Abstract
Objective:
We examined short and long-term outcomes of bariatric surgery in patients with obesity and type 1 diabetes mellitus (T1DM).
Methods:
We reviewed the records of all adults insured by Maccabi Healthcare Services during 2010 -2015, with body mass index (BMI) ⩾30 kg/m2 and T1DM; and compared weight reduction and glucose control according to the performance of bariatric surgery. BMI and glycated hemoglobin (HbA1c) levels were extracted for baseline and every 6 months, for a mean 3.5 years.
Results:
Of 52 patients, 26(50%) underwent bariatric surgery. Those who underwent surgery were more often female and with a longer duration of diabetes. Immediately postoperative, 4(15%) developed diabetic ketoacidosis, while 6(23%) experienced severe hypoglycemic episodes. The mean BMI decreased among surgery patients: from 39.5±4.4 to 30.1±5.0 kg/m2 (p < 0.0001); and increased among those who did not undergo surgery: from 33.6±3.9 to 35.1±4.4 kg/m2 (p = 0.49). The mean HbA1c level decreased during the first 6 months postoperative: from 8.5±0.9% to 7.9±0.9%; however, at the end of follow-up, was similar to baseline, 8.6±2.0% (p = 0.87). For patients who did not undergo surgery, the mean HbA1c increased from 7.9±1.9% to 8.6±1.5% (p = 0.09).
Conclusions:
Among individuals with obesity and T1DM, weight loss was successful after bariatric surgery, but glucose control did not improve. The postoperative risks of diabetic ketoacidosis and severe hypoglycemic episodes should be considering when performing bariatric surgery in this population.
Keywords: bariatric surgery, diabetes complications, HbA1c, type 1 diabetes, weight loss
Introduction
The prevalence of overweight and obesity among adults has increased dramatically, creating a global epidemic.1 Substantial evidence shows that obesity is strongly associated with several serious medical complications that impair quality of life and lead to increased morbidity, including cardiovascular disease, diabetes mellitus, chronic kidney disease, and a number of cancers.1 A low-calorie diet accompanied by behavioral therapy is often considered futile for weight loss, and specifically for maintaining low weight.2 Over the last decade, bariatric surgery has emerged as a viable treatment option for morbidly obese individuals.3
Type 1 diabetes mellitus (T1DM) is one of the most prevalent chronic diseases of young adults, with a distinct risk factor for cardiovascular disease.4 The onset of atherosclerotic changes has been shown to appear early in T1DM patients.5 Overweight and obesity have been found to be highly prevalent among individuals with T1DM (about 30% of T1DM are overweight or obese)6–8 and to be associated with cardiometabolic risk.9 The co-occurrence of obesity in patients with T1DM can therefore have a deleterious impact.
Weight loss and weight maintenance, after weight loss, pose particular challenges for individuals with T1DM; first, because intensive diabetes mellitus therapy is associated with increased weight gain secondary to the anabolic and lipogenic actions of insulin; and second, hypoglycemic episodes are associated with hyperphagia. Finally, alteration in physical activity levels may contribute to increased weight gain.10 Bariatric surgery was found to be a treatment option in morbidly obese individuals with type 2 diabetes (T2DM) and more recently in T1DM.11,12
Due to extreme changes in feeding patterns and food absorption after bariatric surgery, patients with T1DM need special attention for appropriate adjustment of insulin dose, to prevent such short-term complications as diabetic ketoacidosis (DKA) and severe hypoglycemia, both of which are associated with an increased risk of mortality.13,14 In the current study, we assessed the short and long-term outcomes of bariatric surgery in a cohort of obese patients with T1DM. We compared baseline and long-term outcomes with obese patients with T1DM who did not undergo surgery. In addition, we examined factors that predict the long-term success of weight loss and metabolic control in patients with T1DM who underwent bariatric surgery.
Methods
All obese patients with T1DM who were treated by Maccabi Healthcare Services (MHS) between January 2010 and December 2015 were identified in the MHS database. MHS is the second largest of four health maintenance organizations in Israel, with more than 2 million insured members. Data are aggregated by continuous real-time input from physicians and health service providers, and in addition to diagnoses include anthropometric measurements, laboratory data, and pharmaceutical information. Clinical outcomes and metabolic parameters for the identified patients were retrieved. Ethics approval was granted by the institutional review board of MHS (#45/2015). Patients with T1D who underwent bariatric surgery were contacted by telephone by one of the study team (who was not the personal health provider of the patient). The patients were informed of the purposes of the current study and asked to provide oral informed consent prior to initiation of the telephone interview for data collection from electronic medical records and for the interview. Data from electronic medical records of the patients that did not undergo bariatric surgery were retrieved retrospectively while using coded (anonymized) administrative data. Exemption from informed consent was granted by the Helsinki committee for this group of patients.
Study inclusion criteria were: (1) the diagnosis of T1DM verified by the documented presence of autoantibodies known to be associated with T1DM or diagnosis in early childhood, or documentation of DKA; and the regular use of insulin from the time of diagnosis; (2) body mass index (BMI) above 30 kg/m2. For analysis, the patients were divided into two groups: those who underwent bariatric surgery and those who did not. The procedures were performed in various centers by different surgeons. The baseline was defined as the date of surgery for the bariatric group, and a BMI > 30 kg/m2 from January 2010 for the nonsurgery group.
All patients were followed up in diabetes clinics and were treated with insulin (multiple daily injections or by insulin pump). Data retrieved from the electronic medical record for baseline and for every 6-month period included weight, BMI, glycated hemoglobin (HbA1c) level, purchase of medications (other than insulin), the number and causes of emergency room visits and hospitalizations, and documentations of macro and microvascular complications (retinopathy, nephropathy and neuropathy). In addition, for the follow-up period, data were extracted regarding the number of severe hypoglycemic episodes, as defined by episodes requiring assistance and confirmed by documentation of a blood glucose value <50 mg/dl (2.8 mmol/l), and the number of recorded DKA episodes.
After obtaining oral informed consent, a structured 15–20 min telephone interview was conducted with patients who underwent surgery, by a single dietitian specialized in bariatric surgery (GKS), 18 months on average after the surgery. During the interview, patients were asked about the major indication for the bariatric surgery as they perceived it, the frequency and severity of hypoglycemic episodes after surgery, expectations and satisfaction; and if they would recommend this procedure to other obese patients with T1DM.
Statistical analysis
Data were analyzed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Baseline characteristics were compared using the Chi-squared test and the Fisher’s exact test for qualitative variables and the Student’s t test or Wilcoxon rank sum test for quantitative variables. General linear models with repeated measures were fitted for BMI follow up and for HbA1c follow up row values, and for their deviations from the baseline. Each model compared the treatment groups, while being adjusted for baseline variables (baseline BMI, age, baseline HbA1c, sex, duration of diabetes and follow-up time). BMI and HbA1c changes from the baseline at different time points were estimated based on the fitted models. Goodness-of-fit of the models was checked by the residual plots. All tests were two-tailed and p values below 0.05 were considered statistically significant.
Results
Study population
A total of 52 individuals with T1DM and BMI >30 kg/m2 were identified from the MHS database of 2010–2015. Of them, 26 (50%) underwent bariatric surgery. During this same period, 14,048 patients from MHS underwent bariatric surgery. The baseline characteristics of the study groups are shown in Table 1. Individuals who underwent surgery tended to be older (p = 0.01), with higher BMI (p = 0.001), with longer diabetes duration (p = 0.001), and with higher HbA1c levels (p = 0.02) at baseline than patients who did not undergo surgery. At baseline, more patients in the surgery group were treated with antihypertensive drugs (p = 0.02), whereas more patients in the nonsurgery group were treated with metformin (p = 0.003). At baseline, four patients in the surgery group had proteinuria, one had retinopathy and none had neuropathy. No patient in the nonsurgery group had proteinuria, retinopathy or neuropathy. A total of two patients in the nonsurgery group had coronary heart disease. The following surgeries were performed: sleeve gastrectomy (n = 19, 73%), gastric bypass (n = 4, 15%) and gastric banding (n = 3, 12%).
Table 1.
Baseline characteristics of patients with type 1 diabetes.
| Characteristic | Surgery group (N = 26) | Nonsurgery group (N = 26) | p value |
|---|---|---|---|
| Sex (M/F) | 0.05 | ||
| Male n (%) | 7 (27) | 14 (54) | |
| Female n (%) | 19 (73) | 12 (46) | |
| Age (years) mean ± SD | 40.4 ± 9.8 | 33.1 ± 9.6 | 0.01 |
| Age at diagnosis of T1D: mean ± SD | 20.2 ± 11.2 | 23.1 ± 11.9 | NS |
| Duration of diabetes (years) | 20.2 ± 8.6 | 11.1 ± 9.2 | 0.001 |
| BMI (kg/m2) | 39.5 ± 4.4 | 33.6 ± 3.9 | 0.001 |
| HbA1c (%) | 8.5 ± 0.9 | 7.9 ± 1.9 | 0.02 |
| Comorbidities | |||
| Hypertension | 13 (50%) | 5 (19.2%) | 0.02 |
| Retinopathy | 1 (3.8%) | 0 (0%) | NS |
| Neuropathy | 0 (0%) | 0 (0%) | NS |
| Proteinuria | 4 (15.3%) | 0 (0%) | NS |
| Coronary artery disease | 0 (0%) | 2 (7.7%) | NS |
| Medications n (%) | |||
| Insulin pump | 12 (46) | 14 (54) | NS |
| Multiple daily injections | 14 (54) | 12 (46) | NS |
| Lipid-lowering agents | 13 (50.0%) | 7 (26.9%) | 0.09 |
| Antihypertensive agent | 13 (50%) | 5 (19.2%) | 0.02 |
| Metformin | 3 (11.5%) | 13 (50%) | 0.003 |
BMI, body mass index; HbA1c, glycated hemoglobin; NS, not significant; SD, standard deviation.
Follow-up results
For almost all patients, data were available for at least a 2-year follow-up period. Data of 3.5 years of follow up were available for 14/26 patients from the surgery and for 23/26 from the nonsurgery group.
A marked decrease in BMI was documented 6 months after the bariatric surgery, from 39.5 ± 4.4 to 31.3 ± 4.4 kg/m2 (p < 0.0001; Figure 1). Weight continued to decrease and reached the lowest point at 2 years from surgery, at a mean BMI of 27.5 ± 3.6 kg/m2. An increase in BMI was documented thereafter, which reached a mean 30.1 ± 5.0 kg/m2 after 3.5 years. During the follow-up period, a slight increase in BMI was documented among patients who did not undergo surgery, from 33.6 ± 3.9 at baseline to 35.1 ± 4.4 kg/m2 after 3.5 years (Figure 1). A multivariate analysis showed a significant difference (p < 0.0001) between the study groups in BMI trends at follow up: while there was no significant change in BMI in the nonsurgery group, a significant reduction in BMI was observed for the surgery group at 2 years and 3.5 years follow up. Lower BMI at baseline and longer follow-up duration were significantly associated with lower BMI during follow up. No significant associations were observed of change in BMI with sex, diabetes duration or HbA1c at baseline. Comparing baseline with BMI level at 3.5 years, the adjusted model revealed a significant mean reduction of 9.3 kg/m2 (p < 0.0001) for the bariatric group, and a small, not statistically significant, increase in BMI in the reference group (p = 0.49).
Figure 1.

BMI during follow up.
BMI of obese T1DM patients up to 3.5 years following bariatric surgery and of a reference group of obese T1DM patients. The vertical lines show standard deviations.
BMI, body mass index; T1DM, type 1 diabetes mellitus.
Mean HbA1c levels during the study period are shown in Figure 2. At 6 months, individuals in the surgery group had a 0.6 ± 1.0% reduction in HbA1c. However, thereafter, HbA1c increased and was not significantly different from baseline at the end of the follow-up period (8.5 ± 0.9%, 7.9 ± 0.9% and 8.6 ± 2.0% at before surgery, 6 months after surgery and 3.5 years after surgery, respectively). No mean change in HbA1c level was documented in the nonsurgery group (7.9 ± 1.9% and 8.6 ± 1.5% at baseline and 3.5 years later, respectively). In a multivariate analysis, no difference was observed in the trend of HbA1c levels at follow up between the study groups. Comparing the HbA1c level at baseline and at 3.5 years in the adjusted model revealed no difference in the bariatric group (0.04%, p = 0.87), whereas the nonsurgery group had a borderline significant increase in HbA1c level of 0.4% (p = 0.09).
Figure 2.
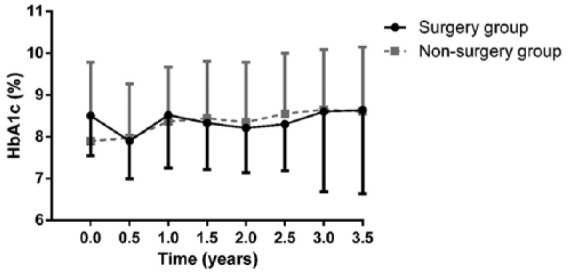
HbA1c during follow up.
HbA1c of obese T1DM patients up to 3.5 years following bariatric surgery and of a reference group of obese T1DM patients. The vertical lines show standard deviations.
HbA1c, glycated hemoglobin; T1DM, type 1 diabetes mellitus.
During follow up, antihypertensive agents were discontinued in four patients of the surgery group; while one patient from the nonsurgery group started antihypertensive agents. Lipid-lowering agents were initiated during follow up in three patients from the nonsurgery group, and in no additional patients of the surgery group. Overall, three patients in the nonsurgery group initiated metformin, compared with one from the surgery group (at the end of follow up: 16/26 and 4/26 in the nonsurgery and surgery groups were using metformin, respectively, p = 0.01)
In the surgery group, systolic and diastolic blood pressure decreased significantly (p < 0.001), and high-density lipoprotein (HDL) levels increased significantly (p = 0.004); mean triglyceride levels decreased, though the change did not reach clinical significance (p = 0.06). Change were not observed in the nonsurgery group in blood pressure, and HDL and triglyceride levels. No significant change was observed in low-density lipoprotein levels and total cholesterol levels in either group.
Adverse events and complications
Overall, four patients in the surgery group were transferred to the intensive care unit due to DKA within the first 48 h after surgery, as a result of a low insulin dose given postoperatively, while no sepsis or other serious complications were documented. Recurrent hypoglycemic episodes (without hospitalizations) were reported by six (23%) patients from the surgery group shortly after discharge from the hospital due to incorrect postoperative adjustment of insulin dose. During follow up, seven patients (27%) were hospitalized at least once, compared with four (15%) of the nonsurgery group. The indications for hospitalization in the surgery group were as follows: stenosis of the proximal stomach (n = 1), leak (n = 1), intestinal obstruction (n = 1), dehydration (n = 2), and severe hypoglycemia (n = 2). In the nonsurgery group: indications for hospitalization were DKA (n = 1), severe hypoglycemia (n = 1) and coronary artery disease (n = 2). Overall, one female patient had a severe hypoglycemic episode at 3 years after sleeve gastrectomy, at the age of 57 years. The episode occurred after consumption of alcohol, which resulted in severe permanent neurologic damage.
Proteinuria was resolved in one patient from the surgery group during the follow-up period. Retinopathy developed in one additional patient in the nonsurgery group during the follow up.
Follow-up interviews in patients who underwent surgery
At 1 to 4 years following surgery, an attempt was made to interview patients in the surgery group. Of 26 patients in this group, 20 completed the structured follow-up interview. Overall, one patient moved from Israel and could not be located, one had a stroke and was unable to respond and four refused to be interviewed. Participants were asked whether they would recommend bariatric surgery to other patients with T1DM and obesity. A total of 13 (65%) responded that they definitely would, 5 (25%) responded that they definitely would not and 2 (10%) were not sure as to whether they would recommend the surgery.
The reasons the study participants reported for undergoing bariatric surgery were: poor glycemic control (n = 2, 10%), obesity (n = 4, 20%), a combination of obesity and poor glycemic control (n = 13, 65%) and misdiagnosis of T2DM (n = 1, 5%). Improvement in blood pressure and lipid profile and reducing the risks of diabetes complications were not reported as reasons for undergoing surgery in this cohort.
Discussion
Individuals with T1DM comprise a small minority of the patients who undergo bariatric surgery, yet they require special medical attention due to their underlying disease. This is the first study that compared clinical outcomes of individuals with T1DM who underwent bariatric surgery to those of individuals with T1DM who did not undergo surgery, rather than with outcomes of individuals with T2DM or without diabetes. A considerable proportion of patients in the bariatric group had DKA episodes that were related to the surgery, recurrent hypoglycemic events, or hospitalizations due to surgical complications. Overall, 15% developed DKA and were transferred to the intensive care unit within the first 48 h of surgery. This concurs with other reports in which 10–20% of bariatric patients with T1DM developed DKA episodes.15–17 Overall, one quarter of the patients in the current study had recurrent hypoglycemic episodes (without hospitalizations) shortly after discharge from the hospital, due to incorrect adjustment of insulin dose after the surgery. Similarly, Rottenstreich and colleagues reported an increased frequency of hypoglycemic episodes during the first few months after surgery in 29% of their cohort of T1DM patients.16 Although most episodes of severe hypoglycemia resolve without apparent permanent injury, we report severe permanent neurologic damage in one patient, secondary to a severe hypoglycemic episode associated with alcohol consumption, which occurred 3 years after sleeve gastrectomy. Compared with the nonsurgery group, the rate of hospitalization was almost double among patients in the surgery group. Hospitalizations in the former were due to surgical complications and in the latter due to diabetes and coronary artery disease.
Marked decreases in weight and BMI were demonstrated in the current cohort 6 months after bariatric surgery. Maximal weight reduction occurred after 2 years, the mean BMI decreased from 39.5 to 27.5 kg/m2, a difference of 12 units. Though BMI increased in the third year after surgery, at the end of the follow up, the mean BMI was 9 units less than the mean BMI before surgery; and 8/15 (65%) were categorized as normal weight or overweight. This contrasts with a small increase in BMI during the same period, in obese patients with T1DM treated at the same clinics, who did not undergo surgery. Although the duration of follow up differs between studies, there is general agreement that bariatric surgery is effective as a weight loss intervention for patients with T1DM.15,17–20
Among the patients who underwent bariatric surgery in the current study, a decrease in mean HbA1c level was observed in the first 6 months after surgery, parallel to the marked weight reduction. This reduction in HbA1c is probably due to strict caloric restriction postoperatively. However, from 12 months after surgery until the end of the follow-up period, HbA1c levels were not significantly different from baseline or from levels of individuals who did not undergo surgery. None of our patients had an HbA1c level below 7%, which is recommended as a target HbA1c by the American Diabetes Association (ADA).21 Previous literature is inconsistent regarding HbA1c levels after bariatric surgery in T1DM patients. Some authors reported a marked decrease in HbA1c,15,22 while others did not show significant improvement.16,19,20,23,24 The reasons for this discrepancy are not clear, and do not appear to be related to patient age, disease duration or weight loss. In two large pediatric clinical registries from the United States and Europe, higher BMI z-scores were significantly related with higher HbA1c levels.25 We suggest that the lack of improvement in HbA1c after bariatric surgery reflects behavioral issues and difficulties in complying with diabetes management and is not weight related. Psychosocial factors such as depressive symptoms, low social support, poor self-esteem, and negative body image were found to be associated with poor glycemic control, as well as with higher BMI.26 Solving the obesity issue by bariatric surgery does not necessarily provide a solution to these psychological issues. Notably, the mean HbA1c level in the nonsurgery group gradually increased during the follow-up period. Although this trend had only borderline significance, it may reflect deterioration, over time, of glycemic control among patients with T1DM and obesity.
Our data could not detect the impact of surgery on macrovascular or microvascular complications, due to the small sample size. Nevertheless, a significant decrease in both systolic and diastolic blood pressure, as well as in HDL levels, was observed among the surgery group, whereas no change was observed in the nonsurgery group.
Patients stated that their rationale for undergoing surgery was to lose weight while improving diabetes control. Several patients expressed some degree of disappointment from the lack of improvement in glucose control and from difficulties in insulin dose adjustment during the first few months after surgery. The latter were due to substantial changes in nutritional habits, which resulted in several patients experiencing multiple mild-to-moderate hypoglycemic episodes due to insulin overdose, and others experiencing pronounced hyperglycemia due to underdosing of insulin.
Despite the high rate of adverse events and hospitalizations of postbariatric surgery, and despite the lack of improvement in glycemic control, 65% of the patients who responded to an interview reported that they would recommend bariatric surgery to other patients with T1DM and obesity. This suggests that decreased weight is a major positive outcome. Nevertheless, 25% were not satisfied and 10% expressed neutral opinions. Similarly, in a recent review of the perceptions of obese patients of the outcomes of bariatric surgery, the responses were categorized as positive (63%), neutral (25%), and negative (12%);27 Among patients with T2DM who underwent bariatric surgery, the vitality and physical component scores remained low.28 These findings emphasize the need to fully inform patients of expected outcomes before deciding on bariatric surgery.
An important limitation of this study is that we interviewed only the patients in the bariatric group, and only at a single point in time following the operation, which differed among the patients. This study design raises the possibility of recall bias. In addition, baseline BMI and age differed between the surgery and nonsurgery groups. The mean BMI of the surgery group was similar to the mean preoperative BMI reported for all patients who underwent bariatric surgery in Israel between 2013 and 2016, 42.14 ± 5.21 kg/m2.29 The mean BMI of the nonsurgery group was significantly lower. Since all patients with T1DM and BMI ⩾ 30 kg/m2 from the MHS database were included in the study, the older age, higher BMI, and greater proportion of females among those who underwent surgery reflect the characteristics of patients who select surgery. We were not able to compare outcomes according to type of surgery due to the relatively small number of patients who underwent gastric banding and gastric bypass.
The strengths of this study are that it is the first report to compare obese T1DM patients who underwent surgery with obese patients with T1DM treated in the same clinics who did not undergo surgery. The inclusion of data from different diabetes clinics and various surgeons increases its generalizability. This study provides relatively long follow up, with longitudinal data every 6 months, of weight, HbA1c levels, medications and hospitalizations on a relatively large cohort of patients with T1DM. Of note, despite the increasing prevalence of obesity among adults from Israel, only a relatively small proportion of the adults in our database with T1DM had obesity. This is in concordance with our previous observation of a low prevalence of obesity among young adults with T1DM. This may be secondary to continual follow up by nutritionists.7
In conclusion, bariatric surgery resulted in significant and long-standing weight loss and improvements in blood pressure and lipid profile in individuals with T1DM and obesity. However, glycemic control did not improve and adverse events were documented: a considerable risk of DKA, severe hypoglycemic episodes requiring hospitalization, and a two-fold increase in hospitalizations compared with individuals with T1DM who did not undergo surgery. A number of considerations should be heeded in regard to patients with T1DM who undergo bariatric surgery, including careful selection, preparation and discussion of realistic expectations; consultation with a diabetologist before and immediately after the surgery; frequent follow-up visits at diabetes clinics to adjust insulin dose and prevent hypoglycemic episodes; and attention to psychological issues, as needed. Bariatric surgeons should be adequately informed of the particular considerations of T1DM patients, since these comprise only a minority of the patients who undergo surgery.
Acknowledgments
The authors are grateful to Ms Cindy Cohen for her excellent editorial assistance.
ZL, GKS and DJ conceived the study. ZL, AR and NS designed the study protocol. ZL, IZP, LLG and OPH drafted this manuscript. ZL, GKS, DJ, AR, NS, IZP, LLG and OPH critically revised the manuscript. ZL, GKS, DJ, AR, NS, IZP, LLG and OPH read and approved the final manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Zohar Landau  https://orcid.org/0000-0002-0434-9563
https://orcid.org/0000-0002-0434-9563
Contributor Information
Zohar Landau, Pediatric Endocrinology Unit, Wolfson Medical Center, 65 Halochamim Street, Holon, 58100, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Maccabi Juvenile Diabetes Center, Raanana, Israel.
Galit Kowen-Sandbank, Maccabi Juvenile Diabetes Center, Raanana, Israel.
Daniela Jakubowicz, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Diabetes Unit, Wolfson Medical Center, Holon, Israel.
Asnat Raziel, Assia Medical Group, Assuta Medical Center, Tel Aviv, Israel.
Nasser Sakran, Assia Medical Group, Assuta Medical Center, Tel Aviv, Israel; Department of Surgery A, Emek Medical Center, Afula, Israel; Rappaport Faculty of Medicine, Technion Israel Institute of Technology, Haifa, Israel.
Inna Zaslavsky-Paltiel, Gertner Institute for Epidemiology and Health Policy Research, Women and Children’s Health Research, Tel Hashomer, Israel.
Liat Lerner-Geva, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Gertner Institute for Epidemiology and Health Policy Research, Women and Children’s Health Research, Tel Hashomer, Israel.
Orit Pinhas-Hamiel, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Maccabi Juvenile Diabetes Center, Raanana, Israel; Pediatric Endocrine and Diabetes Unit, Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel.
References
- 1. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Havrankova J. Is the treatment of obesity futile? YES. Can Fam Physician 2012; 58: 508–510. [PMC free article] [PubMed] [Google Scholar]
- 3. Bray GA, Frühbeck G, Ryan DH, et al. Management of obesity. Lancet 2016; 387: 1947–1956. [DOI] [PubMed] [Google Scholar]
- 4. Snell-Bergeon JK, Nadeau K. Cardiovascular disease risk in young people with Type 1 diabetes. J Cardiovasc Transl Res 2012; 5: 446–462. [DOI] [PubMed] [Google Scholar]
- 5. Jarvisalo MJ, Raitakari M, Toikka JO, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation 2004; 109: 1750–1755. [DOI] [PubMed] [Google Scholar]
- 6. Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for diabetes in youth study. Pediatr Diabetes 2010; 11: 4–11. [DOI] [PubMed] [Google Scholar]
- 7. Pinhas-Hamiel O, Levek-Motola N, Kaidar K, et al. Prevalence of overweight, obesity and metabolic syndrome components in children, adolescents and young adults with type 1 diabetes mellitus. Diabetes Metab Res Rev 2015; 31: 76–84. [DOI] [PubMed] [Google Scholar]
- 8. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the TlD exchange clinic registry. Diabetes Care 2015; 38: 971–978. [DOI] [PubMed] [Google Scholar]
- 9. van Vliet M, Van der Heyden JC, Diamant M, et al. Overweight is highly prevalent in children with type 1 diabetes and associates with cardiometabolic risk. J Pediatr 2010;156: 923–929. [DOI] [PubMed] [Google Scholar]
- 10. McNay EC, Teske JA, Kotz CM, et al. Long-term, intermittent, insulin-induced hypoglycemia produces marked obesity without hyperphagia or insulin resistance: a model for weight gain with intensive insulin therapy. AJP Endocrinol Metab 2013; 304: E131–E138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chow A, Switzer NJ, Dang J, et al. A systematic review and meta-analysis of outcomes for type 1 diabetes after bariatric surgery. J Obes 2016; 2016: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashrafian H, Harling L, Toma T, et al. Type 1 diabetes mellitus and bariatric surgery: a systematic review and meta-analysis. Obes Surg 2016; 26: 1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gibb FW, Teoh WL, Graham J, et al. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia 2016; 59: 2082–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akirov A, Grossman A, Shochat T, et al. Mortality among hospitalized patients with hypoglycemia: insulin-related and non-insulin related. J Clin Endocrinol Metab 2016; jc.2016-2653. [DOI] [PubMed] [Google Scholar]
- 15. Brethauer SA, Aminian A, Rosenthal RJ, et al. Bariatric surgery improves the metabolic profile of morbidly obese patients with type 1 diabetes. Diabetes Care 2014; 37: e51–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rottenstreich A, Keidar A, Yuval JB, et al. Outcome of bariatric surgery in patients with type 1 diabetes mellitus: our experience and review of the literature. Surg Endosc 2016; 30: 5428–5433. [DOI] [PubMed] [Google Scholar]
- 17. Maraka S, Kudva YC, Kellogg TA, et al. Bariatric surgery and diabetes: implications of type 1 versus insulin-requiring type 2. Obesity (Silver Spring) 2015; 23: 552–557. [DOI] [PubMed] [Google Scholar]
- 18. Kirwan JP, Aminian A, Kashyap SR, et al. Bariatric surgery in obese patients with type 1 diabetes. Diabetes Care 2016; 39: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lannoo M, Dillemans B, Van Nieuwenhove Y, et al. Bariatric surgery induces weight loss but does not improve glycemic control in patients with type 1 diabetes. Diabetes Care 2014; 37: e173–e174. [DOI] [PubMed] [Google Scholar]
- 20. Robert M, Belanger P, Hould FS, et al. Should metabolic surgery be offered in morbidly obese patients with type I diabetes? Surg Obes Relat Dis 2015; 11: 798–805. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(Suppl. 1): S55–S64. [DOI] [PubMed] [Google Scholar]
- 22. Faucher P, Poitou C, Carette C, et al. Bariatric surgery in obese patients with type 1 diabetes: effects on weight loss and metabolic control. Obes Surg 2016; 26: 2370–2378. [DOI] [PubMed] [Google Scholar]
- 23. Al Sabah S, Al Haddad E, Muzaffar TH, et al. Laparoscopic sleeve gastrectomy for the management of type 1 diabetes mellitus. Obes Surg 2017; 27: 3187–3193. [DOI] [PubMed] [Google Scholar]
- 24. Vilarrasa N, Rubio MA, Miñambres I, et al. Long-term outcomes in patients with morbid obesity and type 1 diabetes undergoing bariatric surgery. Obes Surg 2017; 27: 856–863. [DOI] [PubMed] [Google Scholar]
- 25. DuBose SN, Hermann JM, Tamborlane WV, et al. Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr 2015; 167: 627–632.e4. [DOI] [PubMed] [Google Scholar]
- 26. Driscoll KA, Corbin KD, Maahs DM, et al. Biopsychosocial aspects of weight management in type 1 diabetes: a review and next steps. Curr Diab Rep 2017; 17: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coulman KD, MacKichan F, Blazeby JM, et al. Patient experiences of outcomes of bariatric surgery: a systematic review and qualitative synthesis. Obes Rev 2017; 18: 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet (London, England) 2015; 386: 964–973. [DOI] [PubMed] [Google Scholar]
- 29. Sakran N, Sherf-Dagan S, Blumenfeld O, et al. Incidence and risk factors for mortality following bariatric surgery: a nationwide registry study. Obes Surg 2018; 28: 2661–2669. [DOI] [PubMed] [Google Scholar]


