Abstract
Depression is common but under-diagnosed in cancer survivors. This study characterized depressive symptoms over one year in cancer survivors and examined disease-related and psychosocial predictors of depression severity. Participants (n = 122; Mage 65.33, SD = 9.17, 98.4% male) with head and neck, esophageal, gastric, or colorectal cancers were recruited through tumor registries at two regional Veterans Administration Medical Centers. Self-report measures assessing depressive symptoms (PHQ-9), combat-related PTSD symptoms (PC-PTSD), and health-related quality of life (PROMIS) were administered at six, twelve, and eighteen months after diagnosis. Symptoms consistent with major depression were endorsed by approximately one-quarter of the sample at six (24%), twelve (22%), and eighteen (26%) months post diagnosis, with 12% of participants reporting consistently significant depressive symptoms. In multivariate modeling, significant predictors of depression at eighteen months included prior depressive symptoms (β = .446, p < 0.001) and current pain interference (β = .231, p = .003). The present findings suggest that major depression is common and persistent one year following cancer diagnosis. Attention to pain management and routine monitoring of mood symptoms is critical to reducing risk of depression in cancer survivors.
Keywords: Cancer, colorectal, depression, esophageal, head and neck, survivor
Introduction
Psychological distress following initial diagnosis of cancer is well-documented in the literature (Linden, Vodermaier, MacKenzie, & Greig, 2012; Mehnert et al., 2014), but less is known about the manifestation of psychological distress throughout the course of cancer survivorship (Cardoso, Graca, Klut, Trancas, & Papoila, 2016). The detection of clinically significant depressive symptoms among cancer survivors is an imperative given its association with morbidity and mortality (Mohile et al., 2011; Pinquart & Duberstein, 2010; Spiegel & Giese-Davis, 2003). However, despite recognition that adjustment unfolds over time in cancer patients (Brennan, 2001; Stommel, Kurtz, Kurtz, Given, & Given, 2004), mood disorders remain under-diagnosed and under-treated in cancer survivors (Institute of Medicine, 2008; Jones & Doebbeling, 2007).
Most of what is known about depression in cancer survivors comes from research focusing on breast cancer survivors (Jean & Syrjala, 2017). Most survivors do well, but a subgroup has persisting depressive symptoms (Jean & Syrjala, 2017). There are several studies examining the longitudinal trajectory of cancer all of which show persistence of depression. Among esophageal cancer patients who underwent treatment, the rate of clinically significant depression was 20% at baseline, ~36% at six months, and ~28% at 1 year following diagnosis (Bergquist, Ruth, & Hammerlid, 2007). Stommel and colleagues (Stommel et al., 2004) found that while symptoms of low mood and anhedonia leveled off over one year, depressive symptoms characterized by the absence of well-being and positive affect remained elevated and stable. Deshields and colleagues (Deshields, Tibbs, Fan, & Taylor, 2006) found persistently elevated depressive symptoms over three years among breast cancer patients.
Risk factors for depression in cancer survivors are multifactorial. Consistent evidence points to the role of physical symptoms and functioning in predicting depression in cancer survivors (de Leeuw et al., 2001; Stommel et al., 2004). Stommel and colleagues (Stommel et al., 2004) found that physical functioning and symptom severity predicted depressive symptoms over time in older adults with breast, colon, lung, or prostate cancer. Additional disease-related factors have been identified as risk factors of depression, including cancer site, cancer stage, and presence of co-morbid conditions (Cardoso et al., 2016; de Leeuw et al., 2001; Kurtz, Kurtz, Stommel, Given, & Given, 2002; Pereira, Figueiredo, & Fincham, 2012a; Stommel et al., 2004). Psychosocial factors have also been examined with low social support (de Leeuw et al., 2001; Kurtz et al., 2002; Uchitomi et al., 2000) and prior depression and anxiety symptoms found to be significant predictors of depression in cancer survivors (de Leeuw et al., 2001; Korfage, Essink-Bot, Janssens, Schröder, & de Koning, 2006). Despite the supporting literature, few studies have examined the impact of multiple disease and psychosocial factors on depressive symptoms in the same model over time. Finally, pain in cancer survivorship has been increasingly recognized as a significant problem following treatment (Moye et al., 2014; Polomano & Farrar, 2006). Despite the documented association between chronic pain and mood disorders in the general population (Kroenke et al., 2011), few studies have examined the impact of pain on depression symptoms in cancer survivors.
Longitudinal examination of depressive symptoms in cancer survivors is essential, to identify factors important to survivors’ well-being and reduce the substantial morbidity and mortality associated with depression. The first aim of the study was to characterize rates of individual depressive symptoms and major depression at six, twelve, and eighteen months following cancer diagnosis in a unique sample drawn from the Veterans Health Administration. The second aim of the study was to extend prior findings by examining empirically and conceptually derived predictors of depression including patient demographics, disease characteristics, and psychosocial factors in a single model.
Methods
Participants
Participants were recruited from the tumor registries from VA Medical Centers in Boston and Houston for an observational cohort study, and interviewed at six (n = 170), twelve (n = 145), and eighteen months post diagnosis (n = 122). Sample size was determined on the basis of power analyses; complete protocol methods including non-responder information are described elsewhere (Naik et al., 2013). Eligibility criteria includes a diagnosis of head and neck, esophageal, gastric, or colorectal cancer; treatment comprised of surgery, chemotherapy, radiation therapy, or a combination of these; and considered ‘cancer survivors.’ A broad definition of cancer survivor was applied, consistent with the National Cancer Institute (NCI), stating that ‘an individual is considered a cancer survivor from the time of diagnosis, through the balance of his or her life.’ In this study we operationalized this definition and recruited participants of all cancer stages as long as the individual was not referred to hospice and palliative care as identified through a case by case chart review. This analysis focuses on individuals with complete data at all three time periods (n = 122). Three classes of oral-digestive cancers (head and neck, eso-gastric, and colorectal) were targeted as these cancers are relatively prevalent but understudied in cancer survivors, and share some common features such as potentially disrupting eating and digestive function. Participants who had a dementia disorder or psychotic spectrum disorder were excluded. Participants were mostly male (98.4%), aged ranged from 41–88 years (M = 65.33, SD = 9.17), and a majority were 60 years of age or older (75%). Table 1 presents complete demographic data for the baseline sample.
Table 1.
Sample Characteristics.
| Variable | Statistic |
||
|---|---|---|---|
| M | SD | ||
| Age | 41–88 | 65.33 | 9.17 |
| Comorbidities | 1–9 | 3.4 | 1.87 |
| Combat PTSD symptoms | 0–4 | 0.59 | 1.27 |
| Treatment length | 1–18 | 6.84 | 5.93 |
| PROMIS Social Role Interference (T1) | 4–20 | 13.05 | 5.21 |
| PROMIS Pain Interference (T1) | 4–20 | 8.14 | 5.18 |
| n | % | ||
| Education | High school or less | 57 | 46.7 |
| College (some or graduate) | 65 | 53.3 | |
| Race | African American and other | 25 | 20.5 |
| Caucasian | 97 | 79.5 | |
| Cancer Type | Head and Neck | 42 | 34.4 |
| Colorectal | 71 | 58.2 | |
| Eso-gastric | 9 | 7.4 | |
| Cancer Stage | Stage I | 34 | 27.9 |
| Stage II | 35 | 28.7 | |
| Stage III | 29 | 23.8 | |
| Stage IV | 24 | 19.7 | |
| Treatment Type | Surgery | 96 | 78.7 |
| Chemotherapy | 68 | 55.7 | |
| Radiation | 46 | 37.7 | |
| Previous MH Treatment | Yes | 44 | 36.1 |
Note. Combat PTSD = Primary Care PTSD Screen; PROMIS = Patient-Reported Outcomes Measurement Information System.
Measures
Demographics
Participants reported their age, gender, racial/ethnic identity, and level of education.
Baseline health characteristics
To assess physical comorbidity, a comorbidity score was created via electronic medical record extraction. Outpatient ICD-9 data were extracted and sorted to pull those diagnoses used in the Charlson Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987). A score of 1 was assigned for each relevant diagnosis. As a measure of social support, participants rated their current level of emotional support by answering ‘how often do you have someone you can count on to listen to you when you need to talk?’ rated on a 5 point scale from rated 0 (none of the time) to 4 (all of the time) from the RAND Medical Outcomes Study social support survey (Hays, Sherbourne, & Mazel, 1995). To provide a measure of current combat-related Post Traumatic Stress Disorder (PTSD) symptoms, participants who experienced military combat completed the 4-item Primary Care PTSD Screen (PC-PTSD; Prins et al., 2004). Finally, to assess previous mental health treatment history participants answered (yes/no) to ‘have you ever had treatment for depression or anxiety, like counseling or medication?’
Cancer information
Information about the cancer diagnosis, including type and stage, and about treatment, specifically whether the individual received surgery, chemotherapy, or radiation, was obtained from the participants’ medical record.
Depression – current
The Patient Health Questionnaire (PHQ-9; Kroenke, Spitzer, & Williams, 2001) was used to measure current depressive symptoms in the past two weeks. This 9-item self-report scale is based on the DSM-IV diagnostic criteria for major depressive disorder (American Psychiatric Association, 2000). Participants indicated how much they had been bothered by each item using a 4-point Likert scale ranging from 0 (‘not at all’) to 3 (‘nearly every day’). In the present sample, the internal-consistency reliability at the six month interview was α = .88; twelve months α = .85; eighteen months α = .88. Consistent with the established scoring protocol for diagnosis and treatment recommendations (Kroenke et al., 2001), participants were divided into groups based on their PHQ-9 total score as follows: 0–5 ‘not depressed’; 5–9, ‘minimal symptoms’ – support, educate, return in 1 month; 10–14, ‘major depression, mild’ (or minor depression or dysthymia) – consider support, antidepressant, or psychotherapy; 15–19, ‘major depression, moderately-severe’ – start antidepressant or psychotherapy; 20+, ‘major depression, severe’ – start antidepressant and psychotherapy. In analyses, a cut-off score of 10 or higher was used to indicate provisional major depression diagnosis.
Health-related quality of life
The Patient-Reported Outcomes Measurement Information System (PROMIS-29 v1.0; PROMIS Health Organization and the PROMIS Cooperative Group) 29 item version was used to measure health related quality of life. The pain impact subscale (4 items) asked participants to rate the extent to which during the past seven days pain interferes with daily activities, work, social activities, and enjoyment of life. In the present sample, the internal-consistency reliability of the pain interference subscale was α = .97 at the six, twelve, and eighteen month interview. Satisfaction with social role (4 items) asked participants to rate the extent to which they are satisfied with their ability to perform in social roles (e.g. work, household responsibilities). In the present sample, the internal-consistency reliability for the social role interference at the six month interview was α = .95; twelve months α = .94; eighteen months α = .96. For both subscales, participants responded on a Likert scale from 1 (not at all) to 5 (very much), for a possible range of 4–20. Total scores were calculated for each subscale.
Procedures
Participants completed face-to-face interviews at six, twelve, and eighteen months following their cancer diagnosis. Most interviews occurred in person at a location convenient for the participant, usually at the local VA medical center or the patient’s home. This study was approved by the Institutional Review Boards of the VA Boston Healthcare System and the Houston VA Medical Center.
Statistical analyses
Descriptive statistics were used to describe the prevalence of depressive symptoms and clinically significant depression (PHQ-9 score > 10) at all time points. Change in depression symptoms between six and eighteen month item and total mean scores was examined using paired t-tests. Bivariate correlations between depression symptom severity (PHQ-9 scores) at all time points and study variables were calculated, to examine which variables were related to depression to include in subsequent regression analysis. Finally, to determine which sample characteristics were most associated with depression at eighteen months, after controlling for six month depression, hierarchical multivariate linear regression was used selecting only those variables significantly associated with eighteen month depression in bivariate analyses. Baseline demographic and health characteristics were entered in the first block, depression severity score at time 1 was entered in the second block, and pain and social role interference were entered in the third block. Missing data points were imputed through mean substitution. Analyses were conducted in Statistical Package for the Social Science (SPSS; version 21; IBM Corp, 2012)
Results
Descriptive data
As noted in Table 1, participants had head and neck, eso-gastric, or colorectal cancer, with relatively equal representation across cancer stages I-IV. On average participants had three comorbidities, and most described a high degree of social support. About one-third (35%) described previous mental health treatment; one-fifth (20%) reported current combat PTSD symptoms.
Satisfaction with social role and pain impact scores at 6 months appear in Table 1. Social role satisfaction did not change over time (six months M = 13.05, SD = 5.21; twelve months M = 13.45, SD = 4.87; eighteen months M = 12.83, SD = 5.66; t = 0.50, p = .62 (six to eighteen month comparison)). Similarly, pain impact scores did not change over time (six months M = 8.14, SD = 5.18; twelve months M = 8.04, SD = 4.85; eighteen months M = 8.68, SD = 5.35; t = 1.25, p = .21 (twelve to eighteen month comparison)).
Prevalence of depressive symptoms
Item scores
The most frequently endorsed symptoms were feeling tired or having low energy and trouble falling or staying asleep, endorsed by 50–60% of the sample over time (Table 2). More than a third of those interviewed reported some degree of depressed mood (38% at 6 months) or anhedonia (33% at six months). In paired t-tests, mean symptom scores did not change from six months to eighteen months post diagnosis (Table 2).
Table 2.
Paired t-test comparing change in PHQ-9 item means at 6 and 18 months.
| Frequency (%) |
Change T1 to T3a |
|||||
|---|---|---|---|---|---|---|
| During the past 2 weeks how often have you been bothered by any of the following problems: | Months post diagnosis | Never | Several Days | > ½ or nearly every dayb | t (1,121) | p |
| 1. Little interest or pleasure in doing things | 6 12 18 |
66.7 70.0 63.4 |
16.3 17.1 20.3 |
17.1 12.5 16.3 |
−0.39 | .699 |
| 2. Feel down, depressed or hopeless | 6 12 18 |
62.6 63.3 57.7 |
21.1 24.2 23.6 |
16.3 12.5 18.7 |
−0.90 | .371 |
| 3. Trouble falling or staying asleep, or sleeping too much | 6 12 18 |
48.8 59.2 52.8 |
16.3 13.3 18.7 |
35.0 27.5 28.5 |
1.86 | .066 |
| 4. Feeling tired or having little energy | 6 12 18 |
39.8 37.5 37.4 |
21.1 32.5 27.6 |
39.0 30.0 35.0 |
1.26 | .210 |
| 5. Poor appetite or overeating | 6 12 18 |
58.5 68.3 59.3 |
17.9 15.0 18.7 |
23.6 16.7 22.0 |
−0.15 | .881 |
| 6. Feel bad about yourself – or that you are a failure or have let yourself or your family down | 6 12 18 |
77.2 79.2 78.0 |
9.8 12.5 12.2 |
13.0 8.3 9.8 |
1.05 | .295 |
| 7. Trouble concentrating on things, such as reading the newspaper or watching television | 6 12 18 |
72.4 70.8 69.9 |
13.8 15.8 17.1 |
13.8 13.3 13.0 |
−0.32 | .749 |
| 8. Moving or speaking so slowly that other people could have noticed. Or the opposite – being so fidgety or restless that you have been moving around a lot more than usual | 6 12 18 |
72.4 69.2 72.4 |
13.0 18.3 15.4 |
14.6 12.5 12.2 |
0.53 | .598 |
| 9. Thoughts that you would be better off dead, or of hurting yourself | 6 12 18 |
89.3 96.7 88.5 |
5.7 1.7 9.0 |
4.9 1.7 2.5 |
0.15 | .880 |
Note. PHQ-9 = Patient Health Questionnaire-9.
Paired t-test comparing 6 months mean to 18 months mean (items rated on 4 point scale).
The categories of more than half of the days and nearly every day are combined in this table.
Total score
Six months after diagnosis, approximately one-quarter of the sample (24%, n = 29) scored above the PHQ-9 cut score of 10 or above consistent with major depression indicating the need for psychotherapy or medication (Figure 1), with 12% in the moderate to severe range and 12% in the mild range (M = 5.92, SD = 6.40). Similar rates were observed at twelve months after diagnosis (22%) (M = 5.16, SD = 5.72), and eighteen months after diagnosis (26%) (M = 5.72, SD = 6.13). Of these, 12% (n = 14) had elevated symptom scores at all time points; at each time point the remaining number of individuals with elevated scores were those with elevated scores at one or two time points.
Figure 1.
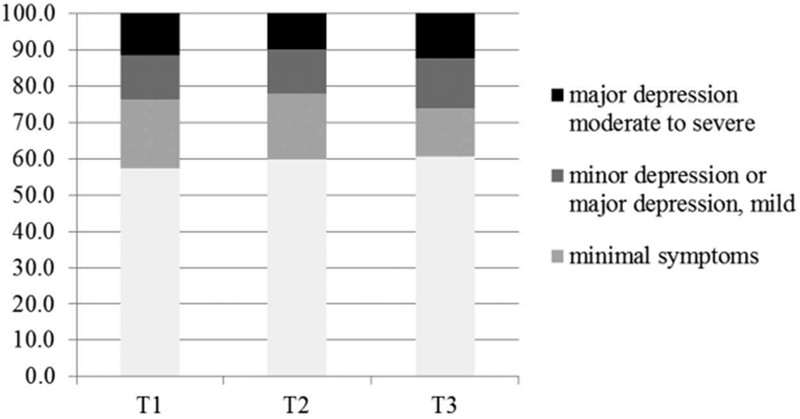
Prevalence rates of depression scores by severity level across time points.
Predictors of depression severity at eighteen months
First, bivariate correlations between depression severity at all time points and study variables were conducted. Younger age, lower emotional support, having received treatment for depression previously, and endorsing combat-PTSD symptoms at six months post diagnosis were associated with the total depression (PHQ) score at all time periods (Table 3). Education and physical comorbidities were not. In addition, cancer stage and type, and treatment length and type were not associated with total depression score at any time point. Participants’ report of social role dissatisfaction and pain interference with daily activities was associated with reported depression at all time points.
Table 3.
Bivariate Correlation between Depression Severity Scores and Study Variables.
| Depression (PHQ-9) Total Score |
||||
|---|---|---|---|---|
| Potential Risk Factors | 6 months | 12 months | 18 months | |
| Demographic and Health Variables | Age | −.32** | −.31** | −.30* |
| Education | −.04 | −.13 | −.13 | |
| MOS Social Support | −.21* | −.33** | −.19* | |
| Previous Treatment | .41** | .41** | .25* | |
| Comorbidity | .03 | −.04 | −.16 | |
| Combat PTSD symptoms | .44* | .33** | .35** | |
| Cancer factors | Tumor Stage | .06 | .07 | .01 |
| Type | −.10 | −.12 | −.10 | |
| Treatment factors | Chemotherapy | .11 | .13 | .08 |
| Radiation therapy | .07 | .13 | .02 | |
| Length of Treatment in Months | .03 | −.01 | .06 | |
| Patient Reported Outcomes at 6 months | PROMIS Social Role Interference | −.58** | −.52** | −.42** |
| PROMIS Pain Interference | .63** | .60** | .43** | |
| Patient Reported Outcomes at 12 months | PROMIS Social Role Interference | −.54** | −.64** | −.36** |
| PROMIS Pain Interference | .47** | .59** | .33** | |
| Patient Reported Outcomes at 18 months | PROMIS Social Role Interference | −.47** | −.43** | −.48** |
| PROMIS Pain Interference | .50** | .55** | .56** | |
Note. PHQ-9 = Patient Health Questionnaire-9; MOS Social Support = RAND Medical Outcomes Study social support survey; Combat PTSD = Primary Care PTSD Screen; PROMIS = Patient-Reported Outcomes Measurement Information System.
p < 0.05
p < 0.001.
Table 4 presents the results of the multivariate longitudinal model. In multiple linear regression, only report of pain at eighteen months (β = .23, p = .003) and depression at six months (β = .44, p < .001) were associated with depression at eighteen months, R2 = .57, F = 20.58, p < .001.
Table 4.
Prediction of Depression Severity Score at 18 months by Hierarchical Multiple Linear Regression.
| β | p | Semi-partial r2 | R2 | F change | ||
|---|---|---|---|---|---|---|
| 1 | (Constant) | .000 | .25 | 9.16* | ||
| Age | −.24 | .005 | .07 | |||
| MOS Social Support | −.14 | .087 | .03 | |||
| Previous MH Treatment | .04 | .671 | .00 | |||
| Combat PTSD symptoms | .35 | .000 | .13 | |||
| 2 | (Constant) | .017 | .50 | 56.38* | ||
| Age | −.10 | .174 | .02 | |||
| MOS Social Support | −.12 | .077 | .03 | |||
| Previous MH Treatment | −.03 | .682 | .00 | |||
| Combat PTSD symptoms | .10 | .233 | .01 | |||
| PHQ-9 (6 month) | .61 | .000 | .34 | |||
| 3 | (Constant) | .027 | .57 | 8.64* | ||
| Age | −.09 | .204 | .01 | |||
| MOS Social Support | −.12 | .101 | .02 | |||
| Previous MH Treatment | −.06 | .381 | .01 | |||
| Combat PTSD symptoms | .12 | .118 | .02 | |||
| PHQ-9 (6 month) | .45 | .000 | .20 | |||
| PROMIS Social Role Interference (18 months) | −.13 | .081 | .03 | |||
| PROMIS Pain Interference (18 month) | .23 | .003 | .08 |
Note. MOS Social Support = RAND Medical Outcomes Study social support survey; Combat PTSD = Primary Care PTSD Screen; PHQ-9 = Patient Health Questionnaire-9; PROMIS = Patient-Reported Outcomes Measurement Information System.
p < 0.05.
Discussion
We explored the prevalence of depressive symptoms and clinically significant depression and predictors of depression severity in cancer survivors up to eighteen months following initial diagnosis. Consistent with prior research (Krebber et al., 2014), major depression was found to be common in cancer survivors affecting approximately one in four participants and rates remained stable across one year. While rates of psychological distress are known to increase at time of diagnosis (Krebber et al., 2014; Linden et al., 2012), these findings are notable in that the majority of participants had completed treatment, yet rates remained elevated over time. However, most participants who reported major depression did not report elevations across all measurement times.
Low energy and sleep disturbance were the most common symptoms of depression endorsed, consistent with overlap between somatic symptoms of depression and treatment-related symptoms (Raison & Miller, 2003). Sleep disturbance can be caused by cancer treatment (Savard & Morin, 2001); therefore, we cannot eliminate the possibility that for some participants, endorsement of sleep disturbance reflects treatment side effects rather than mood dysfunction. Nonetheless, it is important to recognize that although rates of fatigue, diminished energy and appetite are high, that depression after cancer treatment is not purely somatic in nature, but distinguished by the presence of mood disturbance.
In this sample endorsement of ‘thoughts that you would be better off dead, or of hurting yourself ranged from a low of 3.4% (time 2) to a high of 11.5% (time 3). In cancer survivors, endorsement of this item usually indicates suicidal ideation, but for some may indicate a tendency to think about death and dying normatively given disease status (Walker et al., 2011). Depression screening measures may be useful for identifying possible depression and cancer survivors, but a clinical evaluation is needed to distinguish a clinical diagnosis from symptoms comorbid with cancer that are not indicative of major depression.
The role of pain in the current study, while modest in strength of the effect, is notable and concerning for several reasons. Chronic pain interference reflects participants’ perception of the extent to which pain interferes with social, occupation, or leisure roles. Part of adjustment after cancer entails returning to daily roles (Zebrack, 2000). Past research has found that occupying salient roles is integral to older adults’ concept of meaning and purpose in life (Krause, 2004). For cancer survivors with chronic pain, performing salient roles is impeded. This is particularly troubling given the importance of meaning-making and purpose in life on emotional well-being in cancer patients (Park, Chmielewski, & Blank, 2010; Vehling et al., 2011), and that depression, in particular, is marked by diminished purpose in life (Hedberg, Gustafson, Alèx, & Brulin, 2010).
In the current sample, it is unclear whether cancer caused pain interference or whether pain was related to medical comorbidities, or a combination of both. On average, participants were diagnosed with an additional three chronic health conditions. Whether pain preceded or resulted directly from cancer treatment, prior research indicates that chronic pain is highly prevalent in cancer survivors, and older adults are particularly vulnerable to the experience of chronic pain (Burton, Fanciullo, Beasley, & Fisch, 2007; Moye et al., 2014). More research is needed to better understand the experience of chronic pain in cancer survivors, in order to maintain and enhance psychological health.
Prior depression predicted future depression symptoms in our sample. This finding is consistent with the cancer literature (de Leeuw et al., 2001; Korfage et al., 2006) and our general understanding of the course of mood disorders (Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012). This finding points to the need for early interventions to support cancer survivors at risk for depression given premorbid vulnerability.
There are several limitations to this study. The study did not use a structured diagnostic interview to determine diagnosis. Meta-analysis of 211 studies examining the prevalence of depression in cancer patients using self-report and (semi)-structured interviews found that prevalence rates of depression one year after treatment were higher with self-report instruments (21%) compared to interviews (9%) (Krebber et al., 2014). It is possible that rates of depression were overestimated in the current study (Krebber et al., 2014). However, any overestimation is offset by the demographics of the current sample consisting largely of men, given lower rates of depression in men compared to women (Nolen-Hoeksema, 2001). Our sample included veterans, who were predominantly male, and included individuals with oral-digestive cancers only. Findings may not generalize to other populations. Nonetheless, our sample, which included adults with digestive tract cancers, reflects a vulnerable sub-group with particularly high rates of depression compared to other cancer types (Krebber et al., 2014). In addition, our focus on a mostly male sample complements the cancer survivor research literature that focuses often on female survivors of breast cancer. Our sample lacked racial and ethnic diversity. Future research with racially and ethnically diverse samples is warranted, given that differing rates of depression and health-related quality of life have emerged between racial and ethnic groups (Luckett et al., 2011).
Conclusions
One in four cancer survivors reported major depression. Pain interference and prior depression increased risk for future depression in cancer survivors. Early detection of depression with particular attention to the presence of mood symptoms is essential. Pain control is important for psychological well-being.
Acknowledgments
We are indebted to the Veterans who have participated in our research studies and allow us to contribute to their healthcare.
Funding
Funding was provided by the Department of Veterans Affairs Rehabilitation Research and Development Service #5I01RX000104-02. This material is the result of work supported with resources and the use of facilities at the Boston VA Medical Center and the Houston VA Health Services Research & Development Center of Excellence (HEP90-020) at the Michael E. DeBakey VA Medical Center, Houston, TX.
Footnotes
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 USC. 105, no copyright protection is available for such works under US Law.
Declaration of interest
The authors have no conflict of interest relating to this study or this manuscript.
Prior Presentations
Portions of this paper were previously presented at the 6th Biennial Cancer Survivorship Research Conference, Arlington, VA. June 2012.
Notes on contributor
The authors have no conflict of interest relating to this study or this manuscript.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: Author. [Google Scholar]
- Bergquist H, Ruth M, & Hammerlid E (2007). Psychiatric morbidity among patients with cancer of the esophagus or the gastro-esophageal junction: A prospective, longitudinal evaluation. Diseases of the Esophagus, 20(6), 523–529. [DOI] [PubMed] [Google Scholar]
- Brennan J (2001). Adjustment to cancer—Coping or personal transition? Psycho-Oncology, 10 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- Burton AW, Fanciullo GJ, Beasley RD, & Fisch MJ (2007). Chronic pain in the cancer survivor: A new frontier. Pain Medicine, 8(2), 189–198. [DOI] [PubMed] [Google Scholar]
- Cardoso G, Graca J, Klut C, Trancas B, & Papoila A (2016). Depression and anxiety symptoms following cancer diagnosis: A cross-sectional study. Psychology, Health & Medicine, 21(5), 562–570. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, & MacKenzie CR (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40(5), 373–383. [DOI] [PubMed] [Google Scholar]
- de Leeuw JR, De Graeff A, Ros WJ, Blijham GH, Hordijk GJ, & Winnubst JA (2001). Prediction of depression 6 months to 3 years after treatment of head and neck cancer. Head & Neck, 23(10), 892–898. [DOI] [PubMed] [Google Scholar]
- Deshields T, Tibbs T, Fan MY, & Taylor M (2006). Differences in patterns of depression after treatment for breast cancer. Psycho-Oncology, 15(5), 398–406. [DOI] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, & Mazel R (1995). User’s manual for the Medical Outcomes Study (MOS) core measures of health-related quality of life. Rand Corporation. [Google Scholar]
- Hedberg P, Gustafson Y, Alèx L, & Brulin C (2010). Depression in relation to purpose in life among a very old population: A five-year follow-up study. Aging & Mental Health, 14(6), 757–763. [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2012). IBM SPSS statistics for windows, version 21.0. Armonk, NY: Author. [Google Scholar]
- Institute of Medicine. (2008). Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jean CY, & Syrjala KL (2017). Anxiety and Depression in Cancer Survivors. Medical Clinics, 101(6), 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LE, & Doebbeling CC (2007). Suboptimal depression screening following cancer diagnosis. General Hospital Psychiatry, 29(6), 547–554. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, & Wittchen HU (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research, 21(3), 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfage IJ, Essink-Bot ML, Janssens ACJW, Schröder FH, & De Koning HJ (2006). Anxiety and depression after prostate cancer diagnosis and treatment: 5-year follow-up. British Journal of Cancer, 94(8), 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause N (2004). Stressors arising in highly valued roles, meaning in life, and the physical health status of older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 59(5), S287–S297. [DOI] [PubMed] [Google Scholar]
- Krebber AMH, Buffart LM, Kleijn G, Riepma IC, Bree R, Leemans CR, & Verdonck-de Leeuw IM (2014). Prevalence of depression in cancer patients: A meta-analysis of diagnostic interviews and self-report instruments. Psycho-Oncology, 23(2), 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: Validity of a brief depression severity measure. The Journal of General Internal Medicine, 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, & Tu W (2011). Reciprocal relationship between pain and depression: A 12-month longitudinal analysis in primary care. The Journal of Pain, 12(9), 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz ME, Kurtz JJ, Stommel MM, Given CC, & Given BB (2002). Predictors of depressive symptomatology of geriatric patients with colorectal cancer. Supportive Care in Cancer, 10(6), 494–501. [DOI] [PubMed] [Google Scholar]
- Linden W, Vodermaier A, MacKenzie R, & Greig D (2012). Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. Journal of Affective Disorders, 141(2), 343–351. [DOI] [PubMed] [Google Scholar]
- Luckett T, Goldstein D, Butow PN, Gebski V, Aldridge LJ, McGrane J, King MT (2011). Psychological morbidity and quality of life of ethnic minority patients with cancer: A systematic review and meta-analysis. The Lancet Oncology, 12(13), 1240–1248. [DOI] [PubMed] [Google Scholar]
- Mehnert A, Brähler E, Faller H, Härter M, Keller M, Schulz H, Reuter K (2014). Four-week prevalence of mental disorders in patients with cancer across major tumor entities. Journal of Clinical Oncology, 32(31), 3540–3546. [DOI] [PubMed] [Google Scholar]
- Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, & Dale W (2011). Association of cancer with geriatric syndromes in older medicare beneficiaries. Journal of Clinical Oncology, 29(11), 1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye J, June A, Martin LA, Gosian J, Herman LI, & Naik AD (2014). Pain is prevalent and persisting in cancer survivors: Differential factors across age groups. Journal of Geriatric Oncology, 5(2), 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik AD, Martin LA, Karel MJ, Wachen JS, Mulligan E, Gosian JS, & Moye J (2013). Cancer survivor rehabilitation and recovery: Protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Services Research, 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2001). Gender differences in depression. Current Directions in Psychological Science, 10(5), 173–176. [Google Scholar]
- Park CL, Chmielewski J, & Blank TO (2010). Post-traumatic growth: Finding positive meaning in cancer survivorship moderates the impact of intrusive thoughts on adjustment in younger adults. Psycho-Oncology, 19(11), 1139–1147. [DOI] [PubMed] [Google Scholar]
- Pereira M, Figueiredo A, & Fincham F (2012a). Anxiety, depression, traumatic stress and quality of life in colorectal cancer after different treatments: A study with Portuguese patients and their partners. European Journal of Oncology Nursing, 16, 227–232. [DOI] [PubMed] [Google Scholar]
- Pinquart M, & Duberstein PR (2010). Depression and cancer mortality: a meta-analysis. Psychological Medicine, 40(11), 1797–1810. doi: 10.1017/S0033291709992285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomano RC, & Farrar JT (2006). Pain and Neuropathy in Cancer Survivors: Surgery, radiation, and chemotherapy can cause pain; research could improve its detection and treatment. AJN the American Journal of Nursing, 106, 39–47. [DOI] [PubMed] [Google Scholar]
- Prins A, Ouimette P, Kimerling R, Camerond RP, Hugelshofer DS, Shaw-Hegwer J, et al. (2004). The primary care PTSD screen (PC-PTSD): Development and operating characteristics. Primary Care Psychiatry, 9(1), 9–14. [Google Scholar]
- PROMIS Health Organization and the PROMIS Cooperative Group. Patient Reported Outcomes Measurement Information System (PROMIS). Retrieved from www.nihpromis.org
- Raison CL, & Miller AH (2003). Depression in cancer: New developments regarding diagnosis and treatment. Biological Psychiatry, 54(3), 283–294. [DOI] [PubMed] [Google Scholar]
- Savard J, & Morin CM (2001). Insomnia in the context of cancer: A review of a neglected problem. Journal of Clinical Oncology, 19(3), 895–908. [DOI] [PubMed] [Google Scholar]
- Spiegel D, & Giese-Davis J (2003). Depression and cancer: Mechanisms and disease progression. Biological Psychiatry, 54(3), 269–282. [DOI] [PubMed] [Google Scholar]
- Stommel M, Kurtz ME, Kurtz JC, Given CW, & Given BA (2004). A longitudinal analysis of the course of depressive symptomatology in geriatric patients with cancer of the breast, colon, lung, or prostate. Health Psychology, 23(6), 564. [DOI] [PubMed] [Google Scholar]
- Uchitomi Y, Mikami I, Kugaya A, Akizuki N, Nagai K, Nishiwaki Y, & Okamura H (2000). Depression after successful treatment for nonsmall cell lung carcinoma. Cancer, 89(5), 1172–1179. [DOI] [PubMed] [Google Scholar]
- Vehling S, Lehmann C, Oechsle K, Bokemeyer C, Krüll A, Koch U, & Mehnert A (2011). Global meaning and meaning-related life attitudes: Exploring their role in predicting depression, anxiety, and demoralization in cancer patients. Supportive Care in Cancer, 19(4), 513–520. [DOI] [PubMed] [Google Scholar]
- Walker J, Hansen CH, Butcher I, Sharma N, Wall L, Murray G, & Sharpe M (2011). Thoughts of death and suicide reported by cancer patients who endorsed the “suicidal thoughts” item of the PHQ-9 during routine screening for depression. Psychosomatics, 52(5), 424–427. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ (2000). Cancer survivor identity and quality of life. Cancer Practice, 8(5), 238–242. [DOI] [PubMed] [Google Scholar]


