Abstract
OBJECTIVES
Spiral ganglion (SG) counting is used in experimental studies conducted on age-, noise-, and drug-induced sensorineural hearing loss, as well as in the assessment of cochlear implant performances. Different methods of counting have been reported, but no definite standardization of such procedure has been published. The aim of our study is to identify the best method to count human spiral ganglions (SGs).
MATERIALS and METHODS
By identification of nuclei or nucleoli as described by Schucknect, seven researchers with different experience levels counted SGs in 123 human temporal bones (TBs). Data on time of post-mortem bone removal post-mortem, methods of specimen’s fixation, decalcification, and coloration were collected to test their possible influence on human tissue. Percentage, two-tailed t-test, Spearman’s test, and one-way ANOVA were used to analyze the data.
RESULTS
Nucleoli were identified in 61% of cases, whereas nuclei were recognized in 100% of cases (p<0.005). Nucleoli presence in all four segments in the same temporal bone (TB) was observed in 69 cases (92%), whereas nuclei were identified in all four segments in 103 cases (83.7%) (p<0.001). The junior investigators requested a double check by the seniors in 25 (20.3%) cases for identifying and counting nucleoli, whereas the senior researchers showed no doubts in their identification and count. The only parameter positively affecting nucleoli identification in tissue preparation was bone removal for <12 h with respect to longer post-mortem time (p<0.001).
CONCLUSION
We suggest counting nuclei, rather than nucleoli, for spiral ganglion computation because of easier recognition of nuclei, especially in case of investigator’s limited experience.
Keywords: Spiral ganglion, hearing loss, count method, feasibility, accuracy
INTRODUCTION
The spiral ganglions (SGs) are structures deputed to conduct sound-evoked neural activity from inner ear hair cells (IHCs) to the central nervous system. Approximately 95% of SGs’ auditory nerve fibers form synapses with IHCs, whereas about 5% with outer hair cells (OHCs) [1].
The SGs can be damaged by noise [2–7], drugs [8–11], electromagnetic radiation [12], oxidative stress, and aging [3, 13, 14]. The reduction of SGs may affect the quality of sound perception, and more importantly, word discrimination in humans.
Recently, Liberman and Kujawa [15, 16] have highlighted the extreme vulnerability of the afferent synapses and type I SG neurons that contact IHCs; their data suggest that the most vulnerable structures are the afferent terminals of the IHCs that connect to type I auditory nerve fibers and SGs [10, 15–17].
The identification and count of SGs is widely used in experimental studies conducted on age- [18–21], noise-, and drug-induced sensorineural hearing loss [22], as well as on laboratory tests on otoprotective drugs [23, 24]. The number of preserved SGs is also considered an important factor to improve performances in subjects with cochlear implant [25–27]. The correct identification and count of SGs has a relevant role in basic and clinical research.
A technique for SGs count was first described by Schuknecht [28] in 1978, and then by Merchant [29] and Nadol et al. [30] several years later. The SGs in human temporal bones (TBs) resemble fried eggs; they contain one nucleus (mean diameter: 10 μm) that has a nucleolus inside it (mean diameter: 2.5 μm) [30]. The counting of SGs is usually based on the identification of cells’ nuclei, but because of the large diameter of nuclei, this method is associated with a high risk of cell double-counting. In fact, a nucleus that belongs to the same spiral ganglion (SG) may be present in two different sequential tissue sections (cut at a 10 μm distance between one and the other) because of its diameter. To avoid the bias due to double counting of structure on different planes and to section preparation, some correction coefficients have been identified [29, 31–33]. The correction coefficients consider the sections’ thickness and the diameter of the structure (nucleus or nucleolus). Despite such coefficients, the exact counting of SGs remains imprecise.
Some authors proposed to count nucleoli rather than nuclei because the smaller diameter of nucleoli may reduce the risk of cell double-counting [31].
Nowadays, the choice between counting nuclei or nucleoli is personal, and is mainly related to the habit and experience of the researcher, which makes the comparison among different studies on this topic extremely unreliable.
The differences between the two methods (nuclei versus nucleoli count) have never been reported in the literature. Moreover, the effect of specimen preparation details (such as timing of bone post-mortem removal, tissue fixation, and coloring) has also never been investigated.
The aims of this study were to (a) identify the easier and more accurate method (nuclei versus nucleoli) to count SGs in human TBs, thereby comparing the results obtained by senior and junior researchers, and (b) identify one or more variables in patient characteristics and specimen preparation that may modify the identification of nucleoli in SGs. A possible standardization of SGs counting procedure is proposed.
MATERIALS and METHODS
This study has been approved by the ethical committee of the hospital; all study procedures were conducted in accordance with the declaration of Helsinki and Institutional Review Board regulations. Informed consent was signed by donors before temporal bone (TB) removal.
Seven different researchers with different experience levels analyzed 123 TBs from 67 adults.
The senior researchers (n=3) had 3-year experience in this field, whereas the juniors (n=4) had 1-year experience. All researchers analyzed the same slides.
Bone decalcification was performed using trichloroacetic acid (TCA) or ethylenediaminetetraacetic acid (EDTA), whereas specimen fixation was done using formalin (10%).
A Zeiss light microscope with 20× and 40× magnifications was used. An ocular grid was applied on the microscope for increasing the accurancy during the count of SGs.
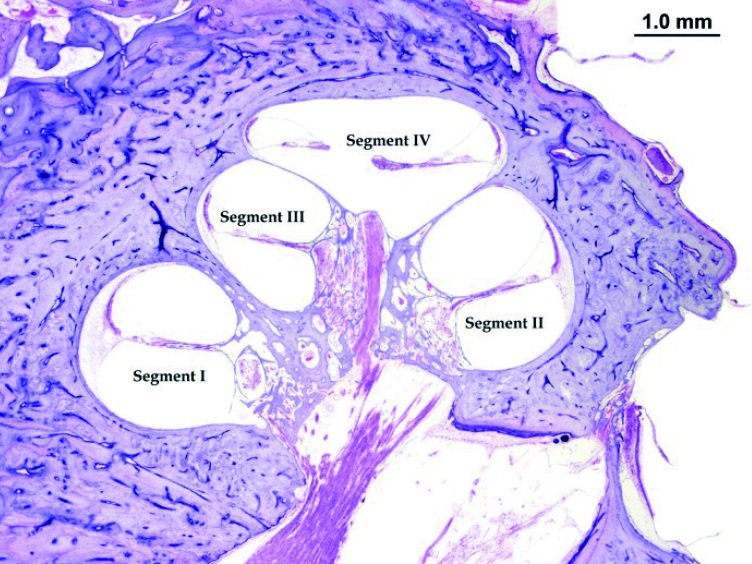
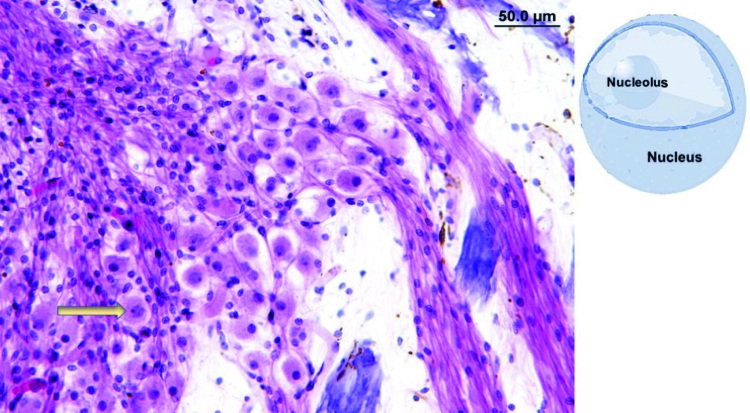
The presence of nucleoli inside the nuclei was counted in the SGs along the cochlear Rosenthal canal (RC), as previously described by Merchant [29] and Nadol et al [30]. The RC was divided into four segments as follows: (a) segment I, from uncus to the half of cochlea basal turn; (b) segment II, from the end of segment I until the beginning of cochlear middle turn; (c) segment III, corresponding to the middle turn of the cochlea; and (d) segment IV, corresponding to the cochlear apex (Figure 1). The identification of nuclei and nucleoli was based on their size and position inside the SG: nuclei are bigger than nucleoli and are less colored; nucleoli are found inside nuclei and have a more intense coloration (Figure 2). The prevalent structure (nucleus or nucleolus) in the four segments of RC was recorded; to define a structure as prevalent, it was necessary that it is detectable in >55% of observed SGs.
Figure 1.
Segments division of human cochlea: segment I: basal turn; segment II: basal/middle turn; segment III: middle turn; and segment IV: apex. Hematoxylin-eosin staining.
Figure 2.
Violet tissue coloration (hematoxylin-eosin staining). Magnification 20Å~. The arrow shows the nucleolus visible inside the nucleus. Top right corner: sche- matic representation of the structures.
Nucleolus identification was recorded with a score: 1 if present and 0 if not.
All the TBs studied were stained by hematoxylin-eosin (HE) method.
The seven researchers were aware about the two different count methods, and they analyzed the same sections using a double-blind approach: each investigator counted SGs separately from the other, and nobody knew the number of the specimen and the patient name. For each slice, SGs count was performed by using one or the other method (nuclei and nucleoli) based on the structure that was more identifiable at a 20× magnification. The structures were assessed in each RC segment. To define their presence in the examined cochlea, it was necessary to identify the structure (nucleolus or nucleus) in at least three of the four segments of the RC.
The results of the analysis of each researcher were grouped as per researchers’ experience into senior and junior; then the overall results for different levels of experience were compared to evaluate the difference in the ability to identify nuclei or nucleoli.
If, at the end of the count, the results of the junior researchers’ did not match with those of the seniors, a double check was performed by the senior researcher to confirm the observed data (Table 1); the double-blinded method was used only in the first counting round; further, when the count was done, all researchers were made aware of the specimen’s details (number and patient name) to allow the comparison of the collected results.
Table 1.
The table is an example of the data collection and shows the identification of nucleoli (1) or nuclei (0) in the different segment of human cochlea. When the researcher reported a doubt result, the data was collected as 0/1. In the last column on the right the general identification of nucleoli or nuclei by considering the TB in toto. In case of presence of nucleoli (or nuclei) in at least 3 on 4 segments of TB we considered an overall of nucleoli (or nuclei).
| Segment I | Segment II | Segment III | Segment IV | General identification in all segment | |||||
|---|---|---|---|---|---|---|---|---|---|
| Junior | Master | Junior | Master | Junior | Master | Junior | Master | Junior | Master |
| 0 | 0 | 0/0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0/1 | 1 | 0/1 | 1 | 0/1 | 1 | 0 | 1 |
| 0/1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 0/1 | 1 | 0/1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 0/1 | 1 | 1 | 1 |
| 0/1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 0 | 1 | 0 | 0 | 0 | 0 | 0/1 | 0 | 0 | 0 |
| 0/1 | 1 | 0 | 0 | 0 | 0 | 0/1 | 0 | 0 | 0 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 0/1 | 0 | 0/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0/1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0/1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Segment I: First Segment of Rosental Canal
Segment II: Second Segment of Rosental Canal
Segment III: Third segment of Rosental Canal
Segment IV: Fourth segment of Rosental Canal
Causes of death, post-mortem time of bone removal, and patients’ age were analyzed and eventually correlated with nuclei and nucleoli counting.
Based on the identification of the prevalent structure (nucleus or nucleolus), TBs were divided into two groups: (a) group 1 included the TBs in which nucleoli were identified more frequently than nuclei with a prevalence of 55% in the slide, and (b) group 2 included the TBs with a prevalence of nuclei over nucleoli of >55%. Such groups were analyzed to assess if differences in individual patient or specimen preparation characteristics were present between the groups.
Statistical analysis
It was performed using STATA® (Statacorp, College Station, TX 77845, Stati Uniti). Percentage was calculated, and mean and standard deviation were identified for numerical values. One-way ANOVA was used to compare the variance between the results obtained by the researchers. Nucleoli count for each segment (I, II, III, and IV) from both groups of researchers were evaluated to understand if there was a variance. Two-tailed t-test was used to evaluate the difference between nucleoli and nuclei identification in the count method. Spearman’s test was used to identify the correlation between the method used and presence of nucleoli; time of bone removal (<12 h or >12 h) and nucleoli identification; sex and nucleoli identification and age and nucleoli presence. Chi-square test was used to evaluate if the causes of death may determine differences in nucleoli identification. A p<0.05 was considered statistically significant.
RESULTS
Nucleoli versus Nuclei Count
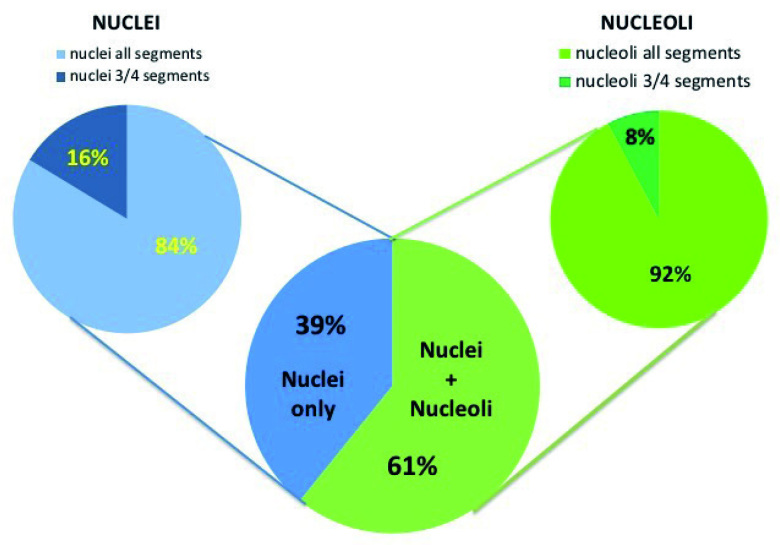
Nuclei were identified in all cases (100%), whereas nucleoli were identified in 61% (75/123) of cases (t-test p<0.005). When sorting by the number of RC segments (3/4, 4/4), 83.7% (103/123) of nuclei and 92% (69/75) of nucleoli were identified in 4/4 segments (t-test: p<0.001), whereas 16.3% (20/123) of nuclei and 8% (6/75) of nucleoli were observed in 3/4 of segments (Figure 3).
Figure 3.
Percentage of nuclei/nucleoli identification in the different specimens. The subgroup images show the cochlear segments where nuclei/nucleoli were observed. Interrupted red line shows that in case of visible nucleoli, nuclei are always recognizable.
Differences between junior and senior researchers were observed in nucleoli count. Junior investigators requested a double check by senior researchers in 18.7% of cases to confirm the identification of nucleoli: in 11.4% of cases for segment I (n=14), 1.6% for segment II (n=2), 1.6% for segment III (n=2), and 4.1% for segment IV (n=7). The senior researcher group had no doubts in nucleoli identification in any segment analysis. The difference between the results obtained by junior and senior researchers was statistically significant only for segment I (ANOVA: p=0.03), whereas non-significant difference was found for segment II (ANOVA: p=1), segment III (ANOVA: p=0.5), and segment IV (ANOVA: p=0.7). Considering the overall (segments I–IV) nucleoli count for each cochlea, the difference between junior and senior researchers was not statistically significant (ANOVA p=0.5). For identifying nucleoli, 20× magnification was used in 89.4% of cases (n=110), whereas 40× magnification was necessary in 10.6% of cases (n=13).
Specimen Characteristics
No significant correlation was found between decalcification methods used (TCA or EDTA) and the capacity to identify nucleoli (Spearman: P=0.9).
The coloration method was always the same, but the operator changed during the years, leading to a variation related to human bias. Post-mortem time of bone removal was <12 hours in the nucleoli group (mean: 5.9; SD: 2.4) in 54% of cases, whereas it was <12 hours in 47% the nuclei group (mean: 7.8; SD: 2.8). Post-mortem time was equally distributed between the two groups (t-test: p >0.05), which made our sample homogeneous. A higher identification rate of nuclei and nucleoli was found in case of bone removal <12 h rather than longer post-mortem time (>12 h) (Spearman: p<0.001).
Individual Characteristics of Subjects
In this study, the authors included 123 TBs extracted from 67 adults with a mean age of 69 years (range: 19–92) (Figure 4). The variation in the expected number of TBs (134) was due to the limited availability of both TBs from the same subject in some cases, in fact sometimes only a single TB was available.
Figure 4.
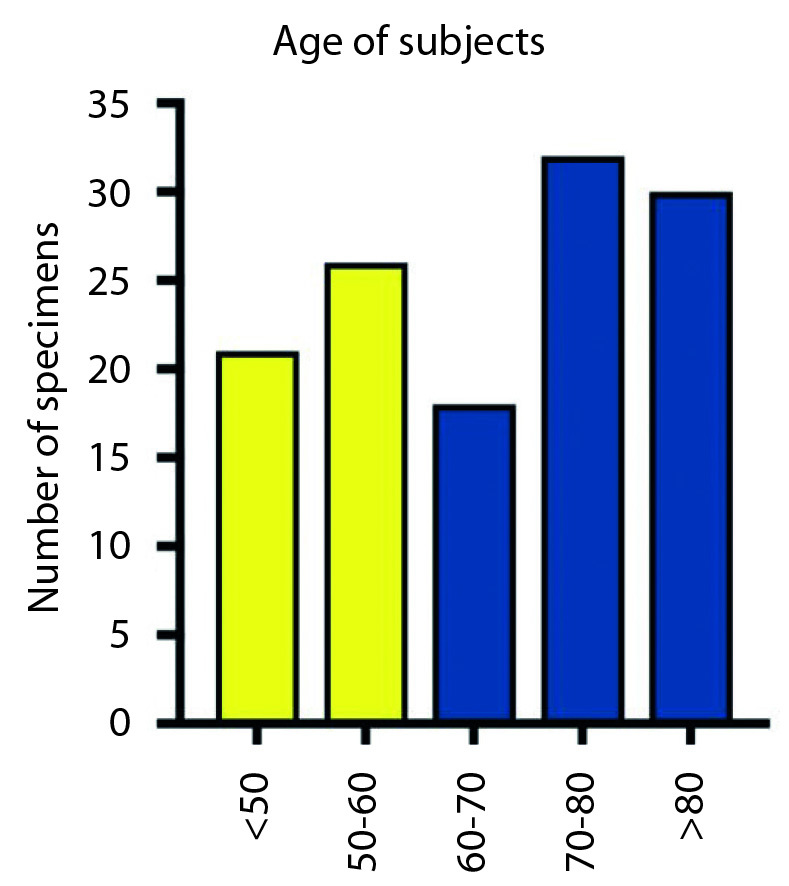
Age distribution in the overall sample; the yellow bars indicate the number of subjects with age <60 years, and the blue the subjects with age >60 years. The subjects were divided by decades to facilitate the understanding of sample age distribution.
The age was not statistically correlated with the visibility and the identification of nucleoli (Spearman: p=0.24).
Sample was equally divided with respect to sex (36 men: 52%; 33 women: 48%). Female sex was statistically correlated with the visibility and identification of nucleoli (Spearman: p=0.01).
Cardiovascular diseases were the most common cause of death in group 2 (35%), followed by cancer (30%) and infection (19%). In group 1, cancer (35%) and cardiovascular diseases (35%) were the most common causes of death, followed by infections in 14% of cases (Figure 5).
Figure 5.
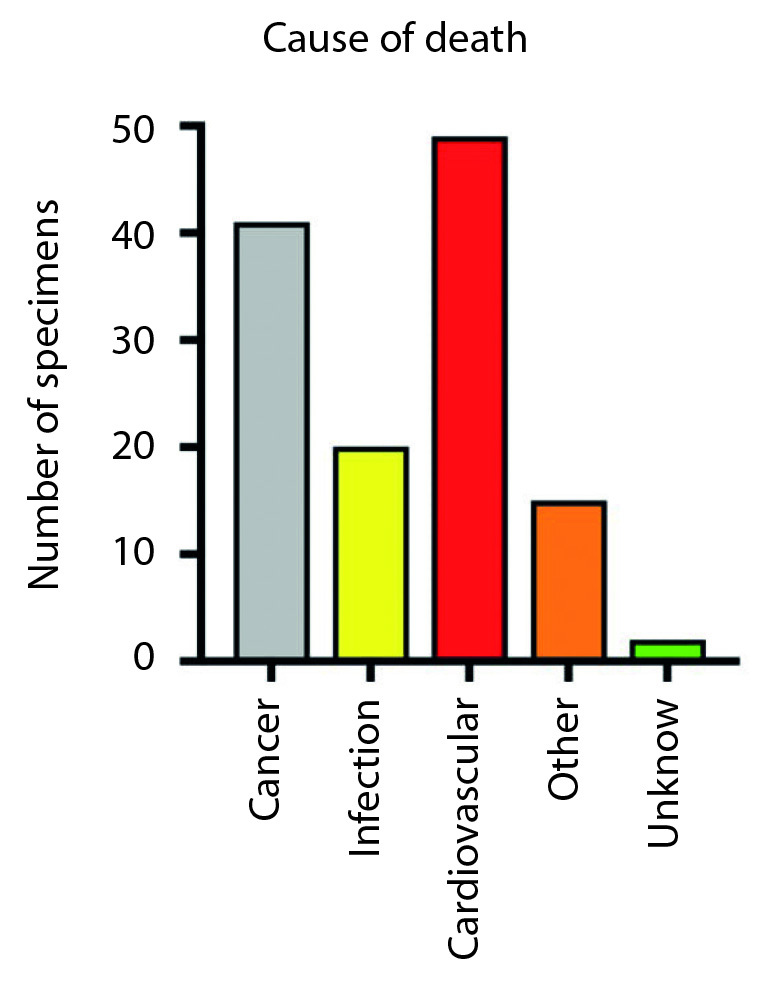
The image shows the overall number of temporal bones and the distribution of the different causes of death. Cardiovascular diseases (red bar) and cancer (grey bar) were the most common causes of death.
No significant correlation was found between the different causes of death and the nucleolus identification (χ: p=0.7)
DISCUSSION
Main Difficulties in Nucleoli Identification
Our study shows no statistically significant differences in counting nucleoli or nuclei between expert and junior researchers; however, in 18.7% of cases, junior researchers requested a double check by seniors to confirm the identification of nucleoli. The nuclei identification and count did not show percentage of doubt because of the larger size that simplifies the identification [29, 30, 34] in particular by the junior researchers.
The bigger size of nuclei allows their identification on lower magnification compared with that necessary for nucleoli, and researchers did not need to change magnification during the count, thereby simplifying and speeding up the count process.
Alternatively, in this study, nucleoli identification was sometimes extremely difficult for younger researchers; however, nuclei were always visible even when the nucleoli were identified (100%). Because of the small volume of the nucleoli and their different position on the specimens (higher or lower in the section), it was often necessary to change microscope focus to identify them, and eventually use a 40× magnification. Such magnification could explain why nucleoli were slightly more identifiable in all four segments compared to nuclei (92% versus 83.7%). Furthermore, we considered the presence of nucleoli as on/off. Therefore, when nuclei were identified in all four segments (83.7%), it was understood that the remaining structures were nucleoli. It is also relevant to state that every time nucleoli were observed, the nuclei were also identifiable, meaning that nuclei were always observable in all four segments in 100% of TBs.
Differences between Junior and Senior Researchers
The junior researcher group required a double check from senior investigators to identify nucleoli, especially in the basal (segment I) and apical (segment IV) turn of the cochlea; this was probably related to the bone conformation of these segments which makes nucleoli identification more difficult in such areas. The senior researchers never showed doubts in identifying the nucleoli. This confirms the hypothesis that experience is the most relevant element in such analysis. Our data reported an overall identification rate of 61% for nucleoli and 100% for nuclei in the observed specimens. However, the statistical analysis comparing the results obtained by the researchers showed no significant difference in this count.
Variables Associated with TBs Preparation
The analysis of the methodology (fixation and decalcification) used to prepare TBs showed no significant influence of TBs preparation methods on nucleoli/nuclei identification rate, supporting the idea that this factor is not relevant to make nucleoli identification easier. The only parameter able to modify nucleoli identification rate in TB preparation technique was bone removal within 12 h after death. This agrees with the study by Kujawa et al. [15] who attributed such result to the oxidative phenomena acting on human tissue and inducing its deterioration along with increasing post-mortem time [22,35].
Variables Associated with Patients’ Characteristics
Specific human tissue features, such as tissue acidity, may affect color absorption and therefore, nucleoli identification on histologic examination.
Our results show that it was easier to identify nucleoli in women than in men. We speculate that this could be correlated to the difference in food habits between women and men [36]. In fact, women prefer fruits and vegetables, which have an acid pH. This habit may affect the systemic pH concentration [37] and may explain the reason why nucleoli were more detectable in women than in men.
The acid-base reaction is also affected by protein concentration: some proteins are acidic as aspartate for example; therefore, an increase in protein concentration can explain the variation (more intense on hematoxylin reaction) in nucleoli coloration [38, 39]. Stan et al. [31] showed that RNA integrity is the best indicator of human tissue preservation. This parameter overlaps protein concentration and affects pH, and consequently, tissue coloration. Gadaleta et al. [40] showed a decrease in protein concentration in nuclear structures during the aging process [40], although we did not observe any correlation between nucleoli identification rate and patients’ age.
Nicolas et al. [41] and Orsolic et al [42] also showed that cancer can increase protein concentration within nucleoli. Our sample showed that a high number of subjects died of cancer (70%), although the percentage of death caused by cancer was similar in both groups (nucleoli and nuclei). Furthermore, we did not find any significant difference in death causes between nucleoli and nuclei groups. We believe this can be related to the limited number of subjects involved in this study, which did not reach a sufficient power for statistical analysis.
Another factor potentially affecting nucleoli/nuclei coloration is the operator: eosin concentration may differ among various technicians, which can determine a change in color intensity from pink to violet. In the violet coloration, nucleoli appear darker than nuclei, which makes them visible on a low-magnification microscope as well. When the coloration is blue, nucleoli identification is more difficult, thus requiring a higher magnification (Figure 6). The standardization of preparation techniques should be underlined to make the results of different studies comparable.
Figure 6.
Pink tissue coloration (hematoxylin-eosin staining). Magnification 20×. No nucleoli are detectable.
Study Limitations
The lack of standardization in the TB preparation may have introduced a bias in nuclei/nucleoli counting procedure of our study. In particular, HE coloration has some limitations that are operator dependent: (a) the quantity of eosin varies and depends on the operator’s experience and habits; (b) variations of hematoxylin concentration can modify color nuances from blue to violet, thus affecting nuclei/nucleoli identification. The same is valid for the decalcification method that may be affected by subject characteristics and operator experience and habits.
CONCLUSION
The identification of nucleoli in human TBs may be more difficult than nuclei because of the difference in coloration and size of these structures. In this study, nuclei identification was possible in all the cases. Because of the risk of cell double-counting, the correction factor suggested by Schuknecht [28] and Nadol et al. [30, 32] should be applied to determine the correct number of SG cells when using the nuclei identification procedure. Based on the results of this study, we suggest using nuclei count because of their easier recognition in all tissue conditions, especially when the researcher has a limited experience.
Acknowledgements
Thanks to the Temporal Bone Bank of the Mass Eye and Ear that hosted a part of this study. Special thanks to Prof Joseph Nadol jr and Dr Felipe Santos who introduced me to this particular technique and stimulate my interest in the field. Thanks to Mrs Diane Jones, Barbara Burgess, Meng Yu Zhen, and Jennifer O’Malley for helping in the understanding of methods to prepare the tissue and for supporting me during this study.
Footnotes
Ethics Committee Approval: The study was hosted at MEEI and because it was done on previously collected human temporal bones present in the Temporal Bone Bank of the Otopathology Laboratory, no ethics committee approval was necessary.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – A.D.S.; Design – A.D.S., M.R., R.I.; Supervision – G.R., G.B.; Resource – A.D.S., A.D.V., F.T.; Materials – A.D.S., M.R., R.I.; Data Collection and/or Processing – A.D.S., M.R., R.I., A.D.V., F.T., L.D.; Analysis and/or Interpretation – A.D.S., M.R., R.I., G.R.; Literature Search – L.D.; Writing – A.D.S.; Critical Reviews – M.R., G.R., G.B.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Thiers FA, Nadol JB, Jr, Liberman MC. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J Assoc Res Otolaryngol. 2008;9:477–89. doi: 10.1007/s10162-008-0135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniel E. Noise and hearing loss: a review. J Sch Health. 2007;77:225–31. doi: 10.1111/j.1746-1561.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- 3.Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 4.Bauer P, Korpert K, Neuberger M, Raber A, Schwetz F. Risk factors for hearing loss at different frequencies in a population of 47,388 noise-exposed workers. J Acoust Soc Am. 1991;90:3086–98. doi: 10.1121/1.401418. [DOI] [PubMed] [Google Scholar]
- 5.Fetoni AR, Mancuso C, Eramo SL, Ralli M, Piacentini R, Barone E, et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169:1575–88. doi: 10.1016/j.neuroscience.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Fetoni AR, Ralli M, Sergi B, Parrilla C, Troiani D, Paludetti G. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29:70–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Fetoni AR, Garzaro M, Ralli M, Landolfo V, Sensini M, Pecorari G, et al. The monitoring role of otoacoustic emissions and oxidative stress markers in the protective effects of antioxidant administration in noise-exposed subjects: a pilot study. Med Sci Monit. 2009;15:PR1–8. [PubMed] [Google Scholar]
- 8.Sheppard A, Hayes SH, Chen GD, Ralli M, Salvi R. Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital. 2014;34:79–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res. 2013;302:113–20. doi: 10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, et al. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265:63–9. doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei L, Ding D, Salvi R. Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience. 2010;168:288–99. doi: 10.1016/j.neuroscience.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo WQ, Hu YJ, Yang Y, Zhao XY, Zhang YY, Kong W, et al. Sensitivity of spiral ganglion neurons to damage caused by mobile phone electromagnetic radiation will increase in lipopolysaccharide-induced inflammation in vitro model. J Neuroinflammation. 2015;12:105. doi: 10.1186/s12974-015-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han C, Someya S. Mouse models of age-related mitochondrial neurosensory hearing loss. Mol Cell Neurosci. 2013;55:95–100. doi: 10.1016/j.mcn.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munzel T, Daiber A, Steven S, Tran LP, Ullmann E, Kossmann S, et al. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Eur Heart J. 2017;38:2838–49. doi: 10.1093/eurheartj/ehx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res. 2017;349:138–47. doi: 10.1016/j.heares.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330(Pt B):191–9. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadol JB., Jr Schuknecht: “Presbycusis.” (Laryngoscope 1955;65:402–419) Laryngoscope. 1996;106:1327–9. doi: 10.1097/00005537-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- 20.Nadol JB., Jr Degeneration of cochlear neurons as seen in the spiral ganglion of man. Hear Res. 1990;49:141–54. doi: 10.1016/0378-5955(90)90101-T. [DOI] [PubMed] [Google Scholar]
- 21.Johnsson LG, Hawkins JE., Jr Sensory and neural degeneration with aging, as seen in microdissections of the human inner ear. Ann Otol Rhinol Laryngol. 1972;81:179–93. doi: 10.1177/000348947208100203. [DOI] [PubMed] [Google Scholar]
- 22.Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, et al. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurioka T, Matsunobu T, Niwa K, Tamura A, Satoh Y, Shiotani A. Activated protein C rescues the cochlea from noise-induced hearing loss. Brain Res. 2014;1583:201–10. doi: 10.1016/j.brainres.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Kurioka T, Matsunobu T, Satoh Y, Niwa K, Endo S, Fujioka M, et al. ERK2 mediates inner hair cell survival and decreases susceptibility to noise-induced hearing loss. Sci Rep. 2015;5:16839. doi: 10.1038/srep16839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulet J, White M, Bruce IC. Temporal considerations for stimulating spiral ganglion neurons with cochlear implants. J Assoc Res Otolaryngol. 2016;17:1–17. doi: 10.1007/s10162-015-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou N, Pfingst BE. Evaluating multipulse integration as a neural-health correlate in human cochlear-implant users: Relationship to forward-masking recovery. J Acoust Soc Am. 2016;139:EL70–5. doi: 10.1121/1.4943783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuknecht HF. Further observations on the pathology of presbycusis. Arch Otolaryngol. 1964;80:369–82. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- 28.Schuknecht HF. Delayed endolymphatic hydrops. Ann Otol Rhinol Laryngol. 1978;87:743–8. doi: 10.1177/000348947808700601. [DOI] [PubMed] [Google Scholar]
- 29.Merchant SN, NJj . Schuknecht’s pathology of the Ear. 3th edition. 2010. pp. 36–42. [Google Scholar]
- 30.Nadol JB, Jr, Burgess BJ, Reisser C. Morphometric analysis of normal human spiral ganglion cells. Ann Otol Rhinol Laryngol. 1990;99:340–8. doi: 10.1177/000348949009900505. [DOI] [PubMed] [Google Scholar]
- 31.Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadol JB., Jr Quantification of human spiral ganglion cells by serial section reconstruction and segmental density estimates. Am J Otolaryngol. 1988;9:47–51. doi: 10.1016/S0196-0709(88)80007-3. [DOI] [PubMed] [Google Scholar]
- 33.Robert ME, Linthicum FH., Jr Empirical Derivation of Correction Factors for Human Spiral Ganglion Cell Nucleus and Nucleolus Count Units. Otolaryngol Head Neck Surg. 2016;154:157–63. doi: 10.1177/0194599815603964. [DOI] [PubMed] [Google Scholar]
- 34.Rosbe KW, Burgess BJ, Glynn RJ, Nadol JB., Jr Morphologic evidence for three cell types in the human spiral ganglion. Hear Res. 1996;93:120–7. doi: 10.1016/0378-5955(95)00208-1. [DOI] [PubMed] [Google Scholar]
- 35.Lagadic-Gossmann D, Huc L, Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 2004;11:953–61. doi: 10.1038/sj.cdd.4401466. [DOI] [PubMed] [Google Scholar]
- 36.Schwalfenberg Gerry K. The alkaline diet: is there evidence that an alkaline pH diet benefits health? J Environ Public Health. 2012;2012:727630. doi: 10.1155/2012/727630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifter JL, Chang HY. Disorders of acid-base balance: new perspectives. Kidney Dis (Basel) 2017;2:170–86. doi: 10.1159/000453028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alberts BJA, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th edition Garland Science; New York: 2002. [Google Scholar]
- 39.Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265–74. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 40.Gadaleta MN, Petruzzella V, Renis M, Fracasso F, Cantatore P. Reduced transcription of mitochondrial DNA in the senescent rat. Tissue dependence and effect of L-carnitine. Eur J Biochem. 1990;187:501–6. doi: 10.1111/j.1432-1033.1990.tb15331.x. [DOI] [PubMed] [Google Scholar]
- 41.Nicolas E, Parisot P, Pinto-Monteiro C, de Walque R, De Vleeschouwer C, Lafontaine DL. Involvement of human ribosomal proteins in nucleolar structure and p53-dependent nucleolar stress. Nat Commun. 2016;7:11390. doi: 10.1038/ncomms11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orsolic I, Jurada D, Pullen N, Oren M, Eliopoulos AG, Volarevic S. The relationship between the nucleolus and cancer: Current evidence and emerging paradigms. Semin Cancer Biol. 2016;37–38:36–50. doi: 10.1016/j.semcancer.2015.12.004. [DOI] [PubMed] [Google Scholar]