Abstract
OBJECTIVES
While an accurate placement in cochleostomy is critical to ensure appropriate insertion of the cochlear implant (CI) electrode into the scala tympani (ST), the choice of preferred cochleostomy sites widely varied among experienced surgeons. We present a novel technique for precise yet readily applicable localization of the optimum site for performing ST cochleostomy.
MATERIALS and METHODS
Twenty fresh frozen temporal bones were dissected using the mastoidectomy-posterior tympanotomy approach. Based on the facial nerve and the margins of the round window membrane (RWM), the cochleostomy site was chosen to insert the electrode into the ST while preserving the surrounding intracochlear structures.
RESULTS
There is a limited safe area suitable for the ST implantation in the area inferior and anterior to the RWM. There is a higher risk of scala vestibuli (SV) insertion anterior to that area. Posterior to that area, the cochlear aqueduct (CA) and inferior cochlear vein (ICV) are liable for the injury.
CONCLUSION
For atraumatic CI, precise and easy localization of the site of cochleostomy play a pivotal role in preserving intracochlear structures. Accurate setting of the vertical and horizontal orientations is mandatory before choosing the site of cochleostomy. The facial nerve and the margins of the RWM offer a very helpful clue for such localization; meanwhile, it is readily identifiable in the surgical field.
Keywords: Cochlear implantation, cochleostomy, scala tympani, hearing preservation, residual hearing
INTRODUCTION
As the first clinically available artificial sensory organ in medicine, cochlear implants (CIs) represent a significant 20th-century surgical innovation [1]. It is mainly used to (re)habilitate patients with profound or severe hearing impairment who could not gain benefit from hearing aids [2]. Previously, assuming that ipsilateral hearing was compromised during surgery, implanting patients with residual hearing was prevented. However, improving the implants by refining the surgical procedures allowed the indication criteria to include many patients with substantial residual hearing [3, 4]. Soft CIs were introduced to preserve residual hearing during CI where scala tympani (ST) cochleostomy was described [5]. While an accurate placement of the cochleostomy is critical to ensure ST insertion, the choice of preferred cochleostomy sites varies widely among experienced surgeons [6]. We present a novel technique for precise yet readily applicable localization of the optimum site for performing ST cochleostomy.
MATERIALS and METHODS
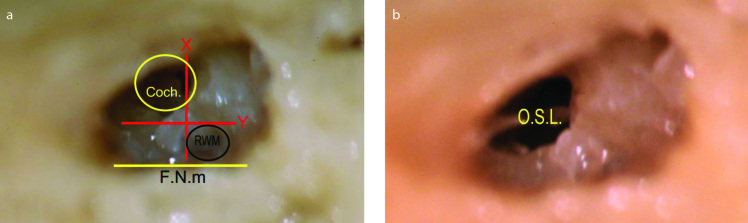
With the approval of the Institutional Review Board in Mansoura Faculty of Medicine, a dissection study of 20 (7 left and 13 right) fresh-frozen temporal bones was performed by the first author with the aim of performing atraumatic ST cochleostomy. The dissection was initiated with an intact wall mastoidectomy followed by posterior tympanotomy (PT) with skeletonization of the chorda tympani (ChT) and the mastoid segment of the facial nerve (F.N.m), thereby achieving the maximum possible width for a more convenient evaluation of the target area of the cochleostomy. In addition, when the skeletonized F.N.m lies exactly transversely across the surgical field, it forms a vertical reference for the precise judgment of the site of cochleostomy (Figure 1). Then, the true round window membrane (RWM) was exposed until its annulus by drilling the bony overhang and removing the false membrane if present. When the annulus was clearly seen, imaginary tangents to the anterior and inferior parts of the annulus were considered parallel (Y) and vertical (X) to the F.N.m, respectively (Figure 2).
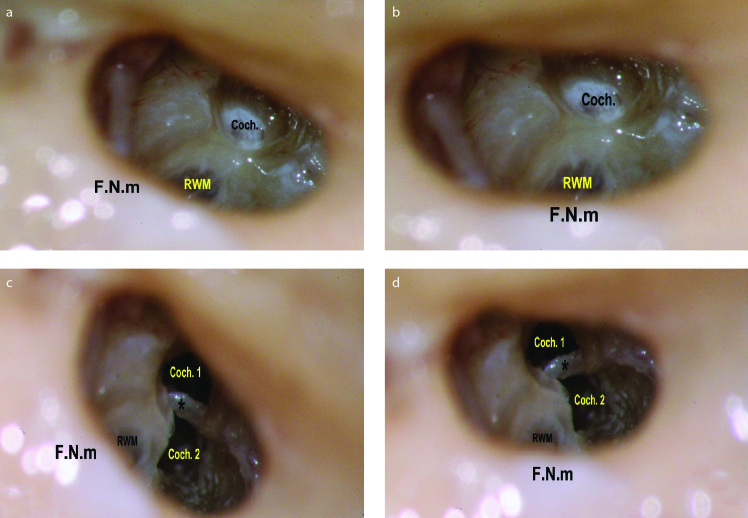
Figure 1. a–d.
The importance of proper orientation of the F.N.m. as seen during performing a right cochleostomy. This figure illustrates the importance of proper orientation of the mastoid segment of the facial nerve (F.N.m). It should lie exactly transversely across the surgical field, for accurate interpretation of the site of cochleostomy. (a) When the nerve was oblique across the field, misinterpretation of the site of the cochleostomy (Coch.) occurred, giving the impression of being anteroinferior to the round window membrane (RWM). Notice the preservation of the endosteal layer, to be later opened with a micro pick, not directly by the drill. (b) After proper transverse positioning of the nerve across the field, the site that seemed to be anteroinferior appears clearly now to be rather anterior to the RWM. N.B.: The rotation is obtained by editing the photo. (c) The endosteum was removed to discover that this cochleostomy site (Coch. 1) had led to the scala vestibuli supero-lateral to the osseous spiral lamina and basilar membrane (*). Then, a second cochleostomy (Coch. 2) was performed in an attempt to gain access to the scala tympani. During drilling, the cochleostomy seemed to be inferior to the RWM. (d) However, rotating the photo to have the facial nerve across the field demonstrated that the second cochleostomy lies anteroinferior to the RWM (rather than inferior).
Figure 2.
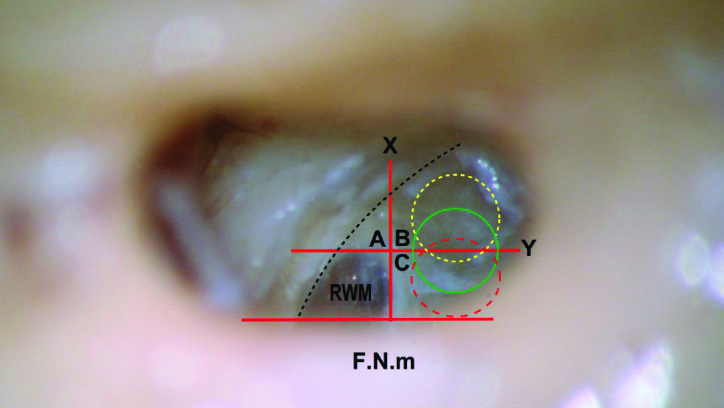
The intermediate and safe-range cochleostomy. When the annulus of the right round window membrane (RWM) is exposed, imaginary tangents are considered touching the anterior and inferior parts of the annulus. Y line: the anterior tangent that is parallel to the mastoid segment of the facial nerve (F.N.m). X line: the inferior tangent that is vertical to the F.N.m. The X and Y lines divide the area anterior and inferior to the RWM into three areas: A, B, and C. Area A is the area anterior to the RWM, anterior to the Y line, and superior to the X line. Anterior cochleostomy shall lie in area A. Area B is the area anteroinferior to the RWM, inferior to the X line, and anterior to the Y line. Without the presence of a precise definition, cochleostomy in any part of area B can be designated as an anteroinferior cochleostomy. Area C is the area inferior to the RWM, inferior to the X line, and posterior to the Y line. Inferior cochleostomy shall be performed in area C. The green circle that is centered on the Y line and inferior to the X line marks the site of our recommended intermediate cochleostomy position. The term intermediate describes its interposition between the areas of the famous anteroinferior and inferior cochleostomies. The yellow fine-dashed circle represents the most anterior limit of the safe cochleostomy range, through which atraumatic scala tympani implantation can be performed, whereas the red coarse-dashed one represents the most posterior limit of that range. The dashed parabola represents the estimated course of the spiral ligament and osseous spiral lamina. Therefore, the area anterosuperior to this dashed parabola corresponds to the scala vestibuli, and the area postero-inferior to it corresponds to the scala tympani.
To lead to the ST, the proposed cochleostomy site must be inferior to the X line. In relation to the Y line, different sites were chosen in different samples to verify the eventuality of each site to open ST. Under ample irrigation, low-speed drilling was performed using a 1-mm diamond burr until the preserved endosteal layer was exposed (Figure 1a) for later opening using a micro pick. Through the cochleostomy, the site of the osseous spiral lamina (OSL) was evaluated to appreciate the scala reached using each cochleostomy position. Electrode insertion was then performed with minimal pressure until the stopper reached the cochleostomy edge. The mastoid cavity was sealed to stabilize the electrode during the subsequent steps. Then, the intracochlear scalar position of the electrode was verified after performing a canaloplasty, thereby removing the tympanic membrane and ossicles and eventually transcanal drilling of the basal cochlear turn. In two specimens, lateral temporal bone resection was performed to provide sufficient space for performing the cochlear drill-out. Two bony rings were preserved around the cochleostomy and RWM. The membranous labyrinth of the basal cochlear turn was preserved to be opened with a hook along its upper and lower margins, preserving the OSL. The relation of the OSL to the cochleostomy, RWM, and inserted electrode was then appreciated.
RESULTS
The first performed cochleostomy was intended to be inferior to the X line and anterior to the Y line, as described above. After removing the endosteum, the cochleostomy was found to purely lead to the scala vestibuli (SV) of the basal cochlear turn and superior to the OSL. Thereafter, a second cochleostomy was performed to open the ST. During drilling, the second cochleostomy seemed inferior to the RWM and led to the ST (Figure 1c). Upon revising the dissection photos, the importance of the F.N.m was recognized as a vertical reference for localizing the cochleostomy site. Having the F.N.m accurately transversely crossing the field, the initial cochleostomy that seemed anteroinferior to the RWM appeared rather anterior (Figure 1b), and the second cochleostomy appeared anteroinferior rather than inferior (Figure 1d). Subsequently, transverse orientation of the F.N.m became a routine step before deciding the cochleostomy site.
In the second specimen, most of the cochleostomy was inferior to the X line and anterior to the Y line (Figure 3a). This cochleostomy equally led to the ST and SV, with the OSL and the basilar membrane (BM) bisecting the opening (Figure 3b).
Figure 3. a, b.
Left (traumatic) anteroinferior cochleostomy, leading to the scala tympani and the scala vestibuli. (a) Left anteroinferior cochleostomy. F.N.m: Facial nerve, mastoid segment. Coch.: Cochleostomy. (b) Cochleostomy led to the area of junction between the scala tympani and scala vestibuli.
OSL: osseous spiral lamina
In the third specimen, the cochleostomy was completely inferior to the X line and nearer but anterior to the Y line. It led mainly to the ST and partially to the SV (Figure 4). The OSL and BM crossed the upper part of the opening, indicating an improvement of the cochleostomy position, but further optimization is still required.
Figure 4.
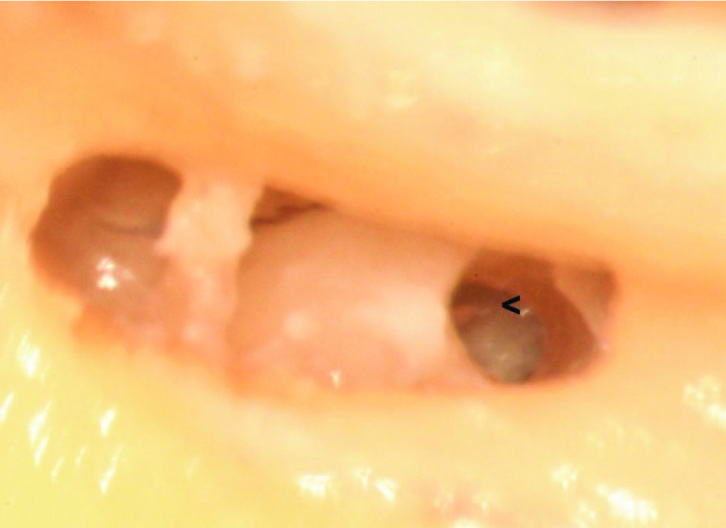
Right (traumatic) anteroinferior cochleostomy, leading mainly to the scala tympani and partially to the scala vestibuli. The osseous spiral lamina (<) is seen in the anterior one-fourth of the cochleostomy. Notice that the narrow space between the facial and chorda tympani nerves, together with the posterior rotation of the cochlea, prevented simultaneous visualization of the round window and the cochleostomy.
In the fourth specimen, the cochleostomy was inferior to the X line. The Y line formed a posterior tangent to the drilled cochleostomy. The cochleostomy purely led to the ST but immediately below and flush with the OSL and BM (Figure 5).
Figure 5.
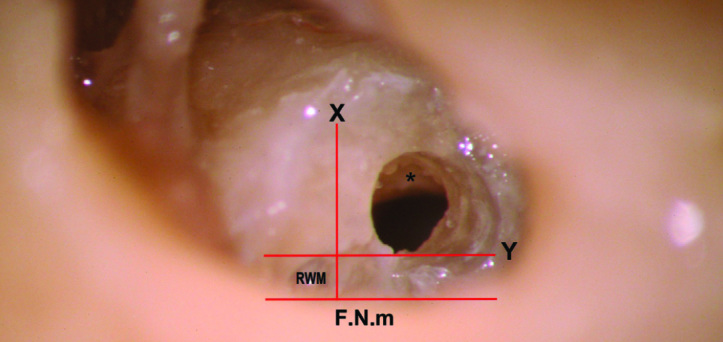
Right anteroinferior cochleostomy, touching the Y line and leading to the scala tympani immediately under the osseous spiral lamina (*). F.N.m: facial nerve, mastoid segment. RWM: anteroinferior part of the true round window membrane; the rest of the membrane is hidden medial to the facial nerve.
In the fifth specimen, approximately 80% of the 1-mm burr was anterior to the Y line, as that shown in Figure 2 (yellow fine-dashed circle). This relative posterior shift was translated into more separation between the cochleostomy and OSL. However, the proximity of the electrode array and OSL can potentially result in an insertional trauma; therefore, it was decided upon the next dissections that the cochleostomy site would be modified to further protect the OSL.
In the specimens 6–11 and 13–20, the cochleostomy site was located inferior to the X line and exactly centered on the Y lines, as that shown in Figure 2 (solid-line and green circle). The burr had to drill through the crista fenestra before reaching the endosteal layer. Using this site, ST was purely reached in all 14 specimens. Meanwhile, the OSL and BM were not seen through the cochleostomy (Figure 6), indicating that these structures were kept intact and sufficiently distant from the cochleostomy site and consequently the inserted electrode. The ST position of the electrode was confirmed after performing the cochlear drill-out procedure. No gross trauma to the intracochlear structure was detected.
Figure 6.
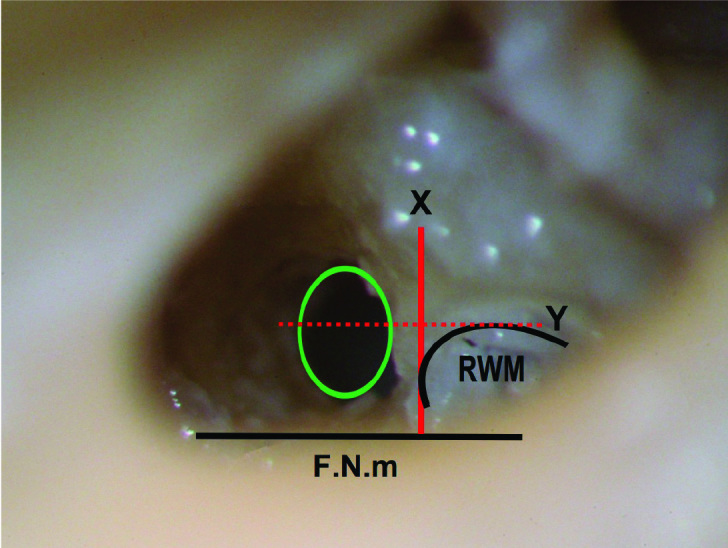
Left intermediate cochleostomy, the green circle is centered on the Y line and inferior to the X line. The cochleostomy purely led to the scala tympani; the osseous spiral lamina is not seen through the lumen of cochleostomy.
F.N.m: facial nerve, mastoid segment; RWM: round window membrane
In specimen 12, despite locating the cochleostomy exactly as that in the latter specimens, the electrode entered the SV. A cochlear drill-out showed steeply vertical and posteriorly located OSL and BM. A posterior enlargement of the cochleostomy was made to expose the ST postero-inferior to the OSL, thereby determining the ideal cochleostomy site in this particular specimen (Figure 7a). Thereafter, a concern arose about the integrity of the cochlear aqueduct (CA) and inferior cochlear vein (ICV). Further, the area inferior to the RWM was drilled to explore the latter structures, which were found very close to the posterior margin of the posterior extension of the cochleostomy (Figure 7b).
Figure 7. a, b.
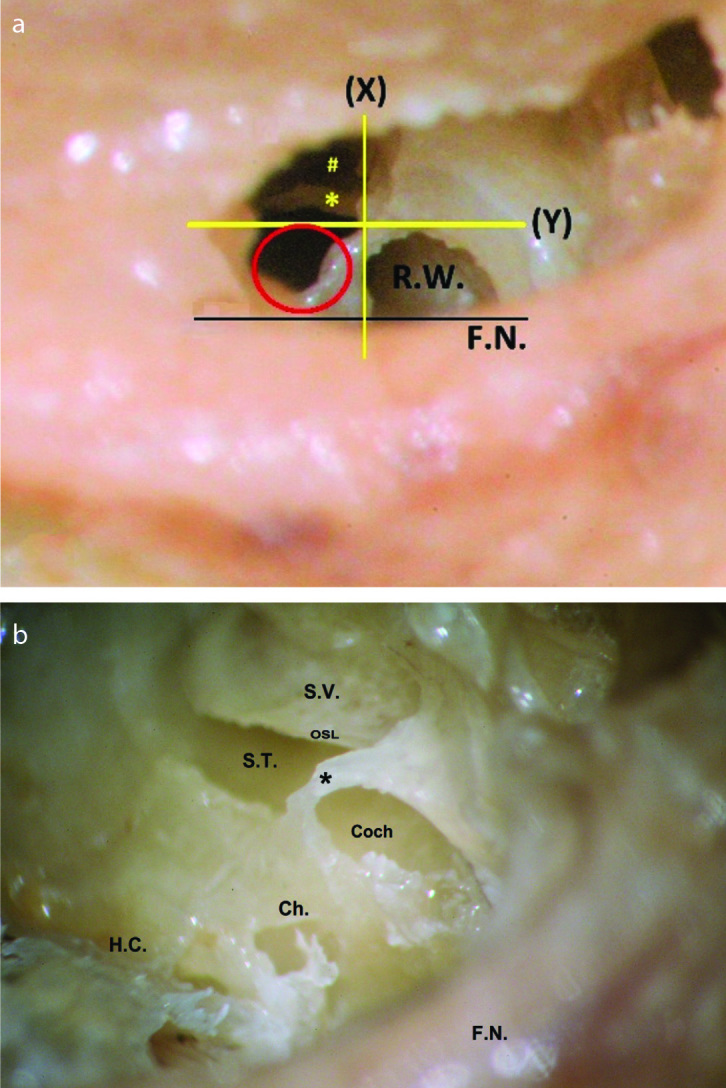
Frank inferior cochleostomy. (a) Posterior enlargement of the initial “intermediate cochleostomy” that was centered on the Y line, in an attempt to expose the scala tympani (ST) postero-inferior to the osseous spiral lamina (OSL) (*). (#) denotes the defect anterior to the OSL, after drilling the initial cochleostomy, which resulted in scala vestibuli (SV) insertion. Notice the position of the red circle (marking the ideal cochleostomy site, frank inferior cochleostomy in this case), in relation to the Y line, touching the line posteriorly. In addition, notice the steep vertical orientation of the OSL. RW: round window, the membrane was removed. F.N.: facial nerve. (b) The same specimen after lateral temporal bone resection and cochlear drill-out viewed from posterosuperior-lateral view. The basal cochlear turn has been opened to show the OSL, ST, and SV, with preservation of a bony rim (*) around the Coch. The channels for the cochlear aqueduct and the inferior cochlear vein (Ch.) seem very close to the posterior margin of the posterior extension.
FN: facial nerve; HC: hypotympanic cells
DISCUSSION
It is widely accepted that hearing preservation CI requires atraumatic electrode insertion into the ST, which is associated with superior audiologic outcomes and better speech perception performance [7, 8, 9]. The two major techniques for electrode insertion into the cochlea are the RW insertion and the cochleostomy approaches. The optimal approach for standard CI electrode insertion is highly debated [10, 11, 12]. Even among the advocates of the cochleostomy approach, the choice of preferred cochleostomy sites widely varies among experienced surgeons [6]. A survey of CI surgeons confirmed this inter-surgeon variability; even some experienced CI surgeons preferred a site superior to the RW [13]. As subtle differences in the cochleostomy site may change the destination of the electrode from the ST to SV, a recommendation of future studies accurately documenting the exact location of the cochleostomy was recently given [14].
The variability in the cochleostomy site may be explained by using the RW niche as the landmark for the cochleostomy [15]. In addition, relating the site of cochleostomy to the RWM are broad descriptions not suitable for this microscopic technique, thus requiring more specification. Meanwhile, the selection of the cochleostomy site in relation to the RWM is liable to inter-surgeon diversity, particularly with different vectors of vision through the PT. In other words, for this microsurgical procedure, where merely each fraction of a millimeter makes a significant difference, precise objective and easily recognized surgical landmarks are lacking. We present a clear clinically applicable description for choosing the cochleostomy site, thereby increasing the chance of atraumatic ST implantation.
Surgical intervention during cochleostomy necessitates detailed submacroscopical knowledge of cochlear morphology [16]. Accordingly, cochlear drill-out was conducted after electrode insertion. Not only the electrode position could be verified but also the complex relationships among structures comprising the hook region of the cochlea could be thoroughly appreciated from a surgical perspective, which is a great advantage of this study. Direct visualization of this area cannot be achieved in life surgeries because of the tiny cochleostomy and the presence of the endolymph. Similarly, a histological sectioning of the temporal bones after experimental implantation lacks such benefit. Previous works presented valuable descriptions and diagrams of the anatomy of the hook region of the cochlea [17–20]. In addition to these efforts, our style of study (cochlear drill-out after electrode insertion) will help a CI surgeon to build a mental map from the surgical perspective to address this microscopic yet complex area, which is dealt with surgically but not actually explored visually.
The first specimen showed the importance of adjusting the vertical and horizontal references for accurate judgement of the site of cochleostomy. A CI surgeon inspects a small part of the bigger tympanic cavity through the PT, along a tilted visual vector from posterior-superior-laterally to anterior-inferior-medially. This peculiar position may affect the accurate interpretation of the relationships of the structures of surgical importance to each other. In this concern, some landmarks may offer the necessary directional clues with varying degrees of reliability: the patient head orientation, supramastoid crest, and relation of the oval and RWs. We suggest the F.N.m as the vertical reference after radiological exclusion of any anomalous course. Skeletonizing the F.N.m will maximize the PT and counteract the not uncommon tendency of leaving a thick bone covering the F.N.m for its safety; this thick bone would shift the vector of vision and shaft of the burr more anteriorly, which invites the risk of SV insertion. Having the skeletonized F.N.m precisely transversely running across the field gives an accurate vertical reference. The failure to appreciate the exact orientations together with the oblique vector of vision will lead to the misinterpretation of the correct site to perform the ideal cochleostomy, resulting in SV insertion (Figure 1).
Besides illustrating the extreme importance of setting the orientations prior to choosing the cochleostomy site, the unintended mistake during dissecting the first specimen yielded some additional benefits. First, the cochleostomy that appeared anteroinferior to the RWM was essentially almost anterior to it. Cochleostomy in this site led to the SV insertion. In addition, cochlear drill-out of all specimens revealed that the areas anterior and superior to the RWM are related to the SV. Consequently, these areas are excluded as routes for the ST. Second, the falsely apparent anteroinferior cochleostomy may explain the higher incidence of the SV insertion reported with cochleostomy compared with that of the RW route [7, 21]. Third, in accordance with Figure 1c and 1d, the inferior-site cochleostomy reported by Briggs and colleagues [22] would be perceived as anteroinferior to the RWM, after correcting the obliquity of the F.N.m, which was evident in the figure they presented to show their preferred cochleostomy site.
After exclusion of the sites anterior and superior to the RWM as routes for the ST, as stated above and in accordance with earlier works, [17, 23] the anteroinferior and inferior sites will then remain available for further analysis. While some authors broadly described cochleostomies inferior or anteroinferior to the RWM as most favorable, [6] more chose the site anteroinferior to the RWM [23–27]. Fewer authors suggested the inferior site for cochleostomy, [21, 28, 29] but the choice was argued due to the risks of damaging the CA and ICV [17]. The latter citations sample the wide variability in surgeon approaches to the basal cochlear turn, [25] a debate that promotes searching for a standardized approach.
In the second specimen, the OSL bisected the opening, denoting cochleostomy malposition. Despite being fractions of a millimeter, the deflections required correction in both vertical (to lie inferior to the X line) and horizontal scales (should be more posterior).
In the third specimen, the cochleostomy, which was located below the X line and anterior but closer to the Y line, had led mainly to the ST and partially to the SV. Although the ST can still be implanted, the BM may rupture during electrode insertion. Consequently, a posterior shift of the cochleostomy site toward the Y line seemed appropriate.
In the fourth specimen, where the cochleostomy was slightly posteriorly shifted to touch the Y line, pure ST opening could be achieved. Despite the better positioning of the opening, concern about frictional trauma between the electrode bands and BM made it wise to further shift the cochleostomy site posteriorly.
The fifth specimen showed a pure ST opening; however, the close proximity of the electrode to the OSL may render it vulnerable to insertional trauma with changing the vector of insertion of the electrode or with more bulky electrodes. Therefore, to minimize the possibility of insertional trauma, a further posterior shift was decided.
In specimens 6–11 and 13–20, the cochleostomy site was performed inferior to the X line and centered on the Y line. This cochleostomy site was termed intermediate cochleostomy position (ICP) because it lied between the popular anteroinferior and inferior cochleostomy positions. It seemed optimum for pure ST opening, meanwhile perfectly protecting the OSL and BM from both the traumas of cochleostomy drilling and electrode insertion. This site that was chosen in 14 bones appeared logical to be the appropriate site for the first five bones by virtue of the posterior shift of the site of drilling in relation to the Y line. In other words, this cochleostomy site would be ideal for 19 bones out of the 20 (95%).
Only specimen 12 did not follow the rule after drilling the ICP. Cochlear drill-out revealed the electrode in the SV after passing supero-lateral to a steep vertically oriented OSL and BM immediately anterior to the Y line (Figure 7a). This posteriorly shifted OSL augmented the lateral surface area of the SV at the expense of the ST, explaining the SV insertion of the electrode. Such orientation of the OSL and BM will significantly impact the choice of the cochleostomy site and the insertion vector of the electrode. The ideal vector of insertion in such cases is better directed rather inferomedially in relation to the vector of the basal cochlear turn. Having such importance, the orientation of the OSL and BM is better to be evaluated through preoperative radiology; future formulation of a specific protocol will be required for this purpose. However, earlier studies may offer some help [30]. In the same specimen 12, a further posterior extension of the initial cochleostomy led to the ST inferomedial to the OSL. The latter posterior extension is equivalent to what we can call Frank inferior cochleostomy (FIC), touching the Y line. FIC by definition would be inferior to the RW, completely posterior to the Y line, and obviously inferior to the X line. FIC seems logical to be ideal for ST insertion in all specimens because it will guarantee an atraumatic opening of the ST and inferomedial to the OSL, regardless of its orientation. However, some drawbacks prevented choosing this site as the recommended optimum cochleostomy site. The first concern is the integrity of the CA and ICV because a damage of the latter will affect the viability of the spiral ganglion cells, which will negatively impact the CI performance [31]. In this specimen, drilling inferior to the RWM revealed that the canals for the CA and ICV were very closely related to the posterior extension or FIC. However, more anatomical studies are recommended to precisely determine the safe area available for drilling inferior to the RWM and posterior to the Y line. Secondly, drilling in this area requires more removal of bone in the limited chorda-facial angle, which is a challenging task. In addition, the more posterior the cochleostomy, the higher the risk to injure the F.N.m, particularly with the rotating burr shaft, a more likely sequela in the setting of the posteriorly rotated RW. Moreover, a high jugular bulb is more liable to trauma with more posterior drilling. Another concern is that a more posterior cochleostomy will shift the vector of electrode insertion toward the modiolus rather than following the lumen of the ST along the basal cochlear turn, inviting a higher incidence of intracochlear insertional trauma.
CONCLUSION
We would recommend the ICP as the site of choice for atraumatic ST implantation, although it had a less theoretical success rate (95%) in comparison with the theoretical (100%) success for the FIC. Supporting this recommendation are the high success rate (95%), technical feasibility, and easier preservation of the F.N.m, CA, and ICV. The FIC should be reserved for cochleae with the steep orientation of OSL and BM, which is shown on a preoperative radiologic evaluation.
In various anatomic constraints, hindering drilling the cochleostomy in the ICP; for the purpose of flexibility and easier applicability, we define the “safe cochleostomy range.” This is the safe range of cochleostomy positions that can be relied upon for atraumatic ST implantation (Figure 2). This range is dependent on the Y line, which is the anterior tangent of the RWM, parallel to the F.N.m. The 1-mm burr can start drilling in any point between two positions anteriorly, where most (approximately 80%) of the burr lie anterior to the Y line, till posteriorly where a similar portion lie posterior to this line. More anteriorly, the safety of the OSL and the related structures is less guaranteed. Similarly, the integrity of the CA and ICV becomes questionable further posteriorly.
Finally, all CI surgeons should enrich their armamentarium to include mastering with ease the RW insertion and cochleostomy approaches, in addition to subtotal petrosectomy. Having these tools readily available in hands, a CI surgeon will be ready to adapt the approach to the patient and not vice versa.
Footnotes
This study was presented at the Otology on the Nile Congress; Future of Otology and Audiology, 28–29 of December, 2017, Cairo, Egypt.
Ethics Committee Approval: Ethics committee approval was received for this study from the Institutional Review Board of Faculty of Medicine, Mansoura University (R/17.09.14).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.B., Y.S.; Design - A.B., Y.S.; Supervision – Y.S., M.S., K.M., A.E., M.G.; Resource – A.B., M.S.; Materials – A.B., M.G.; Data Collection and/or Processing – A.B.; Analysis and/or Interpretation – A.B.; Literature Search - A.B., Y.S.; Writing – A.B.; Critical Reviews - A.B., Y.S., K.M., A.E., M.G., M.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Alice B, Silvia M, Laura G, Patrizia T, Roberto B. Cochlear implantation in the elderly: surgical and hearing outcomes. BMC Surg. 2013;13(Suppl 2):S1. doi: 10.1186/1471-2482-13-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsot-Dupuch K, Meyer B. Cochlear implant assessment: imaging issues. Eur J Radiol. 2001;40:119–32. doi: 10.1016/S0720-048X(01)00380-1. [DOI] [PubMed] [Google Scholar]
- 3.Campbell A, Dillon M, Buchman C, Adunka O. Hearing preservation cochlear implantation. Curr Otorhinolaryngol Rep. 2013;1:69–79. doi: 10.1007/s40136-013-0012-y. [DOI] [Google Scholar]
- 4.Kim LS, Jeong SW, Lee YM, Kim JS. Cochlear implantation in children. Auris Nasus Larynx. 2010;37:6–17. doi: 10.1016/j.anl.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Lehnhardt E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO. 1993;7:356–9. [PubMed] [Google Scholar]
- 6.Meshik X, Holden TA, Chole RA, Hullar TE. Optimal cochlear implant insertion vectors. Otol Neurotol. 2010;31:58–63. doi: 10.1097/MAO.0b013e3181b76bb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connell BP, Hunter JB, Wanna GB. The importance of electrode location in cochlear implantation. Laryngoscope Investig Otolaryngol. 2016;1:169–74. doi: 10.1002/lio2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basura G, Adunka O, Buchman C. Scala tympani cochleostomy for cochlear implantation. Oper Tech Otolayngol Head Neck Surg. 2010;21:218–22. doi: 10.1016/j.otot.2010.08.001. [DOI] [Google Scholar]
- 9.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–9S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 10.Jiam NT, Limb CJ. The impact of round window vs cochleostomy surgical approaches on interscalar excursions in the cochlea: Preliminary results from a flat-panel computed tomography study. World J Otorhinolaryngol Head Neck Surg. 2016;2:142–7. doi: 10.1016/j.wjorl.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bento RF, Danieli F, Magalhães AT, Gnansia D, Hoen M. Residual hearing preservation with the Evo® cochlear implant electrode array: Preliminary results. Int Arch Otorhinolaryngol. 2016;20:353–8. doi: 10.1055/s-0036-1572530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard C, Fayad JN, Doherty J, Linthicum FH., Jr Round window versus cochleostomy technique in cochlear implantation. Otol Neurotol. 2012;33:1181–7. doi: 10.1097/MAO.0b013e318263d56d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adunka OF, Buchman CA. Scala tympani cochleostomy I: results of a survey. Laryngoscope. 2007;117:2187–94. doi: 10.1097/MLG.0b013e3181453a6c. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Friedmann DR, Treaba C, Peng R, Roland JT., Jr Does cochleostomy location influence electrode trajectory and intracochlear trauma? Laryngoscope. 2015;125:966–71. doi: 10.1002/lary.24986. [DOI] [PubMed] [Google Scholar]
- 15.Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29(7):920–8. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tóth M, Alpár A, Bodon G, Moser G, Patonay L. Surgical anatomy of the cochlea for cochlear implantation. Ann Anat. 2006;188:363–70. doi: 10.1016/j.aanat.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Li PM, Wang H, Northrop C, Merchant SN, Nadol JB., Jr Anatomy of the round window and hook region of the cochlea with implications for cochlear implantation and other endocochlear surgical procedures. Otol Neurotol. 2007;28:641–8. doi: 10.1097/mao.0b013e3180577949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atturo F, Barbara M, Rask-Andersen H. On the anatomy of the hook region of the human cochlea and how it relates to cochlear implantation. Audiol Neurootol. 2014;19:378–85. doi: 10.1159/000365585. [DOI] [PubMed] [Google Scholar]
- 19.Stidham KR, Roberson JB., Jr Cochlear hook anatomy: evaluation of the spatial relationship of the basal cochlear duct to middle ear landmarks. Acta Otolaryngol. 1999;119:773–7. doi: 10.1080/00016489950180414. [DOI] [PubMed] [Google Scholar]
- 20.Erixon E, Högstorp H, Wadin K, Rask-Andersen H. Variational anatomy of the human cochlea. Otol Neurotol. 2009;30:14–22. doi: 10.1097/MAO.0b013e31818a08e8. [DOI] [PubMed] [Google Scholar]
- 21.Wanna GB, Noble JH, Carlson ML, Gifford RH, Dietrich MS, Haynes DS, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124:S1–S7. doi: 10.1002/lary.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briggs R, Tykocinski M, Stidham K, Roberson J. Cochleostomy site: Implications for electrode placement and hearing preservation. Acta Otolaryngol. 2005;125:870–6. doi: 10.1080/00016480510031489. [DOI] [PubMed] [Google Scholar]
- 23.Briggs RJ, Tykocinski M, Saunders E, Hellier W, Dahm M, Pyman B, et al. Surgical implications of perimodiolar cochlear implant electrode design: avoiding intracochlear damage and scala vestibuli insertion. Cochlear Implants Int. 2001;2:135–49. doi: 10.1179/cim.2001.2.2.135. [DOI] [PubMed] [Google Scholar]
- 24.Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 25.Friedland DR, Runge-Samuelson C. Soft cochlear implantation: rationale for the surgical approach. Trends Amplif. 2009;13(2):124–38. doi: 10.1177/1084713809336422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majdani O, Bartling SH, Leinung M, Stöver T, Lenarz M, Dullin C, et al. A true minimally invasive approach for cochlear implantation. Otol Neurotol. 2008;29:120–3. doi: 10.1097/mao.0b013e318157f7d8. [DOI] [PubMed] [Google Scholar]
- 27.Roland P, Gstöttner W, Adunka O. Method for hearing preservation in cochlear implant surgery. Oper Tech Otolayngol Head Neck Surg. 2005;16:93–100. doi: 10.1016/j.otot.2005.03.003. [DOI] [Google Scholar]
- 28.Adunka OF, Radeloff A, Gstoettner WK, Pillsbury HC, Buchman CA. Scala tympani cochleostomy II: Topography and histology. Laryngoscope. 2007;117:2195–200. doi: 10.1097/MLG.0b013e3181453a53. [DOI] [PubMed] [Google Scholar]
- 29.Briggs R, Tykocinski M, Xu J, Risi F, Svehla M, Cowan R, et al. Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiol Neurootol. 2006;11:42–8. doi: 10.1159/000095613. [DOI] [PubMed] [Google Scholar]
- 30.Gibson D, Gluth MB, Whyte A, Atlas MD. Rotation of the osseous spiral lamina from the hook region along the basal turn of the cochlea: results of a magnetic resonance image anatomical study using high-resolution DRIVE sequences. Surg Radiol Anat. 2011;34(8):781–5. doi: 10.1007/s00276-011-0896-5. [DOI] [PubMed] [Google Scholar]
- 31.Guo R, Zhang H, Chen W, Zhu X, Liu W, Rask-Andersen H. The inferior cochlear vein: surgical aspects in cochlear implantation. Eur Arch Otorhinolaryngol. 2015;273:355–61. doi: 10.1007/s00405-015-3549-1. [DOI] [PubMed] [Google Scholar]