Abstract
OBJECTIVE
The purpose of this study was to examine the anti-ototoxic impact of Ginkgo biloba extract and lycopene on the model of cisplatin-induced ototoxicity in rats.
MATERIALS and METHODS
Thirty-two Wistar albino rats were examined with the distortion product otoacoustic emission (DPOAE) test (MADSEN Capella2 ; GN Otometrics, ICS Medical, Chicago USA), and they were randomly divided into four groups. Group 1 (n=8) was defined as the healthy control group. Cisplatin was given intraperitoneally as single dose of 12 mg/kg to group 2 (n=8), group 3 (n=8), and group 4 (n=8). Group 2 was determined as ototoxic control group. G. biloba extract (100 mg/kg) was given to group 3, and 20 mg/kg lycopene was given to group 4 with orogastric feeding tube daily for 10 days. DPOAE test was repeated on day 10 on all the groups. Finally, histopathological examination was performed. The study has been lead in agreement with the principles by the Institutional Animal Care and Use Committee Review Board at Kocaeli University Medical Center (KOÜ HADYEK- 1/9–14). The animals were treated in accordance with protocols approved by this committee.
RESULTS
When DPOAE tests were compared, there was no significant difference in the four groups before the application (p>0.05). At the end of day 10, in groups 2 to 4, statistically significant changes were observed (p<0.05). According to the cisplatin group, a significant increase in the DP-grams on G. biloba and lycopene groups was observed (p<00.5). Corti organ and spiral ganglion neurons of groups 1, 3, and 4 were observed to have weak expression. Strong reactions were determined in organum spirale and some spiral ganglions of the cisplatin group. The striae vascularis damage on group 2 was found to be more significant more compared with groups 3 and 4.
CONCLUSION
There is a protective effect of G. biloba and lycopene on cisplatin-dependent ototoxic rat model.
Keywords: Cisplatin, ototoxicity, Ginkgo biloba, lycopene
INTRODUCTION
Cisplatin is an antineoplastic agent often used in malign neoplasms such as those in gastrointestinal systems, urinary system, and head and neck. Although it has successful results against cancer, there are restrictions in clinical use due to serious side effects such as gastrointestinal, peripheral neuropathic, nephropathic, bone marrow toxicity, and ototoxicity [1, 2]. The increase in calcium rate is due to inner cellular calcium channel blockage in cochlear cells in cisplatin-induced ototoxicity; and increase electrolyte in imbalance and lipid peroxidation is due to the disruption in cell membrane, antioxidant system disruption, and emergence of free radicals such as oxygen and nitrogen [3–6]. As a result of these, progressive and irreversible sensorineural hearing loss in high frequencies and continuous or discontinuous tinnitus develops. In the studies conducted, it was shown that outer hairy cells in cisplatin cochlea, spiral ganglion cells (SGC), and cochlea basal and mid-turn parts in cochlea and Reissner’s membrane and stria vascularis are affected [3, 7, 8].
In literature, different antioxidant agents such as vitamin E [9], N-acetylcysteine [10], dexamethasone [11], lycopene [12], and Ginkgo biloba [13] used in cisplatin-induced ototoxicity have been mentioned.
Lycopene and G. biloba are also antioxidant agents. G. biloba contains biloba extract, EGb 761, 24% flavone glycosides, 6% terpene lactones, and 7% proanthocyaniclines which are strong antioxidants (14). In different in vivo and in vitro studies, it was shown that G. biloba prevents oxidative stress and has a role in the cleaning of free radicals [15, 16].
Beta-carotene obtained from lycopene in tomatoes and tropical fruits, vegetables, and microorganisms is a pigment with acyclic isomer [17]. Apart from being an antioxidant scavenger, lycopene is also responsible for proinflammatory inhibition.
The aim of this study is to investigate the protective effects of lycopene and G. biloba in cisplatin-induced ototoxicity and compare their advantages over each other.
MATERIALS and METHODS
Animals and Groups
In this study, 32 healthy, 5- to 17-mo-old, adult male Wistar Hannover rats weighing between 460 and 550 g (mean: 510 g) were used. After transportation, the animals were maintained in the central animal laboratory for 10 days. All rats had free access to commercial food and water and were maintained in an environment with controlled temperature (25°C–27°C) and 11/12-hour dark and light cycles. All rats were evaluated by microscopic examination before the distortion product otoacoustic emission (DPOAE) testing. Animals that had ear disease and tympanic membrane problems were excluded.
The rats with normal hearing were divided into four groups randomly as control (group 1 (n=8)), cisplatin (group 2 (n=8)), G. biloba (group 3 (n=8)), and lycopene group (group 4 (n=8)). Group 1 received single intraperitoneal injection of 1 mL of saline and served as a control group. Groups 2 to 4 received a single dose of 12 mg/kg cisplatin (cisplatin, Koçak Farma, İstanbul, Turkey) intraperitoneally; group 2 served as positive control group. In group 3, 20 mg/kg lycopene (Lycopene, Health Products, China) in 1 mg/kg olive oil administrated by gavage daily for 15 days following cisplatin injections. Group 4 was given 100 mg/kg G. biloba extract (G. biloba leaf extract, Solgar, İstanbul, Turkey) with gavage daily.
Anesthesia
All rats were anesthetized with ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injections. Controlling the depth of anesthesia was determined with pedal reflex. During the experiment, half dose of these anesthetic agents was used, if required. Body temperature was maintained with warm animal blanket.
DPOAE test
Distortion product otoacoustic emission (DPOAE) measurements were made using the Otometrics Madsen Capella DP + TE analyzer (MADSEN Capella2; GN Otometrics, ICS Medical, Chicago USA) that works under the OTOsuite software platform. DPOAE measurements were determined as DP-grams. The intensity levels of the DPOAE measurements were recorded as L1 for the f1 frequency (65 dB SPL) and L2 for the f2 frequency (55 dB SPL). (f2/f1 ratio = 1.22). DPOAE measurements recorded the following frequencies: 996, 1416, 2001, 2832, 4003, 4755, 5654, 6728, and 7998 Hz. Because of the internal noise, frequencies less than 1 kHz were not recorded. DPOAE testing was considered positive for signal-to-noise ratio of 5 dB SPL.
Histopathological Study
Distortion product otoacoustic emission test was repeated on day 15 in all groups, and immediately after that, euthanasia was performed and cochleas were dissected. Cochleas were kept in 10% formalin and fixation was provided. They were decalcified in 10% ethylenediaminetetraacetic acid (EDTA) solution after operation. Specimens were prepared with paraffin blocks, pathologist took serial longitudinal cross-sections one blinded, and the specimens were histopathologically examined with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS version 22 Inc.; IBM Corp., Chicago, IL, USA). Also, data (mean decibels of each individual DPOAE frequencies) were compared between groups with one-way ANOVA test. All differences associated with a chance probability of 0.05 or less were considered statistically significant.
RESULTS
Histomorphologic Results
When the cochlea dissection of rats was examined, low expression was observed in organ of corti inner and outer hair cells in control, G. biloba, and lycopene groups as a result of TUNEL staining. A statistically significant difference was not observed among these three groups in TUNEL-positive cell numbers (Figure 1).
Figure 1.
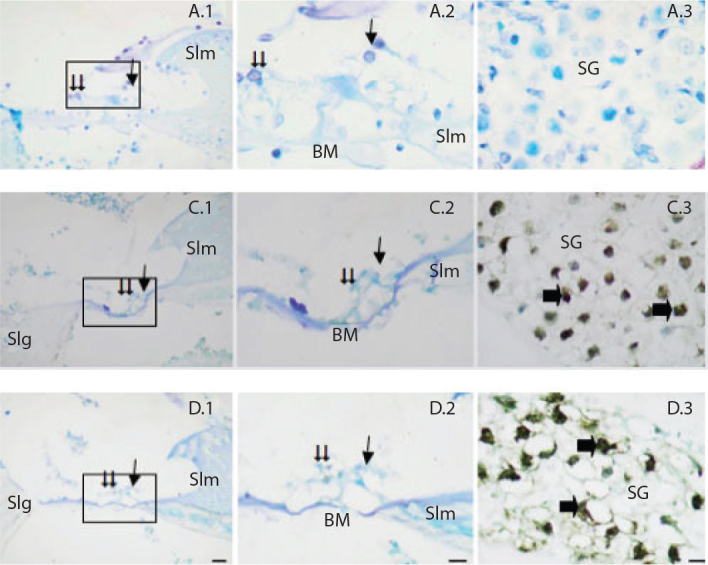
TUNEL staining method in the cochlear sections of the rat groups. Single arrow Inner hair cells, double arrow Outer hair cells.
Slm: spiral limbus; Slg: spiral ligament; BM: basilar membrane; SG: spiral ganglion; Single arrow: TUNEL-positive ganglion neurons. Control (A), Gingko (C), Lycopene (D). Organum spirale ×40 (1), Organum spirale ×100 (2), Ganglion ×100 (3). Methyl green ground fixation, (1) Bar=10 μm, (2,3) Bar = 5 μm.
While strong expression in inner and outer hairy cells of organ of corti was observed in the cisplatin group, cells showing a rather strong TUNEL-positive reaction were detected (Figure 2).
Figure 2.
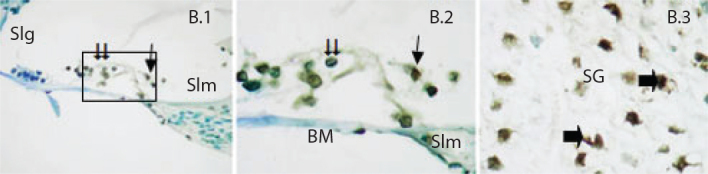
TUNEL staining method in the cochlear sections of the rat groups. Single arrow Inner hair cells, double arrow Outer hair cells.
Slm: spiral limbus; Slg: spiral ligament; BM: basilar membrane; SG: spiral ganglion; Single arrow: TUNEL-positive ganglion neurons. Cisplatin (B). Organum spirale ×40 (1), Organum spirale ×100 (2), Ganglion ×100 (3). Methyl green ground fixation, (1) Bar=10 μm, (2,3) Bar=5 μm.
Negative neurons in spiral ganglions and strong TUNEL-positive neurons in cisplatin, G. biloba, and lycopene groups stood out.
While 2.3% TUNEL-positive inner and outer hairy cells in cochlea cross-sections were observed in the control group, it was 2.6% and 2.5% in the G. biloba and lycopene groups, and no significant difference was observed among the three groups. This ratio was 1.5% in the cisplatin group, which was statistically significantly higher than the other groups.
Spiral ganglion ratio was 4.4% in the control group, 21% in the G. biloba group, and 20.5% in the lycopene group, whereas it was 23% in the cisplatin group. Significantly high spiral ganglion damage was observed in other groups compared with the control group.
DPOAE Measurement Results
Mean decibels of each individual DPOAE frequencies were compared between groups, and statistically significant differences were present in all frequencies in the cisplatin group compared with other groups.
While DP-grams were observed to be lower in the cisplatin group, they were significantly higher in the other groups. No significant difference in DP-grams was observed in the G. biloba and the lycopene groups when compared with the rats without cisplatin treatment (Figure 3).
Figure 3.
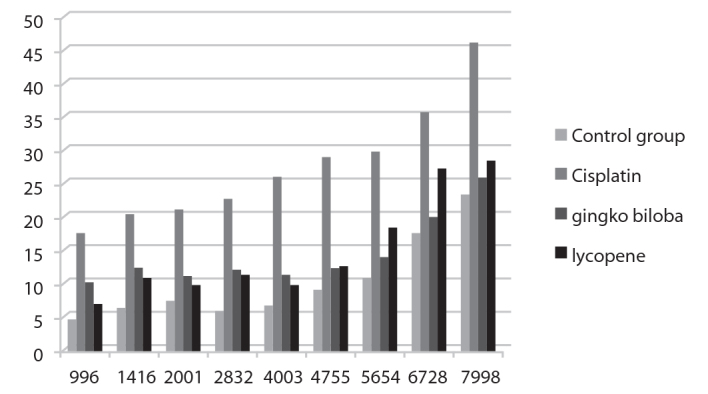
The DP-gram results of the rat groups.
The interesting fact is that while lycopene was more effective in low frequencies (996, 1416, 2001, 2832, 4003 Hz), G. biloba had a more significant effect in higher frequencies (5654, 6728, 7998 Hz) (Figure 4).
Figure 4.
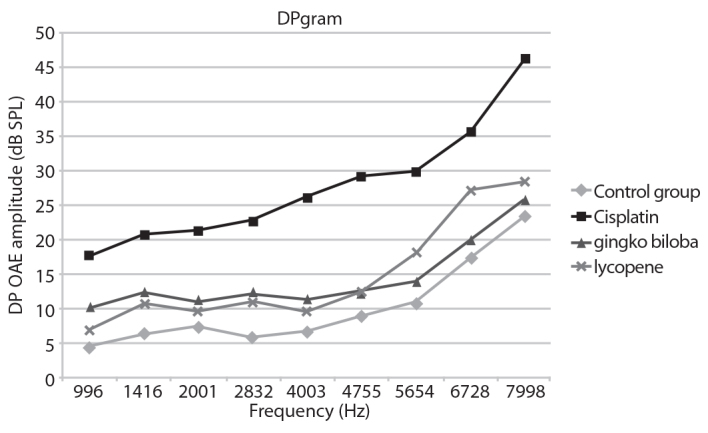
The DP-gram results of the rat groups.
Statistical Analyses
Mean decibels of each individual DPOAE frequencies were compared between groups with one-way ANOVA test. Group 2 had significantly higher mean decibel at a frequency of 4003 Hz, when compared with other groups (degree of freedom=3, p=0.022). There were no statistically significant differences between groups at other levels of DPOAE frequencies, ranging from 996 to 7998 (Table 1).
Table 1.
Distortion product otoacoustic emission measurement results (mean ± SD) and statistical analysis of the rat groups
| Frequency | Negative control | Cycsplatin group | Gingko biloba group | Lycopene group | p |
|---|---|---|---|---|---|
| 996 | 4.61 | 17.65 | 10.39±3.18 | 6.85±6.33 | 0.169 |
| 1416 | 6.43 | 20.69 | 12.46±3.55 | 10.85±4.96 | 0.143 |
| 2001 | 7.43 | 21.29 | 11.3±3.53 | 9.78±4.69 | 0.098 |
| 2832 | 5.85 | 22.73 | 12.28±3.9 | 11.04±5.63 | 0.123 |
| 4003 | 6.66 | 26.19 | 11.43±4.9 | 9.65±4 | 0.022 |
| 4755 | 9.04 | 29.11 | 12.4±7.65 | 12.46±6.47 | 0.183 |
| 5654 | 10.86 | 29.93 | 14±9.7 | 18.1±7.39 | 0.324 |
| 6728 | 17.53 | 35.65 | 20.08±12.87 | 26.1±9.71 | 0.490 |
| 7998 | 23.56 | 46.25 | 26.02±11.09 | 28.41±9.66 | 0.350 |
SD: standard deviation
Mean decibels of each individual DPOAE frequencies were compared between groups with one-way ANOVA test. However, there were no statistically significant differences between two treatment branches in terms of therapeutic effect (Table 2).
Table 2.
Comparison of mean decibels at different DPQAE frequencies between Gingko and Lycopene groups
| Frequency | Gingko group | Lycopene group | t | p |
|---|---|---|---|---|
| 996 | 10.39±3.18 | 6.85±6.33 | 1.413 | 0.065 |
| 1416 | 12.46±3.55 | 10.85±4.96 | 0.748 | 0.660 |
| 2001 | 11.3±3.53 | 9.78±4.69 | 0.735 | 0.911 |
| 2832 | 12.28±3.9 | 11.04±5.63 | 0.511 | 0.211 |
| 4003 | 11.43±4.9 | 9.65±4 | 0.794 | 0.348 |
| 4755 | 12.4±7.65 | 12.46±6.47 | −0.018 | 0.466 |
| 5654 | 14±9.7 | 18.1±7.39 | −0.951 | 0.281 |
| 6728 | 20.08±12.87 | 26.1±9.71 | −1.057 | 0.148 |
| 7998 | 26.02±11.09 | 28.41±9.66 | −0.459 | 0.498 |
DPQAE: distortion product otoacoustic emission
In conclusion, G. biloba and lycopene treatments showed beneficial outcomes in rats treated with cisplatin to induce experimental ototoxicity, as assessed by DPOAE at different frequencies. However, the results were statistically significant only at a frequency of 4003 Hz, and these two treatment branches were not superior to the other. Our results should be further consolidated with large-scale controlled studies to clearly delineate the effect of G. biloba and lycopene on reversing chemotherapy-induced ototoxicity.
DISCUSSION
In this study, we attempted to demonstrate whether there was a relationship between cisplatin-induced ototoxicity and G. biloba, lycopene.
The main targets of cisplatin cover three main areas: hairy cells of organ of corti, SGCs, and stria vascularis. The cells in these three areas are damaged because of the apoptosis by free oxygen radicals caused by cisplatin [7].
Cisplatin-related hearing losses start in 3 to 4 days after the first dose and generally occurs more at higher frequencies. It may be a temporary or a permanent loss. It is observed bilaterally in general [18]. In a study by Pollera et al. [19], the hearing loss after the first dose of cisplatin occurred in the first 48 h and above 4 kHz. Cisplatin ototoxicity starts on the third day and reaches the maximum level on the 10th day [20]. In our study, DPOAE measurements were made on the 15th day after cisplatin was given. When the DPOAE measurements were compared with baseline values, we demonstrated cisplatin-induced ototoxicity between 1 and8 kHz in line with the literature.
The cisplatin dose aimed in this study was determined according to the Cardinaal et al. [21] study. Cardinaal et al. [21] proved that ototoxic effect was not observed up to 10 mg/kg cumulative cisplatin dose and it was effective over this dose. It was shown that cisplatin-induced ototoxicity was effective when at least 1.5 mg/kg/day dose was applied for 8 days (cumulative dose 12 mg/kg). In our study, rats received a single dose of 12 mg/kg cisplatin intraperitoneally. We used DPOAEs to show the ototoxic effect in cochlea.
Different agents were used in different studies over the years to provide protection against cisplatin ototoxicity. Materials with antioxidant effects were examined in the first place. N-acetylcysteine, vitamin E, aminoguadine, Korean red ginseng, G. biloba, and lycopene are antioxidants that were used to prevent or decrease cisplatin-induced ototoxicity in animal experiments [22–28].
Huang et al. [27] examined the effect of G. biloba in cisplatin-induced ototoxicity by auditory brainstem response (ABR). While a significant shift was observed in ABR values in rats untreated with G. biloba, a significant shift was not observed in ABR test in the treated rats. Again, while a significant loss was observed in outer hairy cells in electron microscopy in the same study, outer hairy cells remained intact in those undergoing G. biloba treatment. In our study, hearing thresholds were in a better condition in G. biloba using rats when compared with those not undergoing treatment. Also, in our study, low expression was observed in inner and outer hairy cells of organs of corti in G. biloba using rats and that these cells were protected, and apoptotic outer cells less than non-treatment group.
In a study by Ozkırıs et al. [12], outer hairy cell number was preserved in rats using lycopene in cisplatin-induced ototoxicity, and higher DP-gram values were present in DPOAE measurements when compared with cisplatin group not undergoing treatment.
Parallel to this, low expression in inner and outer hairy cells of organs of corti in rats undergoing lycopene treatment and preservation of cells was observed in our study. It was observed to be significantly higher in DPOAE measurements.
In our study, there was much difference in the number of stained SGCs between group 1 and groups 3 and 4. SGCs are degenerative and did not survive. Because when SGCs are damaged, satellite and Schwan cells around the neuron are also damaged. In this study, hair cells were protected from apoptosis by G. biloba and lycopene. But in literature, studies show that SGCs cannot survive, unlike hair cells. Animal studies and models show that that supporting cell dysfunction can cause SGC degeneration in the absence of hair cell pathology.
In the animal study conducted by Sugawara et al. [29], there was a correlation between supporting cells and SGC dead cells. In another animal study, Gurgen et al. [30] showed that SGC degeneration independently formed hair cells. The human temporal bone study by Suzuka and Schuknecht [31] demonstrated the same. With these studies, it can be suggested that cochlea may be mediated by supporting cells instead of hair cells.
This study shows that lycopene and G. biloba extract decreased ototoxicity in cisplatin-induced ototoxic rats, and cell protective effect was observed in cochlea. While no significant difference was observed between cell protective characteristics of both the agents, G. biloba was more significantly effective at higher frequencies (5654, 6728, 7998 Hz), whereas lycopene was more effective at low frequencies in DPOAE measurements (996, 1416, 2001, 2832, 4003 Hz).
We want to discover that which agent was more effective at cisplatin ototoxicity. But we found that both of them are together more beneficial in ototoxicity. They are antioxidant and inhibit mitochondrial apoptosis pathways. Lycopene is obtained from vegetables easily, such as a diet rich in carotenoid-containing food such as tomatoes. Patients can organize their diet this way.
The main limitation of our study was the relatively small size of our series. Our study findings may potentially have been influenced by rat’s confounding factors. Finally, this was a single-institution study. Because of these restrictions, associations should be interpreted with caution. Although we still consider our results as preliminary, they warrant a larger comprehensive study, one that would include more animals or human studies to evaluate more precisely the role of G. biloba and lycopene.
Further randomized, prospective, controlled trials on larger series are necessary for making more precise interpretations.
CONCLUSION
This experimental animal study made us consider that lycopene and G. biloba may constitute a better choice protecting outer hairy cells in cisplatin-induced ototoxicity and have a complementary effect at both low and high frequencies when used together.
Acknowledgements
The English in this document has been checked by at least two professional editors, both native speakers of English. Thanks to Si-Ser Hearing Center for their support for distortion product-evoked otoacoustic emission test (MADSEN Capella2; GN Otometrics, ICS Medical, Chicago USA).
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of the Institutional Animal Care and Use Committee Review Board at Kocaeli University Medical Center (KOÜ HADYEK- 1/9–14). The animals were treated in accordance with protocols approved by this committee.
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - F.Ö.; Design - H.E.Ö.; Supervision - S.G.; Resources - S.G.G.; Materials - S.B.; Data Collection and/or Processing - E.E.; Analysis and/or Interpretation - E.E.; Literature Search - E.E., F.Ö.; Writing Manuscript - E.E.; Critical Review - A.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: Kocaeli Derince Training and Research Hospital has provided financial support for this work.
REFERENCES
- 1.Boulikas T, Vougiouka M. Recent clinical trial using cisplatin, carboplatin and their combination chemotherapy drugs. Oncol Rep. 2004;11:559–95. doi: 10.3892/or.11.3.559. [DOI] [PubMed] [Google Scholar]
- 2.Rabik CA, Dolan ME. Molecular mechanism of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravi R, Somani SM, Rybak LP. Mechanism of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol. 1995;76:386–94. doi: 10.1111/j.1600-0773.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee JE, Nakagawa T, Kim TS, Endo T, Shiga A, Iguchi F, et al. Role of reactive radicals in degeneration of the auditory system of mice following cisplatin treatment. Acta Otolaryngol. 2004;124:1131–5. doi: 10.1080/00016480410017521. [DOI] [PubMed] [Google Scholar]
- 5.Liang F, Schulte BA, Qu C, Hu W, Shen Z. Inhibition of the calcium and voltage dependent big conductance potassium channel ameliorates cisplatin-induced apopitosis in spiral ligament fibrocytes of the cochlea. Neuroscience. 2005;135:261–71. doi: 10.1016/j.neuroscience.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 6.Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/S0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 7.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanism of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–67. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Sockalingam R, Freeman S, Cherny TL, Sohmer H. Effect of high dose cisplatin on auditory brainstem responses and otoacoustic emissions in laboratory animals. Am J Otol. 2000;21:521–7. [PubMed] [Google Scholar]
- 9.Kalkanis JG, Whitworth C, Rybak LP. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114:538–42. doi: 10.1097/00005537-200403000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Dickey DT, Muldoon LL, Doolittle ND, Peterson DR, Kraemer DF, Neuwelt EA. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother Pharmacol. 2008;62:235–41. doi: 10.1007/s00280-007-0597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes AL, Hussain N, Pafford R, Parham K. Dexamethasone otoprotection in a multidose cisplatin ototoxicity mouse model. Otolaryngol Head Neck Surg. 2014;150:115–20. doi: 10.1177/0194599813511948. [DOI] [PubMed] [Google Scholar]
- 12.Ozkırış M, Kapusuz Z, Karaçavuş S, Saydam L. The effects of lycopene on cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013;270:3027–33. doi: 10.1007/s00405-013-2352-0. [DOI] [PubMed] [Google Scholar]
- 13.Cakil B, Basar FS, Atmaca S, Cengel SK, Tekat A, Tanyeri Y. The protective effect of Ginkgo biloba extract against experimental cisplatin ototoxicity: animal research using distortion product otoacoustic emissions. J Laryngol Otol. 2012;126:1097–101. doi: 10.1017/S0022215112002046. [DOI] [PubMed] [Google Scholar]
- 14.Clostre F. Form the body to the cell membrane:the different levels of pharmacological action of gingko biloba extract. Press Med. 1986;15:1529–38. [PubMed] [Google Scholar]
- 15.Maitra I, Marcocci L, Droy-Lefaix MT, Packer L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem Pharmacol. 1995;49:1649–55. doi: 10.1016/0006-2952(95)00089-I. [DOI] [PubMed] [Google Scholar]
- 16.Choung YH, Kim SW, Tian C, Min JY, Lee HK, Park SN, et al. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope. 2011;121:1294–302. doi: 10.1002/lary.21756. [DOI] [PubMed] [Google Scholar]
- 17.Buyuklu M, Kandemir F, Ozkaraca M, Set T, Bakirci E, Topal E, Ileriturk M, Turkmen K. Benefical effects of lycopene against contrast medium-induced oxidative stress, inflammation, autophagy, and apoptosis in rat kidney. Hum Exp Toxicol. 2015;34:487–96. doi: 10.1177/0960327114542964. [DOI] [PubMed] [Google Scholar]
- 18.Rybak LP, Rankumar V. Ototoxicity. Kidney Int. 2007;72:931–5. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- 19.Pollera CF, Marolla P, Nardi M, Ameglio F, Cozzo L, Bevere F. Very high-dose cisplatin-induced ototoxicity: a preliminary report on early and long-term effects. Cancer Chemother Pharmacol. 1988;21:61–4. doi: 10.1007/BF00262741. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res. 2000;28:73–80. doi: 10.1034/j.1600-079x.2000.280104.x. [DOI] [PubMed] [Google Scholar]
- 21.Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Dose-dependent effect of 8-day cisplatin administration upon the morphology of the albino guinea pig cochlea. Hear Res. 2000;144:135–46. doi: 10.1016/S0378-5955(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 22.Riga MG, Chelis L, Kakolyris S, Papadopoulos S, Stathakidou S, Chamalidou E, et al. Transtympanic injections of N-acetylcysteine for the prevention of cisplatin-induced ototoxicity: a feasible method with promising efficacy. Am J Clin Oncol. 2013;36:1–6. doi: 10.1097/COC.0b013e31822e006d. [DOI] [PubMed] [Google Scholar]
- 23.Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–8. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 24.Kalkanis JG, Whitworth C, Rybak LP. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114:538–42. doi: 10.1097/00005537-200403000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Kelly TC, Whitworth CA, Husain K, Rybak LP. Aminoguanidine reduces cisplatin ototoxicity. Hear Res. 2003;186:10–6. doi: 10.1016/S0378-5955(03)00303-4. [DOI] [PubMed] [Google Scholar]
- 26.Choung YH, Kim SW, Tian C, Min JY, Lee HK, Park SN, et al. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope. 2011;121:1294–302. doi: 10.1002/lary.21756. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Whitworth CA, Rybak LP. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol Neurotol. 2007;28:828–33. doi: 10.1097/MAO.0b013e3180430163. [DOI] [PubMed] [Google Scholar]
- 28.Ozkırış M, Kapusuz Z, Karaçavuş S, Saydam L. The effects of lycopene on cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013;270:3027–33. doi: 10.1007/s00405-013-2352-0. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–47. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gürgen SG, Gürgen O, Kırkım G, Kolatan HE, Gürkan S, Eskiizmir G. The Effect of Erythropoietin on S100 Protein Expression in Cochlea After Acoustic Overstimulation: An Experimental Study. J Clin Anal Med. 2015;6:304–8. doi: 10.4328/JCAM.2009. [DOI] [Google Scholar]
- 31.Suzuka Y, Schuknecht HF. Retrograde cochlear neuronal degeneration in human subjects. Acta Otolaryngol Suppl. 1988;450:1–20. doi: 10.3109/00016488809098973. [DOI] [PubMed] [Google Scholar]


