Abstract
Background
Although people with haematological malignancies have to endure long phases of therapy and immobility, which is known to diminish their physical performance level, the advice to rest and avoid intensive exercises is still common practice. This recommendation is partly due to the severe anaemia and thrombocytopenia from which many patients suffer. The inability to perform activities of daily living restricts them, diminishes their quality of life and can influence medical therapy.
Objectives
In this update of the original review (published in 2014) our main objective was to re‐evaluate the efficacy, safety and feasibility of aerobic physical exercise for adults suffering from haematological malignancies considering the current state of knowledge.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2018, Issue 7) and MEDLINE (1950 to July 2018) trials registries (ISRCTN, EU clinical trials register and clinicaltrials.gov) and conference proceedings. We did not apply any language restrictions. Two review authors independently screened search results, disagreements were solved by discussion.
Selection criteria
We included randomised controlled trials (RCTs) comparing an aerobic physical exercise intervention, intending to improve the oxygen system, in addition to standard care with standard care only for adults suffering from haematological malignancies. We also included studies that evaluated aerobic exercise in addition to strength training. We excluded studies that investigated the effect of training programmes that were composed of yoga, tai chi chuan, qigong or similar types of exercise. We also excluded studies exploring the influence of strength training without additive aerobic exercise as well as studies assessing outcomes without any clinical impact.
Data collection and analysis
Two review authors independently screened search results, extracted data and assessed the quality of trials. We used risk ratios (RRs) for adverse events, mortality and 100‐day survival, standardised mean differences (SMD) for quality of life (QoL), fatigue, and physical performance, and mean differences (MD) for anthropometric measurements.
Main results
In this update, nine trials could be added to the nine trials of the first version of the review, thus we included eighteen RCTs involving 1892 participants. Two of these studies (65 participants) did not provide data for our key outcomes (they analysed laboratory values only) and one study (40 patients) could not be included in the meta‐analyses, as results were presented as changes scores only and not as endpoint scores. One trial (17 patients) did not report standard errors and could also not be included in meta‐analyses. The overall potential risk of bias in the included trials is unclear, due to poor reporting.
The majority of participants suffered from acute lymphoblastic leukaemia (ALL), acute myeloid leukaemia (AML), malignant lymphoma and multiple myeloma, and eight trials randomised people receiving stem cell transplantation. Mostly, the exercise intervention consisted of various walking intervention programmes with different duration and intensity levels.
Our primary endpoint overall survival (OS) was only reported in one of these studies. The study authors found no evidence for a difference between both arms (RR = 0.67; P = 0.112). Six trials (one trial with four arms, analysed as two sub‐studies) reported numbers of deceased participants during the course of the study or during the first 100 to 180 days. For the outcome mortality, there is no evidence for a difference between participants exercising and those in the control group (RR 1.10; 95% CI 0.79 to 1.52; P = 0.59; 1172 participants, low‐certainty evidence).
For the following outcomes, higher numbers indicate better outcomes, with 1 being the best result for the standardised mean differences. Eight studies analysed the influence of exercise intervention on QoL. It remains unclear, whether physical exercise improves QoL (SMD 0.11; 95% CI ‐0.03 to 0.24; 1259 participants, low‐certainty evidence). There is also no evidence for a difference for the subscales physical functioning (SMD 0.15; 95% CI ‐0.01 to 0.32; 8 trials, 1329 participants, low‐certainty evidence) and anxiety (SMD 0.03; 95% CI ‐0.30 to 0.36; 6 trials, 445 participants, very low‐certainty evidence). Depression might slightly be improved by exercising (SMD 0.19; 95% CI 0.0 to 0.38; 6 trials, 445 participants, low‐certainty evidence). There is moderate‐certainty evidence that exercise probably improves fatigue (SMD 0.31; 95% CI 0.13 to 0.48; 9 trials, 826 patients).
Six trials (435 participants) investigated serious adverse events. We are very uncertain, whether additional exercise leads to more serious adverse events (RR 1.39; 95% CI 0.94 to 2.06), based on very low‐certainty evidence.
In addition, we are aware of four ongoing trials. However, none of these trials stated, how many patients they will recruit and when the studies will be completed, thus, potential influence of these trials for the current analyses remains unclear.
Authors' conclusions
Eighteen, mostly small RCTs did not identify evidence for a difference in terms of mortality. Physical exercise added to standard care might improve fatigue and depression. Currently, there is inconclusive evidence regarding QoL, physical functioning, anxiety and SAEs .
We need further trials with more participants and longer follow‐up periods to evaluate the effects of exercise intervention for people suffering from haematological malignancies. To enhance comparability of study data, development and implementation of core sets of measuring devices would be helpful.
Plain language summary
The role of aerobic physical exercise for adults with haematological malignancies
What is the aim of this review?
The aim of this Cochrane Review was to find out whether aerobic physical exercise can improve health, or play a supporting role for adult patients suffering from haematological malignancies. We collected and analysed all relevant studies to answer this question and found 18 relevant studies, of whom 14 reported our pre‐defined patient‐relevant outcomes.
Key messages
Aerobic physical exercise probably has a positive effect on fatigue and depression of patients with haematological malignancies. Evidence related to mortality, quality of life, and serious adverse events is still unclear.
What was studied in the review?
Haematological malignancies are tumours of the blood‐forming system, such as lymphomas, leukaemias, myelomas, myelodysplastic syndromes and myeloproliferative diseases. These diseases represent approximately seven per cent of new cancer diagnoses worldwide. Treatment strategies include wait‐and‐watch approaches, chemotherapy, radiotherapy, immunotherapy and stem cell transplantation, as well as supportive care to prevent, control or treat complications and side effects. Although these patients have to endure long phases of therapy and immobility, which has a negative effect on their physical performance level, it is still common practice to recommend rest and to avoid intensive exercise. There are several studies and approaches that try to establish another strategy and to include physical exercise, especially aerobic physical exercise, into the treatment strategy of haematological malignancies. In detail, these exercise programmes consist of aerobic, resistance and flexibility components, partly home‐based. Some prefer it to be integrated in daily living. A common method is also the use of tools such as bicycle ergometers or stretch bands as well as walking exercises. Aerobic physical exercise might improve oxygen supply to muscles and tissues of the body.
What are the main results of the review?
The review authors of this review update identified nine new trials which could be added to the nine trials of the first version of this review. Of these 18 trials, 14 trials provided sufficient data to be meta‐analysed. Although six trials reported how many participants died during the study period or during the first 100 days, there is no evidence for differences in this outcome between the exercise group and the control group.
Eight trials measured quality of life, physical functioning and anxiety and did not show any evidence for a difference between additional exercise and usual care. There might be a benefit for the exercise group in terms of fatigue and depression.
The evidence for serious adverse events is based on very low certainty, therefore results are still uncertain.
In addition, we are aware of four ongoing trials. However, none of these trials stated, how many patients they will recruit and when the studies will be terminated, thus, potential influence of these trials for the current analyses remains unclear.
How up‐to‐date is the review?
The review authors searched for studies that had been published up to July 2018.
Summary of findings
Summary of findings for the main comparison. Physical exercise versus no physical exercise for adults with haematological malignancies.
| Physical exercise versus no physical exercise for adults with haematological malignancies | ||||||
| Patient or population: Adults with haematological malignancies Settings: Inpatient or outpatient Intervention: Physical exercise versus no physical exercise | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control group without exercise | Physical exercise | |||||
| Mortality | 149 per 1.000 |
164 per 1.000 (117 to 226) |
RR 1.10 (0.79 to 1.52) |
1172 (6 RCTs) | ⊕⊕⊝⊝ low1,2 | |
| Quality of Life Scale from: ‐1 to 1 with 1 indicating best outcome | The mean QoL score in the intervention group was 0.11 higher (better) (‐0.03 to 0.24 higher) | SMD 0.11 higher (‐0.03 to 0.24 higher) | 1259 (8 RCTs) |
⊕⊕⊝⊝ low2,3 | ||
|
Physical functioning/QoL
Scale from: ‐1 to 1 with 1 indicating best outcome |
The mean physical functioning/QoL score in the intervention group was 0.15 higher (better) (‐0.01 to 0.32 higher) |
SMD 0.15 higher (‐0.01 to 0.32 higher) | 1329 (8 RCTs) | ⊕⊕⊝⊝ low2,3 | ||
|
Depression/QoL
Scale from: ‐1 to 1 with 1 indicating best outcome |
The mean depression/QoL score in the intervention group was 0.19 higher (better) (0 to 0.38 higher) |
SMD 0.19 higher (0.00 to 0.38 higher) | 445 (6 RCTs) | ⊕⊕⊝⊝ low1,3 | ||
|
Anxiety/QoL
Scale from: ‐1 to 1 with 1 indicating best outcome |
The mean anxiety/QoL score in the intervention group was 0.03 higher (better) (‐0.30 to 0.36 higher) |
SMD 0.03 higher (‐0.30 to 0.36 higher) | 445 (6 RCTs) | ⊕⊝⊝⊝ very low2,3,4 | ||
|
Fatigue
Scale from: ‐1 to 1 with 1 indicating best outcome |
The mean fatigue score in the intervention group was 0.31 higher (better) (0.13 higher to 0.48 higher) |
SMD 0.31 higher (0.13 higher to 0.48 higher) |
826 (9 RCTs) | ⊕⊕⊕⊝ moderate3 | ||
| Serious adverse events | 174 per 1.000 |
242 per 1.000 (164 to 359) |
RR 1.39 (0.94 to 2.06) |
435 (6 RCTs) | ⊕⊝⊝⊝ very low5,6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Small number of participants/events leads to downgrading (1 point) for imprecision.
2Confidence interval including clinically relevant benefits or harms leads to downgrading (1 point) for imprecision.
3Outcome assessor (participant) not blinded in participant‐reported outcome (QoL questionnaires) leads to downgrading by one point for risk of bias.
4 High heterogeneity leads to downgrading by one point due to inconsistency.
5 Baseline imbalances, especially usage of erythropoietin and thalidomide unknown in both intervention arms leads to downgrading by one point due to inconsistency.
6Very small number of participants and events, very wide confidence interval to downgrading (2 points) for imprecision.
Background
Description of the condition
A haematological malignancy is a tumour of the myeloid or lymphatic cell lines affecting blood, bone marrow or the lymph nodes with possible involvement of other organs. Lymphomas, leukaemias, myelomas, myelodysplastic syndromes and myeloproliferative diseases are all haematological malignancies and account for nearly 10% of new cancer diagnoses in the USA (Howlader 2012). The global age‐adjusted incidence rate of haematological malignancies is 40.3 new cases per 100,000 men and women per year. Individual scores are leukaemia (12.6), lymphoma (22.4) and myeloma (5.6) with all their various subcategories (Altekruse 2009).
Depending on the type and stage of the neoplastic disease, the clinical course can be indolent or aggressive with different patterns of treatment behaviour and treatment response. Various treatment options are available for people with haematological malignancies, extending from watch‐and‐wait approaches to single‐ or multi‐agent chemotherapy, radiotherapy, immunotherapy and autologous or allogeneic stem cell transplantation. Best supportive care is provided to make people more comfortable and to prevent, control or treat complications and side effects (Cullen 2001).
The prevailing advice for patients is to rest and avoid intensive exercise, without taking note of the unfavourable consequences of omitting physical exertion. This advice is mainly based on the properties of cytopenia (reduction in the numbers of any of the blood cell elements) from which most patients suffer. A low performance status due to severe anaemia and thrombocytopenia can potentially lead to haemorrhages, while the reduced immune status due to leukopenia increases the risk for infections (Tosetto 2009).
Description of the intervention
One important challenge in treating people with haematological cancer is physical deconditioning. It is highly prevalent in this population and is the result of various circumstances such as the oncologist’s advice to rest, cardiotoxic, neurotoxic or pulmo‐toxic anti‐cancer therapy, anaemia, thrombocytopenia or cachexia. Exercise has been introduced to improve physical functioning and to increase the ability to cope with activities of daily living. Some evidence suggests that physical exercise, especially aerobic exercise that aims to improve the oxygen system, increases cardiorespiratory fitness, muscle strength and physical well‐being in people with haematological cancer (Coleman 2012; Courneya 2009; Moyer‐Mileur 2009; Thorsen 2005).
People undergoing intensive chemotherapy suffer from unintended effects of the therapy such as inflammation due to long‐lasting immunosuppression and leukopenia. Apart from this, the inability to perform normal physical activity is a decisive limiting factor in the treatment of people with haematological malignancies. For them, this implies detrimental effects on their quality of life, as several studies have shown (Broers 2000; Fife 2000). Nevertheless, physical exercise programmes still occupy a minor role in the treatment concepts of haematological malignancies. Furthermore, we lack reliable data from randomised controlled trials (RCTs) about risk factors, feasibility and outcomes of exercise in people with haematological malignancies, particularly with regard to overall survival (OS).
The first study of therapeutic exercise in the follow‐up treatment of people suffering from breast cancer explicitly showed a positive physical and psychological effect (Schule 1983). Owing to the positive impact of this and further studies, exercise therapy has become a part of oncological treatment concepts (Dimeo 1996; Mock 1994; Peters 1994). The former opinion that exercise as part of health‐orientated therapy, concomitant with or immediately after medical therapy, could be harmful and should not be started before complete remission is achieved, has proved to be incorrect (Andrykowski 1989; Dimeo 1996). The most intensively investigated types of cancer are breast, colorectal and prostate cancers, where large prospective phase III trials are active and clear recommendations for activity were given (Courneya 2013; Dieli‐Conwright 2014; Doyle 2006). Important factors such as quality of life, physical functioning, depression and many other factors could be improved in those patients performing exercise (McCullough 2014).
Another essential burden for people with cancer is cancer‐related fatigue. It is defined as debilitating symptoms of physical, emotional and cognitive tiredness or exhaustion related to cancer or cancer treatment (NCCN 2014). Cancer‐related fatigue is very common during or after treatment and is reported by 60% to 90% of people with cancer (Wagner 2004). In recent meta‐analyses physical exercise has resulted in some reduction of cancer‐related fatigue in people with solid tumours (Velthuis 2010).
Aside from this recent development, the extent of physical exercise for people suffering from blood cancers remains unclear. Previous studies suggest that aerobic exercise can be safely carried out immediately after high‐dose chemotherapy and can partially prevent loss of physical performance (Dimeo 1996; Dimeo 1997). Data from Dimeo 1997 suggest that exercise mediates better maximal physical performance at discharge and shorter durations of neutropenia, thrombopenia and hospitalisation.
How the intervention might work
There is some evidence for a protective role of physical activity for cancer, in particular colon, breast (postmenopausal) and endometrial cancers (Parent 2011). A 20% to 40% reduced risk of several cancer types is reported in the current literature (Parent 2011). The precise/further underlying mechanisms for physical activity in reducing cancer risk remain to be elucidated. Several biological mechanisms have been suggested, which could equally apply to many cancer entities (Friedenreich 2001). These include a decrease in obesity and central adiposity, hormone level and growth factor modulation, modification of carcinogen activation and improvement in immune function (Li 2010a). Li 2010b reported immunomodulation due to physical activity as an increase of human natural killer activity and enhanced expression of intracellular anti‐cancer proteins in lymphocytes.
Why it is important to do this review
This is the updated version of the first systematic review taking into consideration the evidence from randomised comparisons on the impact of physical exercise in adults with haematological malignancies. The main question stated is whether physical exercise in addition to standard care is beneficial regarding OS, fatigue and quality of life compared to standard care alone. Further questions elucidate the role of physical exercise in terms of physical strength, well‐being and adverse effects.
In order to obtain conclusive evidence on the impact of physical exercise, we have performed a systematic review and meta‐analysis. A summary of all results will help us to choose the best available physical exercise approach and to reach conclusions about safety and effectiveness.
Objectives
To evaluate the efficacy, safety and feasibility of aerobic physical exercise for adults suffering from haematological malignancies.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised controlled trials (RCTs) for inclusion. We included both full‐text and abstract publications.
Types of participants
We included trials on adults (18 years and over) with confirmed diagnoses of haematological malignancies. We did not apply gender or ethnicity restrictions. We considered all subtypes and stages of haematological malignancies, including newly‐diagnosed patients and those with relapsed or drug‐resistant disease. If trials had consisted of mixed populations with different conditions or types of cancer, we would have used data only from the haematological malignancy subgroups. If subgroup data for these participants had not been provided (after contacting the authors of the trial), we would have excluded the trial if fewer than 80% of participants had haematological malignancies.
Types of interventions
The main intervention was aerobic physical exercise in addition to standard care, compared to standard care alone. We only included studies that evaluated the response of the participant to aerobic exercise, intending to improve the oxygen system. Accordingly, we included studies that chose exercise interventions such as moderate cycling, walking, Nordic walking, running, swimming and other related forms of sport. These kinds of sports are easy to regulate with regards to load control. We also included studies that analysed further physical exercise programmes, such as moderate strength training in addition to the aerobic exercise programme. We did not include training programmes that were composed of yoga, tai chi chuan, qigong and similar types of exercise. We also excluded studies solely exploring the influence of strength training. Additionally, we excluded studies assessing outcomes without any clinical impact.
Types of outcome measures
We included all trials fitting the above mentioned inclusion criteria, irrespective of outcomes reported
Primary outcomes
We predefined overall survival (OS) as the primary efficacy outcome. As this outcome was reported in one trial only, but mortality was reported in six trials, we also evaluated mortality.
Secondary outcomes
We analysed the following outcomes as secondary outcomes:
quality of life;
fatigue;
physical performance (e.g. aerobic capacity, cardiovascular fitness);
anthropometric measurements (e.g. weight, body mass index);
adverse events.
Search methods for identification of studies
Electronic searches
We adapted the search strategies as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We applied no language restriction, to reduce the language bias. There were no restrictions by date or by publication status (e.g. abstract, conference proceedings, unpublished data, dissertations, etc).
We searched the following databases and sources.
-
Databases of medical literature:
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2018, Issue 7) (for search strategy, see Appendix 1);
MEDLINE (1950 to 30 July, 2018) (for search strategy, see Appendix 2).
-
Conference proceedings of annual meetings (1990 to 2018) of the following societies for abstracts if not included in CENTRAL:
American Society of Hematology (ASH) (2011 to 2017);
American Society of Clinical Oncology (ASCO) (2011 to 2018);
European Hematology Association (2011 to 2018).
-
Databases of ongoing trials:
-
for the original version of the review:
meta‐register of controlled trials: www.controlled‐trials.com/mrct/.
-
for the update: we electronically searched in the database of ongoing trials up to 01 July 2018
ISRCTN: http://www.isrctn.com;
EU clinical trials register: https://www.clinicaltrialsregister.eu/ctr‐search/search;
Clinicaltrials.gov: https://clinicaltrials.gov/.
-
Searching other resources
Handsearching of references:
we checked references of all identified trials and relevant review articles for further literature.
Data collection and analysis
Selection of studies
Three review authors (LK, NS; NB and NS for the first version of the review) independently screened the results of the search strategies for eligibility for this review by reading relevant abstracts. In case of disagreement, we obtained the full‐text publication (Higgins 2011b).
We documented the study selection process in a flow chart as recommended in the PRISMA statement (Moher 2009) showing the total numbers of retrieved references and the numbers of included and excluded studies.
Data extraction and management
Two review authors (LK, NS; NB and NS for the first version of the review) independently extracted the data according to the guidelines proposed by Cochrane (Higgins 2011b). We used a standardised data extraction form containing the following items.
General information: author, title, source, publication date, country, language, duplicate publications.
Quality assessment: sequence generation, allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting, other potential sources of bias.
Study characteristics: trial design, aims, setting and dates, source of participants, inclusion and exclusion criteria, comparability of groups, subgroup analysis, statistical methods, power calculations, treatment cross‐overs, compliance with assigned treatment, length of follow‐up, time point of randomisation.
Participant characteristics: underlying disease, stage of disease, histological subtype, additional diagnoses; age, gender, ethnicity; number of participants recruited, allocated, evaluated; participants lost to follow‐up; type of treatment (multi‐agent chemotherapy, intensity of regimen, number of cycles), additional radiotherapy, type and dosage of monoclonal antibodies, bone marrow transplantation.
Interventions: type, duration and intensity of physical exercise; standard care; duration of follow‐up.
Outcomes: OS, aerobic capacity, cardiovascular fitness, anthropometric measurements, quality of life, fatigue, adverse events.
We used both full‐text versions and abstracts including additional information (for example slides) of eligible studies to retrieve the data. We extracted trials reported in more than one publication on one form only. Where these sources did not provide sufficient information, we had planned to contact the authors for additional details.
Assessment of risk of bias in included studies
To assess quality and risk of bias, two review authors (NS, LK) independently assessed the risk of bias for each study using the following criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a):
Sequence generation
Allocation concealment
Blinding (participants, personnel, outcome assessors)
Incomplete outcome data
Selective outcome reporting
Other potential sources of bias.
For every criterion, we made a judgement using one of three categories.
'Low risk': if the criterion was adequately fulfilled in the study, i.e. the study was at a low risk of bias for the given criterion;
'High risk': if the criterion was not fulfilled in the study, i.e. the study was at high risk of bias for the given criterion;
'Unclear': if the study report did not provide sufficient information to allow for a judgement of 'Yes' or 'No' or if the risk of bias was unknown for one of the criteria listed above.
Measures of treatment effect
We estimated treatment effect measures of individual studies as relative effect measures (RR) with 95% confidence intervals (CI) for dichotomous data. For survival data, we estimated treatment effects by extracting hazard ratios (HR) of individual studies and analysing these using the methods described by Parmar (Parmar 1998) and Tierney (Tierney 2007).We calculated continuous outcomes as mean differences (MD) or in case of different scales in various studies as standardised mean differences (SMDs) with 95% CIs for each trial.
Dealing with missing data
As suggested in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), there were many potential sources of missing data which we had to take into account, at a study level, outcome level, and summary data level. Firstly, it was important to distinguish between 'missing at random' and 'not missing at random'. We did not identify any missing data.
Assessment of heterogeneity
In meta‐analyses with at least three trials, we assessed heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1. In that case, we used the I² statistic to quantify possible heterogeneity (I² > 30% moderate heterogeneity, I² > 75% considerable heterogeneity) (Deeks 2011). We explored potential causes of heterogeneity by sensitivity and subgroup analyses where possible.
Assessment of reporting biases
In a meta‐analysis with at least 10 trials, we would have explored potential reporting bias by generating a funnel plot and statistically testing this by conducting a linear regression test (Sterne 2011). A P value less than 0.1 would have been considered significant for this test. However, we only included maximum nine trials in one meta‐analysis, so this test was not performed.
Data synthesis
We performed analyses according to the recommendations of chapter nine of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We used aggregated data for analysis. For statistical analysis, we entered data into the Cochrane statistical package Review Manager (RevMan) 5.3. One review author entered data into RevMan software and an another review author checked it for accuracy. Due to variation of types of haematological malignancies of participants and the different duration and intensity of the physical intervention, we performed meta‐analyses using a random‐effects model.
If appropriate, we calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH).
We used the software GRADEpro 3.2 to create 'Summary of Finding' tables as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schunemann 2011).
We ranked the following outcomes as the most patient‐relevant outcomes and presented them in the 'Summary of findings' table: overall survival (OS)/mortality, quality of life, physical functioning/quality of life (QoL), depression/QoL, anxiety/QoL, fatigue, serious adverse events.
Subgroup analysis and investigation of heterogeneity
We considered the following characteristics for subgroup analyses, but data were too sparse to perform subgroup analyses.
Age of included patients
Type of therapy of underlying disease (chemotherapy versus radiotherapy versus no treatment)
Type, duration, intensity of physical exercise
We analysed subgroups for patients receiving stem cell transplantation versus non stem cell transplantation as treatment of their underlying disease.
Sensitivity analysis
We analysed quality components (high risk of bias versus low risk of bias). We considered analysing full‐text publications versus abstract publications, but all the included trials were reported as full texts.
Results
Description of studies
Results of the search
Our literature search led to 4314 new publications to screen for this update. We excluded 4231 publications because they did not correspond with our inclusion criteria or were duplicates. We retrieved the remaining 51 publications as full‐text or abstract publications for further evaluation. Of these 72 publications, we finally excluded 34. At the end of our screening procedure, nine new studies (17 publications) could be added to the nine studies (21 references) from the first version of the review, leading to 18 studies with 38 publications in total.
We did not meta‐analyse data from four of these 18 included trials, which evaluated outcomes that are not patient‐relevant (laboratory values only) (Cunningham 1986; Kim 2006), or reported data in a way that could not be included in the meta‐analysis (Alibhai 2014; Bryant 2018). Cunningham 1986 investigated the influence of training on muscle strength or muscle protein status. Kim 2006 investigated the effect of physical exercises on lymphocyte and T‐cell subsets. One trial (40 patients) reported change‐scores only (Alibhai 2014); another trial evaluating 17 patients did not report standard difference or standard error (Bryant 2018), therefore both trials could not be included in meta‐analyses.
For the first version of the review, nine studies with 20 publications were included in the analysis of the review.
Additionally, currently four RCTs are ongoing (Abildgaard 2018; Courneya 2017; Oberste 2016; Walsh 2005). As the authors of these trials do not report, how many participants they will recruit and when the trial will be terminated, the potential influence of these trials for the current analyses is unclear. The study by Walsh 2005 and colleagues started in 2005 and according to the study registry, clinicaltrials.gov it still seems to be active and recruiting patients. One study has been published as an abstract only and is awaiting classification as it remains unclear, due to missing information, whether this trial fits our pre‐defined inclusion criteria (Wehrle 2018).
The overall number of references screened, identified, selected, excluded and included is documented according to the PRISMA flow diagram (Figure 1).
1.
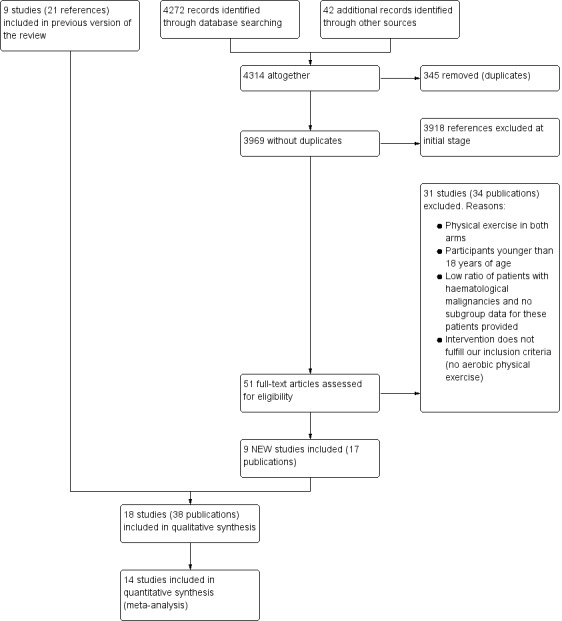
Flow diagram.
Included studies
Eighteen trials in 38 publications, including a total of 1892 participants (range 18 to 711), fulfilled the inclusion criteria (Alibhai 2014; Baumann 2010; Bryant 2018; Chang 2008; Coleman 2003; Coleman 2012; Courneya 2009; Cunningham 1986; DeFor 2007; Furzer 2016; Jacobsen 2014; Jarden 2016; Kim 2006; Knols 2011; Mello 2003; Persoon 2017; Streckmann 2014; Wiskemann 2015).
We did not meta‐analyse two (65 participants) of these 18 RCTs because they evaluated laboratory values only and did not report the pre‐specified outcomes of this review (Cunningham 1986; Kim 2006). One further trial (17 participants) did not provide data in a sufficient way to be meta‐analysed (without confidence intervals or standard deviations) (Bryant 2018). Another trial (40 participants) reported change scores instead of endpoint scores, prohibiting data to be meta‐analysed (Alibhai 2014).
We summarise the features of the included trials in the Characteristics of included studies table.
Nine trials reported no periods for trial recruitment. The earliest trial started recruitment in 2002 (Baumann 2010) until 2004, and the latest trials stopped in 2015 (Bryant 2018). All trials were published as full‐text publications.
Design
Sixteen of the included trials were two‐armed randomised controlled trials (RCTs), one was a three‐armed RCT (Cunningham 1986) and one was a four‐armed RCT (Jacobsen 2014). In order to include this last trial into our review, we divided it into two subgroups and analysed it correspondingly. One subgroup (Jacobsen 2014a) included participants with the goal to exercise three to five times a week for 20 to 30 minutes at 50% to 75% of estimated heart rate reserve compared with participants offered usual care, without any structured or supervised training and without encouragement to do physical exercise. The other subgroup (Jacobsen 2014b) evaluated participants with the advice to exercise three to five times a week for 20 to 30 minutes at 50% to 75% of estimated heart rate reserve and to perform stress management training consisting of paced abdominal breathing, progressive muscle relaxation with guided imagery, and coping self‐statements. Participants in the control group received usual care and performed only the stress management training.
Sample sizes
The smallest trial (Mello 2003) randomised 18 participants and the largest trial 711 participants (Jacobsen 2014). Seven trials provided sample size calculation (Alibhai 2014; Coleman 2012; Jacobsen 2014; Jarden 2016; Kim 2006; Knols 2011; Streckmann 2014). However, Coleman 2012 provided different calculations in various publications and Streckmann 2014 was stopped early due to slow recruitment.
Location
Six trials were conducted in the USA (Bryant 2018; Coleman 2003; Coleman 2012; Cunningham 1986; DeFor 2007; Jacobsen 2014); two trials were conducted in Canada (Alibhai 2014; Courneya 2009), one in Taiwan (Chang 2008), one in Switzerland (Knols 2011), one in Australia (Furzer 2016), one in Denmark (Jarden 2016), one in the Netherlands (Persoon 2017), one in Brazil (Mello 2003), one in South Korea (Kim 2006) and three in Germany (Baumann 2010; Streckmann 2014; Wiskemann 2015).
Participants
A total of 1892 men and women with haematological malignancies were randomly allocated either to a physical exercise group plus standard care or to a standard care alone group. The type of underlying haematological malignancy differed between studies. Two studies only included people with acute myeloid leukaemia (Alibhai 2014; Chang 2008). Three studies involved people with acute leukaemia (Bryant 2018; Cunningham 1986; Jarden 2016). In two studies all evaluated participants suffered from multiple myeloma (Coleman 2003; Coleman 2012). Two studies randomised participants with lymphomas (Courneya 2009; Streckmann 2014), one study included explored people with multiple myeloma or lymphomas (Persoon 2017). In the trials by Baumann 2010, DeFor 2007, Furzer 2016, Jacobsen 2014, Kim 2006, Knols 2011, Mello 2003 and Wiskemann 2015, participants suffered from various haematological diseases (mainly acute myeloid leukaemia or acute lymphatic leukaemia).
In nine trials, participants received stem cell transplantation (Alibhai 2014; Baumann 2010; Coleman 2003; Coleman 2012; Cunningham 1986; DeFor 2007; Knols 2011; Mello 2003; Wiskemann 2015). In three trials, participants received autologous blood stem cell transplantation (Coleman 2003; Coleman 2012; Persoon 2017), and in another four trials participants received allogeneic stem cell transplantation (Cunningham 1986; DeFor 2007; Mello 2003; Wiskemann 2015). In two trials, participants received either autologous or allogeneic transplantation, depending on the underlying disease and donor availability (Baumann 2010; Knols 2011). In one trial, only some of the participants received stem cell transplantation (Jarden 2016).
Interventions
In all included trials, physical exercise was performed in addition to standard care and compared with standard care alone. The intensity and the extent of the physical exercise intervention differed between the studies.
Alibhai 2014: participants in the exercise group were offered home‐based exercise three to five days per week. Training was supposed to be performed at a moderate intensity for 30 minutes per session. It consisted of aerobic, resistance and flexibility components and required no or minimal equipment, such as a stability ball and resistance bands, which were provided. Additionally, participants were invited to attend weekly group‐based booster sessions.
Baumann 2010: participants in the exercise arm were offered endurance training on a bicycle ergometer, for 10 to 20 minutes twice a day. Moreover, they participated twice a day in training activities for daily living to maintain mobility. Mostly, this training consisted of walking, stepping and stretching. The exercise programme started six days before transplantation, for five days a week, and lasted until one day before hospital discharge. People in the control group attended a low‐intensity programme of active and passive mobilisation, starting one day after transplantation until hospital discharge.
Bryant 2018: participants in the exercise arm took part in an individualised prescriptive exercise intervention two to four times per week for a period of the induction chemotherapy/in‐hospital recovery. Each session was divided into two parts, of which one took part in the morning, the other one in the afternoon. There was a break of at least 36 hours between sessions.
Chang 2008: the exercise intervention consisted of a three‐week walking programme of 12 minutes walking for five days a week. The control group did not perform any physical exercise programme. All participants in both arms received chemotherapy with cytarabine and idarubicin.
Coleman 2003: exercise consisted of an aerobic component (usually walking, but depending on the fitness and preferences of the participant, perhaps running or cycling) and strength resistance training (using exercise stretch bands). This programme was home‐based. The exercise programme started three months before and ended three months after stem cell transplantation. The control group received best‐practice usual care in terms of activity and rest provided by their physician.
Coleman 2012: participants in the exercise group received individualised exercise and a set of exercise stretch bands with varying resistance. Strength resistance training was included to strengthen muscles so participants could improve the aerobic component of the exercise programme. People in the control group were advised to remain as active as possible and to try to walk 20 minutes a day. Duration of this short‐term study was 15 weeks. The first 70 participants who were eligible for long‐term participation (i.e. response to erythropoietin) continued in the study for an additional 15 weeks. Participants in both groups (exercise and control) received chemotherapy with an intensive treatment protocol (called Total Therapy II) and stem cell transplantation. Fifty per cent of all participants were randomised to receive additionally thalidomide (400 mg daily) during induction, after transplantation consolidation, and maintenance therapy. Furthermore, 76% (N = 102 participants) received erythropoietin.
Courneya 2009: the exercise programme consisted of bicycle ergometer training three times a week for 12 weeks. Intensity began at 60% of the peak power output and was increased by 5% each week to 75% by the fourth week. Duration began at 15 to 20 minutes for the first four weeks and increased by five minutes a week to 40 to 45 minutes in the ninth week. Additionally, participants in the physical exercise group performed one session a week of interval training. Participants in the control group were asked not to increase exercise above baseline. In both groups, some participants received chemotherapy. These participants may have started treatment before enrolment, but needed to have at least eight weeks of planned treatment remaining. Some participants had already received chemotherapy and some were off treatment.
Cunningham 1986: the exercise programme consisted of the following exercise: 15 repetitions of bicep‐tricep curls, bench press, shoulder retractors, straight leg raises, hip extension, hip abduction and sit ups. This was performed three or five times a week, depending on the assignment to one of the exercise groups, for a period of 35 days.
DeFor 2007: participants in the exercise group were asked to walk for at least 15 minutes twice a day on a treadmill that was placed in their hospital room. After discharge, participants in the exercise group were asked to walk once a day for at least 30 minutes. Participants were told to walk at a comfortable speed and to discontinue the workout if they felt any discomfort or dizziness or if the medical staff advised them to do so. This regimen continued until 100 days post transplant. Participants in the control group were not asked to perform any formal exercise, and were not provided with a treadmill unless the participant or staff requested it. In both arms, there was a subset of participants receiving non‐myeloablative conditioning and a subset receiving myeloablative conditioning before allogeneic stem cell transplantation. The authors reported that the activity level prior to transplantation did not differ between the two arms (P = 0.45), but that more participants in the intervention arm (93%) exercised during hospital stay compared to the control arm (58%; P = 0.01).
Furzer 2016: participants in the exercise group were asked to attend a mixed training consisting of cardiovascular training and endurance training. Each session included warm‐up and cool‐down prior to cardiovascular training at 50% of heart rate max, with a maximum duration of 30 minutes per session. Participants used monitors to maintain prescribed heart rate. Exercise progression was achieved by 1) increasing heart rate intensity (up to 70% heart rate max) and 2) decreasing duration (10‐15 minutes) while additionally increasing heart rate intensity (up to 85% hear ratio max). The endurance component consisted of eight exercises targeting the major muscle groups (three sets of 10‐15 repetitions), a progression in weight was possible.
Jacobsen 2014: see Jacobsen 2014a; Jacobsen 2014b
Jacobsen 2014a: Participants randomised to the exercise arm were given a packet of materials, including a videotape, a brochure, a workbook and an electronic step counter in order to be able to perform and track a home‐based exercise program with an emphasis on walking. The intervention was carried out before a planned haematopoietic cell transplantation (HCT). The goal was to exercise 3 to 5 times a week for 20 to 30 minutes per session at 50% to 75% of estimated heart rate reserve. Trained site personnel served as interventionists and introduced the program as well as giving advice concerning proper technique and overcoming potential barriers.
Jacobsen 2014b: participants randomised to the interventional arm were given an packet of materials, including a videotape, a brochure, a workbook and an electronic step counter in order to be able to perform and track a home‐based exercise program with an emphasis on walking. Additionally, they were given a relaxation CD. The intervention was carried out before a planned HCT. Concerning the exercise program, the goal was to exercise three to five times a week for 20 to 30 minutes per session at 50% to 75% of estimated heart rate reserve. Trained site personnel served as interventionists and introduced the program as well as giving advice concerning proper technique and overcoming potential barriers. The stress‐management goal targeted paced abdominal breathing, progressive muscle relaxation with guided imagery, and coping self‐statements to decrease and manage stress.
Jarden 2016: participants allocated to the exercise arm received a 12‐week exercise program, three times week, for 60 to 70 minutes per session. Sessions consisted of stationary cycling for 20‐25 minutes, six dynamic resistance exercises using hand weights in two sets of 12 repetitions and nutrition support. Additionally, counselling sessions were conducted at week zero, six and 12.
Kim 2006: participants randomised to the exercise group performed an exercise program every day for thirty minutes over a period of six weeks. Sessions consisted of preliminary exercise for 10 minutes, relaxation breathing for 10 minutes and finish exercise for 10 minutes. The preliminary exercises were performed in this sequence: concentrate the attention on lower abdomen for three minutes; put left ankle on right knee for three minutes; put right ankle on left knee for two minutes; and bend both knees for two minutes. The finishing exercises were performed in this sequence: resting and relaxing of body and mind for two minutes; stroking down hair and face for two minutes; right and left rotating of both ankles for two minutes; stretching of legs and arms for two minutes; and stretching out on a bed for two minutes.
Knols 2011: participants were randomised to a 12‐week outpatient programme of physical exercise, consisting of supervised aerobic and strength exercises, or to a usual care group without any advice for physical exercise. The physical exercise was performed twice weekly in a physiotherapy practice or fitness centre. Participants started with 10 minutes ergometer cycling or walking treadmill, followed by progressive resistance training.
Mello 2003: participants allocated to the exercise arm received a six‐week exercise program, carried out five times a week for 40 minutes. It involved exercises for shoulder, elbow, hip, knee and ankle, as well as stretching exercises and a treadmill walking program. Participants allocated to the control group received usual care.
Persoon 2017: participants were randomised to a 18‐week exercise programme consisting of high‐intensity resistance and interval training. Participants did training on specialised resistance training equipment and bicycle ergometers. In weeks one to 12, participants performed resistance and interval training twice a week for 60 minutes per training session. In weeks 13 to 18, the intensity of exercise was decreased to one session a week with a duration of 60 minutes. Participants in the control group received usual care.
Streckmann 2014: participants in the exercise arm attended an aerobic endurance training programme, consisting of cardiovascular activation on a bicycle dynamometer and 10 to 30 minutes walk on a treadmill or bicycle ergometer at the end of the training. Participants were also offered sensorimotor training, progressively increasing in task difficulty, and a strength training of four resistance exercises carried out for one minute. Participants in the control group received physiotherapy.
Wiskemann 2015: participants started the exercise intervention on an outpatient basis before allogeneic haematopoietic stem cell transplantation (in general one to four weeks before admission to the hospital), proceeded during the inpatient period and continued the intervention until six to eight weeks after discharge from the hospital. The outpatient intervention was continued as a self‐directed activity at home, whereas the inpatient period was partly supervised twice a week and adapted to the conditions of an isolation unit. The intervention consisted of three endurance training sessions (up to five during hospitalisation) and two resistance training sessions a week. Endurance training in the outpatient setting was recommended as rapid walking for 20 to 40 minutes. In the inpatient setting the participants performed bicycling and treadmill walking instead of the walking intervention. Additionally, participants performed strength training with and without stretch bands. Participants in the control group were told that moderate physical activity is favourable during the treatment process, without further advice. During the inpatient period, physiotherapy was offered up to three sessions a week (average duration of one session: 30 minutes). For this period, the control group had the same access to stationary bicycles and treadmills as the intervention group (not reported, how many participants exercised). All participants received allogeneic stem cell transplantation.
Outcomes
Primary outcome measure
Our primary outcome OS was only reported in one of the included trials (Wiskemann 2015). Mortality was reported by six trials. One of them assessed 180‐day mortality (Jacobsen 2014). One study assessed 100‐day mortality (DeFor 2007). Baumann 2010 and Wiskemann 2015 reported the number of participants who died during hospital stay; all deaths occurred as a transplant‐related complication. Mello 2003 reported that ten patients died but did not provide any data in which arm the patients died.
Secondary outcome measures
Nine studies reported quality of life (QoL) (Alibhai 2014; Baumann 2010; Courneya 2009; Furzer 2016; Jacobsen 2014; Jarden 2016; Persoon 2017; Streckmann 2014; Wiskemann 2015). Eleven studies mentioned fatigue (Alibhai 2014; Baumann 2010; Chang 2008; Coleman 2012; Courneya 2009; Furzer 2016; Jarden 2016; Knols 2011; Persoon 2017; Streckmann 2014; Wiskemann 2015), however only eight reported data in a similar way to be combined for QoL and nine to be analysed for fatigue. Thirteen trials assessed physical performance data (Alibhai 2014; Baumann 2010; Chang 2008; Coleman 2003; Coleman 2012; Courneya 2009; Furzer 2016; Jarden 2016; Knols 2011; Mello 2003; Persoon 2017; Streckmann 2014; Wiskemann 2015). Anthropometric measurements were captured by three studies (Courneya 2009; Furzer 2016; Knols 2011). Six trials reported serious adverse events or adverse events (Chang 2008; Coleman 2012; Courneya 2009; Jarden 2016; Persoon 2017; Streckmann 2014). Some studies explored further outcomes that are irrelevant for this systematic review, but could be partly relevant for clinical practice. Baumann 2010 reported lung function, Chang 2008 explored the time to recovery after transplantation, DeFor 2007 investigated physical and emotional well‐being at discharge and 100 days posttransplant, and Streckmann 2014 reported movement co‐ordination and balance control (see Characteristics of included studies).
Conflict of interest
One study was supported by the Lance Armstrong Foundation (Courneya 2009), one study by the National Heart, Lung, and Blood Institute and the National Cancer Institute (Jacobsen 2014), one study by the SolarisCare foundation (Furzer 2016), one study by The Center for Integrated Rehabilitation of Cancer Patients, The Novo Nordic Foundation, The University Hospitals’ Centre for Health Research (UCSF), The Lundbeck Foundation, The Novo Nordic Foundation for Clinical Nursing Research and The Danish Cancer Society (Jarden 2016), one by the Zurich cancer league and the Federal Authorities of the Swiss Confederation, Federal Department of Defence, Civil Protection and Sport (Knols 2011), and one study by AMGEN (Streckmann 2014).
Excluded studies
In total, we excluded 31 studies (36 references). Four studies included participants younger than 18 years (Hartman 2009; Marchese 2004; Moyer‐Mileur 2009; Tanir 2013). We excluded four studies because exercise was offered in both arms (PETRA study; Schumacher 2015a; Shelton 2009; Vallerand 2018). In one trial, a multimodal intervention was offered, including a structured exercise programme, progressive relaxation, and psycho‐education (Jarden 2009). We excluded 15 studies because of the involvement of participants suffering from non‐haematological cancers, such as breast cancer, testicular cancer or gynaecological cancer did not report subgroup data (Broderick 2013; Forbes 2017; Grabenbauer 2016; Kampshoff 2015; Kanera 2017; Midtgaard 2013; Oechsle 2014; Peoples 2017; Stacey 2016; Thorsen 2005; Toohey 2016; Tran 2016; Valle 2013; van Waart 2015; Zimmer 2014). We excluded one study because it remained unclear which types of malignancies involved participants had been diagnosed with (Mayo 2014).
We excluded five studies because the applied exercise interventions did not correspond to our inclusion criteria (Cohen 2004; Hacker 2011; Hacker 2016; Prinsen 2013; Yeh 2016). Cohen 2004 explored the influence of a Tibetan yoga intervention on psychological adjustment and sleep quality. Hacker 2011 and Hacker 2016 explored the effect of strength training on physical activity, muscle strength, health status perception, and quality of life. Prinsen 2013 explored the influence of cognitive behavioural therapy on physical activity, physical fitness and fatigue. Yeh 2016 and colleagues evaluated qigong for patients with non‐Hodgkin lymphoma.
Risk of bias in included studies
Overall, the risks of bias were unclear. For detailed information see the 'Risk of bias' tables of included trials and Figure 2 and Figure 3.
2.
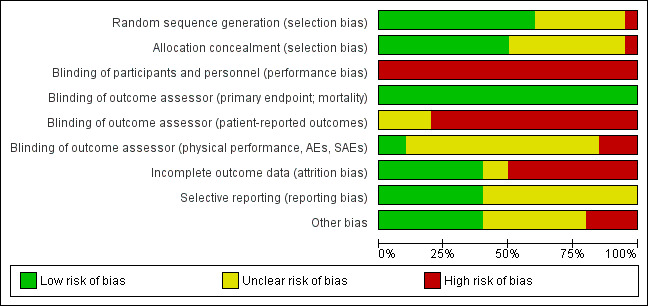
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
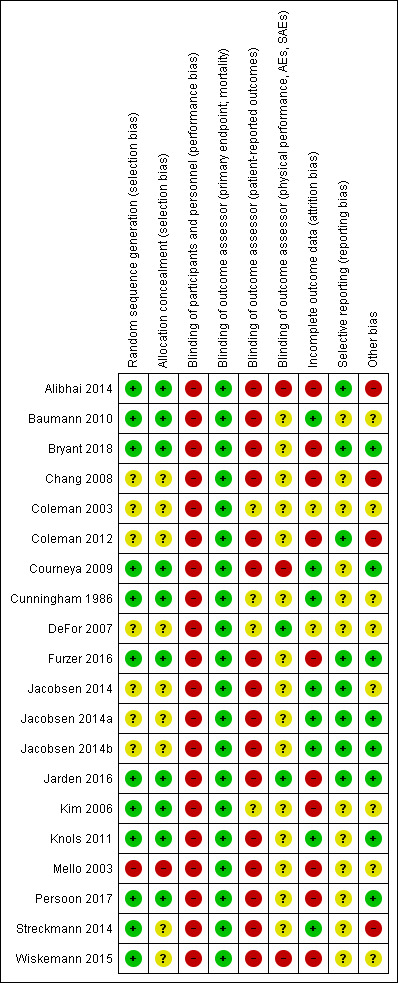
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For one study, Mello 2003, we judged both, sequence generation and allocation concealment as high, as it remains unclear when and how patients have been randomised.
For 12 studies, we rated the random sequence generation as adequate (Alibhai 2014; Baumann 2010; Bryant 2018; Courneya 2009; Cunningham 1986; Furzer 2016; Jarden 2016; Kim 2006; Knols 2011; Persoon 2017; Streckmann 2014; Wiskemann 2015), thus we judged the potential risk of bias as 'low'. Five studies reported randomisation procedure, but did not give details, therefore we judged risk of bias as unclear for five studies (Chang 2008; Coleman 2003; Coleman 2012; DeFor 2007; Jacobsen 2014).
Allocation concealment was adequate for 10 trials (Alibhai 2014; Baumann 2010; Bryant 2018; Courneya 2009; Cunningham 1986; Furzer 2016; Jarden 2016; Kim 2006; Knols 2011; Persoon 2017), and unclear for seven studies (Chang 2008; Coleman 2003; Coleman 2012; DeFor 2007; Jacobsen 2014; Streckmann 2014; Wiskemann 2015).
Blinding
Performance bias
When exploring the influence of physical exercise intervention on people suffering from haemological malignancies, it is not feasible to blind participants or physicians. Consequently, in all 18 studies we judged the potential risk of bias for blinding of participants and physicians as 'high'.
Detection bias
As the outcome of mortality is not influenced by the outcome assessor, we judged risk of bias for outcome assessor blinding for as low.
Thirteen studies measured participant‐reported outcomes for quality of life or fatigue. As it is not feasible to blind the intervention exercise, the participants were aware of the assigned intervention when they filled out the questionnaires. We therefore judged the risk of bias for outcome assessor blinding for those trials that assessed participant‐reported outcomes as high (Alibhai 2014; Baumann 2010; Bryant 2018; Chang 2008; Coleman 2012; Courneya 2009; Furzer 2016; Jacobsen 2014; Jarden 2016; Knols 2011; Mello 2003: Persoon 2017; Streckmann 2014; Wiskemann 2015).
Eleven studies did not report whether the outcome assessors for physical performance or adverse events were blinded, so we judged their risk of bias as 'unclear' (Baumann 2010; Bryant 2018; Chang 2008; Coleman 2003; Coleman 2012; Furzer 2016; Jacobsen 2014; Knols 2011; Mello 2003; Persoon 2017; Streckmann 2014). Two studies did not report outcomes or interest but laboratory values only; risk of bias for these trials is unclear (Cunningham 1986; Kim 2006).
In three studies we judged the assessor bias at 'high' risk (Alibhai 2014; Courneya 2009; Wiskemann 2015), as these trials explicitly stated that outcome assessors were not blinded. In Courneya 2009, the outcome assessors were not always blinded to group assignment, but they were trained in standardising testing procedures. In Wiskemann 2015, the assessors were not blinded to randomisation.
In two studies, the assessor was unaware of the randomised assignment (DeFor 2007; Jarden 2016), and we therefore judged the risk of bias as 'low'.
Incomplete outcome data
For two studies, we judged the risk of attrition bias as 'unclear' as they did not report whether all randomised participants were analysed (Coleman 2003; DeFor 2007). In 10 studies not all the randomised participants were considered in the outcome analysis. Consequently, we judged the risk of attrition bias as 'high' (Alibhai 2014; Bryant 2018; Chang 2008; Coleman 2012; Furzer 2016; Jarden 2016; Kim 2006; Mello 2003; Persoon 2017; Wiskemann 2015). In six studies we could not detect any risk of attrition bias, with all randomised participants analysed in the arm to which they were assigned, so we judged the risk of attrition bias as 'low' for these studies (Baumann 2010; Courneya 2009; Cunningham 1986; Jacobsen 2014; Knols 2011; Streckmann 2014).
Selective reporting
For 12 of the 18 included studies, there is no protocol available at www.controlled‐trials.com/mrct/, so we were not able to judge the potential risk of reporting bias (Baumann 2010; Chang 2008; Coleman 2003; Courneya 2009; Cunningham 1986; DeFor 2007; Kim 2006; Knols 2011; Mello 2003; Persoon 2017; Streckmann 2014; Wiskemann 2015), and we therefore rated the potential risk of reporting bias as 'unclear'. For six studies, a protocol was registered (Alibhai 2014; Bryant 2018; Coleman 2012; Furzer 2016; Jacobsen 2014; Jarden 2016). All planned outcomes are reported. According to this, we judged the potential for reporting bias as 'low'.
Other potential sources of bias
In one study the distribution of participants between exercise and control group is unbalanced due to five out of 17 allocated to the control immediately crossing over to the exercise group (Alibhai 2014), we judge risk of other bias as high.
In Chang 2008, the distribution of gender is unbalanced in the exercise and in the control group. In consequence of this distribution, we judged the potential risk of bias as 'high'; however, the unequal distribution could be due to the small number of participants randomised.
In Coleman 2012, 50% of participants received thalidomide. It is was neither reported whether the thalidomide administration was equally distributed between both arms, nor were subgroup analyses provided for participants receiving or not receiving thalidomide. We therefore judged the potential risk of bias as 'high'. Moreover, in an abstract publication of the trial, Coleman 2012 reported that all participants (in both the exercise and control group) received erythropoietin. In the study description published as full text, the authors reported that erythropoietin was administered to only 102 of 135 study participants, meaning that some participants did not receive erythropoietin therapy. We therefore judged the potential risk of bias as 'high'.
Streckmann 2014: due to a slow recruitment rate, the trial was stopped early. The authors planned to randomise 240 people, but randomised only 61 participants. They argued that physiological parameters are more important than the primary outcome (QoL). We therefore judged the potential risk of bias as 'high'. Moreover, there is a serious baseline imbalance for the outcome of QoL, favouring the control group. We therefore excluded this trial for the outcome QoL in a sensitivity analysis.
Six studies received financial support by various organisations. After evaluating and reviewing we do not expect any bias due to this (Courneya 2009; Furzer 2016; Jacobsen 2014; Jarden 2016; Knols 2011; Persoon 2017).
One study was finalised before the last six participants were enrolled (Coleman 2003). This premature termination was due to time and funding constraints. There is no indication that the premature stopping could have been due to other reasons. On the basis of this abandonment, we judged the potential risk of bias as 'unclear'.
For the remaining studies we do not have any hint for or against other bias and judged potential risk of bias as unclear (Baumann 2010; Cunningham 1986; DeFor 2007; Kim 2006; Mello 2003; Wiskemann 2015).
Effects of interventions
See: Table 1
We did not include two trials in the meta‐analyses which evaluated outcomes which are not patient‐relevant and did not report the pre‐specified outcomes of this review (Cunningham 1986; Kim 2006). These trials reported laboratory values only. One trial involving 40 patients reported change‐scores instead of endpoint scores (Alibhai 2014), another trial with 17 patients did not report standard difference or standard error (Bryant 2018), therefore both trials could not be included in meta‐analyses.
Overall survival (OS)/mortality
The only study investigating our primary outcome OS was Wiskemann 2015. The study authors did not find evidence for participants exercising compared to participants receiving usual care only (risk ratio (RR) = 0.67, P = 0.112). As this was the only trial investigating survival there were no data to pool, therefore a meta‐analysis was not conducted.
Instead, six trials (N = 1172) which reported the number of deceased participants (Baumann 2010; Courneya 2009; DeFor 2007; Jacobsen 2014; Jarden 2016; Wiskemann 2015) were meta‐analysed. We found no statistically significant difference between exercise and control arms (RR 1.10; 95% confidence interval (CI) 0.79 to 1.52; P = 0.59; Analysis 1.2). Heterogeneity is small (I² = 29%) (see Figure 4). The certainty of the evidence is low, because of the small number of patients with an event and a confidence interval that includes both: clinically relevant benefits and harms. We downgraded two points for imprecision.
1.2. Analysis.
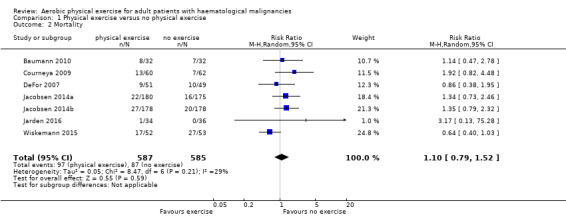
Comparison 1 Physical exercise versus no physical exercise, Outcome 2 Mortality.
4.
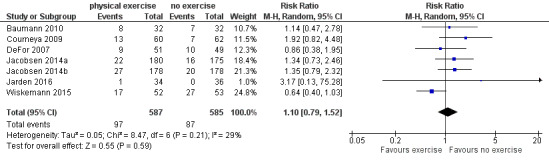
Forest plot of comparison: 1 Physical exercise versus no physical exercise, outcome: 1.2 Mortality.
The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.1) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.3) did not provide any evidence for differences between those groups (test for subgroup differences not significant).
1.1. Analysis.
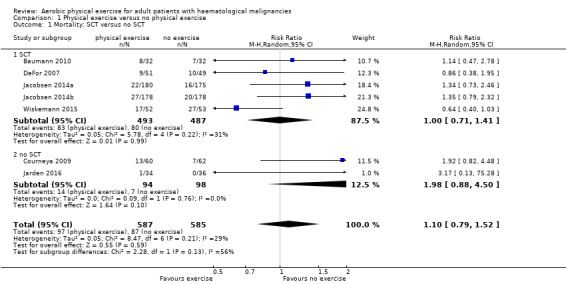
Comparison 1 Physical exercise versus no physical exercise, Outcome 1 Mortality: SCT versus no SCT.
1.3. Analysis.
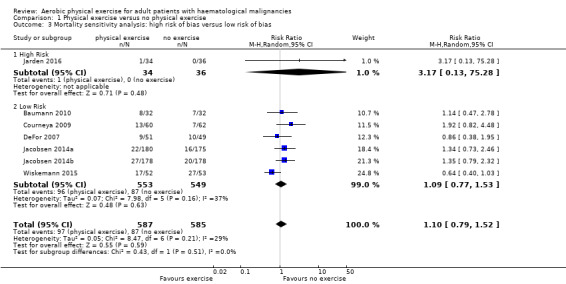
Comparison 1 Physical exercise versus no physical exercise, Outcome 3 Mortality sensitivity analysis: high risk of bias versus low risk of bias.
Quality of life (QoL)
Eight studies measured the outcome quality of life in a comparable way. One of them (Jacobsen 2014) evaluated QoL using the SF‐36 survey, its Mental Component Summary score (MCS) was incorporated in our review as an indicator for general QoL. We found no evidence for a difference (standardised mean difference (SMD) 0.11, 95% CI ‐0.03 to 0.24; 1259 participants; Analysis 1.4) with small heterogeneity (I² = 26%) (see Figure 5). We found no indications of subgroup differences between participants receiving stem cell transplantation (SCT) or chemotherapy only (Analysis 1.5). Moreover, the sensitivity analysis for high versus low risk of overall bias (Analysis 1.6) did not provide any evidence for differences between these groups. The certainty of the evidence is low, due to a confidence interval that includes both, improvement and worsening of QoL (one point downgraded for imprecision) and unblinded outcome assessors (participants) for the participant‐reported outcome (QoL questionnaires) (one point downgraded for risk of bias).
1.4. Analysis.
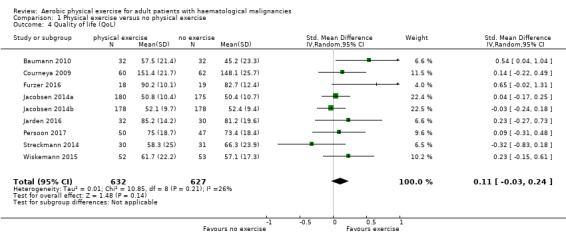
Comparison 1 Physical exercise versus no physical exercise, Outcome 4 Quality of life (QoL).
5.
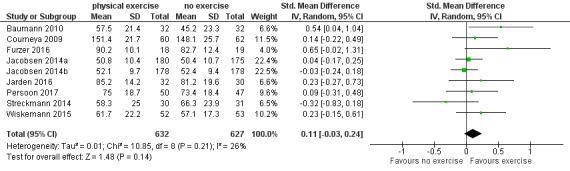
Forest plot of comparison: 1 Physical exercise versus no physical exercise, outcome: 1.4 Quality of life (QoL).
1.5. Analysis.
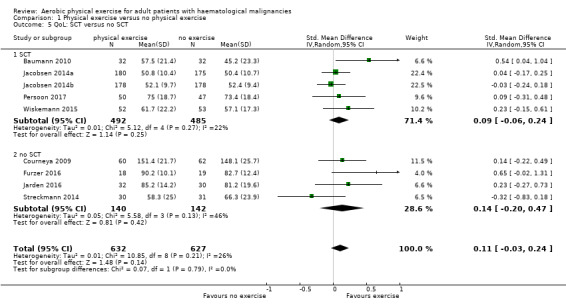
Comparison 1 Physical exercise versus no physical exercise, Outcome 5 QoL: SCT versus no SCT.
1.6. Analysis.
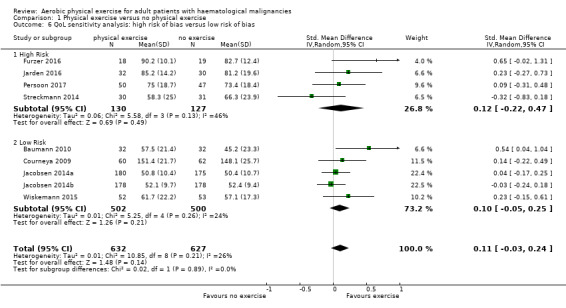
Comparison 1 Physical exercise versus no physical exercise, Outcome 6 QoL sensitivity analysis: high risk of bias versus low risk of bias.
Subscale physical functioning
Eight trials with 1329 participants evaluated physical functioning. There is no significant advantage for participants in the exercise arm (SMD 0.15, 95% CI ‐0.01 to 0.32; Analysis 1.7). Heterogenity is moderate (I² = 48%). The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.8) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.9) did not provide any evidence for differences between those groups. Again, the certainty of the evidence is judged to be low, because of the confidence interval that includes both, improvement and worsening of physical functioning (one point downgraded for imprecision) and unblinded outcome assessors (participants) for the participant‐reported outcome (one point downgraded for risk of bias).
1.7. Analysis.
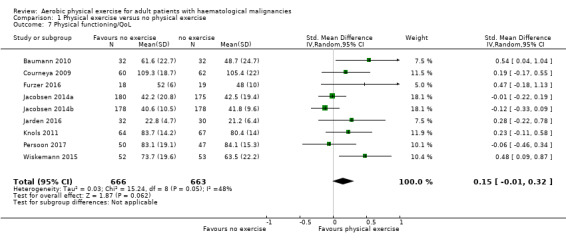
Comparison 1 Physical exercise versus no physical exercise, Outcome 7 Physical functioning/QoL.
1.8. Analysis.
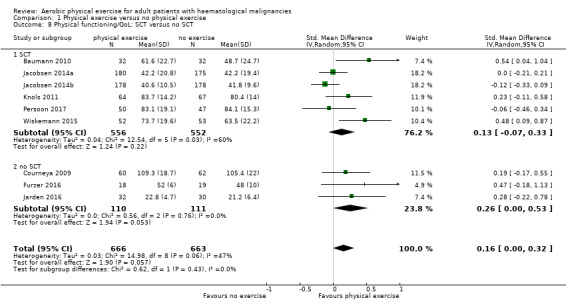
Comparison 1 Physical exercise versus no physical exercise, Outcome 8 Physical functioning/QoL: SCT versus no SCT.
1.9. Analysis.
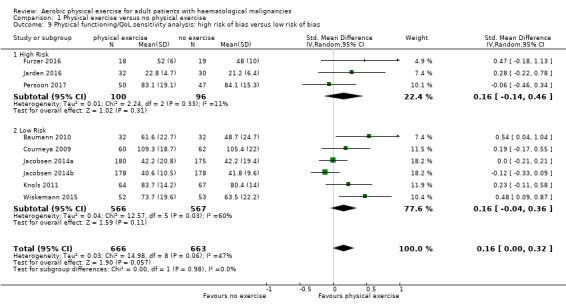
Comparison 1 Physical exercise versus no physical exercise, Outcome 9 Physical functioning/QoL sensitivity analysis: high risk of bias versus low risk of bias.
Subscale depression
The pooled result of six trials (N = 445) for depression show a small effect for patients exercising (SMD 0.19, 95% CI 0.0 to 0.38; I2 = 0%; Analysis 1.10), without any hints for heterogeneity. The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.11) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.12) did not provide any evidence for differences between those groups. As for QoL and the subscale physical functioning, the certainty of the evidence for the outcome depression is low: we downgraded one point for imprecision because of the small number of participants (445) and potential risk of bias (one point downgraded) because of the unblinded outcome assessor (participants for the participant‐reported outcomes).
1.10. Analysis.
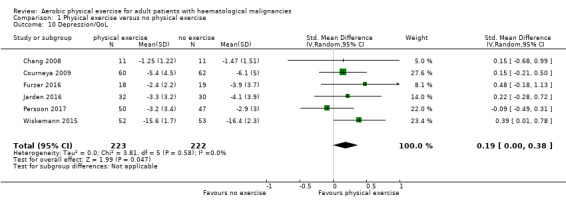
Comparison 1 Physical exercise versus no physical exercise, Outcome 10 Depression/QoL.
1.11. Analysis.
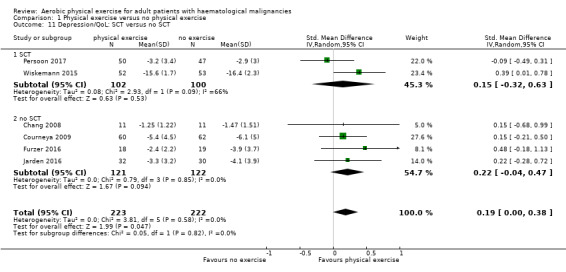
Comparison 1 Physical exercise versus no physical exercise, Outcome 11 Depression/QoL: SCT versus no SCT.
1.12. Analysis.
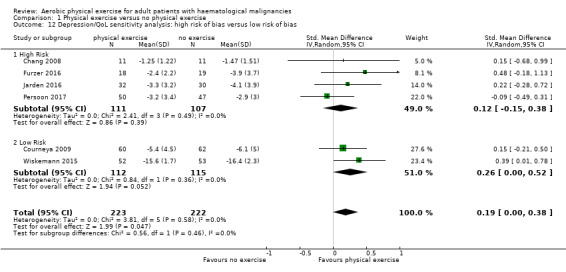
Comparison 1 Physical exercise versus no physical exercise, Outcome 12 Depression/QoL sensitivity analysis: high risk of bias versus low risk of bias.
Subscale anxiety
Anxiety was evaluated by six trials with 445 participants. There is substantial heterogeneity for this analysis (I² = 63%), but no evidence for differences between the exercise arm and the standard treatment arm (SMD 0.03, 95% CI ‐0.30 to 0.36; Analysis 1.13). The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.14) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.16) did not provide any evidence for differences between those groups. As for the aforementioned outcomes, we downgraded certainty of the evidence for a confidence interval including both, potential benefit and harm (one point downgraded for imprecision) and potential risk of bias (one point downgraded for risk of bias) because of the unblinded outcome assessor (participants for the participant‐reported outcomes) and in addition, one point downgraded for inconsistency (high statistical heterogeneity). This results in a very low certainty of the evidence.
1.13. Analysis.
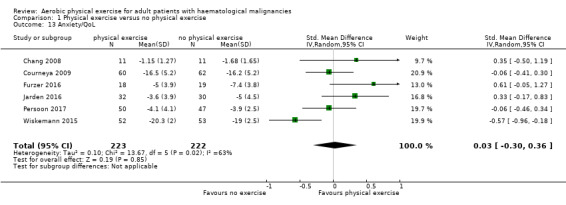
Comparison 1 Physical exercise versus no physical exercise, Outcome 13 Anxiety/QoL.
1.14. Analysis.
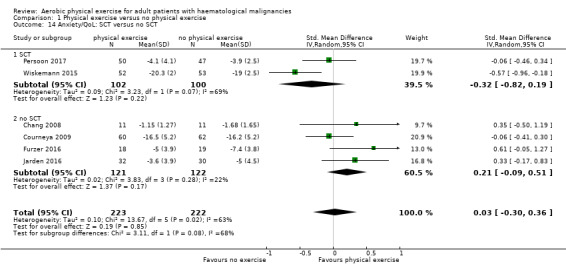
Comparison 1 Physical exercise versus no physical exercise, Outcome 14 Anxiety/QoL: SCT versus no SCT.
1.16. Analysis.
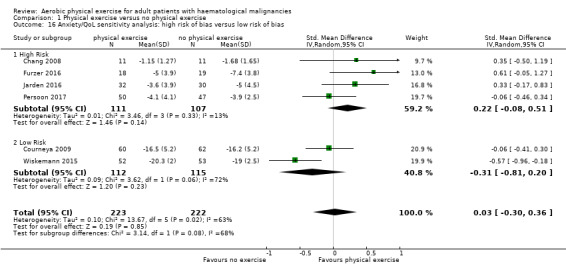
Comparison 1 Physical exercise versus no physical exercise, Outcome 16 Anxiety/QoL sensitivity analysis: high risk of bias versus low risk of bias.
One trial (Alibhai 2014) delivered data for QoL, physical functioning and depression, but had to be excluded from the meta‐analysis due to the fact that only chance values were reported instead of endpoint values. There were no relevant differences between the results of this and the aforementioned meta‐analysed outcomes.
Fatigue
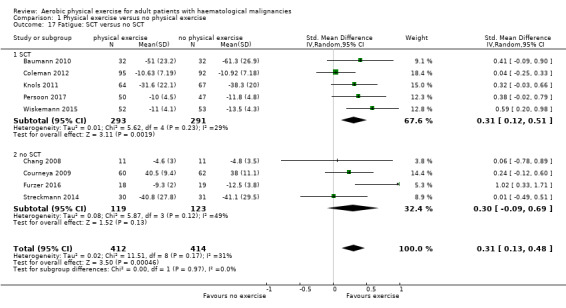
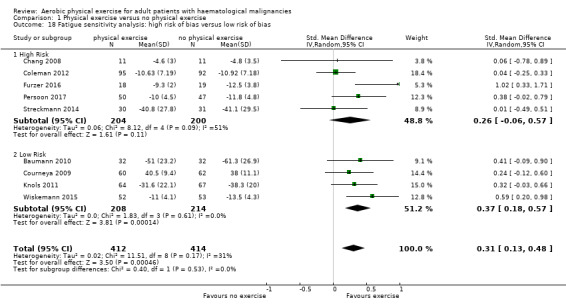
Nine studies (N = 826) assessed fatigue and found a statistically significant advantage for those participants exercising (SMD 0.31, 95% CI 0.13 to 0.48; P = 0.0005; Analysis 1.15), with moderate heterogeneity (I² = 31%) (see Figure 6). The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.17) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.18) did not provide any evidence for differences between those groups. The certainty of the evidence is moderate, as we downgraded one point for risk of bias due to the unblinded outcome assessment.
1.15. Analysis.
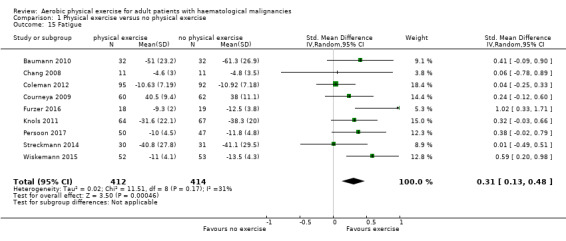
Comparison 1 Physical exercise versus no physical exercise, Outcome 15 Fatigue.
6.
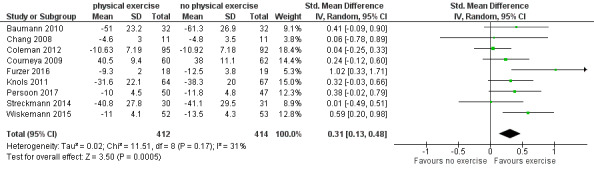
Forest plot of comparison: 1 Physical exercise versus no physical exercise, outcome: 1.15 Fatigue.
1.17. Analysis.
Comparison 1 Physical exercise versus no physical exercise, Outcome 17 Fatigue: SCT versus no SCT.
1.18. Analysis.
Comparison 1 Physical exercise versus no physical exercise, Outcome 18 Fatigue sensitivity analysis: high risk of bias versus low risk of bias.
Alibhai 2014 had to be excluded from the meta‐analysis because of reporting of chance scores instead of endpoint values. There was no hint for different results of this trial compared to the pooled analysis.
Physical performance (e.g. aerobic capacity, cardiovascular fitness)
Thirteen studies evaluated physical performance (Alibhai 2014; Baumann 2010; Chang 2008; Coleman 2003; Coleman 2012; Courneya 2009; Furzer 2016; Jarden 2016; Knols 2011; Mello 2003; Persoon 2017; Streckmann 2014; Wiskemann 2015). However, all studies used different concepts, measuring instruments and outcome definitions, and we therefore have not pooled the data.
Alibhai 2014 assessed functional endurance using the six‐minute walk test. Endurance improved in both exercise and control group over the course of the intervention. There were no significant differences reported between the groups for the six‐minute walk test (mean difference (MD) 34.6; P = 0.99), as well as for grip strength (MD 0.16 kg; P = 0.56) and sit and reach test (MD 1.3 cm; P = 0.29).
Baumann 2010 reported statistically significant differences in the inter‐group comparison for repeated measurements for endurance (P = 0.004), endurance time (P = 0.004) and relative endurance (P = 0.031) between the exercise and the control group, favouring the exercise arm. There were no statistically significant intra‐group changes in the exercise arm between admission and discharge, but there were significant changes between these data in the control group. Endurance between these two time points decreased from 86.5 Watt (W) to 60 W (P = 0.001) and endurance time reduced from 5.4 minutes to 3.3 minutes (P < 0.001) in the control group.
Chang 2008 assessed physical performance by a 12‐minute walking test. In this test, participants were encouraged to walk at a speed to reach their specific heart rate, predefined by the study protocol. At baseline there were no statistically significant differences between the two study arms.The authors reported a statistically significant decrease in 12‐minute walking distance for the control group (estimate ‐119.1 metre (m); 95% CI ‐207.1 to ‐31.0 m; P = 0.008). On the other hand, the 12‐minute walking distance for participants in the exercise programme increased over time.
Coleman 2003 investigated the outcomes strength changes and treadmill minutes. Strength changes were tested by four strength tests using Keiser pneumatic equipment. Treadmill minutes, in detail the measurement of aerobic exercise capacity, were measured by a modified Balke protocol. Comparison between exercise and control groups did not achieve statistical significance, either for strength change or for treadmill minutes. The authors provided no further data.
In Coleman 2012 all participants performed a six‐minutes walking test before and after intervention. The mean values for the walking test showed a tendency for improved performance in the short‐term exercise group, but not in the short‐term control group. Aerobic capacity, measured by the six‐minute walking test, decreased over time in both arms, but less so in the exercise group. No further precise data were published for this outcome.
Courneya 2009 measured VO₂ peak power output, VO₂ peak (mL/kg/min) and ventilatory threshold (L/min). In all three measures, the exercise group was statistically significantly superior to the control group.
Furzer 2016 measured cardiovascular fitness and muscle strength. Significant improvements for both measures were found from baseline to 12 weeks (P ≤ 0.001) comparing exercise group to usual care. Additionally, after usual care participants had started exercising from week 12 to week 24, the usual care group showed significantly improved cardiovascular fitness (P = 0.018) and MS (P ≤ 0.001), too.
Jarden 2016 assessed physical capacity and functional performance by measuring six‐minute walking distance, VO2 max, sit‐to‐stand test and biceps curls. Improvements in all measures found in the intervention group differed significantly from the usual care group in favour of the intervention group (P ≤ 0.001)
Knols 2011 reported statistically significantly improved six‐minute walking test results (P = 0.011), increased walking speed (P = 0.000) and improved knee extension for the exercise arm compared to the standard care arm from baseline to follow‐up examination three months after programme completion. The authors found no difference for grip strength between the two arms (P = 0.624).
Mello 2003 reported a significant benefit for the exercise arm measuring maximal isometric voluntary strength from four muscle groups of the upper limbs and five muscle groups of the lower limbs.
Persoon 2017 reported improvements in fitness and reduced levels of fatigue for both the exercise and control group without significant differences between the groups.
Streckmann 2014 reported that the aerobic performance level increased statistically significantly in the exercise group over time compared to the control group with deteriorating activity levels (P = 0.03). This is true for balance control, with improving balance control in the exercise arm and reducing control in the standard arm (dynamic control P = 0.007; static control P = 0.02).
In the trial by Wiskemann 2015, participants in the exercise group achieved statistically significantly more metres in the six‐minute walking test six to eight weeks after discharge; no more detailed data was published.
Anthropometric measurements
Three studies (N = 964) provided data for body weight (Courneya 2009; Jacobsen 2014; Knols 2011). There was no significant difference between exercise and control groups (MD 0.44 kg; 95% CI ‐1,94 to 2,82; P = 0.72; Analysis 1.19), without evidence for heterogeneity (I2 = 0%). The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.20) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.21) did not provide any evidence for differences between those groups.
1.19. Analysis.
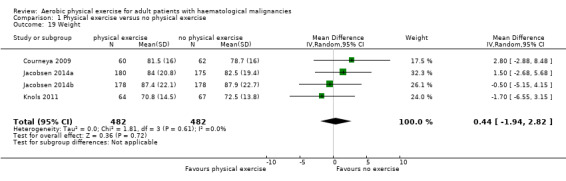
Comparison 1 Physical exercise versus no physical exercise, Outcome 19 Weight.
1.20. Analysis.
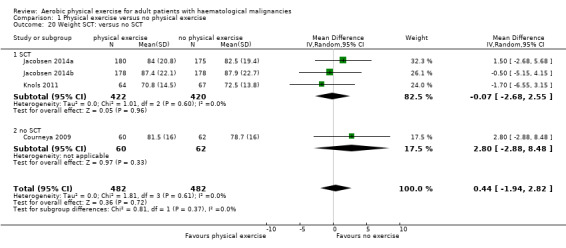
Comparison 1 Physical exercise versus no physical exercise, Outcome 20 Weight SCT: versus no SCT.
1.21. Analysis.
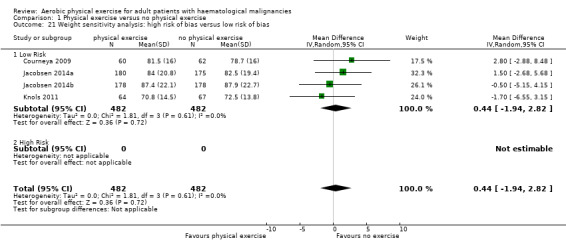
Comparison 1 Physical exercise versus no physical exercise, Outcome 21 Weight sensitivity analysis: high risk of bias versus low risk of bias.
Alibhai 2014 had to be excluded from the meta‐analysis due to reporting change values instead of endpoint values for the outcome body weight. No significant differences between the groups were reported.
Three studies (Courneya 2009; Furzer 2016; Knols 2011, N = 290) measured lean body mass. There was no statistically significant difference between the groups for this outcome (MD 1.26 kg; 95% CI ‐1,22 to 3,74; P = 0.69; I2 = 0%; Analysis 1.22). The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.23) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.24) did not provide any evidence for differences between those groups.
1.22. Analysis.
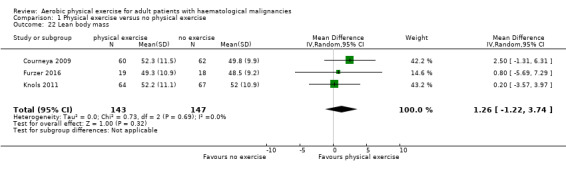
Comparison 1 Physical exercise versus no physical exercise, Outcome 22 Lean body mass.
1.23. Analysis.
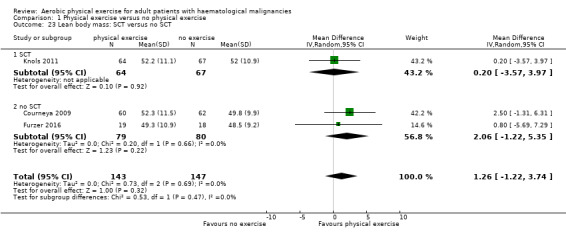
Comparison 1 Physical exercise versus no physical exercise, Outcome 23 Lean body mass: SCT versus no SCT.
1.24. Analysis.
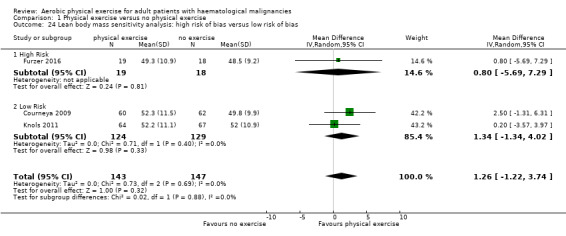
Comparison 1 Physical exercise versus no physical exercise, Outcome 24 Lean body mass sensitivity analysis: high risk of bias versus low risk of bias.
Adverse events
Six studies (435 participants) reported serious adverse events (SAEs) (Alibhai 2014; Chang 2008; Coleman 2012; Courneya 2009; Furzer 2016; Persoon 2017) and were pooled in one analysis. There is uncertain evidence whether exercise is related to more serious adverse events (RR 1.39; 95% CI 0.94 to 2.06; P = 0.10; I2 = 0%); Analysis 1.25), without heterogeneity. The certainty of the evidence is very low as we downgraded one point for inconsistency because of baseline imbalances, especially usage of erythropoietin and thalidomide remains unknown in both study arms of one included study. In addition, we downgraded two points for imprecision, as only a very small number of SAEs were observed, leading to very wide confidence intervals.
1.25. Analysis.
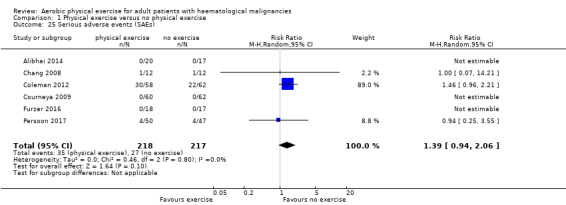
Comparison 1 Physical exercise versus no physical exercise, Outcome 25 Serious adverse events (SAEs).
The subgroup analysis for stem cell transplantation versus chemotherapy only (Analysis 1.26) and the sensitivity analysis for high versus low risk of overall bias (Analysis 1.27) did not provide any evidence for differences between those groups.
1.26. Analysis.
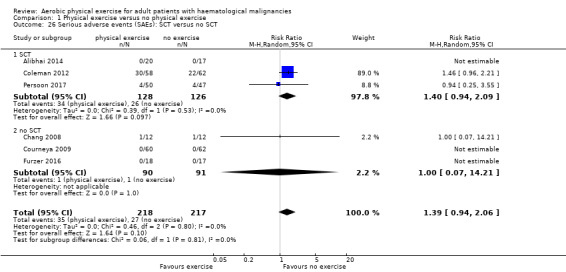
Comparison 1 Physical exercise versus no physical exercise, Outcome 26 Serious adverse events (SAEs): SCT versus no SCT.
1.27. Analysis.
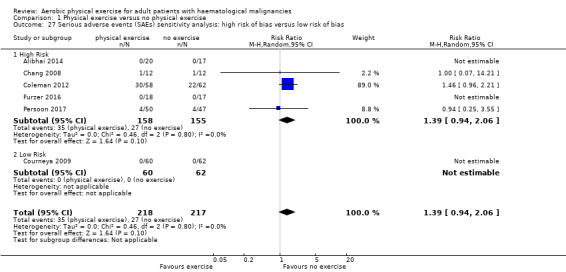
Comparison 1 Physical exercise versus no physical exercise, Outcome 27 Serious adverse events (SAEs) sensitivity analysis: high risk of bias versus low risk of bias.
Chang 2008 reported that one participant in each arm (8%) dropped out of the study due to a SAE. The participant in the exercise group experienced severe bleeding, and the participant in the control group a severe infection.
In the trial by Coleman 2012, the most common SAEs were fever, hyponatraemia, pneumonia, hyperglycaemia, deep vein thrombosis, infection and neutropenia. In the short‐term groups, 15 out of 23 participants (65%) experienced one or more SAEs, while the corresponding rate in the control group was eight out of 28 participants (28%). Regarding the long‐term study group, 15 out of 35 participants (43%) in the exercise group experienced at least one SAE and 14 out of 34 (41%) in the control group. As the authors reported variations in cancer treatment between both study arms (whether erythropoietin or thalidomide, or both were administered or not), the reasons for the differences between study arms remain unclear.
Courneya 2009 reported that no SAE occurred in either arm, but three (5%) adverse events (back, hip and knee pain) related to the exercise programme. One participant with knee pain withdrew from the exercise programme, and the other two participants proceeded with a modified exercise programme. In the control group (N = 62) no adverse events were reported (RR 7.23; 95% CI 0.38 to 137.05; P = 0.19).
Alibhai 2014 and Furzer 2016 each reported that no SAE occurred in either arm.
Persoon 2017 reported four SAEs in each arm.
Streckmann 2014 (61 participants) reported that the number of cancer‐related side effects was statistically significantly reduced in the exercise group by 2.1 compared to baseline (P = 0.043). In the control group, the side effects were reduced by only 0.4 (P = 0.514).
Discussion
Summary of main results
For this review we evaluated the safety, feasibility and efficacy of aerobic physical exercise for adults with haematological malignancies. We included 18 randomised controlled trials (RCTs) of which 14 could be included in the analyses with the following results.
Only one small trial (105 participants) reported overall survival (OS), without any evidence for a difference between both arms. Additionally, instead of measuring the OS, six trials evaluated mortality (1172 patients). For this outcome there is no evidence for a difference between the exercise and control arms.
The quality of life (QoL) was measured in eight studies, but it remains uncertain, whether physical exercise improves QoL (1259 participants). There is no evidence for a difference between participants exercising and those participants receiving standard care only for the subscales physical functioning (1329 participants) and anxiety (445 participants).
Nine trials evaluated fatigue (826 participants). There is moderate‐quality evidence that exercise has a positive effect on fatigue. Additionally, there is low‐certainty evidence, that exercise might improve depression (subscale from QoL instruments).
Thirteen trials evaluated physical performance. Since they used different concepts, measuring instruments and outcomes, the data could not be pooled. Eight trials reported significant benefits for the exercise arm.
Four studies reported anthropometric measurements, without delivering evidence for differences in body weight (964 participants) and lean body mass (290 participants).
Serious adverse events (SAEs) were evaluated in six trials (435 participants). Due to very low‐certainty evidence, it remains unclear, whether there is a disadvantage for participants in the exercise arm.
Two trials could not be analysed as they did not report any of our pre‐specified patient‐relevant outcomes but laboratory values only. One trial (40 participants) had to be excluded from the meta‐analysis for the endpoints QoL with subscales, fatigue and body weight due to reporting change scores instead of endpoint scores, another trial (17 patients) did not report standard differences or errors.
Overall completeness and applicability of evidence
Interpreting the results of this meta‐analysis, the following aspects should be considered.
The 18 included studies, consisting of 1892 participants, may not be adequately powered to detect small differences, especially concerning outcomes with few events.
Two of these trials reported laboratory values only, two further trials of this set of included trials could not be meta‐analysed due to missing standard differences or errors or reporting of change scores only.
Data appear deficient, in particular for the outcome OS. We noticed a lack of data, which requires further research.
The differences in exercise programme (type, duration and follow‐up), supportive care and medical treatment between studies could have influenced the outcomes.
Four RCTs are currently ongoing, but as the authors of these trials do not report how many participants they will recruit and when the trial will be terminated, the potential influence of these trials for the current analyses remains uncertain. One of these studies started in 2003 and according to the study registry, clinicaltrials.gov it still seems to be active and recruiting patients.
Although there are statistical differences in the fatigue and depression scores, especially the clinical significance for the outcome depression remains uncertain, as the confidence interval of the standardised mean difference (SMD) is very close to the zero effect. As the outcome fatigue was measured with different instruments in various RCTs, we had to meta‐analyse the SMD instead of the mean difference. (MD) The clinical meaning of an advantage in the SMD is always difficult to interpret, because no clinically relevant minimal important difference is known for the standardised value. However, as the effect is quite large, we assume the effect is clinically meaningful.
Quality of the evidence
Overall, we judged the potential risk of bias of the 18 included trials as unclear. All the included trials were reported as randomised and as open‐label studies. In seven of the 18 included studies, the scientific quality of allocation concealment remained unclear. The open‐label design and unclear allocation concealment could lead to selection, performance or detection biases. In the included studies, blinding of participants as well as blinding of physicians in the context of physical exercise was impossible. Consequently, we judged the risk of performance bias as high for all studies. As the outcome mortality is not influenced by the outcome assessor, we judged risk of detection bias for this outcome as low. As it is not feasible to blind the intervention exercise, we judged the risk of detection bias for participant‐reported outcomes as high. For the other reported outcomes, most studies did not report whether outcome assessors were blinded, and we therefore judged risk of detection bias for these outcomes as unclear. For seven trials we judged the potential risk of attrition bias as high, because not all participants randomised were analysed.
We judged the certainty of the evidence body as low to moderate for most outcomes, because of an open‐label design, unblinded outcome assessment and a small number of events, leading to wide confidence intervals and imprecision of the results. For the outcome anxiety, we judged the certainty of the evidence body as very low, due to the aforementioned reasons and additionally high statistical heterogeneity, leading to relevant inconsistency. For SAEs, we also judged the certainty as very low, because of baseline imbalances. Especially the usage of erythropoietin and thalidomide was unknown for both arms of one trial and both agents are known to have a high potential for SAEs. This led to downgrading by one point for inconsistency. In addition, very wide confidence intervals led to downgrading (two points) for imprecision.
Potential biases in the review process
We tried to avoid bias by doing all relevant processes in duplicate. We are not aware of any obvious deficiencies in our process of conducting the review. With sensitive search strategies and handsearching of conference proceedings, we tried to avoid retrieval bias.
As the number of included studies is too low to perform tests for publication bias, we cannot be sure that we obtained all relevant studies. Moreover, as this type of intervention, aerobic physical exercise, is usually evaluated in investigator‐initiated trials, there is no manufacturer or company available to ask for missing data. Additionally, for an intervention like physical exercise there might be less need to be registered in advance in clinical trials registries, as this applies more cogently to RCTs of pharmaceutical interventions. We included four trials in the review, but without sufficient data to be used in the meta‐analyses. Two of these trials reported laboratory values only, the other two trials reported data without confidence intervals or standard deviations. Addtionally, the four RCTs which are currently ongoing did not report when they will be terminated and how many participants they plan to recruit. Thus, the potential influence of these trials for the current meta‐analyses remains unclear. One of these four studies if of special concern, as it started in 2003 and according to the study registry, clinicaltrials.gov, it still seems to be active and recruiting patients (last access 9 September 2018). All these points could have induced publication bias.
Agreements and disagreements with other studies or reviews
This is the update of a review published first in 2014, based on RCTs assessing the efficacy, safety and feasibility of aerobic exercise for adult patients suffering from haematological malignancies.
We identified two recent systematic reviews and meta‐analyses which are comparable to our analyses, as they also included patients with haematological malignancies and evaluated exercise interventions.
Zhou 2016 and colleagues investigated the effects of exercise in paediatric or adult patients with acute leukaemia. The authors included nine trials with 314 participants (eight RCTs and one quasi‐experimental design).Two of these trials have been conducted in adults and were also included in this review update (Alibhai 2014; Chang 2008), the remaining trials evaluated children. The review authors came to the conclusion that exercise compared to no exercise has positive effects on cardiorespiratory fitness, muscle strength and functional mobility. However, as they did not assess the certainty of the evidence, they did not take the very small number of participants, the mixed participant population (children and adults) and the clinical and statistical heterogeneity into account while interpreting their data. As the review authors came to their conclusions because of significant P values only, they might have overrated their results. The review did not find significant results for participants exercising for the outcomes fatigue, anxiety, depression or quality of life and concluded that these outcomes are not improved. Again, the interpretation of results based on P values only could be misleading, as a non‐significant P value in such a small population could be explained by the small sample size which is not large enough to detect significant differences.
Liang 2018 and colleagues assessed the effect of exercise for participants with haematological malignancies who were also haematopoietic stem cell transplantation recipients. The authors included RCTs only and included 10 trials with 607 participants. Eight of these trials are also included in the current version of this review update, two trials they included have been excluded in our review, as patients did not practice aerobic exercise, but strength training only (Hacker 2011; Hacker 2016). As discussed before, these review authors also interpreted their findings based on significant or non‐significant P values only, which might overestimate an imprecise, indirect or inconsistent effect. Liang 2018's results for the outcome fatigue are in line with our review, showing an advantage for those patients exercising compared to them not exercising. They also concluded that muscle strength and quality of life is improved for participants exercising. We did not find evidence for improved quality of life, due to the small sample size. However, as the effect estimate in our analysis is in favour for participants exercising, further studies and a larger number of included participants might change the currently non‐significant result to a significant result favouring the exercise arm. As mentioned before, Liang 2018's conclusion that exercise has no effect on participants' cardiorespiratory fitness, upper muscle strength, psychosocial fitness and adverse events could be misleading, as no effect is not the same as no evidence for a difference and could be explained by a small sample population only.
Authors' conclusions
Implications for practice.
Currently, there is moderate‐ to very low‐quality evidence available for the benefits and harms of aerobic physical exercise in adults with haematological malignancies. Aerobic physical exercise in addition to standard care probably improves fatigue and depression. There is currently no evidence for differences in terms of mortality, quality of life, physical functioning and anxiety between people exercising and the control group. Certainty of the evidence related to serious adverse events (SAEs) is very low, exercise might increase number of SAEs.
Implications for research.
To establish the most effective type and intensity of physical exercise, further trials with more participants and longer follow‐up periods are needed. To enhance comparability of study data, we require the development and implementation of core sets of measuring devices. As neither the number of planned patients nor potential completion date is published for the four ongoing trials, the potential impact of these trials for a future update remains unclear.
What's new
| Date | Event | Description |
|---|---|---|
| 3 December 2018 | New citation required but conclusions have not changed | New citation, nine new trials included to the nine trials of the first version, three new ongoing studies added and one study awaiting assessment |
| 3 December 2018 | New search has been performed | Updated, update search 01.07.2018 |
Notes
Some parts of the methods are taken from the Cochrane Haematological Malignancies template.
Acknowledgements
We would like to thank Lise Estcourt, Angela Aldin and Tina Jakob of Cochrane Haematological Malignancies Editorial Base, Bastian van Tresckow (Editor), Freerk Baumman (Peer‐reviewer) and Céline Fournier (Consumer Referee) for their comments and review improvements which sufficiently improved the current version of the review. He would like to thank Heather Meawwell for copy‐editing and improving readability of this review.
We like to thank the authors Andrea Will, Klaus‐Dieter Wolkewitz and Andreas Engert for their contribution of the original version. We would like to thank Sabine Kluge and Kathrin Bauer of the Cochrane Haematological Malignancies Group (CHMG) Editorial Base, Ben Djulbegovic and Sven Trelle (Editors), and Céline Fournier (Consumer Referee) for their comments and review improvements of the original version.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor Exercise Movement Techniques explode all trees |
| #2 | MeSH descriptor Exercise explode all trees |
| #3 | MeSH descriptor Exercise Therapy explode all trees |
| #4 | MeSH descriptor Physical Education and Training explode all trees |
| #5 | MeSH descriptor Physical Fitness explode all trees |
| #6 | MeSH descriptor Physical Exertion explode all trees |
| #7 | MeSH descriptor Physical Endurance explode all trees |
| #8 | physical therap* modalit* |
| #9 | physiotherap* |
| #10 | (human NEAR/1 physical NEAR/1 conditioning*) |
| #11 | (training NEAR/1 program*) |
| #12 | (muscular* NEAR/ fitness*) |
| #13 | exertion* |
| #14 | (physical NEAR/1 (activit* or behaviour* or behavior* or conditioning* or education* or exercis* or habit* or intervention* or program* or recreation* or stud* or train* or effort* or exertion* or fitness*)) |
| #15 | (physical NEAR/1 (activit* or behaviour* or behavior* or conditioning* or education* or exercis* or habit* or intervention* or program* or recreation* or stud* or train* or effort* or exertion* or fitness*)) |
| #16 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 |
| #17 | MeSH descriptor Gymnastics explode all trees |
| #18 | gymnastic* |
| #19 | (calisthenic* or callisthenic*) |
| #20 | pilates* |
| #21 | (resistanc* NEAR/2 (training* or exercise*)) |
| #22 | (muscular* NEAR/ fitness*) |
| #23 | (physical* NEAR/ (activit* or fitness* or exercise*)) |
| #24 | (physical* NEAR/ (condition* or effort4 or train*)) |
| #25 | MeSH descriptor Tai Ji explode all trees |
| #26 | MeSH descriptor Yoga explode all trees |
| #27 | (tai chi or tai ji or ji quan tai) |
| #28 | yoga* |
| #29 | (aerobic* NEAR/ train*) |
| #30 | stretching* |
| #31 | (#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) |
| #32 | MeSH descriptor Sports explode all trees |
| #33 | MeSH descriptor Running explode all trees |
| #34 | MeSH descriptor Walking explode all trees |
| #35 | MeSH descriptor Walking explode all trees |
| #36 | MeSH descriptor Swimming explode all trees |
| #37 | MeSH descriptor Bicycling explode all trees |
| #38 | MeSH descriptor Dancing explode all trees |
| #39 | MeSH descriptor Mountaineering explode all trees |
| #40 | sport* |
| #41 | athletic* |
| #42 | running* |
| #43 | running* |
| #44 | ambulation* |
| #45 | jogging* |
| #46 | swimming* |
| #47 | bicycling* |
| #48 | cycling* |
| #49 | mountaineer* |
| #50 | danc* |
| #51 | (ramble* or rambling*) |
| #52 | rowing* |
| #53 | #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 |
| #54 | (#16 OR #31 OR #53) |
| #55 | MeSH descriptor Hematologic Diseases explode all trees |
| #56 | MeSH descriptor Hematologic Neoplasms explode all trees |
| #57 | (hematolog* NEAR/1 malignan*) |
| #58 | (hematolog* NEAR/1 neoplas*) |
| #59 | (haematolog* NEAR/1 malignan*) |
| #60 | (haematolog* NEAR/1 neoplas*) |
| #61 | MeSH descriptor Bone Marrow Diseases explode all trees |
| #62 | MeSH descriptor Lymphoma explode all trees |
| #63 | MeSH descriptor Leukemia explode all trees |
| #64 | MeSH descriptor Leukemia explode all trees |
| #65 | hodgkin* |
| #66 | lymphogranulomato* |
| #67 | lymphom* |
| #68 | histiocy* |
| #69 | granulom* |
| #70 | non‐hodgkin* |
| #71 | nonhodgkin* |
| #72 | reticulosis |
| #73 | reticulosarcom* |
| #74 | (burkitt* NEAR/ (lymph* or tumor* or tumour*)) |
| #75 | lymphosarcom* |
| #76 | brill‐symmer* |
| #77 | plasm**ytom* |
| #78 | myeloma* |
| #79 | sezary |
| #80 | (leukem* or leukaem*) |
| #81 | myelodysplas* |
| #82 | (aplast* NEAR/ (anem* or anaem*)) |
| #83 | (#55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82) |
| #84 | (#54 AND #83) |
Update search: Cochrane Central Register of Controlled Trials 30.07.2018
ID Search
#1 MeSH descriptor: [Exercise Movement Techniques] explode all trees
#2 MeSH descriptor: [Exercise] explode all trees
#3 MeSH descriptor: [Exercise Therapy] explode all trees
#4 MeSH descriptor: [Physical Education and Training] explode all trees
#5 MeSH descriptor: [Physical Fitness] explode all trees
#6 (muscular* near/1 fitness*)
#7 exertion*
#8 MeSH descriptor: [Gymnastics] explode all trees
#9 gymnastic*
#10 (calisthenic* or callisthenic*)
#11 pilates*
#12 (resistanc* near/2 (training* or exercise*))
#13 (muscular* near/1 fitness*)
#14 (physical* near/1 (activit* or fitness* or exercise*))
#15 (physical* near/2 (condition* or effort* or train*))
#16 ((aerobic* or isometric*) near/2 exercise*)
#17 MeSH descriptor: [Sports] explode all trees
#18 sport*
#19 MeSH descriptor: [Walking] explode all trees
#20 walking*
#21 MeSH descriptor: [Jogging] explode all trees
#22 jogging*
#23 MeSH descriptor: [Swimming] explode all trees
#24 swimming*
#25 MeSH descriptor: [Bicycling] explode all trees
#26 bicycling* or cycling*
#27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 MeSH descriptor: [Neoplasms by Histologic Type] explode all trees
#29 MeSH descriptor: [Neoplasms by Site] explode all trees
#30 neoplas*
#31 tumor* or tumour*
#32 Krebs or cancer*
#33 malignan*
#34 (carcino* or karzino*)
#35 karzinom*
#36 sarcom*
#37 leukaem* or leukem* or leucem*
#38 lymphom*
#39 melano*
#40 metastas*
#41 mesothelio* or mesotelio*
#42 carcinomatos*
#43 (gliom* or glioblastom*)
#44 osteo*sarcom*
#45 (blastom* or neuroblastom*)
#46 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45
#47 #27 and #46 in Trials
#48 #47 Publication Year from 2014 to 2017
Appendix 2. MEDLINE search strategy
| 1 | exp EXERCISE MOVEMENT TECHNIQUES/ |
| 2 | exp EXERCISE/ |
| 3 | EXERCISE THERAPY/ |
| 4 | exp "PHYSICAL EDUCATION AND TRAINING"/ |
| 5 | PHYSICAL FITNESS/ |
| 6 | PHYSICAL EXERTION/ |
| 7 | exp PHYSICAL ENDURANCE/ |
| 8 | physical therap$ modalit$.tw,kf,ot. |
| 9 | physiotherap$.tw,kf,ot. |
| 10 | (human adj1 physical adj1 conditioning$).tw,kf,ot. |
| 11 | (training adj1 program$).tw,kf,ot. |
| 12 | (muscular$ adj fitness$).tw,kf,ot. |
| 13 | exertion$.tw,kf,ot. |
| 14 | (physical adj1 (activit$ or behaviour$ or behavior$ or conditioning$ or education$ or exercis$ or habit$ or intervention$ or program$ or recreation$ or stud$ or train$ or effort$ or exertion$ or fitness$)).tw,kf,ot. |
| 15 | ((activit$ behaviour$ or behavior$ or education$ or intervention$ or lifestyle$ or program$ or recreation$ or stud$ or therap$ or train$ or aerobic$ or isometric$ or physical$ or warm‐up$ or habit$) adj2 exercise$).tw,kf,ot. |
| 16 | or/1‐15 |
| 17 | GYMNASTICS/ |
| 18 | gymnastic$.tw,kf,ot. |
| 19 | (calisthenic$ or callisthenic$).tw,kf,ot. |
| 20 | pilates$.tw,kf,ot. |
| 21 | (resistanc$ adj2 (training$ or exercise$)).tw,kf,ot. |
| 22 | (muscular$ adj fitness$).tw,kf,ot. |
| 23 | (physical$ adj (activit$ or fitness$ or exercise$)).tw,kf,ot. |
| 24 | (physical$ adj (condition$ or effort4 or train$)).tw,kf,ot. |
| 25 | TAI JI/ |
| 26 | YOGA/ |
| 27 | (tai chi or tai ji or ji quan tai).tw,kf,ot. |
| 28 | yoga.tw,kf,ot. |
| 29 | (aerobic$ adj train$).tw,kf,ot. |
| 30 | stretching$.tw,kf,ot. |
| 31 | or/17‐30 |
| 32 | exp SPORTS/ |
| 33 | RUNNING/ |
| 34 | exp WALKING/ |
| 35 | JOGGING/ |
| 36 | SWIMMING/ |
| 37 | BICYCLING/ |
| 38 | DANCING/ |
| 39 | MOUNTAINEERING/ |
| 40 | sport$.tw,kf,ot. |
| 41 | athletic$.tw,kf,ot. |
| 42 | running$.tw,kf,ot. |
| 43 | walking$.tw,kf,ot. |
| 44 | ambulation$.tw,kf,ot. |
| 45 | jogging$.tw,kf,ot. |
| 46 | swimming$.tw,kf,ot. |
| 47 | bicycling$.tw,kf,ot. |
| 48 | cycling$.tw,kf,ot. |
| 49 | mountaineer$.tw,kf,ot. |
| 50 | danc$.tw,kf,ot. |
| 51 | (ramble$ or rambling$).tw,kf,ot. |
| 52 | rowing$.tw,kf,ot. |
| 53 | or/32‐52 |
| 54 | 16 or 31 or 53 |
| 55 | HEMATOLOGIC DISEASES/ |
| 56 | exp HEMATOLOGIC NEOPLASMS/ |
| 57 | (hematolog$ adj1 malignan$).tw,kf,ot. |
| 58 | (hematolog$ adj1 neoplas$).tw,kf,ot. |
| 59 | (haematolog$ adj1 malignan$).tw,kf,ot. |
| 60 | (haematolog$ adj1 neoplas$).tw,kf,ot. |
| 61 | exp BONE MARROW DISEASES/ |
| 62 | exp LYMPHOMA/ |
| 63 | exp LEUKEMIA/ |
| 64 | hodgkin$.tw,kf,ot. |
| 65 | lymphogranulomato$.tw,kf,ot. |
| 66 | lymphom$.tw,kf,ot. |
| 67 | histiocy$.tw,kf,ot. |
| 68 | granulom$.tw,kf,ot. |
| 69 | non‐hodgkin$.tw,kf,ot. |
| 70 | nonhodgkin$.tw,kf,ot. |
| 71 | reticulosis.tw,kf,ot. |
| 72 | reticulosarcom$.tw,kf,ot. |
| 73 | (burkitt$ adj (lymph$ or tumo?r$)).tw,kf,ot. |
| 74 | lymphosarcom$.tw,kf,ot. |
| 75 | brill‐symmer$.tw,kf,ot. |
| 76 | plasm##ytom$.tw,kf,ot. |
| 77 | myeloma$.tw,kf,ot. |
| 78 | sezary.tw,kf,ot. |
| 79 | leuk?em$.tw,kf,ot. |
| 80 | myelodysplas$.tw,kf,ot. |
| 81 | aplast$ an?em$.ti,kf,ot. |
| 82 | or/55‐81 |
| 83 | randomized controlled trial.pt. |
| 84 | controlled clinical trial.pt. |
| 85 | randomized.ab. |
| 86 | placebo.ab. |
| 87 | drug therapy.fs. |
| 88 | randomly.ab. |
| 89 | trial.ab. |
| 90 | groups.ab. |
| 91 | or/83‐90 |
| 92 | humans.sh. |
| 93 | 91 and 92 |
| 94 | 54 and 82 |
| 95 | 54 and 82 and 93 |
Update search: 30.07.2018
| # | Searches |
| 1 | exp Exercise/ |
| 2 | exp Exercise Movement Techniques/ |
| 3 | exp Exercise Therapy/ |
| 4 | exp "Physical Education and Training"/ |
| 5 | exp Physical Fitness/ |
| 6 | exp Sports/ |
| 7 | sport$.tw,kf,ot. |
| 8 | exp Walking/ |
| 9 | walking$.tw,kf,ot. |
| 10 | exp jogging/ |
| 11 | jogging$.tw,kf,ot. |
| 12 | exp swimming/ |
| 13 | swimming$.tw,kf,ot. |
| 14 | exp Bicycling/ |
| 15 | (bicycling$ or cycling$).tw,kf,ot. |
| 16 | exp Gymnastics/ |
| 17 | gymnastic$.tw,kf,ot. |
| 18 | (calisthenic$ or callisthenic$).tw,kf,ot. |
| 19 | (resistan$ adj2 (training$ or exercise$)).tw,kf,ot. |
| 20 | (pilates$ adj5 exercise$).tw,kf,ot. |
| 21 | (resistanc$ adj2 (training$ or exercise$)).tw,kf,ot. |
| 22 | ((aerobic$ or isometric$) adj2 exercise$).tw,kf,ot. |
| 23 | (muscular$ adj fitness$).tw,kf,ot. |
| 24 | exertion$.tw,kf,ot. |
| 25 | pilates$.tw,kf,ot. |
| 26 | (physical$ adj (activit$ or fitness$ or exercise$)).tw,kf,ot. |
| 27 | (physical$ adj (conditioning$ or effort$)).tw,kf,ot. |
| 28 | or/1‐27 |
| 29 | exp NEOPLASMS BY HISTOLOGIC TYPE/ |
| 30 | exp NEOPLASMS BY SITE/ |
| 31 | neoplas$.tw,kf,ot. |
| 32 | tumo?r$.tw,kf,ot. |
| 33 | (Krebs or cancer$).tw,kf,ot. |
| 34 | malignan$.tw,kf,ot. |
| 35 | (carcino$ or karzino$).tw,kf,ot. |
| 36 | karzinom$.tw,kf,ot. |
| 37 | sarcom$.tw,kf,ot. |
| 38 | leuk#?m$.tw,kf,ot. |
| 39 | lymphom$.tw,kf,ot. |
| 40 | melano$.tw,kf,ot. |
| 41 | metastas$.tw,kf,ot. |
| 42 | (mesothelio$ or mesotelio$).tw,kf,ot. |
| 43 | carcinomatos$.tw,kf,ot. |
| 44 | (gliom$ or glioblastom$).tw,kf,ot. |
| 45 | osteo?sarcom$.tw,kf,ot. |
| 46 | (blastom$ or neuroblastom$).tw,kf,ot. |
| 47 | or/29‐46 |
| 48 | randomized controlled trial.pt. |
| 49 | controlled clinical trial.pt. |
| 50 | randomi?ed.ab. |
| 51 | placebo.ab. |
| 52 | drug therapy.fs. |
| 53 | randomly.ab. |
| 54 | trial.ab. |
| 55 | groups.ab. |
| 56 | or/48‐55 |
| 57 | exp animals/ not humans/ |
| 58 | 56 not 57 |
| 59 | 28 and 47 and 58 |
| 60 | limit 59 to ed=20140129‐20172008 |
Data and analyses
Comparison 1. Physical exercise versus no physical exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality: SCT versus no SCT | 7 | 1172 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.79, 1.52] |
| 1.1 SCT | 5 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.71, 1.41] |
| 1.2 no SCT | 2 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.88, 4.50] |
| 2 Mortality | 7 | 1172 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.79, 1.52] |
| 3 Mortality sensitivity analysis: high risk of bias versus low risk of bias | 7 | 1172 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.79, 1.52] |
| 3.1 High Risk | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [0.13, 75.28] |
| 3.2 Low Risk | 6 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.77, 1.53] |
| 4 Quality of life (QoL) | 9 | 1259 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.03, 0.24] |
| 5 QoL: SCT versus no SCT | 9 | 1259 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.03, 0.24] |
| 5.1 SCT | 5 | 977 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.06, 0.24] |
| 5.2 no SCT | 4 | 282 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.20, 0.47] |
| 6 QoL sensitivity analysis: high risk of bias versus low risk of bias | 9 | 1259 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.03, 0.24] |
| 6.1 High Risk | 4 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.22, 0.47] |
| 6.2 Low Risk | 5 | 1002 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 7 Physical functioning/QoL | 9 | 1329 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.01, 0.32] |
| 8 Physical functioning/QoL: SCT versus no SCT | 9 | 1329 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.00, 0.32] |
| 8.1 SCT | 6 | 1108 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.07, 0.33] |
| 8.2 no SCT | 3 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.00, 0.53] |
| 9 Physical functioning/QoL sensitivity analysis: high risk of bias versus low risk of bias | 9 | 1329 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.00, 0.32] |
| 9.1 High Risk | 3 | 196 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.14, 0.46] |
| 9.2 Low Risk | 6 | 1133 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
| 10 Depression/QoL | 6 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.00, 0.38] |
| 11 Depression/QoL: SCT versus no SCT | 6 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.00, 0.38] |
| 11.1 SCT | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.32, 0.63] |
| 11.2 no SCT | 4 | 243 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.04, 0.47] |
| 12 Depression/QoL sensitivity analysis: high risk of bias versus low risk of bias | 6 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.00, 0.38] |
| 12.1 High Risk | 4 | 218 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.15, 0.38] |
| 12.2 Low Risk | 2 | 227 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.00, 0.52] |
| 13 Anxiety/QoL | 6 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.30, 0.36] |
| 14 Anxiety/QoL: SCT versus no SCT | 6 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.30, 0.36] |
| 14.1 SCT | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.82, 0.19] |
| 14.2 no SCT | 4 | 243 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.09, 0.51] |
| 15 Fatigue | 9 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.13, 0.48] |
| 16 Anxiety/QoL sensitivity analysis: high risk of bias versus low risk of bias | 6 | 445 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.30, 0.36] |
| 16.1 High Risk | 4 | 218 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.08, 0.51] |
| 16.2 Low Risk | 2 | 227 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.81, 0.20] |
| 17 Fatigue: SCT versus no SCT | 9 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.13, 0.48] |
| 17.1 SCT | 5 | 584 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.12, 0.51] |
| 17.2 no SCT | 4 | 242 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.09, 0.69] |
| 18 Fatigue sensitivity analysis: high risk of bias versus low risk of bias | 9 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.13, 0.48] |
| 18.1 High Risk | 5 | 404 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.57] |
| 18.2 Low Risk | 4 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.18, 0.57] |
| 19 Weight | 4 | 964 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐1.94, 2.82] |
| 20 Weight SCT: versus no SCT | 4 | 964 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐1.94, 2.82] |
| 20.1 SCT | 3 | 842 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐2.68, 2.55] |
| 20.2 no SCT | 1 | 122 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐2.88, 8.48] |
| 21 Weight sensitivity analysis: high risk of bias versus low risk of bias | 4 | 964 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐1.94, 2.82] |
| 21.1 Low Risk | 4 | 964 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐1.94, 2.82] |
| 21.2 High Risk | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Lean body mass | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 1.26 [‐1.22, 3.74] |
| 23 Lean body mass: SCT versus no SCT | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 1.26 [‐1.22, 3.74] |
| 23.1 SCT | 1 | 131 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐3.57, 3.97] |
| 23.2 no SCT | 2 | 159 | Mean Difference (IV, Random, 95% CI) | 2.06 [‐1.22, 5.35] |
| 24 Lean body mass sensitivity analysis: high risk of bias versus low risk of bias | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 1.26 [‐1.22, 3.74] |
| 24.1 High Risk | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐5.69, 7.29] |
| 24.2 Low Risk | 2 | 253 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐1.34, 4.02] |
| 25 Serious adverse events (SAEs) | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.94, 2.06] |
| 26 Serious adverse events (SAEs): SCT versus no SCT | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.94, 2.06] |
| 26.1 SCT | 3 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.94, 2.09] |
| 26.2 no SCT | 3 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.21] |
| 27 Serious adverse events (SAEs) sensitivity analysis: high risk of bias versus low risk of bias | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.94, 2.06] |
| 27.1 High Risk | 5 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.94, 2.06] |
| 27.2 Low Risk | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alibhai 2014.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 40)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and relevant for this review
Reported and not relevant for this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized using opaque numbered sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote:"Subjects were randomized using opaque numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | High risk | Quote:"Outcomes assessors were not blinded to treatment arm" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two participants of the exercise group and one of the control group dropped out which makes a ratio of 8%. Reported reasons were quote: "early discontinuation due to relapse" respectively "lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | High risk | Out of 17 participants allocated to the control group 5 immediately crossed over to the exercise group |
Baumann 2010.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 64)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and relevant for this review
Reported and not relevant for this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"randomization was achieved....using computer‐generated numbers" |
| Allocation concealment (selection bias) | Low risk | Quote:"randomization was achieved....using computer‐generated numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"All 64 randomized patients represented the intent‐to‐treat population" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
Bryant 2018.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 17)
Mean age
Stage of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported
Not reported but relevant
Reported but not relevant
Primary outcome
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"The randomization sequence was generated by the study’s statistician. The statistician and research outcome assessors were blinded to the randomization allocation" |
| Allocation concealment (selection bias) | Low risk | Quote:"The randomization sequence was generated by the study’s statistician. The statistician and research outcome assessors were blinded to the randomization allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Quote:"The randomization sequence was generated by the study’s statistician. The statistician and research outcome assessors were blinded to the randomization allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote:"1 dropped before the intervention started" (intervention group) |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | Low risk | None |
Chang 2008.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 24)
Mean age
Stage of disease
Country
|
|
| Interventions | Exercise group
Control group
All participants received cytarabine plus idarubicin |
|
| Outcomes |
Reported and analysed in this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two patients out of 24 (9%) dropped out because of severe complications. It is unclear whether these complications are related to the intervention Quote: "One patient dropped out of each group due to severe complications" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Gender distribution unbalanced between arms |
Coleman 2003.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 24)
Mean age
Stage of disease
Country
|
|
| Interventions | Exercise group
Control group
All participants received high‐dose chemotherapy including tandem transplantation. Half of them were randomised to receive thalidomide during induction, posttransplantation consolidation, and maintenance therapy |
|
| Outcomes |
Reported and analysed in this review
Reported but not relevant for this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessor (patient‐reported outcomes) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Study was finalised before the last 6 participants were enrolled due to funding constraints |
Coleman 2012.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 187)
Mean age
Stage of disease
Therapy
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
Reported but not relevant for this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 participants who dropped out were not analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | High risk | 50% of participants received thalidomide. In the study analysis this drug administration was not considered and no subgroup data were provided for participants receiving or not receiving thalidomide |
Courneya 2009.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 122)
Mean age
Stage of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
Reported but not relevant for this review
|
|
| Notes | Financially supported by Lance Armstrong Foundation. No conflicts of interest reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completing baseline tests, participants were stratified by major disease type and current treatment status and were randomly assigned to aerobic exercise training or usual care by using a computer‐generated program. The allocation sequence was generated independently and concealed in opaque envelopes from the study coordinator who assigned participants to groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was generated independently and concealed in opaque envelopes from the study coordinator who assigned participants to groups." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | High risk | Quote: "Outcomes assessors were not always blinded to group assignment but were trained in standardising testing procedures" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were considered in study analysis in conformity with their randomised group assignment |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Study supported by Lance Armstrong Foundation. Any bias due to this support is not expected. No other sources of potential bias were reported |
Cunningham 1986.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 30)
Mean age
Stage of disease
Country
|
|
| Interventions | Exercise groups
Control group
|
|
| Outcomes |
Reported and analysed in this review
Reported but not relevant for this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized (by computer‐generated random permutations of a number unknown to investigators)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomized (by computer‐generated random permutations of a number unknown to investigators)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | Unclear risk | Not reported |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
DeFor 2007.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 100)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
Reported but not relevant for this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | Unclear risk | Outcome not reported |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Low risk | 100‐day assessment was performed in a clinic that was separate from the hospital so the physician was unaware of the randomised assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
Furzer 2016.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 44)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
Reported but not relevant for this review
|
|
| Notes | Financially supported by the SolarisCare foundation. The authors report no competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were block randomised" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were block randomised" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Not reported |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven participants out of 44 (16%) dropped out due to different reasons (withdrew, illness, disease relapse), four having been part of the exercise group and three of the usual care group. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | Low risk | Financially supported by the SolarisCare foundation. Any bias due to this support is not expected |
Jacobsen 2014.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 711)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
|
|
| Notes | Financially supported by the National Heart, Lung, and Blood Institute and the National Cancer Institute. The authors report no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized using a factorial design" |
| Allocation concealment (selection bias) | Unclear risk | Quote:"patients were randomized using a factorial design" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Survival and missing data rates were similar across the study arms" |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | Unclear risk | Financially supported by the National Heart, Lung, and Blood Institute and the National Cancer Institute. No competing interests were reported |
Jacobsen 2014a.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 355)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
|
|
| Notes | Financially supported by the National Heart, Lung, and Blood Institute and the National Cancer Institute. Any bias due to this support is not expected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized using a factorial design" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomized using a factorial design" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Survival and missing data rates were similar across the study arms" |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | Low risk | Financially supported by the National Heart, Lung, and Blood Institute and the National Cancer Institute. Any bias due to this support is not expected |
Jacobsen 2014b.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 356)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes | see Jacobsen 2014 | |
| Notes | see Jacobsen 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized using a factorial design" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomized using a factorial design" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Survival and missing data rates were similar across the study arms" |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | Low risk | Financially supported by the National Heart, Lung, and Blood Institute and the National Cancer Institute. Any bias due to this support is not expected |
Jarden 2016.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 70)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
|
|
| Notes | Financially supported by The Center for Integrated Rehabilitation of Cancer Patients, The Novo Nordic Foundation, The University Hospitals’ Centre for Health Research (UCSF), The Lundbeck Foundation, The Novo Nordic Foundation for Clinical Nursing Research and The Danish Cancer Society. The authors report no competing interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized... using the computerized Clinical Trial Management System" |
| Allocation concealment (selection bias) | Low risk | Quote: "A block design with allocation weight of 1:1 will be used to generate treatment allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Low risk | Quote: "however the outcome assessors...be blinded to the participants study allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Out of 70 participants 62 completed study requirements (89%). 8 participants dropped out, 2 from the IG and 6 from the CG |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol are reported |
| Other bias | Low risk | Financially supported by The Center for Integrated Rehabilitation of Cancer Patients, The Novo Nordic Foundation, The University Hospitals’ Centre for Health Research (UCSF), The Lundbeck Foundation, The Novo Nordic Foundation for Clinical Nursing Research and The Danish Cancer Society. Any bias due to this support is not expected |
Kim 2006.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 35)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported but not relevant
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized by random‐permuted block design using a random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomized by random‐permuted block design using a random number table" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | Unclear risk | Not reported |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Seven subjects were lost before completing the post‐test. One died of cerebral haemorrhage, two died of acute renal failure, four refused before completing the post‐test" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
Knols 2011.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 131)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
|
|
| Notes | Financially supported by Zürcher Krebsliga ("Zurich cancer league") and Eidgenössische Sportkommission (Federal Authorities of the Swiss Confederation, Federal Department of Defence, Civil Protection and Sport) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "minimization procedure was used to achieve an optimal balance between groups for the factors age, sex and type of transplantation" |
| Allocation concealment (selection bias) | Low risk | Quote: "opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "was carried out on intention to treat analysis" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Financially supported by Zürcher Krebsliga ("Zurich cancer league") and Eidgenössische Sportkommission (Federal Authorities of the Swiss Confederation, Federal Department of Defence, Civil Protection and Sport). Any bias due to this support is not expected |
Mello 2003.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 18)
Mean age
Stage/type of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Unclear which patients and when patients have been randomised |
| Allocation concealment (selection bias) | High risk | Methods of randomisation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 18 out of 32 patients have been evaluated. Ten patients have died but it is unclear in which treatment arm |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available |
| Other bias | Unclear risk | Not reported |
Persoon 2017.
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
|
| Participants | Eligibility criteria
Participants (N = 109)
Mean age
Stage of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported
Not reported but relevant
Reported but not relevant
Primary outcome
|
|
| Notes | This study was supported by the Alpe d’HuZes/KWF Fund. The authors declare that no competing interests exist | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was concealed, took place after completion of baseline assessments (T0) and was performed by an independent data manager using a validated software program." |
| Allocation concealment (selection bias) | Low risk | Quote: "...proceeded using block randomization with block sizes varying randomly between 2, 4 and 6." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported. The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Body composition was assessed by the sports physician and it remains unclear if he was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "However, we had a relatively high number of missing values" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | This study was supported by the Alpe d’HuZes/KWF Fund. The authors declare that no competing interests exist |
Streckmann 2014.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 61)
Mean age
Stage of disease
Country
|
|
| Interventions | Exercise group
Control group
|
|
| Outcomes |
Reported and analysed in this review
Reported but not relevant for this review
|
|
| Notes | Financially supported by a grant by AMGEN, the authors report no competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "carried out by an independent randomization office" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | Outcome not reported |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Intention to treat strategies for substituting missing values" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Study stopped early, due to low recruitment. Quote: "At this point, we considered physiological parameters much more relevant to evaluate the intervention than QoL, hence the study was stopped early" Statistically significant baseline imbalances for QoL, favouring the control arm |
Wiskemann 2015.
| Methods | Randomisation
Recruitment period
Median follow‐up time
Sample size calculation
|
|
| Participants | Eligibility criteria
Participants (N = 105)
Mean age
Disease
Country
|
|
| Interventions | Exercise group
Control group
All participants received allogeneic stem cell transplantation |
|
| Outcomes |
Reported and relevant for this review
|
|
| Notes | Conflict of interest not reported, funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised by the minimisation procedure stratified by age, disease, and sex for each centre to an exercise or a control group“ |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (primary endpoint; mortality) | Low risk | The review authors judge that the outcome mortality in this unblinded trial is unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (patient‐reported outcomes) | High risk | Blinding in this context is not feasible |
| Blinding of outcome assessor (physical performance, AEs, SAEs) | High risk | Quote: "The testers were not blinded to randomisation but not involved in the therapeutic supervision of the patients.“ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 112 participants were randomly assigned to both study arms. In study analysis only 105 participants were included. Moreover, it is not reported, how many patients in the control arm performed exercise |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Not reported |
ALL: acute lymphoblastic leukaemia; AML: acute myeloid leukaemia;ASCT: autologous stem cell transplant; BMT: bone marrow transplant; B‐NHL: B‐cell non‐Hodgkin's lymphoma; CLL: chronic lymphoblastic leukaemia; CML: chronic myeloid leukaemia; DCEP: dexamethasone, cyclophosphamide, etoposide, and cisplatin;EPO: erythropoietin;Hb: haemoglobin; HCT: haematopoietic cell transplantation; HL: Hodgkin's lymphoma; MDS: myelodysplastic syndrome; NHL: non‐Hodgkin's lymphoma; OS: overall survival; T‐NHL: T‐cell non‐Hodgkin's lymphoma;QoL: quality of life
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Broderick 2013 | Only 7% of the included participants suffer from haematological malignancies |
| Cohen 2004 | Study investigated influence of Tibetan yoga intervention on psychological adjustment and sleep quality. This intervention does not correspond to our prescribed exercise intervention |
| Forbes 2017 | In this study, participants suffering from different cancer types were included, no subgroup analyses for those with haematological malignancies were provided |
| Grabenbauer 2016 | In this study, participants suffering from different cancer types were included, no subgroup analyses for those with haematological malignancies were provided |
| Hacker 2011 | Exercise intervention only consisted of strength training. This intervention does not correspond to our predefined types of exercise intervention |
| Hacker 2016 | Exercise intervention only consisted of strength training. This intervention does not correspond to our predefined types of exercise intervention |
| Hartman 2009 | Study included participants up to the age of 18 years |
| Jarden 2009 | Control group received standard treatment care and physiotherapy. Intervention consisted of physical exercise together with psycho‐education and progressive relaxation |
| Jones 2014 | In this study, participants suffering from cancer in general were included, no subgroup analyses for those with haematological malignancies were provided |
| Kampshoff 2015 | Only 10 % of the included participants suffer from haematological malignancies |
| Kanera 2017 | In this study, participants suffering from different cancer types were included, no subgroup analyses for those with haematological malignancies were provided |
| Marchese 2004 | Study included participants up to the age of 18 years |
| Mayo 2014 | In this study, it is not made clear which kind of malignancies have been diagnosed |
| Midtgaard 2013 | In this study, only 13% of the participants suffer from haematological malignancies |
| Moyer‐Mileur 2009 | Study included participants up to the age of 18 years |
| Oechsle 2014 | In this study only 75% of the participants suffer from haematological malignancies |
| Peoples 2017 | In this study, participants suffering from different cancer types were included, no subgroup analyses for those with haematological malignancies were provided |
| PETRA study | Study did not compare an exercise group with a standard care group. In this study the control group receives a relaxation program |
| Prinsen 2013 | Intervention is cognitive behavioural therapy. This intervention does not correspond to our prescribed exercise intervention |
| Schumacher 2015a | Both study arms were asked to exercise, either with a physiotherapist or with Nintendo Wii |
| Shelton 2009 | Study did not compare an exercise group with a standard care group. In this study both groups conducted physical exercise intervention |
| Stacey 2016 | In this study, participants suffering from different cancer types were included, no subgroup analyses for those with haematological malignancies were provided |
| Tanir 2013 | Study included participants up to the age of 18 years |
| Thorsen 2005 | In this study, participants suffering from different cancer types (lymphomas, breast, gynaecologic or testicular cancer) were included, no subgroup analyses for those with haematological malignancies were provided |
| Toohey 2016 | In this study, participants suffering from different cancer types were included, no subgroup analyses for those with haematological malignancies were provided |
| Tran 2016 | In this study participants suffering from different cancer types were included, no subgroup analyses for those with haematological malignancies were provided |
| Valle 2013 | In this study, only 30% percent of the participants suffer from haematological exercises |
| Vallerand 2018 | Study did not compare an exercise group with a standard care group. In this study both, intervention and control group, were asked to exercise |
| van Waart 2015 | In this study, only participants suffering from breast cancer were included |
| Yeh 2016 | Intervention is qigong. This intervention does not correspond to our prescribed exercise intervention |
| Zimmer 2014 | In this study, only 75% of the participants were suffering from haematological malignancies |
Characteristics of studies awaiting assessment [ordered by study ID]
Wehrle 2018.
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
| Participants | Eligibility criteria
Participants (N = 29)
Mean age
Stage of disease
Country
|
| Interventions | The intervention took place during induction chemotherapy with three exercise sessions per week for 30 to 45 minutes each Endurance group
Resitance group
Control group
|
| Outcomes |
Reported
Not reported but relevant
|
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Abildgaard 2018.
| Trial name or title | Early initiated individualized physical training in newly diagnosed multiple myeloma patients; effects on physical function, physical activity, quality of life, pain, and bone disease. |
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
| Participants | Eligibility criteria
Participants (N = not reported)
Mean age
Stage of disease
Country
|
| Interventions | Exercise group
Control group
|
| Outcomes |
Reported
Not reported but relevant
Reported but not relevant
Primary outcome
|
| Starting date | May 2015 |
| Contact information | Niels Abildgaard, Zealand University Hospital, Roskilde, Denmark |
| Notes |
Courneya 2017.
| Trial name or title | Improving quality of life in hematologic cancer survivors by closing the exercise intention—behavior gap: a phase II randomized controlled trial of a theory‐based, telephone‐delivered exercise counselling intervention |
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
| Participants | Eligibility criteria
Participants (N = 66)
Mean age
Stage of disease
Country
|
| Interventions | Exercise group
Control group
|
| Outcomes |
Reported
Not reported but relevant
Reported but not relevant
Primary outcome
|
| Starting date | February 2017 |
| Contact information | Kerry Courneya, University of Alberta, Canada |
| Notes |
Oberste 2016.
| Trial name or title | The effect of a chemotherapy accompanying 4‐week aerobic endurance exercise intervention on incidence and severity of cancer related cognitive impairments in leukemia patients. A randomized controlled trial |
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
| Participants | Eligibility criteria
Participants (N = 83)
Mean age
Stage of disease
Country
|
| Interventions | Exercise group
Placebo group
Control group
|
| Outcomes |
Reported
Not reported but relevant
Reported but not relevant
Primary outcome
|
| Starting date | Not reported |
| Contact information | Department of Molecular and Cellular Sport Medicine, Institute of Cardiovascular Research and Sports Medicine, German Sport University Cologne, Cologne, Germany |
| Notes | Will be financially supported by the Marga und Walter Boll Stiftung. The authors report no conflict of interest |
Walsh 2005.
| Trial name or title | Randomised controlled trial to investigate the effects of an exercise programme on physical performance and quality of life after a bone marrow transplant |
| Methods | Randomisation
Recruitment period
Median follow‐up time
|
| Participants | Eligibility criteria
Participants (N = not reported)
Mean age
Stage of disease
Country
|
| Interventions | Exercise group 1
Exercise group 2
Control group
|
| Outcomes |
Reported
Not reported but relevant
Reported but not relevant
Primary outcome
|
| Starting date | November 2003, no further information when the trial will be terminated in study registry clinicaltrials.gov. The status still ongoing (last access 09.12.2018) |
| Contact information | Alfred Hospital Physiotherapy Dept, Melbourne, Australia |
| Notes | Financially supported by Bayside Health |
AML: acute myeloid leukaemia; BDNF: brain‐derived neurotrophic factor; GvHD: graft‐versus‐host‐diease;MDS: myelodysplastic syndrome; QoL: quality of life; VEGF: Vascular endothelial growth factor Vascular endothelial growth factor
Differences between protocol and review
We changed the title and added "aerobic" to reflect that we only included trials that evaluated physical exercise intended to improve the oxygen system.
As our primary outcome overall survival was reported in one trial only, we also analysed mortality.
In meta‐analyses with at least 10 trials, we would have explored potential publication bias by generating a funnel plot and by using a linear regression test (Sterne 2011). We would have considered a P value of less than 0.1 significant for this test.
Contributions of authors
Linus Knips: development and writing of updated review
Nils Bergenthal: development and writing of review
Fiona Streckmann: content input, provided unpublished data
Ina Monsef: development of the search strategy
Thomas Elter: clinical expertise and content input
Nicole Skoetz: clinical, statistical and methodological expertise and advice
Sources of support
Internal sources
Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany.
External sources
No sources of support supplied
Declarations of interest
Linus Knips: none known
Nils Bergenthal: none known
Fiona Streckmann: none known
Ina Monsef: none known
Thomas Elter: none known
Nicole Skoetz: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Alibhai 2014 {published data only}
- Alibhai SM, Durbano H, Breunis S, Brandwein H, Timilshina JM, Tomlinson N. A phase II exercise randomized controlled trial for patients with acute myeloid leukemia undergoing induction chemotherapy. Leukemia Research 2015;11:no pagination. [DOI] [PubMed] [Google Scholar]
- Alibhai SM, O'Neill S, Fisher‐Schlombs K, Breunis H, Timilshina N, Brandwein JM, et al. A pilot phase II RCT of a home‐based exercise intervention for survivors of AML. Supportive Care in Cancer 2014;22(4):881‐9. [DOI] [PubMed] [Google Scholar]
- Alibhai SMH, Durbano S, Timilshina N, Breunis H, Brandwein J, Tomlinson G, et al. A phase II exercise RCT for AML patients undergoing induction chemotherapy. Supportive Care in Cancer 2014, issue 1 suppl. 1:S202‐s203.
Baumann 2010 {published data only}
- Baumann FT, Kraut L, Schule K, Bloch W, Fauser AA. A controlled randomized study examining the effects of exercise therapy on patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplantation 2010;45(2):355‐62. [PUBMED: 19597418] [DOI] [PubMed] [Google Scholar]
- Baumann FT, Zopf EM, Nykamp E, Kraut L, Schule K, Elter T, et al. Physical activity for patients undergoing an allogeneic hematopoietic stem cell transplantation: benefits of a moderate exercise intervention. European Journal of Haematology 2011;87(2):148‐56. [PUBMED: 21545527] [DOI] [PubMed] [Google Scholar]
Bryant 2018 {published data only}
- Bryant AL, Deal AM, Battaglini CL, Phillips B, Pergolotti M, Coffman E, et al. The effects of exercise on patient‐reported outcomes and performance‐based physical function in adults with acute leukemia undergoing induction therapy. Integrative Cancer Therapies 2018;17(2):263‐70. [PUBMED: 28627275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2008 {published data only}
- Chang PH, Lai YH, Shun SC, Lin LY, Chen ML, Yang Y, et al. Effects of a walking intervention on fatigue‐related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: a randomized controlled trial. Journal of Pain and Symptom Management 2008;35(5):524‐34. [PUBMED: 18280104] [DOI] [PubMed] [Google Scholar]
Coleman 2003 {published data only}
- Coleman EA, Coon S, Hall‐Barrow J, Richards K, Gaylor D, Stewart B. Feasibility of exercise during treatment for multiple myeloma. Cancer Nursing 2003;26(5):410‐9. [PUBMED: 14710804] [DOI] [PubMed] [Google Scholar]
- Coleman EA, Hall‐Barrow J, Coon S, Stewart CB. Facilitating exercise adherence for patients with multiple myeloma. Clinical Journal of Oncology Nursing 2003;7(5):529‐34, 540. [PUBMED: 14603549] [DOI] [PubMed] [Google Scholar]
Coleman 2012 {published data only}
- Coleman EA, Anaissie E, Coon SK, Stewart CB, Shaw J, Barlogie B. A randomized trial of home‐based exercise for patients receiving aggressive treatment and epoetin alfa for multiple myeloma: Hemoglobin (Hb), transfusion, fatigue and performance as outcomes [abstract]. Journal of Clinical Oncology 2004:731.
- Coleman EA, Coon SK, Kennedy R, Lockhart K, Anaissie EJ, Barlogie B. Benefits of exercise in combination with epoetin alfa for multiple myeloma [Abstract No. 8605]. Journal of Clinical Oncology. 2006:494. [DOI] [PMC free article] [PubMed]
- Coleman EA, Coon SK, Kennedy RL, Lockhart KD, Stewart CB, Anaissie EJ, et al. Effects of exercise in combination with epoetin alfa during high‐dose chemotherapy and autologous peripheral blood stem cell transplantation for multiple myeloma. Oncology Nursing Forum 2008;35(3):E53‐61. [PUBMED: 18467280] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman EA, Goodwin JA, Kennedy R, Coon SK, Richards K, Enderlin C, et al. Effects of exercise on fatigue, sleep, and performance: a randomized trial. Oncology Nursing Forum 2012;39(5):468‐77. [PUBMED: 22940511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Courneya 2009 {published data only}
- Courneya KS, Friedenreich CM, Franco‐Villalobos C, Crawford JJ, Chua N, Basi S, et al. Effects of supervised exercise on progression‐free survival in lymphoma patients: an exploratory follow‐up of the HELP Trial. Cancer Causes & Control 2015;26:269‐76. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Jones LW, Peddle CJ, Sellar CM, Reiman T, Joy AA, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: a randomized controlled trial. Oncologist 2008;13(9):1012‐20. [PUBMED: 18779540] [DOI] [PubMed] [Google Scholar]
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Friedenreich CM, Peddle CJ, et al. Moderator effects in a randomized controlled trial of exercise training in lymphoma patients. Cancer Epidemiology, Biomarkers & Prevention 2009;18(10):2600‐7. [PUBMED: 19815635] [DOI] [PubMed] [Google Scholar]
- Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. Journal of Clinical Oncology 2009;27(27):4605‐12. [PUBMED: 19687337] [DOI] [PubMed] [Google Scholar]
- Courneya KS, Sellar CM, Trinh L, Forbes CC, Stevinson C, McNeely ML, et al. A randomized trial of aerobic exercise and sleep quality in lymphoma patients receiving chemotherapy or no treatments. Cancer Epidemiology, Biomarkers & Prevention 2012;21(6):887‐94. [PUBMED: 22523181] [DOI] [PubMed] [Google Scholar]
- Courneya KS, Stevinson C, McNeely ML, Sellar CM, Friedenreich CM, Peddle‐McIntyre CJ, et al. Effects of supervised exercise on motivational outcomes and longer‐term behavior. Medicine & Science in Sports & Exercise 2012;44(3):542‐9. [PUBMED: 21814149] [DOI] [PubMed] [Google Scholar]
- Courneya KS, Stevinson C, McNeely ML, Sellar CM, Friedenreich CM, Peddle‐McIntyre CJ, et al. Predictors of follow‐up exercise behavior 6 months after a randomized trial of supervised exercise training in lymphoma patients. Psycho‐Oncology 2012;21(10):1124‐31. [PUBMED: 21766483] [DOI] [PubMed] [Google Scholar]
- Courneya KS, Stevinson C, McNeely ML, Sellar CM, Peddle CJ, Friedenreich CM, et al. Predictors of adherence to supervised exercise in lymphoma patients participating in a randomized controlled trial. Annals of Behavioral Medicine 2010;40(1):30‐9. [PUBMED: 20563764] [DOI] [PubMed] [Google Scholar]
Cunningham 1986 {published data only}
- Cunningham BA, Morris G, Cheney CL, Buergel N, Aker SN, Lenssen P. Effects of resistive exercise on skeletal muscle in marrow transplant recipients receiving total parenteral nutrition. Journal of Parenteral and Enteral Nutrition 1986;10(6):558‐63. [PUBMED: 3098997] [DOI] [PubMed] [Google Scholar]
DeFor 2007 {published data only}
- DeFor TE, Burns LJ, Gold EM, Weisdorf DJ. A randomized trial of the effect of a walking regimen on the functional status of 100 adult allogeneic donor hematopoietic cell transplant patients. Biology of Blood and Marrow Transplantation 2007;13(8):948‐55. [PUBMED: 17640599] [DOI] [PubMed] [Google Scholar]
Furzer 2016 {published data only}
- Furzer BJ, Ackland TR, Wallman KE, Petterson AS, Gordon SM, Wright KE, et al. A randomised controlled trial comparing the effects of a 12‐week supervised exercise versus usual care on outcomes in haematological cancer patients. Supportive Care in Cancer 2016; Vol. 24, issue 4:1697‐707. [DOI] [PubMed]
Jacobsen 2014 {published data only}
- Jacobsen PB, Le‐Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biology of Blood & Marrow Transplantation 2014;20:1530‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobsen 2014a {published data only}
- Jacobsen PB, Le‐Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biology of Blood & Marrow Transplantation 2014;20:1530‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobsen 2014b {published data only}
- Jacobsen PB, Le‐Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biology of Blood & Marrow Transplantation 2014;20:1530‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarden 2016 {published data only}
- Jarden M, Moller T, Bang Christensen K, Birgens H, Kjeldsen L, Adamsen L. Patient activation through counseling and exercise ‐ Acute leukemia (PACE‐AL) trial ‐ A randomized controlled trial. Supportive Care in Cancer. 2015, issue 1 suppl 1:s296. [DOI] [PMC free article] [PubMed]
- Jarden M, Moller T, Kjeldsen L, Birgens H, Birgens K, Adamsen L. Effect of exercise and counseling integrated in the clinical management of acute leukemia on physical function and quality of life during consolidation chemotherapy: a multicenter randomized trial. Haematologica. 2016:594. [DOI] [PMC free article] [PubMed]
- Jarden M, Moller T, Kjeldsen L, Birgens H, Christensen JF, Bang Christensen K, et al. Patient Activation through Counseling and Exercise‐‐Acute Leukemia (PACE‐AL)‐‐a randomized controlled trial. BMC Cancer 2013;13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarden M, Møller T, Christensen KB, Kjeldsen L, Birgens HS, Adamsen L. Multimodal intervention integrated into the clinical management of acute leukemia improves physical function and quality of life during consolidation chemotherapy: a randomized trial ‘PACE‐AL’. Haematologica 2016;101:e316‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim SD, Kim HS. A series of bed exercises to improve lymphocyte count in allogeneic bone marrow transplantation patients. European Journal of Cancer Care 2006;15(5):453‐7. [PUBMED: 17177902] [DOI] [PubMed] [Google Scholar]
Knols 2011 {published data only}
- Knols RH, Bruin ED, Uebelhart D, Aufdemkampe G, Schanz U, Stenner‐Liewen F, et al. Effects of an outpatient physical exercise program on hematopoietic stem‐cell transplantation recipients: a randomized clinical trial. Bone Marrow Transplantation 2011;46(9):1245‐55. [PUBMED: 21132025] [DOI] [PubMed] [Google Scholar]
Mello 2003 {published data only}
- Mello M, Tanaka C, Dulley FL. Effects of an exercise program on muscle performance in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplantation 2003;32(7):723‐8. [PUBMED: 13130321] [DOI] [PubMed] [Google Scholar]
Persoon 2017 {published data only}
- Persoon S, ChinAPaw MJ, Buffart LM, Liu RD, Wijermans P, Koene HR, et al. Randomized controlled trial on the effects of a supervised high intensity exercise program in patients with a hematologic malignancy treated with autologous stem cell transplantation: Results from the EXIST study. PLOS One 2017;12(7):e0181313. [PUBMED: 28727771] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoon S, Chinapaw MJ, Buffart LM, Brug J, Kersten MJ, Nollet F. Lessons learnt from a process evaluation of an exercise intervention in patients treated with autologous stem cell transplantation. European Journal of Cancer Care 2018;27(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoon S, Kersten MJ, Chinapaw MJ, Buffart LM, Burghout H, Schep G, et al. Design of the EXercise Intervention after Stem cell Transplantation (EXIST) study: a randomized controlled trial to evaluate the effectiveness and cost‐effectiveness of an individualized high intensity physical exercise program on fitness and fatigue in patients with multiple myeloma or (non‐) Hodgkin's lymphoma treated with high dose chemotherapy and autologous stem cell transplantation. BMC Cancer 2010;10:671. [PUBMED: 21134270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Streckmann 2014 {published and unpublished data}
- Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihort G, et al. Exercise program improves therapy‐related side‐effects and quality of life in lymphoma patients undergoing therapy. Annals of Oncology 2014;25(2):493‐9. [PUBMED: 24478323] [DOI] [PubMed] [Google Scholar]
Wiskemann 2015 {published data only}
- Wiskemann J, Dreger P, Schwerdtfeger R, Bondong A, Huber G, Kleindienst N, et al. Effects of a partly self‐administered exercise program before, during, and after allogeneic stem cell transplantation. Blood 2011;117(9):2604‐13. [PUBMED: 21190995] [DOI] [PubMed] [Google Scholar]
- Wiskemann J, Kleindienst N, Kuehl R, Dreger P, Schwerdtfeger R, Bohus M. Effects of physical exercise on survival after allogeneic stem cell transplantation. International Journal of Cancer 2015;137(11):2749‐56. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Broderick 2013 {published data only}
- Broderick JM, Guinan E, Kennedy MJ, Hollywood D, Courneya KS, Culos‐Reed SN, et al. Feasibility and efficacy of a supervised exercise intervention in de‐conditioned cancer survivors during the early survivorship phase: the PEACH trial. Journal of Cancer Survivorship 2013;7:551‐62. [DOI] [PubMed] [Google Scholar]
Cohen 2004 {published data only}
- Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul‐Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer 2004;100(10):2253‐60. [PUBMED: 15139072] [DOI] [PubMed] [Google Scholar]
Forbes 2017 {published data only}
- Forbes CC, Blanchard CM, Mummery WK, Courneya KS. A pilot study on the motivational effects of an internet‐delivered physical activity behaviour change programme in Nova Scotian cancer survivors. Psychology & Health 2017;32(2):234‐52. [DOI] [PubMed] [Google Scholar]
Grabenbauer 2016 {published data only}
- Grabenbauer A, Grabenbauer AJ, Lengenfelder R, Grabenbauer GG, Distel LV. Feasibility of a 12‐month‐exercise intervention during and after radiation and chemotherapy in cancer patients: impact on quality of life, peak oxygen consumption, and body composition. Radiation Oncology 2016;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacker 2011 {published data only}
- Hacker ED, Collins E, Park C, Peters T, Patel P, Rondelli D. Strength training to enhance early recovery after hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 2017;23(4):659‐69. [DOI] [PubMed] [Google Scholar]
- Hacker ED, Larson J, Kujath A, Peace D, Rondelli D, Gaston L. Strength training following hematopoietic stem cell transplantation. Cancer Nursing 2011;34(3):238‐49. [PUBMED: 21116175] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacker 2016 {published data only}
- Hacker ED, Collins E, Park C, Peters T, Rondelli D. Strength training to enhance early recovery following hematopoietic stem cell transplantation: a randomized controlled trial. Journal of Pain and Symptom Management.. 2016, issue 6:e143‐4.
Hartman 2009 {published data only}
- Hartman A, Winkel ML, Beek RD, Muinck Keizer‐Schrama SM, Kemper HC, Hop WC, et al. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatric Blood & Cancer 2009;53(1):64‐71. [PUBMED: 19283791] [DOI] [PubMed] [Google Scholar]
Jarden 2009 {published data only}
- Jarden M, Nelausen K, Hovgaard D, Boesen E, Adamsen L. The effect of a multimodal intervention on treatment‐related symptoms in patients undergoing hematopoietic stem cell transplantation: a randomized controlled trial. Journal of Pain and Symptom Management 2009;38(2):174‐90. [PUBMED: 19345060] [DOI] [PubMed] [Google Scholar]
Jones 2014 {published data only}
- Jones LW, Douglas PS, Khouri MG, Mackey JR, Wojdyla D, Kraus WE, et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF‐ACTION randomized trial. Journal of Clinical Oncology 2014;32:2496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kampshoff 2015 {published data only}
- Kampshoff CS, Chinapaw MJ, Brug J, Twisk JW, Schep G, Nijziel MR, et al. Randomized controlled trial of the effects of high intensity and low‐to‐moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Medicine 2015;13:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampshoff CS, Mechelen W, Schep G, Nijziel MR, Witlox L, Bosman L, et al. Participation in and adherence to physical exercise after completion of primary cancer treatment. International Journal of Behavioral Nutrition & Physical Activity 2016;13(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanera 2017 {published data only}
- Kanera IM, Willems RA, Bolman CA, Mesters I, Verboon P, Lechner L. Long‐term effects of a web‐based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2017;14(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marchese 2004 {published data only}
- Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatric Blood & Cancer 2004;42(2):127‐33. [PUBMED: 14752875] [DOI] [PubMed] [Google Scholar]
Mayo 2014 {published data only}
- Mayo NE, Moriello C, Scott SC, Dawes D, Auais M, Chasen M. Pedometer‐facilitated walking intervention shows promising effectiveness for reducing cancer fatigue: a pilot randomized trial. Clinical Rehabilitation 2014;28:1198‐209. [DOI] [PubMed] [Google Scholar]
Midtgaard 2013 {published data only}
- Midtgaard J, Christensen JF, Tolver A, Jones LW, Uth J, Rasmussen B, et al. Efficacy of multimodal exercise‐based rehabilitation on physical activity, cardiorespiratory fitness, and patient‐reported outcomes in cancer survivors: a randomized, controlled trial. Annals of Oncology 2013;24(9):2267‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyer‐Mileur 2009 {published data only}
- Moyer‐Mileur LJ, Ransdell L, Bruggers CS. Fitness of children with standard‐risk acute lymphoblastic leukemia during maintenance therapy: response to a home‐based exercise and nutrition program. Journal of Pediatric Hematology/Oncology 2009;31(4):259‐66. [PUBMED: 19346877] [DOI] [PubMed] [Google Scholar]
Oechsle 2014 {published data only}
- Oechsle K, Aslan Z, Suesse Y, Jensen W, Bokemeyer C, Wit M. Multimodal exercise training during myeloablative chemotherapy: a prospective randomized pilot trial. Supportive Care in Cancer 2014;22(1):63‐9. [DOI] [PubMed] [Google Scholar]
Peoples 2017 {published data only}
- Peoples A, Peppone L, Lin PJ, Cole C, Heckler C, Janeslins M, et al. Influence of exercise on biomarkers of muscle immune response andmitochondrial damage and their relationship with cancer‐related fatigue (crf): a URCC NCORP study. Supportive Care in Cancer. 2017, issue 2 Supplement 1:S116.
PETRA study {published data only}
- Kuehl R, Scharhag‐Rosenberger F, Schommer K, Schmidt ME, Dreger P, Huber G, et al. Exercise intensity classification in cancer patients undergoing allogeneic HCT. Medicine & Science in Sports & Exercise 2015;47(5):889‐95. [DOI] [PubMed] [Google Scholar]
- Wiskemann J, Kuehl R, Dreger P, Huber G, Kleindienst N, Ulrich CM, et al. Physical Exercise Training versus Relaxation in Allogeneic stem cell transplantation (PETRA Study) ‐ Rationale and design of a randomized trial to evaluate a yearlong exercise intervention on overall survival and side‐effects after allogeneic stem cell transplantation. BMC Cancer 2015;15:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prinsen 2013 {published data only}
- Prinsen H, Bleijenberg G, Heijmen L, Zwarts MJ, Leer JW, Heerschap A, et al. The role of physical activity and physical fitness in postcancer fatigue: a randomized controlled trial. Supportive Care in Cancer 2013;21(8):2279‐88. [DOI] [PubMed] [Google Scholar]
- Prinsen H, Dijk JP, Zwarts MJ, Leer JW, Bleijenberg G, Laarhoven HW. The role of central and peripheral muscle fatigue in postcancer fatigue: a randomized controlled trial. Journal of Pain & Symptom Management 2015;49:173‐82. [DOI] [PubMed] [Google Scholar]
Schumacher 2015a {published data only}
Shelton 2009 {published data only}
- Shelton ML, Lee JQ, Morris GS, Massey PR, Kendall DG, Munsell MF, et al. A randomized control trial of a supervised versus a self‐directed exercise program for allogeneic stem cell transplant patients. Psycho‐Oncology 2009;18(4):353‐9. [PUBMED: 19117328] [DOI] [PubMed] [Google Scholar]
Stacey 2016 {published data only}
- Stacey FG, James EL, Chapman K, Lubans DR. Social cognitive theory mediators of physical activity in a lifestyle program for cancer survivors and carers: findings from the ENRICH randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2016;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanir 2013 {published data only}
- Tanir MK, Kuguoglu S. Impact of exercise on lower activity levels in children with acute lymphoblastic leukemia: a randomized controlled trial from Turkey. Rehabilitation Nursing Journal 2013;38(1):48‐59. [PUBMED: 23365005] [DOI] [PubMed] [Google Scholar]
Thorsen 2005 {published data only}
- Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health‐related quality of life in young and middle‐aged cancer patients shortly after chemotherapy. Journal of Clinical Oncology 2005;23(10):2378‐88. [PUBMED: 15800330] [DOI] [PubMed] [Google Scholar]
Toohey 2016 {published data only}
- Toohey K, Pumpa KL, Arnolda L, Cooke J, Yip D, Craft PS, et al. A pilot study examining the effects of low‐volume high‐intensity interval training and continuous low to moderate intensity training on quality of life, functional capacity and cardiovascular risk factors in cancer survivors. PeerJ 2016; Vol. 4:e:2613. [DOI] [PMC free article] [PubMed]
Tran 2016 {published data only}
- Tran H, Lin C, Yu F, Frederick A, Mieras M, Baccaglini L. A multicenter study on the relative effectiveness of a 12‐week physical training program for adults with an oncologic diagnosis. Support Care Cancer 2016;24(9):3705‐13. [DOI] [PubMed] [Google Scholar]
Valle 2013 {published data only}
- Valle CG, Tate D F, Mayer DK, Allicock M, Cai J. A randomized trial of a Facebook‐based physical activity intervention for young adult cancer survivors. Journal of Cancer Survivorship 2013;7:355‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vallerand 2018 {published data only}
- Vallerand JR, Rhodes RE, Walker GJ, Courneya KS. Feasibility and preliminary efficacy of an exercise telephone counseling intervention for hematologic cancer survivors: a phase II randomized controlled trial. Journal of Cancer Survivorship 2018;12(3):357‐70. [DOI] [PubMed] [Google Scholar]
van Waart 2015 {published data only}
- Waart H, Stuiver MM, Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. Journal of Clinical Oncology 2015;33(17):1918‐27. [DOI] [PubMed] [Google Scholar]
Yeh 2016 {published data only}
- Chuang TY, Yeh ML, Chung YC. A nurse facilitated mind‐body interactive exercise (Chan‐Chuang qigong) improves the health status of non‐Hodgkin lymphoma patients receiving chemotherapy: Randomised controlled trial. International Journal of Nursing Studies 2017;69:25‐33. [DOI] [PubMed] [Google Scholar]
- Yeh ML, Chung YC. A randomized controlled trial of qigong on fatigue and sleep quality for non‐Hodgkin's lymphoma patients undergoing chemotherapy. European Journal of Oncology Nursing 2016;23:81‐6. [DOI] [PubMed] [Google Scholar]
Zimmer 2014 {published data only}
- Zimmer P, Baumann FT, Bloch W, Schenk A, Koliamitra C, Jensen P, et al. Impact of exercise on pro inflammatory cytokine levels and epigenetic modulations of tumor‐competitive lymphocytes in Non‐Hodgkin‐Lymphoma patients‐randomized controlled trial. European Journal of Haematology 2014;93:527‐32. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Wehrle 2018 {published data only}
References to ongoing studies
Abildgaard 2018 {published data only}
- Larsen RF, Jarden M, Minet LR, Frolund UC, Abildgaard N. Supervised and home‐based exercise in patients newly diagnosed with multiple myeloma‐a randomized controlled feasibility study. Supportive Care in Cancer. 2018; Vol. 26, issue 2 Supplement 1:S282.
Courneya 2017 {published data only}
- Improving quality of life in hematologic cancer survivors by closing the exercise intention—behavior gap: a phase II randomized controlled trial of a theory‐based, telephone‐delivered exercise counselling intervention. Ongoing study February 2017.
Oberste 2016 {published data only}
- Oberste M, Elter T, Bloch W, Baumann F, Zimmer P. The effect of a chemotherapy accompanying 4‐week aerobic endurance exercise intervention on incidence and severity of cancer related cognitive impairments in leukemia patients. A randomized controlled trial. Oncology Research and Treatment. 2016:152.
- Zimmer P, Oberste M, Bloch W, Schenk A, Joisten N, Hartig P, et al. Impact of aerobic exercise training during chemotherapy on cancer related cognitive impairments in patients suffering from acute myeloid leukemia or myelodysplastic syndrome ‐ Study protocol of a randomized placebo‐controlled trial. Contemporary Clinical Trials 2016;49:1‐5. [PUBMED: 27261170] [DOI] [PubMed] [Google Scholar]
Walsh 2005 {published data only}
- Randomised controlled trial to investigate the effects of an exercise programme on physical performance and quality of life after a bone marrow transplant. Ongoing study November 2003, no further information when the trial will be terminated in study registry clinicaltrials.gov. The status still ongoing (last access 09.12.2018).
Additional references
Altekruse 2009
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review 1975‐2007. seer.cancer.gov/csr/1975_2007/ (accessed 2nd May 2014).
Andrykowski 1989
- Andrykowski MA, Henslee PJ, Barnett RL. Longitudinal assessment of psychosocial functioning of adult survivors of allogeneic bone marrow transplantation. Bone Marrow Transplantation 1989;4(5):505‐9. [PUBMED: 2790328] [PubMed] [Google Scholar]
Broers 2000
- Broers S, Kaptein AA, Cessie S, Fibbe W, Hengeveld MW. Psychological functioning and quality of life following bone marrow transplantation: a 3‐year follow‐up study. Journal of Psychosomatic Research 2000;48(1):11‐21. [PUBMED: 10750625] [DOI] [PubMed] [Google Scholar]
Courneya 2013
- Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, et al. Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. Journal of the National Cancer Institute 2013;105:1821‐32. [DOI] [PubMed] [Google Scholar]
Cullen 2001
- Cullen M. 'Best supportive care' has had its day. Lancet Oncology 2001;2(3):173‐5. [PUBMED: 11902569] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dieli‐Conwright 2014
- Dieli‐Conwright CM, Mortimer JE, Schroeder ET, Courneya K, Demark‐Wahnefried W, Buchanan A, et al. Randomized controlled trial to evaluate the effects of combined progressive exercise on metabolic syndrome in breast cancer survivors: rationale, design, and methods. BMC Cancer 2014;14:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dimeo 1996
- Dimeo F, Bertz H, Finke J, Fetscher S, Mertelsmann R, Keul J. An aerobic exercise program for patients with haematological malignancies after bone marrow transplantation. Bone Marrow Transplantation 1996;18(6):1157‐60. [PUBMED: 8971388] [PubMed] [Google Scholar]
Dimeo 1997
- Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment‐related complications after high‐dose chemotherapy. Blood 1997;90(9):3390‐4. [PUBMED: 9345021] [PubMed] [Google Scholar]
Doyle 2006
- Doyle C, Kushi LH, Byers T, Courneya KS, Demark‐Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA: a Cancer Journal for Clinicians 2006;56:323‐53. [DOI] [PubMed] [Google Scholar]
Fife 2000
- Fife BL, Huster GA, Cornetta KG, Kennedy VN, Akard LP, Broun ER. Longitudinal study of adaptation to the stress of bone marrow transplantation. Journal of Clinical Oncology 2000;18(7):1539‐49. [PUBMED: 10735903] [DOI] [PubMed] [Google Scholar]
Friedenreich 2001
- Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiology, Biomarkers & Prevention 2001;10(4):287‐301. [PUBMED: 11319168] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman A, Schuenemann H. GRADEpro. Version 3.2 for Windows. Cochrane IMS, 2008.
Higgins 2011a
- Higgins JP, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howlader 2012
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al. SEER Cancer Statistics Review, 1975‐2009 (Vintage 2009 Populations), National Cancer Institute. seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site (accessed 2nd May 2014).
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J (editors). Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Li 2010a
- Li Q. Effect of forest bathing trips on human immune function. Environmental Health and Preventive Medicine 2010;15(1):9‐17. [PUBMED: 19568839] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2010b
- Li Q, Kobayashi M, Inagaki H, Hirata Y, Li YJ, Hirata K, et al. A day trip to a forest park increases human natural killer activity and the expression of anti‐cancer proteins in male subjects. Journal of Biological Regulators and Homeostatic Agents 2010;24(2):157‐65. [PUBMED: 20487629] [PubMed] [Google Scholar]
Liang 2018
- Liang Y, Zhou M, Wang F, Wu Z. Exercise for physical fitness, fatigue and quality of life of patients undergoing hematopoietic stem cell transplantation: a meta‐analysis of randomized controlled trials. Japanese Journal of Clinical Oncology 2018;48(12):1046‐57. [DOI] [PubMed] [Google Scholar]
McCullough 2014
- McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. Journal of the National Cancer Institute 2012;106(4):dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mock 1994
- Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney‐Tedder S, et al. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 1994;21(5):899‐908. [PUBMED: 7937251] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
NCCN 2014
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Cancer‐Related Fatigue. Version 1.2014. www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed May 2nd 2014).
Parent 2011
- Parent ME, Rousseau MC, El‐Zein M, Latreille B, Desy M, Siemiatycki J. Occupational and recreational physical activity during adult life and the risk of cancer among men. Cancer Epidemiology 2011;35(2):151‐9. [PUBMED: 21030330] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Peters 1994
- Peters C, Lotzerich H, Niemeier B, Schule K, Uhlenbruck G. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer Research 1994;14(3A):1033‐6. [PUBMED: 8074446] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schule 1983
- Schule K. The rank value of sports and movement therapy in patients with breast or pelvic cancer [Zum Stellenwert der Sport‐ und Bewegungstherapie bei Patientinnen mit Brust‐ oder Unterleibskrebs.]. Die Rehabilitation 1983;22(1):36‐9. [PUBMED: 6836164] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH (editors). Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Adressing reporting biases. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tosetto 2009
- Tosetto A, Balduini CL, Cattaneo M, Candia E, Mariani G, Molinari AC, et al. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thrombosis Research 2009;124(5):e13‐8. [PUBMED: 19631969] [DOI] [PubMed] [Google Scholar]
Velthuis 2010
- Velthuis MJ, Agasi‐Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer‐related fatigue during cancer treatment: a meta‐analysis of randomised controlled trials. Clinical Oncology 2010;22(3):208‐21. [PUBMED: 20110159] [DOI] [PubMed] [Google Scholar]
Wagner 2004
- Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. British Journal of Cancer 2004;91(5):822‐8. [PUBMED: 15238987] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2016
- Zhou Y, Zhu J, Gu Z, Yin X. Efficacy of exercise interventions in patients with acute leukemia: A meta‐analysis. PLOS One 2016;11(7):e0159966. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Bergenthal 2011
- Bergenthal N, Engert A, Wolkewitz KD, Monsef I, Kluge S, Skoetz N. The role of physical exercise for adult patients with haematological malignancies. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD009075; CD009075] [DOI] [PubMed] [Google Scholar]


