Abstract
Rheumatoid arthritis (RA) mainly affects various joints of the body, including the temporomandibular joint (TMJ), and it involves an infiltration of autoantibodies and inflammatory leukocytes into articular tissues and the synovium. Initially, the synovial lining tissue becomes engaged with several kinds of infiltrating cells, including osteoclasts, macrophages, lymphocytes, and plasma cells. Eventually, bone degradation occurs. In order to elucidate the best therapy for RA, a comprehensive study of RA pathogenesis needs to be completed. In this article, we discuss a Fas-deficient condition which develops into RA, with an emphasis on the role of sphingosine 1-phosphate (S1P)/S1P receptor 1 signaling which induces the migration of osteoclast precursor cells. We describe that Fas/S1P1 signaling via NF-κB activation in osteoclasts is a key factor in TMJ-RA severity and we discuss a strategy for blocking nuclear translocation of the p50 NF-κB subunit as a potential therapy for attenuating osteoclastogenesis.
Keywords: Temporomandibular joint, Rheumatoid arthritis, Osteoclast, Fas, S1P1, NF-κB
1. Introduction
Rheumatoid arthritis (RA) develops when the synovial lining of joints in the body are infiltrated by leukocytes, plasma cells, lymphocytes, endothelial cells, and activated macrophages that mediate induction of a chronic inflammatory state (Fig. 1) [1], [2]. Bone erosions often accompany the development of an inflammatory state in many rheumatic diseases and these destructive bone lesions develop when a break in cortical bone has occurred. Loss of adjacent trabecular and cortical bone structures proximal to a bone lesion generally occur due to osteoclastic bone resorption, a reduction in articular cartilage, and bone marrow edema [3], [4]. The latter can be detected with magnetic resonance imaging (MRI) [5], [6].
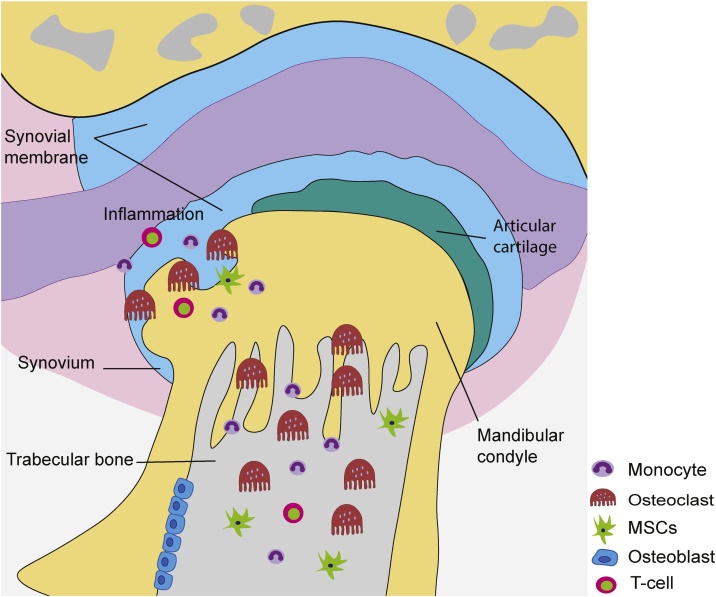
Fig. 1.
Bone destruction associated with TMJ-RA. RANKL is produced by T cells and inflamed synovium (synovitis) tissue. The presence of this cytokine in the TMJ leads to the local destruction of cartilage and eventually severe subchondral trabecular bone loss as a result of the recruitment and enhanced differentiation of osteoclast progenitor cells (monocytes) concomitant with a decrease in the recruitment and differentiation of osteoblast progenitor cells and MSCs.
In RA, the size and number of erosions that are present in affected joints may be an indicator of the extent of damage that has occurred. Bone erosions represent the contribution of the bone marrow compartment to the destruction of bone and the promotion of cytokines involved in pro-inflammation [7], [8]. Osteoclasts are multinucleated myeloid lineage cells that are able to resorb bone, and these cells are essential for the remodeling and regeneration of bone. In degenerative diseases such as arthritis, osteoporosis, and cancer bone metastasis, the presence of an excessive number of osteoclasts has been found to contribute to bone destruction [9].
Receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) stimulate the generation of osteoclast cells [10], [11], [12], [13]. In response to sphingosine 1-phosphate (S1P) signaling, osteoclasts then attach to regions of bone undergoing resorption [14]. In RA when S1P is expressed at higher levels, sphingosine kinase (Sphk) 1 is also present at higher levels and this leads to resistance to Fas-mediated death signaling (Fig. 2) [15], [16]. The activity and number of osteoclasts are modulated by rates of cell death and cell differentiation [17].
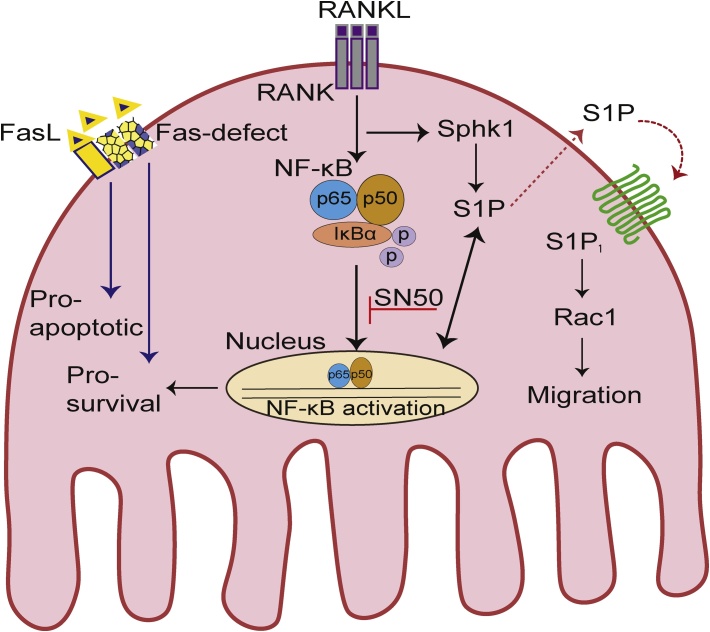
Fig. 2.
A proposed model of the mechanism and action by which crosstalk between the Fas and S1P/S1P1 signaling pathways regulate osteoclastogenesis. Upon binding of RANK to RANKL, IκBα proteins are rapidly phosphorylated. As a result, the p50 and p65 subunits of NF-κB are able to translocate to the nucleus, bind DNA, and activate gene transcription. Overexpression of Sphk1 and S1P1 accelerates the migration of osteoclast progenitor cells, while defects in Fas mitigate apoptosis to promote osteoclast survival and massive bone destruction.
Communication between osteoclasts and osteoblasts or T cells is also mediated by RANKL and S1P receptor 1 (S1P1), and this signaling promotes cell regulation, proliferation, migration, apoptosis, expression of inflammatory genes [18], [19], [20], chemotaxis [21], and differentiation [22], [23]. Events downstream of RANKL binding to osteoclasts involves the activation of various signaling pathways, including nuclear factor of activated T cells cytoplasmic 1 (NFATc1), Akt/PKB, NF-κB, JNK, p38, and ERK, to induce the differentiation, activity, and survival of osteoclasts [14], [24]. In addition, RANKL binding of osteoclast progenitor cells leads to upregulation of Fas expression via NF-κB, thereby resulting in osteoclast cells being targets of Fas-stimulated apoptosis [25]. To promote cell death, Fas-stimulated apoptosis in osteoclasts involving the release of cytochrome c from mitochondria and activation of both caspase 3 and caspase 9 [17].
To date, the predominant strategies for decreasing bone resorption include the addition of estrogen, tamoxifen, or certain bisphosphonates to accelerate the death rate of osteoclasts or decrease osteoclast formation [17], [26], [27], [28], [29], [30]. In this review, we discuss our findings that osteoclast activity-dependent Fas/S1P1 signaling via activation of NF-κB mediates the development of RA and bone resorption in the TMJ. In addition, a prospective therapeutic intervention involving administration of SN50 to block signaling by p50 NF-κB to attenuate osteoclastogenesis in the pathogenesis of temporomandibular joint rheumatoid arthritis (TMJ-RA) is discussed.
2. TMJ-RA
The TMJ is needed for sliding and hinge movements of the jaw. Consequently, it is the most frequently used joint in the body [31]. The TMJ is composed of an articular disc and the mandibular condyle which provide upper and lower articular cavities between the condyle and the glenoid fossa [32]. Unlike other synovial joints, the condyle of the mandible of the TMJ produces less type I collagen [33], its superficial layer does not produce type II collagen, and its articular surfaces consist of fibrous tissue rather than hyaline cartilage [34]. In addition, mandibular condyle cartilage originates from cranial neural crest cells and is considered a secondary cartilage of the chondroskeleton [35].
Clinically, the TMJ is involved in 4–80% of RA cases [36], [37], [38]. This wide range is due on the different examinations performed, the different selection of the patient populations and age distribution, the duration of the RA occurs, the different diagnostic criteria for classifying joint involvement, and the imaging techniques used [5], [37], [38], [39], [40], [41], [42], [43]. However, more than 50% of patients with RA complaints about TMJ discomfort [36], [44], [45].
Manifestation of RA in the TMJ can include swelling, pain, impaired movement, and crepitation. These characteristics are similar to the manifestations of RA in other joints, and these symptoms usually correlate well with radiographic change observed in joints. Specifically in the TMJ, radiographic changes can include erosion, the presence of a subcortical cyst, and a gradual decrease in joint space due to granulation, deossification, pencil head, or spiked deformity of the condylar head [41], [44].
Several studies were found in TMJ-RA. Proteoglycan-induced arthritis mice showed a degradation of cartilage matrix in TMJ due to the effects of the cytokines from the inflamed joints [46]. The ratio of RANKL and OPG was significantly increased in TMJ of collagen-induced acetate rats [47]. The pathway of TMJ involvement in human RA was found to be the same as in other joints based on the analysis of histological findings [48]. However, it should be emphasized that due to the originates difference of the cartilage of TMJ and other joints [35], the pathogenesis of RA in TMJ may differ from the other joints.
Therefore, the histomorphology and the comprehensive analysis of TMJ-RA pathogenesis need to be completed although clinical findings in TMJ-RA are similar to RA in other joints [41]. Especially for the dentist, the difficulties associated with diagnosing TMJ-RA patients [49] posing challenges to diagnose and recover the effects of TMJ-RA.
3. Fas-deficiency in RA pathogenesis
Fas antigen is expressed in various tissues, including the thymus. Fas antigen also shares structural homology with many cell-surface receptors (e.g., tumor necrosis factor (TNF) receptors) and has been shown to mediate apoptosis [50]. In human RA, FLICE-inhibitory protein (FLIP) an antiapoptotic protein, has been detected in synovial tissues and fibroblast-like synoviocytes (FLS) culture. It has been reported that FLS of RA is sensitized to promote Fas mediated-apoptosis by the down-regulation of FLIP [51].
In mice, the Fas antigen gene maps to chromosome 19 and mice that carry a lymphoproliferation mutation (MRL/lpr mice) have been found to have defects in the Fas antigen gene [52], [53]. An aberrant transcription factor in MRL/lpr mice causes premature termination of Fas transcription, and this results in aberrant splicing of Fas mRNA (Fig. 2) [54]. The clinical symptoms exhibited by MRL/lpr mice include hypergammablobulinaemia, production of anti-DNA antibodies, rheumatoid factor (RF), arthritis, and glomerulonephritis. Moreover, the latter closely resembles human autoimmune systemic lupus erythematosus (SLE) [55].
MRL/lpr mice are suitable as a model to study an immune complex disease, to determine the etiology, and to evaluate the therapies. Several studies were done using MRL/lpr mice, such as the effect of the radiation therapy to ameliorate the autoimmune disease [56], the therapeutic effects of Artemisinin analog SM934 to ameliorate lupus syndromes [57], allergic inflammation in blepharitis [58], the analysis pathogenesis of Graft-versus-host disease (GVHD)-like wasting syndrome by defects in Fas-mediated lymphocyte apoptosis [59], and histopathological analysis of spontaneous arthritis [60], [61].
The pathogenesis of RA in MRL/lpr mice particularly involves interactions between osteoclast cells and immune cells (e.g., activated dendritic cells (DCs) with peripheral T cells) via RANKL activation. Thus, a possible therapeutic treatment for lymphoproliferative and autoimmune arthritis has been tested in MRL/lpr mice with induction of Fas-independent apoptosis of CD4+ T cells via TRAIL/TRAIL-R2 which was achieved by performing multiple transfers of activated and Fas-deficient DCs [62], [63]. It has been reported that osteoprotegerin (OPG) induces apoptosis in osteoclast and osteoclast precursor via Fas/FasL pathway. OPG has been shown to trigger the increase of Bax/Bcl-2 ratio and the level of activated cleaved-caspase 9 and caspase 3 in dose-dependent manner [64].
When staining for tartrate-resistant acid phosphatase (TRAP) was performed in long bones and the TMJs of MRL/lpr mice and control mice, the former contained a greater number of osteoclasts. This observation suggests that mutation of Fas leads to prolonged survival of osteoclasts (Fig. 2) [17], [65]. Correspondingly, defects in Fas have been associated with a greater number of osteoclasts and subsequent reductions in bone mineral density, bone volume, and trabecular thickness [17].
4. The role of S1P/S1P1 signaling in osteoclasts under RA conditions
S1P, a cell-derived lysophospholipid growth factor [66], is the final metabolite of the sphingolipid pathway and it is generated from Sphk and S1P lyase [67]. S1P binds G protein-coupled receptors (GPCRs) at the cell surface, particularly the S1P1–5 receptors (Fig. 3) [68], to mediate a wide variety of essential cellular processes: cell differentiation, survival, proliferation, migration, and invasion, as well as angiogenesis and trafficking of immune cells [69], [70], [71], [72]. While S1P1–3 are widely expressed in various tissues, expression of S1P4 and S1P5 is restricted to immune cells and cells of the central nervous system [73], [74].
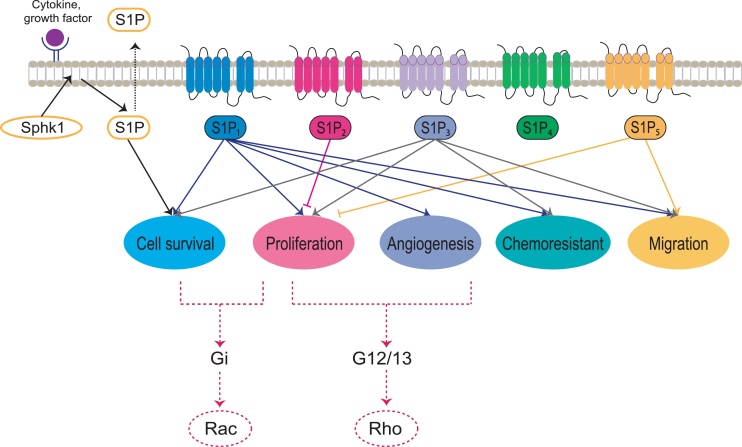
Fig. 3.
The molecular mechanism of S1P signaling via its receptors. Extracellular molecules, including pro-inflammatory cytokines, growth factors, and S1P itself, can induce intracellular signaling that leads to the activation of kinases that phosphorylate Sphk1. S1P is subsequently generated following phosphorylation of Sphk, and S1P localizes to the cell membrane from the cytosol to convert sphingosine to S1P via phosphorylation events. S1P1 is linked to Gi and is a potent activator of the Rac signaling pathway, while S1P2 and S1P3 are linked to G12/13 and activate the Rho signaling pathway.
Potential roles for S1P in angiogenesis, cancer, and autoimmune diseases such as RA have been reported [73]. In RA synoviocytes, S1P has been shown to enhance expression of prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2) in response to the pro-inflammatory cytokines, TNF-α and interleukin (IL)-1β [18]. These cytokines production induce expression of matrix metalloproteinases (MMPs) and activate osteoclasts, thereby resulting in bone resorption and soft tissue damage [75]. Osteoclasts themselves also express S1P, and this molecule stimulates the migration of both osteoblasts and mesenchymal stem cells (MSCs). Accordingly, S1P that derives from osteoclasts may attract osteoblasts to areas of bone resorption as one of the first steps in the process of replacing bone that is lost in a damaged area [76].
S1P signaling via S1P1 regulates T cell development and enhances synoviocyte proliferation, inflammatory cytokine expression, and osteoclastogenesis in bone homeostasis [23], [77], [78], [79]. Meanwhile, expression of Sphk1 has been shown to be higher in osteoclasts that have cathepsin K deleted. These osteoblasts also exhibited a higher RANKL/OPG ratio which corresponded with a greater number of osteoclasts present [80].
Differentiation and maturation of osteoclasts require signaling via Sphk1/S1P/S1P3 as previously demonstrated in assays of Runx2 expression and alkaline phosphatase activity [81]. Transforming growth factor (TGF)-β/Smad3 signaling has also been shown to affect cartilage homeostasis by influencing S1P/S1P receptor signaling and chondrocyte migration. Correspondingly, in mice with Smad3-deficient chondrocytes, only the Sphk1/S1P/S1P3 signaling axis was found to play an important role in degradation of the mandibular condylar [82].
The S1P/S1P1 signaling axis controls the migration of osteoclast precursor cells [21], [22], [23], [83] from bone tissues into blood circulation [21] and it may also induce synovial hyperplasia in RA. RANKL stimulation has been found to decrease expression of S1P1 in an NF-κB-dependent manner (Fig. 2) yet not in a NFATc1-dependent manner [21], [23]. Furthermore, RANKL expression during osteoclastogenesis that is induced by the Sphk1/S1P1 signaling axis is the result of interactions between macrophages and bone marrow derived stromal cells (BMSCs) [84].
However, in our recent study, only expression of Sphk1 and S1P1 in MRL/lpr mice increased in the mandibular condyle, and these increases were accompanied by an increase in Rac1 activity (Fig. 3) [21], [65]. Correspondingly, treatment with a S1P1 agonist led to a significant reduction in inflammation and joint destruction, consistent with the predicted actions of the agonist in retaining osteoclast precursor cells [85]. Taken together, these findings suggest that targeting the S1P/S1P1 signaling axis represents a potential treatment for RA [18].
During the development of RA, expression of S1P and S1P1–5 by osteoclasts [65], osteoblasts [81], chondrocytes [82], and MSCs [76], [86] is considered to be essential for modulating cell migration, cell survival, and cytokine or chemokine production during bone formation (Fig. 1) [65], [87]. Therefore, it will be important for future studies to evaluate the role of the S1P/S1P receptor system among the cell-cell interactions that mediate bone homeostasis in TMJ-RA pathogenesis (Fig. 4).
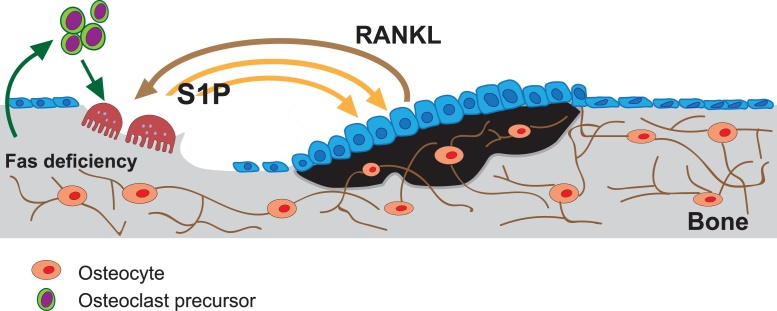
Fig. 4.
Schematic representation of the bone remodeling process. When Fas is defective, a greater amount of S1P is released by osteoclasts (grey arrows) and this leads to increased production of RANKL by osteoblasts (black arrow). As a result, greater stimulation of osteoblasts and the migration of osteoclast precursor cells occurs, and differentiation of osteoclasts is enhanced. S1P that is produced by osteoclasts serves to recruit osteoblasts to bone resorption sites as an initial step in the process to replace lost bone. An overall increase in the level of S1P then stimulates bone formation.
5. Role of the NF-κB in osteoclasts
NF-κB is an inducible transcription factor that binds a particular DNA sequence that is present in a large number of target genes, especially genes that contribute to immune responses and pathogen defense. In autoimmune diseases, such as RA, NF-κB has an essential role in the differentiation, survival, activation, and development of osteoclasts [88], [89], [90].
Two important proteins in osteoclastogenesis are RANK and its ligand, RANKL (also referred to as TNFRSF11A and TRANCE, respectively). In addition to activating mature osteoclasts, RANKL can combine with M-CSF to regulate osteoclast differentiation from monocyte/macrophage precursor cells [14], [89], [91]. Numerous studies have demonstrated that differentiation of osteoclast precursor cells requires induction of NF-κB activity by RANKL [88], [92], [93], while activation of macrophages and osteoclasts requires NF-κB signaling as well [88], [94].
Phosphorylation of the p50-Rel A dimer, the most common form of NF-κB, leads to ubiquitination of IκB proteins (Fig. 2). Poly-ubiquitination of IκB proteins identifies them for rapid degradation by 26S proteasomes, thereby allowing NF-κB dimers to undergo nuclear translocation and activate the transcription of various target genes [95]. However, prior to their degradation, the phosphorylation of IκB proteins by the IκB kinase (IKK) complex facilitates NF-κB activation. The IKK complex consists of a regulatory component, IKKγ or NF-κB essential modulator (NEMO), and catalytic subunits (IKKα and IKKβ) [95], [96], [97], [98]. In MRL/lpr mice, phosphorylation of IκBα, an endogenous inhibitory molecule of NF-κB activation and nuclear translocation of the p50 and p65 subunits of NF-κB, is significantly enhanced compared with control mice [65], [99]. These results suggest that osteoclast activation which occurs with RA involves a role for the classical pathway of NF-κB activation (Fig. 2).
Correspondingly, in both in vivo and in vitro studies a cell-permeable peptide spanning a NEMO-binding domain has been shown to inhibit osteoclastogenesis and activation of NF-κB induced by RANKL [88]. Therefore, it is proposed that a potential strategy for preventing osteoclastogenesis and effectively treating the inflammation associated with bone resorption is to selectively inhibit NF-κB in osteoclasts [65], [88].
6. Blocking nuclear translocation of NF-κB in osteoclasts as therapeutic target of RA
Different strategies have been proposed and tested to inhibit the activation or function of NF-κB. One approach has employed decoy NF-κB sites or their analogs to interfere with the binding of NF-κB to DNA [100]. However, such molecules are polar and rather large, thereby potentially hindering their uptake and cellular bioavailability [96]. Conversely, considering the large interaction surface between NF-κB and DNA, it may be difficult for non-polar molecules that are small in size to specifically disrupt the binding of NF-κB to DNA [96]. A similar argument could be made for molecules designed to inhibit NF-κB protein dimerization [96], [101].
Alternatively, it has been proposed that small peptides that can readily cross the cell membrane could inhibit the activation of NF-κB by blocking translocation of the NF-κB complex to the nucleus. This approach was tested by generating a synthetic peptide that contains the nuclear localization sequence of the p50 NF-κB subunit and a hydrophobic region to facilitate translocation across a cell membrane. This peptide, named SN50, was shown to competitively inhibit the nuclear translocation of NF-κB (Fig. 2) [96], [100], [102].
Moreover, both in vivo and in vitro, SN50 was found to effectively inhibit TNF-α and lipopolysaccharide (LPS)-induced inflammatory responses [102]. SN50 has also been shown to block the translocation of many other transcriptional factors to the nucleus, and to abrogate activation of caspase-3 [96], [100], [103], [104]. In mice, administration of SN50 effectively suppressed NF-κB activation that was dependent on TNF-α/JNK signaling [105], as well as MMP production in alveolar macrophages [106]. Furthermore, in resting normal human peripheral blood derived T cells, the addition of SN50 abolished osteoclast differentiation and induced apoptosis [107], [108].
Based on the results presented here, blocking nuclear translocation of the p50 NF-κB subunit appears to be an effective approach for attenuating the number of osteoclasts present and reducing expression of Sphk1 and S1P1. In particular, it has recently been demonstrated that SN50 targeting of p50 NF-κB translocation to the nuclei of bone marrow macrophages (BMMs) in RA model of MRL/lpr mice inhibits RANKL-induced osteoclastogenesis (Fig. 2) [65].
Subchondral trabecular bone loss in the mandibular condyle was also ameliorated, and this was accompanied by lower expression levels of the osteoclast marker, TRAP, as well as cathepsin K, vascular endothelial growth factor (VEGF), MMP-9, and RANKL. Moreover, treatment with SN50 increased expression of OPG and reduced Sphk1 expression and S1P1 signaling [65]. Taken together, these findings suggest that SN50 is an effective inhibitor of osteoclastogenesis and the preceding migration of osteoclast precursor cells.
7. Conclusions
Osteoclcastogenesis is regulated by multiple signal transduction pathways. To maintain bone homeostasis, osteoclast cells must achieve a balance regulation of formation, function, and trafficking of its precursors. Here, we proposed a model in which crosstalk between Fas and S1P/S1P1 signaling occurs in osteoclast precursor cells and it is dependent on activation of NF-κΒ. Moreover, this signaling affects the migratory behavior of osteoclast precursor cells which is crucial for the pathogenesis of RA.
The TMJ has special characteristics that distinguish it from other joints in the human body. Correspondingly, disorders, symptoms, and disease distribution involving the TMJ are unlike those of other joint disorders. As a result, treatments specific for the TMJ may be needed. However, given that the TMJ is part of a dynamic tissue that undergoes constant remodeling as a result of bone resorption and bone formation processes that are mediated by osteoclasts and osteoblasts, respectively, continued efforts are needed to better understand RA pathogenesis for the identification of effective new therapies.
Author contributions
H. and T.I. conceived the idea.
I.H. and T.I made substantial contributions to the conception, designed, drafted, revised, and was involved in the final approval of the manuscript to be submitted for publication.
T.I. and E.T. reviewed the manuscript for important intellectual content.
All authors approved the final version to be published.
Acknowledgments
This work was supported by the grants provided by JSPS KAKENHI (Grant Numbers. 25713063, 15K15757, 17K19758, 18H03011 to T.I.), The Ichiro Kanehara Foundation, Suzuken Memorial Foundation, The Nakatomi Foundation, Smoking Research Foundation, Takeda Science Foundation, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, Sumitomo Denko Foundation, Mitsui Sumitomo Insurance Welfare Foundation to T.I., and Otsuka Toshimi Scholarship Foundation, Fujii-Otsuka Scholarship to I.H.
Acknowledgments
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Karmakar S., Kay J., Gravallese E.M. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am. 2010;36(2):385–404. doi: 10.1016/j.rdc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X., Chang Y., Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediators Inflamm. 2016;2016:1–9. doi: 10.1155/2016/6813016. 6813016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez-Boj E., Nobauer-Huhmann I., Hanslik-Schnabel B., Dorotka R., Wanivenhaus A.H., Kainberger F. Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum. 2007;56(4):1118–1124. doi: 10.1002/art.22496. [DOI] [PubMed] [Google Scholar]
- 4.Schett G. Bone marrow edema. Ann N Y Acad Sci. 2009;1154:35–40. doi: 10.1111/j.1749-6632.2009.04383.x. [DOI] [PubMed] [Google Scholar]
- 5.Helenius L.M., Tervahartiala P., Helenius I., Al-Sukhun J., Kivisaari L., Suuronen R. Clinical, radiographic and MRI findings of the temporomandibular joint in patients with different rheumatic diseases. Int J Oral Maxillofac Surg. 2006;35(11):983–989. doi: 10.1016/j.ijom.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Narvaez J.A., Narvaez J., Aguilera C., De Lama E., Portabella F. MR imaging of synovial tumors and tumor-like lesions. Eur Radiol. 2001;11(12):2549–2560. doi: 10.1007/s003300000759. [DOI] [PubMed] [Google Scholar]
- 7.Colebatch A.N., Edwards C.J., Ostergaard M., van der Heijde D., Balint P.V., D'Agostino M.A. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):804–814. doi: 10.1136/annrheumdis-2012-203158. [DOI] [PubMed] [Google Scholar]
- 8.Schett G., David J.P. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol. 2010;6(12):698–706. doi: 10.1038/nrendo.2010.190. [DOI] [PubMed] [Google Scholar]
- 9.Jin Z., Li X., Wan Y. Minireview: nuclear receptor regulation of osteoclast and bone remodeling. Mol Endocrinol. 2015;29(2):172–186. doi: 10.1210/me.2014-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyce B.F. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 12.Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 13.Tsurukai T., Udagawa N., Matsuzaki K., Takahashi N., Suda T. Roles of macrophage-colony stimulating factor and osteoclast differentiation factor in osteoclastogenesis. J Bone Miner Metab. 2000;18(4):177–184. doi: 10.1007/s007740070018. [DOI] [PubMed] [Google Scholar]
- 14.Boyce B.F. Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J Bone Miner Res. 2013;28(4):711–722. doi: 10.1002/jbmr.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snider A.J. Sphingosine kinase and sphingosine-1-phosphate: regulators in autoimmune and inflammatory disease. Int J Clin Rheumtol. 2013;8(4) doi: 10.2217/ijr.13.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pi X., Tan S.Y., Hayes M., Xiao L., Shayman J.A., Ling S. Sphingosine kinase 1-mediated inhibition of Fas death signaling in rheumatoid arthritis B lymphoblastoid cells. Arthritis Rheum. 2006;54(3):754–764. doi: 10.1002/art.21635. [DOI] [PubMed] [Google Scholar]
- 17.Wu X., McKenna M.A., Feng X., Nagy T.R., McDonald J.M. Osteoclast apoptosis: the role of Fas in vivo and in vitro. Endocrinology. 2003;144(12):5545–5555. doi: 10.1210/en.2003-0296. [DOI] [PubMed] [Google Scholar]
- 18.Kitano M., Hla T., Sekiguchi M., Kawahito Y., Yoshimura R., Miyazawa K. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54(3):742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 19.Takeshita H., Kitano M., Iwasaki T., Kitano S., Tsunemi S., Sato C. Sphingosine 1-phosphate (S1P)/S1P receptor 1 signaling regulates receptor activator of NF-kappaB ligand (RANKL) expression in rheumatoid arthritis. Biochem Biophys Res Commun. 2012;419(2):154–159. doi: 10.1016/j.bbrc.2012.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel S., Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31(Pt 6):1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- 21.Ishii M., Egen J.G., Klauschen F., Meier-Schellersheim M., Saeki Y., Vacher J. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458(7237):524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu J., Kim H.J., Chang E.J., Huang H., Banno Y., Kim H.H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25(24):5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kikuta J., Kawamura S., Okiji F., Shirazaki M., Sakai S., Saito H. Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc Natl Acad Sci U S A. 2013;110(17):7009–7013. doi: 10.1073/pnas.1218799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng X. RANKing intracellular signaling in osteoclasts. IUBMB Life. 2005;57(6):389–395. doi: 10.1080/15216540500137669. [DOI] [PubMed] [Google Scholar]
- 25.Wu X., Pan G., McKenna M.A., Zayzafoon M., Xiong W.C., McDonald J.M. RANKL regulates Fas expression and Fas-mediated apoptosis in osteoclasts. J Bone Miner Res. 2005;20(1):107–116. doi: 10.1359/JBMR.041022. [DOI] [PubMed] [Google Scholar]
- 26.Hughes D.E., Dai A., Tiffee J.C., Li H.H., Mundy G.R., Boyce B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2(10):1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 27.Kameda T., Mano H., Yuasa T., Mori Y., Miyazawa K., Shiokawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186(4):489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneda T., Ishimaru N., Arakaki R., Kobayashi M., Izawa T., Moriyama K. Estrogen deficiency accelerates murine autoimmune arthritis associated with receptor activator of nuclear factor-kappa B ligand-mediated osteoclastogenesis. Endocrinology. 2004;145(5):2384–2391. doi: 10.1210/en.2003-1536. [DOI] [PubMed] [Google Scholar]
- 29.Arnett T.R., Lindsay R., Kilb J.M., Moonga B.S., Spowage M., Dempster D.W. Selective toxic effects of tamoxifen on osteoclasts: comparison with the effects of oestrogen. J Endocrinol. 1996;149(3):503–508. doi: 10.1677/joe.0.1490503. [DOI] [PubMed] [Google Scholar]
- 30.Halasy-Nagy J.M., Rodan G.A., Reszka A.A. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone. 2001;29(6):553–559. doi: 10.1016/s8756-3282(01)00615-9. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki A., Iwata J. Mouse genetic models for temporomandibular joint development and disorders. Oral Dis. 2016;22(1):33–38. doi: 10.1111/odi.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka E., Detamore M.S., Mercuri L.G. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 33.Benjamin M., Ralphs J.R. Biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- 34.Hinton R.J., Serrano M., So S. Differential gene expression in the perichondrium and cartilage of the neonatal mouse temporomandibular joint. Orthod Craniofac Res. 2009;12(3):168–177. doi: 10.1111/j.1601-6343.2009.01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen G., Darendeliler M.A. The adaptive remodeling of condylar cartilage—a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84(8):691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- 36.Ogus H. Rheumatoid arthritis of the temporomandibular joint. Br J Oral Surg. 1975;12(3):275–284. doi: 10.1016/0007-117x(75)90058-x. [DOI] [PubMed] [Google Scholar]
- 37.Goupille P., Fouquet B., Goga D., Cotty P., Valat J.P. The temporomandibular joint in rheumatoid arthritis: correlations between clinical and tomographic features. J Dent. 1993;21(3):141–146. doi: 10.1016/0300-5712(93)90023-j. [DOI] [PubMed] [Google Scholar]
- 38.Hajati A.K., Alstergren P., Nasstrom K., Bratt J., Kopp S. Endogenous glutamate in association with inflammatory and hormonal factors modulates bone tissue resorption of the temporomandibular joint in patients with early rheumatoid arthritis. J Oral Maxillofac Surg. 2009;67(9):1895–1903. doi: 10.1016/j.joms.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delantoni A., Spyropoulou E., Chatzigiannis J., Papademitriou P. Sole radiographic expression of rheumatoid arthritis in the temporomandibular joints: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):e37–e40. doi: 10.1016/j.tripleo.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y.C., Hsu M.L., Yang J.S., Liang T.H., Chou S.L., Lin H.Y. Temporomandibular joint disorders in patients with rheumatoid arthritis. J Chin Med Assoc. 2007;70(12):527–534. doi: 10.1016/S1726-4901(08)70055-8. [DOI] [PubMed] [Google Scholar]
- 41.Sodhi A., Naik S., Pai A., Anuradha A. Rheumatoid arthritis affecting temporomandibular joint. Contemp Clin Dent. 2015;6(1):124–127. doi: 10.4103/0976-237X.149308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleissner C., Kaesser U., Dehne F., Bolten W.W., Willershausen B. Temporomandibular joint function in patients with longstanding rheumatoid arthritis − I. Role of periodontal status and prosthetic care — a clinical study. Eur J Med Res. 2003;8(3):98–108. [PubMed] [Google Scholar]
- 43.Narvaez J.A., Narvaez J., Roca Y., Aguilera C. MR imaging assessment of clinical problems in rheumatoid arthritis. Eur Radiol. 2002;12(7):1819–1828. doi: 10.1007/s00330-001-1207-z. [DOI] [PubMed] [Google Scholar]
- 44.Ruparelia P.B., Shah D.S., Ruparelia K., Sutaria S.P., Pathak D. Bilateral TMJ Involvement in Rheumatoid Arthritis. Case Rep Dent. 2014;2014:262430. doi: 10.1155/2014/262430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gynther G.W., Holmlund A.B., Reinholt F.P., Lindblad S. Temporomandibular joint involvement in generalized osteoarthritis and rheumatoid arthritis: a clinical, arthroscopic, histologic, and immunohistochemical study. Int J Oral Maxillofac Surg. 1997;26(1):10–16. doi: 10.1016/s0901-5027(97)80838-7. [DOI] [PubMed] [Google Scholar]
- 46.Ghassemi-Nejad S., Kobezda T., Rauch T.A., Matesz C., Glant T.T., Mikecz K. Osteoarthritis-like damage of cartilage in the temporomandibular joints in mice with autoimmune inflammatory arthritis. Osteoarthritis Cartilage. 2011;19(4):458–465. doi: 10.1016/j.joca.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W.W., Xu Z.M., Li Z.Q., Zhang Y., Han B. RANKL, OPG and CTR mRNA expression in the temporomandibular joint in rheumatoid arthritis. Exp Ther Med. 2015;10(3):895–900. doi: 10.3892/etm.2015.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno T., Kagawa T., Kanou M., Ishida N., Fujii T., Fukunaga J. Pathology of the temporomandibular joint of patients with rheumatoid arthritis—case reports of secondary amyloidosis and macrophage populations. J Craniomaxillofac Surg. 2003;31(4):252–256. doi: 10.1016/s1010-5182(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 49.Moen K., Bertelsen L.T., Hellem S., Jonsson R., Brun J.G. Salivary gland and temporomandibular joint involvement in rheumatoid arthritis: relation to disease activity. Oral Dis. 2005;11(1):27–34. doi: 10.1111/j.1601-0825.2004.01054.x. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe-Fukunaga R., Brannan C.I., Copeland N.G., Jenkins N.A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 51.Palao G., Santiago B., Galindo M., Paya M., Ramirez J.C., Pablos J.L. Down-regulation of FLIP sensitizes rheumatoid synovial fibroblasts to Fas-mediated apoptosis. Arthritis Rheum. 2004;50(9):2803–2810. doi: 10.1002/art.20453. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T., Sakai Y., Miyawaki S., Shimizu A., Koiwai O., Ohno K. A molecular genetic linkage map of mouse chromosome 19, including the lpr, Ly-44, and Tdt genes. Biochem Genet. 1991;29(7-8):325–335. doi: 10.1007/BF00554140. [DOI] [PubMed] [Google Scholar]
- 53.Watson M.L., Rao J.K., Gilkeson G.S., Ruiz P., Eicher E.M., Pisetsky D.S. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 1992;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adachi M., Watanabe-Fukunaga R., Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen P.L., Eisenberg R.A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 56.Ootsuyama A., Okazaki R., Norimura T. Effect of extended exposure to low-dose radiation on autoimmune diseases of immunologically suppressed MRL/MpTn-gld/gld mice. J Radiat Res. 2003;44(3):243–247. doi: 10.1269/jrr.44.243. [DOI] [PubMed] [Google Scholar]
- 57.Hou L.F., He S.J., Li X., Yang Y., He P.L., Zhou Y. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63(8):2445–2455. doi: 10.1002/art.30392. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi S., Futatsugi-Yumikura S., Fukuoka A., Yoshimoto T., Nakanishi K., Yonehara S. Fas deficiency in mice with the Balb/c background induces blepharitis with allergic inflammation and hyper-IgE production in conjunction with severe autoimmune disease. Int Immunol. 2013;25(5):287–293. doi: 10.1093/intimm/dxs109. [DOI] [PubMed] [Google Scholar]
- 59.Chu J.L., Ramos P., Rosendorff A., Nikolic-Zugic J., Lacy E., Matsuzawa A. Massive upregulation of the Fas ligand in lpr and gld mice: implications for Fas regulation and the graft-versus-host disease-like wasting syndrome. J Exp Med. 1995;181(1):393–398. doi: 10.1084/jem.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haraldson T., Jonsson R., Tarkowski A. Spontaneous temporomandibular joint arthropathy in MRL-lpr/lpr mice. J Oral Pathol. 1988;17(8):386–389. doi: 10.1111/j.1600-0714.1988.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 61.Ratkay L.G., Tait B., Tonzetich J., Waterfield J.D. Lpr and MRL background gene involvement in the control of adjuvant enhanced arthritis in MRL-lpr mice. J Autoimmun. 1994;7(5):561–573. doi: 10.1006/jaut.1994.1041. [DOI] [PubMed] [Google Scholar]
- 62.Izawa T., Ishimaru N., Moriyama K., Kohashi M., Arakaki R., Hayashi Y. Crosstalk between RANKL and Fas signaling in dendritic cells controls immune tolerance. Blood. 2007;110(1):242–250. doi: 10.1182/blood-2006-11-059980. [DOI] [PubMed] [Google Scholar]
- 63.Izawa T., Kondo T., Kurosawa M., Oura R., Matsumoto K., Tanaka E. Fas-independent T-cell apoptosis by dendritic cells controls autoimmune arthritis in MRL/lpr mice. PLoS One. 2012;7(12):1–12. doi: 10.1371/journal.pone.0048798. e48798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W., Xu C., Zhao H., Xia P., Song R., Gu J. Osteoprotegerin induces apoptosis of osteoclasts and osteoclast precursor cells via the Fas/Fas ligand pathway. PLoS One. 2015;10(11):1–14. doi: 10.1371/journal.pone.0142519. e0142519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutami I.R., Izawa T., Mino-Oka A., Shinohara T., Mori H., Iwasa A. Fas/S1P1 crosstalk via NF-kappaB activation in osteoclasts controls subchondral bone remodeling in murine TMJ arthritis. Biochem Biophys Res Commun. 2017;490(4):1274–1281. doi: 10.1016/j.bbrc.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15(5):513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Spiegel S., Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11(6):403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spiegel S., Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4(5):397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 69.Schwab S.R., Cyster J.G. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8(12):1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 70.Cuvillier O. Sphingosine in apoptosis signaling. Biochim Biophys Acta. 2002;1585(2-3):153–162. doi: 10.1016/s1388-1981(02)00336-0. [DOI] [PubMed] [Google Scholar]
- 71.Kluk M.J., Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582(1-3):72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 72.Takabe K., Paugh S.W., Milstien S., Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez T., Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92(5):913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 74.Dev K.K., Mullershausen F., Mattes H., Kuhn R.R., Bilbe G., Hoyer D. Brain sphingosine-1-phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther. 2008;117(1):77–93. doi: 10.1016/j.pharmthera.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Jovanovic D.V., Martel-Pelletier J., Di Battista J.A., Mineau F., Jolicoeur F.C., Benderdour M. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages: a possible role in rheumatoid arthritis. Arthritis Rheum. 2000;43(5):1134–1144. doi: 10.1002/1529-0131(200005)43:5<1134::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 76.Quint P., Ruan M., Pederson L., Kassem M., Westendorf J.J., Khosla S. Sphingosine 1-phosphate (S1P) receptors 1 and 2 coordinately induce mesenchymal cell migration through S1P activation of complementary kinase pathways. J Biol Chem. 2013;288(8):5398–5406. doi: 10.1074/jbc.M112.413583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goetzl E.J., Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest. 2004;114(11):1531–1537. doi: 10.1172/JCI23704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosen H., Goetzl E.J. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 79.Pettus B.J., Bielawski J., Porcelli A.M., Reames D.L., Johnson K.R., Morrow J. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17(11):1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 80.Lotinun S., Kiviranta R., Matsubara T., Alzate J.A., Neff L., Luth A. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013;123(2):666–681. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brizuela L., Martin C., Jeannot P., Ader I., Gstalder C., Andrieu G. Osteoblast-derived sphingosine 1-phosphate to induce proliferation and confer resistance to therapeutics to bone metastasis-derived prostate cancer cells. Mol Oncol. 2014;8(7):1181–1195. doi: 10.1016/j.molonc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mori H., Izawa T., Tanaka E. Smad3 deficiency leads to mandibular condyle degradation via the sphingosine 1-phosphate (S1P)/S1P3 signaling axis. Am J Pathol. 2015;185(10):2742–2756. doi: 10.1016/j.ajpath.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 83.Ishii T., Shimazu Y., Nishiyama I., Kikuta J., Ishii M. The role of sphingosine 1-phosphate in migration of osteoclast precursors; an application of intravital two-photon microscopy. Mol Cells. 2011;31(5):399–403. doi: 10.1007/s10059-011-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiao L., Zhou Y., Zhu L., Yang S., Huang R., Shi W. SPHK1-S1PR1-RANKL axis regulates the interactions between macrophages and BMSCs in inflammatory bone loss. J Bone Miner Res. 2018 doi: 10.1002/jbmr.3396. [DOI] [PubMed] [Google Scholar]
- 85.Kikuta J., Iwai K., Saeki Y., Ishii M. S1P-targeted therapy for elderly rheumatoid arthritis patients with osteoporosis. Rheumatol Int. 2011;31(7):967–969. doi: 10.1007/s00296-010-1634-8. [DOI] [PubMed] [Google Scholar]
- 86.Price S.T., Beckham T.H., Cheng J.C., Lu P., Liu X., Norris J.S. Sphingosine 1-Phosphate receptor 2 regulates the migration, proliferation, and differentiation of mesenchymal stem Cells. Int J Stem Cell Res Ther. 2015;2(2):1–14. doi: 10.23937/2469-570x/1410014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao C., Fernandes M.J., Turgeon M., Tancrede S., Di Battista J., Poubelle P.E. Specific and overlapping sphingosine-1-phosphate receptor functions in human synoviocytes: impact of TNF-alpha. J Lipid Res. 2008;49(11):2323–2337. doi: 10.1194/jlr.M800143-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Jimi E., Aoki K., Saito H., D'Acquisto F., May M.J., Nakamura I. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10(6):617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 89.Jones D.H., Kong Y.Y., Penninger J.M. Role of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis. 2002;61(Suppl. 2):ii32–ii39. doi: 10.1136/ard.61.suppl_2.ii32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown K.D., Claudio E., Siebenlist U. The roles of the classical and alternative nuclear factor-kappaB pathways: potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res Ther. 2008;10(4):212. doi: 10.1186/ar2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xing L., Schwarz E.M., Boyce B.F. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 92.Iotsova V., Caamano J., Loy J., Yang Y., Lewin A., Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 93.Franzoso G., Carlson L., Xing L., Poljak L., Shores E.W., Brown K.D. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spehlmann M.E., Eckmann L. Nuclear factor-kappa B in intestinal protection and destruction. Curr Opin Gastroenterol. 2009;25(2):92–99. doi: 10.1097/MOG.0b013e328324f857. [DOI] [PubMed] [Google Scholar]
- 95.Ghosh S., Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 96.Karin M., Yamamoto Y., Wang Q.M. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3(1):17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 97.Karin M., Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12(1):85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 98.Novack D.V. Role of NF-kappaB in the skeleton. Cell Res. 2011;21(1):169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oura R., Arakaki R., Yamada A., Kudo Y., Tanaka E., Hayashi Y. Induction of rapid T cell death and phagocytic activity by Fas-deficient lpr macrophages. J Immunol. 2013;190(2):578–585. doi: 10.4049/jimmunol.1103794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta S.C., Sundaram C., Reuter S., Aggarwal B.B. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799(10–12):775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alkalay I., Yaron A., Hatzubai A., Orian A., Ciechanover A., Ben-Neriah Y. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1995;92(23):10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin Y.Z., Yao S.Y., Veach R.A., Torgerson T.R., Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270(24):14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 103.Tan X., Wen X., Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J Am Soc Nephrol. 2008;19(9):1741–1752. doi: 10.1681/ASN.2007060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salmond R.J., Pitman R.S., Jimi E., Soriani M., Hirst T.R., Ghosh S. CD8+ T cell apoptosis induced by Escherichia coli heat-labile enterotoxin B subunit occurs via a novel pathway involving NF-kappaB-dependent caspase activation. Eur J Immunol. 2002;32(6):1737–1747. doi: 10.1002/1521-4141(200206)32:6<1737::AID-IMMU1737>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 105.Saika S., Miyamoto T., Yamanaka O., Kato T., Ohnishi Y., Flanders K.C. Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor-kappaB, in treatment of corneal alkali burns in mice. Am J Pathol. 2005;166(5):1393–1403. doi: 10.1016/s0002-9440(10)62357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoshida M., Korfhagen T.R., Whitsett J.A. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol. 2001;166(12):7514–7519. doi: 10.4049/jimmunol.166.12.7514. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y.M., Lu T.L., Hsu P.N., Tang C.H., Chen J.H., Liu K.C. Ribosome inactivating protein B-chain induces osteoclast differentiation from monocyte/macrophage lineage precursor cells. Bone. 2011;48(6):1336–1345. doi: 10.1016/j.bone.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 108.Kolenko V., Bloom T., Rayman P., Bukowski R., Hsi E., Finke J. Inhibition of NF-kappa B activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and -3. J Immunol. 1999;163(2):590–598. [PubMed] [Google Scholar]