Abstract
OBJECTIVES
The aim of the present study was to investigate the relationships between tumor size, hearing, and vestibular outcomes in patients with vestibular schwannomas (VSs).
MATERIALS and METHODS
Adult patients (n=124) with unilateral extrameatal VS prior to surgery were included in the study. This was a retrospective cohort study of preoperative audiovestibular investigations including audiometry, discrimination test, caloric test, cervical vestibular evoked myogenic potential (c-VEMP), and ocular vestibular evoked myogenic potential (o-VEMP).
RESULTS
The difference between lesioned and non-lesioned ear was significant for all audiovestibular outcomes. The mean caloric deficit was 74%. No tumor sided o-VEMPs were elicited. Caloric deficit correlated with hearing loss measured with pure tone average and discrimination score. c-VEMP deficit was significantly associated with severe hearing loss and larger tumors.
CONCLUSION
The presence of VS leads to a significant deterioration of audiovestibular function in all objective measures. Caloric test and o-VEMPS are sensitive though unspecific measures of VSs. Increasing tumor size is not directly associated with hearing loss and only somewhat to vestibular deficit. However, audiovestibular findings are correlated.
Keywords: Hearing loss, vestibular disorders, acoustic neuroma, surgery, treatment, diagnostics
INTRODUCTION
Vestibular schwannomas (VSs) are benign tumors originating from the Schwann cells of the vestibular branch of the eighth cranial nerve. Recent studies have shown that the incidence of sporadic VS tumors is increasing steadily and is currently estimated to be approximately 20 cases per million/year [1]. Asymmetric sensorineural hearing loss and tinnitus are the most common symptoms, but vertigo is also frequently occurring, indicating the affection of vestibular system function. Despite being of vestibular nerve origin, magnetic resonance imaging (MRI) screening protocols for VS are based on hearing acuity rather than vestibular function [2]. Several studies have shown an association between VS and loss of vestibular function as evaluated by, for example, caloric response [3–6] and vestibular evoked myogenic potentials (VEMPs) [3, 5–8]. However, our knowledge remains limited regarding vestibular function in relation to tumor size and hearing acuity. In addition, little is known on tumor affection of the different parts of the vestibular system, as well as a potential relationship between the function of the individual parts of the vestibular system, i.e., the neuroepithelia of the saccule, utricle, and cristae of the semicircular canals.
Periodic MRI evaluations of tumor progression are based on size [9]. Further knowledge on alternative measures regarding tumor size and loss of audiovestibular function seems warranted.
The aim of the present study was to investigate the relationship between the tumor size and the outcomes of audiovestibular tests in patients with sporadic VS, as well as the potential relationships between the degree of hearing loss and the degree of vestibular function loss, as evaluated by a vestibular function test panel.
MATERIALS and METHODS
Data on tumor size, hearing, and vestibular function were retrieved retrospectively from the files of 127 consecutive patients operated at our tertiary referral center by the translabyrinthine (TLA) or retrolabyrinthine (RLA) approach for sporadic VS. Indication for surgery was either tumor size or tumor growth. The study was conducted in accordance with the Declaration of Helsinki. Informed consent and ethics committee approval were not applicable as the study was based on retrospective file review.
The tumor size was determined by the largest extrameatal tumor diameter on the latest preoperative MRI and classified according to the consensus on reporting VS size by Kanzaki et al. [10]. According to the system, the extrameatal part of the tumor can be small (1–10 mm), medium (11–20 mm), moderately large (21–30 mm), large (31–40 mm), and giant (>40 mm). Isolated intrameatal tumors have an extrameatal extension of 0 and comprise their own subgroup of tumors. However, to enable subgroup analysis of small tumors, the intrameatal tumor group and small tumor group were merged.
Hearing acuity was determined the day before surgery by pure tone audiometry and speech discrimination score (DS). The pure tone average (PTA) was determined by the average of the thresholds of 250, 500, 1000, 2000, 4000, and 8000 Hz.
The vestibular test panel (also performed the day before surgery) consisted of caloric test with bithermal irrigation and quantified according to slow phase velocities (Aqua Stim; Interacoustics, Middelfart, Denmark). Unilateral weakness (UW) was graded as defined by Tringali et al. [11] as UW <20% was considered normal, UW between 20% and 70% signified moderate hypofunction, and UW >70% implied severe hypofunction. Cervical vestibular evoked myogenic potential (c-VEMP) for test of saccular function and ocular vestibular evoked myogenic potential (o-VEMP) for test of utricular function consisted of air-conducted clicks of 100 dBnHL (decibel normal hearing level) presented consecutively to each ear (Eclipse; Interacoustics, Middelfart, Denmark). The VEMP results were assessed binarily to determine whether a potential was elicited or not. All vestibular tests were performed by the same investigator.
Statistical Analysis
GraphPad Prism version 7.0 for Mac OS X (GraphPad Software, San Diego, CA, USA) was applied for data analyses [12]. Mann–Whitney tests for non-parametric data were performed to compare groups. Spearman coefficients (rs) and chi-square (χ2) tests were calculated for correlation analysis. A p-value <0.05 was defined as significant.
RESULTS
Three tumors were excluded from the analysis because the tumor was found to belong to the facial nerve. All included tumors (n=124) were isolated intrameatal tumors (n=4) and tumors with extrameatal extension (n=120). The cohort consisted of 66 (53%) women and 58 (47%) men. Tumors were right-sided in 63 (51%) cases and left-sided in 61 (49%) cases. The mean age of the patients was 55 (Standard deviation, SD 13.4) years at surgery. The mean extrameatal size of all tumors was 19 (SD 5.9) mm. Regarding size, 11 tumors were graded as small, 61 tumors were graded as medium, and 52 tumors were graded as moderately large.
Hearing Acuity
The mean DS on tumor side was 52% compared with 96% on the contralateral side, resulting in a mean DS difference of 44% between the two ears. Of the patients, 90% had an asymmetrical DS, of which 97% were worst on the tumor ear. The mean PTAs were 55 (SD 27.1 dB) dBHL (decibel hearing loss) for tumor ears and 20 (SD 16.4 dB) dBHL for non-tumor ears (Table 1, Fig. 1). There were no significant differences in hearing acuity (PTA and DS) according to tumor grade (Table 2).
Table 1.
Vestibular and auditory results for lesioned side and non-lesioned side
| Side | PTA (mean) | DS (mean) | Caloric hypofunction | c-VEMP elicited | o-VEMP elicited |
|---|---|---|---|---|---|
| Tumor | 55 db | 52% | 98%* | 22% | 0% |
| No tumor | 20 db | 96% | 1% | 81% | 37% |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Differences between the sides are significant.
Mean caloric hypofunction was 72%. Of the caloric findings, 92% were considered abnormal (>20% hyporeflexia) of which all were tumor sided.
PTA: pure tone average; DS: discrimination score; c-VEMP: cervical evoked vestibular myogenic potential; o-VEMP: ocular evoked vestibular myogenic potential
Figure 1.
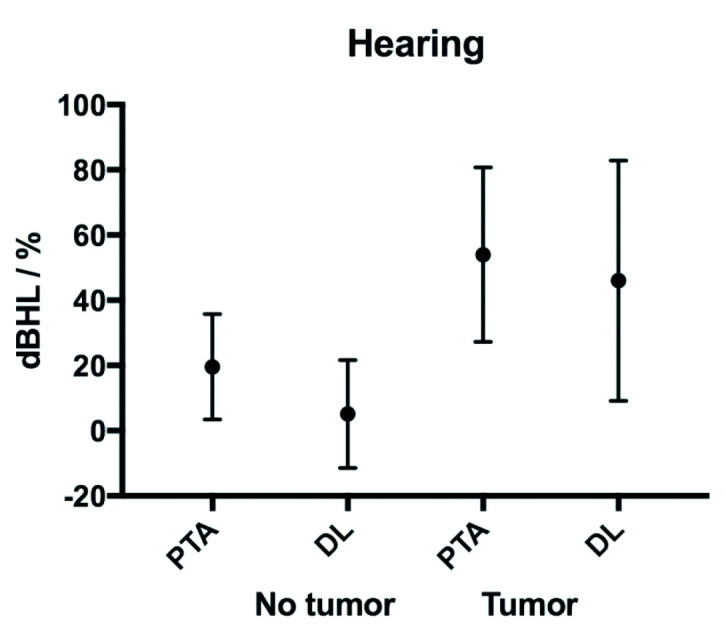
Audiometric findings according to side. X axis displays PTA and DL (mean with SD) for lesioned side and non-lesioned side. Y axis displays the degree of hearing loss in dB hearing level and % for PTA and DL, respectively.
PTA: pure tone average; DL: discrimination loss (100–discrimination score).
Table 2.
Audiovestibular function according to tumor grade
| Grade | Size | PTA | DS | Caloric | c-VEMP (%) | o-VEMP (%) |
|---|---|---|---|---|---|---|
| Intrameatal (n=4) | 0 | 34 | 69 | 51 | 50 | 0 |
| Small (n=7) | 9 | 48 | 53 | 63 | 43 | 0 |
| Medium (n=61) | 16 | 59 | 51 | 72 | 14 | 0 |
| Moderately large (n=52) | 24 | 54 | 51 | 75 | 26 | 0 |
| All (n=124) | 19 | 55 | 52 | 72 | 22 | 0 |
PTA: pure tone average; DS: discrimination score; c-VEMP: cervical evoked vestibular myogenic potential; o-VEMP: ocular evoked vestibular myogenic potential
Vestibular Function
All patients except two had vestibular hypofunction as evaluated by caloric response on the tumor ear. One patient had bilateral normal response, and one patient had hypofunction to the non-tumor ear. The mean caloric hypofunction was 72%. Of the caloric findings, 95% were abnormal (>20% hyporeflexia) of which all were tumor sided.
Of the c-VEMPs, 66% were asymmetrical. Of these, all but four represented negative c-VEMPs on the tumor ear and positive c-VEMPs on the contralateral ear. The remaining 34% had symmetrical c-VEMPS, either bilaterally positive or bilaterally negative. Of these, 22% elicited a c-VEMP response on the tumor ear compared with 81% on the healthy ears. Then, 37% of the o-VEMPs were asymmetrical, in all cases by a negative response on the tumor ear and a positive response on the non-tumor ear. The symmetrical o-VEMPs (67%) were all bilaterally negative. Thus, no tumor sided o-VEMPs were elicited (Fig. 2).
Figure 2.
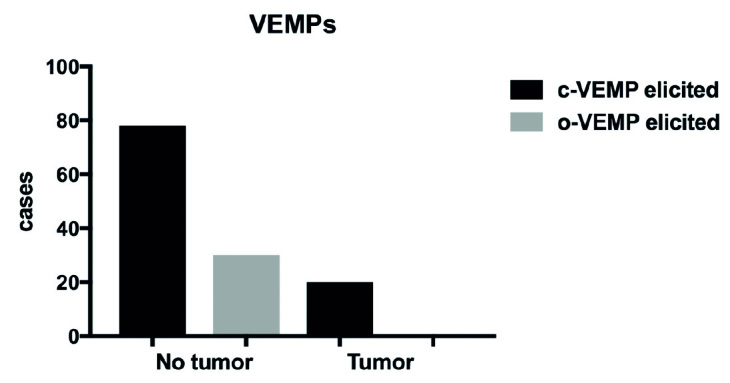
VEMPs according to side. Tumors tended to eliminate VEMP signals: 21% of c-VEMPs and 0% of o-VEMPs were elicited on tumor side.
c-VEMP: cervical evoked vestibular myogenic potential; o-VEMP: ocular evoked vestibular myogenic potential.
Correlations between Tests
Using Spearman coefficients, there was a strong correlation between PTA and DS (rs=0.86, p<0.001). The correlations between PTA and DS against the caloric response were weak (rs=0.25 and rs=0.27). There were no correlations between tumor size and audiometric tests. In addition, there was no correlation between tumor size and caloric function.
In the intrameatal and small tumor subgroup, 45% of the tumors elicited a c-VEMP potential compared with 14% in the medium tumor group (p=0.02) and 26% in the moderately large tumor group (p=0.19).
Patients with an absence of c-VEMPs showed higher PTA thresholds (two-tailed p=0.07 and one-tailed p=0.035), though the same group did not have significantly lower DS. Similarly, there was no association to severe caloric deficit. Since all o-VEMPs were negative on the tumor side, tests of correlations between o-VEMPs and other tests were not possible.
Surgical Approach
There were significantly more small- and medium-sized tumors than larger tumors in the RLA group (χ2, p=0.02 and p=0.003, respectively). There was no difference in approach between small- and medium-sized tumors (p=0.82). The mean PTA and DS in the RLA group were 32 dBHL and 94% compared with 72 dBHL and 53% in the TLA group (p<0.0001 and p<0.0001). The mean caloric hypofunction was UW=41% in the RLA group compared with UW=65% in the TLA group (p=0.001). There was no difference between surgical approach and elicitation of VEMPs (p=0.4).
DISCUSSION
Vestibular schwannomas are known to cause unilateral damage to both auditory and vestibular functions [3, 5, 9, 13–15]. For all individuals in the present study, there was a highly significant difference between lesioned and non-lesioned side for all auditory and vestibular tests. Our patient population represents a selected subgroup of patients with VS, since they have been elected for surgery because of accelerated tumor growth or symptom complaints. The difference between the ears is expected to be somewhat greater among this particular patient group than the background VS population that also counts smaller asymptomatic tumors [9, 16]. The mean tumor side PTA and DS corresponded to a class C hearing loss according to the American Academy of Otolaryngology–Head and Neck Surgery classification [17].
Almost all patients (98%) had a lesioned side caloric hypofunction. The mean UW of 72% corresponds to a severe hypofunction according to a previous classification [11].
There was absence of o-VEMPs on the tumor side with positive contralateral o-VEMPs in 37% of the cases, indicating superior vestibular nerve (SVN) affection on the lesioned side. Most tumors are located on the inferior vestibular nerve (IVN) and as a consequence affecting c-VEMP signals. The results indicate that SVN is affected in all cases of VS though most tumors originate from IVN [3, 18]. The aim for both c- and o-VEMP tests is to evaluate the corresponding end organ, which is the saccule and utricle, respectively. Data suggest that these organs appear to have different sensitivity for the presence of a tumor. However, these organs are supplied by afferents from different parts of the vestibulocochlear nerve. A recent histopathological study has documented that VS in one part of the nerve leads to loss of ganglions in the corresponding end organ, potentially leading to deteriorating function [19]. SVN and related blood supply are anatomically susceptible to entrapment due to the relationship to the internal auditory canal wall [20]. Hearing loss could also be a result of external compression of the cochlear nerve. Anterograde degeneration of end organs may also be present [19, 20]. Our clinical results are in agreement with these observations, although specific perioperative tumor location (SVN or IVN origin) was not available. It seems unfeasible to predict tumor location on the basis of VEMPs alone, which is in accordance with other findings [21]. Being inherently sensitive to tumor, it may be possible to assemble a battery of audiovestibular tests that could indicate tumor growth, instead of the rising patient population enrolled for routine periodic MRI. In recent literature, the video head impulse test has proven effective in detecting peripheral vestibular disorders [22], including VSs [23–25]. However, the specific clinical value and correlation with other vestibular test findings need further investigation.
The positive correlation between the auditory tests was expected [26–28]. However, tumor size failed to correlate with PTA and DS. Previous histopathological studies have shown that >80% of the ganglions have to be lost before a shift in PTA threshold occurs, and that the loss of ganglions affects discrimination and PTA disproportionally [29]. Contrary to our results, other studies have shown a correlation between caloric hypofunction and size [5, 11, 21]. An explanation for the missing correlation in our study could be the relatively small size interval of tumors of which 91% had a diameter of 11–30 mm. This interval differs from the other studies that have a larger distribution of small tumors and a larger range of sizes [5, 11]. In the present study, the medium and moderately large groups were similarly affected on all audiovestibular parameters (Table 2), which could indicate a ceiling effect on the loss of function for many tumors in this size range.
There was an association between good hearing (PTA) and c-VEMP potential, indicating that pure tone hearing loss is associated with loss of vestibular function as measured with VEMP. However, the association between hearing and canal paresis was weak.
Canal paresis was more prevalent in the group lacking c-VEMP potential; however, the difference was not significant. As found in previous studies [6, 30], four patients without canal paresis was missing c-VEMP, which may be explained by the location of the tumor since c-VEMP and caloric test investigate the inferior and superior nerve pathways, respectively. Preserved c-VEMP was associated with smaller tumors, which is in agreement with other studies [5, 25].
The difference in PTA, DS, and size between the RLA and TLA groups was expected since surgical approach is determined by the presence of good hearing of smaller tumors [14]. It is interesting, however, that even though caloric deficit had only weak to non-existing association with hearing and size, the deficit was significantly lower in the RLA group. In contrary, VEMPs were independent of surgical approach.
A weakness of MRI in assessing VS size is the neglect of the intrameatal lesion that might cause vestibular deficits [9, 10, 24]. In addition, a limitation of the study is the varying time between the last MRI and the audiovestibular tests, the latter being performed the day before surgery. Thus, the true size of a tumor may be underestimated. These issues could explain the missing correlations between deficit and tumor size.
CONCLUSION
The present study correlates tumor size with audiovestibular tests by horizontal comparisons of individuals that may respond differently to tumors of the same size due to anatomical and physiological differences. The study finds a significant deterioration of audiovestibular function in the presence of VS in all objective measures. Both caloric test and VEMPs are sensitive yet unspecific measures regarding tumor side. In the present study, increasing tumor size has only a limited association with loss of peripheral vestibular function and has no significant effect on hearing loss.
Though inconsistently affected, audiovestibular function is highly sensitive to the presence of VSs; however, the interdependent factors between audiovestibular parameters remain to be fully understood. Future studies should appreciate a vertical perspective linking individual tumor progression and function.
Acknowledgements
The authors would like to thank vestibular assistant Gerd Hansen for her important work.
Footnotes
Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects”, (amended in October 2013).
Informed Consent: Informed consent for surgical intervention was received for all cases. Approval was obtained by the National Board of Health.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – N.W., S.H., M.N.M., P.C.T.; Design – N.W., S.H.; Supervision – S.H., M.N.M., P.C.T.; Resource – N.W., P.C.T.; Materials – S.H., P.C.T.; Data Collection and/or Processing – N.W., M.N.M.; Analysis and/or Interpretation – N.W., M.N.M.; Literature Search – N.W., M.N.M,; Writing – N.W., M.N.M., P.C.T.; Critical Reviews – M.N.M., P.C.T.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Stangerup SE, Tos M, Thomsen J, Caye-Thomasen P. True incidence of vestibular schwannoma? Neurosurgery. 2010;67:1335–40. doi: 10.1227/NEU.0b013e3181f22660. [DOI] [PubMed] [Google Scholar]
- 2.Gimsing S. Vestibular schwannoma: when to look for it? J Laryngol Otol. 2010;124:258–64. doi: 10.1017/S0022215109991423. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsumi T, Tsunoda A, Noguchi Y, Komatsuzaki A. Prediction of the nerves of origin of vestibular schwannomas with vestibular evoked myogenic potentials. Am J Otol. 2000;21:712–5. [PubMed] [Google Scholar]
- 4.Linthicum FHJ, Churchill D. Vestibular test results in acoustic tumor cases. Arch Otolaryngol. 1968;88:604–7. doi: 10.1001/archotol.1968.00770010606007. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Yamada C, Inoue R, Kashio A, Saito Y, Nakanishi W. Analysis of vestibular testing in patients with vestibular schwannoma based on the nerve of origin, the localization, and the size of the tumor. Otol Neurotol. 2008;29:1029–33. doi: 10.1097/MAO.0b013e3181845854. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki S, Takai Y, Ito K, Murofushi T. Abnormal vestibular evoked myogenic potentials in the presence of normal caloric responses. Otol Neurotol. 2005;26:1196–9. doi: 10.1097/01.mao.0000194890.44023.e6. [DOI] [PubMed] [Google Scholar]
- 7.Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: Past, present and future. Clin Neurophysiol. 2010;121:636–51. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Ushio M, Iwasaki S, Murofushi T, Sugasawa K, Chihara Y, Fujimoto C, et al. The diagnostic value of vestibular-evoked myogenic potential in patients with vestibular schwannoma. Clin Neurophysiol. 2009;120:1149–53. doi: 10.1016/j.clinph.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Stangerup SE, Caye-Thomasen P. Epidemiology and Natural History of Vestibular Schwannomas. Otolaryngol Clin NA. 2012;45:257–68. doi: 10.1016/j.otc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24:642–9. doi: 10.1097/00129492-200307000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Tringali S, Charpiot A, Ould MB, Dubreuil C, Ferber-Viart C. Characteristics of 629 vestibular schwannomas according to preoperative caloric responses. Otol Neurotol. 2010;31:467–72. doi: 10.1097/MAO.0b013e3181cdd8b7. [DOI] [PubMed] [Google Scholar]
- 12.Graphad Software. www.graphpad.com.
- 13.Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): Clinical presentation. Neurosurgery. 1997;40:1–10. doi: 10.1097/00006123-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Stangerup S, Caye-Thomasen P, Tos M, Thomsen J. Change in hearing during “wait and scan” management of patients with vestibular schwannoma. J Laryngol Otol. 2008;122:673–81. doi: 10.1017/S0022215107001077. [DOI] [PubMed] [Google Scholar]
- 15.Tos M, Charabi S, Thomsen J. Clinical-Experience with Vestibular Schwannomas - Epidemiology, Symptomatology, Diagnosis, and Surgical Results. Eur Arch Otorhinolaryngol. 1998;255:1–6. doi: 10.1007/s004050050012. [DOI] [PubMed] [Google Scholar]
- 16.Stangerup SE, Thomsen J, Tos M, Cayé-Thomasen P. Long-term hearing preservation in vestibular schwannoma. Otol Neurotol. 2010;31:271–5. doi: 10.1097/MAO.0b013e3181c34bda. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Otolaryngology-Head and Neck Surgery Foundation. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma) Otol Head Neck Surg. 1995;113:179–80. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 18.Khrais T, Romano G, Sanna M. Nerve origin of vestibular schwannoma: a prospective study. J Laryngol Otol. 2008;122:128–31. doi: 10.1017/S0022215107001028. [DOI] [PubMed] [Google Scholar]
- 19.M⊘ller MN, Hansen S, Caye-Thomasen P. Peripheral vestibular system disease in vestibular schwannomas. Otol Neurotol. 2015;36:1547–53. doi: 10.1097/MAO.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 20.Goebel JA, O’Mara W, Gianoli G. Anatomic considerations in vestibular neuritis. Otol Neurotol. 2001;22:512–8. doi: 10.1097/00129492-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Ushio M, Iwasaki S, Chihara Y, Kawahara N, Morita A, Saito N, et al. Is the nerve origin of the vestibular schwannoma correlated with vestibular evoked myogenic potential, caloric test, and auditory brainstem response? Acta Otolaryngol. 2009;129:1095–100. doi: 10.1080/00016480802552543. [DOI] [PubMed] [Google Scholar]
- 22.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: Diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–41. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blödow A, Pannasch S, Walther LE. Detection of isolated covert saccades with the video head impulse test in peripheral vestibular disorders. Auris Nasus Larynx. 2013;40:348–51. doi: 10.1016/j.anl.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Batuecas-Caletrio A, Santa Cruz-Ruiz S, Mu-oz-Herrera A, Perez-Fernandez N. The map of dizziness in vestibular schwannoma. Laryngoscope. 2015;125:2784–9. doi: 10.1002/lary.25402. [DOI] [PubMed] [Google Scholar]
- 25.Taylor RL, Kong J, Flanagan S, Pogson J, Croxson G, Pohl D, et al. Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. J Neurol. 2015;262:1228–37. doi: 10.1007/s00415-015-7697-4. [DOI] [PubMed] [Google Scholar]
- 26.Massick DD, Welling DB, Dodson EE, Scholfield M, Nagaraja HN, Schmalbrock P, et al. Tumor growth and audiometric change in vestibular schwannomas managed conservatively. Laryngoscope. 2000;110:1843–9. doi: 10.1097/00005537-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Kirchmann M, Karnov K, Hansen S, Dethloff T, Stangerup SE, Caye-Thomasen P. Ten-year follow-up on tumor growth and hearing in patients observed with an intracanalicular vestibular schwannoma. Neurosurgery. 2016;80:49–56. doi: 10.1227/NEU.0000000000001414. [DOI] [PubMed] [Google Scholar]
- 28.Van Linge A, Borsboom GJJM, Wieringa MH, Goedegebure A. Hearing loss progresses faster in patients with growing intracanalicular vestibular schwannomas. Otol Neurotol. 2016;37:1442–8. doi: 10.1097/MAO.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 29.Schuknecht HF. Auditory and cytocochlear correlates of inner ear disorders. Otolaryngol Head Neck Surg. 1994;110:530–8. doi: 10.1177/019459989411000610. [DOI] [PubMed] [Google Scholar]
- 30.Murofushi T, Matsuzki M, Mizuno M. Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1998;124:509–12. doi: 10.1001/archotol.124.5.509. [DOI] [PubMed] [Google Scholar]


