Abstract
Primary sclerosing cholangitis (PSC) is a rare disease of stricturing and destruction of the biliary tree with a complex genetic and environmental etiology. Most patients have co-occurring inflammatory bowel disease. Children generally present with uncomplicated disease, but undergo a variable progression to end-stage liver disease. Within ten years of diagnosis, 50% of children will develop clinical complications including 30% requiring liver transplantation. Cholangiocarcinoma is a rare but serious complication affecting 1% of children. Ursodeoxycholic acid and oral vancomycin therapy used widely in children as medical therapy, and may be effective in a subset of patients. Gamma glutamyltransferase is a potential surrogate endpoint for disease activity, with improved survival in patients who achieve a normal value. Endoscopic retrograde cholangiopancreatography is a necessary adjunct to medical therapy to evaluate mass lesions or dominant strictures for malignancy, and also to relieve biliary obstruction. Liver transplantation remains the only option for patients who progress to end-stage liver disease. We review special considerations for patients before and after transplant, and in patients with inflammatory bowel disease. There is presently no published treatment algorithm or guideline for the management of children with PSC. We review the evidence for drug efficacy, dosing, duration of therapy, and treatment targets in PSC, and provide a framework for endoscopic and medical management of this complex problem.
Keywords: Liver transplant, Pediatric, Ursodeoxycholic acid, Oral vancomycin, Oral vancomycin therapy, Endoscopic retrograde cholangiopancreatography
Core tip: This review provides an evidence-based framework for endoscopic and medical management of children with primary sclerosing cholangitis.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease characterized by cholestasis and progressive stricturing and destruction of the intrahepatic and extrahepatic biliary tree. PSC is rare in the general pediatric population, with an incidence and prevalence of 0.2 and 1.5 cases per 100000 children, respectively. PSC is common in children with inflammatory bowel disease (IBD), affecting at least 10% of children with ulcerative colitis[1].
The etiology of PSC is complex and involves both genetic and environmental factors. Multiple abnormalities along the “gut-liver axis” have been identified including defects in: immune regulation, hepatobiliary protection mechanisms, bile acid metabolism, microbiome and intestinal permeability. Patients undergo a variable progression through hepatobiliary fibrosis, cirrhosis, and end-stage liver disease (ESLD) with a greatly increased risk for cholangiocarcinoma (CCA). In pediatrics most cases of PSC initially present without complications. Fewer than 5% have ESLD or dominant biliary strictures (DS) at diagnosis. Within ten years of diagnosis, 50% of children will develop clinical complications including 30% requiring liver transplantation (LT)[2].
There is presently no medical therapy to delay the progression of liver disease or the onset of clinical complications in PSC. The disease is recognized as having one the largest unmet needs in hepatology[3]. Ursodeoxycholic acid (UDCA) and oral vancomycin therapy (OVT) are used widely in children but the slowly progressive nature of PSC has hindered adequately-powered clinical trials. Advanced endoscopy plays an important role in palliation of PSC, with endoscopic retrograde cholangiopancreatography (ERCP) often being necessary to stent and balloon dilate biliary strictures. LT remains the only option for PSC patients with ESLD[4]. Here we review common and emerging treatment strategies for PSC in children, and their role in management based on recent literature.
URSODEOXYCHOLIC ACID
One aspect of PSC pathogenesis appears to be an abnormal bile acid pool[5-7]. HydroPHOBIC bile acids may be hepatotoxic, and high concentrations present in PSC appear to be cytotoxic within the biliary tree. PSC patients may lack an effective “bicarbonate umbrella” buffer layer between cholangiocytes and the biliary lumen[8], compounding this effect. UDCA is a hydroPHILIC bile acid with cytoprotective effects that is readily absorbed orally. UDCA increases levels of hydroPHILIC bile acids in bile[9,10] and decreases histocompatibility antigen display by hepatocytes[11]. UDCA is effective for adults with primary biliary cholangitis, another immune-mediated disease targeting bile ducts[12,13]. Its role in PSC is controversial however.
Clinical trials have consistently shown that UDCA is more effective than placebo in lowering serum alkaline phosphatase, a potential surrogate marker of disease activity in PSC[14-18]. No benefit to patient survival in treated vs. untreated patients has been shown however. In one trial of high-dose UDCA (25-30 mg/kg/d), outcomes were worse in treated cases prompting early cessation to avoid patient harm[19]. Ultimately the lack of a clear survival benefit and the potential for harm at certain doses prompted the American Association for the Study of Liver Diseases to recommend against the use of UDCA in PSC[20]. Controversy remains however since even the largest trials to date have been substantially under-powered[21] which may be why they failed to show a survival benefit. Further complicating the challenge for clinicians, in a prospective study evaluating the effects of UDCA withdrawal from chronically treated PSC patients, deterioration in serum liver tests occurred and patients reported increased pruritus[22]. Experts feel there is at least some role for a six month therapeutic trial of UDCA, with continuation of the drug in patients with a substantial biochemical response[23]. There are no practice guidelines for PSC in pediatrics, and UDCA is prescribed chronically in over 80% of patients with PSC[2].
The largest retrospective analysis of PSC outcomes showed that survival was similar in UDCA-treated and untreated children[24]. Patients who normalized their gamma-glutamyltransferase (GGT) however (achieving a level of < 50 IU/L by one year), or reduced it from baseline by at least 75%, had improved survival compared to patients who did not. This survival benefit was similar whether a patient normalized GGT on UDCA treatment or spontaneously with no treatment. Untreated patients who achieved biochemical normalization had similar survival to UDCA-treated patients. Retrospective studies and post-hoc analyses of clinical trial data in adults have consistently shown that patients who fully normalize serum alkaline phosphatase (ALP) have dramatically better survival outcomes[25-29]. Even in the clinical trial suggesting that high-dose UDCA was detrimental overall[16], the subset of patients who normalized their ALP on treatment experienced favorable survival and no complications[21].
While there is a ceiling on the appropriate dose of UDCA, with 25-30 mg/kg/d demonstrating harm[30], there is not agreement on what constitutes a minimum effective dosage. Unpublished data from the Pediatric PSC Consortium suggest that 15 mg/kg/d is the most common dosage used in children. Doses as low as 9 mg/kg/d effectively reduced ALP and GGT in a small pediatric series[31]. In a randomized trial comparing three doses of UDCA, patients in a “low dose” 10 mg/kg UDCA group experienced statistically significant reduction in ALP and GGT over two years, similar to the response seen in the “standard” 20 mg/kg and “high” 30mg/kg dose groupings. With capsule strength limitations, the actual dose received in the “low dose” group was 7.4-13.2 mg/kg/d[15]. A just-completed, NIH-funded pediatric study of UDCA withdrawal showed that liver biochemistry was normal in patients entering the study at 13 mg/kg/d of UDCA or greater. A randomized-controlled trial of UDCA at doses of 13-15 mg/kg/d showed significant reduction in ALP and aspartate aminotransferase over one year[14]. It appears that any UDCA doses as low as 7 mg/kg/d may be reasonable with the most data supportive of approximately 13-23 mg/kg/d.
PSC patients are heterogeneous, and differences between patients who do or do not normalize biochemistry on UDCA are presently unknown. There are likely several factors: Earlier disease stage (before DS or extensive hepatic fibrosis have set in for instance), genotypic or phenotypic differences, or the presence of specific changes in a patient’s microbiome or bile acid pool that are amenable to UDCA therapy in some but not all patients. Regardless, there are clearly patients who are responsive to UDCA, and it seems reasonable and safe to attempt a treatment trial at low or medium doses (approximately 15-25 mg/kg/d). In our experience, the maximal effect of UDCA on serum biochemistry is achieved within 8-12 wk, often sooner. This seems a reasonable length of time to try UDCA therapy. Long-term therapy should be reserved for those who show a dramatic biochemical response and normalization of GGT, or for rare children with substantial pruritus that resolves with treatment.
Approximately one third of children with PSC will normalize their serum biochemistry spontaneously. This is especially true in patients who were asymptomatic and identified only via screening bloodwork. These patients appear to undergo such changes frequently, possibly due to presence of an earlier stage of the disease where the inflammatory process waxes and wanes. Sorting out which UDCA-responders truly require lifelong therapy is difficult. The rate of disease progression in pediatrics, regardless of treatment with UDCA or not, is low and thus there is little urgency to initiate UDCA immediately nor is there a necessity to continue the medicine indefinitely. Patients can reasonably wait for two serial GGT values > 50, separated by 2-3 mo before initiating therapy, to reduce the incidence of treatment for highly fluctuating enzymes that spontaneously normalize. A recent clinical trial evaluated UDCA withdrawal from children with PSC who had been on chronic therapy with normal biochemistry. Upon complete withdrawal of the medication for 12 wk, 15/22 patients (68%) did not have a flare (GGT > 100) including 7/22 (32%) who maintained GGT < 29[32]. To prevent unnecessary chronic medication use, it is reasonable to attempt therapeutic withdrawal with regular monitoring of serum biochemistry to ensure each child truly needs chronic UDCA.
ORAL VANCOMYCIN THERAPY
The gut microbiome has been implicated in PSC pathogenesis[33-37]. The interaction between host immunity and dysbiosis remains poorly understood however. PSC patients are known to have reduced bacterial diversity and microbiome profiles that are distinct from healthy controls and from patients with isolated IBD. Enterococcus, Fusobacterium and Lactobacillus species are over-represented in the stool of PSC patients. An operational taxonomic unit of the Enterococcus genus was associated with elevated serum ALP levels, a disease severity marker in adult patients[38]. Even the oral microbiome is abnormal in PSC, with dysbiosis shown in the saliva[39]. Because of this, several antimicrobial agents have been used and studied in the treatment of PSC including rifaximin[40], tetracycline[41], minocycline[42] and metronidazole[43,44], with mixed results. OVT has gained the most traction in pediatric PSC on the basis of positive effects noted in a small, uncontrolled case series of 14 patients[45]. We approach OVT for PSC with hope, based on many promising (but unpublished) personal anecdotes from patient and clinicians, and also caution, given the paucity of published data and lack of any large, controlled clinical trials.
Vancomycin works against gram positive bacteria by inhibiting cross-linking of cell wall substrates. When given orally, the drug has minimal systemic absorption[46]. While the drug is potent against clostridium difficile and other gram positive organisms within the gastrointestinal tract, vancomycin may also function as an immunomodulator. OVT use in children with PSC was shown to increase transforming growth factor beta levels and peripheral T-regulatory cell counts[47]. OVT is presently used in at least 7% of patients with PSC. Practice patterns at different centers vary widely. Most commonly OVT is reserved for select patients with persistently elevated biochemical markers who failed trials of UDCA. At some centers however, OVT is used as primary therapy in virtually all new PSC patients, regardless of biochemical markers[48]. There is immense interest in this therapy amongst the patients, parents, and medical providers. Damman et al[4] provided an excellent review of the promising but small body of published evidence that OVT may be an effective therapy for PSC. Two randomized pilot trials in adults showed efficacy in reducing serum markers of cholestasis over 12 wk in patients receiving 125 mg or 250 mg four times daily[44,49]. Metronidazole was also effective for most endpoints however, and a placebo response was seen for virtually all markers of cholestasis.
Pediatric data is limited to two small case series, published from the same group. Each contains 14 pediatric PSC patients, six of whom were described in both series[45,47]. Vancomycin was administered at 50 mg/kg/d (maximum 1500 mg daily), divided into three doses. In the original publication, after 1-2 mo of therapy, all patients had lower GGT: 9/14 (64%) normalized GGT to 50 or below but 5/14 (36%) did not, including all four patients noted to be cirrhotic before treatment. Bilirubin, an important marker of long-term prognosis even when mildly elevated[2], was not improved in any patient[45]. GGT increased when OVT was stopped, and decreased again once OVT was resumed. In the second series, highly subjective and nonspecific improvements were noted in histology and/or cholangiography in all patients after at least 3 mo of therapy. In patients with PSC-IBD, transforming growth factor beta levels increased, and subsets of T regulatory white blood cells increased on OVT[47]. Most patients were treated continuously and indefinitely. No patients were described to develop ESLD or DS. Mean follow-up time was short at less than a year and a half however. The vast majority of children will not experience liver complications within that timeframe, regardless of therapy. In a large cohort of children with PSC, only 6% of patients progressed to ESLD or DS within one and a half years, while 94% did not[2], making it difficult to infer any relative causality of OVT preventing adverse liver outcomes in these combined series of 22 unique patients.
OVT is a promising potential therapy for PSC, but more data is needed. Important questions remain as to the optimal dose, its efficacy in different stages of hepatic and biliary fibrosis, its efficacy in patients with or without IBD, and whether it should be used as primary or as salvage therapy. Although vancomycin-resistant enterococcus (VRE) has not yet been described in PSC patients with history of chronic OVT exposure, it is unclear how much surveillance testing has been done. It seems inevitable that VRE may become a problem with more widespread, long-term use. Many of these questions will be addressed in a multicenter, randomized-controlled trial that is presently being planned in children. Until more data is available, we recommend somewhat judicious use of the drug, limiting OVT to non-cirrhotic PSC patients who failed an 8-12 wk trial of UDCA, with persistently elevated GGT. Based on the case series and anecdotal data, patients will respond within 8-12 wk, often sooner. Those patients who experience a brisk reduction and ultimate normalization of GGT within this time period can be considered for longer-term therapy with at least semi-annual evaluation for vancomycin-resistant enterococcus. The length of safe treatment with OVT is presently undefined, and providers should approach this on a case-by-case basis with patients and families. OVT is not a panacea. In cases where serum biochemistry does not respond to OVT within 12 wk, we recommend cessation of the drug. Patients in this category may well be cirrhotic. Careful monitoring for the sequelae of ESLD is necessary, and pre-transplant workup should begin in patients with portal hypertension.
MANAGEMENT OF IBD AND THE ROLE OF IMMUNOSUPPRESSION FOR PSC
Most children with PSC have IBD[2,50]. The two disorders share pathophysiologic mechanisms along the “gut-liver axis”[51]. The hypothesized pathogenic mechanism for PSC include an inappropriate and dysregulated immune response in genetically susceptible individuals[52-54], defects in the normal mechanisms that protect the liver from the toxicity of bile acids[5-7], a pathogenic distortion in the fecal microbiome leading to the accumulation of toxic bile acid species and a subsequent inappropriate inflammatory response[33-37], migration of gut-activated mucosal lymphocytes to the liver[55], disruption of the intestinal epithelial barrier due to inflammation[56-58], and an inappropriate inflammatory response to bacterial products and toxic bile acids delivered to the liver through the inflamed gut via the portal vein[59-61].
It is unknown whether treating a patient’s IBD and inducing a sustained endoscopic remission also treats the patient’s PSC. There are no prospective assessments of whether patients with PSC-IBD in sustained endoscopic or histologic IBD remission have improvement in liver biochemistry, imaging or histopathology. A PSC-IBD phenotype, independent of the IBD disease activity, tends to impart a favorable long-term prognosis of ductular disease. Children with PSC-IBD are nearly half as likely to experience a liver complication in follow-up compared to those with PSC alone[2]. Likewise, when PSC occurs concomitantly with IBD, the intestinal disease course is generally milder than in patients with IBD without PSC[62]. Presently it is not understood why when both diseases co-occur they tend to behave more mildly than when either is present individually. Over time PSC and IBD activity tend to wax and wane independently from one another[51]. Liver biochemistry abnormalities are not correlated with IBD activity in general, whether from PSC or another source[63]. Fecal calprotectin and GGT measurements over time were not correlated in children[64], and the severity of ductular disease was not strongly associated with the severity of colitis in adults[65]. Patients who underwent colectomy prior to a PSC diagnosis had a better liver prognosis however[66], implying at least some aspect of colonic disease that drives biliary disease. Longer duration of IBD, even after colectomy, was associated with a greater risk of CCA[67]. Even after LT for PSC on aggressive immunosuppression, exacerbation of existing IBD is common[68,69]. One quarter of patients without known IBD before transplant for PSC will develop it de-novo after transplant[70].
Immunosuppressive and biologic medications, independent of their effect on concomitant IBD or Autoimmune hepatitis (AIH), have not shown a benefit for PSC. Small pilot trials have been performed in adult PSC patients with a variety of agents including: Prednisone[71], budesonide[72,73], methotrexate[74,75], mycophenolate mofetil[76,77], tacrolimus[78], infliximab[79], and etanercept[80], with none demonstrating particular efficacy. When biologic agents are used to treat IBD in PSC-IBD patients, there is no benefit to the liver. Aspartate aminotransferase, alanine aminotransferase, bilirubin, elastography and cholangiography were not improved on adalimumab, infliximab or vedolizumab in PSC-IBD patients[55,81]. Of note, IBD patients without known PSC who were treated with vedolizumab were more likely to develop PSC than those treated with anti-tumor necrosis factor agents, an effect that was particularly pronounced in those with a Crohn disease phenotype[82]. Pediatric studies of immunosuppression for PSC are limited, but also show a lack of efficacy. Azathioprine and prednisone in a prospective study of children with PSC-AIH overlap failed to halt progression of ductular disease[83]. Separate data suggest that PSC-IBD patients may be less-tolerant of azathioprine, with a higher rate of adverse hepatobiliary or pancreatic reactions[84]. Retrospective data show infliximab is not helpful for PSC in children with PSC-IBD[85]. PSC and PSC-AIH patients have similar long-term survival despite most of the latter group receiving systemic immunosuppression[2].
Ultimately the IBD in PSC-IBD should be treated to a goal of deep remission per standards in that field, to prevent bowel surgery and colorectal malignancy. Treatment of IBD does not consistently improve PSC however. Clinicians should be aware that PSC-IBD patients are at risk of subclinical endoscopic and histologic disease activity, even when symptom free and even after colectomy[86,87]. PSC-IBD patients have an approximately four times greater risk of colorectal cancers compared to patients with IBD alone[88]. While rarely seen in children with IBD in general, the increased risk likely applies to pediatric patients as well. In the absence of formal screening guidelines, annual or biannual surveillance colonoscopy seems warranted, especially in teenage patients or those with several years of active colonic disease. Fluorescein-enhanced, probe-based confocal laser endomicroscopy (pCLE) and chromoendoscopy to obtain targeted biopsies from areas suspicious for dysplasia were far superior to sequential random biopsies using routine screening endoscopy. In one study of screening endoscopy in adults with PSC-IBD, 90% of dysplastic biopsies were discovered on pCLE vs only 10% randomly[89]. Pediatric providers are far less experienced than their adult counterparts in colonoscopic screening for dysplasia and polyps, and most have no knowledge of pCLE and chromoendoscopy. We recommend collaboration with adult endoscopists for cancer screening when possible, particularly at critical points in care such as prior to LT.
NEW MEDICAL THERAPIES AND FUTURE DIRECTIONS
Not all patients respond to UDCA or OVT, but new therapies are constantly under investigation. The future seems bright for children and families dealing with PSC. After no large clinical trials in pediatric PSC for decades, three different multicenter clinical trials enrolling pediatric patients are in the late stages of planning for OVT a combination agent HTD1801, and sulfasalazine. There are several active areas of investigation in the field with promising clinical data from adults with PSC. Norursodeoxycholic acid is a synthetic homologue of UDCA which may enhance the protective bicarbonate buffer between cholangiocytes and bile acids in the biliary lumen. A dose-dependent improvement in ALP and GGT was seen in a 12-wk phase 2 adult multicenter trial with a good safety profile[7], and a phase III trial is ongoing. Obeticholic acid is an activator of the farnesoid X receptor, central to bile acid metabolism and homeostasis, currently approved to treat adults with primary biliary cholangitis[90]. A phase II trial of obeticholic acid is underway in adults with PSC. Inhibition of bile acid reuptake in the ileum is also being explored as a therapeutic mechanism[91].
Since PSC has a multifactorial etiology, the possibility of combination therapy with multiple agents has been explored. A combination of all-trans retinoic acid, an inhibitor of bile acid synthesis previously shown to have some benefit in adults with PSC[92], with the anti-inflammatory chemokine receptor antagonist cenicriviroc shown to have benefit in non-alcoholic steatohepatitis[93], demonstrated synergy in treating an animal model of cholestasis[94]. When metronidazole[43] or all-trans retinoic acid[92] were combined with UDCA, biochemical improvements were demonstrated compared to UDCA alone. Personalized medicine with combination therapy tailored to an individual patient’s microbiome, metabolome, bile acid pool and genome may be the future of PSC therapy.
ENDOSCOPIC THERAPY
Close collaboration with an advanced endoscopist, skilled in ERCP is necessary when managing children with PSC. While once used as a diagnostic tool to visualize the focal stricturing and saccular dilation of bile ducts that produce the classic “beaded” appearance of PSC, advancements in magnetic resonance cholangiopancreatography have largely replaced its role in initial PSC diagnosis. ERCP is now mainly a tool to relieve biliary obstruction related to strictures, and to obtain tissue or brushings when CCA is suspected.
The first successful ERCP in a 3.5-mo old child utilizing an adult duodenoscope was reported in 1976[95]. Since then, multiple studies have demonstrated the feasibility, safety and utility of ERCP in the pediatric population with reported success and complication rates comparable to those quoted in the adult literature[96-100]. Pediatric expertise with ERCP is limited however. Pediatric-trained advanced endoscopists are currently practicing at very few institutions in the world, thus necessitating close collaboration with adult GI colleagues for most providers. To ensure optimal care for pediatric patients in adult medical centers it is necessary to establish these relationships in advance of when a PSC patient needs a procedure. Teams should establish protocols to ensure safe sedation of children in an adult hospital, to ensure insurance coverage for patients, and to plan for transportation of patients to and from procedures.
Pediatric and adult patients undergoing ERCP for PSC are at significantly higher risk than the normal population for post-ERCP bacterial cholangitis. Cholangitis in these patients may be life threatening. Prophylactic antibiotics per the American College of Gastroenterology PSC guidelines show antibiotic therapy as a rationale precaution to prevent post-ERCP cholangitis although this is a conditional recommendation based on low quality of evidence. Typically pre-operative antibiotics will be started and followed by a 3-5 d course using either a quinolone or cephalosporin, although to date no prospective studies have determined the best antibiotic regimen[101].
The main role of ERCP is dealing with DS which is defined in adult patients as a common bile duct measuring < 1.5 mm in diameter at is most narrow site or a hepatic duct measuring less than < 1.0 mm in diameter[102]. There is no standard definition of DS in pediatrics, though these definitions are likely applicable to all but the smallest children. For most patients with DS, endoscopic intervention can relieve the cholangitis and associated pruritus. Balloon dilation and deployment of intraductal stents are two available options, with balloon dilation generally favored. Balloon dilation of DS has been shown to prolong development of ESLD in adult PSC patients[103]. A recent European multicenter randomized trial (DILSTENT) compared balloon dilation vs stenting for DS and found that short-term stenting was not superior to balloon dilation. In addition, stenting was associated with far more frequent treatment-related serious adverse events including: post ERCP pancreatitis, cholangitis and cholecystitis[104,105]. Routine stenting is not currently recommended. Many patients require multiple repeated ERCP sessions to achieve stricture palliation.
ERCP is the key tool for diagnosis of CCA. Of adult PSC patients with DS, 26% ultimately developed CCA[19]. CCA is far rarer in children than in adults with PSC. It developed in fewer than 1% of children in a large series, who were all over age 15 with large duct involvement[2]. CCA should be considered in all PSC patients with a DS. The typical presenting symptoms of CCA are due to obstruction of the biliary tree including worsening jaundice, pruritus, clay colored stool, and dark urine. Unexplained chronic abdominal pain and weight loss in a child with PSC, even in the absence of jaundice, should prompt evaluation as well. In a small case series, 2 of 4 teenage male patients with CCA had pulmonary metastasis at the time of diagnosis, raising the possibility of a more rapidly progressive phenotype in children[106]. In adult patients with PSC, screening liver ultrasound and serum CA 19-9 are recommended every 6-12 mo[20]. These recommendations can reasonably be applied to children over aged 15, with abnormalities being referred to ERCP.
The ability to diagnose CCA with ERCP is limited. Endoscopic techniques commonly used to detect malignancy with DS include brushings for cytology, fluorescence in situ hybridization (FISH) and biopsies for pathology. The specificities of these tests are very high, however even when combined, the sensitivities approach only 60% thus having a low negative predictive value and making them inadequate as a sole means to exclude CCA[107,108]. Cholangioscopy is being increasingly utilized to directly visualize the ductal lumen and target biopsies to areas of irregular mucosa, increasing yield over blind sampling[109]. Combining cytology, FISH and cholangioscopy raises the sensitivity and specificity of ERCP for CCA to more than 90%[102,108].
MANAGEMENT OF PRURITUS
Although relatively common in adult PSC patients, pruritus is rare in children. Chronic pruritus was noted in only 4/120 (3%) children in a large single-center series[50]. In patients with ESLD from PSC with severe biliary stricturing present, the associated pruritus can impair sleep and have a major impact on quality of life. Intractable pruritus can be an indication for LT. The etiology of pruritus among cholestatic liver disease, including PSC, is not well understood. The accumulation of bile acids, increased endogenous opioid production, and elevations in lysophosphatidic acid levels, are some of the better-understood mechanisms for the development of pruritus. Data on currently available medications for pruritus is largely anecdotal, and each of several options is effective for only a proportion of patients.
Among patients with PSC presenting with pruritus, initial evaluation for the presence of a DS should be pursued. Endoscopic treatment may be necessary before the use of medical therapy. Formal treatment guidelines for medical therapy of pruritus in adult PSC have recommended bile acid sequestrants, antibiotics such as rifampin, opioid antagonists including naloxone, and selective serotonin reuptake inhibitors such as sertraline, though the strength of evidence for many of these therapies is lacking[20]. Limited case reports have reported mixed results with the use of probiotics[110], ondansetron[111], dronabinol[112], phototherapy, plasmapheresis and dialysis[113]. UDCA has a role in pruritus management as previously mentioned[23], but not all patients respond. Treatment is tailored to the individual patient, often requiring multiple trials of different agents to achieve control of symptoms.
Recently, two European groups have shown significant improvement in pruritus with bezafibrate in patients with PBC who were poor responders to UDCA[114,115]. The FITCH study (http://www.clinicaltrials.gov, NCT02701166) is an ongoing phase 3, multicenter, double-blind, randomized placebo-controlled trial to evaluate the effect of bezafibrate among those with primary biliary cholangitis or primary/secondary sclerosing cholangitis. Studies of maralixibat (http://www.clinicaltrials.gov, NCT02061540) and curcumin (http://www.clinicaltrials.gov, NCT02978339) are active as well.
TREATMENT TARGETS AND ENDPOINTS
The validation of biomarkers as surrogate endpoints is needed in pediatric PSC. Presently there is no accepted surrogate endpoint that reliably predicts clinical outcomes in PSC. There is no formal agreement on which biochemical, radiographic or histologic markers represent the best way to prove remission or stratify a patient as “low-risk” or “high-risk” for progression to liver outcomes. Validation of a surrogate marker of disease activity is critical for clinical trial design as well. Since PSC progresses slowly, over years or even decades, it is preferable to power a large clinical trial to show normalization of a biomarker over six months, rather than a reduction in clinical events over 5-10 years.
In 2014, the International Primary Sclerosing Cholangitis Study Group initiated a Delphi process to identify candidate surrogate endpoints. ALP, vibration controlled transient elastography (VCTE), liver histology, ALP and liver histology in combination, and serum total bilirubin were chosen for future study and validation[116]. Childhood-onset PSC was not specifically considered in this Delphi process however, and ALP is not a useful biomarker for pediatric liver disease. Normal ALP varies widely in children and adolescents. Values of over 500 IU/L are normal in boys and girls aged 12-13 due to rapid growth and bone turnover[117,118], and values in the thousands are normal in young children with benign transient hyperphosphatasemia[119]. Measurement of liver-specific isozyme levels of ALP is not routine in clinical practice. GGT levels are instead measured routinely in pediatric clinical practice. GGT has no source from bone, which avoids the confounding effect of skeletal growth seen with ALP.
There is increasing evidence of the utility of GGT as a candidate surrogate endpoint in pediatric PSC. GGT at diagnosis of PSC in children correlates with long-term outcomes, but ALP does not[2]. GGT response paralleled other markers including ALP in a clinical trial of norursodeoxycholic acid[7]. GGT reduction at one-year predicted long-term outcome in pediatric PSC[24]. ALP and GGT elevation in PSC represent a similar phenomenon of cholestasis and relative biliary obstruction and inflammation. As described earlier, when ALP normalizes in adults with PSC, prognosis is excellent[21]. GGT normalization thus seems to be the most practical treatment target for children. The larger the reduction in GGT better the overall prognosis, with a > 75% reduction representing the best response. Patients who normalize GGT to less than 50 IU/L have the best prognosis overall[24]. More research is needed to determine the optimal GGT response in a shorter timeframe. In practice, clinical experience suggests that an optimal GGT response is seen within 8-12 wk, with patients reaching a nadir of potential GGT at that point, with little to no further improvement with ongoing therapy.
Serum bilirubin has prognostic utility in pediatric PSC[2], but the prevalence of DS and marked elevations of bilirubin in children is low, making it a less useful biomarker to monitor treatment when compared to adults. Not enough is known about VCTE in pediatric liver diseases to support its use in monitoring treatment. While VTCE measurements likely improve when a patient responds to a particular therapy, the time course of a measureable effect is presently unknown. Similarly, histopathology may be useful to follow relative fibrosis and inflammation over time, but liver biopsy is invasive, and the time course of improvement on a therapy is unknown. Several other biomarkers correlate with PSC prognosis when done at the time of diagnosis in adults. The enhanced liver fibrosis score is a noninvasive measurement of three serum byproducts of hepatic fibrogenesis. In PSC, enhanced liver fibrosis score at diagnosis is a strong predictor of prognosis[120]. This tool is not widely available clinically and there are not yet data in children. MRI elastography and MRI cholangiography are areas of active research in pediatric PSC[121], and may serve as an additional study to prove “deep remission” of the biliary tree on therapy when done at diagnosis and repeated 1-2 years later.
LIVER TRANSPLANTATION
LT is the only effective therapy for PSC that has progressed to cirrhosis with end stage liver disease. PSC is one of the leading indications for LT, accounting for 5% of all LT in the United States[122]. Outcomes after transplantation for PSC are favorable and comparable to other pediatric liver disorders with > 90% patient and graft survival at 5 years[123,124].
Complications of portal hypertension and end stage liver disease such as: Recurring variceal bleeding, abdominal ascites, bacterial peritonitis and hepatic encephalopathy are the most common clinical reason to consider LT transplantation for PSC. The Model for End Stage Liver Disease (MELD) score is a useful prognostic marker in PSC. Similar to other liver diseases, pediatric PSC patients who meet minimal listing criteria should benefit from LT. Unfortunately, many children with PSC are more ill than their MELD score reflects. Chronic pruritus can be particularly intractable and debilitating in ESLD from PSC which negatively impacts quality of life and school performance. Within the United States organ allocation system, hepatologists may need to appeal to regional review boards for additional allocation priority beyond a patient’s MELD score in such cases. Adolescent patients with PSC frequently experience much longer wait times pre-LT compared to other pediatric liver conditions. Long wait times are one reason patients with PSC are more likely to receive living related donation LT[125]. Preliminary data from the pediatric PSC consortium suggests transplant evaluation be undertaken for adolescent patients with MELD > 10 given need for transplant in the next 1-2 years[126]. A MELD > 20 has shown significantly decreased short-term survival after LT[127] in a retrospective study. This was most likely due to the patient becoming more ill pre-LT or the MELD score poorly reflecting the actual disease severity. For these reasons, early LT evaluation and referral after developing complications of cirrhosis such as esophageal varices is warranted, even if the MELD score does not appear excessively elevated.
Hepatobiliary cancer is an uncommon indication for LT in children, but these cases do occur. Approximately 1% of pediatric-onset PSC patients will develop CCA, primarily in teenage males. Neoadjuvant chemoradiation followed by LT offers favorable outcomes for select patients[128], possibly better than outcomes achieved with primary surgical resection without transplantation. There were no confirmed cases of hepatocellular carcinoma in a large cohort of pediatric PSC patients[2], but theoretically this risk exists with any chronic liver disease. Consultation with a referral center experienced in LT for hepatobiliary cancers is strongly recommended to assist with surgical planning, prior to undergoing any resection operation locally.
LT in PSC has unique perioperative considerations. Many patients have recurring bouts of bacterial cholangitis prior to transplant, with repeated or chronic exposures to broad spectrum antibiotics. Multidrug resistant organisms are of particular concern. Additionally, due to high rates of co-existing IBD and autoimmune hepatitis in children with PSC, many patients enter transplantation on immunosuppressive medications creating additional risk for opportunistic and atypical infections. With increasing use of OVT for PSC in children, care should be taken to screen patients for VRE. Infectious disease consultation is recommended to determine optimal perioperative antimicrobial prophylaxis.
Management of IBD surrounding LT for PSC is complex. No immunosuppression protocol has proven to be the most effective at controlling IBD pre-LT and accepted IBD management is most commonly recommended. Similarly, choice of immunosuppression regimen post-LT is complex. No single regimen is more effective. Cyclosporine and azathioprine were noted to have protective effects for IBD post-LT for PSC, whereas mycophenolate and tacrolimus were detrimental[129]. Aminosalicylates may provide a protective benefit from IBD recurrence. Nevertheless, despite significant immunosuppression post-LT, > 50% of patients will have active IBD disease warranting additional therapy[130]. Furthermore, no consensus exists regarding optimal timing of colectomy related to LT. While data suggests colectomy pre-LT may prevent PSC recurrence, adequate data to suggest routine colectomy in all patients does not exist[131]. Timing of colectomy should be personalized with factors such as severity of portal hypertension and severity of underlying IBD taken into consideration. The presence of dysplastic lesions in the colon is an absolute indication for colectomy prior to LT.
Transplantation can occur with deceased and living donors with similar success. Some data suggest living-related donation may offer superior survival[132]. This may be related to shorter wait-times and being less ill at the time of transplant, or because of immunologic similarities and lower rates of PSC recurrence. Roux-en-Y choledochojejunostomy and duct-to-duct biliary anastomosis showed similar one-year patient and graft survival in a meta-analysis[133]. PSC patients have an increased risk of vascular thrombosis after LT[134] and require careful postoperative observation and anticoagulant prophylaxis.
PSC frequently reoccurs (rPSC) in the transplanted liver. rPSC is diagnosed when PSC-like ductal lesions and cholestasis occur six months or more after transplant. Care must be taken to exclude ductular lesions from vascular complications (hepatic artery stenosis or thrombosis), anastomotic biliary strictures, and CMV infections. rPSC occurs in 16% of adult transplant patients at a median of 6-years[135]. In children, the five-year recurrence risk after LT for PSC is 23%. Graft survival after recurrence is poor: 53% after five years[136]. The underlying etiology is unknown, but associated risk factors include younger age at PSC diagnosis and/or transplant, the coexistence of IBD, and thymoglobulin induction[137]. One study demonstrated rituximab may prevent disease recurrence, including with ABO-incompatible LT donation[138]. Prevention of rPSC requires ongoing study as factors such as colectomy (noted above) and the optimal induction and maintenance immunosuppressive regimens.
After LT, rates of colorectal carcinoma are particularly high[139]. Colorectal cancers occur in nearly 20% of all transplant recipients during follow-up. LT patients with IBD need annual endoscopic surveillance for colorectal cancer.
CONCLUSION
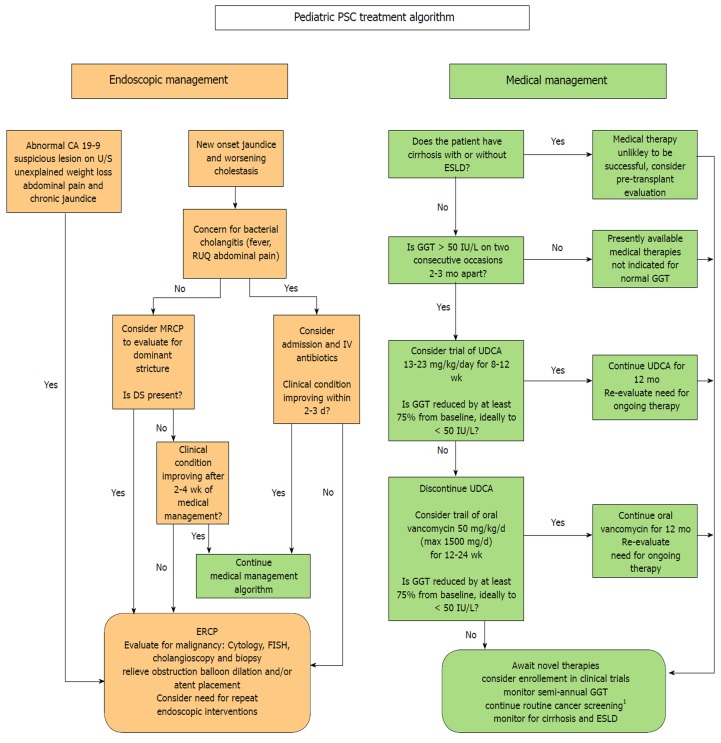
There are presently no specific guidelines for the treatment of children with PSC. Data is limited and much work needs to be done to identify a consistently effective therapy and to define the best surrogate biomarkers for treatment response. At least some patients respond to UDCA or OVT, and the vast majority of children with PSC already try one or both of these therapies. In an effort to offer providers and patients a framework for a standardized approach to treatment we suggest the following evidence-based treatment algorithm, detailed in Figure 1, to be updated as more data becomes available in the coming years.
Figure 1.
Endoscopic and medical management algorithm for pediatric primary sclerosing cholangitis. 1consider CA 19-9 and abdominal ultrasound every 6-12 mo for all patients over 15 and surveillance colonoscopy every 1-2 yr for patients with primary sclerosing cholangitis-inflammatory bowel disease.
Footnotes
Conflict-of-interest statement: The authors report no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: August 29, 2018
First decision: October 8, 2018
Article in press: January 6, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tenca A, Xia Q S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
Contributor Information
Trevor J Laborda, Department of Pediatrics, University of Utah, Salt Lake City, UT 84113, United States.
M Kyle Jensen, Department of Pediatrics, University of Utah, Salt Lake City, UT 84113, United States.
Marianne Kavan, Department of Pediatrics, University of Utah, Salt Lake City, UT 84113, United States.
Mark Deneau, Department of Pediatrics, University of Utah, Salt Lake City, UT 84113, United States. mark.deneau@hsc.utah.edu.
References
- 1.Deneau M, Jensen MK, Holmen J, Williams MS, Book LS, Guthery SL. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013;58:1392–1400. doi: 10.1002/hep.26454. [DOI] [PubMed] [Google Scholar]
- 2.Deneau MR, El-Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, Chan A, Cotter J, Doan S, El-Youssef M, Ferrari F, Furuya KN, Gottrand M, Gottrand F, Gupta N, Homan M, Kamath BM, Kim KM, Kolho KL, Konidari A, Koot B, Iorio R, Ledder O, Mack C, Martinez M, Miloh T, Mohan P, O'Cathain N, Papadopoulou A, Ricciuto A, Saubermann L, Sathya P, Shteyer E, Smolka V, Tanaka A, Varier R, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Jensen MK. The natural history of primary sclerosing cholangitis in 781 children: A multicenter, international collaboration. Hepatology. 2017;66:518–527. doi: 10.1002/hep.29204. [DOI] [PubMed] [Google Scholar]
- 3.Dyson JK, Webb G, Hirschfield GM, Lohse A, Beuers U, Lindor K, Jones DE. Unmet clinical need in autoimmune liver diseases. J Hepatol. 2015;62:208–218. doi: 10.1016/j.jhep.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Damman JL, Rodriguez EA, Ali AH, Buness CW, Cox KL, Carey EJ, Lindor KD. Review article: the evidence that vancomycin is a therapeutic option for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2018;47:886–895. doi: 10.1111/apt.14540. [DOI] [PubMed] [Google Scholar]
- 5.Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, Mol CA, Ottenhoff R, van der Lugt NM, van Roon MA. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 6.Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP, Beuers U. A biliary HCO3- umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 7.Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Färkkilä M, Schramm C, Spengler U, Chapman R, Bergquist A, Schrumpf E, Nevens F, Trivedi P, Reiter FP, Tornai I, Halilbasic E, Greinwald R, Pröls M, Manns MP, Trauner M European PSC norUDCA Study Group. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Barrena MG, Barcena-Varela M, Banales JM. New evidence supporting the biliary bicarbonate umbrella theory. Clin Res Hepatol Gastroenterol. 2017;41:126–128. doi: 10.1016/j.clinre.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Galle PR, Theilmann L, Raedsch R, Otto G, Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990;12:486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- 10.Stiehl A, Rudolph G, Raedsch R, Möller B, Hopf U, Lotterer E, Bircher J, Fölsch U, Klaus J, Endele R. Ursodeoxycholic acid-induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology. 1990;12:492–497. doi: 10.1002/hep.1840120308. [DOI] [PubMed] [Google Scholar]
- 11.Calmus Y, Gane P, Rouger P, Poupon R. Hepatic expression of class I and class II major histocompatibility complex molecules in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology. 1990;11:12–15. doi: 10.1002/hep.1840110104. [DOI] [PubMed] [Google Scholar]
- 12.Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, Wiesner RH, Anderson ML, Lange SM. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–1290. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 13.Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology. 1996;110:1515–1518. doi: 10.1053/gast.1996.v110.pm8613058. [DOI] [PubMed] [Google Scholar]
- 14.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691–695. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 15.Cullen SN, Rust C, Fleming K, Edwards C, Beuers U, Chapman RW. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48:792–800. doi: 10.1016/j.jhep.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, Bell H, Gangsøy-Kristiansen M, Matre J, Rydning A, Wikman O, Danielsson A, Sandberg-Gertzén H, Ung KA, Eriksson A, Lööf L, Prytz H, Marschall HU, Broomé U. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464–1472. doi: 10.1053/j.gastro.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558–1562. doi: 10.1111/j.1572-0241.2001.03777.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman MH, Webster GJ, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051–1058. doi: 10.1097/MEG.0b013e3283554bbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 21.Ponsioen CY, Lindor KD, Mehta R, Dimick-Santos L. Design and Endpoints for Clinical Trials in Primary Sclerosing Cholangitis. Hepatology. 2018;68:1174–1188. doi: 10.1002/hep.29882. [DOI] [PubMed] [Google Scholar]
- 22.Wunsch E, Trottier J, Milkiewicz M, Raszeja-Wyszomirska J, Hirschfield GM, Barbier O, Milkiewicz P. Prospective evaluation of ursodeoxycholic acid withdrawal in patients with primary sclerosing cholangitis. Hepatology. 2014;60:931–940. doi: 10.1002/hep.27074. [DOI] [PubMed] [Google Scholar]
- 23.Tabibian JH, Lindor KD. Ursodeoxycholic acid in primary sclerosing cholangitis: if withdrawal is bad, then administration is good (right?) Hepatology. 2014;60:785–788. doi: 10.1002/hep.27180. [DOI] [PubMed] [Google Scholar]
- 24.Deneau MR, Mack C, Abdou R, Amin M, Amir A, Auth M, Bazerbachi F, Marie Broderick A, Chan A, DiGuglielmo M, El-Matary W, El-Youssef M, Ferrari F, Furuya KN, Gottrand F, Gupta N, Homan M, Jensen MK, Kamath BM, Mo Kim K, Kolho KL, Konidari A, Koot B, Iorio R, Martinez M, Mohan P, Palle S, Papadopoulou A, Ricciuto A, Saubermann L, Sathya P, Shteyer E, Smolka V, Tanaka A, Valentino PL, Varier R, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Miloh T. Gamma Glutamyltransferase Reduction Is Associated With Favorable Outcomes in Pediatric Primary Sclerosing Cholangitis. Hepatol Commun. 2018;2:1369–1378. doi: 10.1002/hep4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindström L, Hultcrantz R, Boberg KM, Friis-Liby I, Bergquist A. Association between reduced levels of alkaline phosphatase and survival times of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2013;11:841–846. doi: 10.1016/j.cgh.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to < 1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–334. doi: 10.1016/j.jhep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Rupp C, Rössler A, Halibasic E, Sauer P, Weiss KH, Friedrich K, Wannhoff A, Stiehl A, Stremmel W, Trauner M, Gotthardt DN. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40:1292–1301. doi: 10.1111/apt.12979. [DOI] [PubMed] [Google Scholar]
- 28.de Vries EM, Wang J, Leeflang MM, Boonstra K, Weersma RK, Beuers UH, Geskus RB, Ponsioen CY. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: evaluation of prognostic value. Liver Int. 2016;36:1867–1875. doi: 10.1111/liv.13110. [DOI] [PubMed] [Google Scholar]
- 29.Stanich PP, Björnsson E, Gossard AA, Enders F, Jorgensen R, Lindor KD. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309–313. doi: 10.1016/j.dld.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imam MH, Sinakos E, Gossard AA, Kowdley KV, Luketic VA, Edwyn Harrison M, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, DeCook AC, Enders F, Lindor KD. High-dose ursodeoxycholic acid increases risk of adverse outcomes in patients with early stage primary sclerosing cholangitis. Aliment Pharmacol Ther. 2011;34:1185–1192. doi: 10.1111/j.1365-2036.2011.04863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilger MA, Gann ME, Opekun AR, Gleason WA., Jr Efficacy of ursodeoxycholic acid in the treatment of primary sclerosing cholangitis in children. J Pediatr Gastroenterol Nutr. 2000;31:136–141. doi: 10.1097/00005176-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Black D MC, Kerkar N, Miloh T, Sundaram S, Anand R, Gupta A, Alonso E, Arnon R, Bulut P, Karpen S, Lin C, Rosenthal P, Ryan M, Squires R, Valentino P, Schneider B. Initial Results of the WUPPSC Study - Prospective Multicenter Withdrawal of Ursodeoxycholic Acid in Pediatric Primary Sclerosing Cholangitis. Gastroenterology. 2018;154:S1209. [Google Scholar]
- 33.Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414–423. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- 34.Rossen NG, Fuentes S, Boonstra K, D'Haens GR, Heilig HG, Zoetendal EG, de Vos WM, Ponsioen CY. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J Crohns Colitis. 2015;9:342–348. doi: 10.1093/ecco-jcc/jju023. [DOI] [PubMed] [Google Scholar]
- 35.Grant AJ, Lalor PF, Hübscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 36.Kevans D, Tyler AD, Holm K, Jørgensen KK, Vatn MH, Karlsen TH, Kaplan GG, Eksteen B, Gevers D, Hov JR, Silverberg MS. Characterization of Intestinal Microbiota in Ulcerative Colitis Patients with and without Primary Sclerosing Cholangitis. J Crohns Colitis. 2016;10:330–337. doi: 10.1093/ecco-jcc/jjv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kummen M, Holm K, Anmarkrud JA, Nygård S, Vesterhus M, Høivik ML, Trøseid M, Marschall HU, Schrumpf E, Moum B, Røsjø H, Aukrust P, Karlsen TH, Hov JR. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611–619. doi: 10.1136/gutjnl-2015-310500. [DOI] [PubMed] [Google Scholar]
- 38.Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, Ferrante M, Van Assche G, Van der Merwe S, Vermeire S, Raes J. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65:1681–1689. doi: 10.1136/gutjnl-2015-311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwasawa K, Suda W, Tsunoda T, Oikawa-Kawamoto M, Umetsu S, Takayasu L, Inui A, Fujisawa T, Morita H, Sogo T, Hattori M. Dysbiosis of the salivary microbiota in pediatric-onset primary sclerosing cholangitis and its potential as a biomarker. Sci Rep. 2018;8:5480. doi: 10.1038/s41598-018-23870-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabibian JH, Gossard A, El-Youssef M, Eaton JE, Petz J, Jorgensen R, Enders FB, Tabibian A, Lindor KD. Prospective Clinical Trial of Rifaximin Therapy for Patients With Primary Sclerosing Cholangitis. Am J Ther. 2017;24:e56–e63. doi: 10.1097/MJT.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mistilis SP, Skyring AP, Goulston SJ. Effect of long-term tetracycline therapy, steroid therapy and colectomy in pericholangitis associated with ulcerative colitis. Australas Ann Med. 1965;14:286–294. doi: 10.1111/imj.1965.14.4.286. [DOI] [PubMed] [Google Scholar]
- 42.Silveira MG, Torok NJ, Gossard AA, Keach JC, Jorgensen RA, Petz JL, Lindor KD. Minocycline in the treatment of patients with primary sclerosing cholangitis: results of a pilot study. Am J Gastroenterol. 2009;104:83–88. doi: 10.1038/ajg.2008.14. [DOI] [PubMed] [Google Scholar]
- 43.Färkkilä M, Karvonen AL, Nurmi H, Nuutinen H, Taavitsainen M, Pikkarainen P, Kärkkäinen P. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology. 2004;40:1379–1386. doi: 10.1002/hep.20457. [DOI] [PubMed] [Google Scholar]
- 44.Tabibian JH, Weeding E, Jorgensen RA, Petz JL, Keach JC, Talwalkar JA, Lindor KD. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2013;37:604–612. doi: 10.1111/apt.12232. [DOI] [PubMed] [Google Scholar]
- 45.Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J Pediatr Gastroenterol Nutr. 2008;47:61–67. doi: 10.1097/MPG.0b013e31816fee95. [DOI] [PubMed] [Google Scholar]
- 46.Moellering RC., Jr Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42 Suppl 1:S3–S4. doi: 10.1086/491708. [DOI] [PubMed] [Google Scholar]
- 47.Abarbanel DN, Seki SM, Davies Y, Marlen N, Benavides JA, Cox K, Nadeau KC, Cox KL. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J Clin Immunol. 2013;33:397–406. doi: 10.1007/s10875-012-9801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deneau MR, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, El-Youssef M, Ferrari F, Furuya KN, Gottrand F, Gupta N, Homan M, Kamath BM, Lin H, Mo Kim K, Kolho KL, Konidari A, Koot B, Iorio R, Jensen MK, Mack C, Martinez M, Ricciuto A, Smolka V, Tanaka A, Valentino PL, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Miloh T. Variability in Diagnostic and Therapeutic Strategies for Pediatric Primary Sclerosing Cholangitis between Centers in the Pediatric PSC Consortium. Hepatology. 2018:68. [Google Scholar]
- 49.Rahimpour S, Nasiri-Toosi M, Khalili H, Ebrahimi-Daryani N, Nouri-Taromlou MK, Azizi Z. A Triple Blinded, Randomized, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Oral Vancomycin in Primary Sclerosing Cholangitis: a Pilot Study. J Gastrointestin Liver Dis. 2016;25:457–464. doi: 10.15403/jgld.2014.1121.254.rah. [DOI] [PubMed] [Google Scholar]
- 50.Valentino PL, Wiggins S, Harney S, Raza R, Lee CK, Jonas MM. The Natural History of Primary Sclerosing Cholangitis in Children: A Large Single-Center Longitudinal Cohort Study. J Pediatr Gastroenterol Nutr. 2016;63:603–609. doi: 10.1097/MPG.0000000000001368. [DOI] [PubMed] [Google Scholar]
- 51.Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 52.Ji SG, Juran BD, Mucha S, Folseraas T, Jostins L, Melum E, Kumasaka N, Atkinson EJ, Schlicht EM, Liu JZ, Shah T, Gutierrez-Achury J, Boberg KM, Bergquist A, Vermeire S, Eksteen B, Durie PR, Farkkila M, Müller T, Schramm C, Sterneck M, Weismüller TJ, Gotthardt DN, Ellinghaus D, Braun F, Teufel A, Laudes M, Lieb W, Jacobs G, Beuers U, Weersma RK, Wijmenga C, Marschall HU, Milkiewicz P, Pares A, Kontula K, Chazouillères O, Invernizzi P, Goode E, Spiess K, Moore C, Sambrook J, Ouwehand WH, Roberts DJ, Danesh J, Floreani A, Gulamhusein AF, Eaton JE, Schreiber S, Coltescu C, Bowlus CL, Luketic VA, Odin JA, Chopra KB, Kowdley KV, Chalasani N, Manns MP, Srivastava B, Mells G, Sandford RN, Alexander G, Gaffney DJ, Chapman RW, Hirschfield GM, de Andrade M UK-PSC Consortium; International IBD Genetics Consortium; International PSC Study Group, Rushbrook SM, Franke A, Karlsen TH, Lazaridis KN, Anderson CA. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49:269–273. doi: 10.1038/ng.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, Andreassen OA, Weersma RK, Weismüller TJ, Eksteen B, Invernizzi P, Hirschfield GM, Gotthardt DN, Pares A, Ellinghaus D, Shah T, Juran BD, Milkiewicz P, Rust C, Schramm C, Müller T, Srivastava B, Dalekos G, Nöthen MM, Herms S, Winkelmann J, Mitrovic M, Braun F, Ponsioen CY, Croucher PJ, Sterneck M, Teufel A, Mason AL, Saarela J, Leppa V, Dorfman R, Alvaro D, Floreani A, Onengut-Gumuscu S, Rich SS, Thompson WK, Schork AJ, Næss S, Thomsen I, Mayr G, König IR, Hveem K, Cleynen I, Gutierrez-Achury J, Ricaño-Ponce I, van Heel D, Björnsson E, Sandford RN, Durie PR, Melum E, Vatn MH, Silverberg MS, Duerr RH, Padyukov L, Brand S, Sans M, Annese V, Achkar JP, Boberg KM, Marschall HU, Chazouillères O, Bowlus CL, Wijmenga C, Schrumpf E, Vermeire S, Albrecht M UK-PSCSC Consortium, Rioux JD, Alexander G, Bergquist A, Cho J, Schreiber S, Manns MP, Färkkilä M, Dale AM, Chapman RW, Lazaridis KN; International PSC Study Group, Franke A, Anderson CA, Karlsen TH; International IBD Genetics Consortium. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670–675. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Folseraas T, Melum E, Rausch P, Juran BD, Ellinghaus E, Shiryaev A, Laerdahl JK, Ellinghaus D, Schramm C, Weismüller TJ, Gotthardt DN, Hov JR, Clausen OP, Weersma RK, Janse M, Boberg KM, Björnsson E, Marschall HU, Cleynen I, Rosenstiel P, Holm K, Teufel A, Rust C, Gieger C, Wichmann HE, Bergquist A, Ryu E, Ponsioen CY, Runz H, Sterneck M, Vermeire S, Beuers U, Wijmenga C, Schrumpf E, Manns MP, Lazaridis KN, Schreiber S, Baines JF, Franke A, Karlsen TH. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J Hepatol. 2012;57:366–375. doi: 10.1016/j.jhep.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tse CS, Loftus EV, Jr, Raffals LE, Gossard AA, Lightner AL. Effects of vedolizumab, adalimumab and infliximab on biliary inflammation in individuals with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:190–195. doi: 10.1111/apt.14829. [DOI] [PubMed] [Google Scholar]
- 56.Björnsson E, Cederborg A, Akvist A, Simren M, Stotzer PO, Bjarnason I. Intestinal permeability and bacterial growth of the small bowel in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2005;40:1090–1094. doi: 10.1080/00365520510023288. [DOI] [PubMed] [Google Scholar]
- 57.Tornai T, Palyu E, Vitalis Z, Tornai I, Tornai D, Antal-Szalmas P, Norman GL, Shums Z, Veres G, Dezsofi A, Par G, Par A, Orosz P, Szalay F, Lakatos PL, Papp M. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J Gastroenterol. 2017;23:5412–5421. doi: 10.3748/wjg.v23.i29.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513–519. doi: 10.1016/0016-5085(91)90224-9. [DOI] [PubMed] [Google Scholar]
- 59.Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 60.Zweers SJ, Shiryaev A, Komuta M, Vesterhus M, Hov JR, Perugorria MJ, de Waart DR, Chang JC, Tol S, Te Velde AA, de Jonge WJ, Banales JM, Roskams T, Beuers U, Karlsen TH, Jansen PL, Schaap FG. Elevated interleukin-8 in bile of patients with primary sclerosing cholangitis. Liver Int. 2016;36:1370–1377. doi: 10.1111/liv.13092. [DOI] [PubMed] [Google Scholar]
- 61.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, Zhang L, Shi R, Wang G, Pandak WM, Sirica AE, Hylemon PB, Zhou H. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60:908–918. doi: 10.1002/hep.27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sokol H, Cosnes J, Chazouilleres O, Beaugerie L, Tiret E, Poupon R, Seksik P. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497–3503. doi: 10.3748/wjg.14.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mendes FD, Levy C, Enders FB, Loftus EV, Jr, Angulo P, Lindor KD. Abnormal hepatic biochemistries in patients with inflammatory bowel disease. Am J Gastroenterol. 2007;102:344–350. doi: 10.1111/j.1572-0241.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 64.Shiau H, Ihekweazu FD, Amin M, Fofanova T, Miloh T, Kellermayer R. Unique Inflammatory Bowel Disease Phenotype of Pediatric Primary Sclerosing Cholangitis: A Single-Center Study. J Pediatr Gastroenterol Nutr. 2017;65:404–409. doi: 10.1097/MPG.0000000000001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stockbrügger RW, Olsson R, Jaup B, Jensen J. Forty-six patients with primary sclerosing cholangitis: radiological bile duct changes in relationship to clinical course and concomitant inflammatory bowel disease. Hepatogastroenterology. 1988;35:289–294. [PubMed] [Google Scholar]
- 66.Nordenvall C, Olén O, Nilsson PJ, von Seth E, Ekbom A, Bottai M, Myrelid P, Bergquist A. Colectomy prior to diagnosis of primary sclerosing cholangitis is associated with improved prognosis in a nationwide cohort study of 2594 PSC-IBD patients. Aliment Pharmacol Ther. 2018;47:238–245. doi: 10.1111/apt.14393. [DOI] [PubMed] [Google Scholar]
- 67.Gulamhusein AF, Eaton JE, Tabibian JH, Atkinson EJ, Juran BD, Lazaridis KN. Duration of Inflammatory Bowel Disease Is Associated With Increased Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis and IBD. Am J Gastroenterol. 2016;111:705–711. doi: 10.1038/ajg.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956–1971. doi: 10.3748/wjg.v21.i6.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mosli M, Croome K, Qumosani K, Al-Judaibi B, Beaton M, Marotta P, Chandok N. The effect of liver transplantation for primary sclerosing cholangitis on disease activity in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2013;9:434–441. [PMC free article] [PubMed] [Google Scholar]
- 70.Mouchli MA, Singh S, Boardman L, Bruining DH, Lightner AL, Rosen CB, Heimbach JK, Hasan B, Poterucha JJ, Watt KD, Kane SV, Raffals LE, Loftus EV., Jr Natural History of Established and De Novo Inflammatory Bowel Disease After Liver Transplantation for Primary Sclerosing Cholangitis. Inflamm Bowel Dis. 2018;24:1074–1081. doi: 10.1093/ibd/izx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lindor KD, Wiesner RH, Colwell LJ, Steiner B, Beaver S, LaRusso NF. The combination of prednisone and colchicine in patients with primary sclerosing cholangitis. Am J Gastroenterol. 1991;86:57–61. [PubMed] [Google Scholar]
- 72.van Hoogstraten HJ, Vleggaar FP, Boland GJ, van Steenbergen W, Griffioen P, Hop WC, van Hattum J, van Berge Henegouwen GP, Schalm SW, van Buuren HR. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomized double-blind pilot study. Belgian-Dutch PSC Study Group. Am J Gastroenterol. 2000;95:2015–2022. doi: 10.1111/j.1572-0241.2000.02267.x. [DOI] [PubMed] [Google Scholar]
- 73.Angulo P, Batts KP, Jorgensen RA, LaRusso NA, Lindor KD. Oral budesonide in the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:2333–2337. doi: 10.1111/j.1572-0241.2000.02323.x. [DOI] [PubMed] [Google Scholar]
- 74.Knox TA, Kaplan MM. A double-blind controlled trial of oral-pulse methotrexate therapy in the treatment of primary sclerosing cholangitis. Gastroenterology. 1994;106:494–499. doi: 10.1016/0016-5085(94)90610-6. [DOI] [PubMed] [Google Scholar]
- 75.Lindor KD, Jorgensen RA, Anderson ML, Gores GJ, Hofmann AF, LaRusso NF. Ursodeoxycholic acid and methotrexate for primary sclerosing cholangitis: a pilot study. Am J Gastroenterol. 1996;91:511–515. [PubMed] [Google Scholar]
- 76.Sterling RK, Salvatori JJ, Luketic VA, Sanyal AJ, Fulcher AS, Stravitz RT, Contos MJ, Mills AS, Shiffman ML. A prospective, randomized-controlled pilot study of ursodeoxycholic acid combined with mycophenolate mofetil in the treatment of primary sclerosing cholangitis. Aliment Pharmacol Ther. 2004;20:943–949. doi: 10.1111/j.1365-2036.2004.02214.x. [DOI] [PubMed] [Google Scholar]
- 77.Talwalkar JA, Angulo P, Keach JC, Petz JL, Jorgensen RA, Lindor KD. Mycophenolate mofetil for the treatment of primary sclerosing cholangitis. Am J Gastroenterol. 2005;100:308–312. doi: 10.1111/j.1572-0241.2005.40484.x. [DOI] [PubMed] [Google Scholar]
- 78.Van Thiel DH, Carroll P, Abu-Elmagd K, Rodriguez-Rilo H, Irish W, McMichael J, Starzl TE. Tacrolimus (FK 506), a treatment for primary sclerosing cholangitis: results of an open-label preliminary trial. Am J Gastroenterol. 1995;90:455–459. [PMC free article] [PubMed] [Google Scholar]
- 79.Hommes DW, Erkelens W, Ponsioen C, Stokkers P, Rauws E, van der Spek M, ten Kate F, van Deventer SJ. A double-blind, placebo-controlled, randomized study of infliximab in primary sclerosing cholangitis. J Clin Gastroenterol. 2008;42:522–526. doi: 10.1097/MCG.0b013e3181662426. [DOI] [PubMed] [Google Scholar]
- 80.Epstein MP, Kaplan MM. A pilot study of etanercept in the treatment of primary sclerosing cholangitis. Dig Dis Sci. 2004;49:1–4. doi: 10.1023/b:ddas.0000011827.87103.2e. [DOI] [PubMed] [Google Scholar]
- 81.Franceschet I, Cazzagon N, Del Ross T, D'Incà R, Buja A, Floreani A. Primary sclerosing cholangitis associated with inflammatory bowel disease: an observational study in a Southern Europe population focusing on new therapeutic options. Eur J Gastroenterol Hepatol. 2016;28:508–513. doi: 10.1097/MEG.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 82.Dubinsky MC, Cross RK, Sandborn WJ, Long M, Song X, Shi N, Ding Y, Eichner S, Pappalardo B, Ganguli A, Wang A. Extraintestinal Manifestations in Vedolizumab and Anti-TNF-Treated Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018 doi: 10.1093/ibd/izy065. [DOI] [PubMed] [Google Scholar]
- 83.Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, Mieli-Vergani G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544–553. doi: 10.1053/jhep.2001.22131. [DOI] [PubMed] [Google Scholar]
- 84.Pallavicino F, Pellicano R, Reggiani S, Simondi D, Sguazzini C, Bonagura AG, Cisarò F, Rizzetto M, Astegiano M. Inflammatory bowel diseases and primary sclerosing cholangitis: hepatic and pancreatic side effects due to azathioprine. Eur Rev Med Pharmacol Sci. 2013;17:84–87. [PubMed] [Google Scholar]
- 85.Cardile S, Candusso M, Papadatou B, Bracci F, Knafelz D, Torre G. Lack of efficacy of infliximab in the treatment of primary sclerosing cholangitis in inflammatory bowel diseases in childhood. Eur J Gastroenterol Hepatol. 2017;29:736. doi: 10.1097/MEG.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 86.Ricciuto A, Fish J, Carman N, Walters TD, Church PC, Hansen BE, Crowley E, Siddiqui I, Nguyen GC, Kamath BM, Griffiths AM. Symptoms Do Not Correlate With Findings From Colonoscopy in Children With Inflammatory Bowel Disease and Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2018;16:1098–1105.e1. doi: 10.1016/j.cgh.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 87.Broomé U, Löfberg R, Lundqvist K, Veress B. Subclinical time span of inflammatory bowel disease in patients with primary sclerosing cholangitis. Dis Colon Rectum. 1995;38:1301–1305. doi: 10.1007/BF02049156. [DOI] [PubMed] [Google Scholar]
- 88.Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:2501–2502. doi: 10.1056/NEJMc1613273. [DOI] [PubMed] [Google Scholar]
- 89.Dlugosz A, Barakat AM, Björkström NK, Öst Å, Bergquist A. Diagnostic yield of endomicroscopy for dysplasia in primary sclerosing cholangitis associated inflammatory bowel disease: a feasibility study. Endosc Int Open. 2016;4:E901–E911. doi: 10.1055/s-0042-111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levy C. Evolving role of obeticholic acid in primary biliary cholangitis. Hepatology. 2018;67:1666–1668. doi: 10.1002/hep.29726. [DOI] [PubMed] [Google Scholar]
- 91.Miethke AG, Zhang W, Simmons J, Taylor AE, Shi T, Shanmukhappa SK, Karns R, White S, Jegga AG, Lages CS, Nkinin S, Keller BT, Setchell KD. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–523. doi: 10.1002/hep.27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Assis DN, Abdelghany O, Cai SY, Gossard AA, Eaton JE, Keach JC, Deng Y, Setchell KD, Ciarleglio M, Lindor KD, Boyer JL. Combination Therapy of All-Trans Retinoic Acid With Ursodeoxycholic Acid in Patients With Primary Sclerosing Cholangitis: A Human Pilot Study. J Clin Gastroenterol. 2017;51:e11–e16. doi: 10.1097/MCG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu DC, Mennone A, Vig P, Boyer J. Cenicriviroc, a cytokine receptor antagonist, potentiated the effects of all-trans retinoic acid by reducing neutrophils and T cells in the liver of rodent cholestatic models. Hepatology. 2017;66:938–939. [Google Scholar]
- 95.Waye JD. Endoscopic retrograde cholangiopancreatography in the infant. Am J Gastroenterol. 1976;65:461–463. [PubMed] [Google Scholar]
- 96.Vegting IL, Tabbers MM, Taminiau JA, Aronson DC, Benninga MA, Rauws EA. Is endoscopic retrograde cholangiopancreatography valuable and safe in children of all ages? J Pediatr Gastroenterol Nutr. 2009;48:66–71. doi: 10.1097/MPG.0b013e31817a24cf. [DOI] [PubMed] [Google Scholar]
- 97.Rosen JD, Lane RS, Martinez JM, Perez EA, Tashiro J, Wagenaar AE, Van Haren RM, Kumar A, Sola JE. Success and safety of endoscopic retrograde cholangiopancreatography in children. J Pediatr Surg. 2017;52:1148–1151. doi: 10.1016/j.jpedsurg.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 98.Keane MG, Kumar M, Cieplik N, Thorburn D, Johnson GJ, Webster GJ, Chapman MH, Lindley KJ, Pereira SP. Paediatric pancreaticobiliary endoscopy: a 21-year experience from a tertiary hepatobiliary centre and systematic literature review. BMC Pediatr. 2018;18:42. doi: 10.1186/s12887-017-0959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonasso PC, Gurien LA, Staszak J, Gowen ME, Troendle DM, Odiase E, Lazar L, Ruan W, Barth BA, Williams RF, Dassinger MS. In-Hospital Pediatric ERCP is Associated with Shorter Hospitalization for Children with Choledocholithiasis. J Pediatr Gastroenterol Nutr. 2018 doi: 10.1097/MPG.0000000000002102. [DOI] [PubMed] [Google Scholar]
- 100.Troendle DM, Barth BA. ERCP can be safely and effectively performed by a pediatric gastroenterologist for choledocholithiasis in a pediatric facility. J Pediatr Gastroenterol Nutr. 2013;57:655–658. doi: 10.1097/MPG.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 101.Lindor KD, Kowdley KV, Harrison ME American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646–59; quiz 660. doi: 10.1038/ajg.2015.112. [DOI] [PubMed] [Google Scholar]
- 102.Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151–156. doi: 10.1016/s0168-8278(01)00251-3. [DOI] [PubMed] [Google Scholar]
- 103.Kaya M, Petersen BT, Angulo P, Baron TH, Andrews JC, Gostout CJ, Lindor KD. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059–1066. doi: 10.1111/j.1572-0241.2001.03690.x. [DOI] [PubMed] [Google Scholar]
- 104.Aabakken L, Karlsen TH, Albert J, Arvanitakis M, Chazouilleres O, Dumonceau JM, Färkkilä M, Fickert P, Hirschfield GM, Laghi A, Marzioni M, Fernandez M, Pereira SP, Pohl J, Poley JW, Ponsioen CY, Schramm C, Swahn F, Tringali A, Hassan C. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. 2017;49:588–608. doi: 10.1055/s-0043-107029. [DOI] [PubMed] [Google Scholar]
- 105.Ponsioen CY, Arnelo U, Bergquist A, Rauws EA, Paulsen V, Cantú P, Parzanese I, De Vries EM, van Munster KN, Said K, Chazouillères O, Desaint B, Kemgang A, Färkkilä M, Van der Merwe S, Van Steenbergen W, Marschall HU, Stotzer PO, Thorburn D, Pereira SP, Aabakken L. No Superiority of Stents vs Balloon Dilatation for Dominant Strictures in Patients With Primary Sclerosing Cholangitis. Gastroenterology. 2018;155:752–759.e5. doi: 10.1053/j.gastro.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 106.Liu R, Cox K, Guthery SL, Book L, Witt B, Chadwick B, Adler DG. Cholangiocarcinoma and high-grade dysplasia in young patients with primary sclerosing cholangitis. Dig Dis Sci. 2014;59:2320–2324. doi: 10.1007/s10620-014-3152-0. [DOI] [PubMed] [Google Scholar]
- 107.Njei B, McCarty TR, Varadarajulu S, Navaneethan U. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139–1151. doi: 10.1111/apt.13817. [DOI] [PubMed] [Google Scholar]
- 108.Barkin JA, Levy C, Souto EO. Endoscopic Management of Primary Sclerosing Cholangitis. Ann Hepatol. 2017;16:842–850. doi: 10.5604/01.3001.0010.5274. [DOI] [PubMed] [Google Scholar]
- 109.Imani Fooladi AA, Mahmoodzadeh Hosseini H, Nourani MR, Khani S, Alavian SM. Probiotic as a novel treatment strategy against liver disease. Hepat Mon. 2013;13:e7521. doi: 10.5812/hepatmon.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jones EA, Molenaar HA, Oosting J. Ondansetron and pruritus in chronic liver disease: a controlled study. Hepatogastroenterology. 2007;54:1196–1199. [PubMed] [Google Scholar]
- 111.Neff GW, O'Brien CB, Reddy KR, Bergasa NV, Regev A, Molina E, Amaro R, Rodriguez MJ, Chase V, Jeffers L, Schiff E. Preliminary observation with dronabinol in patients with intractable pruritus secondary to cholestatic liver disease. Am J Gastroenterol. 2002;97:2117–2119. doi: 10.1111/j.1572-0241.2002.05852.x. [DOI] [PubMed] [Google Scholar]
- 112.Bolier AR, Peri S, Oude Elferink RP, Beuers U. The challenge of cholestatic pruritus. Acta Gastroenterol Belg. 2012;75:399–404. [PubMed] [Google Scholar]
- 113.Reig A, Sesé P, Parés A. Effects of Bezafibrate on Outcome and Pruritus in Primary Biliary Cholangitis With Suboptimal Ursodeoxycholic Acid Response. Am J Gastroenterol. 2018;113:49–55. doi: 10.1038/ajg.2017.287. [DOI] [PubMed] [Google Scholar]
- 114.Corpechot C, Chazouilleres O, Rousseau A. A 2-year multicenter, double-blind, randomized, placebo-controlled study of bezafibrate for the treatment of primary biliary cholangitis in patients with inadequate response to ursodeoxycholic acid therapy. Hepatology. 2017:66. [Google Scholar]
- 115.Ponsioen CY, Chapman RW, Chazouillères O, Hirschfield GM, Karlsen TH, Lohse AW, Pinzani M, Schrumpf E, Trauner M, Gores GJ. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: Review and results from an International PSC Study Group consensus process. Hepatology. 2016;63:1357–1367. doi: 10.1002/hep.28256. [DOI] [PubMed] [Google Scholar]
- 116.Laboratories MM. Alkaline Phosphatase, Total and Isozymes, Serum: Clinical and Interpretive. Hepatology. 2017;64:256–258. [Google Scholar]
- 117.Turan S, Topcu B, Gökçe İ, Güran T, Atay Z, Omar A, Akçay T, Bereket A. Serum alkaline phosphatase levels in healthy children and evaluation of alkaline phosphatase z-scores in different types of rickets. J Clin Res Pediatr Endocrinol. 2011;3:7–11. doi: 10.4274/jcrpe.v3i1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Otero JL, González-Peralta RP, Andres JM, Jolley CD, Novak DA, Haafiz A. Elevated Alkaline Phosphatase in Children: An Algorithm to Determine When a "Wait and See" Approach is Optimal. Clin Med Insights Pediatr. 2011;5:15–18. doi: 10.4137/CMPed.S6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vesterhus M, Hov JR, Holm A, Schrumpf E, Nygård S, Godang K, Andersen IM, Naess S, Thorburn D, Saffioti F, Vatn M, Gilja OH, Lund-Johansen F, Syversveen T, Brabrand K, Parés A, Ponsioen CY, Pinzani M, Färkkilä M, Moum B, Ueland T, Røsjø H, Rosenberg W, Boberg KM, Karlsen TH. Enhanced liver fibrosis score predicts transplant-free survival in primary sclerosing cholangitis. Hepatology. 2015;62:188–197. doi: 10.1002/hep.27825. [DOI] [PubMed] [Google Scholar]
- 120.Serai SD, Trout AT, Miethke A, Diaz E, Xanthakos SA, Dillman JR. Putting it all together: established and emerging MRI techniques for detecting and measuring liver fibrosis. Pediatr Radiol. 2018;48:1256–1272. doi: 10.1007/s00247-018-4083-2. [DOI] [PubMed] [Google Scholar]
- 121.Goldberg D, French B, Thomasson A, Reddy KR, Halpern SD. Waitlist survival of patients with primary sclerosing cholangitis in the model for end-stage liver disease era. Liver Transpl. 2011;17:1355–1363. doi: 10.1002/lt.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jossen J, Annunziato R, Kim HS, Chu J, Arnon R. Liver Transplantation for Children With Primary Sclerosing Cholangitis and Autoimmune Hepatitis: UNOS Database Analysis. J Pediatr Gastroenterol Nutr. 2017;64:e83–e87. doi: 10.1097/MPG.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 123.Miloh T, Anand R, Yin W, Vos M, Kerkar N, Alonso E Studies of Pediatric Liver Transplantation Research Group. Pediatric liver transplantation for primary sclerosing cholangitis. Liver Transpl. 2011;17:925–933. doi: 10.1002/lt.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goldberg DS, French B, Thomasson A, Reddy KR, Halpern SD. Current trends in living donor liver transplantation for primary sclerosing cholangitis. Transplantation. 2011;91:1148–1152. doi: 10.1097/TP.0b013e31821694b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deneau MR, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, El-Youssef M, Ferrari F, Furuya KN, Gottrand F, Gupta N, Homan M, Kamath BM, Lin H, Mo Kim K, Kolho KL, Konidari A, Koot B, Iorio R, Jensen MK, Mack C, Martinez M, Ricciuto A, Smolka V, Tanaka A, Valentino PL, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Miloh T. MELD score and liver transplantation in children with primary sclerosing cholangitis. Pediatric Gastroenterology Nutrition. 2018:67. [Google Scholar]
- 126.Hoffmann K, Hinz U, Hillebrand N, Ganten T, Gotthardt D, Longerich T, Schirmacher P, Schemmer P. The MELD score predicts the short-term and overall survival after liver transplantation in patients with primary sclerosing cholangitis or autoimmune liver diseases. Langenbecks Arch Surg. 2014;399:1001–1009. doi: 10.1007/s00423-014-1237-z. [DOI] [PubMed] [Google Scholar]
- 127.Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98.e3; quiz e14. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jørgensen KK, Lindström L, Cvancarova M, Karlsen TH, Castedal M, Friman S, Schrumpf E, Foss A, Isoniemi H, Nordin A, Holte K, Rasmussen A, Bergquist A, Vatn MH, Boberg KM. Immunosuppression after liver transplantation for primary sclerosing cholangitis influences activity of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:517–523. doi: 10.1016/j.cgh.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 129.Verdonk RC, Dijkstra G, Haagsma EB, Shostrom VK, Van den Berg AP, Kleibeuker JH, Langnas AN, Sudan DL. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. 2006;6:1422–1429. doi: 10.1111/j.1600-6143.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 130.Buchholz BM, Lykoudis PM, Ravikumar R, Pollok JM, Fusai GK. Role of colectomy in preventing recurrent primary sclerosing cholangitis in liver transplant recipients. World J Gastroenterol. 2018;24:3171–3180. doi: 10.3748/wjg.v24.i28.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goldberg DS, French B, Abt PL, Olthoff K, Shaked A. Superior survival using living donors and donor-recipient matching using a novel living donor risk index. Hepatology. 2014;60:1717–1726. doi: 10.1002/hep.27307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wells MM, Croome KP, Boyce E, Chandok N. Roux-en-Y choledochojejunostomy versus duct-to-duct biliary anastomosis in liver transplantation for primary sclerosing cholangitis: a meta-analysis. Transplant Proc. 2013;45:2263–2271. doi: 10.1016/j.transproceed.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 133.Bezinover D, Iskandarani K, Chinchilli V, McQuillan P, Saner F, Kadry Z, Riley TR, Janicki PK. Autoimmune conditions are associated with perioperative thrombotic complications in liver transplant recipients: A UNOS database analysis. BMC Anesthesiol. 2016;16:26. doi: 10.1186/s12871-016-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Montano-Loza AJ, Bhanji RA, Wasilenko S, Mason AL. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther. 2017;45:485–500. doi: 10.1111/apt.13894. [DOI] [PubMed] [Google Scholar]
- 135.Deneau MR, El-Matary W, Valentino PL, Abdou R, Alqoaer K, Amin M, Amir AZ, Auth M, Bazerbachi F, Broderick A, Chan A, Cotter J, Doan S, El-Youssef M, Ferrari F, Furuya KN, Gottrand M, Gottrand F, Gupta N, Homan M, Kamath BM, Mo Kim K, Kolho KL, Konidari A, Koot B, Iorio R, Ledder O, Mack C, Martinez M, Miloh T, Mohan P, O'Cathain N, Papadopoulou A, Ricciuto A, Saubermann L, Sathya P, Shteyer E, Smolka V, Tanaka A, Varier R, Venkat V, Vitola B, Vos MB, Woynarowski M, Yap J, Jensen MK. Recurrence of primary sclerosing cholangitis after liver transplantation in children: data from the Pediatric PSC Consortium. Hepatology. 2017;66:S76. [Google Scholar]
- 136.Soufi N, Bazerbachi F, Deneau M. Post-Transplant Disease Recurrence in Pediatric PSC. Curr Gastroenterol Rep. 2018;20:44. doi: 10.1007/s11894-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 137.Yamada Y, Hoshino K, Fuchimoto Y, Matsubara K, Hibi T, Yagi H, Abe Y, Shinoda M, Kitago M, Obara H, Yagi T, Okajima H, Kaido T, Uemoto S, Suzuki T, Kubota K, Yoshizumi T, Maehara Y, Inomata Y, Kitagawa Y, Egawa H, Kuroda T. Rituximab Induction to Prevent the Recurrence of PSC After Liver Transplantation-The Lessons Learned From ABO-Incompatible Living Donor Liver Transplantation. Transplant Direct. 2018;4:e342. doi: 10.1097/TXD.0000000000000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh S, Edakkanambeth Varayil J, Loftus EV, Jr, Talwalkar JA. Incidence of colorectal cancer after liver transplantation for primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Transpl. 2013;19:1361–1369. doi: 10.1002/lt.23741. [DOI] [PubMed] [Google Scholar]
- 139.Altwegg R, Combes R, Laharie D, De Ledinghen V, Radenne S, Conti F, Chazouilleres O, Duvoux C, Dumortier J, Leroy V, Treton X, Durand F, Dharancy S, Nachury M, Goutorbe F, Lamblin G, Boivineau L, Peyrin-Biroulet L, Pageaux GP. Effectiveness and safety of anti-TNF therapy for inflammatory bowel disease in liver transplant recipients for primary sclerosing cholangitis: A nationwide case series. Dig Liver Dis. 2018;50:668–674. doi: 10.1016/j.dld.2018.02.014. [DOI] [PubMed] [Google Scholar]