Comprehensive genetic analysis uncovers mechanistic insights into sulfate-induced activation of ABA biosynthesis to close stomata.
Abstract
Plants close stomata when root water availability becomes limiting. Recent studies have demonstrated that soil-drying induces root-to-shoot sulfate transport via the xylem and that sulfate closes stomata. Here we provide evidence for a physiologically relevant signaling pathway that underlies sulfate-induced stomatal closure in Arabidopsis (Arabidopsis thaliana). We uncovered that, in the guard cells, sulfate activates NADPH oxidases to produce reactive oxygen species (ROS) and that this ROS induction is essential for sulfate-induced stomata closure. In line with the function of ROS as the second-messenger of abscisic acid (ABA) signaling, sulfate does not induce ROS in the ABA-synthesis mutant, aba3-1, and sulfate-induced ROS were ineffective at closing stomata in the ABA-insensitive mutant abi2-1 and a SLOW ANION CHANNEL1 loss-of-function mutant. We provided direct evidence for sulfate-induced accumulation of ABA in the cytosol of guard cells by application of the ABAleon2.1 ABA sensor, the ABA signaling reporter ProRAB18:GFP, and quantification of endogenous ABA marker genes. In concordance with previous studies, showing that ABA DEFICIENT3 uses Cys as the substrate for activation of the ABSCISIC ALDEHYDE OXIDASE3 (AAO3) enzyme catalyzing the last step of ABA production, we demonstrated that assimilation of sulfate into Cys is necessary for sulfate-induced stomatal closure and that sulfate-feeding or Cys-feeding induces transcription of NINE-CIS-EPOXYCAROTENOID DIOXYGENASE3, limiting the synthesis of the AAO3 substrate. Consequently, Cys synthesis-depleted mutants are sensitive to soil-drying due to enhanced water loss. Our data demonstrate that sulfate is incorporated into Cys and tunes ABA biosynthesis in leaves, promoting stomatal closure, and that this mechanism contributes to the physiological water limitation response.
INTRODUCTION
Global warming causes more frequent extreme weather conditions, provoking longer periods of drought, high temperatures, and low humidity. The sum of these unfavorable conditions decreases the yield of important crops by up to 50% (Lobell et al., 2014). Sessile organisms, like plants, must integrate multiple environmental signals into their physiological processes to optimize the utilization of energy and chemical resources under varying conditions. This allows plants to cope with stress conditions at the expense of biomass production. Stomatal aperture regulation is a prime example of such switches between growth and stress responses in plants (Rosenberger and Chen, 2018). Stomatal aperture increases facilitate water and carbon dioxide exchange to support photosynthesis. Abiotic stress conditions like drought, heat, or intense light as well as biotic stresses close stomata (Tardieu, 2016; Devireddy et al., 2018). Accordingly, the mechanisms that underlie dynamic stomatal movements are crucial for optimizing agricultural production, especially in suboptimal conditions.
Stomatal movement is driven by an osmotic motor that is based on uptake and release of potassium,counteranions chloride, and malate by guard cells (Hedrich, 2012). For stomatal opening, a set of membrane ion channels, transporters, and pumps shuttle ionic osmotica to guard cells (Meyer et al., 2010; Laanemets et al., 2013; Merilo et al., 2013). The slow anion channel of the SLOW ANION CHANNEL (SLAC) type represent a master switch for drought-induced stomatal closure (Vahisalu et al., 2008). The drought stress hormone abscisic acid (ABA) is a key signal inducing stomatal closure. In the guard cells, ABA acts via the PYRABACTIN RESISTANCE/PYRABACTIN LIKE (PYR/PYL)-ABA INSENSITIVE1 (ABI1)-OPEN STOMATA1 (OST1) receptor-phosphatase-kinase core signaling pathway and controls the phosphorylation-dependent activity of SLAC1 (Geiger et al., 2009). In addition, ABA tunes the activity of NADPH oxidases (respiratory burst oxidase protein [RBOH] transcripts RBOHD and RBOHF) for production of reactive oxygen species (ROS) in an OST1-dependent manner (Mustilli et al., 2002; Kwak et al., 2003; Sirichandra et al., 2009; Shang et al., 2016; reviewed in Sierla et al., 2016). Stimulation of plasma membrane Ca2+-permeable channels by ROS is essential for ABA-induced stomatal closure (Pei et al., 2000; Kwak et al., 2003; Sierla et al., 2016). However, the upstream intra- and intercellular signaling cascades that lead to ABA accumulation in guard cells via ABA uptake, ABA degradation (Kuromori et al., 2018), and cell autonomous ABA synthesis (Bauer et al., 2013) remain elusive.
Water uptake from the soil is mainly mediated by roots. Therefore, it had long been accepted that ABA served as a long-distance root-to-shoot drought signal for stomatal closure (Wilkinson and Davies, 2002; Seo and Koshiba, 2011). Recent studies show that ABA produced in roots is neither sufficient nor necessary to drive stomatal closure (Christmann et al., 2007). Rather, in drought conditions, ABA produced in leaf vascular parenchyma participates in stomatal closure. In the vasculature, ABA biosynthesis is supposed to be limited by 9-cis-epoxycarotenoid dioxygenase3 (NCED3) activity, which is a drought-stress-induced isoform of five NCED genes contributing to ABA biosynthesis (Nambara and Marion-Poll, 2005; Endo et al., 2008; Seo and Koshiba, 2011). Importantly, guard cells also harbor the machinery to produce ABA autonomously and it has been shown that this biosynthesis is sufficient to close stomata in response to decreased relative humidity (Bauer et al., 2013).
Together with ABA, sulfate is also reported to be a chemical signal observed under drought stress that enhances the anti-transpiring effect of ABA (Goodger et al., 2005; Ernst et al., 2010). Extracellular sulfate is proposed to gate the R-Type anion channel QUICK ANION CHANNEL1 (QUAC1) and thereby may contribute to feed-forward stomatal closure upon drought (Meyer et al., 2010; Malcheska et al., 2017). Sulfate can also induce the transcription of NCED3 in guard cells but the physiological relevance of the weak NCED3 induction for total ABA production is unclear (Malcheska et al., 2017). Remarkably, sulfide is proposed to be a gasotransmitter involved in stomatal closure (Lisjak et al., 2010; Jin et al., 2013; Honda et al., 2015). Sulfide is primarily produced in leaves by sulfite reductase (SiR), which catalyzes the last and committed step of sulfate reduction (Khan et al., 2010). However, the mode of action of sulfate or sulfide on stomata closure has not been identified due to the dual nature of these compounds as potential signaling molecules and as part of the primary metabolic network (Scuffi et al., 2014; Honda et al., 2015; Wang et al., 2016).
Stomatal closure is also controlled by the activity of the general growth regulator Target of Rapamycin (TOR). The TOR kinase phosphorylates the ABA receptor PYL1 at Ser119 to repress stress responses under favorable conditions. This phosphorylation site is conserved in most PYR/PYL-proteins. Phosphorylation of PYL1 inhibits ABA binding and suppresses OST1 (protein kinase SnRK2.6) activity by releasing constitutively active protein phosphatases of clade 2C (PP2Cs; Wang et al., 2018). Sulfate availability affects TOR kinase activity via the established Glc TOR signaling (Dong et al., 2017). As a result of the multiple connections between sulfur-metabolism and stomatal closure, the molecular mechanism of sulfate-induced stomatal closure remains to be elucidated.
Here we show that sulfate must be incorporated into Cys to trigger stomatal closure. Cys promotes stomatal closure by activating ABA biosynthesis in leaves, which results in significant accumulation of ABA in the guard cells and stomatal closure after petiole feeding of sulfate or Cys. Stomata in epidermal peels are still reactive to external application of sulfate or Cys in the absence of the vasculature. We demonstrate that activation of the second messenger ROS by ABA in the guard cells of epidermal peels is essential for closure of stomata by sulfate or Cys. Cys tunes ABA synthesis by transcriptional induction of NCED3 and, as shown previously, serves as the substrate of the molybdenum cofactor (MoCo) sulfurylase ABA3 activating the last step of ABA biosynthesis. Consequently, sulfate and Cys fail to trigger stomatal closure in NCED3 or ABA3 loss-of-function mutants. In concordance with the decisive function of Cys for tuning ABA biosynthesis, Cys synthesis depleted mutants are sensitive to soil drying. We furthermore reveal that the constitutive closed stomata phenotype of the γ-glutamylcysteine synthetase-depleted cad2-1 mutant is due to Cys accumulation. We conclude from these results that Cys biosynthesis contributes to the regulation of ABA biosynthesis in leaves and that this mechanism is vital for the physiological response to soil drying.
RESULTS
Sulfate Induces Stomatal Closure
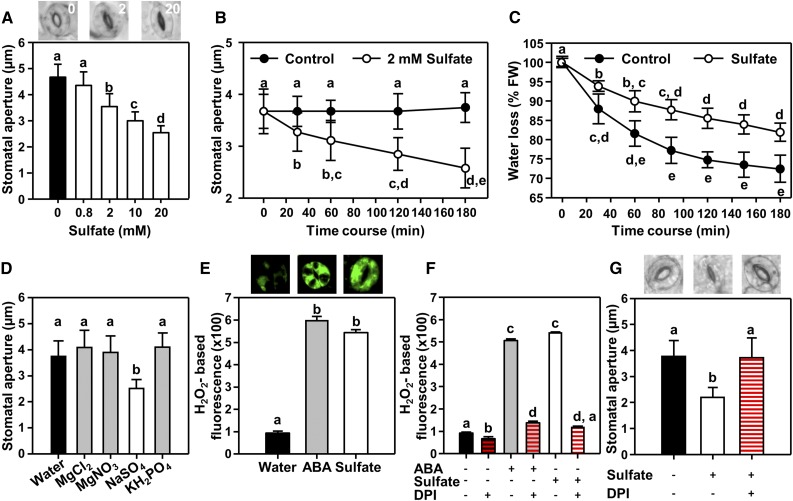
Because limited water accessibility increases the sulfate concentration of the xylem sap in drought-stressed maize (Zea mays) plants from 0.8 mM to 2 mM (Ernst et al., 2010), we tested the impact of increasing sulfate concentrations on maize guard cells in epidermal peels and in detached leaves that were fed with sulfate via petioles. In both approaches, increasing sulfate concentrations from 0 to 2 mM caused significant stomatal closure (P < 0.05), whereas application of 0.8 mM sulfate had no statistically significant impact on maize stomatal closure (Supplemental Figure 1). To allow application of reverse genetics approaches for the study of sulfate-induced stomatal closure in vascular plants, we established a sulfate-dependent stomatal closure assay in Arabidopsis by floating isolated epidermal peels on sulfate solutions (pH 5.5) with various concentrations. A 2-mM sulfate treatment resulted in a significant decrease in stomatal aperture within 180 min and the amplitude of the effect was concentration-dependent (Figure 1A). To test the effect of sulfate in a more physiologically relevant system, we detached leaves from well-watered Arabidopsis (Arabidopsis thaliana) plants and fed those leaves with 2 mM sulfate (pH 5.5) via the petiole. In this system, sulfate reaches the stomata via the xylem, and it significantly decreased stomatal aperture within 30 min. When sulfate treatment was prolonged for 180 min, stomatal aperture reached its minimum and was indistinguishable from the stomatal closure induced by ABA (50 μM, Figure 1B). Application of control solution (pH 5.5) did not affect stomatal closure of detached leaves within the time frame of the experiment. Moreover, we found that specifically sulfate-containing mineral salts lead to stomatal closure. Application of other soil-borne nutrients like nitrate (15 mM MgNO3) and phosphate (15 mM KH2PO4) did not affect stomatal closure (Figure 1D). These results demonstrate that specifically sulfate is sufficient to cause closure of stomata and that, after exogenous application, xylem-delivered sulfate decreases the transpiration of wild-type leaves significantly (P < 0.05; Figure 1C).
Figure 1.
Sulfate Induces Closure of Arabidopsis Stomata in a Dose- and Time-Dependent Manner and by Activation of NADPH Oxidases.
(A) Stomatal apertures of epidermal peels from 5-week-old soil-grown Arabidopsis wild-type plants incubated for 180 min with water containing up to 20 mM MgSO4. The apertures of 50 stomata were determined from peels of five individual plants (n = 5). Images illustrate typical stomatal apertures in response to the treatments. The applied sulfate concentration (mM) is indicated in white in the photographs.
(B) Time course of sulfate-induced stomatal closure in detached leaves fed via the petiole with 2 mM sulfate (white, MgSO4) or water (black, n = 50, from leaves of five individual plants).
(C) Water loss of detached leaves from 5-week-old wild-type plants that were preincubated for 180 min in water (black circles) or 2 mM MgSO4 (white circles, sulfate).
(D) Stomatal apertures of epidermal peels treated with different nutrient salts (15 mM).
(E) Quantification of hydrogen peroxide production after fluorescent-labeling with H2DCF-DA. Epidermal peels were treated with water (control), ABA (50 μM), or sulfate (15 mM) for 180 min before analysis (n ≥ 100).
(F) Impact of the selective NADPH-oxidase inhibitor DPI (10 μM, red dash) on ABA-induced and sulfate-induced production of hydrogen peroxide in guard cells of epidermal peels (ABA, 50 μM, sulfate, 15 mM MgSO4 and DPI, 10 μM, n ≥ 100).
(G) Impact of NADPH-oxidase inhibition by DPI on sulfate-induced stomatal closure (n = 50, from peels of five individual plants). Bars represent mean ± sd in (A) to (E) and (G) and mean ± se in (E) and (F).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
Sulfate Induces the Formation of ROS in Guard Cells by Activation of NADPH Oxidases
In guard cells, the drought-stress hormone ABA induces production of ROS such as hydrogen peroxide, which was shown to trigger stomatal closure (Pei et al., 2000; Hua et al., 2012). To test whether sulfate affects stomatal movement by interfering with ROS signaling, we monitored guard cell ROS production in response to sulfate treatment. ROS were determined by in vivo staining with the H2O2-selective dye 2′,7′dichlorodihydrofluorescein diacetate (H2DCF-DA). Sulfate application (15 mM) significantly (P < 0.05) increased H2O2 levels in guard cells to a similar extent as the stress hormone ABA (Figure 1E). ABA induces ROS formation in guard cells by specific activation of plasma membrane-localized NADPH oxidases via OST1 (Mustilli et al., 2002; Sirichandra et al., 2009). NADPH oxidases can be inhibited selectively by diphenylene iodonium (DPI, 10 μM; Cross and Jones, 1986). The addition of 10 μM DPI to the sulfate- or ABA-containing solution prevented the formation of ROS (Figure 1F) and sulfate-induced stomatal closure (Figure 1G). This finding is in line with the hypothesis that sulfate can mediate stomata closure via activation of NADPH oxidase-dependent ROS formation in guard cells.
SLOW-ANION CHANNEL1 Is Required for Sulfate-Induced Stomata Closure
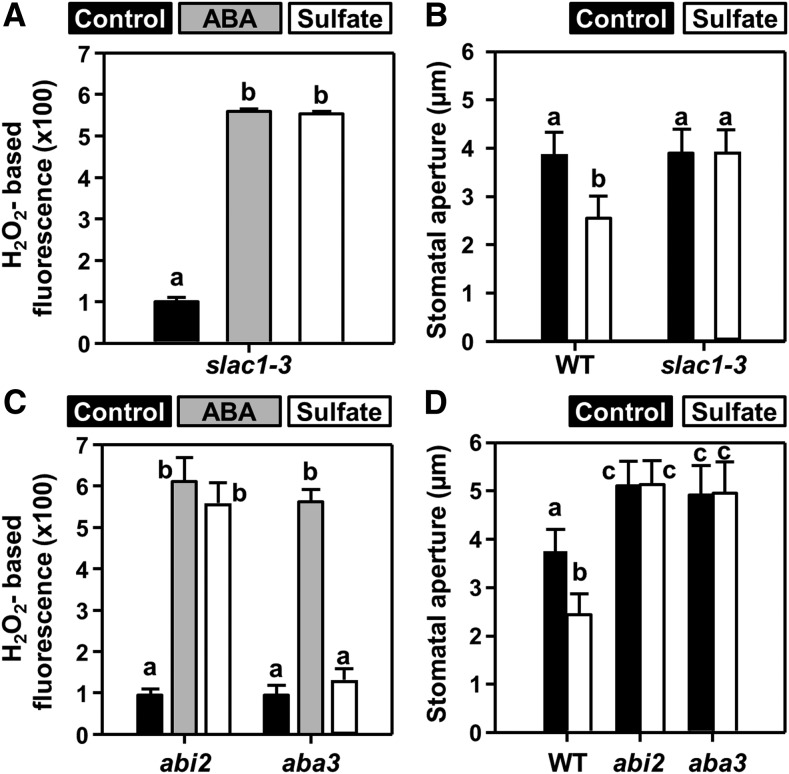
Sulfate directly activates the R-type anion channel QUAC1 in Xenopus oocytes, and the quac1 Arabidopsis mutant fails to close stomata upon sulfate application (Malcheska et al., 2017). SLAC1 is the major guard cell S-type anion channel in Arabidopsis and is not thought to be activated by sulfate (Vahisalu et al., 2008). When epidermal peels of the slac1-3 mutant were exposed to either sulfate or ABA, ROS formation was observed (Figure 2A), but stomata did not close in response to sulfate (Figure 2B) or ABA (Vahisalu et al., 2008; Meyer et al., 2010). These results demonstrate that SLAC1, like QUAC1, is essential for sulfate-induced stomatal closure and suggests that sulfate uses a signaling pathway that addresses both anion channels.
Figure 2.
Sulfate-Induced Stomatal Closure Requires ABA-Signaling Components and ABA-Downstream Effectors.
(A) and (C) Impact of ABA (gray, 50 µM) and sulfate (white, 15 mM MgSO4) on hydrogen peroxide production in guard cells of epidermal peels of five-week-old slac1 (A), and abi2-1 and aba3-1 (C) plants. Data represent mean ± se (n ≥ 100; derived from ≥5 individual plants).
(B) and (D) Impact of sulfate (white, 15 mM MgSO4) on stomatal apertures of the wild-type (WT), slac1 (B) and mutants deficient in ABA sensing (abi2-1, [D]) or ABA production (aba3-1, [D]). Control refers to water. Data represent mean ± sd in (B) and (D) (n ≥ 50, derived from ≥5 individual plants).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
Sulfate-Induced Stomatal Closure Requires ABA Production and ABA Signal Transduction by ABI2
We next addressed the mechanism by which sulfate activates the guard cell S-type anion channel, SLAC1. Like QUAC1, SLAC1 is activated via ABA signaling, a process that requires inhibition of the type-2 protein phosphatase ABI2 (Murata et al., 2001). To test whether sulfate affects SLAC1 via a phosphorylation-dephosphorylation mechanism, a mutant with constitutively active PP2C protein phosphatase ABA2 (ABA insensitive 2-1 [abi2-1]) was exposed to sulfate. This mutant exhibits constitutively open stomata and drought-stress sensitivity. The abi2-1 mutant can produce ABA but the activation of an S-type anion channel by ABA is impaired downstream of ROS production (Pei et al., 1997; Murata et al., 2001). Accordingly, the application of sulfate caused ROS formation in abi2-1 guard cells (Figure 2D) but failed to close stomata (Figure 2D).
In contrast with abi2-1, the aba3-1 mutant cannot synthesize drought-stress–relevant levels of ABA (Bauer et al., 2013). In the aba3-1 mutant, feeding of sulfate did not induce ROS production and failed to close the stomata (Figures 2C and 2D). This result strongly suggests that sulfate-induced ROS production is a consequence of sulfate-induced ABA biosynthesis. To provide an independent line of evidence for the essential function of ABA3 during sulfate-induced stomatal closure, we fed sulfate and ABA via the petiole to detached leaves of the wild-type and the aba3-1 mutant (Supplemental Figure 2). ABA feeding via the petiole decreased water loss of wild-type and the aba3-1 mutant when compared with control conditions. Remarkably, petiole feeding of sulfate did not affect the transpiration rate of the aba3-1 mutant but sulfate decreased the water loss of wild type to the same extent as ABA (Supplemental Figure 2A). These results demonstrate that sulfate-induced stomatal closure requires de novo ABA synthesis and a functional ABA3 protein.
Sulfate Triggers ABA Production in Guard Cells
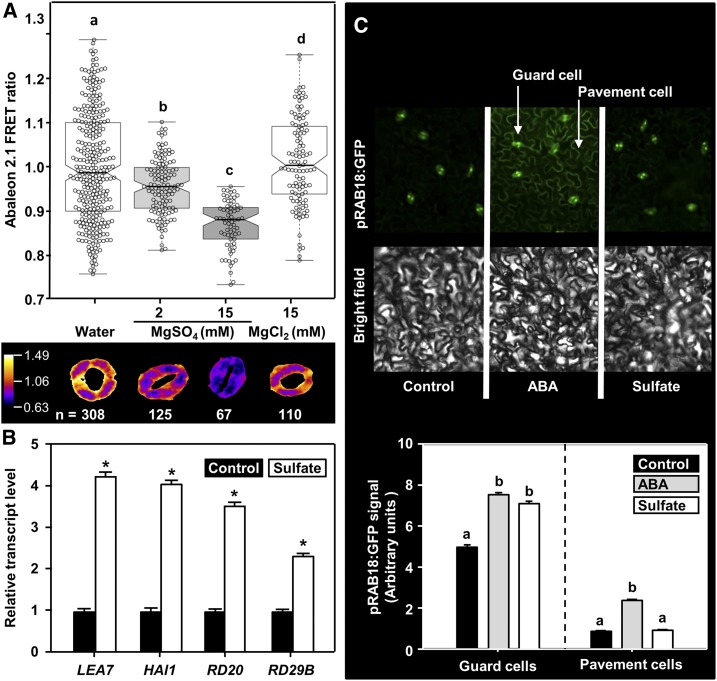
To test whether sulfate affects intrinsic ABA levels, we applied sulfate and monitored the ABA concentration in guard cells employing the genetically encoded ABA sensor ABAleon2.1 that allows direct visualization of changes in cytosolic ABA concentration by noninvasive live cell imaging (Waadt et al., 2014). A decrease of ABAleon2.1 emission ratio can be taken as read-out of an increase in intracellular ABA concentration (Waadt et al., 2014). Treatment of guard cells in epidermal peels with 2 mM MgSO4 decreased the ABAleon2.1 emission ratio by 4% (P < 0.001; Figure 3A). A more pronounced effect (decrease of 13%, P < 0.001) was observed with higher sulfate concentrations (15 mM MgSO4). When leaf peels were treated with 15 mM MgCl2, the ABAleon2.1 emission ratio in guard cells was indistinguishable from the water control (P > 0.25). The sulfate-concentration–dependent induction of ABA biosynthesis in guard cells thus underlies the observed sulfate-dose–dependent stomatal closure (Figures 1A and 1B). Moreover, the transcript levels of four canonical ABA marker genes (LATE EMBRYOGENESIS ABUNDANT7 [LEA7], HIGHLY ABA INDUCED1, RESPONSIVE TO DESSICATION20 [RD20], and RD29B) were determined in water (control) and sulfate-treated epidermal peels by reverse transcription quantitative PCR (RT-qPCR). We found that sulfate application to guard cells in epidermal peels enhanced transcription of all tested ABA-marker genes when compared with the control (Figure 3B). The induction of ABA marker gene transcription by sulfate independently supports the induction of ABA biosynthesis by sulfate. Next, we applied the established ABA-response-reporter ProRAB18:GFP (Kim et al., 2011) to determine activation of ABA-signaling in intact leaves after feeding of sulfate (15 mM MgSO4) via the petiole at cellular resolution. Sulfate feeding resulted in significant expression of green fluorescent protein (GFP) in guard cells (P < 0.001) that was similarly high as that induced by direct feeding of ABA (50 μM) via the petiole (Figure 3C). Petiole feeding of sulfate did not induce GFP expression from the ProRAB18 promoter in pavement cells of the epidermis. Remarkably, xylem-transported ABA was able to reach the pavement cells and induce ProRAB18 promoter-driven GFP expression (P < 0.001, Figure 3C).
Figure 3.
Sulfate Triggers ABA Production in Guard Cells in a Concentration-Dependent Manner.
(A) The upper panel shows the ABAleon2.1 emission ratio. Signals from guard cells treated with water alone (n = 308), or water supplemented with 2 mM MgSO4 (n = 125), 15 mM MgSO4 (n = 67), or 15 mM MgCl2 (n = 110), respectively. Average ABAleon2.1 emission ratio is calculated per stomatal area. Statistical tests are performed with respect to the water control. The lower panel shows a representative stoma in the given treatment. Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
(B) Transcript levels of ABA-responsive genes in sulfate-treated epidermal peels. Epidermal peels were collected from 5-week-old wild-type plants and incubated on water supplemented without (black) or with 2 mM MgSO4 (white, sulfate) for 3 h. RNA was extracted and the steady-state transcript levels of ABA-marker genes (LEA7, HAl1, RD20, and RD29B) were quantified by RT-qPCR. The transcript levels of respective genes from water-treated samples were set to 1. Data represent mean ± sd (n = 3). Asterisks indicate statistical significant differences as determined with the Student’s t test (*P < 0.05).
(C) Impact of petiole-fed ABA or sulfate on the expression of the ABA signaling marker ProRAB18:GFP in detached leaves. Leaves of 25-day-old soil grown ProRAB18:GFP plants were detached and fed via the petiole with ABA (gray, 50 μM) or sulfate (white, 15 mM MgSO4) dissolved in water (black, Control) for 180 min before quantification of the GFP signal. The upper panel displays a representative image of the epidermis containing guard and pavement cells. Bright field image of the same area is shown for orientation. The lower panel depicts the quantification of GFP-signal intensities in guard- or pavement cells after the treatment. Data represent mean ± se (guard cells: n = 666 for water, n = 534 for sulfate, n = 894 for ABA, from five individual leaves, pavement cells n = 20 regions of interest containing multiple pavement cells for each treatment, from five individual leaves).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
ABA Production in Guard Cells Is Sufficient for Sulfate-Induced Stomatal Closure
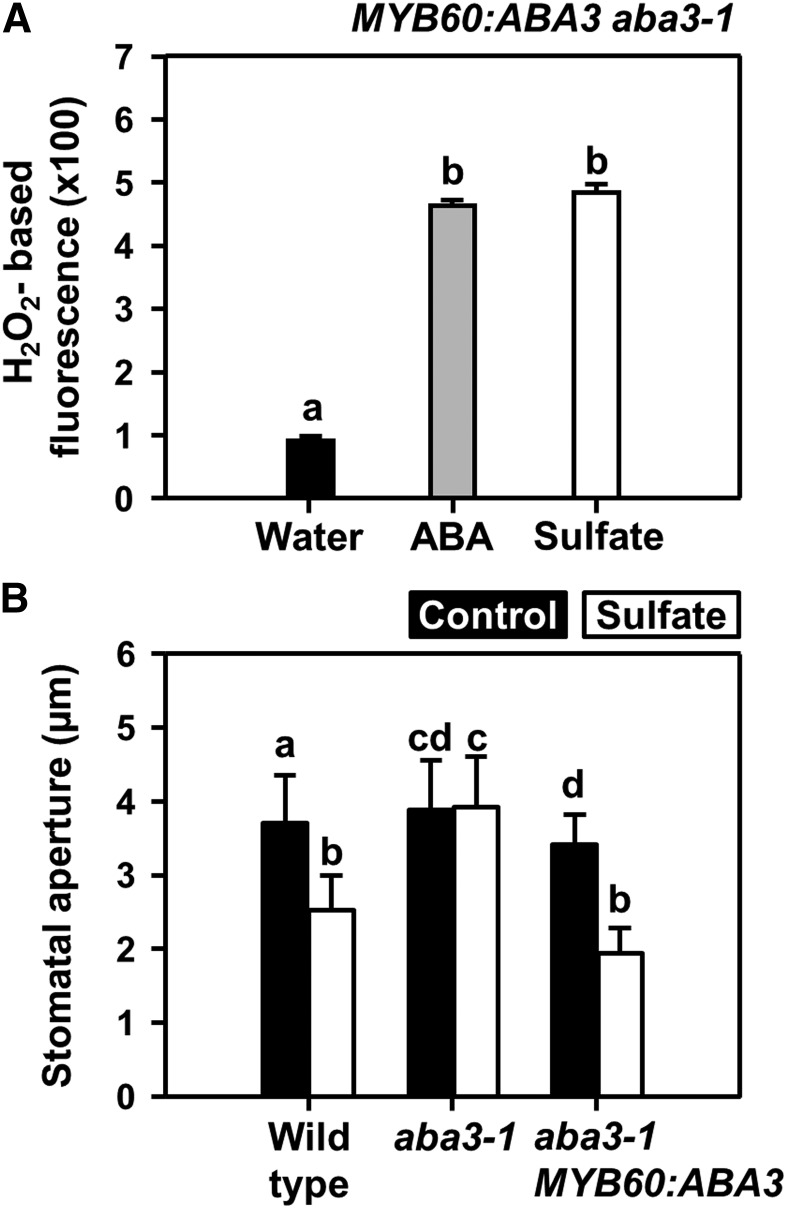
The aba3-1 mutant plants cannot close their stomata and wilt when exposed to dry air, because of a point mutation rendering ABA3 nonfunctional in all cells. When ABA biosynthesis is rescued in stomata by complementation of aba3-1 with functional ABA3 under the control of a guard cell–specific MYB60 promotor, stomata of MYB60:ABA3 aba3-1 plants regain the ability to close upon a decrease in atmospheric relative humidity (Bauer et al., 2013). When exposed to sulfate, the aba3-1 mutant did not produce ROS and did not close the stomata (Figures 2C and 4B). Sulfate application to epidermal peels of MYB60:ABA3 aba3-1 resulted in significant induction of ROS formation in guard cells that was indistinguishable from ROS formation upon ABA application in the wild type (Figures 1E and 4A). Remarkably, ABA biosynthesis is required, and guard-cell autonomous ABA biosynthesis is sufficient for sulfate-induced stomata closure. The degree of stomatal closure induced by sulfate application between the mutant with guard-cell autonomous ABA biosynthesis and the wild type was indistinguishable (Figure 4B). Taken together, these results demonstrate that sulfate can trigger stomatal closure by inducing ABA biosynthesis in guard cells.
Figure 4.
Guard-Cell Autonomous ABA Synthesis in the MYB60:ABA3-Complemented aba3-1 Mutant is Sufficient for Sulfate-Induced Stomatal Closure.
(A) Impact of ABA (gray, 50 μM) and sulfate (white, 15 mM MgSO4) on hydrogen peroxide production in guard cells of the MYB60:ABA3 complemented the aba3 mutant that lacks ABA biosynthesis by ABA3 in other cell types than guard cells.
(B) Impact of sulfate (white, 15 mM MgSO4) on stomata closure of wild type, aba3-1, and the MYB60:ABA3 complemented the aba3-1 mutant. Data represent mean ± sd (n ≥ 50 stomata, derived from ≥5 individual plants).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
Sulfate Assimilation Is Essential for Sulfate-Induced Stomatal Closure
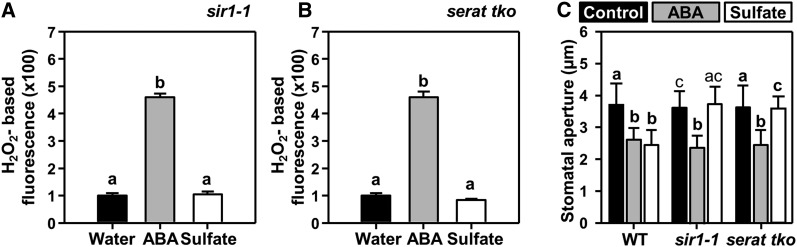
Sulfide, a metabolite in the sulfate assimilation pathway, has been reported to induce stomatal closure (Scuffi et al., 2014; Honda et al., 2015; Wang et al., 2016). To test the requirement of functional sulfur metabolism, we analyzed whether sulfate induces stomatal closure in mutants lacking the ability to produce sulfide (sir1-1) and those unable to incorporate sulfide into Cys (serat tko). The sir1-1 knock-down mutant is characterized by a reduced ability to convert sulfite to sulfide, due to decreased expression of SiR (Khan et al., 2010). Application of sulfate to epidermal peels of sir1-1 failed to induce ROS formation and stomatal closure (Figures 5A and 5C). The triple loss-of-Ser acetyltransferase (SERAT) function mutant (serat tko, lacking the cytosolic [SERAT1;1], the plastidic [SERAT2;1], and the mitochondrial isoform [SERAT2;2] of Ser acetyltransferase) lacks the ability to efficiently produce the scaffold required for fixation of sulfide into Cys (Watanabe et al., 2008). Feeding sulfate to serat tko also did not induce ROS formation and stomatal closure (Figures 5B and 5C). The fact that ABA could induce ROS formation (Figures 5A and 5B) and stomatal closure in both mutants (Figure 5C) excludes the possibility that sir1-1 and serat tko do not respond to sulfate because of nonfunctional ABA signaling. These results implied that metabolic assimilation of sulfate into Cys by SERAT and SiR is mandatory for sulfate-induced stomatal closure.
Figure 5.
Sulfate-Induced Stomatal Closure Requires Sulfate Reduction and Incorporation of Sulfide into Cys.
(A) and (B) Impact of ABA (gray, 50 µM) and sulfate (white, 15 mM MgSO4) on hydrogen peroxide production in guard cells of sir1-1 (A) and serat tko (B) plants. Data represent mean ± se (n ≥ 100; derived from ≥5 individual plants).
(C) Impact of ABA (gray, 50 μM) and sulfate (white, 15 mM MgSO4) on the stomatal apertures of the wild type (WT) and of mutants with a strongly reduced ability to reduce sulfate to sulfide (sir1-1) or incorporate sulfide into Cys (serat tko). Control refers to water. Data represent mean ± sd in (C) (n ≥ 50 stomata, derived from ≥5 individual plants).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
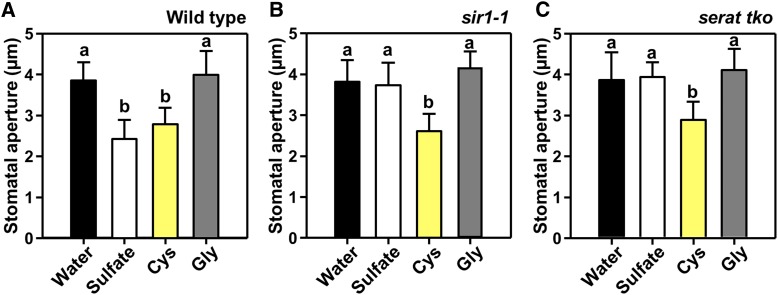
Cys Triggers Stomatal Closure by Inducing ABA Biosynthesis
Cys is a downstream product of SERAT and SiR. Application of Cys to epidermal peels of wild-type leaves induced stomatal closure to an extent comparable to sulfate application (Figure 6A). Cys also induced stomatal closure in the serat tko and sir1-1 mutants, indicating that sulfate failed to close stomata in both mutants due to a lack of or insufficient incorporation of sulfate into Cys (Figures 6B and 6C). Application of Gly did not affect stomata of the wild-type and the mutants (Figure 6). These results demonstrate that assimilatory sulfate incorporation into Cys is an integral part of sulfate-dependent stomatal closure via ABA biosynthesis.
Figure 6.
Exogenous Application of Cys Promotes Stomatal Closure in Cys-Synthesis–Limited Mutants.
(A) to (C) Impact of sulfate (white, 2 mM MgSO4), Cys (yellow, 0.5 mM), and Gly (dark gray, 0.5 mM) on the stomatal apertures of wild-type (A), sir1-1 (B), and serat tko (C) plants. Data represent mean ± sd in (n ≥ 50, derived from ≥5 individual plants).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
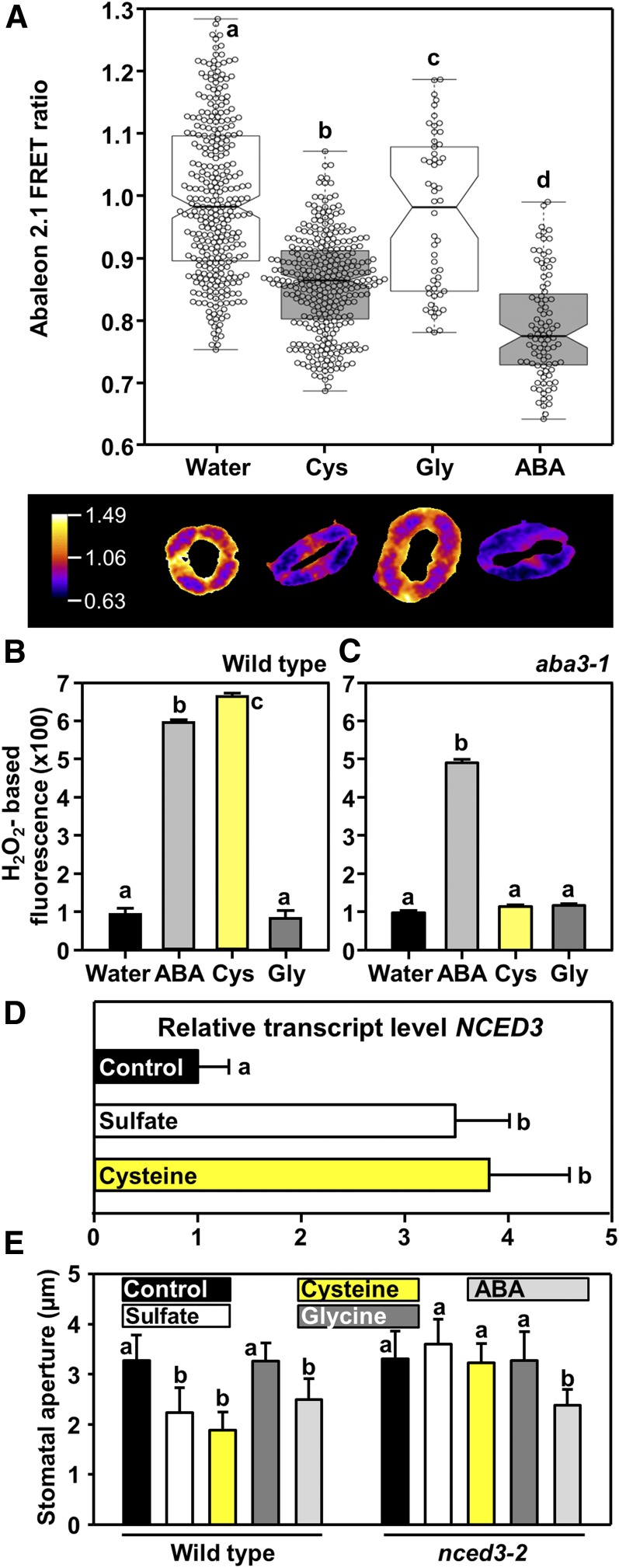
To provide direct evidence for the impact of Cys on ABA biosynthesis in guard cells, we exposed epidermal peels to Cys and monitored ABA concentration changes using ABAleon2.1. Amino acids like Cys enter cells via plasma membrane transporters that are energized by an inward-directed proton gradient (Wipf et al., 2002). Application of 500 μM Cys buffered to pH5.5 decreased the ABAleon2.1 emission ratio by 15% (P < 0.001). When exposed to ABA at a concentration as high as 50 μM, the ABAleon2.1 emission ratio in the stomatal guard cells decreased by 21% (P < 0.001). This indicates that Cys elevates intracellular ABA concentration in guard cells similar to the direct application of the stress hormone (Figure 7A). Moreover, Cys application induced ROS formation in the wild type in a similar manner as addition of ABA, but failed to trigger ROS and stomatal closure in the aba3-1 mutant (Figures 7B and 7C; Supplemental Figure 3). Application of 0.5 mM Gly did not affect ABA levels (Figure 7A), ROS formation (Figures 7B and 7C), or stomatal closure (Supplemental Figure 3), demonstrating that ABA synthesis and stomatal closure in response to Cys application is specific and not due to a pleiotropic stimulation by amino acid treatment. These results demonstrated that Cys or a downstream product of Cys metabolism control ABA synthesis and ABA-dependent regulation of stomatal aperture. Based on these findings, we reinvestigated the impact of sulfur-containing metabolites on NCED3 transcription. We found that petiole feeding of sulfate or Cys induces transcription of NCED3 in leaves (Figure 7D). In agreement with the rate-limiting role of NCED3 in ABA precursor production, a NCED3 loss-of-function mutant (nced3-2) failed to close stomata after application of sulfate or Cys (Figure 7E).
Figure 7.
Exogenous Application of Cys Induces ABA Production in Guard Cells and ROS Formation in an ABA3-Dependent Manner.
(A) The upper panel shows the ABAleon2.1 emission ratio, calculated from guard cells treated with only water (n = 308), 500 μM Cys (n = 311), 500 μM Gly (n = 54), or 50 μM ABA (n = 91), respectively. The average ABAleon2.1 emission ratio is calculated per stomatal area. Statistical tests are performed with respect to the water control. The lower panel shows a representative stomata subjected to the treatment indicated in the x axis label above.
(B) and , (C) Impact of ABA (light gray, 50 μM), Cys (yellow, 0.5 mM), or Gly (dark gray, 0.5 mM) on hydrogen peroxide production in guard cells of wild type (B) and aba3-1 (C) as measured by H2DCF-DA staining. Data represent mean ± sd in (n = 50, derived from ≥5 individual plants).
(D) and (E) Impact of sulfate (white, 15 mM), Cys (yellow, 0.5 mM), Gly (dark gray, 0.5 mM), or ABA (light gray, 50 μM) on transcript levels of NCED3 in leaves of the wild type (D) and stomatal aperture of the wild type and the nced3-2 mutant (E). Data represent mean ± sd (n = 50, derived from ≥5 individual plants for stomatal closure, n = 3 for determination of transcript levels).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
Physiological Relevance of Cys-Mediated Stomatal Closure
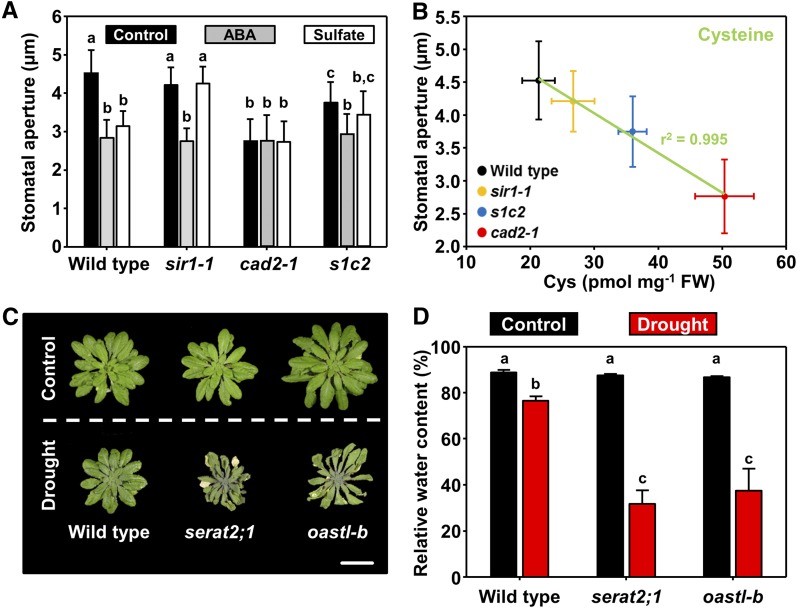
We analyzed the stomatal apertures of the cad2-1, sir1-1, and sir1-1 × cad2-1 (s1c2) mutants to provide direct evidence for the physiological importance of Cys-induced stomatal closure and to exclude the possibility that Cys triggers stomatal closure by affecting the ROS scavenger glutathione (GSH). The cad2-1 mutant suffers from decreased GSH synthesis capacity, overoxidation of the cytosol, and accumulation of Cys (Cobbett et al., 1998; Speiser et al., 2018). The stomata are closed in cad2-1, but the molecular link between decreased GSH synthesis and stomatal closure was enigmatic (Okuma et al., 2011). It has been postulated that lowered levels of GSH in cad2-1 might cause increased ROS levels resulting in stomatal closure. We recently showed that the s1c2 mutant displays even more enhanced oxidation of the cytosolic GSH pool when compared with cad2-1. Furthermore, the s1c2 mutant accumulates less Cys than cad2-1 due to the lowered reduction of sulfite to sulfide, which is the direct precursor Cys (Speiser et al., 2018). In accordance with the lower Cys levels in s1c2 when compared with cad2-1, the stomata were more open in s1c2 when compared with cad2-1 (Figure 8A). Because GSH levels are comparable in s1c2 and cad2-1, GSH is not the trigger of stomatal closure in cad2-1 and s1c2 (Speiser et al., 2018). The s1c2 mutant is still responsive to ABA, eliminating the possibility of disturbed ABA sensing being the cause for the stomatal reopening in s1c2. The data strongly indicate that stomatal closure in cad2-1 is caused by the accumulation of Cys, which stimulates ABA biosynthesis and stomatal closure. Dynamic fitting of the Cys steady-state levels against the stomatal aperture revealed a remarkably high correlation (r2 = 0.995, Figure 8B). The correlation between GSH and the stomatal aperture was lower (r2 = 0.7) in the mutants (Supplemental Figure 4), suggesting that the stomatal closure phenotype of cad2-1 (Okuma et al., 2011) is not due to lowered GSH but a result of Cys accumulation.
Figure 8.
Physiological Relevance of Cys-Induced Stomatal Closure.
(A) Stomatal aperture in detached leaves of wild type and mutants affected in provision of sulfide for Cys synthesis (sir1-1), synthesis of GSH from Cys (cad2-1), and the sir1-1 cad2-1 double mutant (s1c2). Detached leaves were fed with ABA (gray, 50 μM) or sulfate (white, 15 mM MgSO4) dissolved in water (black, Control) for 180 min before analysis. Data represent mean ± sd (n = 50, derived from five individual plants). Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05). Please note that feeding of ABA via the petiole can close the stomata in sir1-1 and s1c2.
(B) Correlation analysis between endogenous foliar Cys steady-state levels and stomatal aperture in wild type (black), sir1-1 (orange), cad2-1 (red), and s1c2 (blue). The data were dynamically fitted with a linear equation (y = m x +b). The negative slope demonstrates that higher endogenous Cys levels correlate with stomatal closure in mutants with functional ABA biosynthesis and ABA response. The regression coefficient was 0.997 and the coefficient of determination (r2) was 0.995, demonstrating the significant correlation between endogenous Cys steady-state levels and stomatal aperture.
(C) and (D) Cys-synthesis–depleted mutants (serat2;1 and oastl-b) are sensitive to soil drying. Five-week-old soil-grown wild type and Cys-synthesis–depleted mutants were subjected to drought stress for 25 d. The serat2;1 and oastl-b mutants suffered from only mild depletion of Cys synthesis capacity and thus grow like the wild type under nonstressed conditions (Heeg et al., 2008; Watanabe et al., 2008). Application of drought resulted in a more pronounced wilting of both Cys-synthesis–depleted mutants when compared with the wild type (C) caused by a significantly greater water loss of both mutants upon soil drying (D). Scale bar = 4 cm. The relative water content of the leaves was determined according to the following equation: (fresh weight – dry weight) / (turgid weight – dry weight). Data represent mean ± se (n = 4 individual plants).
Letters indicate statistically significant differences between groups determined with one-way ANOVA (P < 0.05).
To provide functional evidence for the biological relevance of sulfate-dependent Cys synthesis for ABA production upon soil drying, we challenged two Cys-synthesis–depleted mutants (serat2-1, and O-acetylserine(thiol)lyase isoform-B (oastl-B; Heeg et al., 2008; Watanabe et al., 2008) with water removal. In this experimental setup, temperature, air humidity, and light intensity were kept constant between the control and the stressed plants. Thus the only trigger for stomatal closure was limited water supply due to soil drying. The two Cys-synthesis–depleted mutants displayed enhanced wilting and suffered from lower relative water content than the wild type under this condition (Figures 8C and 8D). Furthermore, the survival after rewatering of both mutants was reduced when compared with the wild type (Supplemental Figure 5). Our findings provide direct evidence for the biological relevance of Cys synthesis to decrease the transpiration rate of leaves when soil drying occurs.
DISCUSSION
Do Stomata Read Xylem-Derived Sulfate as a Signal for Soil Drying?
The existence of a root-to-shoot signal for drought-induced stomatal closure has been controversial (Wilkinson and Davies, 2002; Seo and Koshiba, 2011; Tardieu, 2016; McLachlan et al., 2018). Grafting experiments with ABA-deficient tomato (Solanum lycopersicum) plants provide evidence that the signal for stomatal closure upon soil drying requires ABA biosynthesis in shoots but not in roots. Thus the proposed signal cannot be ABA itself (Holbrook et al., 2002; Christmann et al., 2007). Furthermore, these experiments showed that stomatal closure occurred when the soil dried but before the water potential of leaves was affected. A root-to-shoot drought signal must, therefore, originate in the roots, travel via the xylem, and trigger ABA biosynthesis in leaves. Sulfate is a good candidate for this signal because it exclusively enters the plant via the roots, and according to our findings, gains competence as an inductor of stomatal closure after its reduction in plastids to sulfide and subsequent incorporation into Cys. Cys itself or a downstream product of Cys metabolism then triggers ABA biosynthesis (see below). Metabolic profiling of maize xylem sap revealed that sulfate was the only detectable xylem-born chemical that consistently showed significantly higher concentrations in the xylem sap during early and late drought, whereas abundance of other nutrients such as phosphate, nitrate, or ammonium decreased in the xylem sap of maize upon soil drying in this analysis (Ernst et al., 2010). This specific drought-induced increase of sulfate in the xylem sap has been found in maize, common hop (Humulus lupulus), and poplar (Populus x canescens; Goodger et al., 2005; Ernst et al., 2010; Korovetska et al., 2014; Malcheska et al., 2017). In maize, early drought stress increases the concentration of sulfate in the xylem to up to 2 mM (Goodger et al., 2005). In petiole feeding experiments, application of 2 mM sulfate leads to closure of stomata of maize (Supplemental Figure 1) and Arabidopsis (Figure 1) in less than half an hour. Consistently, sulfate (2 mM) feeding of petioles decreased stomatal conductance of poplar leaves in the same time-scale (Malcheska et al., 2017). These findings allow speculating that fluctuations of sulfate concentration in the xylem path can serve as a trigger for stomatal closure. In line with these results, ABA steady-state levels of wild-type Arabidopsis seedlings increased upon enhanced external sulfate supply (Cao et al., 2014) and exogenous application of sulfate triggered ABA accumulation in the cytosol of guard cells (Figure 3). The published results on the enhanced xylem transport of sulfate and the here uncovered molecular mechanism for sulfate-induced ABA biosynthesis via Cys suggest that sulfate can work together with other drought-induced root-to-shoot signals like hydraulic signals, strigolactones, and the recently identified peptide CLAVAT3/EMBRYO-SURROUNDING REGION-RELATED25 (CLE25) to adjust the stomatal conductance toward the root water-availability (Takahashi et al., 2018).
How and Where Does Sulfate Trigger Stomata Closure?
Previously, sulfate was shown to activate recombinant plant QUAC1 expressed in Xenopus oocytes. Furthermore, the Arabidopsis quac1 loss-of-function mutant was insensitive to sulfate-induced stomatal closure. Based on these results, sulfate was reported to close stomata by direct activation of the plasma membrane-localized anion channel QUAC1 (Malcheska et al., 2017). Here, we showed that sulfate must be reduced to sulfide and incorporated into Cys to trigger stomatal closure in Arabidopsis. This finding contradicts a significant contribution of sulfate for direct activation of QUAC1 at the plasma membrane but does not exclude a QUAC1 function during sulfate-induced stomatal closure by other mechanisms in Arabidopsis. We also demonstrated that sulfate after incorporation into Cys tunes ABA biosynthesis. QUAC1 is a canonical downstream target of ABA signaling (Imes et al., 2013). Consequently, the failure of quac1 to close stomata upon sulfate application can be explained by the lack of activation of QUAC1 due to sulfate-induced ABA biosynthesis.
The initial steps of ABA biosynthesis take place in the plastid, which is also the exclusive site of sulfate reduction (Khan et al., 2010; Seo and Koshiba, 2011). Accordingly, the action of sulfate on guard-cell ABA synthesis requires translocation of sulfate from the cytosol to the plastids by the plastid-localized group-3 sulfate transporters (SULTR3; Takahashi et al., 2011; Cao et al., 2013). Remarkably, transcription of four out of the five SULTR3 members (3;1, 3;2, 3;4, and 3;5) is enriched in guard cells, indicating more efficient transport of sulfate from the cytosol into the plastids of guard cells when compared with mesophyll cells (Bauer et al., 2013). In plastids, adenosine-5-phosphosulfate reductase (APR; Figure 9) catalyzes the committed step of sulfate reduction. APR2 is tightly regulated at the transcriptional level and possesses significant flux control on the sulfate assimilation pathway (Loudet et al., 2007). Notably, APR2 transcription is also enriched in guard cells (Bauer et al., 2013) and apr2 seedlings accumulated less ABA in response to external sulfate supply when compared with wild-type seedlings (Cao et al., 2014). These findings point to a highly active sulfate metabolism in the guard cell itself, which allowed isolated stomata in epidermal peels to react to external application of sulfate in the same concentration range and with the same temporal kinetics when compared with petiole feeding of sulfate. However, our results do not exclude a significant contribution of sulfate or Cys to the induction of ABA biosynthesis in the vasculature (see next section).
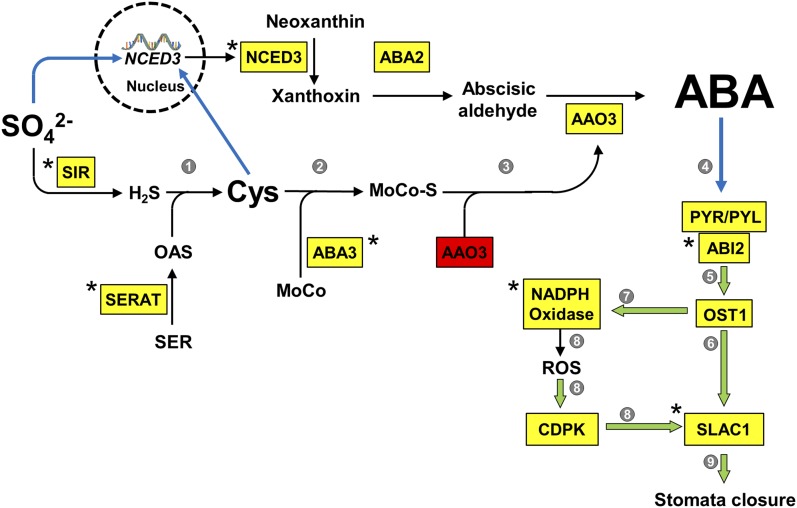
Figure 9.
Model for the Function of Sulfate in ABA Biosynthesis and Stomatal Closure.
Enzymes catalyzing reactions (black arrows) in the biosynthesis pathways of Cys and ABA as well as the sensing of ABA for stomatal closure are shown in yellow boxes. Red box indicates the nonactive apoenzyme, which requires the cofactor for activation. Asterisks indicate enzymes that have been shown by this study to be essential for sulfate/Cys-induced stomatal closure. The stimulating effects of metabolites or enzymes on downstream reactions are depicted as blue arrows or green open arrows, respectively. Numbers in gray circles indicate references for known regulations/processes not experimentally addressed:
1: Synthesis of Cys is limited by provision of O-actylserine and sulfide (Takahashi et al., 2011).
2: Cys is the substrate of the MoCo-sulfurylase ABA3 required for activation of AAO3 (Bittner et al., 2001).
3: Cys level affects AAO activity in vivo (Cao et al., 2014).
4: PYR/PYL acts as an ABA receptor and controls PP2C activity (e.g. ABI1; Park et al., 2009).
5: PP2C activity regulates activation of OST1 in response to ABA (Vlad et al., 2009).
6: OST1 activates SLAC1 by phosphorylation at multiple residues (Geiger et al., 2009; Lee et al., 2009).
7: OST1 phosphorylates RBOHF (NADPH oxidase; Sirichandra et al., 2009).
8: ROS induce stomatal closure in an ABA2-dependent manner (Sierla et al., 2016).
9: SLAC1 is essential for ABA-induced stomatal closure (Vahisalu et al., 2008).
How does Cys trigger ABA biosynthesis?
ABA biosynthesis is limited at two steps: (i) production of xanthoxin by NCED in the vasculature and (ii) sulfurylation of the MoCo of ABSCISIC ALDEHYDE OXIDASE3 (AAO3) by the MoCo sulfurylase ABA3 (Xiong et al., 2001; Wollers et al., 2008).
A previous study demonstrated that sulfate induces the transcription of NCED3 specifically in guard cells (Malcheska et al., 2017), which was supposed to add to the stimulation of ABA biosynthesis upon enhanced sulfate availability by providing a substrate precursor for AAO3 (Nambara and Marion-Poll, 2005). We provided direct genetic evidence that a functional NCED3 gene is essential for stomatal closure after petiole feeding of sulfate and application of sulfate to guard cells in epidermal peels (Figure 7E). Because sulfate cannot close stomata in Cys-depleted mutants (Figures 5 and 6), we hypothesized that sulfate must be incorporated into Cys for induction of NCED3. Indeed, Cys can also induce NCED3 transcription in whole leaves (Figure 7D). The NCED3 induction in whole leaves by sulfate or Cys is more pronounced than the transcriptional induction of NCED3 by sulfate in enriched guard cells (Malcheska et al., 2017), suggesting that NCED3 is also up-regulated in the vasculature. Consequently, our data are entirely consistent with the transcriptional induction of NCED3 and the significant accumulation of NCED3 protein in the vasculature of drought-stressed plants (Endo et al., 2008). NCED3 transcription is also up-regulated by drought stress-induced root-to-shoot transport of the peptide hormone CLE25 (Takahashi et al., 2018). This offers the possibility that root-derived sulfate after assimilation into Cys modulates NCED3 transcription and ABA production in leaves together with other drought-induced signals.
The coexistence of independent signals for root-to-shoot communication during drought has already been hypothesized (Tardieu, 2016; McLachlan et al., 2018) and will add to the spatial and temporal coordination of the multiple sites of ABA synthesis in plants (Kuromori et al., 2018). By adding Cys as a new signal for stomatal closure, our study contributes to the understanding of the integration of the multiple internal signals to optimize the stomatal aperture under diverse environmental challenges. Remarkably, some of these environmental challenges are perceived in leaves and known to up-regulate de novo Cys biosynthesis (e.g. high light stress and pathogen attack; Kruse et al., 2007; Müller et al., 2017). This opens the possibility that Cys-induced stomatal closure is not only triggered by enhanced sulfate transport upon soil drying as described in Ernst et al. (2010) and Malcheska et al. (2017), but also contributes to stomatal closure upon other stresses. It is now timely to evaluate the diverse stomatal closure signals (ABA, CLE25, ethylene precursors, hydraulic signals, strigolactones, and sulfate/Cys) with high temporal resolution and to characterize the contribution of these signals to individual stress responses resulting in stomatal closure (e.g. high light stress, pathogen attack, darkness, low humidity, CO2-level, or soil drying).
NCED3 produces a precursor that is finally converted to ABA by AAO activity. Remarkably, the dominant AAO isoform, AAO3, can be post-translationally activated by the MoCo sulfurylase ABA3. The strong induction of AAO3 mRNA in guard cells by dehydration strongly suggests that AAO3 activity limits ABA biosynthesis (Koiwai et al., 2004). Furthermore, the restriction of ABA3 activity to guard cells is sufficient for stomatal closure in response to decreased relative humidity (Bauer et al., 2013). Cys serves as the substrate for ABA3 during activation of AAO3 (Bittner et al., 2001; Heidenreich et al., 2005). Consequently, total aldehyde oxidase activity (including AAO3 activity) is low in sulfate-depleted wild-type plants and sultr3;1 mutants, which suffer from decreased Cys biosynthesis. Short-term exogenous application of Cys restores the decreased aldehyde oxidase activity in sultr3;1 (Cao et al., 2014). We thus propose that Cys is a limiting factor for ABA biosynthesis in the early stages of drought conditions in guard cells and potentially also in other cell types of the leaf (Figure 9). Restriction of ABA3 activity to guard cells is also sufficient for sulfate/Cys-induced stomatal closure. Remarkably, the aba3-1 mutant is unable to close stomata upon petiole feeding with sulfate, demonstrating that induction of NCED3 by Cys in the absence of functional ABA3 is not sufficient to trigger ABA accumulation in guard cells for stomatal closure. We thus hypothesize that Cys-induced stomatal closure depends on the activation of NCED and ABA3 activity in leaves. Further studies are required to determine whether sulfate/Cys-induced stomata closure depends on differential activation of NCED3 and AAO3 at the multiple ABA production sites. However, the drought-sensitive phenotype of Cys-synthesis–depleted mutants provides functional evidence for biological relevance of Cys synthesis during drought stress-induced production of ABA, because this phenotype is caused by higher transpiration of leaves.
Limitation of ABA biosynthesis by Cys in guard cells also explains the so far not understood closed stomata phenotype of the cad2-1 mutant (Okuma et al., 2011). This knock-down mutant accumulates significantly higher Cys levels due to a reduction in GSH synthesis (Cobbett et al., 1998; Speiser et al., 2018). Remarkably, the s1c2 double mutant, which has lower Cys levels than cad2-1, partially reopens the stomata when compared with cad2-1. These results strongly suggest that the trigger for stomatal closure in cad2-1 was the accumulation of Cys and not the depletion of the ROS-scavenger GSH (Figures 8A and 8B; Supplemental Figure 5).
CONCLUSION
Stomata are the gates of plants to the environment. They allow efficient uptake of CO2 for photosynthesis but also offer pathogens avenues to invade plants and allow water to evaporate. Consequently, the stomatal aperture is tightly controlled by several independent signals of which ABA acts as the master regulator. Independent studies identified sulfate as a molecule transported in early drought from the roots-to-shoots of woody and herbaceous plants. We have shown that sulfate feeding of the petiole leads to stomatal closure by increasing the production of Cys, which stimulates the synthesis of the drought hormone ABA. This is one of the few examples of nutrient signaling where the end product of a primary anabolic pathway acts as a limiting factor in the initiation of hormone signaling by stimulating the biosynthesis of this hormone in stress conditions.
METHODS
Plant Material and Growth
Seeds of Arabidopsis (Arabidopsis thaliana) Col-0 (ecotype Columbia) and the mutants aba3-1 (CS157), abi2-1 (CS23), cad2-1 (Cobbett et al., 1998), nced3-2 (Endo et al., 2008), oastl-b (SALK_021183), quac1 (Meyer et al., 2010), slac1-3 (SALK_099139), serat2;1 (SALK_ 099019), sir1-1 (GABI-Kat 550A09), serat tko (SALK_ 050213 x SALK_ 099019 x Kazusa_KG752, Dong et al., 2017), myb60:aba3-1 (Bauer et al., 2013), and ABAleon2.1 (Waadt et al., 2014) were sown on soil (Tonsubstrat from Ökohum) supplemented with 10% (v/v) vermiculite and 2% quartz sand. Seeds were stratified at 4°C for 2 days in the dark. The plants were subsequently grown under long-day conditions for five weeks before the experiment (16 h light, 150 µmol m−2 s−1 [OSRAM, LUMILUX Cool White] at 22°C and 8 h dark at 18°C for day and night, respectively). Relative humidity was kept at 50%).
Stomatal Aperture Bioassay
Epidermal peels were obtained from the abaxial side of Arabidopsis leaves as described in Ernst et al. (2010) and floated on distilled water for 2 h under constant light. The peels were then transferred to distilled water pH 5.5 supplemented without (control) or with effectors (0.8 mM to 20 mM MgSO4, 15 mM MgCl2, 15 mM Na2SO4, 15 mM MgNO3, 0.5 mM Cys, Gly, and 50 µM ABA) for the indicated periods. Stomata were imaged before and after treatment with a conventional wide-angle microscope (Leica DMIRB). Each experiment was performed at least in triplicate and showed the same results.
Petiole Feeding Bioassay
Leaves were detached from plants by cutting the petioles under distilled water and placing them in an Eppendorf tube containing distilled water for 2 h. Subsequently, the detached leaves were transferred to Eppendorf tubes containing just water (pH 5.5) or water (pH 5.5) supplemented with 2 mM sulfate (MgSO4) or 50 µM ABA and incubated for up to 3 h under constant light conditions. Stomata were imaged at the indicated time points using a conventional wide-angle microscope (Leica DMIRB).
Transpiration Bioassays
A minimum of three equally sized leaves were detached from five individual plants and treated with or without effectors as described in the petiole feeding bioassay. Loss of water was determined by subtraction of fresh weight at the indicated time-point from fresh weight determined directly after detachment of the leaves. Leaves were incubated for up to 180 min at room temperature and short-day conditions (see above).
RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted from 100 mg to 150 mg of leaf epidermal peels incubated in water or water containing sulfate for 3 h using the peqGOLD total RNA kit (VWR). RNA was converted into cDNA using a cDNA synthesis kit (Fermentas), in accordance with the manufacturer’s instructions. The RT-qPCR analysis was performed using SYBR mix (qPCRBIOSyGreen Mix Lo-ROX) in a RotorQ cycler (Qiagen) according to the manufacturer’s guidelines. The absolute amount of transcript for each gene was determined by standard curve analysis. Absolute expression data were normalized against the average expression values of the reference gene TIP41-like (Han et al., 2013). The primers used for RT-qPCR analysis of ABA marker genes are listed in Supplemental Table 1.
H2O2 Quantification In Guard Cell Leaves
The ROS in intact stomata were determined according to Pei et al. (2000). The abaxial side of Arabidopsis leaves was peeled with tweezers and floated on water without and with effectors for 2 h as described above. Subsequently, 50-μM 2′,7′-dichlorodihydrofluorescein diacetate was added to the samples. After 30 min of incubation, the ROS-specific fluorescence was detected using a confocal microscope (Nikon A2). An excitation of 488 nm and an emission of 525 nm were used. Five images were taken for each peel from an individual plant and the fluorescent signal was quantified for all stomata (50–120) using the open source software ImageJ (http://fiji.sc/).
NADPH Oxidase Inhibitor Experiments
To evaluate the importance of NADPH oxidases for sulfate-induced ROS production and sulfate-induced stomata closure, the NADPH oxidase inhibitor DPI (10 μM, Sigma-Aldrich) was applied. Epidermal cells were pretreated with 10 µM DPI, pH 5.5 for 30 min before the application of sulfate, ABA, or water (control) containing DPI for 3 h. ROS formation was then measured as described above. According to the manufacturer's specifications, DPI solutions remain stable for at least 12 h; thus, DPI solutions were prepared immediately before use.
In Vivo Analyses of ABA in Guard Cells with the ABAleon2.1 Sensor
ABAleon2.1 is an established sensor that allows the rapid detection of ABA concentration changes in the cytosol of guard cells in response to external stimuli (Waadt et al., 2014). ABA concentrations as high as 50 μM are used to ensure full response of the ABAleon2.1 sensor in intact plant cells. Sulfate-induced production of ABA was analyzed in leaves obtained from 4-week-old plants, grown on 0.7% Hoagland-Agar medium. For guard cell imaging, the abaxial epidermal layer was peeled from leaves of individual plants. These peels were incubated on water for 2 h and then transferred to 15-mM sulfate solution adjusted to pH 5.5 for 2 h. Experiments were performed in triplicate and a minimum of five guard cells per peel were used. In total, a minimum of 67 and a maximum of 308 guard cells were analyzed for each condition. A quantity of 458 nm (cyan fluorescent protein) and 514 nm (yellow fluorescent protein) laser lines were used in the LSM 510 (Zeiss). C1 channel detects emission between 475 nm and 500 nm upon 458-nm excitation. C2 channel detects emission between 525 nm and 550 nm upon 458-nm excitation. C3 control channel detects 525 nm to 500 nm emission upon 514-nm excitation. All the experiments were done on the same day with exactly the same settings. Therefore, the same water control is used for experiments shown in Figures 3 and 5.
The fluorescence resonance energy transfer (FRET) ratio is calculated as the ratio of C2 to C1. A mask based on a threshold in the C1 channel is applied to restrict the background and used to compare C1 intensity among all images to avoid intensity-based changes in FRET ratio. The data were analyzed via a FIJI macro, programmed in-house according to Waadt et al. (2014). Stomata are selected manually for average FRET ratio measurement in FIJI. The overall image quality was assessed based on the C3 channel to avoid epidermal cell contribution to stomata FRET signal. The data were transferred and pooled in Microsoft Excel for Student’s t test analysis.
In Vivo Analyses of the ABA Response with the ProRAB18:GFP Sensor
The ProRAB18:GFP lines (Kim et al., 2011) were grown on soil for 25 d in short-day chambers. Five hours after onset of light, leaves were detached for petiole feeding with water, 50 μM ABA, or 15 mM sulfate (pH5.5) for 2 h. Intact leaf samples were visualized with a Nikon E wide-field fluorescent microscope connected to a 10× objective. GFP (488-nm excitation/507-nm to 514-nm emission) for reporter visualization, red fluorescent protein (488-nm excitation/562-nm emission) for background, and bright field images have been obtained. For guard cell imaging, stomata with both guard cells in focus were manually selected. After background subtraction, the maximum GFP intensity in the middle of the nuclei was determined. For epidermal pavement cell GFP measurements, regions that exclude guard cells were manually selected and average intensity in the region of interest including multiple pavement cells was measured (NWater = 666 guard cells from five individual leaves, NSulfate = 534 guard cells from four individual leaves, NABA = 894 guard cells from four individual leaves).
Statistical Analysis
Statistical analysis was performed using SigmaPlot 12.5 (Systat). Different data sets were analyzed for statistical significance with the one-way repeated measures analysis of variance (one-way ANOVA), which uses the Holm-Sidak method for all pairwise multiple comparisons. Normality distribution of data points was tested with the Shapiro-Wilk method (p to reject was P > 0.05). Letters indicate significant difference (P < 0.05) in the figures. In the case of comparisons between two sample groups, the Student‘s t test (*P < 0.05) was applied (only in Figure 3B).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ABA3, AT1G16540; ABI2, AT5G57050; AAO3, AT2G27150; HIGHLY ABA INDUCED1, AT5G59220; LEA7, AT1G52690; NCED3, AT3G14440; RD20, AT2g29830; RD29B, AT5G52300; SERAT1;1, AT5G56760; SERAT2;1, AT1G55920; SERAT2;2, AT3G13110; SiR, AT5G04590; SLAC1, AT1G12480; TIP41-like, AT4G34270.
Supplemental Data
Supplemental Figure 1. Physiological relevant fluctuations of sulfate in the xylem of maize affect stomata aperture.
Supplemental Figure 2. Functional ABA3 is a prerequisite for stomata closure after petiole feeding of sulfate.
Supplemental Figure 3. Stomatal closure by cysteine requires functional ABA3.
Supplemental Figure 4. NCED3 is essential for stomata closure after petiole feeding of sulfate.
Supplemental Figure 5. Correlation analysis of foliar GSH levels and stomatal aperture in mutants with depleted cysteine and/or glutathione synthesis.
Supplemental Figure 6. Cysteine-Synthesis–Depleted mutants display enhanced drought-stress sensitivity.
Supplemental Table 1. Oligonucleotides used for RT-qPCR analysis.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Prof. Eiji Nambara (University of Toronto, Canada) for kindly providing the nced3-2 mutant.
This work was supported by the Higher Education Commission of Pakistan and Gomal University (D.I. Khan Scholarship to S.B.); a European Molecular Biology Organization Long Term Fellowship (EMBO ALTF 1478-2014 to V.V.U.); the Sonderforschungsbereich (“Collaborative Research Centers” grant SFB 1036 TP 13); and the Deutsches Forschungsgemeinschaft (grants HE1848/14-1, HE1848/15-1, and HE1848/16-1 to R.H., grants WI3560/1-1 and WI3560/2-1 to M.W., and the “Receptor Light” project B08 TRR166 to D.G. and Ra.H.).
Author Contributions
S.B. performed most of the experiments; H.R. determined the kinetics for sulfate-induced stomatal closure; V.V.U. performed ABA measurements in guard cells with the ABAleon2.1 sensor; R.W. provided material and advice for in vivo ABA measurements; C.H. supervised S.B. for selected aspects of the work; Ra.H., M.M., C.-B.X., and D.G. contributed to the writing of the manuscript and advised H.R; M.W. and Ru.H. wrote the manuscript and supervised S.B. and V.V.U.
Footnotes
Articles can be viewed without a subscription.
References
- Bauer H., et al. (2013). The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 23: 53–57. [DOI] [PubMed] [Google Scholar]
- Bittner F., Oreb M., Mendel R.R. (2001). ABA3 is a molybdenum cofactor sulfurase required for activation of aldehyde oxidase and xanthine dehydrogenase in Arabidopsis thaliana. J. Biol. Chem. 276: 40381–40384. [DOI] [PubMed] [Google Scholar]
- Cao M.J., Wang Z., Wirtz M., Hell R., Oliver D.J., Xiang C.B. (2013). SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J. 73: 607–616. [DOI] [PubMed] [Google Scholar]
- Cao M.J., Wang Z., Zhao Q., Mao J.L., Speiser A., Wirtz M., Hell R., Zhu J.K., Xiang C.B. (2014). Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J. 77: 604–615. [DOI] [PubMed] [Google Scholar]
- Christmann A., Weiler E.W., Steudle E., Grill E. (2007). A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 52: 167–174. [DOI] [PubMed] [Google Scholar]
- Cobbett C.S., May M.J., Howden R., Rolls B. (1998). The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J. 16: 73–78. [DOI] [PubMed] [Google Scholar]
- Cross A.R., Jones O.T. (1986). The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy A.R., Zandalinas S.I., Gómez-Cadenas A., Blumwald E., Mittler R. (2018). Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci. Signal. 11: eaam9514. [DOI] [PubMed] [Google Scholar]
- Dong Y., et al. (2017). Sulfur availability regulates plant growth via glucose-TOR signaling. Nat. Commun. 8: 1174. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Endo A., et al. (2008). Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 147: 1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst L., Goodger J.Q.D., Alvarez S., Marsh E.L., Berla B., Lockhart E., Jung J., Li P., Bohnert H.J., Schachtman D.P. (2010). Sulphate as a xylem-borne chemical signal precedes the expression of ABA biosynthetic genes in maize roots. J. Exp. Bot. 61: 3395–3405. [DOI] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A., Romeis T., Hedrich R. (2009). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodger J.Q., Sharp R.E., Marsh E.L., Schachtman D.P. (2005). Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J. Exp. Bot. 56: 2389–2400. [DOI] [PubMed] [Google Scholar]
- Han B., Yang Z., Samma M.K., Wang R., and Shen W. (2013). Systematic validation of candidate reference genes for qRT-PCR normalization under iron deficiency in Arabidopsis. Biometals 26: 403–413. [DOI] [PubMed] [Google Scholar]
- Hedrich R. (2012). Ion channels in plants. Physiol. Rev. 92: 1777–1811. [DOI] [PubMed] [Google Scholar]
- Heeg C., Kruse C., Jost R., Gutensohn M., Ruppert T., Wirtz M., Hell R. (2008). Analysis of the Arabidopsis O-acetylserine(thiol)lyase gene family demonstrates compartment-specific differences in the regulation of cysteine synthesis. Plant Cell 20: 168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich T., Wollers S., Mendel R.R., Bittner F. (2005). Characterization of the NifS-like domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J. Biol. Chem. 280: 4213–4218. [DOI] [PubMed] [Google Scholar]
- Holbrook N.M., Shashidhar V.R., James R.A., Munns R. (2002). Stomatal control in tomato with ABA-deficient roots: Response of grafted plants to soil drying. J. Exp. Bot. 53: 1503–1514. [PubMed] [Google Scholar]
- Honda K., Yamada N., Yoshida R., Ihara H., Sawa T., Akaike T., and Iwai S. (2015). 8-Mercapto-Cyclic GMP mediates hydrogen sulfide-induced stomatal closure in Arabidopsis. Plant Cell Physiol. 56: 1481–1489 [DOI] [PubMed] [Google Scholar]
- Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., Gong Z. (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes D., Mumm P., Böhm J., Al-Rasheid K.A., Marten I., Geiger D., Hedrich R. (2013). Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J. 74: 372–382. [DOI] [PubMed] [Google Scholar]
- Jin Z., Xue S., Luo Y., Tian B., Fang H., Li H., Pei Y. (2013). Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 62: 41–46. [DOI] [PubMed] [Google Scholar]
- Khan M.S., Haas F.H., Samami A.A., Gholami A.M., Bauer A., Fellenberg K., Reichelt M., Hänsch R., Mendel R.R., Meyer A.J., Wirtz M., Hell R. (2010). Sulfite reductase defines a newly discovered bottleneck for assimilatory sulfate reduction and is essential for growth and development in Arabidopsis thaliana. Plant Cell 22: 1216–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., et al. (2011). Chemical genetics reveals negative regulation of abscisic acid signaling by a plant immune response pathway. Curr. Biol. 21: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koiwai H., Nakaminami K., Seo M., Mitsuhashi W., Toyomasu T., Koshiba T. (2004). Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol. 134: 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korovetska H., Novák O., Jůza O., Gloser V. (2014). Signalling mechanisms involved in the response of two varieties of Humulus lupulus L. to soil drying: I. changes in xylem sap pH and the concentrations of abscisic acid and anions. Plant Soil 380: 375–387. [Google Scholar]
- Kruse C., Jost R., Lipschis M., Kopp B., Hartmann M., Hell R. (2007). Sulfur-enhanced defence: Effects of sulfur metabolism, nitrogen supply, and pathogen lifestyle. Plant Biol (Stuttg) 9: 608–619. [DOI] [PubMed] [Google Scholar]
- Kuromori T., Seo M., Shinozaki K. (2018). ABA transport and plant water stress responses. Trends Plant Sci. 23: 513–522. [DOI] [PubMed] [Google Scholar]
- Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D., Schroeder J.I. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K., Brandt B., Li J., Merilo E., Wang Y.F., Keshwani M.M., Taylor S.S., Kollist H., Schroeder J.I. (2013). Calcium-dependent and -independent stomatal signaling network and compensatory feedback control of stomatal opening via Ca2+ sensitivity priming. Plant Physiol. 163: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.C., Lan W., Buchanan B.B., Luan S. (2009). A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 106: 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisjak M., Srivastava N., Teklic T., Civale L., Lewandowski K., Wilson I., Wood M.E., Whiteman M., Hancock J.T. (2010). A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol. Biochem. 48: 931–935. [DOI] [PubMed] [Google Scholar]
- Lobell D.B., Roberts M.J., Schlenker W., Braun N., Little B.B., Rejesus R.M., Hammer G.L. (2014). Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344: 516–519. [DOI] [PubMed] [Google Scholar]
- Loudet O., Saliba-Colombani V., Camilleri C., Calenge F., Gaudon V., Koprivova A., North K.A., Kopriva S., Daniel-Vedele F. (2007). Natural variation for sulfate content in Arabidopsis thaliana is highly controlled by APR2. Nat. Genet. 39: 896–900. [DOI] [PubMed] [Google Scholar]
- Malcheska F., et al. (2017). Drought enhanced xylem sap sulfate closes stomata by affecting ALMT12 and guard cell ABA synthesis. Plant Physiol. 174: 798–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan D.H., Pridgeon A.J., Hetherington A.M. (2018). How Arabidopsis talks to itself about its water supply. Mol. Cell 70: 991–992. [DOI] [PubMed] [Google Scholar]
- Merilo E., Laanemets K., Hu H., Xue S., Jakobson L., Tulva I., Gonzalez-Guzman M., Rodriguez P.L., Schroeder J.I., Broschè M., Kollist H. (2013). PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol. 162: 1652–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S., Mumm P., Imes D., Endler A., Weder B., Al-Rasheid K.A., Geiger D., Marten I., Martinoia E., Hedrich R. (2010). AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 63: 1054–1062. [DOI] [PubMed] [Google Scholar]
- Müller S.M., Wang S., Telman W., Liebthal M., Schnitzer H., Viehhauser A., Sticht C., Delatorre C., Wirtz M., Hell R., Dietz K.J. (2017). The redox-sensitive module of cyclophilin 20-3, 2-cysteine peroxiredoxin and cysteine synthase integrates sulfur metabolism and oxylipin signaling in the high light acclimation response. Plant J. 91: 995–1014. [DOI] [PubMed] [Google Scholar]
- Murata Y., Pei Z.-M., Mori I.C., Schroeder J. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A.C., Merlot S., Vavasseur A., Fenzi F., Giraudat J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E., Marion-Poll A. (2005). Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 56: 165–185. [DOI] [PubMed] [Google Scholar]
- Okuma E., Jahan M.S., Munemasa S., Hossain M.A., Muroyama D., Islam M.M., Ogawa K., Watanabe-Sugimoto M., Nakamura Y., Shimoishi Y., Mori I.C., Murata Y. (2011). Negative regulation of abscisic acid-induced stomatal closure by glutathione in Arabidopsis. J. Plant Physiol. 168: 2048–2055. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Fung P., Nishimura N., Jensen D.R., Fujii H., Zhao Y., Lumba S., Santiago J., Rodrigues A., Chow T.F., Alfred S.E., Bonetta D., et al. (2009). Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.M., Kuchitsu K., Ward J.M., Schwarz M., Schroeder J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- Rosenberger C.L., Chen J. (2018). To Grow or Not to Grow: TOR and SnRK2 Coordinate Growth and Stress Response in Arabidopsis. Mol. Cell 69: 3–4. [DOI] [PubMed] [Google Scholar]
- Scuffi D., Álvarez C., Laspina N., Gotor C., Lamattina L., García-Mata C. (2014). Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol. 166: 2065–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M., Koshiba T. (2011). Transport of ABA from the site of biosynthesis to the site of action. J. Plant Res. 124: 501–507. [DOI] [PubMed] [Google Scholar]
- Shang Y., Dai C., Lee M.M., Kwak J.M., Nam K.H. (2016). BRI1-Associated Receptor Kinase1 regulates guard cell ABA signaling mediated by Open Stomata1 in Arabidopsis. Mol. Plant 9: 447–460. [DOI] [PubMed] [Google Scholar]
- Sierla M., Waszczak C., Vahisalu T., Kangasjärvi J. (2016). Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171: 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C., Gu D., Hu H.C., Davanture M., Lee S., Djaoui M., Valot B., Zivy M., Leung J., Merlot S., Kwak J.M. (2009). Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583: 2982–2986. [DOI] [PubMed] [Google Scholar]
- Speiser A., Silbermann M., Dong Y., Haberland S., Uslu V.V., Wang S., Bangash S.A.K., Reichelt M., Meyer A.J., Wirtz M., Hell R. (2018). Sulfur partitioning between glutathione and protein synthesis determines plant growth. Plant Physiol. 177: 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F., Suzuki T., Osakabe Y., Betsuyaku S., Kondo Y., Dohmae N., Fukuda H., Yamaguchi-Shinozaki K., Shinozaki K. (2018). A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556: 235–238. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Kopriva S., Giordano M., Saito K., Hell R. (2011). Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 62: 157–184. [DOI] [PubMed] [Google Scholar]
- Tardieu F. (2016). Too many partners in root-shoot signals. Does hydraulics qualify as the only signal that feeds back over time for reliable stomatal control? New Phytol. 212: 802–804. [DOI] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y.F., Nishimura N., Chan W.Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., Schroeder J.I., Kangasjärvi J. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad F., Rubio S., Rodrigues A., Sirichandra C., Belin C., Robert N., Leung J., Rodriguez P.L., Laurière C., Merlot S. (2009). Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R., Hitomi K., Nishimura N., Hitomi C., Adams S.R., Getzoff E.D., and Schroeder J.I. (2014). FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 3: e01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., et al. (2018). Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Mol. Cell 69: 100–112 e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wan R., Shi Y., Xue S. (2016). Hydrogen sulfide activates S-type anion channel via OST1 and Ca2+ modules. Mol. Plant 9: 489–491. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Mochida K., Kato T., Tabata S., Yoshimoto N., Noji M., Saito K. (2008). Comparative genomics and reverse genetics analysis reveal indispensable functions of the serine acetyltransferase gene family in Arabidopsis. Plant Cell 20: 2484–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S., Davies W.J. (2002). ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 25: 195–210. [DOI] [PubMed] [Google Scholar]
- Wipf D., Ludewig U., Tegeder M., Rentsch D., Koch W., Frommer W.B. (2002). Conservation of amino acid transporters in fungi, plants and animals. Trends Biochem. Sci. 27: 139–147. [DOI] [PubMed] [Google Scholar]
- Wollers S., Heidenreich T., Zarepour M., Zachmann D., Kraft C., Zhao Y., Mendel R.R., Bittner F. (2008). Binding of sulfurated molybdenum cofactor to the C-terminal domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J. Biol. Chem. 283: 9642–9650. [DOI] [PubMed] [Google Scholar]
- Xiong L., Ishitani M., Lee H., Zhu J.K. (2001). The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13: 2063–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]