Abstract
We investigated the biological impacts of Indigenous residential school attendance on the adult children of survivors, operationalized through allostatic load (AL); and the extent to which intergenerational trauma, operationalized through adverse childhood experience (ACE) score, mediated this association. Data were collected in-person from a university-based sample of Indigenous adults (N = 90, mean age: 28 years) in a mid-sized city in western Canada between 2015 and 2016. Associations were analyzed in multinominal regression models, with terciled AL and ACE scores as outcomes. The cross-products of coefficients method was used to test mediation. Overall, 42.7% and 33.7% reported their mother and father had attended residential school; respectively. In an adjusted model, maternal, but not paternal, residential school attendance was a risk factor associated with a moderate increase in AL among her adult children. The strength of this association did not change when the analysis was limited to mothers who raised their children. Maternal and paternal residential school attendance were each associated with increased ACE score among adults raised by survivors. However, ACE score did not explain the association between maternal residential school attendance and offspring AL score in mediational analyses. The present findings suggest colonial residential school experiences may have become biologically embedded, passed to subsequent generations, and exhibited through the dysregulation of allostatic systems among the adult children of maternal residential school survivors. Maternal exposure to residential school influenced biological dysregulation among her adult children in ways that could not be further exacerbated by her children's exposure to ACEs. The Canadian Truth and Reconciliation Commission asked governments to acknowledge the impact of residential schools on the current state of Indigenous health. Our findings underline the importance of this call by demonstrating how the residential school experience may get under the skin to impact the health of the next generation.
Keywords: Residential school, Indigenous, Allostatic load, ACEs, Intergenerational trauma
Highlights
-
•
Adults of mothers who attended Indigenous residential school had increased biologic dysregulation.
-
•
Adults of mothers and fathers who attended Indigenous residential school had increased ACEs.
-
•
ACEs did not explain biological dysregulation among the children of maternal residential school survivors.
1. Introduction
Internationally, there remains a paucity of published research on the determinants of health within Indigenous populations, despite wide ranging health inequalities and lower life expectancy across the majority of the more than 370 million Indigenous people who live in 90 countries worldwide (Anderson et al., 2016; King, Smith, & Gracey, 2009). This study examines the impact of residential school, a common social stressor experienced by many Indigenous populations worldwide, on biological health in the next generation.
Residential schools, also referred to as boarding schools, were a colonial method of Indigenous assimilation in the United States, Canada, Australia, New Zealand, and other countries. Attendance at residential school resulted in the widespread abuse and neglect of many Indigenous children, yet the biological consequences of this colonial practice on survivors and their offspring is not well understood. In Canada, the objectives of the residential school system, operating from the 1870s to the 1990s, were to isolate Indigenous children from their families and assimilate them into settler culture (Sinclair, 2015). In the present study we examined the biological impacts of residential school attendance on the adult children of survivors.
The parents of the majority of participants in this study would have attended residential school between the mid-1940s and the early-1970s. During these decades, Canada was undergoing unprecedented economic growth and prosperity, yet there was little change in the functioning of the residential school system during this time (Truth and Reconciliation Commission of Canada, 2015, Truth and Reconciliation Commission of Canada, 2016). Indigenous children in the schools remained poorly housed, fed, clothed, and educated (Truth and Reconciliation Commission of Canada, 2016). There were no clear education goals for children, with the curriculum set by the church running each particular school. Church staff and teachers were poorly trained and not adequately supervised. Indigenous languages, cultures, and spiritual practices were denigrated and suppressed (Truth and Reconciliation Commission of Canada, 2016). Physical abuse, emotional abuse, and hunger were used to control children (Fast & Vézina, 2010), and the sexual abuse of Indigenous children, by both male and female staff, was widespread (Milloy, 2006; Mosby, 2013). Many children were separated from their parents and communities by long distances that made visits home difficult. Nutritional experiments sanctioned by the Canadian government and carried out against the will of Indigenous children between 1942-1952, were designed to take advantage of widespread malnutrition across the majority of Canadian residential schools, in order to understand the impacts of starvation on a child’s body; the findings of which were not used to improve nutrition for Indigenous children in the schools (Mosby, 2013). When the compulsory attendance of Indigenous children in residential school ended in 1948, it was replaced by the forced placement of children in the schools by child welfare agencies (Sinclair, 2015). By 1960, the Canadian government estimated that 50% of Indigenous children in residential school were placed there for the “best interest of the child”, the interpretation of which was wrought with cultural bias in a system dominated by white, middle class workers, boards of directors, administrators, lawyers and judges (Kimelman, 1985). Many other families were given no option but residential school for their children given some form of school attendance was compulsory in Canada, and no other schools were made available in their territory. In 1969, the Canadian government was forced to take over full responsibility for the schools from the churches due to a federal labor board ruling (Truth and Reconciliation Commission of Canada, 2016). Over time, the schools were gradually closed, excluding the few turned over to, and overhauled by, Indigenous communities in the mid to late 1970s.
To date, most research has examined the psychological impacts of residential school attendance on the children of survivors, documenting increased depressive symptoms and suicide ideation and attempts among the adult children and grandchildren of residential school survivors (Bombay et al., 2011, Elias et al., 2012, McQuaid et al., 2017, Bombay et al., 2014). Research has also documented impacts on the physical health of survivors (Running Bear et al., 2018). A recent scoping review called for empirical studies that help us develop a clearer understanding of the aetiology of these effects (Wilk, Maltby, & Cooke, 2017). The present study answers this call by hypothesizing that parental residential school attendance affects the next generation through two mechanisms: (1) altered allostatic load among offspring, a marker of biological dysfunction; and (2) the transmission of intergenerational trauma, a marker of social dysfunction.
1.1. Residential school attendance and offspring allostatic load
The first objective of this study was to assess if parental residential school attendance was associated with increased allostatic load score among the children of survivors. Conceptually, the allostatic load (AL) framework has been used to describe cumulative, multisystem strain on the body produced through the elevated activity of physiologic systems under challenge, and the changes in functioning it can predispose (McEwen and Stellar, 1993, Seeman, 1997). Allostasis describes the physiological processes that allow the body to achieve stability despite environmental change and challenge (McEwen, 1998). Allostatic processes are coordinated by the neuroendocrine system, particularly the hypothalamic-pituitary-adrenal axis (HPA) and the sympathetic-adrenal-medullary (SAM) axis. The primary mediators of allostasis include epinephrine, norepinephrine, cortisol, dehydroepiandrosterone sulfate (DHEA-S), and both pro- and anti-inflammatory cytokines. These mediators interact and coordinate adaptive regulatory processes that enable the body to respond appropriately to environmental challenges. The concept of AL was developed by McEwen (1998) to describe the physiological dysregulation that can occur from high, unremitting stress including hypoactive or hyperactive responses to environmental triggers, an inability to turn off the stress response when it is no longer needed, and ultimately, primary mediators that chronically deviate from their normal range. These influence secondary outcomes such as blood pressure and inflammation, which then go on to influence tertiary outcomes of morbidity and mortality (Beckie, 2012). Thus, high AL can be characterized to represent multi-system physiologic wear and tear and dysregulation.
1.2. Residential school attendance and offspring ACE score
Our secondary objectives were, first to assess if parental residential school attendance was associated with increased intergenerational trauma, operationalized through ACE scores among children raised by survivors; and second to determine the extent to which ACEs mediated potential associations between residential school attendance and offspring AL. An adverse childhood experience (ACE) score is a measure of child emotional, physical and sexual abuse; child emotional and physical neglect; and mental illness, addiction, divorce, incarceration of a family member, and domestic violence in a household experienced before the age of 18. An ACE score typically ranges from zero to 10, with each type of ACE experienced regardless of frequency, counted as one point (Felitti et al., 1998). A growing body of research highlights ACEs as a critical public health issue across populations internationally (Sacks & Murphey, 2018). Direct links have been found between childhood trauma, as measured by an ACE score, and adult onset of chronic disease, mental illness, addiction, and premature death; with the risk for negative outcomes increasing with the number of ACEs experienced (Chartier et al., 2010, Mersky et al., 2013).
One of the most enduring predictors of parenting behavior in published studies is how parents were parented themselves (Knutson, 1995, Roberts et al., 2004, Scaramella et al., 2008, Madden et al., 2015, Lomanowska et al., 2015). Given this research, we would expect that residential schools have had a profound and negative impact on parenting as it is well documented that survivors were taught neglectful and abusive disciplinary practices through observation and direct experience, which were not part of their traditional cultures (Chansonneuve, 2005, Lindstrom et al., 2016). A seminal study published by Felitti et al. (1998) found adults who had experienced four or more ACEs were at particularly high risk for mental illness, addiction, disease and death. Yet it is our opinion, based on evidence published about child experiences within the schools, that the average ACE score of a residential school survivor is at least six, given the majority report emotional, physical and sexual abuse by staff and clergy in the schools; emotional and physical neglect including systemic malnutrition; and forced removal from their families to an institution, which may be akin to the incarceration of a family member (Sinclair, 2015, Mosby, 2013). Thus, this study examined the extent to which parental residential school attendance was associated with ACE score among the children of survivors.
2. Methods
2.1. Indigenous Advisory Committee
An urban Indigenous Advisory Committee made up of key Elders and members of the Indigenous community in the Lethbridge area, located in Treaty 7 territory, was assembled in 2013 as the conceptualization of this project was being developed. The Committee worked with the research team to set study priorities and make data collection decisions. As a group, we determined that in-person data would be collected from post-secondary students who self-identified as First Nations, Métis, Inuit or Indigenous generally. Working with the Committee, it was determined that salivary rather than blood samples would be taken given blood is a sacred element in many Indigenous cultures and must be respected in ways that are often incompatible with scientific research. This belief ties in with Indigenous holistic beliefs about the body, self, culture and nature. As saliva is also a substance that comes from the body, a system was put in place in consultation with the Indigenous Advisory Committee to ensure the wishes of Indigenous participants were honoured. The consent form provided participants the option of having their saliva samples returned to them upon analysis or to have their saliva samples included in an Indigenous ceremony led by an Elder that returned the samples back to the Earth.
2.2. Sampling
Costs associated with obtaining a random sample were not feasible given Indigenous peoples represent less than 5% of the population in this territory. Instead, participants were recruited using posters and ads placed in e-newsletters on campus at the University of Lethbridge. To increase generalizability snowball sampling techniques were avoided. No advertising took place in psychological or health treatment centres. Participant recruitment and data collection for the present paper began in September 2015 and ended in April 2016. The final sample used for this analysis was 114 Indigenous adults.
2.3. Procedure
2.3.1. In office
Study procedures were approved by the Office of Research Ethics at the University of Lethbridge. Written consent was obtained from all participants. After screening for eligibility by email or phone (i.e., that participants identified as Indigenous, were current post-secondary students, and were 18 years or older), participants attended an on-campus study office to complete consent procedures, paper-and pencil surveys, and physical assessments needed to calculate AL score (mean completion time = 90 min). Saliva samples were collected during the first office visit at 3 time points using the passive drool technique. Participants rinsed their mouth with bottled water upon arrival and the first sample was collected after completion of the demographic portion of the questionnaire. Remaining samples were taken 30 and 60 min after questionnaire completion.
Whole saliva samples were collected in a 2 ml microcentrifuge tube using a Saliva Collection Aid (Salimetrics, State College, PA), a collection device specifically designed to improve volume collection and increase participant compliance. During data collection, salivary samples were stored in the in-office freezer and then transferred immediately to a −80 °C freezer. At the end of the session participants were provided with supplies for collecting saliva samples at home.
2.3.2. At home
At home, participants were asked to select 2 consecutive days in which they would have similar wake and sleep times. On each of these days, they were asked to collect a saliva sample at three time points: immediately upon wake-up, 30 min after wake-up, and before bed, and to record the times in which samples were taken on the forms provided. Participants were instructed to place the swab under the tongue for three minutes and then place the swab in a pre-labeled tube and put it in their freezer. When all six samples were collected, the participant contacted the research assistant who had collected their in office data to coordinate sample return. We used cortisol awakening response (CAR) expert consensus guidelines to increase adherence including clearly explaining the importance of adherence to sampling times, emphasizing the importance of collecting S1 immediately upon awakening, encouraging participants to ask questions via text message/email/phone, providing take-home instructions, having participants record data collection time points in a diary log, advising participants to place kits beside the bed for morning collection, and text messaging the evening before sampling to highlight instructions (Stalder et al., 2016). Participants returned the samples in an insulated lunch kit with a freezer pack given to them during the in-office visit. Samples received were immediately transferred to a -80C freezer. Participants were given an honorarium in two installments: $50 for completing in-office measures, and $50 for returning at-home measures.
2.4. Measures
2.4.1. Parental residential school attendance
Participants were asked: “Did you have any family members attend residential school (check all that apply)?” Response options were mother, father, one or both grandmothers, one or both grandfathers, one or more aunts, one or more uncles, or no relatives attended. To better understand the residential school experience, and impacts on the next generation, participants who indicated a relative had attended residential school were asked 6 questions: (1) Would you say that attending a residential school for your family member was a: (response options: very negative, negative, somewhat positive or very positive experience); (2) Do you feel you have been personally impacted in negative ways because your family member(s) attended residential school (response options: yes, no); (3) do you feel that the way you were parented and cared for as a child was influenced by residential school experiences in your family (response options: yes, no); (4) has the way your family handles or copes with stress been impacted by experiences at residential school (response options: yes, no); (5) has the way you handle or cope with stress been impacted by experiences of residential school in your family (response options: yes, no); and (6) are issues related to residential school openly discussed in your family (response options: yes, no).
2.4.2. Allostatic Load (AL)
Allostatic load has been operationalized using a range of biomarkers; there is no consensus on which biomarkers are necessary and studies vary widely in measurements used (Beckie, 2012, Juster et al., 2010). A recent systematic review by Johnson et al. (2017) called for a more critical approach of the calculation of AL to ensure indices used capture the biological effects of psychosocial stress rather than markers of physiologic dysfunction more generally (Johnson, Cavallaro, & Leon, 2017). Taking this into consideration, we operationalized AL using markers from the three biological domains that framed the original AL index. A measure of inflammation was added given universal agreement since that time that biomarkers from the immune system should be included in AL calculations (Segerstrom and Miller, 2004, Juster et al., 2016). Thus, AL score in this study was based on a composite of seven biomarkers across four biological domains including:
-
1.
Cardiovascular: Resting systolic and diastolic blood pressure were measured using a Life Source automated sphygmomanometer (Auto Control Medical, Mississauga, ON). Participants were seated and resting quietly for three sets of two readings taken approximately 10 min apart. The second measure taken in each set was summed and the average of the systolic reading and the diastolic reading were each used in the AL score.
-
2.
Neuroendocrine markers included DHEA-S and CAR. Previous research has confirmed the validity of salivary DHEA-S measurement and its association with psychological stress and health (Duong et al. 2017). DHEA-S was collected through in-office saliva sampling and analyzed using Enzyme-Linked Immunosorbent Assays with kits from Salimetrics (Salimetrics, State College, PA). As per manufacturer’s suggestion for DHEA-S, the three in-office samples were pooled and mixed for analysis. To examine CAR, the wake-up (S1) and 30 minutes post wake-up (S2) samples taken at home on the second day, and the percent change in cortisol between S1 and S2 was calculated. CAR represents the sharp rise in cortisol levels across the first 30-45 min following morning awakening. In healthy adults, the magnitude of CAR ranges between a 50-156% increase in salivary cortisol levels (Clow, Thorn, Evans, & Hucklebridge, 2004). The mean magnitude of CAR was 59.6% in this sample (Table 1).
-
3.
Metabolic markers included body mass index (BMI) and waist circumference. To calculate BMI, height and weight were measured to the nearest 0.5 cm using a Health O Meter mechanical beam scale and height rod. Waist circumference was measured at the top of the iliac crest, to the nearest 0.5 cm.
-
4.
Immune: Previous research has confirmed the validity of salivary CRP measurement and its association with psychological stress and health (Dhingra et al., 2007, Truba et al., 2018, Van Dyke et al., 2017). We measured CRP using the third in-office saliva sample and analyzed the biomarker using the Enzyme-linked Immunosorbent assays (ELISAs) from Salimetrics LLC, Carlsbad, CA, USA.
Table 1.
Mean, range and cut-points used for allostatic load (AL) biomarkers (N = 90).
| Biomarker | Range | Mean (SD) | Cut-point female | Cut-point male |
|---|---|---|---|---|
| 1. Cardiovascular | ||||
| SBP (mm Hg) | 90–145 | 118.0 (13.1) | >126 | >137 |
| DBP (mm Hg) | 57–102 | 77.5 (10.6) | >86 | >86 |
| 2. Neuroendocrine | ||||
| DHEA-S (ug/dL) | 188.5–13069.7 | 3682.3 (2644.3) | <1610.9 | <2376.4 |
| CAR | -85.9–748.7 | 59.6 (153.8) | <34.8 or >139.0 | <34.8 or >139.0 |
| 3. Metabolic | ||||
| BMI (kg/m2) | 19.2–48.5 | 29.5 (7.0) | >34.0 | >34.0 |
| Waist circumference (cm) | 68.9–145.5 | 97.8 (17.2) | >110.7 | >110.7 |
| 4. Immune | ||||
| CRP (pg/ml) | 52.9–2884.8 | 415.2 (497.7) | >394.3 | >403.8 |
| Total AL Score | 0–6 | 2.1 (1.5) |
*BP= SBP= systolic blood pressure; DBP= diastolic blood pressure; DHEA-S= dehydroepiandrosterone-sulfate; CAR= cortisol awakening response; BMI= Body mass index; CRP=C-reactive protein.
Cutpoints for each biomarker are displayed in Table 1. All samples were analyzed in duplicate and samples for each participant were analyzed in the same assay to minimize the effects of inter-assay variability. AL score was based on the distribution of the sample for all biomarkers. To create an allostatic load score, all biomarkers but CAR were divided into sex-specific quartiles with high risk defined by the highest quartile for BMI, WC, BP, and CRP. For DHEA-S the lowest quartile was used. For CAR, the lowest and highest octiles defined high risk based on previous studies associating low and high levels with adverse health outcomes (Juster et al., 2013, Gustafsson et al., 2014).
2.4.3. Mediating variable: Adverse childhood experiences (ACEs)
ACEs were assessed using 10 standard questions based on the original ACE questionnaire (Felitti et al., 1998). Response options were yes (1 point) and no (0 points). ACE score was calculated by summing the number of yes responses (Felitti et al., 1998, Sotero, 2015).
2.4.4. Covariates
We measured offspring age and gender, parental educational attainment (i.e., grade 9 or less, some high school, high school diploma, some college or university, or college and university degree), and perceived socioeconomic status of offspring (i.e., the participant) as a child (i.e., upper income, upper-middle income, middle income, lower-middle income, and lower income). Income was asked in this manner to improve validity, given university students may not know their household income as a child, and given previous Indigenous research documenting low missing values when SES was measured in this way (Currie et al., 2011, Currie et al., 2013).
2.5. Missing data
Data were collected from 114 participants, 11 of which were removed for not complying with guidelines for the collection of at-home salivary samples. An additional 11 participants were removed because they personally attended residential school, the confounding impacts of which would be difficult to control in statistical models. Finally, 2 participants were removed because they did not know if their parents had attended residential school, resulting in a final sample size of N = 90.
2.6. Analysis strategy
To examine associations between parental residential school attendance and offspring allostatic load, AL score was collapsed into terciles given the non-normality of the distribution. Perfectly sized terciles could not be achieved given the distribution of scores. Thus, 38% of the sample had AL scores of 0 to 1 and were categorized into the low AL group, 40% had AL scores of 2 to 3 and were categorized into the moderately elevated AL group, and 22% had scores of 4 to 7 and were categorized into the high AL group. Associations between residential school attendance and offspring AL were examined via multinominal regression models and 95% confidence intervals (CIs) adjusted for covariates selected a priori based on existing literature including current participant age, gender, socioeconomic status as a child, and parental education. Associations between parental residential school attendance and offspring ACE score were examined using multinominal regression adjusted for confounders, given the non-normality of the ACE score distribution. Perfectly sized terciles could not be achieved given the ACE score distribution. Thus, 38% of the sample had ACE scores of 0 to 2 and were categorized into the low ACE group, 39% had ACE scores of 3 to 5 and were categorized into the moderately elevated ACE group, and 23% had ACE scores of 6 or more and were categorized into the highly elevated ACE group. We planned to analyze mediation by ACE score using the cross-products of coefficients method by Hayes & Little.
3. Results
3.1. Sample characteristics
The mean age of the sample was 28 years (SD = 8.4, range = 18 to 57 years). As shown in Table 2, 77% of the sample were female which is consistent with higher numbers of Indigenous women compared to men who are completing post-secondary training in Canada (Arriagada, 2016). Most participants (63%) identified as First Nations. One in two participants were single. Most (60%) grew up in lower-middle or low-income households.
Table 2.
Characteristics of the sample.
| Characteristic | Sample N (%) |
|---|---|
| Total Sample | 90 (100%) |
| Gender | |
| Men | 21 (23.3%) |
| Women | 69 (76.7%) |
| Age | |
| 18–24 | 38 (42.2%) |
| 25–34 | 32 (35.6%) |
| 35+ | 20 (22.2%) |
| Indigenous Group | |
| Aboriginal | 19 (21.1%) |
| First Nation | 57 (63.3%) |
| Metis | 14 (15.6%) |
| Marital Status | |
| Married | 9 (10.0%) |
| Common law | 29 (32.2%) |
| Widowed/Divorced/Separated | 7 (7.8%) |
| Single | 45 (50.0%) |
| Maternal Education | |
| Less than secondary grad | 14 (15.7%) |
| Secondary grad | 14 (15.7%) |
| Some post-secondary | 29 (32.6%) |
| Post-secondary grad | 31 (34.8%) |
| Paternal Education | |
| Less than secondary grad | 24 (27.3%) |
| Secondary grad | 15 (17.0%) |
| Some post-secondary | 19 (21.6%) |
| Post-secondary grad | 22 (25.0%) |
| Socioeconomic Status as a Child | |
| Upper to upper-middle income household | 9 (10.0%) |
| Middle income household | 28 (31.1%) |
| Lower-middle income household | 29 (32.2%) |
| Low income household | 24 (26.7%) |
| Residential School Attendance | |
| Mother attended | 38 (42.7%) |
| Father attended | 30 (33.7%) |
| One or both grandmothers attended | 55 (61.8%) |
| One or both grandfathers attended | 44 (49.4%) |
As shown in Table 2, 42.7% of participants had a mother and 33.7% had a father who attended residential school. Within these subsamples, 85.7% were raised by a mother and 66.7% were raised by a father who attended residential school. More generally, familial exposure to residential school in the sample was high with 85% reporting that at least one of their parents, grandparents, aunts, or uncles had attended. Among those with familial exposure to residential school (n = 77), 28% reported one family member had attended, and 16% reported four or more family members had attended (Mean = 1.88 family members, SD = 1.28). Overall, 97.4% of participants indicated that attending residential school was a negative experience for their relatives. Approximately 8 in 10 Indigenous university students indicated they were personally impacted because one or more family members had attended residential school (79.3%), including 61.6% who indicated that the way they were parented and cared for as children was influenced by residential school experiences in their families. Approximately 85% believed that the way their family handled stress had been impacted by experiences at residential school. Two thirds also believed that the way they personally handled stress was being impacted by their relatives’ experiences at residential school. Despite these problems, most participants (62.8%) indicated that residential school experiences were not openly discussed in their family.
AL scores ranged from 0 to 6 (median = 2.1, SD = 1.5) in the sample. Most participants (62%) had an AL score of 2 or less. Overall, 38% of the sample had AL scores of 0 to 1, 40% had AL scores of 2 to 3, and 22% had scores of 4 to 7.
3.2. Residential school attendance and AL score
The first objective of this paper was to examine if parent residential school attendance was associated with offspring AL score. We also examined grandparent residential school attendance given 61.8% had at least one grandmother who attended, and 49.4% had at least one grandfather who attended. As shown in Table 3, in separate multinominal regression models adjusted for participant age, gender, and SES as a child, grandmother and grandfather residential school attendance were not associated with AL score for their adult grandchildren. Paternal attendance was also not associated with offspring AL score. In an unadjusted model, participants who had a mother attend residential school were 5.0 times more likely to have moderately increased AL scores than participants who did not have a mother attend residential school. After adjustment for offspring age, gender, SES as a child, and maternal education, the strength of the odds ratio increased to 12.6 suggesting confounders had masked the strength of this association in the unadjusted analysis. This association did not extend to the high AL category. That is, while participants who had a mother attend residential school were more likely to have an AL score in the moderately elevated range, there were no more likely to have an AL score in the high range than other participants, suggesting a non-linear association and/or the analysis was underpowered to test this association given there were n = 20 adults in the high AL group.
Table 3.
Unadjusted and adjusted odds ratios of adult offspring allostatic load category by parent and grand-parent residential school attendance (N = 90)*.
| Model 1 |
Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Moderate AL OR (95% CI) | SE | High AL OR (95% CI) | SE | Moderate AL OR (95% CI) | SE | High AL OR (95% CI) | SE |
| Mother attendance | 5.0 [1.70, 14.53] | 0.55 | 0.65 [0.21, 2.03] | 0.59 | 12.6 [2.94, 53.84] | 0.74 | 2.70 [0.59, 12.4] | 0.78 |
| Offspring age | 1.15 [1.04, 1.28] | 0.05 | 1.24 [1.10, 1.39] | 0.06 | ||||
| Offspring gender | 0.63 [0.16, 2.44] | 0.69 | 1.94 [0.33, 11.3] | 0.82 | ||||
| Mother’s education | 2.12 [1.23, 3.68] | 0.28 | 1.61 [0.96, 2.72] | 0.27 | ||||
| Perceived SES as a child | 1.05 [0.55, 2.03] | 0.33 | 1.42 [0.66, 3.04] | 0.39 | ||||
| Father attendance | 0.77 [0.27, 2.21] | 0.54 | 2.81 [0.89, 8.90] | 0.59 | 0.59 [0.18, 1.97] | 0.62 | 1.55 [0.38, 6.37] | 0.72 |
| Offspring age | 1.05 [0.97, 1.14] | 0.04 | 1.18 [1.08, 1.30] | 0.05 | ||||
| Offspring gender | 0.66 [0.20, 2.25] | 0.62 | 1.85 [0.30, 11.5] | 0.84 | ||||
| Father’s education | 1.33 [0.94, 1.89] | 0.18 | 0.89 [0.58, 1.35] | 0.22 | ||||
| Perceived SES as a child | 0.81 [0.45, 1.45] | 0.30 | 0.92 [0.42, 2.02] | 0.40 | ||||
| Grandmother attendance | 2.06 [0.77, 5.54] | 0.51 | 1.24 [0.38, 4.04] | 0.60 | 1.74 [0.61, 4.96] | 0.53 | 0.91 [0.22, 3.75] | 0.72 |
| Offspring age | 1.05 [0.97, 1.13] | 0.04 | 1.18 [1.08, 1.30] | 0.05 | ||||
| Offspring gender | 0.87 [0.26, 2.87] | 0.61 | 1.93 [0.35, 10.8] | 0.88 | ||||
| Perceived SES as a child | 0.69 [0.40, 1.19] | 0.29 | 1.02 [0.51, 2.06] | 0.36 | ||||
| Grandfather attendance | 1.07 [0.41, 2.73] | 0.48 | 1.06 [0.35, 3.23] | 0.56 | 1.19 [0.45, 3.17] | 0.50 | 1.71 [0.68, 7.98] | 0.66 |
| Offspring age | 1.05 [0.98, 1.13] | 0.04 | 1.19 [1.09, 1.30] | 0.05 | ||||
| Offspring gender | 0.73 [0.23, 2.34] | 0.59 | 2.01 [0.37, 10.9] | 0.86 | ||||
| Perceived SES as a child | 0.64 [0.37, 1.11] | 0.28 | 1.02 [0.51, 2.04] | 0.36 | ||||
Significant results (p < 0.05) presented in bold. Outcome variable (AL category – low, moderate, high) used the low AL category as the reference group for analysis. Model 1 presents unadjusted estimates. Model 2 presents estimates adjusted for adult offspring age, offspring gender, perceived socioeconomic status as a child (as reported by offspring), and parental education. Grandparent education was not controlled in modelled because it was not measured.
To better understand these findings, a post hoc analysis limited the sample to participants raised by their biological parents. There was little change in the result. First, the sample was limited to participants raised by their biological mothers (N = 76). As shown in Table 4, in an adjusted analysis, participants raised by a mother who attended residential school (caregiver mother attendance) were 12.3 times more likely to have an AL score in the mid-range, but no more likely to have AL scores in the high range than participants raised by mothers who did not attend residential school. Next, the sample was limited to participants raised by their biological fathers (with or without their biological mothers, N = 72). Paternal residential school attendance continued to have no impact on offspring AL score (analysis not shown). The sample size did not permit limiting the sample to participants who were not raised by their biological parents.
Table 4.
Unadjusted and adjusted odds ratios of adult offspring allostatic load category by parental residential school attendance among those raised by their parents.
| Model 1 |
Model 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Moderate AL OR (95% CI) | SE | High AL OR (95% CI) | SE | Moderate AL OR (95% CI) | SE | High AL OR (95% CI) | SE |
| Caregiver mother attendance | 4.67 [1.48, 14.8] | 0.59 | 0.55 [0.16, 1.89] | 0.60 | 12.3 [2.59, 58.5] | 0.80 | 2.17 [0.43, 11.0] | 0.83 |
| Offspring age | 1.18 [1.05, 1.31] | 0.06 | 1.25 [1.11, 1.41] | 0.06 | ||||
| Offspring gender | 0.65 [0.16, 2.63] | 0.71 | 3.62 [0.48, 27.5] | 1.0 | ||||
| Mother’s education | 0.78 [0.39, 1.53] | 0.35 | 1.06 [0.51, 2.21] | 0.38 | ||||
| Perceived SES as a child | 1.98 [1.09, 3.62] | 0.31 | 1.43 [0.78, 2.62] | 0.31 | ||||
| Caregiver father attendance | 1.29 [0.42, 3.95] | 0.57 | 2.57 [0.68, 9.68] | 0.68 | 0.78 [0.19, 3.15] | 0.71 | 1.49 [0.26, 8.73] | 0.90 |
| Offspring age | 1.05 [0.96, 1.16] | 0.05 | 1.26 [1.11, 1.43] | 0.06 | ||||
| Offspring gender | 0.84 [0.23, 3.13] | 0.67 | 2.11 [0.27, 16.74] | 1.06 | ||||
| Father’s education | 0.76 [0.41, 1.41] | 0.32 | 0.92 [0.35, 2.41] | 0.49 | ||||
| Perceived SES as a child | 1.53 [1.04, 2.26] | 0.20 | 1.03 [0.61, 1.73] | 0.27 | ||||
*(n = 76 and n = 72 for caregiver mother and father attendance; respectively. Significant results (p < 0.05) presented in bold. Outcome variable (AL category – low, moderate, high) used the low AL category as the reference group for analysis. Model 1 presents unadjusted estimates. Model 2 presents estimates adjusted for adult offspring age, offspring gender, parental education, and perceived socioeconomic status as a child (as reported by offspring).
3.3. Residential school attendance and ACE score
The second objective of this study was to examine if parental residential school attendance was associated with elevated ACE scores among offspring raised by one or both of their biological parents. Participants who had a mother or father attend residential school had significantly higher ACE scores (M = 5 ACEs) than participants who did not have a mother or father attend residential school (M = 3 ACEs; independent samples t-test for mothers = 3.67, df =73, p = 0.001; independent samples t-test for fathers = 4.10, df = 53, p = 0.001). The most common ACEs experienced by children raised by mothers or fathers who attended residential school were parental divorce, living with a caregiver with a mental illness, living with a caregiver with an addiction, and living with a caregiver who was emotionally abusive.
3.4. Mediation testing
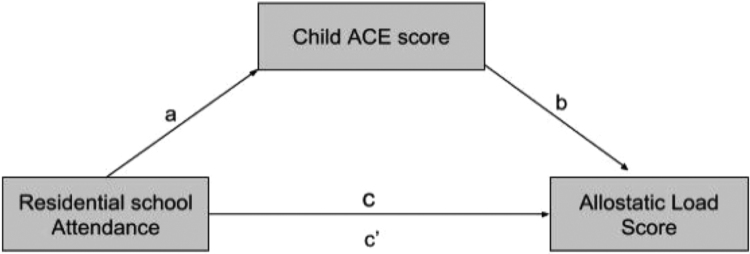
The third objective of this study was to test whether elevated ACE scores could explain the significant association observed between maternal residential school attendance and AL among adults raised by their mothers. Using the Hayes method, we first tested pathway a (Fig. 1) in an adjusted model (Hayes & Little). The association between maternal residential school attendance and offspring ACE score (as an outcome) was tested in a multinominal regression model adjusted for participant age, gender, perceived SES as a child and maternal education. As shown in Table 5, adults raised by a mother who attended residential school were significantly more likely to have highly elevated ACE scores (6+ ACEs), but no more likely to have moderately elevated ACE scores (3–5 ACEs), compared to adults raised by mothers who did not attend residential school (N = 76).
Fig. 1.
Hypothesized mediation pathway.
Table 5.
Adjusted odds ratios of adult offspring ACEs by maternal residential school attendance among those raised by their mothers (n = 76).
| Variables | Moderate ACEs OR (95% CI) | SE | High ACEs OR (95% CI) | SE |
|---|---|---|---|---|
| Caregiver mother attendance | 2.52 [0.66, 9.66] | 0.70 | 4.59 [1.10, 19.2] | 0.73 |
| Age | 0.97 [0.90, 1.05] | 0.39 | 1.00 [0.92, 1.08] | 0.04 |
| Gender | 2.24 [0.56, 8.96] | 0.71 | 2.00 [0.44, 9.11] | 0.77 |
| Mother’s education | 0.96 [0.60, 1.53] | 0.24 | 0.93 [0.56, 1.54] | 0.26 |
| Perceived SES as a child | 2.52 [1.29, 4.91] | 0.34 | 2.41 [1.14, 5.07] | 0.38 |
*Significant results (p < 0.05) presented in bold. Outcome variable (ACE category – low, moderate, high) used the low ACE category (0–2 ACEs) as the reference group for analysis. Model presents estimates adjusted for adult offspring age, offspring gender, maternal education, and perceived socioeconomic status as a child (as reported by offspring).
Next, the association between offspring ACE score and AL score as adults was examined. Participants with low, moderately elevated, and highly elevated ACEs had AL scores of 1.9, 2.3 and 2.1; respectively, suggesting adverse childhood experiences did not have a significant impact on adult AL in this sample. This was confirmed in a multinominal regression model adjusted for participant age, gender, perceived SES as a child, and maternal education (i.e., a test of pathway b, Fig. 1). Results indicate ACE score was not significantly associated with adult AL score (OR = 0.95, 95% CI 0.76, 1.19 and OR = 1.02, 95% CI 0.77, 1.34 for mid and high range AL scores; respectively). The final test of the ab pathway was not conducted given pathway b was not significant, thus ACE score could not mediate the association between maternal residential school attendance and offspring AL. We conducted several post hoc tests to confirm the result. First, participant ACE score was entered as a control variable in the multinominal regression models reported in Table 3, Table 4. Results demonstrated no change in the significance or strength of the association between maternal residential school attendance and offspring AL score when offspring ACE score was controlled (analyses not shown). We also examined post hoc correlations between childhood ACE score and the continuous form of each individual AL marker using Lowess curves and partial correlations, adjusting for age and gender. There were no statistically significant associations between ACE score and the seven adult AL biomarkers examined in this study with or without adjustment for age, nor were non-linear U or inverse-U associations observed that might account for these findings.
4. Discussion
Despite the young age of this adult sample, most (85%) had relatives who attended residential school, highlighting the ongoing impact of colonization on the present generation of Indigenous Canadians. Results of this study go beyond subjective distress to highlight moderate impairment in subclinical indices of physiologic regulation among the adult offspring of maternal residential school survivors. The aggregate measure of physiologic dysregulation used in this study suggests Indigenous adults who had a biological mother who attended residential school were experiencing early and more pronounced wear and tear on stress response systems than Indigenous peers who did not have a biological mother attend residential school. The strength and significance of this association did not change when the sample was limited to maternal survivors who raised their children, nor was it explained by ACE scores among adults raised by mothers who attended residential school.
This research builds on previous studies examining the deleterious biological impacts of residential school attendance on the physical health of surivors, as well as studies documenting the biological impacts of the Dutch famine and the Holocaust on the offspring of survivors (Running Bear et al., 2018; Yehuda & Bierer 2008; Yehuda, Bierer, Andrew, Schmeidler, & Seckl, 2009). The present findings are suggestive of the ways in which childhood trauma and systemic malnutrition in Canadian residential schools may have become biologically embedded, passed to subsequent generations, and exhibited through the dysregulation of allostatic systems in the next generation. Although it is possible that these findings reflect the intergenerational transmission of residential school trauma through epigenetic processes, caution is needed in the interpretation of the results. Blood samples and DNA information were not collected in this study; thus epigenetic processes could not be directly examined. However, these findings do provide strong justification for future research designed to examine the epigenetic impacts of parental residential school attendance on the children of survivors.
Consistent with research across populations highlighting that the most profound influence on parenting is the way an individual was parented themselves, this study found Indigenous adults raised by biological parents who had attended residential school had significantly higher ACE scores than Indigenous adults raised by biological parents who had not attended the schools (Roberts et al., 2004, Lomanowska et al., 2015). While we did not directly measure the experiences of mothers who attended residential school in this study, 97.4% of participants indicated that attending residential school was a negative experience for their family members generally. This is consistent with overwhelming evidence from the testimonies of thousands of residential school survivors across Canada suggest most experienced widespread abuse and neglect (Sinclair, 2015). The weight of this evidence suggests most residential school survivors have ACE scores that far exceed the score of three, which was highlighted by Felitti et al. (1998) in his seminal work on ACEs, as the threshold after which significant increases in behavioral dysfunction, morbidity, and mortality in adulthood become very apparent. The most common ACEs experienced by adults raised by residential school survivors in this study were parental divorce, living with a caregiver with a mental illness, living with a caregiver with an addiction, and living with a caregiver who was emotionally abusive.
ACE score could not explain the association between maternal residential school attendance and increased AL among her adult children. We conducted a number of post hoc tests to confirm the veracity of this result. Findings indicate no significant associations between ACE score and any of the seven adult AL biomarkers examined. It may be that the study was underpowered for this analyses (N = 76); yet our examination of Lowess curves suggests offspring ACE score had little impact on later neuroendocrine (cortisol awakening response, DHEA-S), cardiovascular (systolic and diastolic blood pressure), immune system (CRP) or metabolic (waist circumference, BMI) functioning among the adult children of survivors. It may also be that heightened maternal exposure to ACEs through residential school resulted in biological embedding that influenced AL score among her children in ways that could not be further exacerbated by her children's exposure to ACEs. It may also be that the AL biomarkers used in this study did not adequately capture the impact of ACEs on biological dysfunction. The most consistent neurobiological finding among adults with a history of childhood maltreatment is a reduction in hippocampal volume associated with a deficit in declarative memory, a marker which AL does not typically include (Danese and McEwen, 2012, Smith, 1999). As well, neuroendocrine findings in maltreated adults vary according to the presence or absence of psychiatric disorders, which may explain nonsignificant findings between child ACE score and adult neuroendocrine markers in this study (Danese and McEwen, 2012). That said, several studies have documented elevated basal levels of inflammatory biomarkers among adults who have a history of child maltreatment, which was not repeated in this study (Danese, Pariante, Caspi, Taylor, & Poulton, 2007). Associations between ACE score and metabolic disorders and obesity have also been documented, which were not repeated in the present sample (Huang et al., 2015). All participants in this study were post-secondary students, and it may also be that this subpopulation is resilient given education and health are positively associated.
In summary, the findings of this study suggest mechanisms other than the intergenerational transmission of trauma, operationalized through ACEs, were responsible for the observed association between maternal residential school attendance and offspring AL score. Additional studies with larger, more diverse samples of Indigenous adults are needed to confirm this result. Strengths of this study include guidance by an Indigenous Advisory Committee. Limitations include the use of a cross-sectional design which precludes inferences about causation, and potential response bias due to the use of self-report and take-home salivary measures. As demonstrated by wide confidence intervals in the analysis, this study was underpowered. If the true effect was small or medium-sized, the sample size may have been insufficient to achieve the threshold for statistical significance. For example, we observed medium-sized associations between maternal residential school attendance and high AL among offspring (OR = 2.7) which, given the size of the effect, would have likely been significant with adequate study power. We also found maternal residential school attendance was associated with high ACEs scores (i.e., ACEs of 6 or more), but not moderate ACE scores (i.e., ACEs of 3 to 5) among her offspring (Table 5). This non-linear finding is likely due to an inadequate sample size rather than a true non-linear effect, given the sample was sufficient to achieve the threshold for statistical significance for a large effect (OR = 4.6), but insufficient to achieve significance for a medium-sized effect (OR = 2.5 for ).
4.1. Conclusions
The Canadian Truth and Reconciliation Commission (2015) asked governments to acknowledge the impact of residential schools on the current state of Indigenous health. The present findings underline the importance of this call by demonstrating how the residential school experience may get under the skin to impact the health of the next generation. Findings provide justification for future research designed to examine the epigenetic impacts of the residential school experience among the children of survivors.
Acknowledgements
The authors wish to thank members of the Indigenous Advisory Committee who provided input on the development of the larger project from which this study was based, and the Indigenous students who shared their experiences and perspectives with us. This study was funded by an operating grant from the Institute of Aboriginal Peoples Health within the Canadian Institutes of Health Research (Funding Reference Number: 131590, PI: Currie, CL). Dr. Currie was supported by an Alberta Innovates Translational Health Chair in Aboriginal Health & Well-being during the course of this research.
Acknowledgments
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
This study was reviewed and approved by the Human Research Ethics Board in the Office of Research Ethics at the University of Lethbridge (Protocol #2014-046). Informed written consent was obtained from all participants.
Authors' contributions
KCMR and CC performed the analysis and interpretation of the data, and drafted the manuscript. JC and GM contributed to the interpretation of the data, drafting, and revising of the manuscript. All authors played a role in designing and conceptualizing the study. All authors read and approved the final manuscript.
Financial disclosure statement
This research was funded by an operating grant from the Institute of Indigenous Peoples Health within the Canadian Institutes of Health Research (Funding Reference Number: 131590). The corresponding author was supported by an Alberta Innovates Translational Health Research Chair in Aboriginal Health & Well-being during the course of this research.
References
- Anderson I., Robson B., Connolly M., Al-Yaman F., Bjertness E., King A., Yap L. Indigenous and tribal peoples’ health (The Lancet–Lowitja Institute Global Collaboration): a population study. The Lancet. 2016;388(10040):131–157. doi: 10.1016/S0140-6736(16)00345-7. [DOI] [PubMed] [Google Scholar]
- Arriagada, P. (2016). Women in Canada, a gender-based statistical report. (Statistics Canada). Doi:Catalogue no. 89-503-X.
- Beckie T.M. A systematic review of allostatic load, health, and health disparities. Biological Research For Nursing. 2012;14:311–346. doi: 10.1177/1099800412455688. [DOI] [PubMed] [Google Scholar]
- Bombay A., Matheson K., Anisman H. The impact of stressors on second generation Indian residential school survivors. Transcultural Psychiatry. 2011;48:367–391. doi: 10.1177/1363461511410240. [DOI] [PubMed] [Google Scholar]
- Bombay A., Matheson K., Anisman H. The intergenerational effects of Indian Residential Schools: Implications for the concept of historical trauma. Transcultural Psychiatry. 2014;51:320–338. doi: 10.1177/1363461513503380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansonneuve D. The Aboriginal Healing Foundation; 2005. Reclaiming connections: Understanding residential school trauma among aboriginal people. [Google Scholar]
- Chartier M.J., Walker J.R., Naimark B. Separate and cumulative effects of adverse childhood experiences in predicting adult health and health care utilization. Child Abuse & Neglect. 2010;34:454–464. doi: 10.1016/j.chiabu.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Currie C.L. Enculturation and alcohol use problems among Aboriginal university students. Canadian Journal of Psychiatry. 2011;56:735–742. doi: 10.1177/070674371105601205. [DOI] [PubMed] [Google Scholar]
- Currie C.L., Wild T.C., Schoflocher D.P., Laing L., Veugelers P. Illicit and prescription drug problems among urban Aboriginal adults in Canada: The role of traditional culture in protection and resilience. Social Science & Medicine. 2013;88:1–9. doi: 10.1016/j.socscimed.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Danese A., McEwen B.S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Danese A., Pariante C.M., Caspi A., Taylor A., Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R. Creactive protein, inflammatory conditions, and cardiovascular disease risk. American Journal of Medicine. 2007;120:1054–1062. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong M.T., Bingham B.A., Aldana P.C., Chung S.T., Sumner A.E. Variation in the calculation of allostatic load score: 21 Examples from NHANES. Journal of Racial and Ethnic Health Disparities. 2017;4:455–461. doi: 10.1007/s40615-016-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias B. Trauma and suicide behaviour histories among a Canadian indigenous population: An empirical exploration of the potential role of Canada’s residential school system. Social Science & Medicine. 2012;74:1560–1569. doi: 10.1016/j.socscimed.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Fast E., Vézina D.C.- Historical trauma, race-based trauma and resilience of indigenous peoples: A literature review. First Peoples Child & Family Review. 2010;5:126–136. [Google Scholar]
- Felitti V.J. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Koss M.P., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Gustafsson P.E. Life-course accumulation of neighborhood disadvantage and allostatic load: Empirical integration of three social determinants of health frameworks. American Journal of Public Health. 2014;104:904–910. doi: 10.2105/AJPH.2013.301707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A.F. & Little, T.D. (2017). Introduction to mediation, moderation, and conditional process analysis: a regression-based approach 2nd Ed. New York, NY: Guilford Press.
- Huang H. Adverse childhood experiences and risk of type 2 diabetes: A systematic review and meta-analysis. Metabolism. 2015;64:1408–1418. doi: 10.1016/j.metabol.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Johnson S.C., Cavallaro F.L., Leon D.A. A systematic review of allostatic load in relation to socioeconomic position: Poor fidelity and major inconsistencies in biomarkers employed. Social Science & Medicine. 2017;192:66–73. doi: 10.1016/j.socscimed.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Juster R.-P. John Wiley & Sons, Inc; 2016. Developmental psychopathology; pp. 1–54. [Google Scholar]
- Juster R.-P., McEwen B.S., Lupien S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Juster R.-P., Moskowitz D.S., Lavoie J., D’Antono B. Sex-specific interaction effects of age, occupational status, and workplace stress on psychiatric symptoms and allostatic load among healthy Montreal workers. Stress. 2013;16:616–629. doi: 10.3109/10253890.2013.835395. [DOI] [PubMed] [Google Scholar]
- Kimelman, E. C. (1985). No quiet place: Review Committee on Indian and Metis Adoptions and Placements. Winnipeg, MB. Retrieved from 〈https://digitalcollection.gov.mb.ca/awweb/pdfopener?smd=1&did=24788&md=1〉.
- King M., Smith A., Gracey M. Indigenous health part 2: the underlying causes of the health gap. The Lancet. 2009;374(9683):76–85. doi: 10.1016/S0140-6736(09)60827-8. [DOI] [PubMed] [Google Scholar]
- Knutson J.F. Psychological characteristics of maltreated children: Putative risk factors and consequences. Annual Review of Psychology. 1995;46:401–431. doi: 10.1146/annurev.ps.46.020195.002153. [DOI] [PubMed] [Google Scholar]
- Lindstrom, G., Choate, P., Bastien, L., Weasel Traveller, A., Breaker, S., Breaker, C., Good Striker, W., Good Striker, E. (2016). Nistawatsiman Exploring First Nations Parenting: A Literature Review and Expert Consultation With Blackfoot Elders Calgary, AB: Mount Royal University. Available at: http://cwrp.ca/publications/3110.
- Lomanowska A.M., Boivin M., Hertzman C., Fleming A.S. Parenting begets parenting: A neurobiological perspective on early adversity and the transmission of parenting styles across generations. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Madden V. Intergenerational transmission of parenting: Findings from a UK longitudinal study. European Journal of Public Health. 2015;25:1030–1035. doi: 10.1093/eurpub/ckv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McQuaid R.J. Suicide ideation and attempts among first nations peoples living on-reserve in Canada: The intergenerational and cumulative effects of indian residential schools. Canadian Journal of Psychiatry. 2017;62:422–430. doi: 10.1177/0706743717702075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersky J.P., Topitzes J., Reynolds A.J. Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: A cohort study of an urban, minority sample in the U.S. Child Abuse & Neglect. 2013;37:917–925. doi: 10.1016/j.chiabu.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy J.S. A national crime: The Canadian Government and the residential school system, 1879 to 1986: Volume 11 of Manitoba studies in native history. Univ. of Manitoba Press; 2006. [Google Scholar]
- Mosby I. Administering colonial science: Nutrition research and human biomedical experimentation in Aboriginal communities and residential schools, 1942–1952. Histoire Sociale. 2013;46:145–172. [Google Scholar]
- Roberts R., O’Connor T., Dunn J., Golding J. The effects of child sexual abuse in later family life; mental health, parenting and adjustment of offspring. Child Abuse & Neglect. 2004;28:525–545. doi: 10.1016/j.chiabu.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Running Bear U. The relationship of five boarding school experiences and physical health status among Northern Plains Tribes. Quality of Life Research. 2018;27:153–157. doi: 10.1007/s11136-017-1742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks, V. & Murphey, D. (2018). The prevalence of adverse childhood experiences, nationally, by state, and by race/ethnicity Available at: https://www.childtrends.org/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity.
- Scaramella L.V., Neppl T.K., Ontai L.L., Conger R.D. Consequences of socioeconomic disadvantage across three generations: Parenting behavior and child externalizing problems. Journal of Family Psychology. 2008;22:725–733. doi: 10.1037/a0013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T.E. Price of Adaptation—Allostatic Load and Its Health Consequences. Archives of Internal Medicine. 1997;157:2259. [PubMed] [Google Scholar]
- Segerstrom S.C., Miller G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, M. (2015). Honouring the Truth, Reconciling for the Future: Summary of the Final report of the Truth and Reconciliation Commission of Canada. (Truth and Reconciliation Commission of Canada).
- Smith E.E. Storage and executive processes in the frontal lobes. Science (80) 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sotero, M. (2015). The Effects of Adverse Childhood Experiences on Subsequent Injury in Young Adulthood: Findings from the National Longitudinal Study of Adolescent and Adult Health. UNLV Theses, Dissertations, Professional Papers, and Capstones.
- Stalder T., Kirschbaum C., Kudielka B.M., Adam E.K., Pruessner J.C., Wüst S., Clow A. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Truba T.N., Doan J., Currie C.L., Copeland J.L. Short-term changes in daily movement behaviour influence salivary C-reactive protein in healthy women. Applied Physiology Nutrition and Metabolism-Physiologie Appliquee Nutrition Et Metabolisme. 2018;43:854–856. doi: 10.1139/apnm-2017-0758. [DOI] [PubMed] [Google Scholar]
- Truth and Reconciliation Commission of Canada. (2015). Canada’s Residential Schools: The History, Part 1 Origins to 1939: Final Report of the Truth and Reconciliation Commission of Canada Volume 1. National Centre for Truth and Reconciliation. Montreal and Kingston, Canada. Retrieved from 〈http://nctr.ca/assets/reports/Final Reports/Volume_1_History_Part_1_English_Web.pdf〉.
- Truth and Reconciliation Commission of Canada. (2016). Canada’s residential schools: the history, part 2, 1939 to 2000: the final report of the Truth and Reconciliation Commission of Canada Volume 1. National Centre for Truth and Reconciliation. Retrieved from 〈http://nctr.ca/assets/reports/Final Reports/Volume_1_History_Part_2_English_Web.pdf〉.
- Van Dyke M.E. Socioeconomic status discrimination and C-reactive protein in African-American and White adults. Psychoneuroendocrinology. 2017;82:9–16. doi: 10.1016/j.psyneuen.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk P., Maltby A., Cooke M. Residential schools and the effects on Indigenous health and well-being in Canada—a scoping review. Public Health Rev. 2017;38:8. doi: 10.1186/s40985-017-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R., Bierer L.M., Andrew R., Schmeidler J., Seckl J.R. Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. Journal of Psychiatric Research. 2009;43:877–883. doi: 10.1016/j.jpsychires.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda, R. & Bierer, L. M. in Prog Brain Res. 167, 121–135 (2008). [DOI] [PubMed]