Abstract
OBJECTIVE
Benign paroxysmal positional vertigo (BPPV) is the most frequent peripheral vestibular disorder and is particularly seen among older patients suffering from vertigo. The brief vertigo attacks in and imbalance symptoms of BPPV are caused by freely floating otoconia within the semicircular canals. The aim of this prospective study was to evaluate the role of oxidative stress, using native thiol/disulfide (SH/SS) homeostasis as a novel indicator, in the etiology of BPPV.
MATERIALS and METHODS
The 62 participants in the study included 31 patients with BPPV and, as the control group, 31 healthy individuals without any cochleovestibular disorders.
RESULTS
Patients with BPPV initially had significantly lower native SH levels and significantly lower SH/total thiol (TT) ratios, as well as significantly higher SS/SH and SS/TT ratios, than the healthy controls. After successful treatment of their vertigo, which was confirmed based on the results obtained from the second blood sample, patients with BPPV still had lower SH levels and SH/TT ratios and significantly higher SS/SH and SS/TT ratios than the healthy controls.
CONCLUSION
Our results suggest a role of oxidative stress in the development of BPPV, through both calcium metabolism and the direct toxic effects of free oxygen radicals, including the triggering of apoptosis.
Keywords: Benign paroxysmal positional vertigo, oxidative stress, thiol, disulfide
INTRODUCTION
Benign paroxysmal positional vertigo (BPPV) is the most frequent peripheral vestibular disorder; it is particularly seen among older patients suffering from vertigo [1]. Brief vertigo attacks in and imbalance symptomatic of BPPV are caused by otoconia freely floating within the semicircular canals [2]. Vertigo occurs after specific head movements and has the characteristics of nystagmus, with respect to latency time, fatigability, and transiency. Canalithiasis and cupulolithiasis are the most likely mechanisms underlying BPPV. Although any of the three semicircular canals may be involved, canalithiasis of the posterior semicircular canal is the underlying cause in at least 85% of patients [3–5].
Oxidative stress, which is defined as excessive production of reactive oxygen species (ROS) that is not counterbalanced by adequate endogenous and exogenous antioxidant defenses, causes cellular dysfunction and is a risk factor for microvascular injury [6]. Several studies have shown an elevation in oxidative stress levels in different pathologies, with higher-than-control levels of biomarkers such as modified lipids, proteins, and nucleic acids, reduction in antioxidant capacity, and increased ROS production by leukocytes. In otolaryngology, the relationship between oxidative stress and laryngeal cancer, hearing loss, rhinosinusitis, otitis media, chronic tonsillitis, and other conditions has been investigated [7–10]. In a recent study, it has been revealed that calcium metabolism and its relationship with oxidative stress may play a role in the development of BPPV [7].
The aim of this prospective study was to evaluate the role of oxidative stress, using native thiol thiol/disulfide (SH/SS) homeostasis as a novel indicator, in the etiology of BPPV.
MATERIALS and METHODS
Patients and Controls
This prospective two-center study was conducted from June 2017 to July 2017 at the Department of Otolaryngology Head and Neck Surgery of the Ümraniye Training and Research Hospital and at the Department of Otolaryngology Head and Neck Surgery of İçerenköy Hospital, Bayındır Health Care group. The study was approved by the Ethics Committee of Ethics Committee of Ümraniye Training and Research Hospital (number 2017/76). Written informed consent was obtained from all participants recruited for the study.
Totally, there were 62 participants: 31 patients with BPPV (study group) and 31 healthy individuals without any cochleovestibular disorders (control group).
Patients with hearing loss, a history of otologic surgery, neurologic disorders, smoking, malignancy, autoimmune disorders, hypertension, endocrine disorders including diabetes mellitus and hypothyroidism, cardiovascular disease, a history of antiaggregant therapy, infectious diseases, or other inflammatory conditions were excluded.
Vestibular and Acoustic Evaluation
All participants underwent a complete otorhinolaryngologic examination, including a neuro-otologic examination. The otologic examination included otomicroscopy to visualize the tympanic membrane for vesicles attributable to herpes zoster infection and to determine the presence of chronic ear disease or retraction pockets with cholesteatoma. The Valsalva maneuver and pushing on the tragus cartilage (fistula test) were performed; it was evaluated whether either would induce a vertigo attack, which would have suggested a perilymphatic fistula. All participants underwent pure-tone audiometry. The neurologic examination focused on gait, balance, and coordination. Gait and balance were assessed using Romberg’s sign and the Fukuda stepping test. The presence of cerebellar signs was evaluated to exclude central pathologies.
For the diagnosis of BPPV in patients whose symptoms worsened with sudden head movements, videonystagmography-assisted Dix–Hallpike and supine roll tests (Pagnini–McClure maneuver) were performed. The characteristic pattern of BPPV nystagmus and the history of the disease were recorded for all patients in the study group. Only those with posterior canal BPPV were included. Patients in the study group had geotropic, torsional nystagmus beating toward the undermost ear. The nystagmus duration was less than 60 s, with a latency of a few seconds and a decline in response upon performing repeat maneuvers. Epley’s repositioning maneuver was performed in the treatment of patients with BPPV. The Dix–Hallpike test was repeated for all patients with BPPV 2 days after the diagnosis of posterior canal BPPV; Epley’s repositioning maneuver was repeated if patients complained of vertigo, nausea, or vomiting or if nystagmus was determined during the Dix–Hallpike test. The latter test was performed in control individuals 21 days after the first hospital admission.
Blood Sample Collection
Peripheral venous blood samples were obtained from the patients with BPPV upon first hospital admission (during the vertigo attack) and 21 days after treatment. Blood samples were obtained from the healthy controls during a routine medical examination. Plain tubes were used to collect blood from the patients and controls. The samples were centrifuged for 10 min at 1,500 g, after which the serum was separated and stored at −80°C until further analysis. Serum SH, total thiol (TT), and SS levels were analyzed in these samples, and SS/SH, SS/TT, and SH/TT ratios were calculated according to the method of Erel and Neselioglu [11].
Statistical Analysis
The NCSS 2007 program (NCSS, Kaysville, Utah, USA) was used for statistically evaluating the data. The mean, standard deviation, median, minimum, maximum, frequency, and percentage values served as descriptive statistics. Normally distributed data were compared between the groups with the independent samples t-test. The paired samples t-test was used for within-group analysis of normally distributed data, and Pearson’s chi-squared test was applied to evaluate qualitative data. A p-value of <0.05 was considered to indicate statistical significance. The 95% confidence intervals were also determined.
RESULTS
There was no statistically significant difference between the study and control groups with respect to the mean age (59.65±10.97 and 58.35±4.75 years, respectively) or male:female ratio (14:17 and 14:17, respectively) (Table 1). The demographics of the patients with BPPV and healthy controls are shown in Table 1.
Table 1.
Demographic parameters
| Total | Control group (n=31) | Patient group (n=31) | p | ||
|---|---|---|---|---|---|
| Age (years) | Min–Max | 34–78 | 51–67 | 34–78 | a0.551 |
| Mean±SD | 59.00±8.41 | 58.35±4.75 | 59.65±10.97 | ||
| Gender; n (%) | Female | 34 (54.8) | 17 (54.8) | 17 (54.8) | b0.999 |
| Male | 28 (45.2) | 14 (45.2) | 14 (45.2) |
Independent samples t-test
Pearson’s chi-squared test
SD: Standard deviation
However, the differences between the oxidative parameters in the patient and control groups were statistically significant. At baseline, the patients with BPPV had significantly lower SH levels and SH/TT ratios and significantly higher SS/SH and SS/TT ratios than the healthy controls. After treatment for vertigo, the patients with BPPV still had lower SH levels and SH/TT ratios and significantly higher SS/SH and SS/TT ratios than the healthy controls (Figure 1, 2) (Table 2).
Figure 1.
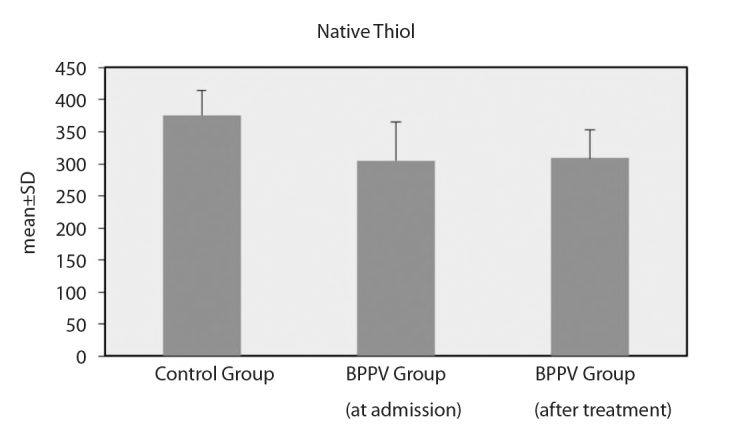
Native thiol levels
Figure 2.
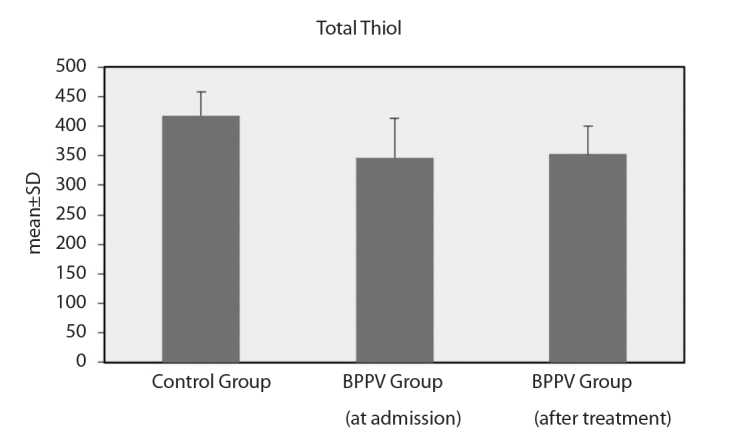
Total thiol levels
Table 2.
Serum native thiol/disulfide and total thiol levels
| 0Control group | 1Patient group (at admission) | 2Patient group (after treatment) | ap0–1 | ap0–2 | cp1–2 | ||
|---|---|---|---|---|---|---|---|
| SH | Min–Max | 280.30–454.80 | 128.60–395.30 | 218.90–394.20 | <0.001** | <0.001** | 0.751 |
| Mean±SD | 375.95±38.95 | 305.22±59.70 | 308.60±45.08 | ||||
| TT | Min–Max | 329.80–514.50 | 162.27–456.51 | 248.68–451.32 | <0.001** | <0.001** | 0.620 |
| Mean±SD | 417.98±40.92 | 347.03±66.03 | 352.88±47.90 | ||||
| SS | Min–Max | 15.70–34.45 | 1.10–38.71 | 7.97–36.71 | 0.956 | 0.504 | 0.545 |
| Mean±SD | 21.01±4.87 | 20.91±9.77 | 22.14±7.96 | ||||
| SS/SH | Min–Max | 4.02–10.78 | 0.45–18.97 | 3.52–22.32 | <0.001** | <0.001** | 0.671 |
| Mean±SD | 5.76±1.72 | 10.23±4.81 | 10.67±4.45 | ||||
| SS/TT | Min–Max | 3.72–8.87 | 0.44–13.75 | 3.29–15.43 | <0.001** | <0.001** | 0.630 |
| Mean±SD | 5.12±1.32 | 8.23±3.42 | 8.59±2.91 | ||||
| SH/TT % | Min–Max | 82.26–92.56 | 72.49–99.11 | 69.14–93.42 | <0.001** | <0.001** | 0.630 |
| Mean±SD | 89.75±2.65 | 83.54±6.84 | 82.83±5.83 |
Independent samples t-test
Paired samples t-test
p<0.01
SD: standard deviation; SH: native thiol; TT: total thiol; SS: disulfide
DISCUSSION
To the best of our knowledge, this is the first study to investigate SH/SS homeostasis as a novel marker of oxidative stress in patients with BPPV. Specifically, we examined SH/SS homeostasis in a study group of patients with BPPV and a control group of healthy individuals. Our study showed a significant difference between the groups.
Free oxygen radicals are naturally generated during every reaction in the body. Normally, these unstable electron-laden chemicals are largely destroyed or removed by the body’s natural antioxidant defense systems. Oxidative stress occurs due to an inadequate response to the formation of free radicals. Among the major non-enzymatic antioxidants able to eliminate oxidative stress in the cell are sulfhydryl group (−SH) containing SHs [11]. Circulating blood albumin binds SH groups via albumin cysteine residues. Reversible SS bonds form with cysteine residues located at the active sites of the protein, thereby reducing the toxicity of ROS [12, 13]. TT levels in cells remain constant to ensure continuous SH/SS homeostasis, reflecting the turnover between SSs and SHs [11].
A global plasma oxidative stress index was recently developed and validated in several diseases. Oxidative stress is related to cardiovascular diseases and their risk factors, such as diabetes, hypertension, and obesity, all of which are highly prevalent in numerous countries all over the world. A relationship between a decline in thiol levels and several systemic diseases has been demonstrated in many studies [14, 15]. While SH/SS homeostasis could previously only be measured by measuring the levels of individual components, Erel and Neselioglu [11] described a new method that allows for the measurement of the levels of these compounds both individually and cumulatively.
Episodes of dizziness are common in the elderly and significantly increase the risk of falls. The incidence of BPPV increases with age. Peripheral vestibular dysfunction, including BPPV, is one of the most common causes of dizziness among the elderly and is one of the most frequent diseases seen in dizziness clinics. The results of our study revealed that oxidative stress may be one of the etiologic factors in the development of BPPV. However, in our study, while SH and TH levels were significantly lower in the study group than in the control group, SS levels did not significantly differ between the groups. Patients with BPPV were treated with Epley’s repositioning maneuver and then followed up (outpatient visits) until nystagmus was resolved. SH and TH levels increased after treatment, but this was not statistically significant. The SS/SH, SS/TT, and SH/TT ratios, which represent corrected values and predominantly indicate oxidative homeostasis, significantly differed between the control and study groups: the patients with BPPV had significantly higher SS/SH and SS/TT ratios and lower SH/TT ratios. These findings are consistent with an increase in oxidative stress in patients with BPPV. However, there was no significant difference in oxidative stress parameters in the patients with BPPV before treatment versus after treatment. Although oxidative stress may play a role in the development of BPPV, the increase in oxidative stress did not respond to the treatments that we administered to our patients. Güçlütürk et al. [16] studied the levels of the antioxidant paraoxonase in patients with BPPV before and after treatment and reported results similar to ours.
In the semicircular canals, the presence of otoconial debris originating from the utriculus and sacculus causes BPPV when the head position is changed quickly and suddenly [2]. Otoconia consist of calcite, a mixture of calcium and carbonates. The structure of otoconia differs from that of teeth and bones because of its carbonate, rather than phosphate, composition. In the inner ear, calcium and carbonate levels are under the control of the calcium channel transport system. Normal levels of calcium and carbonate are important for the maintenance of otoconial function. Vibert et al. [17] designed an osteoporosis model in ovariectomized rats and studied the ultrastructure of their otoconia. The otoconia of these rats were less dense and contained less calcium; similar mechanisms may underlie the pathogenesis of BPPV. Talaat et al. [18] found that patients with recurrent or non-recurrent BPPV had significantly lower levels of vitamin D than control patients, while patients with recurrent BPPV had significantly lower vitamin D levels than those with non-recurrent BPPV. In another study, Talaat et al. [19] found a relationship between the recovery of serum 25-hydroxyvitamin D3 levels and a significant reduction in the rate of BPPV relapse.
Oxidative stress is related to calcium metabolism, with the endoplasmic reticulum being the most important cellular site for calcium storage and protein folding. In the presence of cell stress, the endoplasmic reticulum may initiate an increase in cellular calcium levels, causing rupture of the mitochondrial membrane and apoptosis.
Recent studies have suggested that oxidative stress and inner ear diseases are related. Brosel et al. [20] reported a strong link between oxidative stress, the related apoptosis of cochlear cells, and age-related hearing loss. Dinc et al. [11] found significant differences in SH/SS homeostasis between patients with sudden sensorineural hearing loss and control patients. Tsai et al. [21] reported increased levels of oxidative stress markers in blood samples from patients with BPPV.
Iwasaki and Yamasoba. [22] showed a correlation between age-related decrease in vestibular function and age-related decline in vestibular hair cells and neurons. The underlying mechanism of age-related cell loss in the vestibular end organ is not known, but the cumulative effect of a genetic predisposition and oxidative stress may play an important role. They recommended conducted further studies on the protective effect of antioxidant therapies with respect to vestibular function during aging.
These studies, together with the present results, indicate a role of oxidative stress in the development of BPPV, through both calcium metabolism and the direct toxic effects of free oxygen radicals, which trigger apoptosis. These mechanisms may also have a synergistic effect.
CONCLUSION
Our study was conducted on a small patient group. Further studies with larger samples are needed to evaluate and compare other oxidative stress markers, such as paraoxonase and arylesterase, as well as the total antioxidant status in patients with BPPV.
Footnotes
Ethics Committee Approval: Ethic committee approval was received for this study from Ethics Committee of Ümraniye Training and Research Hospital (Decision No: 2017/76).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.Ş.; Design - E.Ş.; M.E.D.; Supervision - E.Ş.; Resource - E.Ş.; Materials - E.Ş., C.B., Ö.E.; Data Collection and/or Processing - B.Y.Ö.; Analysis and/or Interpretation - C.B., Ö.E.; Literature Search - İ.D.; Writing - İ.D.; Critical Reviews - E.Ş., M.E.D., İ.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Liu Y, Wang W, Zhang AB, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: A network meta-analysis. Laryngoscope. 2016;126:951–5. doi: 10.1002/lary.25688. [DOI] [PubMed] [Google Scholar]
- 2.Fay JL. Benign Paroxysmal Positional Vertigo in 2 Children: A Case Series. Pediatr Phys Ther. 2016;28:355–60. doi: 10.1097/PEP.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 3.Hall SF, Ruby RR, McClure JA. The mechanics of benign paroxysmal vertigo. J Otolaryngol. 1979;8:151–8. [PubMed] [Google Scholar]
- 4.Schuknecht HF. Cupulolthiasis. Arch Otolaryngology. 1969;70:765–78. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Rea PA. Bilateral posterior semi-circular canal obliteration surgery for refractory benign paroxysmal positional vertigo (BPPV) in three patients. Clin Otolaryngol. 2017;42:480–3. doi: 10.1111/coa.12636. [DOI] [PubMed] [Google Scholar]
- 6.Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Radic Res. 2007;41:1364–75. doi: 10.1080/10715760701732830. [DOI] [PubMed] [Google Scholar]
- 7.Güçlütürk MT, Ünal ZN, İsmi O, Çimen MB, Ünal M. The Role of Oxidative Stress and Inflammatory Mediators in Benign Paroxysmal Positional Vertigo. J Int Adv Otol. 2016;12:101–5. doi: 10.5152/iao.2015.1412. [DOI] [PubMed] [Google Scholar]
- 8.Unal M, Tamer L, Pata YS, Kilic S, Degirmenci U, Akbaş Y, et al. Serum levels of antioxidant vitamins, copper, zinc and magnesium in children with chronic rhinosinusitis. J Trace Elem Med Biol. 2004;18:189–92. doi: 10.1016/j.jtemb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Doğruer ZN, Unal M, Eskandari G, Pata YS, Akbaş Y, Cevik T, et al. Malondialdehyde and antioxidant enzymes in children with obstructive adenotonsillar hypertrophy. Clin Biochem. 2004;37:718–21. doi: 10.1016/j.clinbiochem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Ulusoy S, Ayan NN, Dinc ME, Is A, Bicer C, Erel O. A new oxidative stress marker for thiol-disulphide homeostasis in seasonal allergic rhinitis. Am J Rhinol Allergy. 2016;30:53–7. doi: 10.2500/ajra.2016.30.4308. [DOI] [PubMed] [Google Scholar]
- 11.Dinc ME, Ulusoy S, Is A, Ayan NN, Avincsal MO, Bicer C, et al. Thiol/disulphide homeostasis as a novel indicator of oxidative stress in sudden sensorineural hearing loss. J Laryngol Otol. 2016;130:447–52. doi: 10.1017/S002221511600092X. [DOI] [PubMed] [Google Scholar]
- 12.Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326–32. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–86. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JA, Poland B, Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys. 1995;319:1–9. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- 15.Kundi H, Ates I, Kiziltunc E, Cetin M, Cicekcioglu H, Neselioglu S, et al. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am J Emerg Med. 2015;33:1567–71. doi: 10.1016/j.ajem.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Güçlütürk MT, Ünal ZN, İsmi O, Çimen MB, Ünal M. The Role of Oxidative Stress and Inflammatory Mediators in Benign Paroxysmal Positional Vertigo. J Int Adv Otol. 2016;12:101–5. doi: 10.5152/iao.2015.1412. [DOI] [PubMed] [Google Scholar]
- 17.Vibert D, Sans A, Kompis M, Travo C, Muhlbauer RC, Tschudi I, et al. Ultrastructural changes in otoconia of osteoporotic rats. Audiol Neurootol. 2008;13:293–301. doi: 10.1159/000124277. [DOI] [PubMed] [Google Scholar]
- 18.Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Otorhinolaryngol. 2015;272:2249–53. doi: 10.1007/s00405-014-3175-3. [DOI] [PubMed] [Google Scholar]
- 19.Talaat HS, Kabel AM, Khaliel LH, Abuhadied G, El-Naga HA, Talaat AS. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx. 2016;43:237–41. doi: 10.1016/j.anl.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Brosel S, Laub C, Averdam A, Bender A, Elstner M. Molecular aging of the mammalian vestibular system. Ageing Res Rev. 2016;26:72–80. doi: 10.1016/j.arr.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Tsai KL, Cheng YY, Leu HB, Lee YY, Chen TJ, Liu DH, et al. Investigating the role of Sirt1-modulated oxidative stress in relation to benign paroxysmal positional vertigo and Parkinson’s disease. Neurobiol Aging. 2015;36:2607–16. doi: 10.1016/j.neurobiolaging.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki S, Yamasoba T. Dizziness and Imbalance in the Elderly: Age-related Decline in the Vestibular System. Aging Dis. 2014;6:38–47. doi: 10.14336/AD.2014.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]


