Abstract
OBJECTIVES
We aimed to assess the clinical significance of the intensity of transcutaneous vagus nerve stimulation (tVNS) in chronic tinnitus.
MATERIALS and METHODS
Four sessions of tVNS were performed over a 2-week period for 24 patients with unilateral, non-pulsatile chronic tinnitus. The cavum, cymba, and tragus were sequentially stimulated to the maximal sensory thresholds. One month later, after the four sessions, the level of tinnitus distress and changes in stimulus intensity were assessed.
RESULTS
The stimulus intensity did not differ according to sex or laterality. However, a moderate positive correlation between tinnitus distress and the initial stimulus intensity was observed. This correlation was not observed during the subsequent sessions. The stimulus intensity at the cavum changed significantly (p=0.018), and notable differences in tinnitus annoyance were observed between the responders and non-responders (p=0.006).
CONCLUSION
The effect of stimulus intensity on the treatment outcome seems to be limited. An increasing trend in the stimulus intensity for tinnitus annoyance at the cavum was observed in the responders. Therefore, the cavum may be an optimal stimulation site for tVNS.
Keywords: Tinnitus, neuromodulation, vagus nerve, prognosis
INTRODUCTION
The vagus nerve is the longest cranial nerve and is involved in the control of various functions of the entire body, including parasympathetic innervations to the heart, lung, and digestive organs; branchial motor functions, such as swallowing and speaking; and somatic and visceral sensations [1].
Cervical vagus nerve stimulation (VNS) has been approved for the treatment of refractory epilepsy and depression in the Unites States. The possible mechanisms of action include alterations in the activities of the reticular activating system, central autonomic network, limbic system, and diffuse noradrenergic projection system [2]. However, implanting a stimulator on the cervical branch of the vagus nerve is a complicated process, given the invasiveness and high cost of the procedure.
Non-invasive transcutaneous VNS (tVNS) of the auricular branch of the vagus nerve (ABVN) has been recently introduced. A functional magnetic resonance imaging (fMRI) scan has revealed that the deactivation of the limbic system-the amygdala, hippocampus, and parahippocampal gyrus-is observed after tVNS. These findings were similar to those found in the studies investigating cervical VNS [3].
Tinnitus refers to an acoustic perception without external sources. The possible etiologies that may lead to tinnitus include auditory alteration-related conditions (presbycusis, acoustic trauma, noise-induced hearing loss, sudden hearing loss, Meniere’s disease, and otosclerosis), somatosensory-auditory interactions (patulous eustachian tube, middle ear myoclonus, spasm of stapedius muscle or tensor tympani, and typewriter tinnitus), and psychiatric disorders (depression, anxiety, and obsessive-compulsive disorder) [4].
Regarding tinnitus, a promising preliminary study was recently performed to assess the combined effect of tVNS and tailor-made notched classical music [5]. The N1 m wave, which is one of the evoked auditory cortical responses during magnetoencephalography and found in some patients with sensorineural hearing loss, was attenuated, and a tendency toward decreased tinnitus distress was observed after the administration of treatment [5]. The authors stimulated the left tragus slightly above the sensory threshold using a clip-type electrode for 45–60 min each during seven sessions. Alternatively, other studies have described a stimulus intensity that was set to the highest threshold that patients could tolerate using a patch-type electrode [6]. In other studies, the intensity was simply documented as intervals from the minimal to maximal threshold without a detailed description [7, 8]. Although different criteria for stimulation intensity have been used in various studies, the clinical significance and the optimal location of the stimulation site have not been studied extensively.
In this study, we hypothesized that the maximal sensory thresholds at each tVNS site that could be tolerated without any painful sensation are associated with unique tinnitus characteristics and affect the treatment outcomes. Accordingly, we performed a prospective observational study to verify this hypothesis.
MATERIALS and METHODS
Patients
Between July 2015 and June 2016, the patients complaining of unilateral, non-pulsatile, subjective tinnitus lasting for more than 3 months and who visited the university hospital and agreed to participate were recruited for this study. In total, 24 patients (16 men, eight women; mean±standard deviation [SD] age, 44±10.93 years; age range, 24–62 years) were recruited into this prospective study. The mean duration of tinnitus was 31±49 months (range, 3–204 months). The proposed etiologies consisted of somatosensory tinnitus after trauma in seven cases (29.2%), noise-induced hearing loss in six cases (25.0%), sudden deafness in four cases (16.7%), Meniere’s diseases in three cases (12.5%), presbycusis in two cases (8.3%), acoustic trauma in one case (4.2%), and the undiagnosed in one case (4.2%).
The exclusion criteria were as follows: bilateral and/or pulsatile tinnitus, tinnitus under medical treatment, previous diagnosis of neuropsychiatric or dermatological diseases, and the presence of an implanted metal device in the cranial region. The Institutional Review Board of Eulji University approved this study. Written informed consent was obtained from all the patients.
Study Design
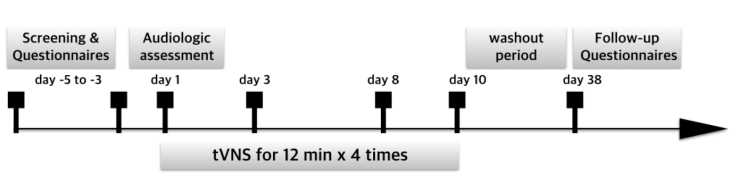
The overall study design is depicted in Figure 1. During the initial visit, the patients were screened and the severity of tinnitus was assessed using questionnaires, including the Tinnitus Handicap Inventory (THI), the Beck Depression Inventory (BDI), and a visual analog scale (VAS) from 0 to 10 (0: no symptoms, 10: maximal symptoms) regarding tinnitus loudness (LD), awareness (AW), annoyance (AN), and its effect on life (EL).
Figure 1.
Schematic overview of the study design.
tVNS: transcutaneous vagus nerve stimulation.
During the second visit, an audiological assessment comprising pure tone audiometry, speech audiometry, tinnitography, otoacoustic emissions, and test of auditory brainstem response was performed. The first tVNS was subsequently performed. All patients participated in the remaining three sessions during the consecutive visits over a 2-week period.
Following a 4-week washout period, the patients’ subjective distress regarding tinnitus was re-assessed using questionnaires. Patients whose final VAS score decreased by 50% or more were categorized as responders, whereas others were considered as non-responders.
The tVNS Protocol
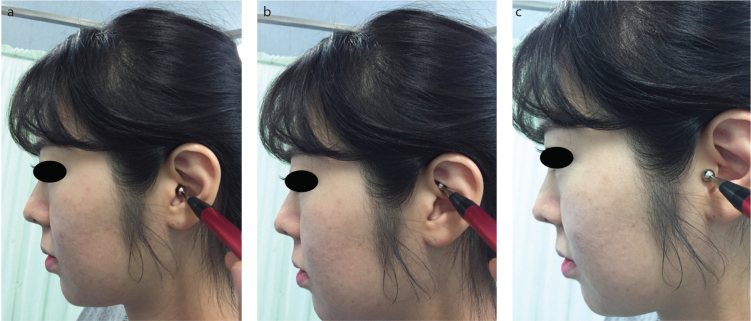
Non-invasive transcutaneous VNS (tVNS) was performed using a transcutaneous electrical nerve stimulation (TENS) device (ES-420, Ito Co., Ltd., Tokyo, Japan). The stimulation conditions were as follows: pulse width, 200 μs; frequency, 30 Hz; stimulation sites (in sequence), the cavum, cymba, and the outer surface of the tragus; and the duration of stimulation, 4 min for each site (Figure 2) [9]. A ball-type electrode was placed on the stimulation site, and the intensity was increased by 1 mA every 5 s until the maximum intensity that the patients could tolerate without feeling pain.
Figure 2. a–c.
Stimulation sites for auricular vagus nerve stimulation. A ball-type electrode was used for sequentially stimulating the cavum, cymba, and outer surface of the tragus. (a) Cymba concha stimulation, (b) cavum concha stimulation, (c) tragus stimulation.
Statistical Analysis
The two-tailed Fisher’s exact test was performed to assess the differences between the categorical variables. The changes in VAS, THI, and BDI scores after treatment were compared using the Wilcoxon signed-rank test. The correlations between the stimulus intensity and VAS were assessed using the Spearman correlation analysis. Repeated measures analysis of variance (ANOVA) was used to assess the differences in the stimulus intensity at each stimulation site over time. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 24.0 (IBM Corp., Armonk, NY, USA) for Macintosh (Apple Inc., Cupertino, CA, USA); p<0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Of the 24 patients, 16 complained of left-sided tinnitus and eight complained of right-sided tinnitus. The mean hearing threshold was 2622 dB in the affected side and 1821 dB in the unaffected side; this difference was not statistically significant (p>0.05). The mean THI and BDI scores were 4519 and 1211, respectively.
Differences in Stimulus Intensity According to Sex, Laterality, and Stimulation Site
The stimulation intensity did not show any significant difference with respect to sex and laterality (p>0.05). With regard to the stimulation site, the mean maximal sensory threshold at the tragus was 5.792.397 mA (range, 1–14 mA); this tended to be higher than that at the cymba (4.982.098 mA; range, 1–14 mA) or cavum (5.101.861 mA, range 1–12 mA; p=0.018).
Changes in VAS, THI, and BDI Scores After Treatment
All the VAS scores indicating tinnitus distress improved after the treatment (p<0.05) (Figure 1). In addition, the THI and BDI scores were significantly reduced to 2715 (p<0.001) and 911 (p=0.004), respectively. Based on the VAS scores, 33.3% (n=8), 62.5% (n=15), 45.8% (n=11), and 41.7% (n=10) of the patients were classified as responders based on LD, AW, AN, and EL, respectively.
Relationship Between Stimulus Intensity and Tinnitus Severity
The repeated measures ANOVA revealed that the stimulus intensity at the cavum differed significantly across sessions (p=0.018). Conversely, no significant change was observed at the cymba or tragus (p>0.05). Moreover, the sum of the stimuli at all stimulation sites did not change significantly (p>0.05).
At the cavum, a moderate positive correlation was observed between stimulus intensity and all VAS scores, except EL, in the first session (LD: r=0.570 and p=0.004; AW: r=0.481 and p=0.017; and AN: r=0.509 and p=0.011). However, only AW showed a moderate positive correlation in the second session (r=0.511 and p=0.011). No significant correlation was found between the VAS scores and stimulus intensity even in the third and fourth sessions. In addition, these trends were similar at both the cymba and tragus (data not shown).
The VAS scores of the responders were subsequently compared. The stimulus intensity at the cavum showed a significant difference between the responders and non-responders in terms of AN (Figure 3; p=0.006). However, no differences were observed at the other stimulation sites or in the VAS scores.
Figure 3.
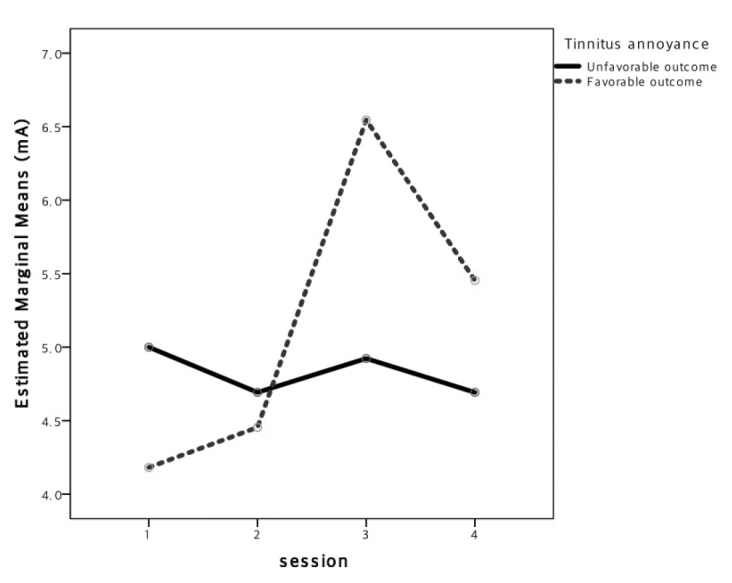
Differences in the stimulus intensity across sessions, according to treatment response. The x-axis refers to multiple sessions and the y-axis refers to mean maximal sensory thresholds. For tinnitus annoyance, a small increasing trend in the stimulus intensity at the cavum was observed in the responders but not in non-responders; the difference was statistically significant (p=0.006).
Lastly, the changes in THI and BDI scores and the percentage changes in the VAS scores between the initial and final observations were calculated (Table 1). Subsequently, the relationship between these scores, stimulation intensity at each site, and sum of stimuli at all sites were assessed using the Spearman’s correlation analysis. No significant correlation was observed between the stimulation intensity at each site and in the changes in the scores. Similarly, the sum of stimuli at all sites was not correlated to the scores, irrespective of the response to treatment (p>0.05).
Table 1.
Demographic information and the treatment outcome for tinnitus patients
| No. | Age, years | Sex | Side | Onset (months) | First session | Second session | Third session | Fourth session | ΔTHI | ΔBDI | ΔLD (%) | Δ AW (%) | Δ AN (%) | Δ EL (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CB | CC | T | SUM | CB | CC | T | SUM | CB | CC | T | SUM | CB | CC | T | SUM | |||||||||||
| 1 | 36 | M | Left | 13 | 3 | 5 | 4 | 12 | 4 | 5 | 4 | 13 | 3 | 5 | 4 | 12 | 7 | 6 | 4 | 17 | 28 | 7 | 50 | 70 | 43 | −33 |
| 2 | 60 | M | Left | 3 | 3 | 1 | 3 | 7 | 2 | 4 | 3 | 9 | 4 | 5 | 4 | 13 | 4 | 6 | 6 | 16 | 54 | 4 | 0 | 50 | 75 | 33 |
| 3 | 55 | M | Left | 18 | 6 | 7 | 6 | 19 | 5 | 7 | 5 | 17 | 6 | 5 | 4 | 15 | 6 | 6 | 5 | 17 | 18 | 3 | −20 | 100 | 0 | 100 |
| 4 | 52 | M | Left | 12 | 6 | 6 | 6 | 18 | 8 | 8 | 5 | 21 | 4 | 8 | 8 | 20 | 5 | 10 | 8 | 23 | 12 | 0 | 25 | 70 | 40 | 50 |
| 5 | 45 | M | Right | 4 | 5 | 8 | 6 | 19 | 2 | 5 | 6 | 13 | 2 | 8 | 5 | 15 | 3 | 4 | 6 | 13 | 4 | 0 | 0 | 0 | 40 | 30 |
| 6 | 62 | F | Left | 12 | 4 | 6 | 4 | 14 | 4 | 4 | 3 | 11 | 4 | 5 | 4 | 13 | 4 | 5 | 5 | 14 | −2 | 0 | −33 | 67 | −33 | 100 |
| 7 | 53 | M | Left | 3 | 7 | 7 | 7 | 21 | 7 | 14 | 7 | 28 | 10 | 12 | 7 | 29 | 5 | 3 | 4 | 12 | 20 | 0 | 13 | −67 | −50 | −17 |
| 8 | 39 | M | Right | 3 | 1 | 3 | 3 | 7 | 2 | 1 | 4 | 7 | 3 | 3 | 2 | 8 | 2 | 5 | 4 | 11 | 4 | 0 | −67 | 71 | −25 | −67 |
| 9 | 50 | F | Right | 24 | 7 | 7 | 8 | 22 | 4 | 7 | 8 | 19 | 6 | 7 | 8 | 21 | 6 | 4 | 8 | 18 | 52 | 0 | 14 | 70 | −40 | 0 |
| 10 | 57 | F | Left | 12 | 4 | 4 | 3 | 11 | 3 | 3 | 4 | 10 | 5 | 3 | 2 | 10 | 3 | 4 | 2 | 9 | 30 | 8 | 80 | 100 | 100 | 100 |
| 11 | 52 | M | Left | 32 | 9 | 10 | 5 | 24 | 10 | 10 | 6 | 26 | 14 | 14 | 12 | 40 | 8 | 8 | 8 | 24 | 4 | 0 | 40 | 50 | 50 | −67 |
| 12 | 42 | M | Right | 84 | 6 | 9 | 9 | 24 | 7 | 11 | 11 | 29 | 9 | 11 | 10 | 30 | 7 | 9 | 8 | 24 | 26 | 17 | 88 | 90 | 86 | 100 |
| 13 | 24 | F | Left | 3 | 6 | 7 | 5 | 18 | 4 | 6 | 6 | 16 | 6 | 6 | 5 | 17 | 6 | 6 | 5 | 17 | 16 | 19 | 50 | −100 | 0 | 20 |
| 14 | 28 | F | Left | 7 | 2 | 3 | 4 | 9 | 2 | 4 | 5 | 11 | 6 | 5 | 6 | 17 | 5 | 6 | 6 | 17 | 4 | 8 | 100 | 100 | 100 | 100 |
| 15 | 24 | F | Right | 3 | 2 | 3 | 4 | 9 | 2 | 4 | 5 | 11 | 6 | 5 | 6 | 17 | 5 | 6 | 6 | 17 | 4 | 0 | 100 | 100 | 100 | 100 |
| 16 | 40 | M | Left | 120 | 4 | 5 | 4 | 13 | 4 | 6 | 4 | 14 | 4 | 5 | 3 | 12 | 3 | 6 | 3 | 12 | 24 | 0 | 33 | 100 | 67 | 0 |
| 17 | 33 | F | Left | 6 | 4 | 4 | 6 | 14 | 5 | 7 | 5 | 17 | 4 | 3 | 5 | 12 | 3 | 4 | 3 | 10 | 6 | 9 | −25 | 0 | −33 | −20 |
| 18 | 52 | M | Right | 3 | 3 | 5 | 4 | 12 | 3 | 5 | 5 | 13 | 4 | 4 | 2 | 10 | 5 | 5 | 3 | 13 | 44 | 0 | 50 | 50 | 50 | 50 |
| 19 | 41 | M | Left | 84 | 6 | 6 | 5 | 17 | 5 | 6 | 4 | 15 | 7 | 8 | 7 | 22 | 5 | 6 | 4 | 15 | 6 | 0 | 0 | 0 | 0 | 40 |
| 20 | 56 | M | Right | 84 | 3 | 5 | 4 | 12 | 3 | 5 | 5 | 13 | 6 | 5 | 4 | 15 | 6 | 5 | 5 | 16 | 30 | 12 | 33 | 0 | 50 | 60 |
| 21 | 50 | M | Left | 6 | 6 | 6 | 4 | 16 | 6 | 9 | 4 | 19 | 4 | 4 | 5 | 13 | 4 | 4 | 5 | 13 | −30 | −6 | 50 | 60 | 0 | 100 |
| 22 | 38 | M | Left | 3 | 6 | 6 | 5 | 17 | 6 | 5 | 4 | 15 | 7 | 6 | 4 | 17 | 7 | 6 | 4 | 17 | 28 | 13 | −40 | −14 | 50 | 25 |
| 23 | 36 | M | Left | 204 | 4 | 5 | 5 | 14 | 5 | 7 | 5 | 17 | 5 | 6 | 6 | 17 | 5 | 5 | 5 | 15 | 28 | 3 | 14 | 17 | 0 | 0 |
| 24 | 45 | F | Right | 3 | 4 | 3 | 5 | 12 | 7 | 4 | 3 | 14 | 7 | 3 | 5 | 15 | 7 | 3 | 5 | 15 | 8 | 1 | 33 | 0 | 100 | 0 |
CB: cymba concha; CC: cavum concha; T: tragus; SUM CB + CC + T; Δ: the change between initial and final measurement; THI: tinnitus handicap inventory; BDI: Beck depression inventory; LD: loudness; AW: awareness; AN: annoyance; EL: effect on life
Adverse Effects
None of the patients complained of chest pain, dizziness, headache, otalgia, or dermatological effects at the stimulation sites.
DISCUSSION
Although few previous studies have evaluated the feasibility of tVNS for treatment of chronic tinnitus, the present study is the first to evaluate the effect of the stimulus intensity of tVNS on the treatment of chronic tinnitus. In this study, we investigated the effect of stimulus intensity on the treatment outcome, which has been frequently overlooked in many previous studies, and identified the optimal stimulation site.
Based on our findings, the effect of stimulus intensity on the treatment outcome seems to be limited, although a positive correlation exists between the initial VAS scores and the stimulus intensity. We conclude that the stimulus intensity may be subsidiary to whether tVNS should be performed.
From an anatomical perspective, there are heterogeneous distributions of nerves in the external ear; the cymba is innervated by ABVN, both ABVN and the greater auricular nerve (GAN) are found in the cavum, and at the tragus, three nerves, ABVN, GAN, and auriculotemporal nerve are observed at the tragus [10]. A recent imaging study reported that stimulating the left cymba induced a widespread activation from the ipsilateral nucleus tractus solitarii (NTS) to the brainstem and to the forebrain along the central vagal afferent pathway [11]. Consistently, other studies have proposed a hypothesis for the mechanism underlying the therapeutic effect of tVNS; it may originate from the noradrenergic and serotonergic interactions between the NTS, locus coeruleus (LC), and raphe nuclei [5].
Alternatively, other studies have compared the four stimulation sites in healthy subjects-the inner tragus, inferoposterior wall of the external auditory meatus, cymba, and ear lobule to ascertain the optimal tVNS site [12]. The authors found that the stimulation of the cymba produced a stronger activation in the NTS and the LC than at the ear lobule (sham), although they also reported a contradictory result that the stimulation of the ear lobule deactivated the Heschl’s gyrus and the superior temporal gyrus, similar to a real stimulation. Collectively, these results suggest that the optimal site for tVNS is, in theory, the cymba.
Conversely, in the present study, the responders could not be differentiated from non-responders using the cymba concha stimulation. Instead, the stimulus intensity showed a significant change at the cavum and the responders exhibited a changing pattern of stimulus intensity across sessions (Figure 3), which was significantly different from our expectations.
With regard to the anatomical perspective, the cavum is innervated by both the ABVN and GAN, whereas the ear lobule, which has been frequently used as a sham stimulation site in many tVNS studies, is innervated by GAN alone. GAN is the superficial branch of the cervical plexus from the C2 and C3 spinal nerves and is divided into anterior and posterior branches. The anterior branch runs into the parotid gland, whereas the posterior branch communicates with ABVN and the posterior auricular branch of the facial nerve [13]. Moreover, a previous study reported that TENS of the C2 dermatome may enhance the inhibitory role of the dorsal cochlear nucleus (DCN) in the central auditory pathway in cases where tinnitus could be modulated by somatosensory events [14]. Collectively, these data led us to conclude that the anatomical interconnection between GAN and ABVN as well as the auditory-somatosensory integration in DCN explain both our findings and the similarity between the fMRI findings for sham and real stimulations [12].
We found that the initial stimulus intensity correlated the most with the VAS scores; however, the correlation was not observed after multiple sessions. The initial correlation may provide evidence supporting a therapeutic effect of tVNS or it may reflect the patients’ expectation for improvement in proportion to subjective tinnitus distress. In addition, the lack of correlation between stimulus intensity and final VAS scores may arise from early onset tolerance due to repeated administration of tVNS [15].
In general, sensory intensity is defined as the point at which patients feel a strong but tolerable sensation without a motor contraction [16]. Thus, the threshold used in this study corresponded to “maximal” sensory stimulus intensity. The frequency is also an important factor; >100 Hz is regarded as high-frequency stimulation and <10 Hz is regarded as low-frequency stimulation [16]. In the current tVNS studies, including the present study, a low-frequency (<30 Hz) was used [6–9, 12, 14].
Apart from the modulating options of frequency and/or intensity, commercially available tVNS devices are also equipped with “on” and “off” functions. The options to adjust the stimulation patterns or waveforms are also available in most TENS devices. A recent study reported that alternating low and high frequencies were helpful in slowing the development of tolerance in arthritic rats [17]. Thus, the modulating stimulation patterns may be an important approach for preventing tolerance.
This study has several limitations. Firstly, four separate 12-min sessions appeared to be inadequate for an outcome assessment. However, because we also wanted to ascertain the optimal stimulation site for the treatment of tinnitus, we selected three tVNS application sites based on the related literature review. Further studies with longer sessions devoting more time to the cavum are needed. Secondly, the study design did not include a control group, and this may have increased the possibility of bias. Thirdly, we did not document the changes in the VAS scores directly before or after every session. If we had compared the VAS scores with stimulus intensity at every session, more details regarding the relationship between the intensity and VAS could have been confirmed. Finally, fMRI or positron emission tomography were not performed. Although these techniques do not always provide direct information regarding tinnitus patients, neural activities may be measured indirectly by detecting changes in perfusion or local metabolism, and this may have provided additional information for our study. Lastly, the effect of tVNS may be influenced by age. A previous study has suggested that sedentary or old populations reduced parasympathetic activity compared with healthy participants [18]. However, only two of the patients included in this study were of 60 years of age or older. Thus, our results may not be greatly affected by aging.
CONCLUSION
The stimulus intensity for tVNS does not differ according to sex or laterality. Although a moderate, positive correlation between intensity and tinnitus distress was noted initially, it did not persist after subsequent sessions. Therefore, the effect of stimulus intensity on the treatment outcome seems to be limited. Among patients with AN, the responders to tVNS showed a small increasing trend in stimulus intensity at the cavum. Conversely, no significant differences according to treatment response or other VAS scores were found at the cymba or tragus. Therefore, these results suggest that the optimal stimulation site for tVNS is the cavum and not the cymba.
Acknowledgements
This research was supported by EMBRI Grants 2017-EMBRI-DJ0002 from the Eulji University and this work was supported by the Basic Science Research Program, through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2017R1C1B5017839 for H.Y.L.).
Footnotes
This study was presented at the 22th congress of Korean society of otorhinolaryngology-Head and Neck Surgery held in Cheongju, South Korea on 29 October 2016
Ethics Committee Approval: The Institutional Review Board of Eulji University approved this study (IRB 2015-04-012).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – H.Y.L.; Design – H.Y.L.; Supervision – H.Y.L.; Resources -; Materials -; Data Collection and/or Processing – W.S.C., H.Y.L.; Analysis and/or Interpretation – S.J.K., D.S.C., H.Y.L.; Literature Search – S.J.K., D.S.C.; Writing Manuscript – W.S.C., S.J.K.; Critical Review – S.J.K., D.S.C.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Blumfeld H. Neuroanatomy through Clinical Cases. 2nd ed. Sunderland: Sinauer Associates, Inc; 2010. pp. 532–3. [Google Scholar]
- 2.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(Suppl 4):S3–14. doi: 10.1212/WNL.59.6_suppl_4.S3. [DOI] [PubMed] [Google Scholar]
- 3.Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114:1485–93. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 4.Cianfrone G, Mazzei F, Salviati M, Turchetta R, Orlando MP, Testugini V, et al. Tinnitus Holistic Simplified Classification (THoSC) Ann Otol Rhinol Laryngol. 2015;124:550–60. doi: 10.1177/0003489415570931. [DOI] [PubMed] [Google Scholar]
- 5.Lehtimäki J, Hyvärinen P, Ylikoski M, Bergholm M, Mäkelä JP, Aarnisalo A, et al. Transcutaneous vagus nerve stimulation in tinnitus: a pilot study. Acta Otolaryngol. 2012;133:378–82. doi: 10.3109/00016489.2012.750736. [DOI] [PubMed] [Google Scholar]
- 6.Shim HJ, Kwak MY, An YH, Kim DH, Kim YJ, Kim HJ. Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. J Audiol Otol. 2015;19:159–67. doi: 10.7874/jao.2015.19.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li TT, Wang ZJ, Yang SB, Zhu JH, Zhang SZ, Cai SJ, et al. Transcutaneous electrical stimulation at auricular acupoints innervated by auricular branch of vagus nerve pairing tone for tinnitus: study protocol for a randomized controlled clinical trial. Trials. 2015;19:101. doi: 10.1186/s13063-015-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreuzer PM, Landgrebe M, Resch M, Husser O, Schecklmann M, Geisreiter F, et al. Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in chronic tinnitus: an open pilot study. Brain Stimul. 2014;7:740–7. doi: 10.1016/j.brs.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Cha WW, Song K, Lee HY. Persistent geotropic direction-changing positional nystagmus treated with transcutaneous vagus nerve stimulation. Brain Stimul. 2016;9:469–70. doi: 10.1016/j.brs.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–7. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 11.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–36. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yakunina N, Kim SS, Nam EC. Optimization of transcutaneous vagus erve stimulation using functional MRI. Neuromodulation. 2017;20:290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. Am J Neuroradiol. 2000;21:568–71. [PMC free article] [PubMed] [Google Scholar]
- 14.Vanneste S, Plazier M, Van de Heyning P, De Ridder D. Transcutaneous electrical nerve stimulation (TENS) of upper cervical nerve (C2) for the treatment of somatic tinnitus. Exp Brain Res. 2010;204:283–7. doi: 10.1007/s00221-010-2304-5. [DOI] [PubMed] [Google Scholar]
- 15.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 16.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492–9. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89:754–60. doi: 10.1016/j.apmr.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7:871–7. doi: 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]