing associations between germline mutations and non-cirrhotic HCC have been scarce. In a recent study by Donati et al[76], germline mutations in telomerase reverse transcriptase gene (hTERT) were associated with shorter telomere lengths and progression of NAFLD to HCC in non-cirrhotic liver. Future studies may identify such germline mutations, which would allow for closer surveillance in these high-risk individuals.
Hepatic adenoma
Hepatic adenomas (HA) is a benign liver tumor that carries a small risk for the development of HCC[60]. The risk of malignant transformation is controversial and has been heavily debated based on available past research studies. Two studies that analyzed available literature, estimated this risk to be approximately 5%[77,78]. Studies on HA in non-cirrhotic livers have been scarce and those that exist have limitations related to overestimation of the risk and biased reviews of resected cases[79,80]. Nonetheless, there is compelling evidence in the literature to reserve resection of adenomas for adenoma diameter > 5 cm with telangiectatic or unclassified subtypes and male sex[77,78,81].
CLINICAL FEATURES
HCC in non-cirrhotic patients is clinically silent in its early stages because of lack of symptoms and surveillance imaging; and higher hepatic reserve in this population[82]. The median age of these patients is 69 yr however, the FLC variant commonly occurs in adolescents and young adults, ranging from 10-35 yr at presentation[11,16]. Unfortunately, these tumors are often found in advanced stages with approximately 25% of non-cirrhotic HCC presenting with extra-hepatic metastasis[16]. When symptoms do occur, they arise due to large tumor burdens from its insidious progression. The most common presenting symptom is abdominal pain (52%). Other symptoms include abdominal distention, weight loss, malaise, anorexia, fatigue, chronic diarrhea, jaundice, chest pain and fever of unknown origin[4,83,84]. Non-cirrhotic HCC can also present in the form of paraneoplastic syndrome of hypercalcemia or hypoglycemia[82,85]. FLC has been known to present with gynecomastia, recurrent deep vein thrombosis, Budd-Chiari syndrome, non-bacterial thrombotic endocarditis, fulminant liver failure or encephalopathy[10].
DIAGNOSIS
Serum alpha-fetoprotein
Alpha-fetoprotein (AFP), a serum glycoprotein is a commonly used tumor marker for HCC[86]. Due to its poor sensitivity, the American Association for the Study of Liver Disease (AASLD) guidelines suggests surveillance with ultrasound with or without AFP in cirrhotic patients. In non-cirrhotic HCC, the sensitivity of AFP further decreases with elevation less common compared to cirrhotic HCC (31%-67% vs 63%-84%). AFP levels in majority of patients with the FLC-variant HCC are normal[87,88]. Levels > 400 ng/mL are essentially diagnostic for non-cirrhotic HCC and are equally prevalent in both groups[89]. This implies that elevated AFP levels may suggest a HCC, but normal levels should never be used to exclude the diagnosis, especially in a patient with high-risk factors. AFP levels however, may have a role in tumor surveillance and prognosis.
Des-gamma-carboxyprothrombin
Des-gamma-carboxyprothrombin (DCP) also known as PIVKA II (protein induced by vitamin K absence) is produced by malignant hepatocytes and its relationship to HCC has been known since 1984[90,91]. DCP has been reported to be more sensitive and specific than AFP for the diagnosis of HCC with a cutoff of > 40 mAU/mL[92]. However, its role in the detection of small tumors and early HCC is unclear as various studies have utilized different cutoff values for DCP and AFP[92-94]. Moreover, it has never been studied in non-cirrhotic patients. DCP might be the answer for early HCC diagnosis in non-cirrhotic patients along with monitoring treatment response and recurrence. Perhaps DCP could compliment AFP as evidenced by improved sensitivities emphasized in several studies[91,93]. Future studies should be directed towards establishing a relationship between elevated DCP and non-cirrhotic HCC.
Imaging
The radiological appearance of HCC in cirrhotic and non-cirrhotic patients is very similar, except HCC in non-cirrhotic livers frequently present as a solitary mass with or without satellite lesions, are much larger in tumor size and are often seen with a central scar[95].
Ultrasonography: Ultrasonography (US) is a non-invasive test that allows determining the size, location, morphology and vascular involvement of the lesion. The appearance of HCC on US is variable and non-specific ranging from hypo or hyperechoic lesions with or without heterogeneity or necrotic areas. This imaging modality is limited in the detection of tumors < 2 cm and tumors in a liver base with a heterogeneous diffuse nodular pattern[82,84].
Contrast-enhanced US (CEUS) could be valuable diagnostic tool because the dye allows its use in patients with nephropathies and those with known adverse reactions to other contrast agents[82]. CEUS shows a typical HCC vascular pattern, although inconsistently when compared to computed tomography (CT) and magnetic resonance imaging (MRI) especially for tumors < 2 cm[96-99]. There is a need for such studies in non-cirrhotic HCC, but for now its role in diagnosis is limited. However, it may have a role in guiding biopsies and monitoring tumor response to treatment with anti-angiogenic properties[100,101].
Computed tomography: CT scan can make the diagnosis of HCC with a high degree of confidence, hence proper technique and contrast administration is crucial for an accurate assessment (Figure 3)[82]. The main diagnostic criteria include hypervascularization on the arterial “wash-in” phase and washout during portal phase of enhancement, which is similar in cirrhotic and non-cirrhotic livers[102,103]. It often presents as a single large well-circumscribed encapsulated hypoattenuating lesion on unenhanced CT, with other tumor features like fat involvement, foci of hemorrhage and necrotic areas more common in non-cirrhotic HCC[104]. The FLC variant may show a similar pattern on contrast enhanced imaging as the classic non-cirrhotic HCC and often demonstrates internal calcifications, central scars and a discontinuous capsule[105].
Figure 3.
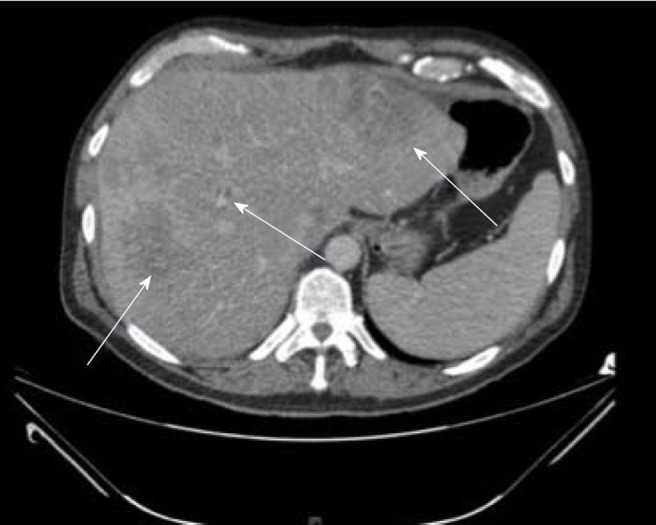
Computed tomography image of a 55-yr-old male with no significant past medical history found to have multifocal hepatocellular carcinoma in the right lobe of liver on imaging done for abdominal pain and jaundice.
Magnetic resonance imaging: MRI is superior to CT for the diagnosis of HCC (Figure 4). Its appearance on T1 sequences varies depending on the degree of fibrosis, necrosis and fat but it more commonly presents as a hypointense lesion. Its appearance on T2 is variable as well but it is commonly a hyperintense lesion. Intracellular fat accumulation, which is present in 10%-17% of non-cirrhotic HCC and 36% of well-differentiated HCC is easier to detect on MRI compared to CT/US and signifies a better prognosis[82,84]. The gadolinium enhancement shows a similar pattern on MRI as described in contrast enhanced CT[82]. About 50% of non-cirrhotic HCC have a central scar that is detectable by MRI[99]. The FLC variant is hypointense on T1, hyperintense on T2 and heterogenous after gadolinium enhancement[106]. The central scar that is frequently seen in this subtype can be hypo or hyperintense on T2 sequences depending on the presence of necrosis and altered vascularity[107]. The differentiation between HCC and other benign liver lesions (focal nodular hyperplasia and hepatocellular adenoma) on MRI has been challenging in a non-cirrhotic liver often requiring unnecessary interventions for histopathological proof. In conclusion, T1 hypointensity, T2 hypo or hyperintensity, lack of central tumor enhancement and presence of satellite lesions are independent imaging factors, with a combined specificity of 98%, can allow MRI guided diagnosis of HCC in non-cirrhotic liver with a high level of confidence[107].
Figure 4.
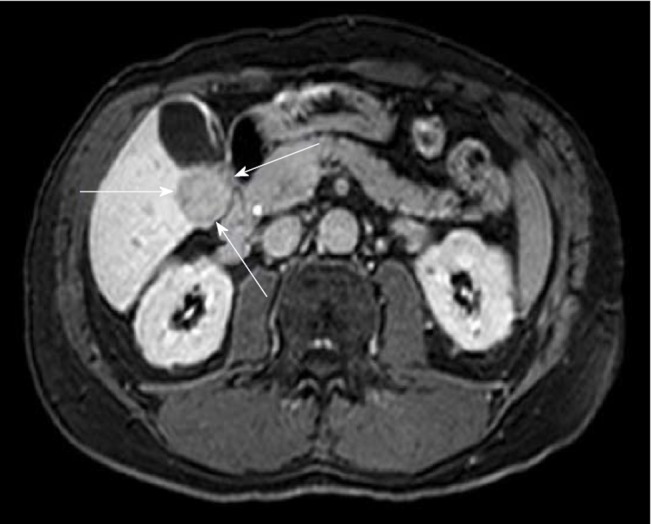
Magnetic resonance imaging (e-THRIVE_BH AX 15 min delay) of 61-yr-old male with hepatitis C virus, without cirrhosis showing a 2.8 cm × 3 cm mass lesion in segment 5 consistent with hepatocellular carcinoma.
Percutaneous liver biopsy
Histological diagnosis via liver biopsy may only be necessary if imaging studies are inconclusive for being compatible with HCC[108-110]. The AASLD does not recommend biopsy for lesions > 1 cm if two different imaging studies yield concordant findings[108]. When performed, they are done via transabdominal technique under CT or US guidance with varying degrees of sensitivity (66%–93% based on tumor size) and 100% specificity and positive predictive value[109]. Liver biopsy may be needed in patients who are not candidates for curative resection, to establish diagnosis for the purpose of systemic therapy or transplantation[109].
MANAGEMENT
Surgical Resection
Surgical resection is the treatment of choice for HCC in non-cirrhotic liver and is considered equally safe in non-cirrhotic and cirrhotic patients[111-113]. Since clinical staging systems like Okuda/Barcelona-Clinic Liver Cancer associated with underlying cirrhosis are not relevant in these patients, primary tumor features are utilized for staging and prognosis. Patients that are typically not candidates for surgical resection are those that have extrahepatic spread of their disease or anatomical constraints related to the tumor. Majority of the patients require a major hepatic resection, often with advanced surgical techniques for inferior vena cava or diaphragmatic resection; or total vascular exclusion[114]. These surgeries are feasible due to the preserved liver function and low perioperative mortality when compared to cirrhotic livers[114]. Common complications associated with such resections include intra-abdominal collections and liver insufficiency[115]. Perioperative morbidity and mortality is low when compared to the cirrhotic liver, 29.5% and 2.7% respectively[115]. Impressive postoperative survival rates of 96%, 87% and 68% after 1, 3 and 5-yr respectively have been reported in patients with tumors without vascular invasion; factors such as portal vein thrombosis, lymph node involvement and tumor recurrence are associated with poor outcomes[112,113].
Tumor recurrence
Tumor recurrence is the major cause of death in non-cirrhotic livers with HCC since no effective post-operative adjuvant chemotherapy exists[116,117]. The recurrence rate of HCC in non-cirrhotic liver is extremely high after surgical resection. Taking into account the best reported figures, the 1, 3 and 5-yr disease free survival rate is 79%, 58% and 54% respectively. Repeat hepatectomy is feasible in these patients due to normal liver function, which allows for good regenerative capacity. A mean time recurrence of 31 mo with a 61% 5-yr survival and 25% 10-yr survival after a first repeat hepatectomy has been reported for non-cirrhotic patients[116]. A good functioning liver also allows for repeat second and third hepatectomies in these patients and can be considered equally safe and effective when compared to the first[84]. However, when surgical resections fail to control recurrent disease or repeat tumors are considered unresectable, patients may need to be evaluated for liver transplantation (LT).
LT
Historically, LT was not routinely recommended in non-cirrhotic HCC due to the lack of precise guidelines or selection criteria for this patient population. Very early reports and studies have demonstrated high tumor recurrences and dismal survival outcomes. A systematic review of all reported cases of LT in non-cirrhotic patients from 1966-1988 reported a 5-yr survival rate of 11.2% for HCC and 39.4% for the FLC variant[118]. McPeake et al[119] reported a 40% recurrence rate for lesions 4-8 cm and 78% in lesions > 8 cm. However, Mergental et al[120] reported a 5-yr survival of 50%-70% after analysis of literature and European Liver Transplant Registry with median tumor sizes of 8 cm. This study recommends that Milan criteria should not be used to exclude patients with non-cirrhotic HCC and identified extrahepatic spread, gross vascular or lymph node involvement, multiple tumors and tumor recurrence < 1 yr after resection as predictors of poor outcome after LT. Based on these findings, an international consensus conference report recommended LT in patients with non-resectable HCC or in patients who experience intrahepatic recurrence after surgical resection; provided these patients have no macrovascular invasion or extrahepatic spread[121]. Decaens et al[122] highlighted the need for prospective studies addressing pre-LT imaging for tumor characteristics, response to treatment performed and kinetics of tumor progression during the waiting period and rate of dropout from tumor progression. It will be interesting to note the recurrence and overall survival (OS) of these patients in future studies after improved patient selection and advances in perioperative management and surgical care.
Systemic therapy
Sorafenib is an Food and Drug Administration (FDA) approved oral multi-tyrosine kinase inhibitor, which is the first line therapy for patients with advanced HCC[123,124]. It is indicated in patients who are not deemed surgical or transplant candidates with preserved liver function. It inhibits tumor growth and has anti-proliferative, anti-angiogenic and pro-apoptotic features[125]. The most common side effects reported include diarrhea and hand-foot skin reactions[126]. Sorafenib has demonstrated survival benefit in cirrhotic HCC, however no studies have formally evaluated outcomes in patients with a non-cirrhotic liver. Given the similarity of angiogenic characteristics between cirrhotic and non-cirrhotic HCC, Sorafenib could have a role in the management of advanced HCC in non-cirrhotic patients[127]. Recently, the FDA approved two new systemic drugs for advanced HCC. Regorafenib, a multikinase inhibitor, and Nivolumab, a PD-1 (programmed cell death protein 1) inhibitor have shown survival benefit in patients who had disease progression despite treatment with Sorafenib[128,129].
Systemic chemotherapy with other agents has been ineffective and has resulted in sub-optimal outcomes in cirrhotic advanced HCC. This has been largely due to the underlying cirrhosis with altered drug metabolism, which can lead to serious toxicity requiring either decrease in the dose or discontinuation[130,131]. However, non-cirrhotic HCC patients with a healthy liver may be able to tolerate these agents. Edeline et al[132] in their study had a 52% disease control rate with ECC (epirubicin, cisplatin and 5-flurouracil) or ECF (epirubicin, cisplastin and capecitabine). Romano et al[130] demonstrated partial response to Docetaxel with long-term survival and without severe toxicity in 3 patients. Gras et al[133] reported a complete response with GEMOX chemotherapy without a 5-yr relapse after discontinuation in a patient with advanced FLC-HCC in a non-cirrhotic liver. These reports highlight the need for further trials to explore the use of systemic chemotherapy to improve prognosis of patients with advanced non-cirrhotic HCC. Systemic chemotherapy may result in downsizing of the tumor allowing such patients to be candidates for curative surgical resection or liver transplant.
There have been few studies that have evaluated the combination of Sorafenib with other systemic chemotherapeutic agents in non-cirrhotic advanced HCC. In patients who experience recurrences after LT, combination of Sorafenib and mTOR inhibitor in conjunction with locoregional treatments improved survival, with 1 and 5-year survival rates of 82% and 33% respectively[134]. This could open potential avenues for research for combination chemotherapy and loco-ablative techniques like radiofrequency ablation (RFA), transarterial chemoembolization (TACE) and radio-embolization commonly employed in cirrhotic HCC; in the management of non-cirrhotic HCC.
Loco-ablative therapies and selective internal radiation therapy
Local ablation therapies like RFA and TACE are considered first line treatment options for unresectable HCC in cirrhotic patients. These techniques along with selective internal radiation therapy (SIRT) have been employed for down staging of tumors and control progression[135,136]. Unfortunately, their role in non-cirrhotic HCC has not been established in studies likely due to the rarity of the disease, benefit of other treatment modalities, poor prognosis and high tumor recurrence. However, for metastatic disease after prior hepatectomy, RFA has been associated with improved progression free survival and OS when compared to transcatheter therapy[135].
A recent case report published by Mafeld et al[136] showed a 94% reduction in tumor size 7 mo after SIRT with Yttrium-90 for unresectable FLC-HCC after which the patient underwent a curative surgical resection. Studies establishing SIRT as a standard of care in regular non-cirrhotic HCC may be difficult given its high 90-d morbidity; complications and lack of long term follow up data in the current case[136]. However, SIRT should be considered for the FLC-variant given limited treatment options for unresectable disease and its grave prognosis.
PROGNOSIS AND SURVEILLANCE
Survival of patients with HCC in non-cirrhotic liver mainly depends on tumor related factors such as tumor size, existence of satellite lesions, lack of tumor capsule, vascular invasion, grading, incomplete resection, HBV infection and the amount of intraoperative blood transfusions[117,137-139]. Poor prognostic factors that affected the OS rate in patients undergoing surgical resection include the need for blood transfusion and advanced age > 65. Factors that affected the recurrence free survival rate included the presence of multiple tumors[117]. FLC-HCC has a 70% 5-yr survival following surgical resection whereas for unresectable disease, the 5-yr survival rate is 0-5% with a median survival of 12 mo[140]. The number of tumors and vascular invasion are considered poor prognostic factors in this variant; the 3-yr recurrence free survival rate is 9% in patients with vascular invasion and 35% without[140].
AFP levels could be better suited as prognostic indicators. Burnett et al[141] used AFP staging based on four levels: < 10 ng/mL, 10 to 150 ng/mL, 150 to 500 ng/mL and > 500 ng/mL; and found these to be appropriate predictors of prognosis in non-cirrhotic HCC. Witjes et al[89] showed that elevated pre-operative AFP levels were associated with worse outcomes and high recurrence rates. Using an AFP cut off of 9 ng/mL, they demonstrated 1 and 3-yr survival rates of 53% and 21% respectively in patients with high AFP and 86% and 75% in patients with low AFP.
The need for effective surveillance needs to be addressed given the high tumor recurrence rate. The most common and significant issue raised is the delayed presentation of HCC in non-cirrhotic patients. There is a further need to direct research towards alternative cost-effective surveillance strategies and risk factor profiling to identify this high-risk population, decrease the tumor burden upon presentation and improve survival outcomes. Fu et al. reported a high association between high relative telomere length (RTL) and risk of HCC in non-cirrhotic chronic hepatitis B patients. Future studies could expand the role of RTL in serum DNA as a non-invasive biomarker for surveillance in non-cirrhotic HCC[142]. Wang et al[143] developed a prognostic scoring system of HCC after hepatectomy based on 11 independent risk factors and classified patients into low, intermediate and high-risk groups with an 80, 27 and 6-mo recurrence free survival respectively in each group. Such categorization is perhaps what is required in non-cirrhotic patients. Alkaline phosphatase (ALP) has also been reported as an independent risk factor that affects recurrence-free and survival rates[139]. High ALP levels could have a potential role in predicting HCC recurrence in non-cirrhotic patients and could be part of guidelines that establish risk for tumor recurrence. Further studies are necessary however, to validate the use of such scoring systems. This might allow for a more cost-effective and economic surveillance in high and intermediate risk groups.
FUTURE CONSIDERATIONS
microRNA
microRNAs (miRNAs) are endogenous non-coding 21-23 nucleotide RNAs that are involved in post-transcriptional regulation and thus play an important role in almost all main cellular pathways including regulation of major tumor-related genes in carcinogenesis[144,145]. miRNAs are also involved in iron metabolism through regulation of genes that control iron homeostasis in hepatocytes[146]. Dysregulation of miRNAs can lead to iron overload which can lead to the generation of reactive oxygen species and cause oxidative stress which damages DNA, lipids and proteins[147]. miRNA could serve as important diagnostic and prognostic biomarkers in non-cirrhotic HCC. Koh et al[148] identified 16 miRNAs that displayed significant change in expression non-tumor and HCC tissues in non-cirrhotic livers. Analysis of miRNA in the serum is an exciting prospect for the diagnosis and/or prognosis of non-cirrhotic HCC.
Early and unique changes in circulating miRNA in the serum could potentially allow it to be a biomarker for the early detection of non-cirrhotic HCC. In a study performed by Zhang et al[149], a 3 mi-RNA panel comprising of miR-92-3p, miR-107, and miR-3126-5p was equally effective as AFP for diagnosis of early HCC. Furthermore, the combination of miRNA panel and AFP had higher sensitivity and specificity than AFP alone, especially in patients with early HCC or low-level AFP. However, the study did not specify presence or absence of cirrhosis in the patient cohort. miRNA could also serve as an important prognostic marker in non-cirrhotic liver. Dysregulation of certain miRNA has been associated with poor disease-free survival after liver resection of HCC[145]. Another study showed low levels of miRNA (miR-181a-5p) and poor disease control after Sorafenib therapy[150]. Again, these studies did not comment on background liver cirrhosis. In a recent study by Mei et al[151] cirrhotic and non-cirrhotic HCC patients were found to have 41 differentially expressed miRNAs. Specifically, two miRNAs (mir-149 and mir-1296) were strongly associated with non-cirrhotic HCC and influenced the TMN tumor staging. Furthermore, increased mir-149 was associated with increased post-operative survival in non-cirrhotic HCC. Huang et al[152] developed 5-panel mi-RNA that capable of assessing risk in HCC patients. The hope is that future studies could identify a similar miRNA signature for non-cirrhotic HCC patients. Hence, miRNAs are an exciting future research prospect and could perhaps be the solution for improved diagnosis, surveillance and prognosis along with therapeutic management of advanced non-cirrhotic HCC.
CONTRIBUTIONS
The current review should help make significant contributions to research progress for HCC in non-cirrhotic liver. It highlights the major risk factors implicated in the development of HCC in a non-cirrhotic liver; especially NAFLD/NASH. It also brings to attention other rare risk factors that would further assist clinicians in decreasing the incidence of cryptogenic HCC. The review has also attempted to drive research towards finding alternative means of diagnosing non-cirrhotic HCC early by exploring other tumor markers like DCP as well as improve surveillance and monitoring of these patients. Finally, the review placed special emphasis on the management of non-cirrhotic HCC by incorporating the latest literature, which would allow researcher to explore other potential avenues especially systematic chemotherapy and loco-ablative techniques.
CONCLUSION
HCC in a non-cirrhotic liver is a complex disease phenomenon with risk factors, pathogenesis, clinical features, management and prognosis that are distinct from the cirrhotic counterpart. Even though considerable progress has been made in the management of this entity, there is a dire need for implementation of surveillance strategies in the patient population at risk, to decrease the disease burden at presentation and improve the prognosis of these patients. The hope is that this review sparks further research to close the knowledge gap and uncover answers to questions that have puzzled experts for decades.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: August 24, 2018
First decision: October 4, 2018
Article in press: December 31, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gencdal G, Jarcuska P, Luo GH, Quarleri J, Sirin G, Zhu YY S- Editor: Yan JP L- Editor: A E- Editor: Tan WW
Contributor Information
Aakash Desai, Department of Internal Medicine, Case Western Reserve University/MetroHealth Medical Center, Cleveland, OH 44109, United States.
Sonia Sandhu, Department of Hematology and Oncology, Cleveland Clinic/Akron General Medical Center, Akron, OH 44307, United States.
Jin-Ping Lai, Department of Pathology, University of Florida, Gainsville, FL 32611, United States.
Dalbir Singh Sandhu, Division of Gastroenterology and Hepatology, Case Western Reserve University/MetroHealth Medical Center, Cleveland, OH 44109, the United States. drdalbir@gmail.com.
References
- 1.Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006;23:47–63. doi: 10.1055/s-2006-939841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Lee DH, Lee JM. Primary malignant tumours in the non-cirrhotic liver. Eur J Radiol. 2017;95:349–361. doi: 10.1016/j.ejrad.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Chan KW, Wang B, Qiao L. Fibrolamellar hepatocellular carcinoma. Am J Gastroenterol. 2009;104:2617–24; quiz 2625. doi: 10.1038/ajg.2009.440. [DOI] [PubMed] [Google Scholar]
- 7.Stipa F, Yoon SS, Liau KH, Fong Y, Jarnagin WR, D’Angelica M, Abou-Alfa G, Blumgart LH, DeMatteo RP. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106:1331–1338. doi: 10.1002/cncr.21703. [DOI] [PubMed] [Google Scholar]
- 8.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 10.Ganeshan D, Szklaruk J, Kundra V, Kaseb A, Rashid A, Elsayes KM. Imaging features of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol. 2014;202:544–552. doi: 10.2214/AJR.13.11117. [DOI] [PubMed] [Google Scholar]
- 11.Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma. 2015;2:151–157. doi: 10.2147/JHC.S75153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perumpail RB, Liu A, Wong RJ, Ahmed A, Harrison SA. Pathogenesis of hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease: Potential mechanistic pathways. World J Hepatol. 2015;7:2384–2388. doi: 10.4254/wjh.v7.i22.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piñero F, Pages J, Marciano S, Fernández N, Silva J, Anders M, Zerega A, Ridruejo E, Ameigeiras B, D'Amico C, Gaite L, Bermúdez C, Cobos M, Rosales C, Romero G, McCormack L, Reggiardo V, Colombato L, Gadano A, Silva M. Fatty liver disease, an emerging etiology of hepatocellular carcinoma in Argentina. World J Hepatol. 2018;10:41–50. doi: 10.4254/wjh.v10.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander J, Torbenson M, Wu TT, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28:848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]
- 15.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317–324. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 16.Schütte K, Schulz C, Poranzke J, Antweiler K, Bornschein J, Bretschneider T, Arend J, Ricke J, Malfertheiner P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. doi: 10.1186/1471-230X-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balakrishnan M, El-Serag HB. Editorial: NAFLD-related hepatocellular carcinoma - increasing or not? With or without cirrhosis? Aliment Pharmacol Ther. 2018;47:437–438. doi: 10.1111/apt.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dongiovanni P, Romeo S, Valenti L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol. 2014;20:12945–12955. doi: 10.3748/wjg.v20.i36.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman R, Hammoud GM, Almashhrawi AA, Ahmed KT, Ibdah JA. Primary hepatocellular carcinoma and metabolic syndrome: An update. World J Gastrointest Oncol. 2013;5:186–194. doi: 10.4251/wjgo.v5.i9.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung C, Yeoh SW, Patrick D, Ket S, Marion K, Gow P, Angus PW. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol. 2015;21:1189–1196. doi: 10.3748/wjg.v21.i4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15 Suppl 4:14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- 24.Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, Kao JH, Chen DS. Role of hepatitis B virus precore/core promoter mutations and serum viral load on noncirrhotic hepatocellular carcinoma: a case-control study. J Infect Dis. 2006;194:594–599. doi: 10.1086/505883. [DOI] [PubMed] [Google Scholar]
- 25.Do AL, Wong CR, Nguyen LH, Nguyen VG, Trinh H, Nguyen MH. Hepatocellular carcinoma incidence in noncirrhotic patients with chronic hepatitis B and patients with cirrhosis of all etiologies. J Clin Gastroenterol. 2014;48:644–649. doi: 10.1097/MCG.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 26.Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology. 2003;124:327–334. doi: 10.1053/gast.2003.50053. [DOI] [PubMed] [Google Scholar]
- 27.Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. 12 The Risk Factors of Hepatocellular Carcinoma (HCC) in Non-Cirrhotic Chronic Hepatitis B Patients in United States Veterans. Gastroenterology. 2016;150:S4. [Google Scholar]
- 28.Shim CW, Park JW, Kim SH, Kim JS, Kim BH, Kim SH, Hong EK. Noncirrhotic hepatocellular carcinoma: Etiology and occult hepatitis B virus infection in a hepatitis B virus-endemic area. Therap Adv Gastroenterol. 2017;10:529–536. doi: 10.1177/1756283X17710247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.el-Refaie A, Savage K, Bhattacharya S, Khakoo S, Harrison TJ, el-Batanony M, Soliman el-S, Nasr S, Mokhtar N, Amer K, Scheuer PJ, Dhillon AP. HCV-associated hepatocellular carcinoma without cirrhosis. J Hepatol. 1996;24:277–285. doi: 10.1016/s0168-8278(96)80005-5. [DOI] [PubMed] [Google Scholar]
- 30.Nash KL, Woodall T, Brown AS, Davies SE, Alexander GJ. Hepatocellular carcinoma in patients with chronic hepatitis C virus infection without cirrhosis. World J Gastroenterol. 2010;16:4061–4065. doi: 10.3748/wjg.v16.i32.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray RB, Meyer K, Ray R. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 2000;271:197–204. doi: 10.1006/viro.2000.0295. [DOI] [PubMed] [Google Scholar]
- 32.Alotaibi AS, Alghamdi W, Marotta P, Qumosani K. A266 Hepatocellular Carcinoma Prevalence In Non-Cirrhotic Hepatitis C Patients. J. Can Assoc Gastroenterol. 2018;1:383–384. [Google Scholar]
- 33.Mattos AA, Marcon Pdos S, Araújo FS, Coral GP, Tovo CV. Hepatocellular carcinoma in a non-cirrhotic patient with sustained virological response after hepatitis c treatment. Rev Inst Med Trop Sao Paulo. 2015;57:519–522. doi: 10.1590/S0036-46652015000600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertolini E, Bassi F, Fornaciari G. Deveopment of hepatocellular carcinoma in a non-cirrhotic, long-term responder to antiviral therapy, chronic hepatitis C patient: what kind of surveillance? Ann Gastroenterol. 2013;26:80–83. [PMC free article] [PubMed] [Google Scholar]
- 35.Huang CF, Yeh ML, Tsai PC, Hsieh MH, Yang HL, Hsieh MY, Yang JF, Lin ZY, Chen SC, Wang LY, Dai CY, Huang JF, Chuang WL, Yu ML. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol. 2014;61:67–74. doi: 10.1016/j.jhep.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Toyoda H, Kumada T, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Ito T. Risk factors of hepatocellular carcinoma development in non-cirrhotic patients with sustained virologic response for chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2015;30:1183–1189. doi: 10.1111/jgh.12915. [DOI] [PubMed] [Google Scholar]
- 37.Testino G, Leone S, Borro P. Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol. 2014;20:15943–15954. doi: 10.3748/wjg.v20.i43.15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 39.Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis. 2004;24:305–315. doi: 10.1055/s-2004-832942. [DOI] [PubMed] [Google Scholar]
- 40.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 41.Murugavel KG, Naranatt PP, Shankar EM, Mathews S, Raghuram K, Rajasambandam P, Jayanthi V, Surendran R, Murali A, Srinivas U, Palaniswamy KR, Srikumari D, Thyagarajan SP. Prevalence of aflatoxin B1 in liver biopsies of proven hepatocellular carcinoma in India determined by an in-house immunoperoxidase test. J Med Microbiol. 2007;56:1455–1459. doi: 10.1099/jmm.0.47151-0. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Besaratinia A, Kim SI, Hainaut P, Pfeifer GP. In vitro recapitulating of TP53 mutagenesis in hepatocellular carcinoma associated with dietary aflatoxin B1 exposure. Gastroenterology. 2009;137:1127–1137, 1137.e1-1137.e5. doi: 10.1053/j.gastro.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu YJ, Yang HI, Wu HC, Liu J, Wang LY, Lu SN, Lee MH, Jen CL, You SL, Santella RM, Chen CJ. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. 2017;141:711–720. doi: 10.1002/ijc.30782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kar P. Risk factors for hepatocellular carcinoma in India. J Clin Exp Hepatol. 2014;4:S34–S42. doi: 10.1016/j.jceh.2014.02.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bottomley SS. Secondary iron overload disorders. Semin Hematol. 1998;35:77–86. [PubMed] [Google Scholar]
- 47.Chung H, Kudo M, Kawasaki T, Kitano M, Minami Y, Suetomi Y, Onda H. Hepatocellular carcinoma associated with secondary haemochromatosis in non-cirrhotic liver: a case report. Hepatol Res. 2003;26:254–258. doi: 10.1016/s1386-6346(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 48.Ikoma N, Shinozaki H, Kozuki A, Ibuki S, Sugano K, Mukai M, Masuda Y, Kobayashi K, Ogata Y. A Case Report of Hepatocellular Carcinoma in a Non-cirrhotic Patient With Liver Iron Overload Associated With Myelodysplastic Syndrome. World J Oncol. 2013;4:248–251. doi: 10.4021/wjon611e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turlin B, Juguet F, Moirand R, Le Quilleuc D, Loréal O, Campion JP, Launois B, Ramée MP, Brissot P, Deugnier Y. Increased liver iron stores in patients with hepatocellular carcinoma developed on a noncirrhotic liver. Hepatology. 1995;22:446–450. [PubMed] [Google Scholar]
- 50.Hussain SP, Raja K, Amstad PA, Sawyer M, Trudel LJ, Wogan GN, Hofseth LJ, Shields PG, Billiar TR, Trautwein C, Hohler T, Galle PR, Phillips DH, Markin R, Marrogi AJ, Harris CC. Increased p53 mutation load in nontumorous human liver of wilson disease and hemochromatosis: oxyradical overload diseases. Proc Natl Acad Sci U S A. 2000;97:12770–12775. doi: 10.1073/pnas.220416097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ioannou GN, Kowdley KV. Iron, HFE mutations, and hepatocellular carcinoma: is hepatic iron a carcinogen? Clin Gastroenterol Hepatol. 2003;1:246–248. [PubMed] [Google Scholar]
- 52.Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma in cirrhotic and noncirrhotic livers. A clinico-histopathologic study of 804 North American patients. Am J Clin Pathol. 1996;105:65–75. doi: 10.1093/ajcp/105.1.65. [DOI] [PubMed] [Google Scholar]
- 53.Zhang YJ. Interactions of chemical carcinogens and genetic variation in hepatocellular carcinoma. World J Hepatol. 2010;2:94–102. doi: 10.4254/wjh.v2.i3.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang YS, Chern HD, Wu JC, Chao Y, Huang YH, Chang FY, Lee SD. Polymorphism of the N-acetyltransferase 2 gene, red meat intake, and the susceptibility of hepatocellular carcinoma. Am J Gastroenterol. 2003;98:1417–1422. doi: 10.1111/j.1572-0241.2003.07452.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang LY, Chen CJ, Zhang YJ, Tsai WY, Lee PH, Feitelson MA, Lee CS, Santella RM. 4-Aminobiphenyl DNA damage in liver tissue of hepatocellular carcinoma patients and controls. Am J Epidemiol. 1998;147:315–323. doi: 10.1093/oxfordjournals.aje.a009452. [DOI] [PubMed] [Google Scholar]
- 56.Hardt A, Stippel D, Odenthal M, Hölscher AH, Dienes HP, Drebber U. Development of hepatocellular carcinoma associated with anabolic androgenic steroid abuse in a young bodybuilder: A case report. Case Rep Pathol. 2012;2012:195607. doi: 10.1155/2012/195607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solbach P, Potthoff A, Raatschen HJ, Soudah B, Lehmann U, Schneider A, Gebel MJ, Manns MP, Vogel A. Testosterone-receptor positive hepatocellular carcinoma in a 29-year old bodybuilder with a history of anabolic androgenic steroid abuse: a case report. BMC Gastroenterol. 2015;15:60. doi: 10.1186/s12876-015-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas DB, Hall AB, Michel M. Non-cirrhotic hepatocellular carcinoma in a young active duty male. Mil Med. 2011;176:475–476. doi: 10.7205/milmed-d-10-00295. [DOI] [PubMed] [Google Scholar]
- 59.Fiel MI, Min A, Gerber MA, Faire B, Schwartz M, Thung SN. Hepatocellular carcinoma in long-term oral contraceptive use. Liver. 1996;16:372–376. doi: 10.1111/j.1600-0676.1996.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim DH, Kim SU, Nam DH, Choi YJ, Park SM, Lee CK, Kim DY. A case of hepatocellular carcinoma within hepatocellular adenoma in a non-cirrhotic male. Korean J Intern Med. 2009;24:147–152. doi: 10.3904/kjim.2009.24.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Britto MR, Thomas LA, Balaratnam N, Griffiths AP, Duane PD. Hepatocellular carcinoma arising in non-cirrhotic liver in genetic haemochromatosis. Scand J Gastroenterol. 2000;35:889–893. doi: 10.1080/003655200750023282. [DOI] [PubMed] [Google Scholar]
- 62.Evert M, Dombrowski F. [Hepatocellular carcinoma in the non-cirrhotic liver] Pathologe. 2008;29:47–52. doi: 10.1007/s00292-007-0953-3. [DOI] [PubMed] [Google Scholar]
- 63.Hiatt T, Trotter JF, Kam I. Hepatocellular carcinoma in a noncirrhotic patient with hereditary hemochromatosis. Am J Med Sci. 2007;334:228–230. doi: 10.1097/MAJ.0b013e3181425209. [DOI] [PubMed] [Google Scholar]
- 64.Köhler HH, Höhler T, Küsel U, Kirkpatrick CJ, Schirmacher P. Hepatocellular carcinoma in a patient with hereditary hemochromatosis and noncirrhotic liver. A case report. Pathol Res Pract. 1999;195:509–513. doi: 10.1016/S0344-0338(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 65.Topic A, Ljujic M, Radojkovic D. Alpha-1-antitrypsin in pathogenesis of hepatocellular carcinoma. Hepat Mon. 2012;12:e7042. doi: 10.5812/hepatmon.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deybach JC, Puy H. Hepatocellular carcinoma without cirrhosis: think acute hepatic porphyrias and vice versa. J Intern Med. 2011;269:521–524. doi: 10.1111/j.1365-2796.2011.02358.x. [DOI] [PubMed] [Google Scholar]
- 67.Luvai A, Mbagaya W, Narayanan D, Degg T, Toogood G, Wyatt JI, Swinson D, Hall CJ, Barth JH. Hepatocellular carcinoma in variegate porphyria: a case report and literature review. Ann Clin Biochem. 2015;52:407–412. doi: 10.1177/0004563214557568. [DOI] [PubMed] [Google Scholar]
- 68.Nakayama M, Okamoto Y, Morita T, Matsumoto M, Fukui H, Nakano H, Tsujii T. Promoting effect of citrulline in hepatocarcinogenesis: possible mechanism in hypercitrullinemia. Hepatology. 1990;11:819–823. doi: 10.1002/hep.1840110517. [DOI] [PubMed] [Google Scholar]
- 69.Fukutomi K, Sakamori R, Furuta K, Shigekawa M, Yamada R, Kodama T, Hikita H, Yakushijin T, Tatsumi T, Honma K, Morii E, Takehara T. Hepatocellular carcinoma in a case of Wilson’s disease with non-cirrhotic liver. Kanzo. 2017;58:519–527. [Google Scholar]
- 70.Thattil R, Dufour JF. Hepatocellular carcinoma in a non-cirrhotic patient with Wilson's disease. World J Gastroenterol. 2013;19:2110–2113. doi: 10.3748/wjg.v19.i13.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oterdoom LH, Verweij KE, Biermann K, Langeveld M, van Buuren HR. Hepatocellular Adenomas and Carcinoma in Asymptomatic, Non-Cirrhotic Type III Glycogen Storage Disease. J Gastrointestin Liver Dis. 2015;24:515–518. doi: 10.15403/jgld.2014.1121.244.had. [DOI] [PubMed] [Google Scholar]
- 72.Krantz ID, Piccoli DA, Spinner NB. Alagille syndrome. J Med Genet. 1997;34:152–157. doi: 10.1136/jmg.34.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Bail B, Bioulac-Sage P, Arnoux R, Perissat J, Saric J, Balabaud C. Late recurrence of a hepatocellular carcinoma in a patient with incomplete Alagille syndrome. Gastroenterology. 1990;99:1514–1516. doi: 10.1016/0016-5085(90)91185-9. [DOI] [PubMed] [Google Scholar]
- 74.Fodor A, Fit AM, Farcau O, Rusu I, Stefanescu H, Procopet B. A Rare Association between Left Lobe Secondary Biliary Cirrhosis and Budd-Chiari Syndrome Secondary to Hepatocellular Carcinoma in the Non-Cirrhotic Right Liver Lobe. J Gastrointestin Liver Dis. 2017;26:421–424. doi: 10.15403/jgld.2014.1121.264.cri. [DOI] [PubMed] [Google Scholar]
- 75.Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011;17:1400–1409. doi: 10.3748/wjg.v17.i11.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donati B, Pietrelli A, Pingitore P, Dongiovanni P, Caddeo A, Walker L, Baselli G, Pelusi S, Rosso C, Vanni E, Daly A, Mancina RM, Grieco A, Miele L, Grimaudo S, Craxi A, Petta S, De Luca L, Maier S, Soardo G, Bugianesi E, Colli F, Romagnoli R, Anstee QM, Reeves HL, Fracanzani AL, Fargion S, Romeo S, Valenti L. Telomerase reverse transcriptase germline mutations and hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancer Med. 2017;6:1930–1940. doi: 10.1002/cam4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farges O, Dokmak S. Malignant transformation of liver adenoma: an analysis of the literature. Dig Surg. 2010;27:32–38. doi: 10.1159/000268405. [DOI] [PubMed] [Google Scholar]
- 78.Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 2010;12:509–522. doi: 10.1111/j.1477-2574.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kudo M. Malignant transformation of hepatocellular adenoma: How frequently does it happen? Liver Cancer. 2015;4:1–5. doi: 10.1159/000367726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witjes CD, Ten Kate FJ, van Aalten SM, Dwarkasing RS, Willemssen FE, Verhoef C, de Man RA, Ijzermans JN. Hepatocellular adenoma as a risk factor for hepatocellular carcinoma in a non-cirrhotic liver: a plea against. Gut. 2012;61:1645–1646. doi: 10.1136/gutjnl-2012-302219. [DOI] [PubMed] [Google Scholar]
- 81.An SL, Wang LM, Rong WQ, Wu F, Sun W, Yu WB, Feng L, Liu FQ, Tian F, Wu JX. Hepatocellular adenoma with malignant transformation in male patients with non-cirrhotic livers. Chin J Cancer. 2015;34:217–224. doi: 10.1186/s40880-015-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castán A, Navarro Y, Sarría L, Larrosa R, Serradilla M, Serrablo A. Radiological diagnosis of hepatocellular carcinoma in non-cirrhotic patients. Hepatoma Research. 2017;3:1. [Google Scholar]
- 83.Brancatelli G, Federle MP, Grazioli L, Carr BI. Hepatocellular carcinoma in noncirrhotic liver: CT, clinical, and pathologic findings in 39 U.S. residents. Radiology. 2002;222:89–94. doi: 10.1148/radiol.2221010767. [DOI] [PubMed] [Google Scholar]
- 84.Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, Yeh MM, Dubinsky TJ, Kolokythas O, Lalwani N. Hepatocellular carcinoma in the noncirrhotic liver. AJR Am J Roentgenol. 2014;203:W34–W47. doi: 10.2214/AJR.13.11511. [DOI] [PubMed] [Google Scholar]
- 85.Newman NB, Jabbour SK, Hon JD, Berman JJ, Malik D, Carpizo D, Moss RA. Hepatocellular Carcinoma Without Cirrhosis Presenting With Hypercalcemia: Case Report and Literature Review. J Clin Exp Hepatol. 2015;5:163–166. doi: 10.1016/j.jceh.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–417. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 87.Chagas AL, Kikuchi L, Herman P, Alencar RS, Tani CM, Diniz MA, Pugliese V, Rocha Mde S, D’Albuquerque LA, Carrilho FJ, Alves VA. Clinical and pathological evaluation of fibrolamellar hepatocellular carcinoma: a single center study of 21 cases. Clinics (Sao Paulo) 2015;70:207–213. doi: 10.6061/clinics/2015(03)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kassahun WT. Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol. 2016;14:151. doi: 10.1186/s12957-016-0903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witjes CD, Polak WG, Verhoef C, Eskens FA, Dwarkasing RS, Verheij J, de Man RA, Ijzermans JN. Increased alpha-fetoprotein serum level is predictive for survival and recurrence of hepatocellular carcinoma in non-cirrhotic livers. Dig Surg. 2012;29:522–528. doi: 10.1159/000348669. [DOI] [PubMed] [Google Scholar]
- 90.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, Dalhgren J, Chia D, Lok AS, Wagner PD, Srivastava S, Schwartz M. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang CS, Lin CL, Lee HC, Chen KY, Chiang MF, Chen HS, Lin TJ, Liao LY. Usefulness of serum des-gamma-carboxy prothrombin in detection of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6115–6119. doi: 10.3748/wjg.v11.i39.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zakhary NI, Khodeer SM, Shafik HE, Abdel Malak CA. Impact of PIVKA-II in diagnosis of hepatocellular carcinoma. J Adv Res. 2013;4:539–546. doi: 10.1016/j.jare.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen H, Chen S, Li S, Chen Z, Zhu X, Dai M, Kong L, Lv X, Huang Z, Qin X. Combining des-gamma-carboxyprothrombin and alpha-fetoprotein for hepatocellular carcinoma diagnosing: an update meta-analysis and validation study. Oncotarget. 2017;8:90390–90401. doi: 10.18632/oncotarget.20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ette AI, Ndububa DA, Adekanle O, Ekrikpo U. Utility of serum des-gamma-carboxyprothrombin in the diagnosis of hepatocellular carcinoma among Nigerians, a case-control study. BMC Gastroenterol. 2015;15:113. doi: 10.1186/s12876-015-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Winston CB, Schwartz LH, Fong Y, Blumgart LH, Panicek DM. Hepatocellular carcinoma: MR imaging findings in cirrhotic livers and noncirrhotic livers. Radiology. 1999;210:75–79. doi: 10.1148/radiology.210.1.r99ja1975. [DOI] [PubMed] [Google Scholar]
- 96.Giorgio A, Ferraioli G, Tarantino L, de Stefano G, Scala V, Scarano F, Coppola C, Del Viscovo L. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced helical CT appearance. AJR Am J Roentgenol. 2004;183:1319–1326. doi: 10.2214/ajr.183.5.1831319. [DOI] [PubMed] [Google Scholar]
- 97.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906. doi: 10.1148/radiol.2443061520. [DOI] [PubMed] [Google Scholar]
- 98.Liu JJ, Li HX, Chen ZB, Yang WP, Zhao SF, Chen J, Bai T, Li H, Li LQ. Consistency analysis of contrast-enhanced ultrasound and contrast-enhanced CT in diagnosis of small hepatocellular carcinoma. Int J Clin Exp Med. 2015;8:21466–21471. [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson SR, Kim TK, Jang HJ, Burns PN. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2007;189:W7–W12. doi: 10.2214/AJR.06.1060. [DOI] [PubMed] [Google Scholar]
- 100.Spârchez Z, Radu P, Kacso G, Spârchez M, Zaharia T, Al Hajjar N. Prospective comparison between real time contrast enhanced and conventional ultrasound guidance in percutaneous biopsies of liver tumors. Med Ultrason. 2015;17:456–463. doi: 10.11152/mu.2013.2066.174.deu. [DOI] [PubMed] [Google Scholar]
- 101.Roccarina D, Garcovich M, Ainora ME, Riccardi L, Pompili M, Gasbarrini A, Zocco MA. Usefulness of contrast enhanced ultrasound in monitoring therapeutic response after hepatocellular carcinoma treatment. World J Hepatol. 2015;7:1866–1874. doi: 10.4254/wjh.v7.i14.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Martino M, Saba L, Bosco S, Rossi M, Miles KA, Di Miscio R, Lombardo CV, Tamponi E, Piga M, Catalano C. Hepatocellular carcinoma (HCC) in non-cirrhotic liver: clinical, radiological and pathological findings. Eur Radiol. 2014;24:1446–1454. doi: 10.1007/s00330-014-3173-2. [DOI] [PubMed] [Google Scholar]
- 103.Lafitte M, Laurent V, Soyer P, Ayav A, Balaj C, Petit I, Hossu G. MDCT features of hepatocellular carcinoma (HCC) in non-cirrhotic liver. Diagn Interv Imaging. 2016;97:355–360. doi: 10.1016/j.diii.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 104.Shriki JE, Seyal AR, Dighe MK, Yeh MM, Jalikis FG, Andeen NK, Lall C, Bhargava P. CT of Atypical and Uncommon Presentations of Hepatocellular Carcinoma. AJR Am J Roentgenol. 2015;205:W411–W423. doi: 10.2214/AJR.14.14000. [DOI] [PubMed] [Google Scholar]
- 105.Ichikawa T, Federle MP, Grazioli L, Madariaga J, Nalesnik M, Marsh W. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology. 1999;213:352–361. doi: 10.1148/radiology.213.2.r99nv31352. [DOI] [PubMed] [Google Scholar]
- 106.McLarney JK, Rucker PT, Bender GN, Goodman ZD, Kashitani N, Ros PR. Fibrolamellar carcinoma of the liver: radiologic-pathologic correlation. Radiographics. 1999;19:453–471. doi: 10.1148/radiographics.19.2.g99mr09453. [DOI] [PubMed] [Google Scholar]
- 107.Fischer MA, Raptis DA, Donati OF, Hunziker R, Schade E, Sotiropoulos GC, McCall J, Bartlett A, Bachellier P, Frilling A, Breitenstein S, Clavien PA, Alkadhi H, Patak MA. MR imaging features for improved diagnosis of hepatocellular carcinoma in the non-cirrhotic liver: Multi-center evaluation. Eur J Radiol. 2015;84:1879–1887. doi: 10.1016/j.ejrad.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 108.Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1632–1651. doi: 10.4254/wjh.v7.i12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jain D. Tissue diagnosis of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S67–S73. doi: 10.1016/j.jceh.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tannapfel A, Dienes HP, Lohse AW. The indications for liver biopsy. Dtsch Arztebl Int. 2012;109:477–483. doi: 10.3238/arztebl.2012.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cipriani F, Fantini C, Ratti F, Lauro R, Tranchart H, Halls M, Scuderi V, Barkhatov L, Edwin B, Troisi RI, Dagher I, Reggiani P, Belli G, Aldrighetti L, Abu Hilal M. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc. 2018;32:617–626. doi: 10.1007/s00464-017-5711-x. [DOI] [PubMed] [Google Scholar]
- 112.Lang H, Sotiropoulos GC, Dömland M, Frühauf NR, Paul A, Hüsing J, Malagó M, Broelsch CE. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. Br J Surg. 2005;92:198–202. doi: 10.1002/bjs.4763. [DOI] [PubMed] [Google Scholar]
- 113.Verhoef C, de Man RA, Zondervan PE, Eijkemans MJ, Tilanus HW, Ijzermans JN. Good outcomes after resection of large hepatocellular carcinoma in the non-cirrhotic liver. Dig Surg. 2004;21:380–386. doi: 10.1159/000081882. [DOI] [PubMed] [Google Scholar]
- 114.Young AL, Adair R, Prasad KR, Toogood GJ, Lodge JP. Hepatocellular carcinoma within a noncirrhotic, nonfibrotic, seronegative liver: surgical approaches and outcomes. J Am Coll Surg. 2012;214:174–183. doi: 10.1016/j.jamcollsurg.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Zhou Y, Lei X, Wu L, Wu X, Xu D, Li B. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol. 2014;23:236–242. doi: 10.1016/j.suronc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 116.Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, Abascal A, Majno P, Engerran L. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225:51–60; discussion 60-2. doi: 10.1097/00000658-199701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hadjittofi C, Athanasopoulos PG, Koti RS, Konstantinidou SK, Davidson BR. Long-term survival with repeated resections of recurrent hepatocellular carcinoma in a non-cirrhotic liver: case report and brief review of the literature. Ann Transl Med. 2016;4:112. doi: 10.21037/atm.2016.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Houben KW, McCall JL. Liver transplantation for hepatocellular carcinoma in patients without underlying liver disease: a systematic review. Liver Transpl Surg. 1999;5:91–95. doi: 10.1002/lt.500050201. [DOI] [PubMed] [Google Scholar]
- 119.McPeake JR, O'Grady JG, Zaman S, Portmann B, Wight DG, Tan KC, Calne RY, Williams R. Liver transplantation for primary hepatocellular carcinoma: tumor size and number determine outcome. J Hepatol. 1993;18:226–234. doi: 10.1016/s0168-8278(05)80250-8. [DOI] [PubMed] [Google Scholar]
- 120.Mergental H, Porte RJ. Liver transplantation for unresectable hepatocellular carcinoma in patients without liver cirrhosis. Transpl Int. 2010;23:662–667. doi: 10.1111/j.1432-2277.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 121.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Decaens T, Laurent A, Luciani A. Liver transplantation for hepatocellular carcinoma in non-cirrhotic livers regardless of the number and size of tumours? J Hepatol. 2012;57:235–236. doi: 10.1016/j.jhep.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Eatrides J, Wang E, Kothari N, Kim R. Role of Systemic Therapy and Future Directions for Hepatocellular Carcinoma. Cancer Control. 2017;24:1073274817729243. doi: 10.1177/1073274817729243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 125.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 126.Mlynarsky L, Menachem Y, Shibolet O. Treatment of hepatocellular carcinoma: Steps forward but still a long way to go. World J Hepatol. 2015;7:566–574. doi: 10.4254/wjh.v7.i3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zeng W, Gouw AS, van den Heuvel MC, Molema G, Poppema S, van der Jagt EJ, de Jong KP. Hepatocellular carcinomas in cirrhotic and noncirrhotic human livers share angiogenic characteristics. Ann Surg Oncol. 2010;17:1564–1571. doi: 10.1245/s10434-009-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nivolumab Approved for Liver Cancer. Cancer Discov. 2017;7:OF3. doi: 10.1158/2159-8290.CD-NB2017-138. [DOI] [PubMed] [Google Scholar]
- 129.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 130.Romano O, Truant S, Sergent-Baudson G, Comet B, Pruvot FR, Hebbar M. Docetaxel therapy for advanced hepatocellular carcinoma developed in healthy liver: report of three cases. J Chemother. 2008;20:518–520. doi: 10.1179/joc.2008.20.4.518. [DOI] [PubMed] [Google Scholar]
- 131.Samonakis DN, Kouroumalis EA. Systemic treatment for hepatocellular carcinoma: Still unmet expectations. World J Hepatol. 2017;9:80–90. doi: 10.4254/wjh.v9.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Edeline J, Raoul JL, Vauleon E, Guillygomac'h A, Boudjema K, Boucher E. Systemic chemotherapy for hepatocellular carcinoma in non-cirrhotic liver: a retrospective study. World J Gastroenterol. 2009;15:713–716. doi: 10.3748/wjg.15.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gras P, Truant S, Boige V, Ladrat L, Rougier P, Pruvot FR, Hebbar M. Prolonged Complete Response after GEMOX Chemotherapy in a Patient with Advanced Fibrolamellar Hepatocellular Carcinoma. Case Rep Oncol. 2012;5:169–172. doi: 10.1159/000338242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Invernizzi F, Iavarone M, Donato MF, Sangiovanni A, Monico S, Manini MA, Maggi U, Antonelli B, Dondossola D, Rossi G, Lampertico P. Early treatment with sorafenib and mTOR inhibitor in recurrent hepatocellular carcinoma after liver transplantation: Safety and survival. Digest Liver Dis. 2018;50:49. [Google Scholar]
- 135.Henry LR, Hostetter RB, Ressler B, Bowser I, Yan M, Vaghefi H, Abad J, Gulec S, Schwarz RE. Liver resection for metastatic disease after y90 radioembolization: a case series with long-term follow-up. Ann Surg Oncol. 2015;22:467–474. doi: 10.1245/s10434-014-4012-z. [DOI] [PubMed] [Google Scholar]
- 136.Mafeld S, French J, Tiniakos D, Haugk B, Manas D, Littler P. Fibrolamellar Hepatocellular Carcinoma: Treatment with Yttrium-90 and Subsequent Surgical Resection. Cardiovasc Intervent Radiol. 2018;41:816–820. doi: 10.1007/s00270-018-1903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, Weiss M, Wolfgang CL, Choti MA, Pawlik TM. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. 2014;21:147–154. doi: 10.1245/s10434-013-3211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bège T, Le Treut YP, Hardwigsen J, Ananian P, Richa H, Campan P, Garcia S. Prognostic factors after resection for hepatocellular carcinoma in nonfibrotic or moderately fibrotic liver. A 116-case European series. J Gastrointest Surg. 2007;11:619–625. doi: 10.1007/s11605-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 139.Yu MC, Chan KM, Lee CF, Lee YS, Eldeen FZ, Chou HS, Lee WC, Chen MF. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440–1449. doi: 10.1007/s11605-011-1537-3. [DOI] [PubMed] [Google Scholar]
- 140.Yamashita S, Vauthey JN, Kaseb AO, Aloia TA, Conrad C, Hassan MM, Passot G, Raghav KP, Shama MA, Chun YS. Prognosis of Fibrolamellar Carcinoma Compared to Non-cirrhotic Conventional Hepatocellular Carcinoma. J Gastrointest Surg. 2016;20:1725–1731. doi: 10.1007/s11605-016-3216-x. [DOI] [PubMed] [Google Scholar]
- 141.Burnett NP, Dunki-Jacobs EM, Callender GG, Anderson RJ, Scoggins CR, McMasters KM, Martin RC. Evaluation of alpha-fetoprotein staging system for hepatocellular carcinoma in noncirrhotic patients. Am Surg. 2013;79:716–722. [PubMed] [Google Scholar]
- 142.Fu X, Wan S, Hann HW, Myers RE, Hann RS, Au J, Chen B, Xing J, Yang H. Relative telomere length: a novel non-invasive biomarker for the risk of non-cirrhotic hepatocellular carcinoma in patients with chronic hepatitis B infection. Eur J Cancer. 2012;48:1014–1022. doi: 10.1016/j.ejca.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang YK, Bi XY, Li ZY, Zhao H, Zhao JJ, Zhou JG, Huang Z, Zhang YF, Li MX, Chen X, Wu XL, Mao R, Hu XH, Hu HJ, Liu JM, Cai JQ. [A new prognostic score system of hepatocellular carcinoma following hepatectomy] Zhonghua Zhong Liu Za Zhi. 2017;39:903–909. doi: 10.3760/cma.j.issn.0253-3766.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 144.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 145.Vasuri F, Visani M, Acquaviva G, Brand T, Fiorentino M, Pession A, Tallini G, D'Errico A, de Biase D. Role of microRNAs in the main molecular pathways of hepatocellular carcinoma. World J Gastroenterol. 2018;24:2647–2660. doi: 10.3748/wjg.v24.i25.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davis M, Clarke S. Influence of microRNA on the maintenance of human iron metabolism. Nutrients. 2013;5:2611–2628. doi: 10.3390/nu5072611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Greene CM, Varley RB, Lawless MW. MicroRNAs and liver cancer associated with iron overload: therapeutic targets unravelled. World J Gastroenterol. 2013;19:5212–5226. doi: 10.3748/wjg.v19.i32.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koh YS, Kim JH, Cai H, Li L-H, Kim H-S, Kim K, Kim N, Shin BA, Lee T, Choi SY, Cho CK. Dysregulated microRNAs in non-cirrhotic hepatocellular carcinoma. Genes Genom. 2013;35:759–765. [Google Scholar]
- 149.Zhang Y, Li T, Qiu Y, Zhang T, Guo P, Ma X, Wei Q, Han L. Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5642. doi: 10.1097/MD.0000000000005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nishida N, Arizumi T, Hagiwara S, Ida H, Sakurai T, Kudo M. MicroRNAs for the Prediction of Early Response to Sorafenib Treatment in Human Hepatocellular Carcinoma. Liver Cancer. 2017;6:113–125. doi: 10.1159/000449475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mei Y, You Y, Xia J, Gong JP, Wang YB. Identifying Differentially Expressed MicroRNAs Between Cirrhotic and Non-Cirrhotic Hepatocellular Carcinoma and Exploring Their Functions Using Bioinformatic Analysis. Cell Physiol Biochem. 2018;48:1443–1456. doi: 10.1159/000492254. [DOI] [PubMed] [Google Scholar]
- 152.Huang YH, Liang KH, Chien RN, Hu TH, Lin KH, Hsu CW, Lin CL, Pan TL, Ke PY, Yeh CT. A Circulating MicroRNA Signature Capable of Assessing the Risk of Hepatocellular Carcinoma in Cirrhotic Patients. Sci Rep. 2017;7:523. doi: 10.1038/s41598-017-00631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Techathuvanan K, Srisajjakul S, Pongpaibul A, Limsrichamrern S, Charatcharoenwitthaya P, Chainuvati S, Tanwandee T, Chotiyaputta W. Comparison between disease free survival of hepatocellular carcinoma after hepatic resection in chronic hepatitis B patients with or without cirrhosis. J Med Assoc Thai. 2015;98:334–342. [PubMed] [Google Scholar]
- 154.Chang CH, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg. 2004;139:320–325; discussion 326. doi: 10.1001/archsurg.139.3.320. [DOI] [PubMed] [Google Scholar]
- 155.Grazi GL, Cescon M, Ravaioli M, Ercolani G, Gardini A, Del Gaudio M, Vetrone G, Cavallari A. Liver resection for hepatocellular carcinoma in cirrhotics and noncirrhotics. Evaluation of clinicopathologic features and comparison of risk factors for long-term survival and tumour recurrence in a single centre. Aliment Pharmacol Ther. 2003;17 Suppl 2:119–129. doi: 10.1046/j.1365-2036.17.s2.9.x. [DOI] [PubMed] [Google Scholar]
- 156.Kew MC. Hepatocellular carcinoma with and without cirrhosis. A comparison in southern African blacks. Gastroenterology. 1989;97:136–139. doi: 10.1016/0016-5085(89)91426-1. [DOI] [PubMed] [Google Scholar]
- 157.Albeldawi M, Soliman M, Lopez R, Zein NN. Hepatitis C virus-associated primary hepatocellular carcinoma in non-cirrhotic patients. Dig Dis Sci. 2012;57:3265–3270. doi: 10.1007/s10620-012-2260-y. [DOI] [PubMed] [Google Scholar]