Abstract
OBJECTIVE
The aim of this study was to evaluate procalcitonin and high sensitive c-reactive protein (hs-CRP) levels in idiopathic sudden sensorineural hearing loss (ISSNHL) patients and assess their correlations with the clinical prognosis.
MATERIALS and METHODS
Twenty-three ISSNHL patients were included in the study (group A). The control group was consisted of 19 patients (group B). Procalcitonin and hs-CRP levels were compared between the groups. The relationship between procalcitonin and hs-CRP levels and the configuration of the audiogram, degree of hearing loss [partial or total (>90 dB)], and status of improvement (improvement of >15 dB in the first month PTA) were evaluated.
RESULTS
The mean age was 47.91±15.73 years (range 21–73 years) and 35.16±15.67 years (range 19–79 years) in groups A and B, respectively. Seven patients (30.4%) had underlying cardiovascular risk factors. Mean procalcitonin levels were 0.057±0.025 μg/L and 0.041±0.016 μg/L in groups A and B, respectively. Mean hs-CRP levels were 0.461±1.335 mg/dL and 0.129±0.125 mg/dL in groups A and B, respectively. Procalcitonin levels were significantly higher in group A than in group B (p=0.018). Procalcitonin levels were significantly lower (0.035±0.013 μg/L vs. 0.061±0.025 μg/L) in patients with low-frequency hearing loss (p=0.04). ROC analysis of procalcitonin values revealed that area under the curve was 0.80 (p=0.005). A cut-off procalcitonin level of 0.45 μg/L yielded a sensitivity of 90% and specificity of 56.2%.
CONCLUSION
In conclusion, as a proinflammatory marker, procalcitonin levels were higher in ISSNHL patients than in healthy controls. The procalcitonin level was significantly lower in upsloping-type hearing loss patients. This finding could be regarded as an indirect indicator of pathogenesis.
Keywords: Procalcitonin, hs-CRP, inflammation, sudden sensorineural hearing loss
INTRODUCTION
Idiopathic sudden sensorineural hearing loss (ISSNHL) is usually defined as a loss over 30 dB in pure-tone audiometry (PTA) in at least 3 consecutive frequencies in one or both ears within a period of 72 h [1, 2]. More than 100 etiologies have been proposed for this disorder; however, vascular and viral inflammatory causes are the two predominant etiologies [3–6]. Procalcitonin is one of the most commonly used proinflammatory biomarkers in the current medical practice [7, 8]. Procalcitonin, which is a peptide procursor of calcitonin, is produced by parafollicular C cells of the thyroid gland and is involed in calcium homeostasis. The procalcitonin level in healthy subjects is <0.01 μg/L [9]. C-reactive protein (CRP) is an acute phase reactant that increases in inflammatory states [10]. There is evidence supporting the use high-sensitivity c-reactive protein (hs-CRP) to monitor insulin resistance and cardiovascular risk in diabetic and nondiabetic subjects [10].
The aim of this study was to evaluate the procalcitonin and hs-CRP levels in ISSNHL patients and assess their correlations with the clinical prognosis.
MATERIALS and METHODS
This study was conducted between March 2014 and October 2015 at an otolaryngology clinic of a tertiary academic center in concordance with international ethical standards and the World Health Organization Declaration of Helsinki. The study was approved by the institutional review board. Informed consent was obtained from all the subjects.
Patient Selection
Twenty-three ISSNHL patients were included in the study (group A). The control group was consisted of 19 patients (group B). Patients with an acute onset (72 h) of sensorineural hearing loss over 30 dB in at least 3 consecutive frequencies in PTA were included in the study. Retrocochlear pathology, a history of malignancy, and pediatric age were the exclusion criteria. Patients with conditions associated with high procalcitonin levels, such as active infection, thyroid disease, renal failure, chronic liver or intestinal disease, and chronic obstructive pulmonary disease, were also excluded.
Procedure
The procalcitonin and hs-CRP levels were measured after the diagnosis of ISSNHL was established and before any treatments were initiated. All the control patients were recruited from the healthy scheduled septoplasty patients, and their procalcitonin and hs-CRP levels were measured along with other routine blood tests during preoperative anesthesia evaluation.
Pure-tone audiometry was repeated a month later. An increase of >15 dB in the average hearing level (arithmetic mean of 500, 1000, 2000, and 4000 Hz frequencies) was considered as an improvement [11]. The configuration of PTA (upslope, downslope, or flat) was noted. The presence of vestibular symptoms and cardiovascular risk factors (hypertension, coronary artery disease, or diabetes mellitus) was also noted.
Outcome measures
Procalcitonin and hs-CRP levels were compared between the groups. The relationship between procalcitonin and hs-CRP levels and the configuration of the audiogram, degree of hearing loss [partial or total (>90 dB)], status of improvement (improvement of >15 dB in the first month PTA) were evaluated.
Statistical Analysis
Statistical analysis was performed using computer software (Statistical Package for the Social Sciences version 22.0, Inc.; Chicago, IL, USA). Chi-square (X2) exact tests were used for the comparison of categorical data. Independent and paired-sample t-tests were used for the analysis of parametric variables, while Wilcoxon and Mann–Whitney U tests were used for the analysis of nonparametric variables based on the distribution pattern of the data. The Shapiro–Wilk test was used for determining the distribution pattern of the data. The distribution of the groups was nonparametric. Correlation analysis was performed via Pearson or Spearman correlation analysis based on the distribution pattern of the data. Data were expressed as “median, interquartile range (IQR)”. Receiver operating characteristic (ROC) analysis was performed. A p-value less than 0.05 was considered statistically significant.
RESULTS
The mean age was 47.91±15.73 years (range 21–73 years) and 35.16±15.67 years (range 19–79 years) in groups A and B, respectively. Of the 42 subjects, 24 were male and 18 were female. Fourteen (60.9%) of the patients were male and 9 (39.1%) were female in group A. Ten (52.6%) of the patients were male and 9 (47.4%) were female in group B. The groups did not differ significantly with regard to the frequency of gender (p>0.05). Seven patients (30.4%) had underlying cardiovascular risk factors (5 had diabetes mellitus; 1 had diabetes mellitus and hypertension; and 1 had diabetes mellitus, hypertension, and coronary artery disease) and 3 (13%) had complaints of vertigo.
Mean procalcitonin levels were 0.057±0.025 μg/L and 0.041±0.016 μg/L in groups A and B, respectively. Mean hs-CRP levels were 0.461±1.335 mg/dL and 0.129±0.125 mg/dL in groups A and B, respectively. Procalcitonin levels were significantly higher in group A than in group B (p=0.018). Hs-CRP levels were also higher in group A; however, this difference was not significant (p=0.287).
There was an improvement in the average PTA findings of 12 (52.2%) patients, while no improvement was recorded in 11 patients (47.8%). The procalcitonin and hs-CRP levels were not statistically different between the patients who did and did not show improvement (p=0.056 and p=0.321, respectively). Procalcitonin levels in patients who did and did not show improvement were 0.048 μg/L and 0.068 μg/L, respectively.
Procalcitonin and hs-CRP levels of the patients who had profound (>90 dB) hearing loss were compared with those of the patients who had less severe hearing loss (<90 dB). Procalcitonin and hs-CRP levels did not differ significantly between patients who had severe hearing loss and those who had less severe hearing loss (p=0.534 and p=0.256, respectively).
The configuration of PTA was downslope in 5 patients (21.7%), upslope in 4 patients (17.4%), and flat in 14 patients (60.9%). Mean procalcitonin levels were 0.035, 0.054 and 0.064 μg/L in upsloping, downsloping, and flat groups, respectively. Neither Kruskal–Wallis nor ANOVA test yielded a statistically significant difference in both procalcitonin and hs-CRP levels. Similarly, pairwise post-hoc analysis with Bonferroni correction in order to overcome type 1 error yielded nonsignificant results. Flat and downsloping PTA configurations were combined and compared with upsloping ones by Mann–Whitney U test. This analysis revealed a significantly lower procalcitonin level in case of the upsloping PTA configuration (0.035±0.013 μg/L vs. 0.061±0.025 μg/L) (p=0.04).
Receiver operating characteristic analysis was performed to assess the relationship between the procalcitonin level and ISSNHL positive state. ROC analysis of procalcitonin values revealed that area under the curve was 0.80 (p=0.005). A cut-off procalcitonin level of 0.45 μg/L yielded a sensitivity of 90% and specificity of 56.2% (Figure 1).
Figure 1.
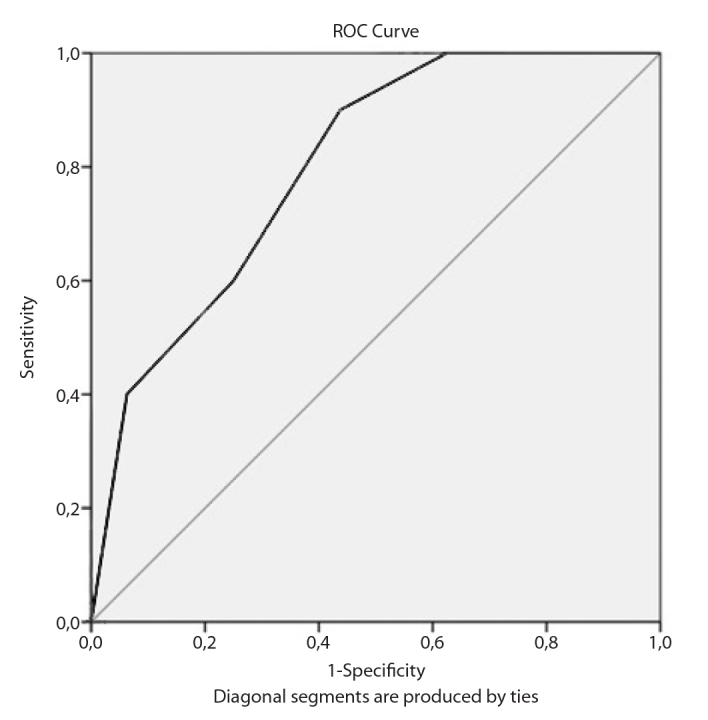
ROC analysis for procalcitonin.
ROC: Receiver operating characteristic
DISCUSSION
It is well known that procalcitonin levels increase significantly in the presence of bacteremia and sepsis [12–14]. Although procalcitonin is primarily used to help guide physicians to support the presence of bacterial infections, previous research has reported slightly elevated procalcitonin levels during localized bacterial infection and viral infection [15]. In a recent review, hs-CRP was reported to be a valid tool to identify people at a risk of cardiovascular events independent of their demographic background [16]. Xie et al. [17] suggested that hs-CRP is associated with internal carotid artery occlusion in ischemic stroke patients. Because vascular and inflammatory conditions are the most postulated etiologies, procalcitonin and hs-CRP levels in ISSNHL patients were assesed.
Masuda et al. [18] investigated whether inflammatory markers, including leukocyte counts, natural killer cell activity (NKCA), interleukin 6 (IL-6) level, tumor necrosis factor level, and high-sensitivity CRP level, are involved in the pathophysiology of idiopathic sensorineural hearing loss. They suggested that neutrophil counts above the reference range could be a useful indicatior of poor prognosis of ISSNHL; however, hs-CRP did not seem to be a useful biomarker [18]. Similarly, hs-CRP levels were not significantly higher in ISSNHL patients than in controls in this study. Hs-CRP levels were also not related to the prognosis, degree of hearing loss, and configuration of PTA. Hs-CRP levels are higher in women and tend to increase with age; however, a similar relationship was observed with procalcitonin levels in large population-based studies [19]. CRP levels were higher in group A, and the mean age in this group was also higher than that in group B. However, this difference was insignificant.
An increase in the mean platelet volume (MPV) is seen in vascular events such as atheroscherosis, acute syndromes, venous and arterial thrombosis, or thromboembolism [20–22]. Previous reports have investigated the relationship between MPV levels and ISSNHL. Ulu et al. [23] reported significantly higher MPV levels in ISSNHL patients and suggested that increased MPV values indicate the possible causes of ischemia or atherosclerosis in ISSNHL patients. However, Karli et al. [24] have reported no significant difference between MPV levels of ISSNHL patients and controls.
A study assesing procalcitonin levels in ISSNHL patients does not exist. However, Kilicaslan et al. [25] investigated the diagnostic and prognostic value of procalctonin levels in patients with Bell’s palsy. The etiology of Bell’s palsy is also unclear similar to that of ISSNHL, and inflammation is thought to play an important role. They reported significantly higher procalcitonin levels in Bell’s palsy patients than in controls [25]. In addition, higher procalcitonin levels have been found to be associated with the severity and prognosis of Bell’s palsy [25].
This study is the first to assess procalcitonin levels in ISSNHL patients. Procalcitonin levels were significantly higher in ISSNHL patients, and this finding may support the inflammatory etiology of ISSNHL of either vascular or infectious origin. Furthermore procalcitonin levels were significantly lower in ISSNHL patients with low-frequency hearing loss. Because cochlear hydrops may be the underlying cause in low-frequency hearing loss, it is postulated that inflammation plays a larger role in the remaining ISSNHL patients. This data may be supported with further studies. A procalcitonin level greater than 0.45 μg/L may support the diagnosis of ISSNHL with a sensitivity of 90% and specificity of 56.2%.
The major limitation of this study may be the small sample size. More reliable results could have been yielded, particularly considering ISSNHL patients with low-frequency hearing loss. Procalcitonin levels were lower in patients with good prognosis; however, this difference could not reach a statistically significant level. In addition, control PTA was obtained a month after the diagnosis. It is known that the sensorineural healing process continues for months. Therefore, the prognostic value could be supported with a longer follow-up.
In conclusion, as a proinflammatory marker, procalcitonin levels were higher in ISSNHL patients in than in healthy controls. The procalcitonin level was higher in patients with poor prognosis; however, this difference could not reach statistical significance. The procalcitonin level was significantly lower in upsloping-type hearing loss patients. This finding could be regarded as an indirect indicator of pathogenesis.
Footnotes
Ethics Committee Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.G., İ.K., H.İ.M.; Design - S.G., G.T., İ.K., H.İ.M.; Supervision - S.G., T.K.; Resources - S.G., G.T., İ.K.; Materials - G.T., H.İ.M.; Data Collection and/or Processing - G.T., H.İ.M.; Analysis and/or Interpretation - S.G., G.T., İ.K.; Literature Search - S.G., G.T.; Writing Manuscript - S.G., G.T.; Critical Review - S.G., T.K.; Other - G.T., İ.K., H.İ.M.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Stachler RJ, Chandrasekhar SS, Archer SM, Rosenfeld RM, Schwartz SR, Barrs DM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:S1–35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 2.Castro TM, Costa LA, Nemezio ME, Fonseca LJ. Bilateral sudden deafness. Braz J Otorhinolaryngol. 2011;77:678. doi: 10.1590/S1808-86942011000500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe BF. Clinical studies in sudden deafness. Adv Otorhinolaryngol. 1973;20:221–8. doi: 10.1159/000393099. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia A, Burchette R, Cueva R. Combination therapy (intratympanic dexamethasone + high-dose prednisone taper) for the treatment of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2008;29:453–60. doi: 10.1097/MAO.0b013e318168da7a. [DOI] [PubMed] [Google Scholar]
- 5.Ciuffetti G, Scardazza A, Serafini G, Lombardini R, Mannarino E, Simoncelli C. Whole-blood filterability in sudden deafness. Laryngoscope. 1991;101:65–7. doi: 10.1288/00005537-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Wilson W, Veltri R, Laird N, Sprinkle P. Viral and epidemiologic studies of idiopathic sudden hearing loss. Otolaryngol Head Neck Surg. 1983;91:653–8. doi: 10.1177/019459988309100612. [DOI] [PubMed] [Google Scholar]
- 7.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patientswith sepsis and infection. Lancet. 1993;341:515–8. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whicher J, Bienvenu J, Monneret G. Procalcitonin as an acute phase marker. Ann Clin Biochem. 2001;38:483–93. doi: 10.1177/000456320103800505. [DOI] [PubMed] [Google Scholar]
- 9.Reinhart K, Karzai W, Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 2000;26:1193–200. doi: 10.1007/s001340000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfützner A, Schöndorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol. 2010;4:706–16. doi: 10.1177/193229681000400326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Arch Otolaryngol Head Neck Surg. 2007;133:573–81. doi: 10.1001/archotol.133.6.573. [DOI] [PubMed] [Google Scholar]
- 12.Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351:17–29. doi: 10.1016/j.cccn.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36:941–52. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- 14.Limper M, de Kruif MD, Duits AJ, Brandjes DP, van Gorp EC. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J Infect. 2010;60:409–16. doi: 10.1016/j.jinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–8. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca FA, Izar MC. High-Sensitivity C-Reactive Protein and Cardiovascular Disease Across Countries and Ethnicities. Clinics (Sao Paulo) 2016;71:235–42. doi: 10.6061/clinics/2016(04)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie D, Hu D, Zhang Q, Sun Y, Li J, Zhang Y. Increased high-sensitivity C-reactive protein, erythrocyte sedimentation rate and lactic acid in stroke patients with internal carotid artery occlusion. Arch Med Sci. 2016;12:546–51. doi: 10.5114/aoms.2014.47879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda M, Kanzaki S, Minami S, Kikuchi J, Kanzaki J, Sato H, et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2012;33:1142–50. doi: 10.1097/MAO.0b013e3182635417. [DOI] [PubMed] [Google Scholar]
- 19.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352:1611–3. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]
- 20.Martin JF, Shaw T, Heggie J, Penington DG. Measurement of the density of human platelets and its relationship to volume. Br J Haematol. 1983;54:337–52. doi: 10.1111/j.1365-2141.1983.00337.x. [DOI] [PubMed] [Google Scholar]
- 21.Machin SJ, Briggs C. Mean platelet volume: a quick, easy determinant of thrombotic risk? J Thromb Haemost. 2010;8:146–7. doi: 10.1111/j.1538-7836.2009.03673.x. [DOI] [PubMed] [Google Scholar]
- 22.Braekkan SK, Mathiesen EB, Nj⊘lstad I, Wilsgaard T, St⊘rmer J, Hansen JB. Mean platelet volume is a risk factor for venous thromboembolism: the Troms⊘ Study, Troms⊘, Norway. J Thromb Haemost. 2010;8:157–62. doi: 10.1111/j.1538-7836.2009.03498.x. [DOI] [PubMed] [Google Scholar]
- 23.Ulu S, Ulu MS, Ahsen A, Yucedag F, Aycicek A, Celik S. Increased levels of mean platelet volume: a possible relationship with idiopathic sudden hearing loss. Eur Arch Otorhinolaryngol. 2013;270:2875–8. doi: 10.1007/s00405-013-2348-9. [DOI] [PubMed] [Google Scholar]
- 24.Karli R, Alacam H, Unal R, Kucuk H, Aksoy A, Ayhan E. Mean platelet volume: is it a predictive parameter in the diagnosis of sudden sensorineural hearing loss? Indian J Otolaryngol Head Neck Surg. 2013;65:350–3. doi: 10.1007/s12070-013-0648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilicaslan S, Uluyol S, Gur MH, Arslan IB, Yagiz O. Diagnostic and prognostic value of procalcitonin levels in patients with Bell’s palsy. Eur Arch Otorhinolaryngol. 2016;273:1615–8. doi: 10.1007/s00405-016-3937-1. [DOI] [PubMed] [Google Scholar]


