Abstract
Background:
Regorafenib is a multi-kinase inhibitor approved for treatment of refractory advanced colorectal cancer. It was found in the clinical trials to have a modest benefit and significant toxicity. Our aim was to assess the outcome in our local clinic practice.
Patients and methods:
Records of patients with confirmed colorectal cancer treated with regorafenib were reviewed. Clinical, pathological, and molecular data were collected. Efficacy and factors of possible prognostic significance were analyzed.
Results:
A total of 78 patients with metastatic colorectal cancer were treated with regorafenib from February 2014 to February 2016 in 4 different institutions (median age: 50.5 years; male: 40 [51.3%]; KRAS mutant: 41 [52%]; right colonic primary: 18 [23%]). A total of 52 patients (66.7%) had Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 to 1, whereas in 25 patients (32.1%) it was >1. In total, 58 patients (74%) had dose reduction. No patient achieved objective response, 15 patients (19%) achieved stable disease, and 56 patients (72%) had progressive disease. With a median follow-up of 6.5 months, the median progression-free survival was 2.8 months (95% confidence interval [CI], 2.5-3.3) and overall survival was 8.0 months (95% CI, 6.2-9.7). Only performance status of ⩽1 had a statistically significant impact on progression-free survival and overall survival in both univariate and multivariate analyses.
Conclusions:
Regorafenib in our clinical practice has equal efficacy to reported data from pivotal registration trials. Our data suggest that performance status is the most important prognostic factor in patients treated with regorafenib, suggesting a careful selection of patients.
Keywords: regorafenib, multi-kinase inhibitor, colorectal cancer, prognosis, metastatic
Introduction
Colorectal carcinoma (CRC) is the second most common malignancy in females and the third most common among males worldwide.1 It is also the second most common cause of cancer-related death across the globe.2 A significant percentage of patients have metastatic disease at initial presentation. Most of these patients have unresectable tumors, rendering the disease incurable in this population.3
The backbone of management for metastatic colorectal cancer (mCRC) has been chemotherapy. The current standard includes fluoropyrimidine-, oxaliplatin-, or irinotecan-based chemotherapy given sequentially or in combination. The introduction of biological agents over the past 20 years, such as monoclonal antibodies targeting vascular endothelial growth factor (VEGF) and epidermal growth factor receptors (EGFR) (in RAS wild-type tumors), provided an additional synergistic mechanism for disease control in this cohort of patients.4,5
Unfortunately, until 2012, there was no standard therapy available after exhaustion of the above-mentioned agents.
In September 2012, the Food and Drug Administration (FDA) approved regorafenib as salvage treatment for mCRC previously treated with chemotherapy with or without anti-VEGF or anti-EGFR therapy. Regorafenib is an oral multi-kinase inhibitor that has been shown to block the activity of multiple protein kinases active in oncogenesis, tumor angiogenesis, as well as in the modulation of the tumor microenvironment.6
FDA approval was based on the results of the phase III CORRECT trial. The CORRECT trial demonstrated an improved median overall survival (OS) of 1.4 months when compared to placebo in patients with mCRC who had progressed on previous therapy.7
A similar study was undertaken to assess the response of mCRC in a broader Asian population which yielded similar results demonstrating a modest survival benefit.8
Despite the side effects profile, the relatively modest incremental benefit in OS, and the high cost of the drug, regorafenib, has been incorporated as a third-line treatment option for mCRC in most clinical practice guidelines.9,10
Data from the Middle East are lacking. Accordingly, we reviewed regorafenib efficacy data in 4 tertiary care centers from the Middle East that represent real-world experience from this region of the world.
Patients and Methods
Procedure and data collection
The medical records of all patients with known diagnosis of metastatic CRC who received regorafenib at 4 governmental institutions in Saudi Arabia, from February 2014 to September 2017, were reviewed. The objective of this study was to determine the efficacy of regorafenib in clinical practice and to determine factors that influence the efficacy of regorafenib in this group of patients. Patients were considered eligible for this analysis if they had histologically confirmed metastatic CRC; received fluoropyrimidine, oxaliplatin, and irinotecan; and received at least one cycle of regorafenib.
Demographic data, prior chemotherapy, targeted therapy, pretreatment laboratory parameters, response rate, disease progression, and survival were collected.
Because of the retrospective nature of the study, we felt that toxicity data would not be accurate and accordingly were not captured.
The study was approved by the respective institutional review board of each institution.
Statistical analysis
This was a retrospective study. Response was assessed retrospectively according to response evaluation criteria in solid tumors (RECIST) v1.1.11 Response in patients with non-measurable disease was categorized according to the decision made by the treating physician.
Progression-free survival (PFS) was calculated from the date of starting regorafenib till the date of radiological progression, death, or last follow-up. Overall survival was calculated from the date of starting regorafenib till death or last follow-up. Patients who were lost to follow-up were censored at the date of their last follow-up. Median follow-up was calculated by reversing the codes for death or censoring using the Kaplan-Meier method.
PFS and OS were analyzed according to prior therapy with bevacizumab, KRAS status, regorafenib starting dose (160 mg vs less than 160 mg), regorafenib dose reduction, sidedness of the primary (Right vs Left), Eastern Cooperative Oncology Group (ECOG) performance status (PS) (0, 1 vs >1), date from metastatic disease to starting regorafenib (equal or less than 12 months vs more than 12 months), number of metastatic sites (one vs more than one), and liver involvement (yes vs no).
Statistical analysis was done using the software package SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics for the continuous variables were reported as mean ± 95% confidence interval (CI) and categorical variables were summarized as frequencies and percentages. Continuous variables were compared by Student’s t-test or analysis of variance (ANOVA) as appropriate, while categorical variables were compared by chi-square test. Kaplan-Meier method was used in survival tables and curves and the different subgroups were compared by the log-rank test. Cox regression model was used for multivariate analysis. Factors evaluated were those who were significant in univariate analysis or had P value of ⩽.2. Sidedness and the number of organ involved were also included in the Cox regression model analysis. The level of statistical significance is set at P <.05.
Results
A total of 78 patients with metastatic CRC treated with regorafenib were identified. Patients’ characteristics are illustrated in Table 1. Note is made of no patient with performance status zero. In total, 62 patients (79%) received prior bevacizumab. Dose reduction was frequent in this group with 60% of patients starting with a reduced dose and 74% had dose reduction (including patients starting with lower dose).
Table 1.
Patients’ characteristics.
| No. (%) | |
|---|---|
| Age, y | |
| Median | 50.5 |
| Range | 25-81 |
| Gender | |
| Male | 40 (51.3) |
| Female | 38 (48.7) |
| ECOG PS | |
| 1 | 52 (66.7) |
| >1 | 25 (32.1) |
| Duration from diagnosis of metastasis to start regorafenib | |
| ⩽12 mo | 13 (17) |
| >12 mo | 65 (83) |
| Sidedness of primary tumor | |
| Right | 18 (23) |
| Left | 60 (77) |
| Number of organs involved | |
| 1 | 15 (19) |
| >1 | 63 (81) |
| Liver metastasis | |
| Yes | 65 (83) |
| No | 13 (17) |
| KRAS gene mutation | |
| Mutant | 41 (52) |
| Wild type | 34 (44) |
| Unknown | 3 (4) |
| Prior bevacizumab | |
| Yes | 62 (79) |
| No | 16 (21) |
| Prior cetuximab | |
| Yes | 30 (38) |
| No | 48 (62) |
| Regorafenib starting dose | |
| 160 mg | 31 (40) |
| <160 mg | 47 (60) |
| Dose reduction | |
| Yes | 58 (74) |
| No | 20 (26) |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group Performance Status.
A total of 68 patients (87%) had measurable disease. The median date to re-evaluation of response from start of regorafenib was 77 days. No patient had objective response to regorafenib. In total, 15 patients (19%) had stable disease and 56 (71%) had disease progression on their first evaluation. Seven patients did not have radiological evaluation after starting regorafenib.
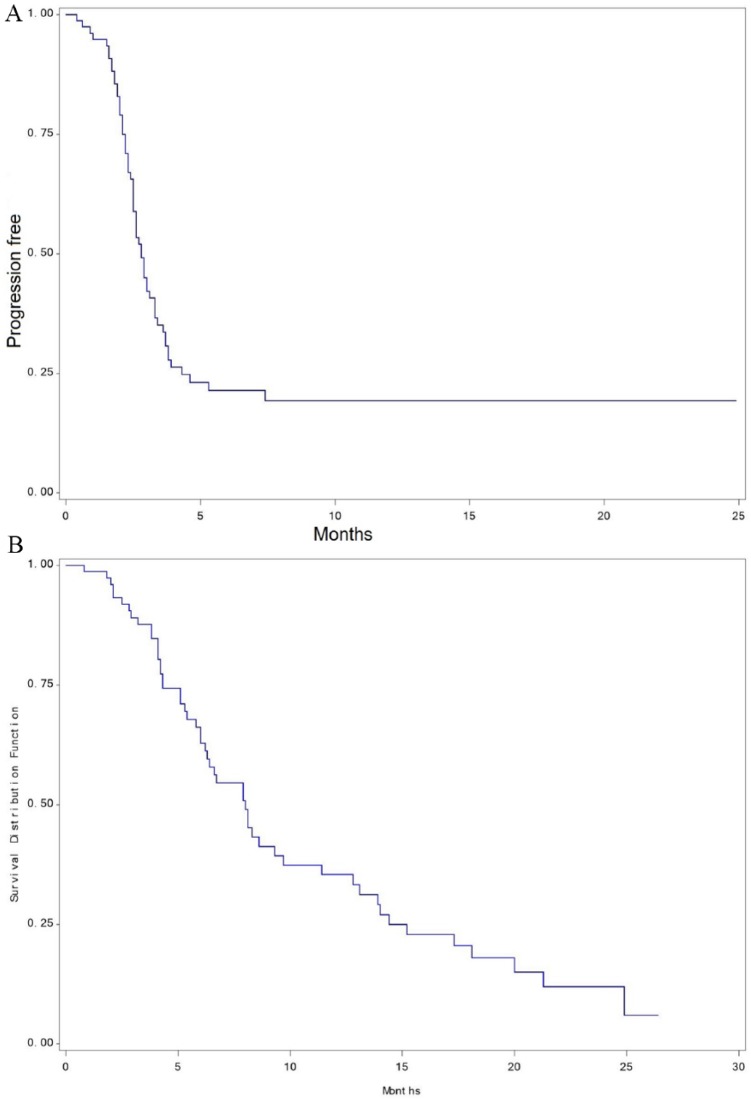
At a median follow-up of 6.5 months, the median PFS was 2.8 months (95% CI, 2.5-3.3), and the OS was 8 months (95% CI, 6.2-9.7; Figure 1).
Figure 1.
(A) Kaplan-Meier curve of progression-free survival of 78 patients treated with regorafenib. (B) Kaplan-Meier curve of overall survival of 78 patients treated with regorafenib.
Univariate analysis of different subgroups showed patients with ECOG PS and dose reduction to be of statistical significance for PFS (P = .0002 and .0012, respectively). Only PS had statistical significance for OS (P = .01; Table 2).
Table 2.
Univariate analysis of different prognostic factors in patients treated with regorafenib.
| Item | PFS (mo) (95% CI) |
P-value | OS (mo) (95% CI) |
P-value |
|---|---|---|---|---|
| Age, y | ||||
| ⩽65 | 2.8 (2.5-3.3) |
.599 | 7.9 (6.0-9.7) |
.628 |
| >65 | 2.95 (0.4-NR) |
8.1 (3.2-NR) |
||
| Gender | ||||
| Male | 3.0 (2.5-3.8) |
.139 | 8.0 (6.0-13.1) |
.986 |
| Female | 2.6 (2.3-3.0) |
7.9 (5.3-12.8) |
||
| ECOG PS | ||||
| 1 | 3.3 (2.6-4.6) | .0002 | 8.6 (6.7-14) | .010 |
| >1 | 2.3 (1.7-3.0) | 5.4 (4.2-7.9) | ||
| Interval from metastasis to regorafenib | ||||
| ⩽12 mo | 3.8 (1.7-NR) | .394 | 6.6 (2.9-NR) | .537 |
| >12 mo | 2.7 (2.5-3.1) | 8.1 (6-12) | ||
| Sidedness of primary tumor | ||||
| Right | 2.9 (2.3-3.3) | .764 | 9.3 (5.1-14.4) | .860 |
| Left | 2.8 (2.4-3.6) | 8.0 (6.0-11.4) | ||
| Number of organs involved | ||||
| 1 | 2.4 (2.0-2.9) | .081 | 9.3 (5.1-15.2) | .633 |
| >1 | 3.0 (2.5-3.7) | 7.9 (5.8-11.4) | ||
| Liver metastasis | ||||
| Yes | 2.8 (2.5-3.3) | .328 | 7.9 (5.8-9.3) | .442 |
| No | 2.6 (1.8-4.6) | 12.8 (5.1-15.2) | ||
| KRAS gene mutation | ||||
| Wild | 2.9 (2.1-3.8) | .995 | 8.1 (5.4-17.3) | .311 |
| Mutant | 2.8 (2.5-3.3) | 6.4 (5.3-9.7) | ||
| Prior bevacizumab | ||||
| Yes | 2.8 (2.5-3.3) | .972 | 8 (6.0-9.7) | .658 |
| No | 2.8 (2.0-5.3) | 6.6 (3.2-NR) | ||
| Prior cetuximab | ||||
| Yes | 2.9 (2.0-7.4) | .280 | 8.1 (5.4-15.2) | .973 |
| No | 2.8 (2.5-3.3) | 8.0 (5.8-12.8) | ||
| Regorafenib starting dose | ||||
| 160 mg | 2.3 (2.1-3.3) | .547 | 12.8 (5.1-18.1) | .149 |
| <160 mg | 3.0 (2.6-3.7) | 6.6 (5.4-8.6) | ||
| Dose reduction | ||||
| Yes | 3.0 (2.6-3.8) | .0012 | 8.0 (6.2-11.4) | .573 |
| No | 2.2 (2.0-2.5) | 6.0 (4.1-13.1) | ||
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; NR, not reached; OS, overall survival; PFS, progression-free survival.
Multivariate analysis using Cox regression model was performed. Only performance status was found to be of statistical significance for both PFS and OS (P = .0097 and .0065, respectively; Table 3).
Table 3.
Multivariate analysis of different prognostic factors in patients treated with regorafenib.
| Item | PFS |
OS |
||||
|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Gender (male vs female) | .6958 | 0.881 | 0.468-1.659 | .6265 | 1.195 | 0.583-2.453 |
| ECOG PS (1 vs >1) | .0097 | 2.683 | 1.270-5.668 | .0065 | 3.104 | 1.374-7.015 |
| Interval from metastasis to regorafenib (⩽1 y vs >1 y) | .9069 | 1.001 | 0.983-1.020 | .0517 | 1.019 | 1.000-1.039 |
| Sidedness (right vs left) | .3840 | 0.741 | 0.377-1.455 | .9111 | 0.958 | 0.453-2.027 |
| Number of organs involved (1 vs >1) | .5410 | 0.910 | 0.672-1.232 | .6957 | 1.062 | 0.785-1.439 |
| Regorafenib starting dose (160 vs <160 mg) | .8384 | 1.084 | 0.500-2.348 | .0716 | 2.244 | 0.931-5.406 |
| Dose reduction (yes vs no) | .1019 | 0.475 | 0.195-1.159 | .0704 | 0.357 | 0.117-1.090 |
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Group Performance Status; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Discussion
This is the first retrospective, multi-institutional study evaluating the efficacy of regorafenib in the Middle East. In this study, we evaluated the outcome of the routine use of regorafenib in clinical practice from 4 governmental institutions.
The median age in our study (50 years) was much younger than that reported in the CORRECT and CONCUR studies (57-60 years) which probably reflect the median age of colorectal cancer reported in our cancer registry.12 In addition, one-third of our patients had PS more than one, a group which was not included in the pivotal CORRECT and CONCUR studies. The characteristics of our patients reflect patients with poor prognostic factors, with 81% having more than one organ involved, 83% with liver metastasis, and 79% received prior bevacizumab.
The median follow-up of 6.5 months reflects the tertiary care practice in these institutions where patients with terminal status prefer to be cared for in their hometown rather than in the treating institution.
None of our patients had an objective response. The median PFS in our study was 2.8 months which was more than the reported PFS in the CORRECT and CONCUR studies. This might reflect the delay in the re-evaluation of response to regorafenib which was 77 days (56 days in the CORRECT and CONCUR trials). Similar results in PFS have been reported with either retrospective single institution or small prospective studies.13,14 The median OS in our cohort was 8 months, which also might be related to the short follow-up duration (median, 6.3 months). This compares favorably with similar reported studies.13,15
Our subgroup analysis confirmed that performance status is one of the most important factors in regorafenib efficacy. All prior pivotal studies included only patients with PS 0 to 1, while one-third of our patients had PS more than one with a significant difference in PFS and OS (P = .0002 and .01, respectively) on both univariate analysis and multivariate analysis (P = .0097 and .0065, respectively). This result is in concordance with the result of the REBACCA study where 10.6% of patients had PS more than 1 and the difference in OS between the groups with PS 0 to 1 compared to >1 was statistically significant (P < .001).16 In addition, performance status was reported to correlate with outcome in the study reported by Angeles et al.15
Despite dose reduction showed prognostic significance on univariate analysis in PFS, this did not translate to benefit on multivariate analysis. Moreover, dose reduction is usually an event that occurs after starting therapy and accordingly cannot be used as a prognostic factor for response to therapy. Interestingly, our data did not show significant difference between those who started on the standard dose of 160 mg versus those who started at a lower dose. This observation is in concordance with the new data of the Re-Dos study where patients who started at a loser dose (and then escalated to higher one) did not have a statistically lower PFS than patients who started at the standard dose of 160 mg with a median survival of 9 months vs 5.9, respectively (P = .0943).17 Other investigators have studied a dosing regimen with 2 weeks on 1 week off, with starting dose reduced to 120 or 80 mg in patients with >1 comorbidity, age ⩾80 years, or PS of 2. In this prospective study, the PFS and OS for the entire group were 4.8 and 8.9 months, respectively.13
The limitations of our study include its retrospective nature, the absence of toxicity evaluation, the small sample size, and the short median follow-up. On the other hand, its strength includes the multicenter nature and being the only study coming from this part of the world.
In conclusion, our data support the efficacy of regorafenib as third-line treatment for patients with mCRC. Performance status probably represents the most significant factor affecting the efficacy of regorafenib.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AA contributed to conception and design of the study. MAE contributed to data collection, analysis of the data, and manuscript writing. MK contributed to data collection. MNZ contributed to design of the study and data collection. AS contributed to data collection. AG, OA, and AA contributed to data collection. AE contributed to statistical analysis. SB contributed to design of the study, coordination between institutions, analysis of data, and manuscript writing.
ORCID iD: Shouki Bazarbashi  https://orcid.org/0000-0002-8913-8158
https://orcid.org/0000-0002-8913-8158
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 3. Segal NH, Saltz LB. Evolving treatment of advanced colon cancer. Annu Rev Med. 2009;60:207–219. [DOI] [PubMed] [Google Scholar]
- 4. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 5. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. [DOI] [PubMed] [Google Scholar]
- 6. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. [DOI] [PubMed] [Google Scholar]
- 7. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. [DOI] [PubMed] [Google Scholar]
- 8. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. [DOI] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Colon cancer 2018 (Version 3). https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Up-dated 2018. Accessed 8 August 2018.
- 10. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D; ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii1–iii9. [DOI] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 12. Bazarbashi S, Al Eid H, Minguet J. Cancer incidence in Saudi Arabia: 2012 data from the Saudi cancer registry. Asian Pac J Cancer Prev. 2017;18:2437–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petrioli R, Chirra M, Messuti L, et al. Efficacy and safety of regorafenib with 2/1 schedule for patients ⩾75 years with metastatic colorectal cancer (mCRC) after failure of 2 lines of chemotherapy. Clin Colorect Cancer. 2018;17:307–312. [DOI] [PubMed] [Google Scholar]
- 14. Unseld M, Filip M, Seirl S, et al. Regorafenib therapy in metastatic colorectal cancer patients: markers and outcome in an actual clinical setting. Neoplasma. 2018;65:599–603. [DOI] [PubMed] [Google Scholar]
- 15. Angeles A, Hung W, Cheung WY. Eligibility of real-world patients with chemo-refractory, K-RAS wild-type, metastatic colorectal cancer for palliative intent regorafenib monotherapy. Med Oncol. 2018;35:114. [DOI] [PubMed] [Google Scholar]
- 16. Adenis A, de la, Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer. 2016;16:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bekaii-Saab TS, Ou F-S, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC)—an ACCRU Network Study. J Clin Oncol. 2018;36:611. [Google Scholar]