Abstract
We investigated the influence of mechanical stretching on the genetic expression pattern of non-collagenous bone matrix proteins in osteoblasts. The cranial sutures of neonatal mice were subjected to ex vivo mechanical stretching. In the non-stretched control group, as osteoblast differentiation progressed, the successive genetic expression of bone sialoprotein (BSP), osteopontin (OPN), and osteocalcin (OCN) was detected using in situ hybridization, in that order. In the stretched group, the sutures were widened, and after 24 hr of cultivation, a large number of osteoblasts and abundant new osteoid were observed on the borders of the parietal bones. All new osteoblasts expressed BSP and some of them expressed OPN, but very few of them expressed OCN. After 48 hr, more extensive presence of osteoid was noted on the borders of the parietal bones, and this osteoid was partially mineralized; all osteoblasts on the osteoid surface expressed BSP, and more osteoblasts expressed OPN than those after 24 hr cultivation. Surprisingly, many of the osteoblasts that did not express OPN, expressed OCN. This suggests that when osteoblast differentiation is stimulated by mechanical stress, the genetic expression pattern of non-collagenous proteins in the newly differentiated osteoblasts is affected.
Keywords: mechanical loading, non-collagenous bone proteins, ossification, sagittal sutures
Introduction
The bone matrix non-collagenous proteins produced and secreted by osteoblasts possess important biological functions, particularly in regulating bone matrix mineralization and osteoblast and osteoclast activities. For example, bone sialoprotein (BSP) is considered to play a critical roles in primary bone formation and modeling, and in the nuclei formation for matrix mineralization.1 BSP also stimulates osteoblast differentiation through its Arg-Gly-Asp (RGD) sequence, and the bone resorption activity of osteoclasts.2,3 Osteopontin (OPN) is thought to regulate hydroxyapatite crystal growth4 and plays important roles in bone remodeling by creating a scaffold for cell adhesion and by regulating the migration and resorption activities of osteoclasts via CD44.5,6 Furthermore, osteocalcin (OCN) contributes to the maturation of hydroxyapatite crystals,7,8 promotes the migration and maturation of osteoclast precursors, and negatively regulates bone formation.9
During the process of embryogenesis, these non-collagenous proteins are expressed sequentially, according to the developmental stage of osteoblasts. For example, during the process of intramembranous ossification in the crania of 16-day-old mouse fetuses, osteoblasts initiate to express BSP, followed by the expressions of OPN and OCN.10 At the very early stage of the mineralization process, OPN, but not OCN, is expressed in osteoblasts.11 OCN mRNA is rarely expressed at the beginning stages of osteoblast differentiation,12 and OCN is detected in the bone matrix after mineralization significantly progressed.13 Therefore, OCN has been considered to be a marker for mature osteoblasts. These observations suggest that the successive expression pattern of non-collagenous proteins is critical for the precise osteoblast differentiation and bone matrix mineralization.
Mechanical stretching of osteoblastic cells in vitro is known to stimulate both the differentiation and activation, and the expression of non-collagenous proteins, such as OPN, and OCN.14,15 In vivo as well, a stretching of mouse cranial sutures increases OPN expression in osteoblasts.16 Moreover, in the distraction osteogenesis operations, in which rapid bone growth is artificially stimulated by applying traction to newly formed bone area, notable changes in the expression of non-collagenous bone matrix proteins were observed. As the results of stretching, abnormal expressions of OPN and OCN were induced in the preosteoblasts and fibroblast-like cells within the region of membranous ossification in the distraction gap.17 These results indicated that mechanical stretching induced changes in the expression pattern of non-collagenous proteins in differentiating osteoblasts. However, few studies have examined such effects of mechanical stretching on the gene expression patterns during the differentiation process of osteoblasts.
We previously reported that mechanical stretching accelerated osteoblast differentiation and membranous ossification in the cultured cranial sagittal sutures of 4-day-old mice.18 Therefore, in this study, we further elucidated the effects of mechanical stretching on the expression patterns of non-collagenous bone matrix proteins in the differentiating osteoblasts that may affect mechanical and/or biological properties of the bone matrix.
Materials and Methods
Organ Culture and Mechanical Stretching
The organ culture system with stretching stimulus using coiled springs has been described previously.18,19 Briefly, the left and right parietal bones of 4-day-old ddY mice were excised, with presenting the intact sagittal suture. In the stretched experimental groups (EXP), these parietal bones were then affixed to an activated coiled spring, creating a continuous stretching stimulus in the direction of enlarging the suture. For the control groups (CON), similarly excised parietal bones were simply affixed to fixed coiled springs, which do not exert any stretch force (Fig. 1). They were cultured for either 24 or 48 hr in 24-well plates (Thermo Fisher Scientific Nunc A/S, Roskilde, Denmark) in BGJb medium (Gibco BRL, Life Technologies, Inc., Rockville, MD) containing 10% fetal bovine serum (FBS; Nichirei Biosciences, Inc., Tokyo, Japan), penicillin G (100 U/mL), and streptomycin (100 mg/mL), at 37C in a 5% CO2 atmosphere. The amount of stretch force applied to the crania by the springs was set as close as possible to 0.2 g in each case. These experiments were repeated more than three times, using two mice for each EXP in an experiment. This study was approved by the Animal Care and Use Committee, Okayama University.
Figure 1.
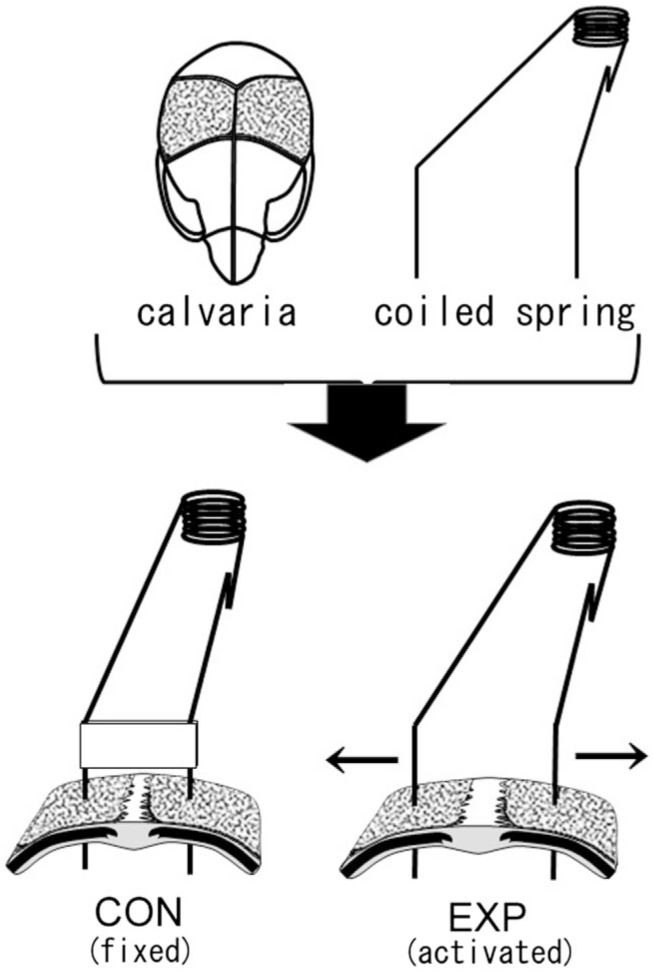
Schematic representation of a mouse calvaria and coiled springs. In the control group (CON), a fixed coiled spring maintains static width in the suture between parietal bones (dotted part of the calvaria). In the experimental group (EXP), an activated coiled spring exerts tensile stress in the sagittal suture and enlarges the suture in the direction of the arrows.
Detection of Alkaline Phosphatase (ALP) Activity and Mineralization Locations
After fixation of the specimens in 4% paraformaldehyde, OCT compound embedding was performed, and 6-μm thick cryosections were prepared. ALP activity was used as a marker for osteoblast differentiation, and its enzymatic activity was detected histochemically using the Azo-dye method.20 To determine the locations of mineralization in these sections, von Kossa (VK) staining21 was performed to detect the presence of calcium. Nuclear staining was done using methylene blue.
In Situ Hybridization (ISH)
Specific riboprobes were used to detect the locations of BSP, OPN, and OCN mRNAs in the serial sections (for full details, please see Ikegame et al.18). Briefly, the cryosections were pretreated with 0.2 N HCl, proteinase K, and acetic anhydride, and then hybridized with probes at a final concentration of 0.5 μg/mL in a hybridization mixture at 55C for 18 to 20 hr. After hybridization, the sections were washed with PBS and treated with ribonuclease. The specific transcripts were detected with antidigoxigenin (DIG)-conjugated ALP antibody according to the manufacturer’s protocol (DIG Detection Kit; Boehringer Mannheim, Mannheim, Germany). The sections were counterstained with nuclear fast red.
Probe Preparation
DIG-labeled sense and antisense single-stranded RNA probes were prepared with a DIG RNA Labeling Kit (Boehringer Mannheim) according to the manufacturer’s instructions. The cDNA sequences used for the probes were: BSP, a 1048-bp fragment containing bases 1 to 104822; OPN, a 588 bp fragment containing bases 215 to 802 in X14882; and OCN, a 470 bp fragment containing bases 1 to 470 in X04142.
Quantification of the Results of ISH in the Stretched Mouse Cranial Sutures
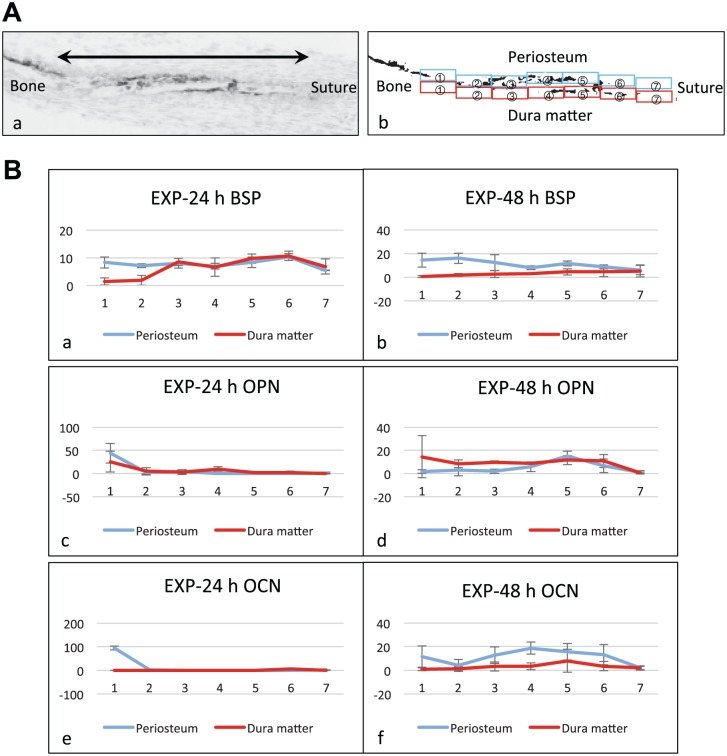
Three binarized images of ISH from three separate experiments were analyzed for each bone matrix protein and time point. The bone surface area on the periosteum or dura matter side from the margin of calcified bone to the tip of osteoid was equally divided into seven measurement areas (Fig. 4A). We calculated the percentage of positive areas for ISH within each measurement area, using ImageJ software from NIH image.
Figure 4.
Quantification of expression pattern of bone matrix proteins. (A) Indication of the measurement areas. (a) A gray scale image of a cryosection hybridized with the antisense probe for bone matrix protein. The bone surface areas from the calcified bone margin (left side: Bone) to the tip of the osteoid (right side: Suture), indicated by the double headed arrow in the gray scale image, were equally divided into seven measurement areas. (b) A binarized image of (a). The measurement areas were numbered from 1 to 7 in the direction from bone to suture side on each of the periosteum side (blue squares) and the dura mater side (red squares). The percentage of the reaction-positive areas for in situ hybridization (ISH) in each measurement area was calculated on binarized images. (B) Graphs showing the quantification results of ISH for the stretched experimental group (EXP) at 24 hr and 48 hr (n=3). The vertical axis and horizontal axis correspond to the percentage of the reaction-positive areas and to the numbers of the measurement areas, respectively. Error bars: Standard deviation. (a and b) Reaction-positive areas for bone sialoprotein (BSP) expression. In EXP-24 h group, they were observed overall both on the periosteum side and the dura matter side (a). In EXP-48 h group, the reaction-positive areas decreased on the dura mater side (b). (c and d) Reaction-positive areas for osteopontin (OPN) expression. In EXP-24 h group, they were observed partially on the dura matter side but were hardly detected on the periosteal side (c). In EXP-48 h group, the reaction-positive areas were observed overall on the dura mater side and partially on the periosteum side (d). (e and f) Reaction-positive areas for osteocalcin (OCN) expression. In EXP-24 h group, OCN was rarely expressed in the newly formed area (e). However, in EXP-48 h group, the reaction-positive areas increased on the periosteum side (f). Particularly, osteoblasts located in the areas 3 and 4 of the periosteum side in EXP-48 h group mainly expressed OCN rather than OPN (d and f).
Results
Precultured Parietal Bones
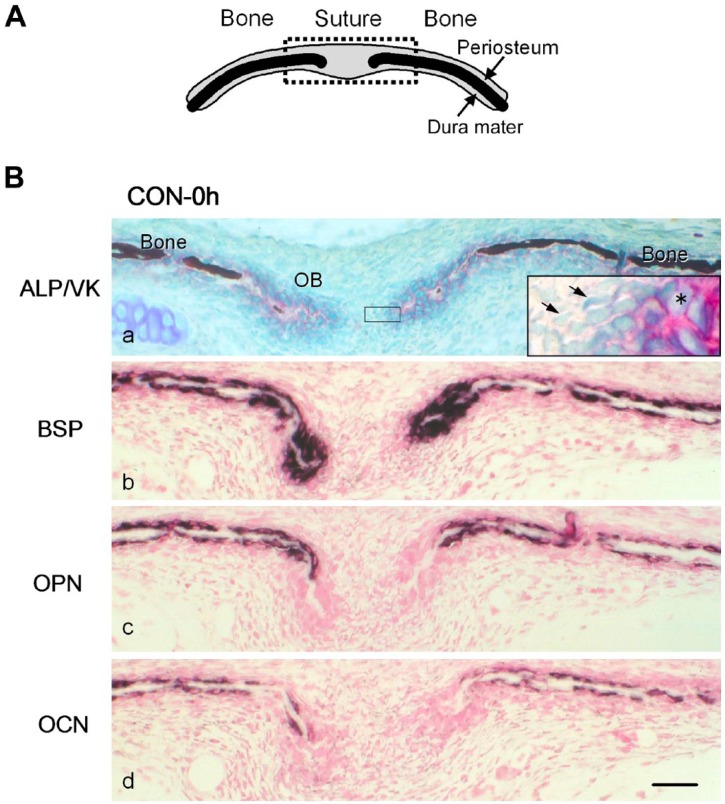
In the excised crania (CON-0 h; schematically illustrated in Fig. 2A), fibroblast-like cells presented in the sagittal suture between the left and right parietal bones, and ALP-positive cuboidal osteoblasts were observed on the surface of the parietal bones. The ends of the parietal bones at the suture side consisted of osteoid (Fig. 2B-a). BSP mRNA was expressed in almost all osteoblasts on the parietal bones (Fig. 2B-b). OPN mRNA was expressed in osteoblasts on the surface of the mineralized areas but not in those on the osteoid surfaces at the ends of the parietal bones (Fig. 2B-c). Osteoblasts expressing OCN mRNA located in approximately the same areas as those expressing OPN mRNA. However, in the area close to the osteoid end, OCN expressing cells were fewer than OPN expressing cells (Fig. 2B-d). No specific signal was observed in the samples with any of the sense probes (data not shown).
Figure 2.
(A) A schematic frontal view of the sagittal suture and parietal bones. The upper and lower sides of the suture and bones are the periosteum and dura mater, respectively. The dotted square indicates the area shown in (B). (B) Distribution of mRNA expression for non-collagenous bone matrices in the frontal sections of the sagittal suture and parietal bones from 4-day-old mice inspected immediately after excision (CON-0 h). (a) Alkaline phosphatase (ALP) activity (red) was detected on the cell surface of cuboidal osteoblasts (OB) on the bone matrix. The mineralized matrix (dark brown) was detected by von Kossa staining (VK). Unmineralized osteoid was observed at the ends of parietal bones. The inset is a higher magnification of the squared area. Asterisk: ALP-positive osteoblasts. Arrows: fibroblast-like cells. (b, c, d) Specific transcripts for bone sialoprotein (BSP) (b), osteopontin (OPN) (c), and osteocalcin (OCN) (d), detected with antisense probes for in situ hybridization, are shown in dark purple. Scale bar: 50 μm. Abbreviation: CON, control group.
Parietal Bones Cultured for 24 hr
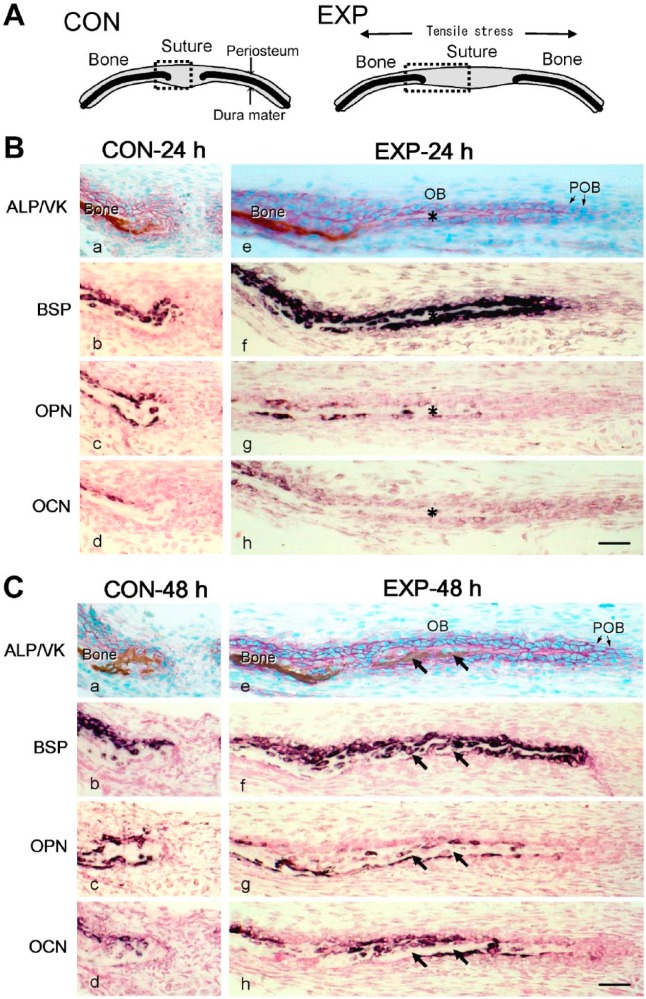
In the non-stretched CON cultured for 24 hr (CON-24 h; schematically illustrated in Fig. 3A), the parietal bone surface was covered with ALP-positive osteoblasts. Mineralization of the osteoid progressed and very small non-mineralized areas remained at the ends of the parietal bones (Fig. 3B-a). BSP mRNA was expressed in most osteoblasts on the periosteal side (Fig. 3B-b). OPN mRNA expression was detected in the osteoblasts located close to the ends of the parietal bones (Fig. 3B-c). OCN mRNA expression was only detected in some of the osteoblasts on the periosteal side and there were almost no expression in the osteoblasts at the ends of the parietal bones (Fig. 3B-d).
Figure 3.
(A) Schematic frontal views of the sagittal suture and parietal bones of the control group (CON) cultured without tension (left), and the experimental group (EXP) cultured with tension (right). Dotted squares indicate the areas shown in (B) and (C). (B and C) Distribution of mRNA expression for non-collagenous bone matrices in the frontal sections of the sagittal suture and parietal bones from 4-day-old mice of CON-24 h or EXP-24 h cultured for 24 hr (B) and CON-48 h or EXP-48 h cultured for 48 hr (C). (a and e) Alkaline phosphatase activity (ALP; red), and mineralized matrix (brown) detected by von Kossa staining (VK). Specific transcripts for bone sialoprotein (BSP) (b and f), osteopontin (OPN) (c and g), or osteocalcin (d and h), detected with antisense probes for in situ hybridization, are shown in dark purple. Asterisks in (B) indicate the osteoid regions formed among the newly differentiated osteoblasts (OB). Arrows in (C) indicate the mineralized area in the newly formed bone matrix. Scale bars: 50 μm. Abbreviation: POB, preosteoblasts.
In the stretched EXP cultured for 24 hr (EXP-24 h; schematically illustrated in Fig. 3A), the suture had widened, and the cells were elongated in the direction of stretching. At the ends of the parietal bones, a large number of oval ALP-positive osteoblasts formed cues in the direction of stretching and osteoid areas were visible between them (Fig. 3B-e). BSP mRNA expression was detected in the osteoblasts on the osteoid and mineralized bone surfaces (Fig. 3B-f). OPN mRNA expression was observed in osteoblasts on the mineralized bone surfaces, and in a few osteoblasts on the osteoid surfaces at the ends of the parietal bones (Fig. 3B-g). OCN mRNA expression was also detected in the osteoblasts on the mineralized bone, but almost no expression was seen in those on the osteoid (Fig. 3B-h).
Parietal Bones Cultured for 48 hr
In the non-stretched CON cultured for 48 hr (CON-48 h), mineralization of bone matrix further progressed at the ends of the parietal bones, and almost no osteoid surfaces remained (Fig. 3C-a). The distributions of the cells expressing ALP activity, BSP, OPN, or OCN were almost the same as those observed in CON-24 h group (Fig. 3C-a, b, c, d).
In the stretched EXP cultured for 48 hr (EXP-48 h), the ALP-positive osteoblasts forming cues at the ends of the parietal bones possessed cuboidal appearance, and between them, more extended matrix areas were observed compared with those in EXP-24 h group. Some part of the matrix was mineralized primarily from the dura mater side (Fig. 3C-e). BSP mRNA expression was detected in osteoblasts on the osteoid at the ends of the parietal bones (Fig. 3C-f). OPN mRNA expression was observed in several osteoblasts located on the osteoid and was seen in more number of osteoblasts on the mineralized matrix (Fig. 3C-g). OCN mRNA was expressed in some of the osteoblasts on the mineralized matrix, whereas abundantly expressed in the osteoblasts on the osteoid, where no OPN mRNA was detected (Fig. 3C-h). Quantified results of ISH showed the gene expression of OPN and OCN initiated from different regions on the newly formed bone in the stretched sutures at 48 hr (Fig. 4).
Discussion
To examine whether mechanical stress affects mRNA expression patterns of non-collagenous bone matrix proteins in developing osteoblasts, we investigated the expression of BSP, OPN, and OCN in the mechanically stretched cranial sagittal suture of neonatal mice. Our study demonstrated that mechanical stretching changed the sequential expression pattern of OPN and OCN in the developing osteoblasts.
In this study, we use the modified organ culture system from the method reported by Hickory and Nanda.23 In the previous report, they found that cell proliferation in the cranial sutures of 2-day-old rats was upregulated by 0.2 g tension, but not by 0.1 g or 0.6 g tension. We used 4-day-old mice calvariae and examined the effects of different ranges of tension on cell proliferation and osteoblastic differentiation in the sutures, and found that 0.2 g tension was most effective for the stimulation.18
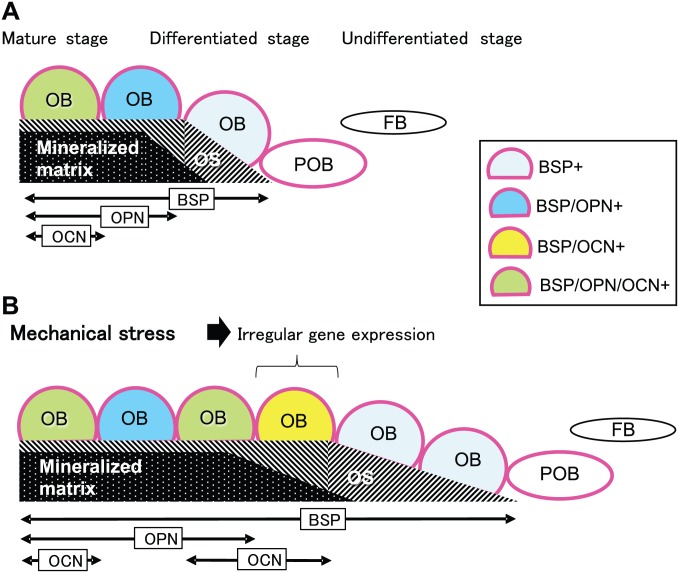
In the non-stretched CON, BSP was already expressed in the osteoblasts on the osteoid surfaces at the ends of the parietal bones, where the new osteoblasts differentiation and bone matrix formation is progressing. OPN expression was detected in the osteoblasts on the mineralized bone matrix, while OCN expression was detected slightly later. These results suggest that under no-stimulation, BSP, OPN, and OCN expression sequentially occurred in that order in parietal bone osteoblasts during their differentiation progressed (Fig. 5A). These gene expression patterns closely resemble the previously reported one during the development of the cranium, mandible, and long bones in fetal mice.11 However, mechanical stretching accelerated osteoblast differentiation and changed the gene expression patterns of OPN and OCN. Quite a few newly differentiated osteoblasts under mechanical stress expressed OCN before the expression of OPN (Fig. 5B). The quantification of the expression patterns of OPN and OCN obtained from 3 separate ISH experiments also revealed the irregular expression patterns of OPN and OCN in the stretched group.
Figure 5.
Illustrated summary of the results. Bidirectional arrows indicate distribution ranges along the parietal bone surface in which the osteoblasts (OB) express bone sialoprotein (BSP), osteopontin (OPN), or osteocalcin (OCN). (A) Normal gene expression pattern of non-collagenous proteins during osteoblast differentiation in the cranial suture. Alkaline phosphatase (Red-colored outline)-positive preosteoblasts (POB) differentiated into osteoid (OS)-forming OB that expressed BSP mRNA. OB then expressed OPN mRNA during the mineralization phase, and finally OCN mRNA at further mature stages. (B) Irregular gene expression pattern of non-collagenous proteins during OB differentiation in the cranial suture stimulated by mechanical stretching. Some OB expressed BSP mRNA, and also OCN mRNA, ahead of OPN mRNA expression (BSP/OCN+). Abbreviation: FB, fibroblast-like cell.
There are several possible explanations for this different gene expression pattern of OPN and OCN observed in the stretched suture. One possible explanation is that the potential variation of gene expression pattern in each osteoblast may become obvious when external forces induce differentiation of a large number of new osteoblasts in a short period. In vitro experiments have shown that osteoblasts possess some diversity in the expression pattern of functional proteins, even in cases where osteoblasts are differentiating side-by-side almost simultaneously.24,25 Another explanation is that mechanical stretching may genetically influence the sequential expression pattern of OPN and OCN by activating signal transduction systems differently. When different degrees of stretching load are applied, intracellular signaling in cultured osteoblastic cells are differently activated. Lighter load induces extracellular signal-related kinase activation and stimulates OPN expression, while heavier load results in c-Jun N-terminal kinase (JNK) and p38 activation, which suppresses OPN expression.26 It is also reported that stretching increased OCN expression via the activation of p38 in bone marrow-derived mesenchymal stem cells.27 Another recent study has demonstrated that the levels of JNK activation during the early stages of osteoblast differentiation determine osteoblast characteristics including OPN or OCN secretion.28 These observations implicated that mechanical stretching elicits various types of intracellular signaling in osteoblasts. Therefore, the stretching of the suture may stimulate the ectopic expression of OCN at the very early stages of osteoblast differentiations before OPN expression. The changes in OCN expression would be able to affect the biological properties of the bone. Recently, OCN is known as a kind of hormone released from bone matrix and playing important roles in energy metabolism, brain function, and so on.9 Therefore, further research on the mechanism to control the OCN expression may contribute to the regulation of metabolic diseases.
Tensile stress-stimulated osteogenesis has been also observed during the process of distraction osteogenesis. In this case, after long bones are cut, the callus is subjected to considerable tension to induce ossification. However, the expression pattern of OPN and OCN mRNA expression was different from that induced by the present mechanical stretching. Sato et al. has reported that OPN and OCN mRNAs were expressed even in fibroblast-like cells and preosteoblasts with tensile stress.17 However, in the present study, we found no BSP, OPN, or OCN expression in fibroblast-like cells or preosteoblasts both in the non-stretched control and the stretched experimental sutures. It therefore seems that distraction osteogenesis is a different osteogenic process, which is not universally found in intramembranous ossification. Further research on the factors which cause such different process of osteoblastic differentiation in distraction osteogenesis should contribute to find the better methods for clinical bone-formation promotion.
In summary, our results, using ex vivo culture system, clearly demonstrated that application of mechanical stretching to the cranial suture stimulates osteoblast differentiation through the different expression patterns of OPN and OCN mRNAs from the regulatory expression patterns observed in the non-stretched sutures.
Acknowledgments
A large portion of the research presented in this article was carried out in the First Department of Oral Anatomy and in the Dental Pharmacology, both at the Niigata University. The authors particularly thank Dr. Hidehiro Ozawa (Professor Emeritus, Niigata University and Matsumoto Dental University) and Dr. Hiroyuki Kawashima (former Professor of Department of Dental Pharmacology, Niigata University), for their guidance and advice during the research.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MI designed the study, carried out all experiments, and drafted the manuscript. HO and SE contributed to the interpretation of the data and approval of the manuscript for publication.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by Grant-in-Aids from Japan Society for the Promotion of Science (No. 14571729, 16K11442).
Contributor Information
Mika Ikegame, Department of Oral Morphology, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan.
Sadakazu Ejiri, Department of Oral Anatomy, School of Dentistry, Asahi University, Gifu, Japan.
Hirohiko Okamura, Department of Oral Morphology, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan.
Literature Cited
- 1. Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993;90(18):8562–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouleftour W, Juignet L, Bouet G, Granito RN, Vanden-Bossche A, Laroche N, Aubin JE, Lafage-Proust MH, Vico L, Malaval L. The role of the SIBLING, Bone Sialoprotein in skeletal biology—contribution of mouse experimental genetics. Matrix Biol. 2016;52–54:60–77. [DOI] [PubMed] [Google Scholar]
- 3. Zhang J, Tu Q, Chen J. Applications of transgenics in studies of bone sialoprotein. J Cell Physiol. 2009;220(1):30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int. 2013;93(4):348–54. [DOI] [PubMed] [Google Scholar]
- 5. Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol. 2001;41:723–49. [DOI] [PubMed] [Google Scholar]
- 6. Staines KA, MacRae VE, Farquharson C. The importance of the SIBLING family of proteins on skeletal mineralization and bone remodeling. J Endocrinol. 2012;214(3):241–55. [DOI] [PubMed] [Google Scholar]
- 7. Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23(3):187–96. [DOI] [PubMed] [Google Scholar]
- 8. Hunter GK, Kyle CL, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J. 1994;300(Pt 3):723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Zhang H, Yang C, Li Y, Dai Z. An overview of osteocalcin progress. J Bone Miner Metab. 2016;34(4):367–79. [DOI] [PubMed] [Google Scholar]
- 10. Chen J, Shapiro HS, Sodek J. Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res. 1992;7(8):987–97. [DOI] [PubMed] [Google Scholar]
- 11. Nakase T, Takaoka K, Hirakawa K, Hirota S, Takemura T, Onoue H, Takebayashi K, Kitamura Y, Nomura S. Alterations in the expression of osteonectin, osteopontin and osteocalcin mRNAs during the development of skeletal tissues in vivo. Bone Miner. 1994;26(2):109–22. [DOI] [PubMed] [Google Scholar]
- 12. Bellows CG, Reimers SM, Heersche JN. Expression of mRNAs for type-I collagen, bone sialoprotein, osteocalcin, and osteopontin at different stages of osteoblastic differentiation and their regulation by 1,25 dihydroxyvitamin D3. Cell Tissue Res. 1999;297(2):249–59. [DOI] [PubMed] [Google Scholar]
- 13. Mark MP, Butler WT, Prince CW, Finkelman RD, Ruch JV. Developmental expression of 44-kDa bone phosphoprotein (osteopontin) and bone gamma-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation. 1988;37(2):123–36. [DOI] [PubMed] [Google Scholar]
- 14. Bhatt KA, Chang EI, Warren SM, Lin SE, Bastidas N, Ghali S, Thibboneir A, Capla JM, McCarthy JG, Gurtner GC. Uniaxial mechanical strain: an in vitro correlate to distraction osteogenesis. J Surg Res. 2007;143(2):329–36. [DOI] [PubMed] [Google Scholar]
- 15. Jekir MG, Donahue HJ. Gap junctions and osteoblast-like cell gene expression in response to fluid flow. J Biomech Eng. 2009;131(1):011005. [DOI] [PubMed] [Google Scholar]
- 16. Morinobu M, Ishijima M, Rittling SR, Tsuji K, Yamamoto H, Nifuji A, Denhardt DT, Noda M. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J Bone Miner Res. 2003;18(9):1706–15. [DOI] [PubMed] [Google Scholar]
- 17. Sato M, Yasui N, Nakase T, Kawahata H, Sugimoto M, Hirota S, Kitamura Y, Nomura S, Ochi T. Expression of bone matrix proteins mRNA during distraction osteogenesis. J Bone Miner Res. 1998;13(8):1221–31. [DOI] [PubMed] [Google Scholar]
- 18. Ikegame M, Ishibashi O, Yoshizawa T, Shimomura J, Komori T, Ozawa H, Kawashima H. Tensile stress induces bone morphogenetic protein 4 in preosteoblastic and fibroblastic cells, which later differentiate into osteoblasts leading to osteogenesis in the mouse calvariae in organ culture. J Bone Miner Res. 2001;16(1):24–32. [DOI] [PubMed] [Google Scholar]
- 19. Ikegame M, Tabuchi Y, Furusawa Y, Kawai M, Hattori A, Kondo T, Yamamoto T. Tensile stress stimulates the expression of osteogenic cytokines/growth factors and matricellular proteins in the mouse cranial suture at the site of osteoblast differentiation. Biomed Res. 2016;37(2):117–26. [DOI] [PubMed] [Google Scholar]
- 20. Burstone MS. Alkaline phosphatase, naphthol AS-BI phosphate method. New York: Academic Press; 1962. [Google Scholar]
- 21. Chaplin AJ, Grace SR. Calcium oxalate and the von Kossa method with reference to the influence of citric acid. Histochem J. 1975;7(5):451–8. [DOI] [PubMed] [Google Scholar]
- 22. Chen JJ, Jin H, Ranly DM, Sodek J, Boyan BD. Altered expression of bone sialoproteins in vitamin D-deficient rBSP2.7Luc transgenic mice. J Bone Miner Res. 1999;14(2):221–9. [DOI] [PubMed] [Google Scholar]
- 23. Hickory WB, Nanda R. Effect of tensile force magnitude on release of cranial suture cells into S phase. Am J Orthod Dentofacial Orthop. 1987;91(4):328–34. [DOI] [PubMed] [Google Scholar]
- 24. Malaval L, Modrowski D, Gupta AK, Aubin JE. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol. 1994;158(3):555–72. [DOI] [PubMed] [Google Scholar]
- 25. Pockwinse SM, Stein JL, Lian JB, Stein GS. Developmental stage-specific cellular responses to vitamin D and glucocorticoids during differentiation of the osteoblast phenotype: interrelationship of morphology and gene expression by in situ hybridization. Exp Cell Res. 1995;216(1):244–60. [DOI] [PubMed] [Google Scholar]
- 26. Matsui H, Fukuno N, Kanda Y, Kantoh Y, Chida T, Nagaura Y, Suzuki O, Nishitoh H, Takeda K, Ichijo H, Sawada Y, Sasaki K, Kobayashi T, Tamura S. The expression of Fn14 via mechanical stress-activated JNK contributes to apoptosis induction in osteoblasts. J Biol Chem. 2014;289(10):6438–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao WL, Zhang DZ, Fan CH, Yu BJ. Intermittent stretching and osteogenic differentiation of bone marrow derived mesenchymal stem cells via the p38MAPK-osterix signaling pathway. Cell Physiol Biochem. 2015;36(3):1015–25. [DOI] [PubMed] [Google Scholar]
- 28. Kusuyama J, Ohnishi T, Matsuguchi T. JNK signaling regulates multifunctional differentiation of osteoblasts. 34th Annual Meeting of the Japanese Society for Bone and Mineral Research, Program & Abstracts; 2016 July 20–23, Osaka, Japan. [Google Scholar]