Abstract
Background
Use of pressure ulcer risk assessment tools or scales is a component of the assessment process used to identify individuals at risk of developing a pressure ulcer. Use of a risk assessment tool is recommended by many international pressure ulcer prevention guidelines, however it is not known whether using a risk assessment tool makes a difference to patient outcomes. We conducted a review to provide a summary of the evidence pertaining to pressure ulcer risk assessment in clinical practice, and this is the third update of this review.
Objectives
To assess whether using structured and systematic pressure ulcer risk assessment tools, in any healthcare setting, reduces the incidence of pressure ulcers.
Search methods
In February 2018 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase; and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
Randomised controlled trials (RCTs) comparing the use of structured and systematic pressure ulcer risk assessment tools with no structured pressure ulcer risk assessment, or with unaided clinical judgement, or RCTs comparing the use of different structured pressure ulcer risk assessment tools.
Data collection and analysis
Two review authors independently performed study selection, data extraction, 'Risk of bias' assessment and GRADE assessment of the certainty of evidence.
Main results
We included two studies in this review (1,487 participants). We identified no new trials for this latest update.
Both studies were undertaken in acute‐care hospitals. In one study, patients were eligible if they had a Braden score of 18 or less. In the second study all admitted patients were eligible for inclusion, once they were expected to have a hospital stay of more than three days and they had been in hospital for no more than 24 hours before baseline assessment took place. In the first study, most of the participants were medical patients; no information on age or gender distribution was provided. In the second study, 50.3% (619) of the participants were male, with a mean age of 62.6 years (standard deviation (SD): 19.3), and 15.4% (190) were admitted to oncology wards.
The two included studies were three‐armed studies. In the first study the three groups were: Braden risk assessment tool and training (n = 74), clinical judgement and training (n = 76) and clinical judgement alone (n = 106); follow‐up was eight weeks. In the second study the three groups were: Waterlow risk assessment tool (n = 411), clinical judgement (n = 410) and Ramstadius risk assessment tool (n = 410); follow‐up was four days. Both studies reported the primary outcome of pressure ulcer incidence and one study also reported the secondary outcome, severity of new pressure ulcers.
We are uncertain whether use of the Braden risk assessment tool and training makes any difference to pressure ulcer incidence, compared to risk assessment using clinical judgement and training (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.53 to 1.77; 150 participants), or compared to risk assessment using clinical judgement alone (RR 1.43, 95% CI 0.77 to 2.68; 180 participants). We assessed the certainty of the evidence as very low (downgraded twice for study limitations and twice for imprecision).
Risk assessment using the Waterlow tool may make little or no difference to pressure ulcer incidence, or to pressure ulcer severity, when compared to risk assessment using clinical judgement (pressure ulcers of all stages: RR 1.10, 95% CI 0.68 to 1.81; 821 participants; stage 1 pressure ulcers: RR 1.05, 95% CI 0.58 to 1.90; 821 participants; stage 2 pressure ulcers: RR 1.25, 95% CI 0.50 to 3.13; 821 participants), or risk assessment using the Ramstadius tool (pressure ulcers of all stages: RR 1.41, 95% CI 0.83 to 2.39; 821 participants; stage 1 pressure ulcers: RR 1.16, 95% CI 0.63 to 2.15; 821 participants; stage 2 pressure ulcers: RR 2.49, 95% CI 0.79 to 7.89; 821 participants). Similarily, risk assessment using the Ramstadius tool may make little or no difference to pressure ulcer incidence, or to pressure ulcer severity, when compared to risk assessment using clinical judgement (pressure ulcers of all stages: RR 0.79, 95% CI 0.46 to 1.35; 820 participants; stage 1 pressure ulcers: RR 0.90, 95% CI 0.48 to 1.68; 820 participants; stage 2 pressure ulcers: RR 0.50, 95% CI 0.15 to 1.65; 820 participants). We assessed the certainty of the evidence as low (downgraded once for study limitations and once for imprecision).
The studies did not report the secondary outcomes of time to ulcer development, or pressure ulcer prevalence.
Authors' conclusions
We identified two studies which evaluated the effect of risk assessment on pressure ulcer incidence. Based on evidence from one study, we are uncertain whether risk assessment using the Braden tool makes any difference to pressure ulcer incidence, compared with training and risk assessment using clinical judgement, or risk assessment using clinical judgement alone. Risk assessment using the Waterlow tool, or the Ramstadius tool may make little or no difference to pressure ulcer incidence, or severity, compared with clinical judgement. The low, or very low certainty of evidence available from the included studies is not reliable enough to suggest that the use of structured and systematic pressure ulcer risk assessment tools reduces the incidence, or severity of pressure ulcers.
Plain language summary
Risk assessment tools used for preventing pressure ulcers
What is the aim of this review?
The aim of this review was to find out what effect the use of risk assessment tools has on the development of new pressure ulcers, among people at risk of pressure ulcer development. Many different pressure ulcer risk assessment tools are used in clinical practice and it is not known which one is the best. Researchers from Cochrane collected and analysed all relevant studies (randomised controlled trials) to answer this question and found two relevant studies.
Key messages
We cannot be certain whether the use of a risk assessment tool makes any difference to the number of new pressure ulcers that develop among people who are at risk. The certainty of evidence ranged from low to very low.
What was studied in the review?
Pressure ulcers (also known as bed sores, pressure sores, pressure injuries and decubitus ulcers) are areas of localised injury to the skin and underlying tissue, usually over a bony part of the body such as the hip or heel. These ulcers develop as a result of pressure, or pressure in combination with shear forces (squeezing and stretching soft tissues between bony structures and the skin). Pressure ulcers mainly occur in people who have limited mobility or nerve damage, such as older people, people with spinal injuries, or long‐term hospital patients. Pressure ulcer risk assessment is part of the process used to identify individuals at risk of developing a pressure ulcer. Use of a risk assessment tool is recommended by many international guidelines on pressure ulcer prevention. Different tools are used for pressure ulcer risk assessment. We wanted to find out which is the most effective in preventing pressure ulcers from developing. We also wanted to find out which risk assessment tools reduced the time for a pressure ulcer to develop and the severity of the pressure ulcer.
What are the main results of the review?
We found two relevant studies, dating from 2009 and 2011. Both of the included studies had three arms. One study compared Braden risk assessment and training, to training and risk assessment using clinical judgement, or risk assessment using clinical judgement alone. The second study compared Waterlow risk assessment to Ramstadius risk assessment, or risk assessment using clinical judgement. The studies involved 1,487 people at risk of developing pressure ulcers. In the first study, no information was provided on age or gender distribution. In the second study, 50.3% (619) of the participants were male, with an average age of 62.6 years. The first study did not state any source of funding. The second study was funded by research grants from the Queensland Nursing Council, the Royal Brisbane and Women’s Hospital Private Practice Fund, the Royal Brisbane and Women’s Hospital Research Foundation and a Queensland Health Nursing Research Grant.
We cannot be certain whether use of a risk assessment tool makes any difference to the prevention of pressure ulcers, compared with the use of clinical judgement. The results of the studies did not show differences in the number of pressure ulcers that developed among the participants and one study did not show a difference in the severity of pressure ulcers that developed. We assessed the certainty of the evidence as low, or very low, because not all the people completed one of the studies, and in both studies the results varied widely, and the staff knew which study group the patient was in. The outcomes for time to pressure ulcer development, and pressure ulcer prevalence, were not reported on by either study.
How up to date is this review?
We searched for studies that had been published up to February 2018.
Summary of findings
Background
Description of the condition
Pressure ulcers (also known as pressure injuries, bed sores, pressure sores and decubitus ulcers) are localised injury to the skin, underlying tissue or both, usually over a bony prominence, as a result of pressure or pressure in combination with shear (NPUAP/EPUAP/PPPIA 2014). They occur in people who do not have the ability to reposition themselves in order to relieve pressure on bony prominences (Moore 2011). This ability is often diminished in the very old, the malnourished and those with an acute illness (Moore 2012). Prevalence rates in long‐term care settings fluctuate from 8.8% to 53.2% and incidence rates vary from 7% to 71.6% (Moore 2011; Scott 2006). The most common anatomical sites for pressure ulcers to occur are the sacrum and the heels, and the majority are grade 1 or grade 2 in severity (Gallagher 2008; Moore 2011; Moore 2012). Furthermore, as age increases, so too does pressure ulcer prevalence and incidence (NPUAP/EPUAP/PPPIA 2014). Changing population demographics, and the predicted rise in the number of older people in the future (US Census Bureau 2018), suggest that there will be a corresponding increase in the number of people with pressure ulcers unless effective preventative measures are put in place.
Pressure ulcers impact negatively on quality of life: it is known that individuals with pressure ulcers frequently experience pain, combined with fear, isolation and anxiety regarding wound healing (Fox 2002; Hopkins 2006; Spilsbury 2007). Importantly, it has also been shown that pressure ulcers are associated with an increased risk of death (Khor 2014), although it is probable that pressure ulcers are usually a consequence of poor health rather than a cause of death. Further prospective cohort studies examined the factors predictive of mortality in older individuals admitted to hospital. Among individuals in intensive care, nursed on a ventilator, pressure ulcer onset was a significant independent predictor of mortality (adjusted hazard ratio = 1.28; 95% confidence interval (CI) 1.003 to 1.65; P = 0.047) (Manzano 2014). Further, a study of data from a general in‐patient population identified that the mortality rate in patients with a pressure ulcer was significantly higher than in patients without a pressure ulcer (9.1% versus 1.8%, odds ratio = 5.08, CI: 5.03 to 5.1, P < 0.001) (Bauer 2016).
Pressure ulcers are a significant financial burden to healthcare systems. A recent systematic review noted that the cost for prevention of pressure ulcers was lower than that for treatment, with the cost of pressure ulcer prevention per patient per day varying between 2.65 € to 87.57 € across all clinical settings. The cost of pressure ulcer treatment per patient per day ranged from EUR 1.71 to EUR 470.49 across different settings (Demarre 2015). It has also been suggested that the length of hospital stay is significantly different between patients with and without pressure ulcers (median seven days (mean 11.1 ± 15) compared to median three days (mean 4.6 ± 6.8), respectively) (Bauer 2016).
Globally, the economic impact of pressure ulcers has yet to be established. However, it is known that pressure ulcers are common and affect patients in both community and hospital settings (Moore 2013). Therefore, it is reasonable to suggest that pressure ulcer prevention strategies that can reduce prevalence and incidence rates will have a positive impact on patients and the health service as a whole (Moore 2011).
Description of the intervention
Use of pressure ulcer risk assessment tools or scales is a component of the assessment process to identify individuals at risk of developing a pressure ulcer (NPUAP/EPUAP/PPPIA 2014). Risk assessments generally use checklists that alert practitioners to the most common risk factors that predispose individuals to pressure ulcer development. These checklists are often developed into risk assessment tools, for example the Norton Scale (Norton 1975), the Waterlow tool (Waterlow 1985) and the Braden tool (Braden 1987). It is argued that there is a lack of consensus regarding which variables are the most important indicators of risk (Gould 2002). Therefore, it is not surprising that there are currently almost 40 risk assessment scales in use, most of which are based on the seminal work of Norton 1975, or have been designed in response to a review of the literature (Defloor 2004). It is clear, however, that the risk factors that predispose an individual to developing a pressure ulcer will vary among patients in different clinical settings (Henoch 2003), and it may not be possible to design one risk assessment tool that will meet the needs of all patients in all clinical settings.
How the intervention might work
Use of a risk assessment tool is recommended by many international guidelines on pressure ulcer prevention (NPUAP/EPUAP/PPPIA 2014; NICE 2001; Rycroft‐Malone 2000). The ideal risk assessment tool should be both reliable and valid, and sensitive and specific (NPUAP 1998). The tool must accurately identify those individuals who are at risk, as well as those not at risk, and do this consistently (Defloor 2005). To date, there is little empirical evidence available concerning the reliability and validity of existing tools (Anthony 2008; Cullum 1995; Defloor 2004; Defloor 2005; Haalboom 1999; McGough 1999; Pancorbo‐Hidalgo 2006; Schoonhoven 2002). Assessing reliability and validity is a real challenge in clinical practice because risk assessment scales are used to identify those who would develop a pressure ulcer should no interventions be put in place. It is common to use different pressure ulcer prevention strategies once risk has been identified, which will therefore appear to alter the predictive ability of the scale (Defloor 2004; Halfens 2000). Different studies using the same risk assessment tools, but in diverse healthcare settings with diverse patient populations and prevention strategies, report varying levels of sensitivity and specificity (Gould 2002). It is of relevance to note that the prevention strategies which were in use in these studies are often not stated (Halfens 2000). Lack of clear knowledge of the sensitivity and specificity of risk assessment tools has far‐reaching implications for practice, because clinical decisions — such as the use, or not, of pressure ulcer preventative strategies — are often made on the basis of the results of risk assessment, although it has been argued that nurses often use their clinical judgement alone in deciding which preventative measures to use (Anthony 2008). Therefore, it is likely that some patients are receiving interventions that they do not require, and conversely others are not receiving interventions that they would benefit from (Defloor 2005). This inappropriate allocation of resources compounds the increasing burden of pressure ulcers, and adds to spiraling healthcare costs. It is important to note that the primary focus of interest for this systematic review is whether or not using a risk assessment tool makes any difference to pressure ulcer incidence, as such the review is not looking at the predictive validity of pressure ulcer risk tools.
Why it is important to do this review
There are three published systematic reviews that explore the effectiveness of pressure ulcer risk assessment tools for the prevention of pressure ulcers. The first review searched from 1962 to 1995 (Cullum 1995), the second review from 1962 to 1999 (McGough 1999), and the third review from 1966 to 2003 (Pancorbo‐Hidalgo 2006). The Royal College of Nursing (UK) guidelines on pressure ulcer prevention were based largely on the results of the review by McGough and colleagues (McGough 1999; NICE 2001). Two reviews restricted their inclusion criteria to studies published only in the English language (Cullum 1995; McGough 1999); the third review restricted the inclusion criteria to four languages: Spanish, English, French and Portuguese (Pancorbo‐Hidalgo 2006). The reviews found no evidence that pressure ulcer risk assessment scales reduce the incidence of pressure ulcers. However, given the time since these reviews were written, and the language restrictions that were imposed, it is possible that other relevant literature was originally overlooked or has been published in the meantime. Therefore it is timely to conduct a review with no language restrictions and recent searches, in order to clarify the role of pressure ulcer risk assessment tools in clinical practice. This is the third update of this review (see Other published versions of this review) and in this version we incorporated GRADE assessment of the certainty of evidence.
Objectives
To assess whether using structured and systematic pressure ulcer risk assessment tools, in any healthcare setting, reduces the incidence of pressure ulcers.
Methods
Criteria for considering studies for this review
Types of studies
For inclusion in the review, we considered randomised controlled trials (RCTs) comparing the use of structured and systematic pressure ulcer risk assessment tools with no structured pressure ulcer risk assessment, or with unaided clinical judgement; or RCTs comparing the use of different structured pressure ulcer risk assessment tools. Studies that randomised individuals (RCTs), or cluster‐randomised trials (cluster‐RCTs) that randomise by groups, were eligible for inclusion.
Types of participants
Studies involving people without pressure ulcers, of any age, in any healthcare setting (primary, secondary and extended care) were eligible for inclusion.
Types of interventions
RCTs making the following comparisons were eligible for inclusion in this review:
pressure ulcer risk assessment using a specific structured and systematic pressure ulcer risk assessment tool compared with no structured pressure ulcer risk assessment tool or unaided clinical judgement;
comparisons between two different structured and systematic pressure ulcer risk assessment tools.
Types of outcome measures
Primary outcomes
The proportion of participants developing new pressure ulcers of any grade, within the study period. For the purpose of this review a pressure ulcer was defined as a localised injury to the skin or underlying tissue (or both), usually over a bony prominence, as a result of pressure, or pressure in combination with shear (NPUAP/EPUAP/PPPIA 2014).
Secondary outcomes
The severity of new pressure ulcers (as assessed using a validated pressure ulcer staging system).
Time to ulcer development, measured as the time of onset of the pressure ulcer within the study follow‐up period.
Pressure ulcer prevalence, defined as number of existing pressure ulcers at the study end point.
Search methods for identification of studies
Electronic searches
We developed the search strategy in consultation with Cochrane Wound's Information Specialist. We searched the following electronic databases to identify reports of relevant clinical trials:
Cochrane Wounds Specialised Register (searched 5 February 2018);
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2018, Issue 1)(searched 5 February 2018);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 5 February 2018);
Ovid Embase (1974 to 5 February 2018);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 5 February 2018).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018). There were no restrictions with respect to language, date of publication or study setting. Details of the search strategies used for the previous versions of the review are given in Moore 2014.
Searching other resources
We searched citations in all retrieved and relevant studies identified by these strategies for further studies. We contacted experts in the wound care field — namely council members of the European Pressure Ulcer Advisory Panel, the European Wound Management Association, the National Pressure Ulcer Advisory Panel and the World Union of Wound Healing Societies — to identify any studies not located through the primary search.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 18 February 2018);
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch) (searched 18 February 2018);
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/ctr‐search/search) (searched 18 February 2018).
Search strategies for clinical trial registries can be found in Appendix 1.
Data collection and analysis
Selection of studies
Two review authors independently assessed titles and, where available, abstracts of the studies identified by the search strategy for their eligibility (as identified in the selection criteria) for inclusion in the review. Two review authors obtained full versions of potentially relevant studies and screened these against the inclusion criteria independently. Any differences in opinion were resolved by discussion and, where necessary, reference to the Wounds Group editorial base. We completed a PRISMA flowchart to summarise this process (Liberati 2009); see Figure 1.
1.
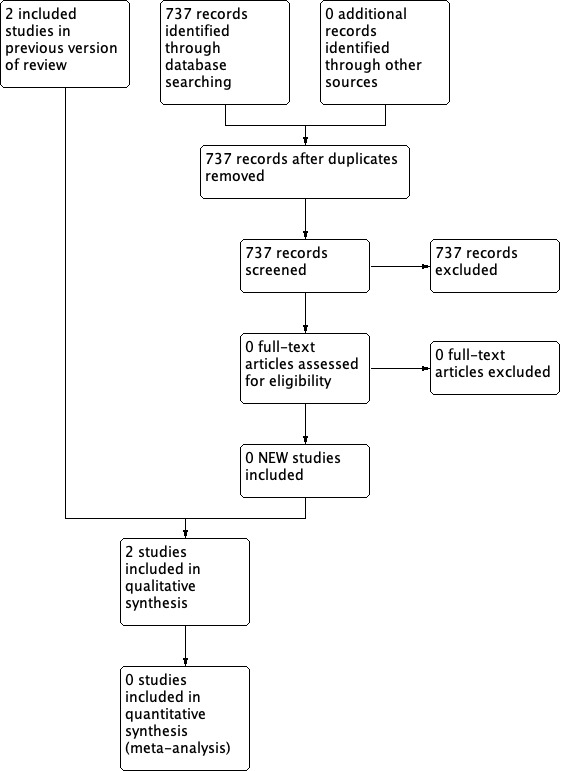
Study flow diagram.
Data extraction and management
One review author extracted and summarised trial data. Data entry was independently checked by a second review author. We extracted and summarised details of the eligible studies using a data extraction sheet. Specifically, we extracted the following information:
author, title, source, date of study;
country, care setting;
inclusion and exclusion criteria;
participant baseline characteristics by group;
design details, study type, sample size;
allocation;
intervention details, concurrent interventions;
if risk assessment was part of a wider assessment programme/package;
frequency of risk assessment, length of follow up;
patient length of stay;
which health professional administered the tool;
outcome measures;
verification of diagnosis;
analysis;
loss to follow‐up;
results and conclusions.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies using the Cochrane tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains: namely, sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 2 for details of criteria on which each judgement was based). For cluster‐RCTs we assessed additional risk of bias domains, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), namely, recruitment bias; baseline imbalance; loss of clusters and incorrect analysis (see Appendix 3 for the risk of bias criteria for cluster‐RCTs). We have presented an assessment of risk of bias using a 'Risk of bias' summary figure and a 'Risk of bias' graph (Figure 2 and Figure 3). These displays of internal validity indicate the weight the reader may give to the results of each study.
2.
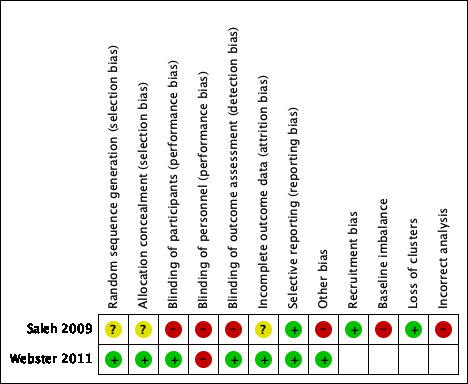
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
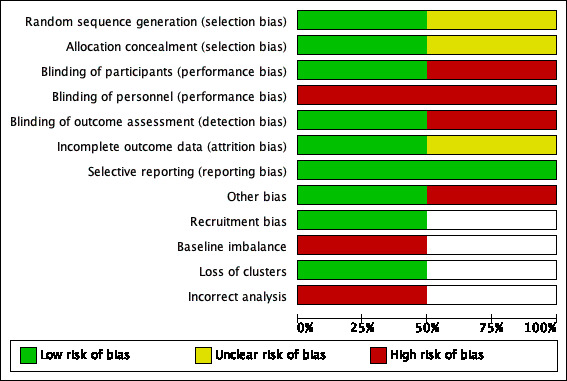
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We conducted data analysis according to Cochrane guidelines. Results for dichotomous variables are presented as risk ratios (RRs) with 95% confidence intervals (CIs). Risk ratio is the rate of the event of interest (e.g. pressure ulcers developed) in the experimental group divided by the rate of this event in the control group, and indicates the chances of pressure ulcer development for people in the experimental group compared with the control group (Higgins 2011). An RR of one means there is no difference in risk between the two study groups, an RR of less than one means the event is less likely to occur in the experimental group than in the control group, and an RR of more than one means the event is more likely to occur in the experimental group than in the control group (Higgins 2011).
Unit of analysis issues
We anticipated the main unit of analysis issues would occur in cluster‐RCTs when allocation occurred at the level of the organisation or the team and data were collected from individual patients. Where a cluster‐RCT has been conducted and correctly analysed, effect estimates and their standard errors may be meta‐analysed using the generic inverse‐variance method in Review Manager 5 (Review Manager 2014). We recorded where a cluster‐RCT had been conducted, but incorrectly analysed, as part of the 'Risk of bias’ assessment. If possible, we would have approximated the correct analyses based on guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2011), using information on:
the number of clusters (or groups) randomised to each intervention group or the average (mean) size of each cluster;
whether the outcome data ignored the cluster design for the total number of participants (e.g. number or proportion of participants with events, or means and standard deviations); and
an estimate of the intracluster (or intraclass) correlation coefficient.
If we could not analyse the study data, we would have extracted and presented, but not further analysed and not included, outcome data in any otherwise relevant meta‐analysis we may have conducted. As meta‐analysis was not relevant in this review, this was not an issue.
Dealing with missing data
If there were missing data, we would have contacted the study authors to request the missing information.
Assessment of heterogeneity
We planned to explore clinical heterogeneity by examining potentially influential factors, e.g. care setting or patient characteristics. Statistical heterogeneity was to be assessed using the I2 statistic (Higgins 2003). This examines the percentage of total variation across studies due to heterogeneity rather than to chance. Values of I2 over 75% indicate a high level of heterogeneity. We intended to carry out statistical pooling on groups of studies which were considered to be sufficiently similar. However, owing to the lack of homogeneity of the studies included (in terms of the interventions evaluated), statistical pooling was not relevant.
Assessment of reporting biases
We assessed reporting bias using guidelines in the Cochrane Handbook for Systematic Reveiws of Interventions (Sterne 2011). If we had identified sufficient studies for a meaningful assessment of publication bias, we would have constructed a funnel plot of primary outcomes to test for asymmetry. If asymmetry was present we would have explored possible causes, including reporting and publication bias. However, as we only included two studies in this review, this was not relevant.
Data synthesis
We entered quantitative data into Review Manager 5.3 (Review Manager 2014) and analysed them using the Review Manager analysis software. Initially we present a structured narrative summary of the studies reviewed. For dichotomous outcomes, we calculated RR, plus 95% CIs. We were unable to pool the data in a meta‐analysis. We analysed the results of individual studies using the fixed‐effect model, due to the limited number of included studies.
'Summary of findings’ tables
We present the main results of the review in 'Summary of findings’ tables. 'Summary of findings' tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011). 'Summary of findings’ tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach (Schünemann 2011). The GRADE approach defines the quality of a body of evidence with regard to the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. To assess the overall body of evidence, we developed 'Summary of findings’ tables using (GRADEpro GDT 2015).
The quality of the body of evidence was assessed against five principle domains: 1) limitations in design and implementation; 2) indirectness of evidence or generalisability of findings; 3) inconsistency of results, for example unexplained heterogeneity and inconsistent findings; 4) imprecision of results where confidence intervals are wide; and 5) publication bias (Schünemann 2011). We present the following outcomes in 'Summary of findings’ tables:
pressure ulcer incidence (the proportion of people developing any new pressure ulcer(s) of any grade);
the severity of new pressure ulcers (as assessed using a validated pressure ulcer staging system);
time to ulcer development, measured as the time of onset of the pressure ulcer within the study follow‐up period;
pressure ulcer prevalence, defined as number of existing pressure ulcers at the study end point.
Subgroup analysis and investigation of heterogeneity
If substantial heterogeneity had existed between studies for the primary outcomes (that is, when the I² statistic exceeds 75%), we would have explored reasons for heterogeneity.
Sensitivity analysis
We planned to perform a sensitivity analysis by excluding those studies assessed as having a high risk and unclear risk of bias, assessed using the Cochrane tool for assessing risk of bias (Higgins 2011). We planned to include those studies assessed as having low risk bias in the key domains of adequate generation of the randomisation sequence, adequate allocation concealment and blinding of outcome assessor for the estimates of treatment effect.
Results
Description of studies
Results of the search
The initial search for this review identified 105 records. Following independent review of the abstracts by the two review authors, we retrieved 10 citations in full. Two review authors independently assessed the papers and applied the inclusion and exclusion criteria. No studies were identified that met the inclusion criteria. Fifty‐two letters were written to wound care experts and 16 replies were received, yielding a response rate of 31%. We identified no further trials through this process. The search for the first update of this review identified 98 records. Following review of the abstracts, we retrieved one citation in full and included this study in the review (Saleh 2009). For the second update, 171 records were identified. Following review of the abstracts one further study met the inclusion criteria and was included in the review (Webster 2011). For the third update, 737 records were retrieved, no studies were assessed as full texts and hence no new excluded studies were identified and there were no new trials to include. Despite searching trials registers we identified no new ongoing studies. See Figure 1 for the flow of studies through the review.
Included studies
Two studies met the inclusion criteria (Saleh 2009; Webster 2011).
Population
The first study was published in 2009 (Saleh 2009). This cluster‐randomised study was conducted among 256 participants within nine wards of a military hospital in Saudi Arabia. Data were collected from all participants with Braden scores of 18 or less across the nine wards; follow‐up was for eight weeks. Participants were nursed on either standard foam mattresses, alternating pressure redistribution devices, gel overlay mattresses or air fluidised mattresses. Repositioning schedules were every two hours, three to four hours, or six hours. The procedure for allocation of mattresses and repositioning schedules are not reported by the study authors.
The second study was published in 2011 (Webster 2011). This randomised controlled trial (RCT) was conducted among 1,231 participants within a tertiary referral teaching hospital in Australia. All patients admitted through the Department of Emergency Medicine, or any of the outpatient departments to an internal medicine ward or an oncology ward at the Royal Brisbane and Women’s Hospital between April 2009 and December 2009, were eligible for inclusion. Patients were excluded if their hospital stay was expected to be less than three days and if they had been in hospital for no more than 24 hours before baseline assessment could occur.
Intervention and comparisons
The study by Saleh and colleagues compared the effect of three different methods of pressure ulcer risk assessment on the incidence of pressure ulcers in hospitalised individuals with a Braden score of 18 or less (Braden 1987; Saleh 2009). The methods of risk assessment were: the Braden pressure ulcer risk assessment tool and training; risk assessment using clinical judgement and training; and risk assessment using clinical judgement alone (see Characteristics of included studies). The Braden pressure ulcer risk assessment tool comprises six sub‐scales: sensory perception, moisture, activity, mobility, nutrition and friction/shear. Each sub‐scale is ranked numerically from 1 to 4; a score of 4 indicates no problem with regard to the specific sub‐scale, whereas a score of 1 indicates a significant problem. The friction and shear sub‐scale is scored 1 to 3. The scores for each of the sub‐scales are totaled to give a final score ranging from 6 to 23; as scores become lower, predicted risk becomes higher (Braden 1987). The study randomly allocated the clinical wards into three groups (Saleh 2009). Group A nurses (the Braden group; n = 74) received a mandatory study day on wound care management, pressure ulcer prevention training programme and specific training on the application of the Braden pressure ulcer risk assessment tool. These nurses were required to implement Braden on their patients in the post‐intervention stage. Group B nurses (the training group; n = 76) were identical to group A but were not required to implement Braden. Group C nurses (the clinical judgement group; n = 106) received only a mandatory study day on wound care management.
In Webster 2011, participants were allocated to either a Waterlow (n = 411), or Ramstadius (n = 410) pressure ulcer risk assessment group, or to a group where risk assessment was undertaken using clinical judgement (n = 410); see Characteristics of included studies. The Waterlow pressure ulcer risk assessment tool comprises seven sub‐scales: build/weight for height; skin type; nutrition; sex/age; continence; mobility; special risks. Each sub‐scale is scored individually according to an allocated score to each component of the sub‐scale, with the scores added to give an overall risk status. As scores become higher, the predicted risk become correspondingly higher (more than 10 = low risk; more than 15 = high risk; more than 20 = very high risk) (Waterlow 1985). The main focus of the Ramstadius pressure ulcer risk assessment tool is on mobility status, it is a non‐numerical tool and begins with the assessment of mobility as yes/no (Ramstadius 2000). If the patient can reposition themselves independently, no further assessment is required and the patient is deemed not to be at risk. Conversely, if problems with mobility are identified, the patient is deemed to be at high risk and further assessment of risk factors — namely age, medication, skin integrity, temperature, decreased blood volume, dyspnoea and presence of an existing pressure ulcer — is undertaken. No scores are given, rather an algorithm is provided to direct interventions which may be appropriate for the specific risk factor. The only requirement of staff in the participating wards was to use only the instrument found in the chart. Otherwise, there were no changes to routine care.
Outcomes
Both studies reported pressure ulcer incidence as the primary outcome.
In Saleh 2009, incidence was recorded as the development of a pressure ulcer during the study period. Pressure ulcers were identified according to the National Pressure Ulcer Advisory Panel (NPUAP; USA) pressure ulcer classification system (NPUAP 1998). Follow‐up was for eight weeks. The study did not identify the grade of pressure ulcer damage specifically for each participant, but rather reported 'pressure ulcer present: yes or no'.
In Webster 2011, research assistants who were trained in pressure ulcer staging, and who were blinded to the screening method, visually inspected participants for evidence of pressure ulcer formation daily (except weekends). Follow‐up was for four days. The primary outcome was defined as development of a new pressure ulcer, or any increase in the stage of an existing ulcer. Follow‐up was discontinued once the study end point was reached. Pressure ulcers were staged according to the NPUAP pressure ulcer staging system (Black 2007).
Excluded studies
The Characteristics of excluded studies table summarises the 10 studies that were excluded from the review.
Risk of bias in included studies
See Figure 2 and Figure 3 for the summary of the risk of bias of the included studies.
Allocation (selection bias)
Methods used for generating the allocation sequence and for concealing the group allocation were unclear in Saleh 2009; the study randomly allocated nine wards into three groups: groups A, B and C. The ward, not the patient, was the unit of randomisation and therefore this is a cluster‐RCT study design. The randomisation resulted in unequal allocation across the groups and no explanation for this was given in the study report. The second study reports that a computer‐generated randomised list, with a phone randomisation method, was used (Webster 2011).
Blinding (performance and detection bias)
The study by Saleh and colleagues did not mention blinding of the participants, personnel or outcome assessor in the study report (Saleh 2009). The study by Webster and colleagues reports that the patient and the outcome assessor were blinded to group assignment (Webster 2011). However, in this study the staff were aware of which group the patient was in ("The only requirement of staff in the participating wards was to use only the instrument found in the chart"; Webster 2011). Thus, the staff delivering pressure ulcer prevention care to the patient were aware of which group the patient was in. For this update we assessed blinding of personnel separately, and we assigned a judgement of high risk of bias for this domain for Webster 2011.
Incomplete outcome data (attrition bias)
In Saleh 2009, it is not reported whether an intention‐to‐treat (ITT) analysis was undertaken. In Webster 2011, it is reported that the number of participants allocated to each group were analysed for the primary outcome at the end of the study.
Selective reporting (reporting bias)
In both included studies, Saleh 2009 and Webster 2011, all outcomes mentioned in the methods section are reported in the results. Webster 2011 was registered with the Australian and New Zealand Clinical Trials Registry and outcomes match those registered. Saleh 2009 was not registered with a trial registry.
Other potential sources of bias
No additional sources of bias were identified in Webster 2011. For the cluster‐RCT, Saleh 2009, see details below.
Recruitment bias
All patients in each cluster, meeting the inclusion criteria were included (Saleh 2009).
Baseline imbalance
The groups in the cluster‐RCT were not comparable at baseline for medical diagnoses, pressure ulcer prevention practices, use of barrier creams and use of vitamin supplementary therapy. The type of mattress the patients lay on was not the same for all participants and the repositioning schedules for each participant was not the same (Saleh 2009).
Loss of clusters
There was no loss of clusters from the cluster‐RCT (Saleh 2009).
Incorrect analysis
The cluster‐RCT did not report if they adjusted for the clustering in the sample size calculation and in the analysis (Saleh 2009).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement and training for the prevention of pressure ulcers.
| Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement and training for the prevention of pressure ulcers | |||||||
| Patient or population: patients at risk of pressure ulcers Setting: hospital setting Intervention: Braden pressure ulcer risk assessment and training Comparison: pressure ulcer risk assessment using clinical judgement and training | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
Absolute effect (95% CI) |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Pressure ulcer risk assessment using clinical judgement and training | Braden pressure ulcer risk assessment and training | ||||||
| Pressure ulcer incidence Visual skin assessment Follow‐up: 8 weeks | Study population | RR 0.97 (0.53 to 1.77) | 7 fewer per 1000 (from 105 fewer to 172 more) | 150 (1 study) | ⊕⊝⊝⊝ Very low1 | We are uncertain if Braden pressure ulcer risk assessment and training, compared with pressure ulcer risk assessment using clinical judgement and training, makes any difference to pressure ulcer incidence. | |
| 224 per 1000 | 217 per 1000 (119 to 396) | ||||||
|
Severity of new pressure ulcers Time to ulcer development Pressure ulcer prevalence |
Not reported | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1 Downgraded twice for study limitations due to high risk of performance and detection bias and unclear risk of selection and attrition bias; downgraded twice for imprecision due to wide confidence intervals, small sample size and no allowance for the use of cluster randomisation.
Summary of findings 2. Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement alone for the prevention of pressure ulcers.
| Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement alone for the prevention of pressure ulcers | |||||||
| Patient or population: patients at risk of pressure ulcers Setting: hospital setting Intervention: Braden pressure ulcer risk assessment and training Comparison: pressure ulcer risk assessment using clinical judgement | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
Absolute effect (95% CI) |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Pressure ulcer risk assessment using clinical judgement alone | Braden pressure ulcer risk assessment and training | ||||||
| Pressure ulcer incidence Visual skin assessment Follow‐up: 8 weeks | Study population | RR 1.43 (0.77 to 2.68) | 65 more per 1000 (from 35 fewer to 254 more) | 180 (1 study) | ⊕⊝⊝⊝ Very low1 | We are uncertain if Braden pressure ulcer risk assessment and training, compared with pressure ulcer risk assessment using clinical judgement alone, makes any difference to pressure ulcer incidence. | |
| 151 per 1000 | 216 per 1000 (116 to 405) | ||||||
|
Severity of new pressure ulcers Time to ulcer development Pressure ulcer prevalence |
Not reported | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1 Downgraded twice for study limitations due to high risk of performance and detection bias and unclear risk of selection and attrition bias; downgraded twice for imprecision due to wide confidence intervals, small sample size and no allowance for the use of cluster randomisation.
Summary of findings 3. Waterlow pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement for the prevention of pressure ulcers.
| Waterlow risk assessment compared with pressure ulcer risk assessment using clinical judgement for the prevention of pressure ulcers | |||||||
|
Patient or population: patients at risk of pressure ulcers
Setting: hospital setting
Intervention: Waterlow pressure ulcer risk assessment Comparison: pressure ulcer risk assessment using clinical judgement | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) |
Absolute effect (95% CI) |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Pressure ulcer risk assessment using clinical judgement | Waterlow pressure ulcer risk assessment | ||||||
| Pressure ulcer incidence Visual skin assessment Follow‐up: 4 days | Study population | RR 1.10 (0.68 to 1.81) | 7 more per 1000 (from 22 fewer to 55 more) | 821 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Waterlow pressure ulcer risk assessment tool may make little or no difference to pressure ulcer incidence when compared to pressure ulcer risk assessment using clinical judgement. | |
| 68 per 1000 | 75 per 1000 (46 to 124) | ||||||
| Severity of new pressure ulcers ‐ Stage 1 | 49 per 1000 |
51 per 1000 (28 to 93) |
RR 1.05 (0.58 to 1.90) | 2 more per 1000 (from 20 fewer to 44 more) | 821 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Waterlow pressure ulcer risk assessment tool may make little or no difference to pressure ulcer severity (stage 1) when compared to pressure ulcer risk assessment using clinical judgement. |
| Severity of new pressure ulcers ‐ Stage 2 | 20 per 1000 |
24 per 1000 (10 to 61) |
RR 1.25 (0.50 to 3.13) |
5 more per 1000 (from 10 fewer to 43 more) | 821 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Waterlow pressure ulcer risk assessment tool may make little or no difference to pressure ulcer severity (stage 2) when compared to pressure ulcer risk assessment using clinical judgement. |
|
Time to ulcer development Pressure ulcer prevalence |
Not reported | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1Downgraded once for study limitations due to high risk of performance bias. Downgraded once for imprecision due to wide confidence intervals.
Summary of findings 4. Ramstadius pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement for the prevention of pressure ulcers.
| Ramstadius pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement for the prevention of pressure ulcers | |||||||
|
Patient or population: patients at risk of pressure ulcers
Setting: hospital
Intervention: Ramstadius pressure ulcer risk assessment Comparison: pressure ulcer risk assessment using clinical judgement | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Absolute effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Pressure ulcer risk assessment using clinical judgement | Ramstadius pressure ulcer risk assessment | ||||||
| Pressure ulcer incidence Visual skin assessment Follow‐up: 4 days | Study population | RR 0.79 (0.46 to 1.35) | 14 fewer per 1000 (from 37 fewer to 24 more) | 820 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Ramstadius pressure ulcer risk assessment tool may make little or no difference to pressure ulcer incidence when compared to pressure ulcer risk assessment using clinical judgement. | |
| 68 per 1000 | 54 per 1000 (31 to 92) | ||||||
| Severity of new pressure ulcers ‐ Stage 1 | 49 per 1000 |
44 per 1000 (23 to 82) |
RR 0.90 (0.48 to 1.68) | 5 fewer per 1000 (from 25 fewer to 33 more) | 820 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Ramstadius pressure ulcer risk assessment tool may make little or no difference to pressure ulcer severity (stage1) when compared to pressure ulcer risk assessment using clinical judgement. |
| Severity of new pressure ulcers ‐ Stage 2 | 20 per 1000 |
10 per 1000 (3 to 32) |
RR 0.50 (0.15 to 1.65) |
10 fewer per 1000 (from 17 fewer to 13 more) | 820 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Ramstadius pressure ulcer risk assessment tool may make little or no difference to pressure ulcer severity (stage 2) when compared to pressure ulcer risk assessment using clinical judgement. |
|
Time to ulcer development Pressure ulcer prevalence |
Not reported | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1Downgraded once for study limitations due to high risk of performance bias. Downgraded once for imprecision due to wide confidence intervals.
Summary of findings 5. Waterlow pressure ulcer risk assessment compared with Ramstadius pressure ulcer risk assessment for the prevention of pressure ulcers.
| Waterlow pressure ulcer risk assessment compared with Ramstadius pressure ulcer risk assessment for the prevention of pressure ulcers | |||||||
|
Patient or population: patients at risk of pressure ulcers
Setting: hospital setting
Intervention: Waterlow pressure ulcer risk assessment Comparison: Ramstadius pressure ulcer risk assessment tool | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Absolute effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Ramstadius pressure ulcer risk assessment tool | Waterlow pressure ulcer risk assessment | ||||||
| Pressure ulcer incidence Visual skin assessment Follow‐up: 4 days | Study population | RR 1.41 (0.83 to 2.39) | 23 more per 1000 (from 8 fewer to 75 more) | 821 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Waterlow risk assessment tool may make little or no difference to pressure ulcer incidence when compared to use of the Ramstadius pressure ulcer risk assessment tool. | |
| 52 per 1000 | 75 per 1000 (45 to 128) | ||||||
| Severity of new pressure ulcers ‐ Stage 1 | 44 per 1000 |
51 per 1000 (28 to 94) |
RR 1.16 (0.63 to 2.15) | 7 more per 1000 (from 16 fewer to 50 more) | 821 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Waterlow risk assessment tool may make little or no difference to pressure ulcer severity (stage 1) when compared to use of the Ramstadius pressure ulcer risk assessment tool. |
| Severity of new pressure ulcers ‐ Stage 2 | 10 per 1000 |
24 per 1000 (10 to 61) |
RR 2.49 (0.79 to 7.89) |
15 more per 1000 (from 2 fewer to 69 more) | 821 (1 study) | ⊕⊕⊝⊝ Low1 | Risk assessment using the Waterlow risk assessment tool may make little or no difference to pressure ulcer severity (stage 2) when compared to use of the Ramstadius pressure ulcer risk assessment tool. |
|
Time to ulcer development Pressure ulcer prevalence |
Not reported | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1Downgraded once for study limitations due to high risk of performance bias. Downgraded once for imprecision due to wide confidence intervals.
See Table 1; Table 2; Table 3; Table 4 and Table 5.
Comparison 1: Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement and training
Primary outcome: pressure ulcer incidence
Following delivery of the training to the staff, one study enrolled 150 participants with a Braden score of 18 or less, from six wards (Saleh 2009). Seventy‐four participants were in the Braden scale group (Group A), and 76 were in the training group (Group B). We are uncertain if Braden pressure ulcer risk assessment and training, compared with pressure ulcer risk assessment using clinical judgement and training, makes any difference to pressure ulcer incidence (Braden group: 22%, n = 16/74; training group: 22%, n = 17/76; risk ratio (RR) 0.97, 95% confidence interval (CI) 0.53 to 1.77; very‐low certainty evidence, downgraded twice for serious risk of bias and twice for serious imprecision). Although the outcome is described as pressure ulcer incidence by the study authors, what is reported is the number of people who developed pressure ulcers. (Analysis 1.1; Table 1).
1.1. Analysis.
Comparison 1 Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement and training, Outcome 1 Pressure ulcer incidence.
Secondary outcomes
The following secondary outcomes were not reported in the study:
severity of new pressure ulcers;
time to pressure ulcer development;
pressure ulcer prevalence.
Comparison 2: Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement alone
Primary outcome: pressure ulcer incidence
Following delivery of the training to the staff, one study enrolled 106 participants from three wards with a Braden score of 18 or less (Saleh 2009). These patients were risk assessed using clinical judgement (Group C). The incidence of pressure ulcers in this clinical judgement group was compared with the 74 participants who were in the Braden scale group (Group A). We are uncertain if Braden pressure ulcer risk assessment and training, compared with pressure ulcer risk assessment using clinical judgement alone, makes any difference to pressure ulcer incidence (Braden group: 22%, n = 16/74; clinical judgement group: 15%, n = 16/106; RR 1.43, 95% CI 0.77 to 2.68; very‐low certainty evidence, downgraded twice for serious risk of bias and twice for serious imprecision). Although the outcome is described as pressure ulcer incidence by the study authors, what is reported is the number of people who developed pressure ulcers. (Analysis 2.1; Table 2).
2.1. Analysis.
Comparison 2 Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement alone, Outcome 1 Pressure ulcer incidence.
Secondary outcomes
The following secondary outcomes were not reported in the study:
severity of new pressure ulcers;
time to pressure ulcer development;
pressure ulcer prevalence.
Comparison 3: Waterlow pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement
Primary outcome: pressure ulcer incidence
One study enrolled 411 participants in the Waterlow group and 410 participants into the group risk assessed using clinical judgement (Webster 2011). Although outcomes are reported as ulcers developed, rather than people developing ulcers, only one ulcer was assessed per person. Risk assessment using the Waterlow pressure ulcer risk assessment tool may make little or no difference to pressure ulcer incidence when compared to risk assessment using clinical judgement (Waterlow group 7.5%, n = 31/411; clinical judgement group: 6.8%, n = 28/410; RR 1.10, 95% CI 0.68 to 1.81; low‐certainty evidence, downgraded once for risk of bias and once for imprecision) (Analysis 3.1; Table 3).
3.1. Analysis.
Comparison 3 Waterlow risk assessment compared with pressure ulcer risk assessment using clinical judgement, Outcome 1 Pressure ulcer incidence.
Secondary outcome: severity of new pressure ulcers
Risk assessment using the Waterlow pressure ulcer risk assessment tool may make little or no difference to pressure ulcer severity when compared to risk assessment using clinical judgement (Stage 1 ulcer severity: Waterlow group: 5.1%, n = 21/411; clinical judgement group: 4.9%, n = 20/410; RR 1.05, 95% CI 0.58 to 1.90; Stage 2 ulcer severity: Waterlow group: 2.4%, n = 10/411; clinical judgement group: 1.9%. n = 8/410; RR 1.25, 95% CI 0.50 to 3.13; low‐certainty evidence, downgraded once for risk of bias and once for imprecision). As above only one ulcer was assessed per person. (Analysis 3.2; Analysis 3.3; Table 3).
3.2. Analysis.
Comparison 3 Waterlow risk assessment compared with pressure ulcer risk assessment using clinical judgement, Outcome 2 Pressure ulcer severity ‐ Stage 1.
3.3. Analysis.
Comparison 3 Waterlow risk assessment compared with pressure ulcer risk assessment using clinical judgement, Outcome 3 Pressure ulcer severity ‐ Stage 2.
The following secondary outcomes were not reported in the study:
time to pressure ulcer development;
pressure ulcer prevalence.
Comparison 4: Ramstadius pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement
Primary outcome: pressure ulcer incidence
One study enrolled 410 participants in the Ramstadius group and 410 participants into the group receiving pressure ulcer risk assessment using clinical judgement. Although outcomes are reported as ulcers developed, rather than people developing ulcers, only one ulcer was assessed per person. Risk assessment using the Ramstadius pressure ulcer risk assessment tool may make little or no difference to pressure ulcer incidence when compared to risk assessment using clinical judgement (Ramstadius group: 5.4%, n = 22/410; clinical judgement group: 6.8%, n = 28/410; RR 0.79, 95% CI 0.46 to 1.35; low‐certainty evidence, downgraded once for risk of bias and once for imprecision) (Analysis 4.1; Table 4).
4.1. Analysis.
Comparison 4 Ramstadius pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement, Outcome 1 Pressure ulcer incidence.
Secondary outcome: severity of new pressure ulcers
Risk assessment using the Ramstadius pressure ulcer risk assessment tool may make little or no difference to pressure ulcer severity when compared to risk assessment using clinical judgement (Stage 1 ulcer severity: Ramstadius group: 4.4%, n = 18/410; clinical judgement group: 4.9%, n = 20/410; RR 0.90, 95% CI 0.48 to 1.68; Stage 2 ulcer severity: Ramstadius group: 1.0%, n = 4/410; clinical judgement group: 1.9%, n = 8/410; RR 0.50, 95% CI 0.15 to 1.65; low‐certainty evidence, downgraded once for risk of bias and once for imprecision). As above only one ulcer was assessed per person. (Analysis 4.2; Analysis 4.3; Table 4).
4.2. Analysis.
Comparison 4 Ramstadius pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement, Outcome 2 Pressure ulcer severity ‐ Stage 1.
4.3. Analysis.
Comparison 4 Ramstadius pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement, Outcome 3 Pressure ulcer severity ‐ Stage 2.
The following secondary outcomes were not reported in the study:
time to pressure ulcer development;
pressure ulcer prevalence.
Comparison 5: Waterlow pressure ulcer risk assessment compared with Ramstadius pressure ulcer risk assessment
Primary outcome: pressure ulcer incidence
One study enrolled 411 participants in the Waterlow group and 410 participants in the Ramstadius group (Webster 2011). Although outcomes are reported as ulcers developed, rather than people developing ulcers, only one ulcer was assessed per person. Risk assessment using the Waterlow pressure ulcer risk assessment tool may make little or no difference to pressure ulcer incidence when compared to use of the Ramstadius pressure ulcer risk assessment tool (Waterlow group: 7.5%, n = 31/411; Ramstadius group: 5.4%, n = 22/410; RR 1.41, 95% CI 0.83 to 2.39; low‐certainty evidence, downgraded once for risk of bias and once for imprecision) (Analysis 5.1; Table 5).
5.1. Analysis.
Comparison 5 Waterlow pressure ulcer risk assessment compared with Ramstadius pressure ulcer risk assessment, Outcome 1 Pressure ulcer incidence.
Secondary outcome: severity of new pressure ulcers
Risk assessment using the Waterlow pressure ulcer risk assessment tool may make little or no difference to pressure ulcer severity when compared to use of the Ramstadius pressure ulcer risk assessment tool (Stage 1 ulcer severity: Waterlow group: 5.1%, n = 21/411; Ramstadius group: 4.4%, n = 18/410; RR 1.16, 95% CI 0.63 to 2.15; Stage 2: Waterlow group: 2.4%, n = 10/411; Ramstadius group: 1.40%, n = 4/410; RR 2.49, 95% CI 0.79 to 7.89; low‐certainty evidence, downgraded once for risk of bias and once for imprecision). As above only one ulcer was assessed per person. (Analysis 5.2; Analysis 5.3; Table 5).
5.2. Analysis.
Comparison 5 Waterlow pressure ulcer risk assessment compared with Ramstadius pressure ulcer risk assessment, Outcome 2 Pressure ulcer severity ‐ Stage 1.
5.3. Analysis.
Comparison 5 Waterlow pressure ulcer risk assessment compared with Ramstadius pressure ulcer risk assessment, Outcome 3 Pressure ulcer severity ‐ Stage 2.
The following secondary outcomes were not reported in the study:
time to pressure ulcer development;
pressure ulcer prevalence.
Discussion
Summary of main results
Two eligible studies were included in this review (Saleh 2009; Webster 2011). The first study found no differences in pressure ulcer incidence when participants were risk assessed using the Braden scale compared with a risk assessment following pressure ulcer prevention training, or when risk assessment was compared with using clinical judgement alone (Saleh 2009). Similarly, the second study found no clear differences in pressure ulcer incidence, or in pressure ulcer severity, when participants were risk assessed using the Waterlow risk assessment tool, the Ramstadius risk assessment tool, or using clinical judgement (Webster 2011).
Pressure ulcer risk assessment tools are widely used in clinical practice, although not necessarily in all healthcare settings (Anthony 2008; Defloor 2005), and as such it is impossible to 'unlearn' the knowledge gained during the experience of using a risk assessment tool. This means that use of an individual's clinical judgement alone, without use of a risk assessment tool, will ultimately be influenced by prior knowledge of risk assessment tools. Thus it is possible that, within the clinical setting, risk assessment follows a structured format similar to that of the current risk assessment tools even in the absence of a paper/electronic version of the tool (Anthony 2008). One therefore might not see a difference in pressure ulcer incidence because the tool does not add to the quality of the clinical judgement. Indeed, Defloor and colleagues argue that if nurses act according to risk assessment scales, 80% of the patients would unnecessarily receive preventive measures (Defloor 2005). Furthermore, use of preventative measures impacts negatively on the predictive ability of the risk assessment tool. One may consider the presence of a pressure ulcer in an individual identified to be at risk to be a success of the risk assessment process; however, this actually indicates a failure of prevention methods (Defloor 2005). It would be interesting to determine what information is gathered using clinical judgement alone, to assess whether this matches the data collected using structured risk assessment. If there were a relationship between the two methods of assessment then a reduction in pressure ulcer incidence, due to the introduction of structured risk assessment, would not be anticipated. Thus, in the studies included in this review, it is unclear what impact prior knowledge of pressure ulcer risk assessment had on the clinical judgement of the participants and this should be borne in mind when considering the generalisability of findings to other healthcare settings.
It has been argued that pressure ulcer risk assessment is in itself not an intervention but rather a precursor to the development of an appropriate plan of care to combat or reduce the impact of the risk factors identified (Ackroyd‐Stolarz 2014). Anthony and colleagues suggest that if a risk assessment tool is working well, then a reduction in the incidence of pressure ulcers should follow (Anthony 2008). Presumably this means that the risk assessment is followed by appropriate risk intervention, and that these interventions are available and effective. It is evident from the literature, however, that this is not always the case (Moore 2012; Moore 2013). Fundamentally, risk assessment alone will make no difference unless it is followed up by an intervention to combat risk, and these interventions need to be available (Jacobson 2016). Interestingly, the method of risk assessment employed in one of our included studies did not influence the interventions offered to patients; indeed, there were no differences in measured processes of care — including use of special mattresses, documentation of an explicit pressure care plan, referral to the specialist skin integrity nurse or referral to a dietician — between the three groups (Webster 2011). Thus, although risk assessment is suggested to be a precursor to planning and implementing care, it appears that this may not always be the case.
Overall completeness and applicability of evidence
One cluster‐randomised controlled trial, which provided only very‐low certainty evidence, has explored the impact of pressure ulcer risk assessment on patient outcomes (Saleh 2009). However, methodological issues with the study make it difficult to draw firm conclusions. A further large randomised controlled trial (Webster 2011), which provided low‐certainty evidence, identified no differences in pressure ulcer incidence, or pressure ulcer severity, when patients were risk assessed using either the Waterlow risk assessment tool, the Ramstadius screening tool, or using clinical judgement. As the studies included here were within two specific clinical settings (military hospital and internal medicine or oncology) there is limited generalisability to other high‐risk groups, for example elderly residents of care homes. Therefore, as yet, there is no high‐certainty evidence from randomised controlled trials to suggest that conducting pressure ulcer risk assessment makes any difference to the number of pressure ulcers that develop. If risk assessment tools/scales continue to be used in clinical practice, in the absence of empirical knowledge regarding their effect on clinical outcomes, issues will arise concerning resource utilisation and this in turn will add to increasing healthcare costs.
Quality of the evidence
Limitations in the study design and implementation
Some methodological issues require consideration and limit the conclusions that can be drawn from this review. In Saleh 2009, randomisation was not at the individual level, but rather at the unit level, where each ward served as the unit of randomisation and all patients within the ward were in the same group. This type of randomisation is called cluster‐randomisation (Medical Research Council 2002). Cluster‐randomised trials increase efficiency and study protocol compliance whilst avoiding contamination (Donner 2004). Contamination is said to occur when an intervention is given to an individual but may affect others within the trial (Puffer 2005), or when the intervention is given by accident to the control group.
The disadvantages of cluster‐randomisation are that all the individuals in the cluster cannot be assumed to be independent of one another and, furthermore, the analysis is not at the level of randomisation but is at the group level (Elley 2004). A way to overcome the disadvantages is to allow for the effects of clustering in the analysis of the data using, for example, regression models (Hahn 2005). Normally, with individual randomisation, one would expect there to be a variance in the responses within study groups. Clustering can exert an effect on this variance yielding a correlation of responses within the clusters. When cluster‐randomisation is used, this needs to be considered during both the sample size calculation and the data analysis. The study by Saleh and colleagues was a small study and the authors did not report that they accounted for the use of cluster‐randomisation in either the sample size calculation, or in the analysis (Saleh 2009). Conversely, the study by Webster and colleagues randomised at the individual level, thereby enhancing the comparability between the study groups (Webster 2011).
Concealment of group allocation was inadequately described in Saleh 2009. Allocation concealment is a randomisation method that prevents the researcher influencing which group, experimental or control, a participant is allocated to (Higgins 2011), therein ensuring that the participant is assigned to a specific study group by chance (Higgins 2011). It has been suggested that lack of a clear description of allocation concealment leads to bias in assessing the outcome of studies (Moher 2001); the size of the effect could be overestimated and so give a false impression of the value of the intervention. In Webster 2011, computer generated, phone randomisation was used, thereby minimising the risk of selection bias.
Blinding of the study is said to be complete if the investigators, the participants, the outcome assessor and the individual analysing the data have no idea which group the participant is allocated to (Higgins 2011). In Saleh 2009, there was no reporting on blinding of the patient, the staff, the data collector or the data analyst. Whilst it would not have been feasible to blind care givers as they must know the allocation because they are conducting the risk assessment, it would have been possible to blind the outcome assessors and data analyst. In Webster 2011, they ensured that the patient was blinded to the group assignment, however the staff were not blinded, and so there is a risk of performance bias on the part of the staff. This study also ensured that the outcome assessor was blinded to group assignment, thereby minimising the risk of detection bias.
Intention‐to‐treat (ITT) analysis means that participants are analysed according to the group they were originally allocated to even if they did not adhere to the study protocol or complete the study. The rationale for using ITT analysis is two‐fold; it maintains treatment groups that are similar (apart from random variation) and therefore validates the use of randomisation and allows for handling of protocol deviations, further protecting the randomisation process (Hollis 1999). In essence, omitting those who do not complete the study from the final analysis may bias the outcomes of the study because those who do not complete may do so because of adverse effects of the intervention (Montori 2001). In Saleh 2009, there is no report of use of ITT; pressure ulcer incidence is reported for all patients in the post‐training groups, but it is not clear whether any randomised patients withdrew. Conversely, in Webster 2011, it was ensured that all the participants allocated to each group were analysed for the primary outcome, thereby minimising the risk of attrition bias.
Baseline data refers to the data collected from each participant before beginning the trial (Friedman 1996). This includes demographic information, medical condition, prognostic factors and, where appropriate, socioeconomic information. This allows the researcher to determine if participants in both arms of the study are comparable at the outset of the study (Friedman 1996), and allows those evaluating the study to determine if the characteristics of those participating in the study are similar to those normally encountered in clinical practice (Friedman 1996). In Webster 2011, details were provided on the baseline characteristics of the participants and they did not identify statistically significant differences at baseline between the study groups. In Saleh 2009, it was reported that overall the groups were not comparable at baseline for medical diagnoses, pressure ulcer prevention practices, use of barrier creams and use of vitamin supplementary therapy. This was not an issue in Webster 2011.
Indirectness of the evidence
Although the review was limited by variations in both the experimental and the control interventions the evidence was not downgraded for indirectness as it covered the population, intervention and outcomes stipulated in the protocol.
Imprecision of results
Both studies were downgraded for imprecision. In Saleh 2009, this is because of the wide confidence intervals, the small sample size and that no allowance was made for the use of cluster randomisation. In Webster 2011, imprecision arose due to the wide confidence intervals. Wide confidence intervals represent a high level of uncertainty around the effect size, consequently, further research is very likely to have an important impact on the confidence of the estimate of effect for risk assessment on pressure ulcer incidence.
Publication bias
We feel confident that our comprehensive electronic searches identified all existing, published randomised controlled trials addressing the review question. There is always the risk that there are unpublished studies available which we have not been able to locate. In line with Cochrane policy, this review will be updated again in future, and any further studies identified that meet the inclusion criteria will be included at that stage.
Potential biases in the review process
During the review process we followed clearly defined process to prevent potential bias. This included a careful literature search and the methods we used were transparent and reproducible. It is possible that trials published in journals that were outside our search strategy may have been missed. This review will be updated in the future, and any studies identified that meet the inclusion criteria will be included at that stage, in line with Cochrane policy.
Agreements and disagreements with other studies or reviews
Use of pressure ulcer risk assessment tools or scales is a component of the assessment process used to identify individuals at risk of developing a pressure ulcer, and is recommended by many international guidelines (AHCPR 1992; NPUAP/EPUAP/PPPIA 2014; NICE 2001). This review identified no RCT evidence to suggest that conducting a structured risk assessment makes any difference to pressure ulcer incidence. This finding is in keeping with previous reviews (Baris 2015; Chou 2013; Cullum 1995; He 2012; McGough 1999; Pancorbo‐Hidalgo 2006) which also found a lack of published literature that reliably assesses whether the use of a risk assessment tool reduces the incidence of pressure ulcers.
Authors' conclusions
Implications for practice.
Due to the low and very low certainty of evidence from the included studies, there is no reliable evidence to suggest that the use of structured and systematic pressure ulcer risk assessment tools reduce the incidence, or severity of pressure ulcers when compared to risk assessment using clinical judgement. Given these uncertainties, practitioners may be influenced by other guidance. For example, the NPUAP/EPUAP/PPPIA 2014 guidelines suggest that risk assessment should be undertaken using a structured approach, that is refined through the use of clinical judgment and informed by knowledge of relevant risk factors.
Implications for research.
Pressure ulcer risk assessment is an integral component of pressure ulcer prevention and is widely utilised in clinical practice. To date, there is no high‐quality evidence from randomised controlled trials to suggest that undertaking structured pressure ulcer risk assessment makes any difference to pressure ulcer incidence, when compared with risk assessment using clinical judgement. As there is limited generalisability of the findings from this review to other high‐risk groups, because both studies included were conducted in the acute care setting, studies in other settings (e.g. nursing homes) could also add knowledge to this field.
There is a need to conduct further research aimed at establishing whether undertaking risk assessment makes any difference to pressure ulcer incidence. Future research should ensure that the following are incorporated:
true randomisation;
adequate allocation concealment;
blinded outcome assessment;
intention‐to‐treat analysis;
baseline comparability of groups;
adequate sample size; and
reporting of studies in accordance with the CONSORT guidelines (Moher 2001).
What's new
| Date | Event | Description |
|---|---|---|
| 11 January 2019 | New citation required but conclusions have not changed | Updated. Conclusions unchanged. |
| 16 February 2018 | New search has been performed | New searches completed for the third update; no new studies identified. |
| 16 February 2018 | Amended | A new co‐author (Declan Patton) has joined Zena Moore, replacing Seamus Cowman who worked on the protocol and the first version of the review. 1. Plain language summary amended to current style format. 2. Information added to the 'Methods' section about 'Summary of findings' tables. 3. Description of studies amended using the headings: 'Population', 'Interventions and comparisons', 'Outcomes'. 4. GRADE 'Summary of findings' tables included and GRADE assessment incorporated into the results. 5. Narrative in 'Quality of the evidence' amended using the headings: 'Limitations in study design and implementation', 'Indirectness of evidence', 'Imprecision of results', 'Publication bias.' |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 19 December 2013 | New search has been performed | New searches completed for the second update, one new study included. |
| 19 December 2013 | New citation required and conclusions have changed | One new study included in the review (Webster 2011). Conclusions updated. |
| 8 November 2010 | New search has been performed | New searches completed for the first update, one new study included in the review and risk of bias assessment completed (Saleh 2009). |
Acknowledgements
We would like to acknowledge the contribution of the peer reviewers for previous versions of this review: Mieke Flour, Susanne Hempel, Jane Nixon, Gill Worthy and Janet Yarrow, and for this current update: Zhenmi Liu, Joan Webster, Teresa Lopez and Jo Dumville. We would also like to thank copy‐editors Elizabeth Royle and Jessica Sharp.
We acknowledge the contribution of Seamus Cowman, who was involved with the conception and design of the original review and was a co‐author of the previous review.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Pressure Ulcer EXPLODE ALL AND INREGISTER 2 (pressure next (ulcer* or sore* or injur* or wound*)) AND INREGISTER 3 (decubit* next (ulcer* or sore*)) AND INREGISTER 4 ((ulcer* or sore*) next decubit*) AND INREGISTER 5 #1 OR #2 OR #3 OR #4 AND INREGISTER 6 MESH DESCRIPTOR Risk Assessment EXPLODE ALL AND INREGISTER 7 MESH DESCRIPTOR Nursing Assessment EXPLODE ALL AND INREGISTER 8 MESH DESCRIPTOR Risk Factors EXPLODE ALL AND INREGISTER 9 (risk* near2 assess*) AND INREGISTER 10 PURAS AND INREGISTER 11 (anderson or braden or norton or knoll or waterlow or medley or maelor or arnold or gosnell or birty or shape or purpose t or glamorgan or ppupet or hunters hill or hunter's hill or ramstadius or sappire or cornell or douglas ward or psps walsall or cubbin or comhon or sunderland or calculate or scipus or dupa or interRAI or chailey) near3 (risk* or tool* or score* or scoring or scale* or instrument* or checklist* or calculat*) AND INREGISTER119 12 (risk* or predict*) near3 (tool* or score* or scoring or scale* or instrument* or checklist* or calculat*) AND INREGISTER153 13 #6 or #7 or #8 OR #9 OR #10 OR #11 or #12 AND INREGISTER 19 #5 AND #13 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Pressure Ulcer] explode all trees #2 (pressure next (ulcer* or sore* or injur* or wound*)):ti,ab,kw #3 (decubit* next (ulcer* or sore*)):ti,ab,kw #4 ((bed next sore*) or bedsore*):ti,ab,kw #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Risk Assessment] explode all trees #7 MeSH descriptor: [Nursing Assessment] explode all trees #8 MeSH descriptor: [Risk Factors] explode all trees #9 (risk* near/2 assess*):ti,ab,kw #10 PURAS:ti,ab,kw #11 (anderson or braden or norton or knoll or waterlow or medley or maelor or arnold or gosnell or birty or shape or purpose t or glamorgan or ppupet or hunters hill or hunter's hill or ramstadius or sappire or cornell or douglas ward or psps walsall or cubbin or comhon or sunderland or calculate or scipus or dupa or interRAI or chailey) near/3 (risk* or tool* or score* or scoring or scale* or instrument* or checklist* or calculat*):ti,ab,kw #12 ((risk* or predict*) near/3 (tool* or score* or scoring or scale* or instrument* or checklist* or calculat*)):ti,ab,kw #13 #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 #5 and #13
Ovid MEDLINE
1 exp Pressure Ulcer/ 2 (pressure adj (ulcer* or sore* or injur* or wound*)).ab,ti. 3 (decubit* adj (ulcer* or sore*)).ab,ti. 4 ((bed adj sore*) or bedsore*).ab,ti. 5 1 or 2 or 3 or 4 6 exp Risk Assessment/ 7 exp Nursing Assessment/ 8 exp Risk Factors/ 9 (risk* adj2 assess*).ab,ti. 10 PURAS.ab,ti. 11 ((anderson or braden or norton or knoll or waterlow or medley or maelor or arnold or gosnell or birty or shape or purpose t or glamorgan or ppupet or hunters hill or hunter's hill or ramstadius or sappire or cornell or douglas ward or psps walsall or cubbin or comhon or sunderland or calculate or scipus or dupa or interRAI or chailey) adj3 (risk* or tool* or score* or scoring or scale* or instrument* or checklist* or calculat*)).ab,ti. 12 ((risk* or predict*) adj3 (tool* or score* or scoring or scale* or instrument* or checklist* or calculat*)).ab,ti. 13 6 or 7 or 8 or 9 or 10 or 11 or 12 14 5 and 13 15 randomised controlled trial.pt. 16 controlled clinical trial.pt. 17 randomi?ed.ab. 18 placebo.ab. 19 clinical trials as topic.sh. 20 randomly.ab. 21 trial.ti. 22 or/15‐21 23 exp animals/ not humans.sh. 24 22 not 23 25 14 and 24
Ovid Embase
1 exp decubitus/ 2 (pressure adj (ulcer* or sore* or injur* or wound*)).ab,ti. 3 (decubit* adj (ulcer* or sore*)).ab,ti. 4 (bedsore* or bed sore*).ab,ti. 5 1 or 2 or 3 or 4 6 exp risk assessment/ 7 exp nursing assessment/ 8 exp risk factor/ 9 (risk* adj2 assess*).ab,ti. 10 PURAS.ab,ti. 11 ((anderson or braden or norton or knoll or waterlow or medley or maelor or arnold or gosnell or birty or shape or purpose t or glamorgan or ppupet or hunters hill or hunter's hill or ramstadius or sappire or cornell or douglas ward or psps walsall or cubbin or comhon or sunderland or calculate or scipus or dupa or interRAI or chailey) adj3 (risk* or tool* or score* or scoring or scale* or instrument* or checklist* or calculat*)).ab,ti. 12 ((risk* or predict*) adj3 (tool* or score* or scoring or scale* or instrument* or checklist* or calculat*)).ab,ti. 13 6 or 7 or 8 or 9 or 10 or 11 or 12 14 5 and 13 15 Randomized controlled trials/ 16 Single‐Blind Method/ 17 Double‐Blind Method/ 18 Crossover Procedure/ 19 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 20 (doubl* adj blind*).ti,ab. 21 (singl* adj blind*).ti,ab. 22 or/15‐21 23 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 24 human/ or human cell/ 25 and/23‐24 26 23 not 25 27 22 not 26 28 14 and 27
EBSCO CINAHL Plus
S28 S14 AND S27 S27 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 S26 TI allocat* random* or AB allocat* random* S25 MH "Quantitative Studies" S24 TI placebo* or AB placebo* S23 MH "Placebos" S22 TI random* allocat* or AB random* allocat* S21 MH "Random Assignment" S20 TI randomi?ed control* trial* or AB randomi?ed control* trial* S19 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S18 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S17 TI clinic* N1 trial* or AB clinic* N1 trial* S16 PT Clinical trial S15 MH "Clinical Trials+" S14 S5 AND S13 S13 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S12 TI ( ((risk* or predict*) n3 (tool* or score* or scoring or scale* or instrument* or checklist* or calculat*)) ) OR AB ( ((risk* or predict*) n3 (tool* or score* or scoring or scale* or instrument* or checklist* or calculat*)) ) S11 TI ( (anderson or braden or norton or knoll or waterlow or medley or maelor or arnold or gosnell or birty or shape or purpose t or glamorgan or ppupet or hunters hill or hunter's hill or ramstadius or sappire or cornell or douglas ward or psps walsall or cubbin or comhon or sunderland or calculate or scipus or dupa or interRAI or chailey) N3 (risk* or tool* or score* or scoring or scale* or instrument* or checklist* or calculat*) ) OR AB ( (anderson or braden or norton or knoll or waterlow or medley or maelor or arnold or gosnell or birty or shape or purpose t or glamorgan or ppupet or hunters hill or hunter's hill or ramstadius or sappire or cornell or douglas ward or psps walsall or cubbin or comhon or sunderland or calculate or scipus or dupa or interRAI or chailey) N3 (risk* or tool* or score* or scoring or scale* or instrument* or checklist* or calculat*) ) S10 TI PURAS OR AB PURAS S9 TI (risk* N2 assess*) OR AB (risk* N2 assess*) S8 (MH "Risk Factors+") S7 (MH "Nursing Assessment") S6 (MH "Risk Assessment") S5 (S1 OR S2 OR S3 OR S4) S4 TI decubit* OR AB decubit* S3 TI ( (bed sore* or bedsore*) ) OR AB ( (bed sore* or bedsore*) ) S2 TI ( (pressure ulcer* or pressure sore* or pressure injur* or pressure wound*) ) OR AB ( (pressure ulcer* or pressure sore* or pressure injur* or pressure wound*) ) S1 (MH "Pressure Ulcer+")
Clinical trial registries (ClinicalTrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform, EU Clinical Trials Register)
Pressure ulcer OR pressure sore OR pressure injury OR bed sore OR decubitus ulcer OR skin ulcer AND Risk assessment OR Braden OR Waterlow OR Ramstadius OR Clinical Judgement OR Norton
Appendix 2. Cochrane tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size.
'As‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 3. Risk of bias criteria ‐ cluster controlled trials
In cluster‐randomised trials, particular biases to consider include recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; and comparability with individually randomised trials.
Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomly assigned, as knowledge of whether each cluster is an 'intervention' or 'control' cluster could affect the types of participants recruited.
Cluster‐randomised trials often randomly assigned all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomly assigned, there is a possibility of chance baseline imbalance between randomly assigned groups, in terms of the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
Occasionally, complete clusters are lost from a trial and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may lead to risk of bias in cluster randomised trials.
Many cluster‐randomised trials are analysed by incorrect statistical methods, without taking the clustering into account. Such analyses create a 'unit of analysis error' and produce overly precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
In a meta‐analysis including both cluster and individually randomised trials, or including cluster randomised trials with different types of clusters, possible differences between the intervention effects estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than vaccine applied to only half of the people. Another example is provided by a Cochrane Review of hip protectors (Hahn 2005). The cluster trials showed a large positive effect, whereas individually randomised trials did not show clear benefit. One possibility is that there was a 'herd effect' in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such 'contamination' would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and 'herd effects' may be different for different types of clusters.
Data and analyses
Comparison 1. Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement and training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.53, 1.77] |
Comparison 2. Braden pressure ulcer risk assessment and training compared with pressure ulcer risk assessment using clinical judgement alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.77, 2.68] |
Comparison 3. Waterlow risk assessment compared with pressure ulcer risk assessment using clinical judgement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.68, 1.81] |
| 2 Pressure ulcer severity ‐ Stage 1 | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.58, 1.90] |
| 3 Pressure ulcer severity ‐ Stage 2 | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.50, 3.13] |
Comparison 4. Ramstadius pressure ulcer risk assessment compared with pressure ulcer risk assessment using clinical judgement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 1 | 820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.35] |
| 2 Pressure ulcer severity ‐ Stage 1 | 1 | 820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.48, 1.68] |
| 3 Pressure ulcer severity ‐ Stage 2 | 1 | 820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.15, 1.65] |
Comparison 5. Waterlow pressure ulcer risk assessment compared with Ramstadius pressure ulcer risk assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.83, 2.39] |
| 2 Pressure ulcer severity ‐ Stage 1 | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.63, 2.15] |
| 3 Pressure ulcer severity ‐ Stage 2 | 1 | 821 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [0.79, 7.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Saleh 2009.
| Methods | Randomised controlled trial, allocation by ward (cluster). No details provided regarding the randomisation process. | |
| Participants | Patients in a military hospital with a Braden score of less than or equal to 18. For the Braden group, 74 patients post‐test. For the training group, 76 patients post‐test. For the clinical judgement group, 106 patients post‐test. | |
| Interventions | Group A: Braden risk assessment and training (n = 74) Group B: risk assessment using clinical judgement and training (n = 76) Group C: risk assessment using clinical judgement alone (n = 106) | |
| Outcomes | Pressure ulcers developed | |
| Notes | The groups were not comparable at baseline for medical diagnoses, pressure ulcer prevention practices, use of barrier creams and use of vitamin supplementary therapy. The type of mattress the patients lay on was not the same for all participants. The repositioning schedules for each participant were not the same. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "for pragmatism, this study randomly allocated nine wards into three groups." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "for pragmatism, this study randomly allocated nine wards into three groups." |
| Blinding of participants (performance bias) | High risk | There is no mention of blinding of the participants. |
| Blinding of personnel (performance bias) All outcomes | High risk | There is no mention of blinding of the staff. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There is no mention of blinding of the data collector or the data analyst. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Pressure ulcer incidence is reported for all patients in the post‐test groups, however a number of patients were excluded and it is unclear if these patients were excluded before the start of the study, i.e. that they did not meet inclusion criteria. This is not specifically stated by the authors. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available but the important outcome measures stated in the methods section are reported in the results. |
| Other bias | High risk | Use of cluster‐randomisation, i.e. wards were the unit of randomisation, not patients. No allowance for this is made in the sample size calculation and the data analysis. The groups were not comparable at baseline for medical diagnoses, pressure ulcer prevention practices, use of barrier creams and use of vitamin supplementary therapy. The type of mattress used was not the same for all participants. The repositioning schedules for each participant was not the same. |
| Recruitment bias All outcomes | Low risk | All patients in each cluster, meeting the inclusion criteria, were included. |
| Baseline imbalance All outcomes | High risk | The groups were not comparable at baseline for medical diagnoses, pressure ulcer prevention practices, use of barrier creams and use of vitamin supplementary therapy. The type of mattress the patients lay on was not the same for all participants. The repositioning schedules for each participant were not the same. |
| Loss of clusters All outcomes | Low risk | No loss of clusters |
| Incorrect analysis | High risk | The authors did not report that they accounted for the use of cluster‐randomisation in either the sample size calculation, or in the analysis |
Webster 2011.
| Methods | A single‐blind randomised controlled trial. | |
| Participants | 1231 patients admitted to internal medicine or oncology wards | |
| Interventions | Participants allocated to either of the following. A. Waterlow (n = 411) B. Ramstadius (n = 410) C. Risk assessment using clinical judgement (n = 410) |
|
| Outcomes | Incidence of hospital acquired pressure ulcers. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomised list was used." |
| Allocation concealment (selection bias) | Low risk | Quote: "A phone randomisation method was used." |
| Blinding of participants (performance bias) | Low risk | Quote: "The patient was blinded to group assignment" |
| Blinding of personnel (performance bias) All outcomes | High risk | Quote: "The only requirement of staff in the participating wards was to use only the instrument found in the chart". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome assessor was blinded to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants allocated to each group were analysed for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | The authors report all outcomes alluded to in the paper. |
| Other bias | Low risk | The study was funded by research grants from the Queensland Nursing Council, the Royal Brisbane and Women’s Hospital Private Practice Fund, the Royal Brisbane and Women’s Hospital Research Foundation and a Queensland Health Nursing Research grant. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anthony 1998 | The study used the wrong population, as some patients already had pressure ulcers. |
| Bale 1995 | Not an RCT |
| Bergstrom 1998 | Not an RCT: random allocation to Braden, but no control group. The authors are focusing on sensitivity and specificity. |
| Chan 1997 | Not an RCT, descriptive statistics only. |
| Defloor 2005 | Clinical trial, random allocation to turning group, but not to risk assessment tool, patients assessed using Braden and Norton. Looked at the sensitivity and specificity of Braden and Norton among the 2 groups: turning and no turning. |
| Gunningberg 1999 | Clinical trial but no random allocation. |
| Gunningberg 2001 | This was an audit of nursing records and not an RCT. |
| Hodge 1990 | Quasi‐experimental |
| Lyne 1999 | This was a retrospective chart analysis and was not an RCT. |
| Salvadena 1992 | Only looked at sensitivity and specificity |
RCT: randomised controlled trial
Differences between protocol and review
Changes to the 'Methods' section have been made as follows: inclusion of cluster‐RCTs, removal of the secondary outcome "sensitivity, specificity and ease of use of the various risk assessment tools", inclusion of additional criteria for assessment of risk of bias in cluster‐RCTs and inclusion of GRADE assessment of the certainty of the evidence.
Contributions of authors
Zena Moore: conceived the review; designed and co‐ordinated the review update; produced the first draft of the review update; contributed to writing or editing the review update; approved the final review update prior to submission; and is a guarantor of the review update.
Declan Patton: designed and co‐ordinated the review update; produced the first draft of the review update; contributed to writing or editing the review update; and approved the final review update prior to submission.
Contributions of editorial base
Nicky Cullum and Liz McInnes: edited the previous versions of the review, advised on methodology, interpretation and review content. Approved the final review prior to submission.
Gill Norman (Editor): edited this updated version of the review, advised on methodology, interpretation and review content. Approved the final review prior to submission.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process. Advised on interpretation and content. Edited this updated review.
Naomi Shaw (Information Specialist): designed the search strategy, ran the searches and edited the search methods section.
Ursula Gonthier (Editorial Assistant): edited the Plain Language Summary and reference sections of this update.
Sources of support
Internal sources
The Faculty Board, Faculty of Nursing & Midwifery, RCSI, Dublin, Ireland.
Royal College of Surgeons in Ireland, Ireland.
External sources
Health Research Board of Ireland, Ireland.
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Zena Moore: has received an honorarium for speaking at professional meetings for Smith & Nephew and Molnlycke.
Declan Patton: none known.
Joan Webster (peer reviewer) is an author of one of the included studies in this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Saleh 2009 {published data only}
- Saleh M, Anthony D, Parboteeah S. The impact of pressure ulcer risk assessment on patient outcomes among hospitalised patients. Journal of Clinical Nursing 2009;18(13):1923‐9. [DOI] [PubMed] [Google Scholar]
Webster 2011 {published data only}
- Webster J, Coleman K, Mudge A, Marquart L, Gardner G, Stankiewicz M, et al . Pressure ulcers: effectiveness of risk‐assessment tools. A randomised controlled trial (the ULCER trial). BMJ Quality and Safety 2011;20(4):297‐306. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anthony 1998 {published data only}
- Anthony D, Barnes J, Unsworth J. An evaluation of current risk assessment scales for decubitus ulcer in general inpatients and wheelchair users. Clinical Rehabilitation 1998;12(2):136‐42. [DOI] [PubMed] [Google Scholar]
Bale 1995 {published data only}
- Bale S, Finlay I, Harding KG. Pressure sore prevention in a hospice. Journal of Wound Care 1995;4(10):465‐8. [DOI] [PubMed] [Google Scholar]
Bergstrom 1998 {published data only}
- Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: a multisite study of the predictive validity of the Braden scale. Nursing Research 1998;47(5):261‐9. [DOI] [PubMed] [Google Scholar]
Chan 1997 {published data only}
- Chan WH, Chow KW, French P, Lai YS, Tse LK. Which pressure sore risk calculator? A study of the effectiveness of the Norton scale in Hong Kong. International Journal of Nursing Studies 1997;34(2):165‐9. [DOI] [PubMed] [Google Scholar]
Defloor 2005 {published data only}
- Defloor T, Grypdonck MF. Pressure ulcers: validation of two risk assessment scales. Journal of Clinical Nursing 2005;14(3):378‐82. [DOI] [PubMed] [Google Scholar]
Gunningberg 1999 {published data only}
- Gunningberg L, Lindholm C, Carlsson M, Sjode PO. Implementation of risk assessment and classification of pressure ulcers as quality indicators for patients with hip fractures. Journal of Clinical Nursing 1999;8(4):396‐406. [DOI] [PubMed] [Google Scholar]
Gunningberg 2001 {published data only}
- Gunningberg L, Lindohlm C, Carlsson M, Sjoden PO. Reduced incidence of pressure ulcers in patients with hip fractures: a 2 year follow up of quality indicators. International Journal for Quality in Health Care 2001;13(5):399‐407. [DOI] [PubMed] [Google Scholar]
Hodge 1990 {published data only}
- Hodge J, Mounter J, Garner G, Rowley G. Clinical trial of the Norton scale in acute care settings. Australian Journal of Advanced Nursing 1990;8(1):39‐48. [PubMed] [Google Scholar]
Lyne 1999 {published data only}
- Lyne P, Papanikolaou P, Lycett E. Pressure sore risk assessment: preliminary report of a study using multivariate methods to define and weight risk factors. Clinical Effectiveness in Nursing 1999;3(3):136‐8. [Google Scholar]
Salvadena 1992 {published data only}
- Salvadalena GD, Snyder ML, Brogdon KE. Clinical trial of the Braden scale on an acute care medical unit. Journal of Enterostomal Therapy Nursing 1992;19(5):160‐5. [PubMed] [Google Scholar]
Additional references
Ackroyd‐Stolarz 2014
- Ackroyd‐Stolarz S. Improving the prevention of pressure ulcers as a way to reduce health care expenditures. Canadian Medical Association Journal 2012;186(10):E370‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
AHCPR 1992
- US Agency for Health Care Policy and Research (AHCPR). Pressure ulcers in adults: prediction and prevention. Clinical practice guidelines number 3. AHCPR publication number 92‐0047. Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services. Rockville, Maryland 1992.
Anthony 2008
- Anthony D, Parboteeah S, Saleh M, Papanikolau P. Norton, Waterlow and Braden scores: a review of the literature and a comparison between the scores and clinical judgement. Journal of Clinical Nursing 2008;17(5):646‐53. [DOI] [PubMed] [Google Scholar]
Baris 2015
- Baris N, Karabacak BG, Alpar SE. The use of the Braden scale in assessing pressure ulcers in turkey: a systematic review. Advances in Skin & Wound Care 2015;28(8):359‐57. [DOI] [PubMed] [Google Scholar]
Bauer 2016
- Bauer K, Rock K, Nazzal M, Jones O, Qu W. Pressure ulcers in the United States' inpatient population from 2008 to 2012: results of a retrospective nationwide study. Ostomy/Wound Management 2016;62:30‐8. [PubMed] [Google Scholar]
Black 2007
- Black J, Baharestani MM, Cuddigan J, Dorner B, Edsberg L, Langemo D, et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Advances in Skin & Wound Care 2007;20(5):269‐74. [DOI] [PubMed] [Google Scholar]
Braden 1987
- Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabilitation Nursing 1987;12(1):8‐16. [DOI] [PubMed] [Google Scholar]
Chou 2013
- Chou R, Dana T, Bougatsos C, Blazina I, Starmer AJ, Reitel K, et al. Pressure ulcer risk assessment and prevention: a systematic comparative effectiveness review. Annals of Internal Medicine 2013;159(1):28‐38. [DOI] [PubMed] [Google Scholar]
Cullum 1995
- Cullum N, Deeks J, Fletcher A. The prevention and treatment of pressure sores. Effective Health Care Bulletin 1995;2(1):1‐16. [Google Scholar]
Defloor 2004
- Defloor T, Grypdonck MF. Validation of pressure ulcer risk assessment scales: a critique. Journal of Advanced Nursing 2004;48(6):613‐21. [DOI] [PubMed] [Google Scholar]
Demarre 2015
- Demarre L, Lanker A, Hecke A, Verhaeghe S, Grypdonck M, Lemey J, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. International Journal of Nursing Studies 2015;52:1754‐74. [DOI] [PubMed] [Google Scholar]
Donner 2004
- Donner A, Klar N. Pitfalls of and controversies in cluster randomisation trials. American Journal of Public Health 2004;94(3):416‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elley 2004
- Elley CR, Chondros P, Kerse NM, Primary Care Alliance for Clinical Trials (PACT) Network. Randomised trials ‐ cluster versus individual randomisation. Australian Family Physician 2004;33(9):759‐63. [PubMed] [Google Scholar]
Fox 2002
- Fox C. Living with a pressure ulcer: a descriptive study of patient's experiences. British Journal of Community Nursing Wound Care 2002;10(6 Suppl):10, 12, 14, 16, 20, 22. [DOI] [PubMed] [Google Scholar]
Friedman 1996
- Friedman LM, Demets DL, Furberg CD. Baseline assessment. In: Friedman LM, Demets DL, Furberg CD editor(s). Fundamentals of Clinical Trials. 3rd Edition. Basel, Switzerland: Birkhäuser, 1996. [Google Scholar]
Gallagher 2008
- Gallagher P, Barry P, Hartigan I, McCluskey P, O'Connor K, O'Connor M. Prevalence of pressure ulcers in three university teaching hospitals in Ireland. Journal of Tissue Viability 2008;17(4):103‐09. [DOI] [PubMed] [Google Scholar]
Gould 2002
- Gould D, Goldstone L, Gammon J, Kelly D, Maidwell A. Establishing the validity of pressure ulcer risk assessment scales: a novel approach using illustrated patient scenarios. International Journal of Nursing Studies 2002;39(2):215‐28. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version Last updated December 2015. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haalboom 1999
- Haalboom JR, Boer J, Buskens E. Risk‐assessment tools in the prevention of pressure ulcers. Ostomy/Wound Management 1999;45(2):20‐34. [PubMed] [Google Scholar]
Hahn 2005
- Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Medical Research Methodology 2005;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halfens 2000
- Halfens RJ, Achterberg T, Bal RM. Validity and reliability of the Braden scale and the influence of other risk factors: a multi‐centre prospective study. International Journal of Nursing Studies 2000;37(4):313‐9. [DOI] [PubMed] [Google Scholar]
He 2012
- He W, Liu P, Chen HL. The Braden Scale cannot be used alone for assessing pressure ulcer risk in surgical patients: a meta‐analysis. Ostomy/Wound Management 2012;58(2):34‐40. [PubMed] [Google Scholar]
Henoch 2003
- Henoch I, Gustafsson M. Pressure ulcers in palliative care: development of a hospice pressure ulcer risk assessment scale. International Journal of Palliative Nursing 2003;9(11):474‐84. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistencies in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopkins 2006
- Hopkins A, Dealey C, Bale S, Defloor T, Worboys F. Patient stories of living with a pressure ulcer. Journal of Advanced Nursing 2006;56(4):345‐53. [DOI] [PubMed] [Google Scholar]
Jacobson 2016
- Jacobson TM, Thompson SL, Halvorson AM, Zeitler K. Enhancing documentation of pressure ulcer prevention interventions: a quality improvement strategy to reduce pressure ulcers. Journal of Nursing Care Quality 2016;31(3):207‐14. [DOI] [PubMed] [Google Scholar]
Khor 2014
- Khor HM, Tan J, Saedon NI, Kamarussaman SB, Chin AV, Poi PJ, et al. Determinants of mortality among older adults with pressure ulcers. Archives of Gerontology and Geriatrics 2014;59:536‐41. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manzano 2014
- Manzano F, Perez‐Perez AM, Martinez‐Ruiz S, Garrido‐Colmenero C, Roldan D, JIiminez‐Quintana Mdel M, et al. Hospital‐acquired pressure ulcers and risk of hospital mortality in intensive care patients on mechanical ventilation. Journal of Evaluation in Clinical Practice 2014;20:362‐8. [DOI] [PubMed] [Google Scholar]
McGough 1999
- McGough A. A systematic review of the effectiveness of risk assessment tools in the prevention and treatment of pressure sores [Masters thesis]. York, UK: University of York, 1999. [Google Scholar]
Medical Research Council 2002
- Medical Research Council. Cluster randomised trials: methodological and ethical considerations. November 2002. www.cebma.org/wp‐content/uploads/Cluster‐randomised‐trials‐Methodological‐and‐ethical‐considerations.pdf (accessed 19 February 2018).
Moher 2001
- Moher D, Schulz KF, Altman DG, CONSORT GROUP (Consolidated Standards of Reporting Trials). The consort statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Annals of Internal Medicine 2001;134(8):656‐62. [DOI] [PubMed] [Google Scholar]
Montori 2001
- Montori VM, Guyatt GH. Intention‐to‐treat principle. Canadian Medical Association Journal 2001;165(10):1339‐41. [PMC free article] [PubMed] [Google Scholar]
Moore 2011
- Moore Z, Cowman S, Conroy RM. A randomised controlled clinical trial of repositioning, using the 30° tilt, for the prevention of pressure ulcers. Journal of Clinical Nursing 2011;20(17‐18):2633‐44. [DOI] [PubMed] [Google Scholar]
Moore 2012
- Moore Z, Cowman S. Pressure ulcer prevalence and prevention practices in care of the older person in the Republic of Ireland. Journal of Clinical Nursing 2012;21(3‐4):362–71. [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore Z, Johansen E, Etten M. A review of PU prevalence and incidence across Scandinavia, Iceland and Ireland (part I). Journal of Wound Care 2013;22:1‐7. [DOI] [PubMed] [Google Scholar]
NICE 2001
- National Institute for Clinical Excellence (NICE). Pressure ulcer risk assessment and prevention. Inherited Clinical Guideline B. April 2001. www.judy‐waterlow.co.uk/downloads/clinical‐guideline‐pressure‐sore‐guidance‐nice.pdf (accessed 19 February 2018).
Norton 1975
- Norton D, McLaren R, Exton‐Smith AN. An Investigation of Geriatric Nursing Problems in Hospital. 2nd Edition. New York: Churchill Livingstone, 1975. [Google Scholar]
NPUAP 1998
- National Pressure Ulcer Advisory Panel (NPUAP). Pressure ulcers prevalence, cost and risk assessment: consensus development conference statement. Decubitus 1998;2(2):24‐8. [PubMed] [Google Scholar]
NPUAP/EPUAP/PPPIA 2014
- National Pressure Ulcer Advisory Panel (NPUAP), European Pressure Advisory Panel (EPUAP), Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and treatment of pressure ulcers: quick reference guide. 2014. www.npuap.org/wp‐content/uploads/2014/08/Updated‐10‐16‐14‐Quick‐Reference‐Guide‐DIGITAL‐NPUAP‐EPUAP‐PPPIA‐16Oct2014.pdf (accessed 19 February 2018).
Pancorbo‐Hidalgo 2006
- Pancorbo‐Hidalgo PL, Garcia‐Fernandez FP, Lopez‐Medina IM, Alvarez‐Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. Journal of Advanced Nursing 2006;54(1):94‐110. [DOI] [PubMed] [Google Scholar]
Puffer 2005
- Puffer S, Torgerson DJ, Watson J. Cluster randomized controlled trials. Journal of Evaluation in Clinical Practice 2005;11(5):479‐83. [DOI] [PubMed] [Google Scholar]
Ramstadius 2000
- Ramstadius B. Preventing institution acquired pressure ulcers. Australian Nursing Journal 2000;7(10):34. [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JP. Chapter 13: Including non‐randomized studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Rycroft‐Malone 2000
- Rycroft‐Malone J, McInnes E. Risk Assessment and Prevention of Pressure Ulcers. Technical Report. London: RCN Publishing, 2000. [Google Scholar]
Schoonhoven 2002
- Schoonhoven L, Haalboom JR, Bousema MT, Algra A, Grobbee DE, Grypdonck MH, et al. Prospective cohort study of routine use or risk assessment scales for prediction of pressure ulcers. BMJ 2002;325(7368):797‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Scott 2006
- Scott JR, Gibran NS, Engrav LH, Mack CD. Rivara FP. Incidence and characteristics of hospitalized patients with pressure ulcers: State of Washington, 1987 to 2000. Plastic and Reconstructive Surgery 2006;117(2):630‐4. [DOI] [PubMed] [Google Scholar]
SIGN 2018
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/search‐filters.html (accessed 6 July 2018).
Spilsbury 2007
- Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon H, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. Journal of Advanced Nursing 2007;57(5):494‐504. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
US Census Bureau 2018
- US Census Bureau. Population estimates, July 1, 2017. www.census.gov/quickfacts/fact/table/US/PST045217 (accessed 6 July 2018).
Waterlow 1985
- Waterlow J. A risk assessment card. Nursing Times 1985;81(49):51‐5. [PubMed] [Google Scholar]
References to other published versions of this review
Moore 2007
- Moore ZEH, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006471] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore ZEH, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database of Systematic Reviews 2008, Issue 7. [DOI: 10.1002/14651858.CD006471.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore ZEH, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD006471.pub3] [DOI] [PubMed] [Google Scholar]


