Abstract
Kidney paired donation (KPD) is an important tool to facilitate living donor kidney transplantation (LDKT). Concerns remain over prolonged cold ischemia times (CIT) associated with shipping kidneys long distances through KPD. We examined the association between CIT and delayed graft function (DGF), allograft survival, and patient survival for 1,267 shipped and 205 non-shipped/internal KPD LDKTs facilitated by the National Kidney Registry in the United States from 2008–2015, compared to 4,800 unrelated, non-shipped, non-KPD LDKTs. Shipped KPD recipients had a median CIT of 9.3 hours (range = 0.25 to 23.9 hours), compared to 1.0 hour for internal KPD transplants and 0.93 hours for non-KPD LDKTs. Each hour of CIT was associated with a 5% increased odds of DGF (adjusted odds ratio: 1.05, 95% CI: 1.02–1.09, p<0.01). However, there was not a significant association between CIT and all-cause graft failure (aHR: 1.01, 95% CI: 0.98–1.04, p=0.4), death-censored graft failure (aHR: 1.02, 95% CI: 0.98–1.06, p=0.4), or mortality (aHR 1.00, 95% CI: 0.96–1.04, p>0.9). This study of KPD-facilitated LDKTs found no evidence that long CIT is a concern for reduced graft or patient survival. Studies with longer follow-up are needed to refine our understanding of the safety of shipping donor kidneys through KPD.
BACKGROUND
The burden of End-Stage Renal Disease (ESRD) is high in the U.S. with approximately 98,000 patients waiting for a kidney transplant (OPTN data as of 5/22/2017). Living donor kidney transplantation (LDKT) is a better alternative to waiting for a deceased donor organ when the recipient candidate has a willing and compatible donor. If the donor and candidate are incompatible, however, kidney paired donation (KPD) provides a means to exchange donors with another incompatible pair so that both candidates can undergo a compatible LDKT. Recent acceptance of the practice of KPD in the U.S. has given rise to national KPD registries that facilitate KPD exchanges between kidney donors and recipients separated by long distances (1). Although these nation-wide exchanges allow more incompatible pairs to participate in LDKT, the long distances between transplant centers result in prolonged cold ischemia time (CIT) for the shipped kidney.
The transplant community varies in whether they support shipping living donor kidneys long distances through KPD programs adding significant CIT. Some national programs, such as in Canada or the Netherlands, never ship extirpated living donor kidneys (2, 3). On the other hand, the National Kidney Registry (NKR) in the U.S. has routinely shipped living donor kidneys since inception in 2008 (4). Our ability to evaluate and compare these different policies on shipping kidneys and establish an evidence-based, standard approach is limited by a paucity of research. Initial preliminary studies of shipped LDKT in KPD programs have suggested minimal to no association between CIT and graft or patient outcomes, however these studies were limited by small sample sizes and minimal follow-up times (5, 6). Additionally, none of these studies identified potential risk factors or predictors of poorer outcomes in shipped KPD kidneys with prolonged CIT. In a slightly different study population, a recent report of non-KPD living donor kidney transplants incurring longer CIT (maximum of 8 hours) in older donors (>50 years old) demonstrated poorer graft survival (7). In larger studies of deceased donor organs, there has been conflicting evidence for the association between long CIT (upwards of 24 hours) and delayed graft function (DGF), poorer allograft survival, or poorer patient survival (8, 9).
In order to address the important clinical and programmatic questions about the benefits and risks of shipping KPD kidneys, this study compares a large cohort of KPD recipients facilitated by the NKR, a large national KPD exchange program, to a national cohort of unrelated LDKTs not shipped or facilitated in a KPD exchange, which was identified from the Scientific Registry of Transplant Recipients (SRTR). This study aims to identify associations between CIT and KPD recipient delayed graft function, allograft failure and patient death. Additionally we sought to identify any associated risk factors for poorer outcomes. In comparison to data used in previous studies, the unique experience of the NKR offers a larger study population and longer CIT from transcontinental shipping.
METHODS
The National Kidney Registry
The NKR is a non-profit, 501c organization comprised of 76 transplant centers within the U.S. participating during this study period. Details of the NKR have been previously described (5). NKR policies are available online at: http://www.kidneyregistry.org. Protocols for evaluating patients, performing the transplant procedures, and post-operative care are outlined by the NKR, however ultimately carried out by the participating transplant centers abiding by, and in concordance with, the individual center protocols. The shipping of kidneys was performed utilizing existing organ procurement organizations (OPO) methodologies in accordance with Organ Procurement and Transplantation Network (OPTN) and United Network for Organ Sharing (UNOS) standards. Cold preservation solution without pumping was used for storage of the kidneys during transport. To date, the NKR has facilitated over 2000 KPD exchanges, greater than 80% of which involve shipping the living donor organ across the U.S.
Study Population
KPD transplants between Feb 1, 2008 and November 30, 2015 were identified from the NKR registry. The NKR registry was linked to the SRTR using the UNOS donor identifier to obtain demographic and clinical variables for the recipients and donors. Any transplant that could not be linked or validated on transplant center, transplant date, ABO, and gender were excluded from the study (5%, n=78). Additionally, as a comparison group, we included the cohort of all living unrelated non-KPD transplants identified from the SRTR that had their transplant at an NKR-participating center, during the same time period, and with short CIT (<1.33 hours, the average CIT of in-center NKR exchanges). NKR exchanges where the kidney was shipped were termed “shipped exchange,” NKR exchanges within the same center were termed “in-center exchanges,” and the additional cohort of living unrelated non-KPD transplants from SRTR were termed “other non-exchange.”
Cold Ischemia Time
In this study, CIT was defined as the hours of cold ischemia time associated with facilitating the transplant. Three records of CIT > 36 hours (exchange) and two records of CIT > 12 hours (in-center exchange) were recoded as unknown CIT as the prolonged CIT in these cases was likely due to confounding recipient factors.
Delayed Graft Function
Delayed graft function (DGF) was ascertained through SRTR and defined as requiring dialysis in the first week after transplantation. We studied whether longer CIT was associated with increased odds of DGF. We adjusted for recipient factors (sex, black race, BMI, diabetes mellitus [DM], primary diagnosis of congenital disease, PRA at transplant, previous transplant, pre-emptive transplant, and years on renal replacement therapy [RRT]), donor factors (living kidney donor profile index [LD KDPI] (10)), and transplant factors (HLA mismatch and year of transplant).
All-Cause Graft Failure
All-cause graft failure (ACGF) was ascertained through the SRTR. Recipients were followed until graft failure, death, or administrative censorship on Nov 30, 2015. We studied whether longer CIT was associated with an increased hazard of ACGF. Adjusted ACGF estimates were based on SRTR risk-adjustment approach. Recipient factors included years of age at transplant, black race, peripheral vascular disease (PVD), DM, PRA at transplant, pre-emptive transplant, years of RRT, public insurance, highest education level, and year of transplant. Donor factors were adjusted for through LD KDPI.
Death Censored Graft Failure
Death Censored Graft Failure (DCGF) was ascertained through SRTR. Recipients were followed until graft failure, censorship for death, or administrative censorship on Nov 30, 2015. We studied whether longer CIT was associated with an increased hazard of DCGF, adjusting for the same recipient and donor factors as ACGF.
Mortality
Mortality was ascertained through SRTR. Recipients were followed until death, or administrative censorship on Nov 30, 2015. We studied whether longer CIT was associated with an increased hazard of mortality. Adjusted mortality estimates were based on SRTR risk-adjustments. Recipient factors included years of age at transplant, sex, black race, PVD, DM, previous transplant, pre-emptive transplant, years of RRT, highest education level of grade school or none, public insurance, and year of transplant. Donor factors were adjusted for through LD KDPI, and donor ABO O.
Donors Older Than 50
We investigated whether CIT was associated differently to DGF, ACGF, DCGF, and mortality based on whether the donor was 50 years or older. This was accomplished using an interaction term in the regression models of CIT and donor age > 50 described in the Statistical Analysis section below.
Data Sources
This study was approved by the UCLA David Geffen School of Medicine Institutional Review Board (IRB protocol #11–003253-CR-00004) as well as the Johns Hopkins Medical Institutions Institutional Review Board (IRB-00048731). The NKR research committee granted access to the administrative NKR database to perform this study. Representatives and employees of the NKR provided data but did not directly participate in the design, analysis, or manuscript preparation for this study.
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (11). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Statistical Analysis
All analyses were conducted in Stata 14.2/MP for Linux (College Station, Texas). For all analyses, p<0.05 was considered statistically significant. Odds of DGF were estimated using a multi-level logistic regression that accounted for transplant center-level variation. Hazard of graft failure and mortality was estimated with Cox regression models with shared frailty to account center-level variation. The shared frailty framework accounts for center-level variation in a manner similar to multi-level generalized linear regression models. We used the log-likelihood ratio (LLR) test to test whether models fit with random-effects parameters (multi-level models) were better fit than regression models without these parameters. In this case, since the LLR compare the multi-level model with random effects to the single-level model, a LLR p-value < 0.05 implies that the association between CIT and post-transplant outcomes varies by center. In addition to these models, the hazard of ACGF, DCGF, and patient mortality stratified by type of LDKT (shipped exchange vs. in-center exchange vs. other non-exchange) were examined with Kaplan-Meier methods. Multiple imputation by chained equations with 10 imputations over 100 iterations was used to handle missing covariates. Missing PRA categories were imputed as a nominal variable; missing CIT, BMI, and LD KDPI were linearly imputed. All methods of handling missing data were compared to case-wise deletion regression models.
RESULTS
Study Population Characteristics
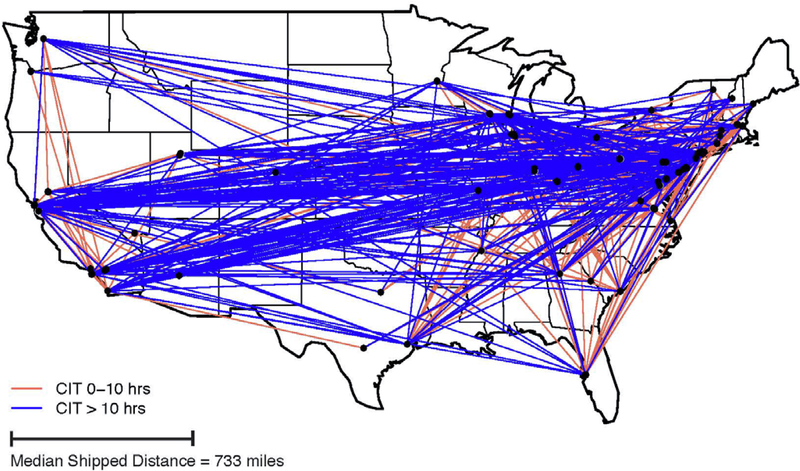
From 2008 to 2015, the 76 transplant centers considered in this study performed 6,272 total LDKTs. Of these, 1,472 (24%) were NKR-facilitated transplants with validated linkages to SRTR data. (Note that this sample does not comprise the total number of NKR-facilitated transplants to date since only transplants conducted up to 2015 were sampled.) Among the total 6,272 LDKTs, 1,267 (20%) were shipped KPD LDKTs and 205 (3%) were non-shipped in-center KPDs arranged by NKR. The remaining 4,800 (77%) were other unrelated, non-KPD LDKT recipients identified from the SRTR with CIT < 1.33 hours. The study sample characteristics of each group are shown in Table 1. The median follow-up was 3.2 years. Of the shipped kidneys, 1,046 (83%) were transported via air and 206 (16%) via ground transportation. The median shipping distance was 733 miles (1.5–2717 mile range). Figure 1 shows the geographic distribution of the 1,267 shipped LDKTs in this sample, coded by shipped KPD with 0–10 (red) and >10 hours (blue) of CIT.
Table 1:
Study Sample Characteristics
| Shipped Exchange | In-Center Exchange | p-valb | Other Non-Exchange | p-valb | |
|---|---|---|---|---|---|
| n=1267 | n=205 | n=4800 | |||
| Cold Ischemia Time (hours) a | 9.3 (6.9–12.2) | 1.0 (0.8–1.5) | <0.001 | 0.9 (0.5–1.0) | <0.001 |
| Recipient: Age a | 50 (39–60) | 50 (39–60) | >0.9 | 50 (41–59) | 0.6 |
| Female | 599 (47%) | 74 (36%) | <0.01 | 1557 (32%) | <0.001 |
| Diabetic | 307 (24%) | 53 (26%) | 0.6 | 1349 (28%) | <0.01 |
| Primary Diagnosis: DM | 232 (18%) | 46 (23%) | 0.07 | 1078 (22%) | <0.001 |
| GN | 412 (33%) | 60 (29%) | 1401 (29%) | ||
| PKD | 158 (12%) | 30 (15%) | 824 (17%) | ||
| Congenital | 48 (3.8%) | 1 (0.5%) | 108 (2.3%) | ||
| Other | 417 (33%) | 68 (33%) | 1389 (29%) | ||
| Years on RRT a | 1.4 (0.2–2.9) | 1.8 (0.6–3.6) | 0.048 | 0.5 (0–1.6) | <0.001 |
| Previous Transplant | 358 (28%) | 35 (17%) | 0.001 | 504 (11%) | <0.001 |
| Pre-emptive Transplant | 306 (24%) | 40 (20%) | 0.1 | 1818 (38%) | <0.001 |
| Black (vs non-black) | 211 (17%) | 50 (24%) | <0.01 | 554 (12%) | <0.001 |
| PRA at Transplant: 0 | 522 (41%) | 119 (58%) | <0.001 | 3291 (69%) | <0.001 |
| 1–10 | 67 (5.3%) | 14 (6.8%) | 327 (6.8%) | ||
| 11–79 | 347 (27%) | 39 (19%) | 661 (14%) | ||
| ≥80 | 324 (26%) | 26 (13%) | 111 (2.3%) | ||
| missing | 7 (0.6%) | 7 (3.4%) | 410 (8.5%) | ||
| BMI a | 27 (23–31) | 26 (23–31) | 0.3 | 27 (24–31) | <0.01 |
| Donor: Age a | 45 (35–52) | 48 (38–56) | <0.01 | 45 (36–53) | 0.02 |
| Female | 789 (62%) | 126 (62%) | 0.8 | 3217 (67%) | <0.01 |
| Black (vs non-black) | 137 (11%) | 24 (12%) | 0.7 | 385 (8.0%) | <0.01 |
| LD KDPI a | 12.2 (−0.84 – 25.0) | 12.0 (−0.51 – 31.3) | 0.4 | 15.2 (1.86 – 30.1) | <0.001 |
| HLA Mismatches: 0 | 9 (0.7%) | 0 | 0.08 | 17 (0.4%) | <0.001 |
| 1 | 29 (2.3%) | 3 (1.5%) | 38 (0.8%) | ||
| 2 | 81 (6.4%) | 10 (4.9%) | 207 (4.3%) | ||
| 3 | 198 (16%) | 38 (19%) | 633 (13%) | ||
| 4 | 340 (27%) | 44 (22%) | 1348 (28%) | ||
| 5 | 404 (32%) | 68 (33%) | 1620 (34%) | ||
| 6 | 182 (14%) | 41 (20%) | 907 (19%) | ||
| missing | 22 (1.7%) | 0 | 30 (0.6%) | ||
| Year of Transplanta | 2013 (2012–2014) | 2012 (2010 – 2014) | <0.001 | 2012 (2010 – 2014) | <0.001 |
median (IQR). CIT missing in 30 (15%) in-center exchanges, 21 (1.7%) of shipped exchanges, and none of the other non-exchanges. Recipient BMI missing in 1 (0.5%) in-center exchange, 4 (0.3%) of shipped exchanges, and 150 (3.2%) of the other non-exchanges. LD KDPI: Live-donor kidney donor profile index. LD KDPI missing in 3 in-center exchanges, 48 shipped exchanges, 242 of other non-exchanges.
P-values are compared to the Shipped Exchange group only.
Figure 1.
Geographic Distribution of Shipped Kidneys
Cold Ischemia Time
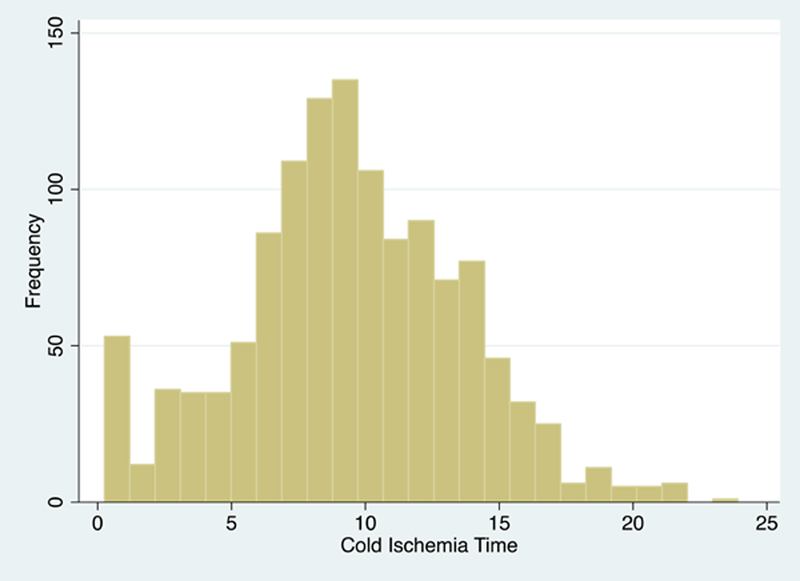
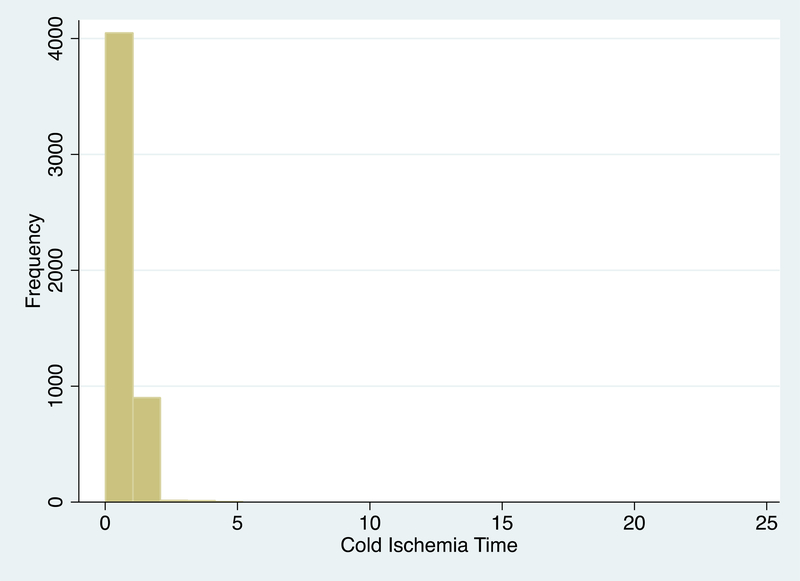
Shipped KPD recipients had a median (IQR) CIT of 9.3 (6.9–12.2) hours that ranged from 0.25 to 23.9 hours, longer than in-center KPD recipients with 1.0 hour (0.8–1.5) of CIT that ranged from 0.22 to 5.2 hours, and other non-exchanges with 0.93 (0.5–1.0) hours of CIT that ranged from 0.01 to 1.33 hours (by study design). (Table 1.) The distribution of CIT is shown in Figure 2 separately for shipped KPD (Figure 2a) and in-center KPD and other non-exchange transplant (Figure 2b). CIT that were missing from SRTR were imputed using CIT reported to NKR in 53 cases. CIT remained missing in 30 (15%) in-center exchanges, in 21 (1.7%) of shipped exchanges, and none of the other non-exchanges. These remaining 51 cases with missing CIT were imputed statistically in each model in subsequent analyses.
Figure 2a:
Cold Ischemia Time of Shipped Kidney Paired Donation Transplants
Figure 2b:
Cold Ischemia Time of In-Center Kidney Paired Donation and Other Non-Exchange Transplants
Delayed Graft Function
Shipped KPD recipients experienced 64 (5.1%) cases of DGF, in-center KPD experienced 7 (3.4%), and other non-KPD LDKT experienced 137 (2.9%) cases of DGF (χ2 test, p=0.001). 5 cases were excluded from analysis as the recipient died before DGF could be ascertained. The odds of DGF varied between transplant centers (p=0.03). After accounting for heterogeneity between centers, recipient characteristics, and donor characteristics, each hour of CIT was associated with a 5% increased odds of DGF (aOR 1.05, 95% CI: 1.02–1.09, p<0.01). Black race, diabetes, primary diagnosis of congenital disease, years on RRT, and LD KDPI were also associated with increased odds of DGF. Pre-emptive transplant and more recent year of transplant were associated with decreased odds of DGF (Table 2). Multivariate imputations were used for missing CIT in 51 (0.8%) cases, missing BMI in 155 (2.5%) cases, missing LD KDPI in 293 (4.8%) cases, and missing PRA at transplant in 424 (7%) cases. In an identically adjusted model where cases with missing data were handled by case-wise deletion (n=5522), CIT remained associated with increased DGF (aOR 1.06 (95% CI: 1.02–1.09, p<0.01).
Table 2.
Risk Factors for Delayed Graft Function among KPD and non-KPD Living Kidney Donor Transplant Recipients
| aORa (95% CI) | p-value | |
|---|---|---|
| Cold Ischemia Time (per hour) | 1.05 (1.02–1.09) | <0.01 |
| Black Recipient | 2.37 (1.71–3.28) | <0.001 |
| Female recipient | 0.74 (0.54–1.03) | 0.07 |
| Recipient BMI (centered at 25) | 1.00 (1.00–1.00) | 0.8 |
| Diabetic Recipient | 1.39 (1.02–1.89) | 0.04 |
| Primary Diagnosis of Congenital Disease | 2.30 (1.07–4.98) | 0.03 |
| PRA at Transplant 0 | REF | - |
| 1–10 | 0.78 | 0.4 |
| 11–79 | 1.00 | >0.9 |
| 80+ | 0.93 | 0.8 |
| Pre-emptive Transplant | 0.30 (0.19–0.49) | <0.001 |
| Previous Transplant | 1.08 (0.69–1.67) | 0.7 |
| Years of RRTb | 1.10 (1.05–1.15) | <0.001 |
| LD KDPIc | 1.01 (1.00–1.02) | <0.01 |
| Year of Transplant | 0.92 (0.86–0.99) | 0.02 |
Adjusted odds ratio
Renal replacement therapy
Live Donor Kidney Donor Profile Index
DGF was modeled in a multilevel logistic regression to adjust for center variation (n=6267). 5 cases of DGF were unknown since the patient died in the first week before DGF could be ascertained. Missing data were handled through multivariate imputation.
All-Cause Graft Failure
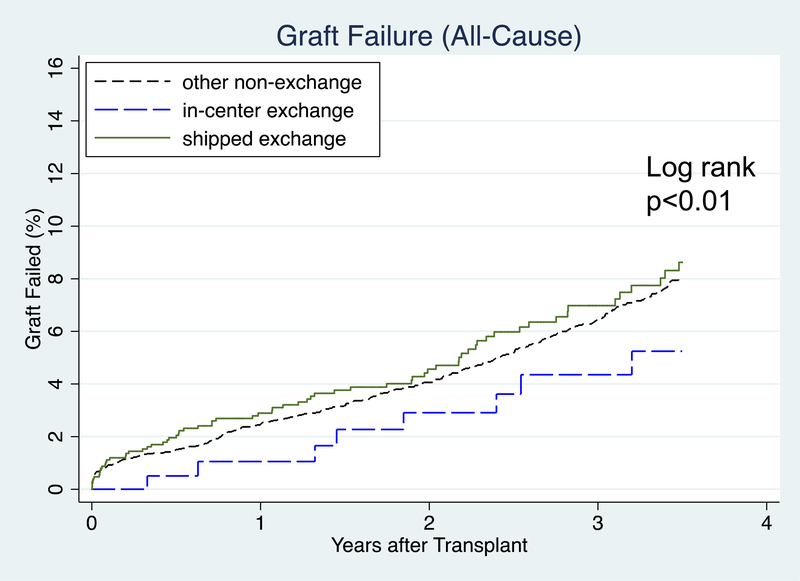
1-year ACGF was 2.9% in shipped KPD, 1.1% in in-center KPD, and 2.5% in other non-KPD. 3-year ACGF was 7.0% in shipped KPD, 4.4% in in-center KPD, and 6.4% in other non-KPD (Figure 3a). After accounting for heterogeneity between centers, recipient characteristics, and donor characteristics, there was not a significant association between CIT and ACGF (aHR 1.01, 95% CI: 0.98–1.04, p=0.4). Each year of recipient age below 40 was associated with a lower hazard of ACGF. Each year of recipient age above 55 was associated with an increased hazard of ACGF. Recipients with public insurance, diabetes, years of RRT, and LD KDPI were associated with an increased hazard of ACGF. Pre-emptive transplants and more recent year of transplant were associated with decreased hazard of ACGF (Table 3). In an identically adjusted model where cases with missing data were handled by case-wise deletion (n=5506), CIT was not associated with increased ACGF (aHR 1.02 (95% CI: 0.98–1.07, p=0.3).
Figure 3a:
Time to Graft Failure (All-Cause) after Transplant
Table 3:
Risk Factors for All-Cause Graft Failure among KPD and non-KPD Living Kidney Donor Transplant Recipients
| aHRa (95% CI) | p-value | |
|---|---|---|
| Cold Ischemia Time (per hour) | 1.01 (0.98–1.04) | 0.4 |
| Recipient Age at Transplant (per year) | 1.00 (0.98–1.02) | 0.8 |
| Per year under 40 | 0.96 (0.93–0.99) | 0.01 |
| Per year above 55 | 1.05 (1.01–1.09) | 0.01 |
| Black Recipient | 1.04 (0.81–1.34) | 0.8 |
| Peripheral Vascular Disease | 1.23 (0.81–1.87) | 0.3 |
| Diabetic Recipient | 1.47 (1.21–1.79) | <0.001 |
| PRA at Transplant 0 | REF | - |
| 1–10 | 0.95 | 0.8 |
| 11–79 | 1.20 | 0.1 |
| 80+ | 0.90 | 0.6 |
| Pre-emptive Transplant | 0.69 (0.55–0.86) | 0.001 |
| Years of RRTb | 1.04 (1.00–1.08) | 0.049 |
| Public Insurance | 1.24 (1.02–1.51) | 0.03 |
| High School (or lower) Education | 1.05 (0.87–1.26) | 0.6 |
| LD KDPIc | 1.01 (1.01–1.01) | <0.001 |
| Year of Transplant | 0.91 (0.86–0.96) | 0.001 |
Adjusted hazard ratio
Renal replacement therapy
Live Donor Kidney Donor Profile Index
All-Cause Graft Failure was modeled in a Cox regression with shared frailties to adjust for center variation (n=6272). Missing data were handled through multivariate imputation.
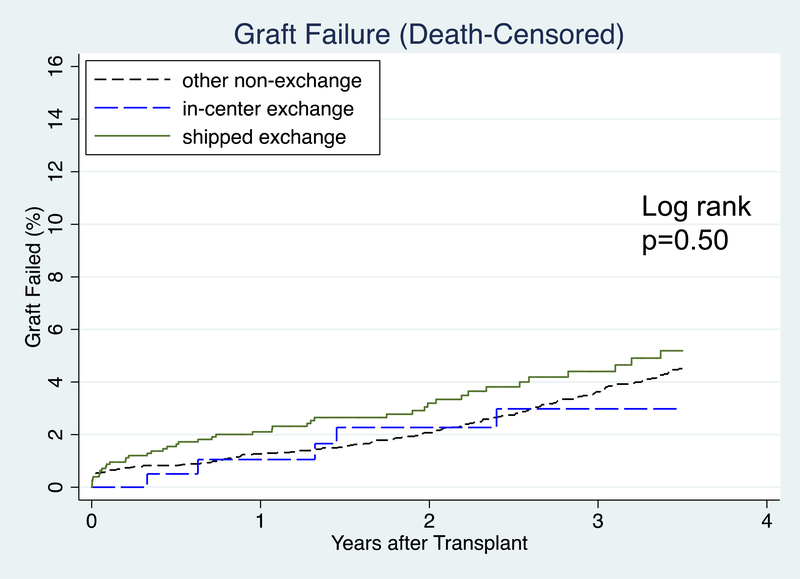
Death-Censored Graft Failure
1-year graft survival was 97.9% in shipped KPD, 99.0% in in-center KPD, and 98.7% in other non-KPD. 3-year graft survival was 95.6% in shipped KPD, 97.0% in in-center KPD, and 96.4% in other non-KPD (Figure 3b). After accounting for heterogeneity between centers, recipient characteristics, and donor characteristics, there was no association found between CIT and death-censored graft failure (aHR 1.02, 95% CI: 0.98–1.06, p=0.4). Recipient public health insurance, PRA 11–79, and LD KDPI were associated with increased hazard of DCGF. Recipient age, pre-emptive transplantation, and more recent year of transplant were associated with lower hazard of DCGF (Table 4). In an identically adjusted model where cases with missing data were handled by case-wise deletion (n=5506), CIT was not associated with increased DCGF (aHR 1.03 (95% CI: 0.99–1.05, p=0.1).
Figure 3b:
Time to Graft Failure (Death-Censored) after Transplant
Table 4:
Risk Factors for Death-Censored Graft Failure among KPD and non-KPD Living Kidney Donor Transplant Recipients
| aHRa (95% CI) | p-value | |
|---|---|---|
| Cold Ischemia Time (per hour) | 1.02 (0.98–1.06) | 0.4 |
| Recipient Age at Transplant (per year) | 0.96 (0.95–0.97) | <0.001 |
| Per year above 55 | 1.02 (0.98–1.06) | 0.3 |
| Black Recipient | 1.28 (0.94–1.74) | 0.1 |
| Peripheral Vascular Disease | 0.68 (0.29–1.55) | 0.4 |
| Diabetic Recipient | 1.07 (0.79–1.44) | 0.7 |
| PRA at Transplant 0 | REF | - |
| 1–10 | 0.97 | 0.9 |
| 11–79 | 1.46 | 0.01 |
| 80+ | 1.08 | 0.7 |
| Pre-emptive Transplant | 0.65 (0.48–0.89) | <0.01 |
| Years of RRTb | 1.01 (0.96–1.07) | 0.7 |
| Recipient Public Insurance | 1.33 (1.02–1.73) | 0.04 |
| High School (or lower) Education | 1.23 (0.96–1.57) | 0.1 |
| LD KDPIc | 1.01 (1.00–1.02) | 0.001 |
| Year of Transplant | 0.92 (0.85–0.99) | 0.03 |
Adjusted hazard ratio
Renal replacement therapy
Live Donor Kidney Donor Profile Index
Death-Censored Graft Failure was modeled in a Cox regression with shared frailties to adjust for center variation (n=6272). Missing data were handled through multivariate imputation.
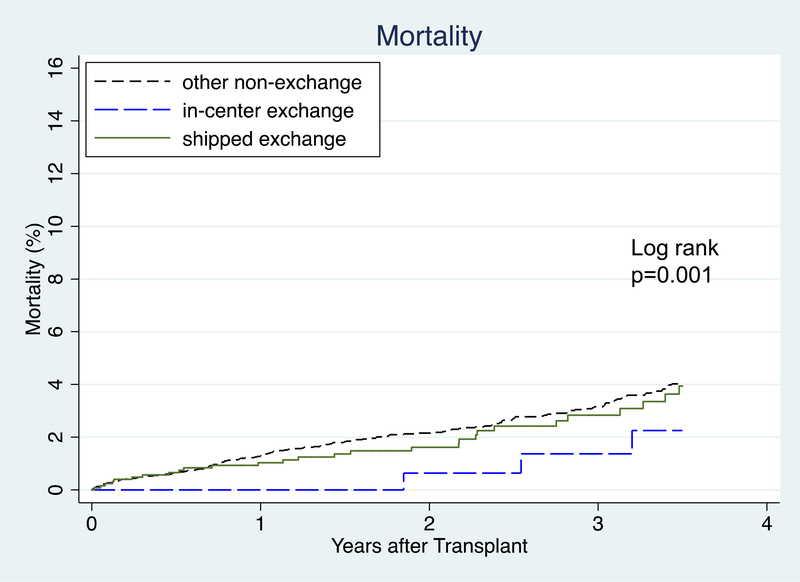
Mortality
1-year patient survival was 99.0% for shipped KPD, 100% for in-center KPD, and 98.7% for other non-KPD. 3-year patient survival was 97.2% for shipped KPD, 98.6% for in-center KPD, and 96.8% for other non-KPD (Figure 4). After accounting for heterogeneity between centers, recipient, and donor factors, there was no association found between CIT and post-transplant mortality (aHR 1.00, 95% CI: 0.96–1.04, p>0.9). Each year of recipient age at transplant above 40, diabetes, PVD, years of RRT, previous transplant, and LD KDPI were associated with increased hazard of mortality. Pre-emptive transplant, black race, and more recent year of transplant were associated with lower hazard of mortality (Table 5). In an identically adjusted model where cases with missing data were handled by case-wise deletion (n=5506), CIT was not associated with increased mortality (aHR 1.05 (95% CI: 0.99–1.11, p=0.1).
Figure 4:
Time to Mortality after Transplant
Table 5:
Risk Factors for Post-Transplant Mortality among KPD and non-KPD Living Kidney Donor Transplant Recipients
| aHRa (95% CI) | p-value | |
|---|---|---|
| Cold Ischemia Time (per hour) | 1.00 (0.96–1.04) | >0.9 |
| Recipient Age at Transplant (per year) | 0.99 (0.95–1.03) | 0.6 |
| Per year above 40 | 1.07 (1.02–1.12) | <0.01 |
| Female Recipient | 0.79 (0.60–1.04) | 0.1 |
| Black Recipient | 0.62 (0.40–0.98) | 0.04 |
| Peripheral Vascular Disease | 1.71 (1.09–2.66) | 0.02 |
| Diabetic Recipient | 1.97 (1.53–2.54) | <0.001 |
| Recipient Previous Transplant | 1.48 (1.04–2.11) | 0.03 |
| Pre-emptive Transplant | 0.64 (0.47–0.87) | <0.01 |
| Years of RRTb | 1.06 (1.01–1.11) | 0.01 |
| Grade School (or None) Education | 0.68 (0.32–1.46) | 0.3 |
| Recipient Public Insurance | 1.10 (0.85–1.41) | 0.5 |
| LD KDPIc | 1.01 (1.00–1.01) | 0.02 |
| Donor ABO O | 0.97 (0.75–1.25) | 0.8 |
| Year of Transplant | 0.91 (0.84–0.98) | 0.02 |
Adjusted hazard ratio
Renal replacement therapy
Live Donor Kidney Donor Profile Index
Mortality was modeled in a Cox regression with shared frailties to adjust for center variation (n=6272). Missing data were handled through multivariate imputation.
Donors Older than 50
There was no modified association between CIT and DGF among those with a donor aged > 50 (interaction p=0.06). CIT remained associated with DGF among those with a donor 50 or younger with aOR 1.07 (95% CI: 1.03–1.11, p<0.001). There was no modified association between CIT and ACGF (p=0.4), DCGF (p=0.3), or mortality (p=0.8) among those with older donors aged > 50.
DISCUSSION
The effect of shipping living donor kidneys on transplant recipient outcomes has been a major concern. In this retrospective cohort study of shipped live donor kidneys to KPD recipients in a large multicenter exchange program, each hour of CIT was associated with a 5% increased odds of DGF. As an example, a transplant recipient with a 3% chance of DGF might experience a 3.1% chance of DGF with 1 additional hour of CIT, a 3.6% chance of DGF with 4 additional hours of CIT, and a 5.3% chance of DGF with 12 additional hours of CIT. CIT was not found to be associated with graft failure or mortality. These results indicate only a minimal association between graft and patient outcomes and shipping living donor kidneys in a large multicenter KPD exchange program.
Similar to this study’s observations with LDKT, in deceased donor kidney transplantation (DDKT) prolonged CIT has been identified as an independent risk factor for DGF (12, 13). The direct impact of CIT induced DGF in the DDKT studies has not consistently predicted graft survival, implicating alternative or multi-factorial etiological factors underlying DGF within deceased donor organs, such as cytokine release with brain death, or other factors, which lead to poorer outcomes (9). Similar to deceased donor organs, prolonged cold storage in living donation appears to be associated with development of DGF, however, this small increase in DGF did not appear associated with graft or patient outcomes in this study. While this study was not specifically designed to investigate “CIT induced DGF” as an etiology of allograft injury leading to poor graft and patient survival, our findings are comparable to those in the study published by Kayler et al., which was specifically designed to address “CIT induced DGF” DDKT outcomes and attempted to control for other confounding factors surrounding the donor circumstance (9). Other large observational studies have found no significant associations between CIT and deceased donor allograft function (14). Finally, transplant centers should be aware of the small increase in risk for DGF that comes with increased shipping times demonstrated by this study, and incorporate that risk into their expectations for the transplant’s performance along with other, more detrimental risk factors, like additional time on RRT. An important line of research in this area includes additional investigation of the DGF’s phenotype (e.g., actual length of dialysis, creatinine values shortly after transplant) (15). Further description of DGF phenotypes associated with shipping kidneys could help transplant centers determine whether or not the small risk of DGF associated with long shipping times is clinically meaningful to them.
Although recent studies reported poorer allograft outcomes with prolonged CIT in living donor recipients not participating in exchange programs, we observed no association between CIT and allograft or patient survival in our shipped KPD cohort. Krishnan et al. found that among Australian recipients of kidneys from donors >50 years of age, CIT of 4–8 hours was associated with an increased odds of death-censored and all-cause graft failure (7). We found no evidence that DGF, graft failure, or mortality differed by donors age > 50 and those younger than 50. These conflicting findings may be explained by differences in the study populations, study designs, and definitions of CIT used. In the Krishnan study, the kidneys were not shipped, and they excluded exchange transplants, transplants with CIT > 8 hours, and ABO-incompatible transplants. Although that study had longer follow-up (median 6.6 years), their maximum CIT was only 8 hours, less than the median CIT of 9 hours and a maximum of 23.9 hours in this study. Other previous studies of living donor exchange programs and shipping kidneys in the U.S. were limited by small sample sizes and shorter CIT, but report similar findings to what this study reports in regards to DGF and graft and patient outcomes (5, 6).
Aside from CIT, previous studies investigating outcomes of LDKT found similar risk factors for DGF, allograft and patient survival to our results (16–18). Routine use of older living donors is increasing in clinical practice and organs from older donors have been repeatedly shown to have worse outcomes in LDKT but remains comparatively better than standard, young deceased donor organs (19). In particular, DGF rates above 5% are seen with donors above the age of 60, which is well above the recent overall DGF rate of 2.75% for LDKT reported in the SRTR Annual report (20). On the other hand, higher donor age, which independently predicted poorer graft survival in this study, suggests that prolonged CIT may prove less harmful than other factors (such as age). Considering these and other acceptable risk factors for poorer outcomes in LDKT, distance between centers, shipping, and potentially prolonged cold storage can be a consideration in optimizing strategies for matching for exchange outcomes. However, this study does not suggest that long shipping times should prevent exchanges from occurring or contribute to the barriers to transplantation.
The results of this study need to be considered in the context of study design. The primary limitation of the study is its limited follow-up time, with a maximum of 7.8 years. Ultimately, the long-term graft survival after shipped KPD entailing long CIT must be investigated, and continued follow-up of the participants in this study is an important next step. Next, retrospective studies like this one are limited by unmeasured confounding variables. While attempts were made to account for other transplant center, recipient, and donor factors that may be associated with post-transplant outcomes, there are other unique unmeasured/unrecognized variables in exchange programs that potentially alter graft and patient outcomes. These include variables such as improved HLA-matching, use of alternate, potentially less aggressive desensitization protocols, and garnering more “high profile” attention as exchange cases in transplant centers. Unmeasured variations between shipping protocols and in-center exchanges also could contribute to the differences in outcomes. These would include differences in packing and handling the organs, variations in operative techniques (donor and recipient), and unfamiliar donor and recipient surgeons working together in out-of-center exchanges. Furthermore, recipients in exchange programs tend to be more complex immunologically (sensitized), have donor specific antibodies, have undergone prior transplantation, or have other extenuating circumstances surrounding their operative procedure. Together, these unmeasured factors could be confounding the outcomes we studied in shipped KPDs. A third limitation of using large administrative databases is with missing data. Additionally, the impact of pumping the organ during transport could not be studied here. In this study, CIT was missing in 51 cases from both SRTR and NKR. Several other recipient and donor factors had small varying degrees of missingness. These missing values were imputed through multivariate imputation. Inferences remained consistent through case-wise deletion and multivariate imputation analysis.
Future studies on KPD exchange programs and the practice of shipping kidneys incurring long cold ischemia times could begin to focus on implementing enhanced matching algorithms for refining donor selection to minimize risk of poor outcomes balanced by patient willingness to assume risks through thorough an informed consent process. Efforts are also needed that focus on issues and barriers with international exchanges between countries that abide by ethical and laboratory standards (21). Further studies are needed particularly in the context of increased acceptance and practice of compatible pair KPD.
The practice of shipping living donor kidneys in KPD exchange programs increases CIT in kidney allografts. This study demonstrated increased odds of DGF for KPD recipients of shipped kidneys, but no associations between CIT and graft or patient survival. These findings support the current practice of shipping living donor organs in efforts to increase overall living donor transplantation, but should be considered along with the caveat that the long-term outcomes of shipping kidneys are not yet known. This study will hopefully guide further research and contribute new evidence around the upper limits of cold time and shipping distance acceptable for KPD programs, belaying some of the fears of transporting living donor kidneys in the international transplant community.
ACKNOWLEDGMENTS
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C, the National Institutes of Health Training Grant T32-DK-07789, and the UCLA Clinical and Translational Science Institute grant [UL1TR000124]. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors alone are responsible for reporting and interpreting these data; the views expressed herein are those of the authors and not necessarily those of the US Government or NKR. Dr. Segev is supported by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
ABBREVIATIONS
- ESRD
end stage renal disease
- LDKT
living donor kidney transplant
- KPD
kidney paired donation
- NKR
national kidney registry
- CIT
cold ischemia time
- DGF
delayed graft function
- RRT
renal replacement therapy
- PVD
peripheral vascular disease
- ACGF
all cause graft failure
- UNOS
United Network for Organ Sharing
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transport Network
- OPO
Organ Procurement Organization
- DM
Diabetes Mellitus
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Rees MA, Kopke JE, Pelletier RP, Segev DL, Rutter ME, Fabrega AJ, et al. A nonsimultaneous, extended, altruistic-donor chain. N Engl J Med. 2009;360(11):1096–101. [DOI] [PubMed] [Google Scholar]
- 2.de Klerk M, Keizer KM, Claas FH, Witvliet M, Haase-Kromwijk BJ, Weimar W. The Dutch national living donor kidney exchange program. Am J Transplant. 2005;5(9):2302–5. [DOI] [PubMed] [Google Scholar]
- 3.Cole EH, Nickerson P, Campbell P, Yetzer K, Lahaie N, Zaltzman J, et al. The Canadian Kidney Paired Donation Program: A National Program to Increase Living Donor Transplantation. Transplantation. 2014. [DOI] [PubMed] [Google Scholar]
- 4.Melcher ML, Leeser DB, Gritsch HA, Milner J, Kapur S, Busque S, et al. Chain transplantation: initial experience of a large multicenter program. Am J Transplant. 2012;12(9):2429–36. [DOI] [PubMed] [Google Scholar]
- 5.Treat EG, Miller ET, Kwan L, Connor SE, Maliski SL, Hicks EM, et al. Outcomes of shipped live donor kidney transplants compared with traditional living donor kidney transplants. Transpl Int. 2014;27(11):1175–82. [DOI] [PubMed] [Google Scholar]
- 6.Segev DL, Veale JL, Berger JC, Hiller JM, Hanto RL, Leeser DB, et al. Transporting live donor kidneys for kidney paired donation: initial national results. Am J Transplant. 2011;11(2):356–60. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan AR, Wong G, Chapman JR, Coates PT, Russ GR, Pleass H, et al. Prolonged Ischemic Time, Delayed Graft Function, and Graft and Patient Outcomes in Live Donor Kidney Transplant Recipients. Am J Transplant. 2016;16(9):2714–23. [DOI] [PubMed] [Google Scholar]
- 8.Debout A, Foucher Y, Trebern-Launay K, Legendre C, Kreis H, Mourad G, et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015;87(2):343–9. [DOI] [PubMed] [Google Scholar]
- 9.Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant. 2011;11(12):2657–64. [DOI] [PubMed] [Google Scholar]
- 10.Massie AB, Leanza J, Fahmy LM, Chow EK, Desai NM, Luo X, et al. A Risk Index for Living Donor Kidney Transplantation. Am J Transplant. 2016;16(7):2077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–74. [DOI] [PubMed] [Google Scholar]
- 13.Lebranchu Y, Halimi JM, Bock A, Chapman J, Dussol B, Fritsche L, et al. Delayed graft function: risk factors, consequences and parameters affecting outcome-results from MOST, A Multinational Observational Study. Transplant Proc. 2005;37(1):345–7. [DOI] [PubMed] [Google Scholar]
- 14.Waki K, Terasaki PI. Paired kidney donation by shipment of living donor kidneys. Clin Transplant. 2007;21(2):186–91. [DOI] [PubMed] [Google Scholar]
- 15.Hall IE, Reese PP, Doshi MD, Weng FL, Schroppel B, Asch WS, et al. Delayed Graft Function Phenotypes and 12-Month Kidney Transplant Outcomes. Transplantation. 2017;101(8):1913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10(10):2279–86. [DOI] [PubMed] [Google Scholar]
- 17.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74(10):1377–81. [DOI] [PubMed] [Google Scholar]
- 18.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344(10):726–31. [DOI] [PubMed] [Google Scholar]
- 19.Englum BR, Schechter MA, Irish WD, Ravindra KV, Vikraman DS, Sanoff SL, et al. Outcomes in Kidney Transplant Recipients From Older Living Donors. Transplantation. 2015. [DOI] [PubMed] [Google Scholar]
- 20.Scientific Registry of Transplant Recipients. OPTN/SRTR 2012 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2014. [Google Scholar]
- 21.Connolly JS, Terasaki PI, Veale JL. Kidney paired donation--the next step. N Engl J Med. 2011;365(9):868–9. [DOI] [PubMed] [Google Scholar]