Abstract
OBJECTIVE
The aim of this report is to evaluate whether cortexin provides any protective activity against ototoxicity of cisplatin.
MATERIALS and METHODS
The study was performed on 30 healthy adult Wistar Albino rats, and rats were randomly divided into three groups of ten. Group I (Control group) was given intraperitoneal (ip) saline solution 1 mL/day. Group II (Cisplatin group) was given ip cisplatin for 2 days at doses of 10 mg/kg. Group III (Cisplatin + Cortexin group) was given ip cisplatin for 2 days at same doses with ip cortexin 2 mg/day for 7 days. Before and on the fourth day of the study, all subjects underwent auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAE) tests. At the end of fourth day, half of the subjects in all three groups were decapitated, and their cochlea were removed for histopathologic examination. On the eighth day, tests of the remaining subjects and histopathological examinations were repeated.
RESULTS
ABR tests on the fourth and eighth days showed elevations in the mean hearing thresholds of Groups II and III compared to Group I (p<0.05). DPOAE tests revealed a loss in emission values on the fourth and eighth days of the study compared to the baseline in Groups II and III. Comparison of Groups II with III showed that emission loss was higher in Group II at both time points, and the difference was more pronounced on the eighth day. Histopathological findings supported these tests.
CONCLUSION
Cortexin provide protective activity against cisplatin-induced ototoxicity.
Keywords: Cisplatin, cortexin, ototoxicity
INTRODUCTION
Ototoxicity is a general term describing the damage that occurs in vestibular and cochlear organs because of exposure to chemical substances or certain therapeutic agents [1]. The medication classes that are currently known to cause ototoxicity may be listed as antibiotics, antineoplastic agents, diuretics, anti-inflammatory agents, chelating agents, antimalarial drugs, ototropic drugs, and various others. Major symptoms induced by ototoxic substances include tinnitus, hearing loss, and dizziness [2, 3].
Cisplatin (cis-diamminedichloroplatinum II) is an effective antineoplastic agent commonly used for the treatment of several malignant diseases including squamous cell carcinoma of the head and neck, ovarian, solid testicle, prostate, bladder, cervix tumors, and non-small cell lung carcinoma [4]. On the other hand, serious side effects of cisplatin, such as ototoxicity, nephrotoxicity, gastrointestinal toxicity, myelinotoxicity, and peripheral neuropathy, limit the clinical use of this agent. Nephrotoxicity and ototoxicity are pointed out as the major dose-limiting side effects [5]. It is thought that the autotoxic side effect of cisplatin is caused by binding to DNA and causing apoptosis and activation of inflammatory cascade pathway leading to oxidative stress in the cell [6]. Reactive oxygen products produced after oxidative stress increase alfahydrate, malandialdehyde, toxic lipid peroxidase levels by allowing lipid peroxidation of protective antioxidant molecules against cochlear tissues [7]. The target regions that are mainly affected by cell damage mechanisms are the spiral ganglion cells, the lateral wall and the hairy cells in the cortical organ [6]. Ototoxic effects of cisplatin are characterized by progressive, irreversible, bilateral high-frequency sensory-neural hearing loss accompanied by tinnitus. Factors effecting the incidence of ototoxicity include the mode of administration, age, serum protein levels, cumulative dose, dietary factors, genetic factors, and a history of cranial radiotherapy [4, 5].
Cortexin is a polypeptide produced from bovine brain, and it was first introduced to clinical use in 1999. Its low molecular weight (7 kilodalton) allows it to pass the blood-brain barrier. Cortexin involves excitatory and inhibitory amino acid neuromediators. The mechanism of action of cortexin is associated by its metabolic activity. Cortexin has GABA-ergic activity, and it regulates the excitatory and inhibitory amino acid balance, as well as serotonin and dopamine levels, while also modulating antioxidant levels and bioelectrical activity in the brain [8].
There is currently no ideal agent to protect the patients from potential side effects of cisplatin, an agent widely used in the field of oncology. The present study aimed to evaluate the potential protective efficacy of concomitant cortexin use against cisplatin-induced ototoxicity in rats, by means of auditory and histopathological examinations.
MATERIALS and METHODS
Experimental Animals
The study was performed on 30 healthy adult Wistar Albino rats weighing between 200 and 240 g. The study was initiated after approval of local ethics committee. A maximum of five rats were placed in each cage at Experimental Research Center and were fed in a standard manner with a mechanism whereby they could reach unlimited special bait and water.
All rats used for the study were anesthetized by intraperitoneal administration of 50 mg/kg ketamine hydrochloride (Ketalar*; Eczacıbaşı Pharmaceuticals, İstanbul, Turkey) in combination with 7.5 mg/kg xylazine (Rompun; Bayer AG, Leverkusen, Germany). An appropriate speculum was placed into the outer ear canal of the subjects under an oto-microscope (Zeiss, Germany) to examine the outer ear canal and the tympanic membrane. Rats with outer and middle ear pathologies were excluded from the study.
Groups
The subjects were randomly divided into three groups of 10 mice each.
Group I (Control Group): The group was administered intraperitoneal (ip) saline solution at a dose of 1 mL/d for 7 days.
Group II (Cisplatin group): The group was administered ip cisplatin (Cisplatin-teva 10 mg 1 flakon, MED-İLAÇ San. ve Tic. A.Ş, İstanbul) for 2 days at a dose of 10 mg/kg, reaching to a cumulative dose of 20 mg/kg.
Group III (Cisplatin + Cortexin group): The group was given ip cisplatin for 2 days at a dose of 10 mg/kg reaching to a total dose of 20 mg/kg, and concomitant ip cortexin (Koptekcnh; Geropharm pharmaceutical company, Saint Petersburg, Russia) at a dose of 2 mg/d for 7 days.
On the fourth and eighth days after study baseline, all rats in all the three study groups were anesthetized by ip administering 50 mg/kg ketamine hydrochloride and 7.5 mg/kg xylazine in combination, and their auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAE) measurements were obtained from the right ear. On the fourth day after ABR and DPOAE measurements, five rats in each of the three groups were decapitated. Similarly, the remaining five rats in all three groups were decapitated on the eighth day after ABR and DPOAE measurements. Following decapitation, cochleas of the subjects were rapidly removed and transferred to histopathology laboratory for histopathological investigations.
Hearing Assessment
The ABR and DPOAE measurements of all subjects were recorded before the study and on the 4th and the 8th days of the study. ABR responses were recorded by subcutaneous needle electrodes. ABR threshold was defined as the lowest intensity level where V wave of ABR could be seen. DPOAE findings were evaluated based on signal to noise (SNR) ratios recorded at frequency bands of 1.5, 2, 3, 4, 5, 6, 7, 8, 10, and 12 kHz. Basal hearing thresholds were detected and subjects with hearing loss were excluded from the study.
Histopathological Assessment
For histopathological investigations, cochlea tissues obtained from the subjects in all groups were fixed by decalcification + 10% phormol solution, processed by a series of routine histologic tracing, and embedded into paraffin blocks. Sections at a thickness of 4–6 μm obtained from the paraffin blocks were stained by hematoxylin-eosin and Masson’s trichrome, and the histopathologic changes (edema, congestion, tissue separation and breakdown, edema in the spiral ganglion cells, vacuolization of the spiral ganglion cells) were identified. On the basis of the histopathological status, the changes were evaluated a none (0), mild (1), moderate (2), and severe (3). Maximum score was defined as 15. Scoring was done for each subject, and mean values were estimated for all groups. TUNEL method was used to identify the cells progressing to apoptosis. For the assessment of TUNEL staining, nuclei stained blue by hematoxylin were evaluated as normal, and nuclei showing brown nuclear staining were evaluated as apoptotic. Apoptotic index (AI) was calculated on the basis of the ratio of apoptotic cells to total (normal + apoptotic) cells.
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences version 22 software (IBM Corp.; New York, USA). One-way analysis of variance (ANOVA) and post-hoc Tukey tests were used to produce histo-scores based on histopathologic scores. A p value of <0.05 was considered statistically significant.
RESULTS
Hearing and histopathologic assessments of all three groups are shown below.
Hearing Assessment
ABR findings
The mean hearing thresholds were similar among the three groups based on ABR tests performed at study baseline (before drug administration) (p>0.05) (Table 1).
Table 1.
Mean hearing thresholds in ABR test (ABR mean dB ± standard deviation)
| Groups | Day 0 | Day 4 | Day 8 |
|---|---|---|---|
| Group I (Control Group) | 10.50±4.37 | 10.50±5.50 | 11.00±4.18 |
| Group II (Cisplatin group) | 10.00±4.08 | 16.50±3.37a | 25.00±3.53ab |
| Group III (Cisplatin + Cortexin group) | 9.50±4.97 | 13.50±3.37 | 16.00±2.23c |
The variables were expressed as mean±standard deviation.
Compared to the Control group, Cisplatin group had significantly elevated hearing thresholds on the 4th and the 8th days (p<0.05).
Cisplatin group had significantly elevated hearing threshold on the 8th day compared to the 4th day (p<0.05).
Comparison of the mean hearing thresholds of Cisplatin+Cortexin group and Cisplatin group on the 8th day indicated that there was a significant decrease in the Cisplatin+Cortexin group (p<0.05).
ABR: auditory brainstem response
Comparison of the control group and cisplatin group on the fourth day of study indicated that there was a significant increase in the mean hearing threshold of cisplatin group (p<0.05). When the control group and cisplatin+cortexin group were compared, a statistically significant increase was noted in the mean hearing threshold of cisplatin+cortexin group (p<0.05). Comparison of cisplatin group with cisplatin+cortexin group, on the other hand, did not indicate any statistically significant difference (p>0.05). ABR test performed on the eighth day of study demonstrated that there was a statistically significant increase in the mean hearing threshold of cisplatin group compared with the control group (p<0.05). When control group was compared with cisplatin+cortexin group, a statistically significant in of cisplatin group with cisplatin+cortexin group showed that there was a statistically significant decrease in the mean hearing threshold of cisplatin+cortexin group (p<0.05). When the mean hearing thresholds of cisplatin group on the fourth and the eighth days were compared, a statistically significant increase was noted in the values recorded on the eighth day (p<0.05). Comparison of mean hearing thresholds of cisplatin+cortexin group recorded on the fourth and the eighth days did not indicate any statistically significant difference (p>0.05).
DPOAE Findings
In neither of the frequencies, the changes on days 0, 4, and 8 were not found to be statistically significantly different in the control group (p>0.05) (Table 2). When the control group was compared with the cisplatin and cisplatin+cortexin groups on the fourth day of study, statistically significant emission loss was noted at all frequencies (p<0.05). In the cisplatin group, no significant differences were noted at 1, 4, 5, 6, 8, and 10 kHz frequencies between the fourth and the eighth days of study (p>0.05), whereas statistically significant emission loss was recorded at all other frequencies (p<0.05). In the cisplatin+cortexin group, no statistically significant differences were found at 1, 2, 5, 6, and 12 kHz frequencies between the fourth and the eighth days of study (p>0.05), whereas statistically significant emission loss was recorded at all other frequencies (p<0.05). Cisplatin ototoxicity occurred on the fourth and the eighth days of the study in cisplatin and cisplatin+cortexin groups, whereas statistically significant emission loss was not noted between these days in cisplatin+cortexin group at 2, 5, and 12 kHz (Table 2).
Table 2.
DPOAE tests’ results
| Frequency | Group I (Control Group) (dB±sd) | Group II (Cisplatin group) (dB±sd) | Group III (Cisplatin + Cortexin group) (dB±sd) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 a | Day 4 a | Day 8 a | Day 0 | Day 4 | Day 8 | Day 0 | Day 4 | Day 8 | |
| 1.5 khz | −5.7±0.41 | −5.4±0.48 | −5.8±0.57 | −7.5±0.53 | −10.9±0.5 b | −11.3±0.4 b | −5.13±0.1 | −11.5±0.33 c | −11.9±0.65 c |
| 2 khz | 9.1±0.75 | 9.5±0.25 | 9±073 | 8.35±0.41 | −6.1±0.83 | −7.2±0.6 | 7.54±0.3 | 3.04±0.65 c | 3.2±0.49 c |
| 3 khz | 7.7±0,.4 | 8.1±0.74 | 7.7±1.07 | 8.3±0.77 | 2±2.29 | −0.64±0.4 | 6.2±2.17 | 3.7±2.6 | 2.7±2.73 |
| 4 khz | 18±0.5 | 18.5±0.86 | 18±2.87 | 16.3±0.9 | 2.2±0.93 b | 1.5±0.25 b | 13.2±0.51 | 10.5±0.88 | 11.7±0.8 |
| 5 khz | 21.7±1.32 | 21±1.85 | 22±2.19 | 23±1.29 | 6.1±0.91 | 4.54±0.33 | 14.9±0.48 | 12.3±0.57 c | 11.9±0.63 c |
| 6 khz | 27.7±3.58 | 25±1.73 | 26.5±1.73 | 31.2±1.55 | 13.2±0.6 b | 12.5±0.52 b | 25.6±1.67 | 21.5±1.3 c | 20.9±0.95 c |
| 8 khz | 32.3±3.69 | 31.5±4.88 | 32.1±2.74 | 35.4±0.91 | 10.4±0.8 b | 9.8±0.54 b | 37.2±0.63 | 23.51.12 | 21.5±1.12 |
| 10 khz | 32.2±2.39 | 32.5±2.2 | 31.8±1.57 | 28.2±1.25 | 5±0.4 b | 5.1±0.84 b | 29.5±1.5 | 27.1±0.43 | 22.2±0.77 |
| 12 khz | 17.15±1.46 | 17.3±4.07 | 17±3.67 | 22.65±1.17 | −0.5±0.08 | 1±0.5 | 27.1±0.5 | 7.95±0.67 c | 8.5±0.58 c |
The variables were expressed as mean±standard deviation. One-way ANOVA test was performed.
No significant difference was noted when the measurements obtained from the control group on Days 0, 4 and 8 were compared (p>0.05).
No significant difference was noted when the measurements obtained from the Cisplatin group on Days 4 and 8 were compared(p>0.05).
No significant difference was noted when the measurements obtained from the cisplatin+cortexin group on Days 4 and 8 were compared (p>0.05).
DPOAE: distortion product otoacoustic emissions; sd: standard deviation
Histopathological Findings
TUNEL staining, performed to identify apoptotic cells, was examined under a light microscope.
TUNEL positivity was not recorded in the control group. Compared with the control group, increased TUNEL positivity was recorded on the fourth day of study in the cisplatin and cisplatin+cortexin groups (p<0.05) (Table 3). Similarly, compared with the control group, a significantly higher TUNEL positivity was observed in the cisplatin (Figure 1) and cisplatin+cortexin (Figure 2) groups on the eighth day of study (p<0.05). Whereas comparison of cisplatin and cisplatin+cortexin groups did not indicate a statistically significant difference between the groups on the fourth day of study (p>0.05), a marked increase in TUNEL positivity was noted in the cisplatin group on the eighth day of study (p<0.05) (Table 3).
Table 3.
Apoptotic index (AI) values between the groups
| Apoptotic index (%) | ||
|---|---|---|
| Day 4 | Day 8 | |
| Group I (Control Group) | 0±0 | 0±0 |
| Group II (Cisplatin group) | 4.16±0.40a | 9.50±0.83ab |
| Group III (Cisplatin + Cortexin group) | 3.66±0.51a | 2.25 ± 0.50ac |
The variables were expressed as mean±standard deviation.
Compared to the control group, a significant level of apoptosis was present in Cisplatin and Cisplatin+Cortexin groups on the 4th and the 8th days of the study (p<0.05).
AI values recorded on the 8th day was significantly higher compared to the values on 4th day in Cisplatin group (p<0.05).
When Cisplatin+Cortexin and Cisplatin groups were compared for Day 8 values, a significant decrease was found in Cisplatin+Cortexin group (p<0.05)
Figure 1.
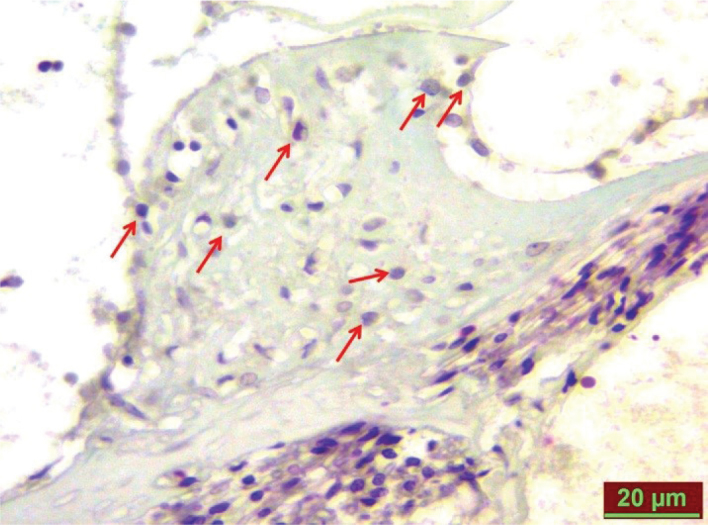
TUNEL-positive cells in the cisplatin group on fay 8 (red arrows).
Figure 2.
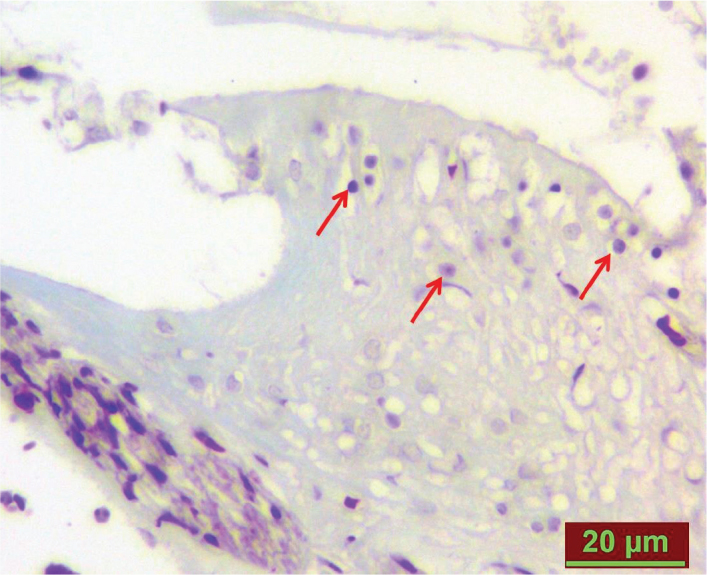
TUNEL-positive cells in the cisplatin+cortexin group on day 8 (red arrows).
Investigation of the triple-stained slides prepared for histopathological examinations under a light microscope showed that the corti organ was normal in the control group. Compared with the control group, mild edema and congestion were noted in the cisplatin and cisplatin+cortexin groups on the fourth day of study. Cisplatin group demonstrated marked congestion and tissue separation and breakdown on the eighth day (Figure 3). Moreover, there was a remarkable level of spiral ganglion cell edema and vacuolization. In the cisplatin+cortexin group, spiral ganglion cell edema and vacuolization were found to be markedly reduced and resembled those of the control group on the eighth day (Figure 4).
Figure 3.
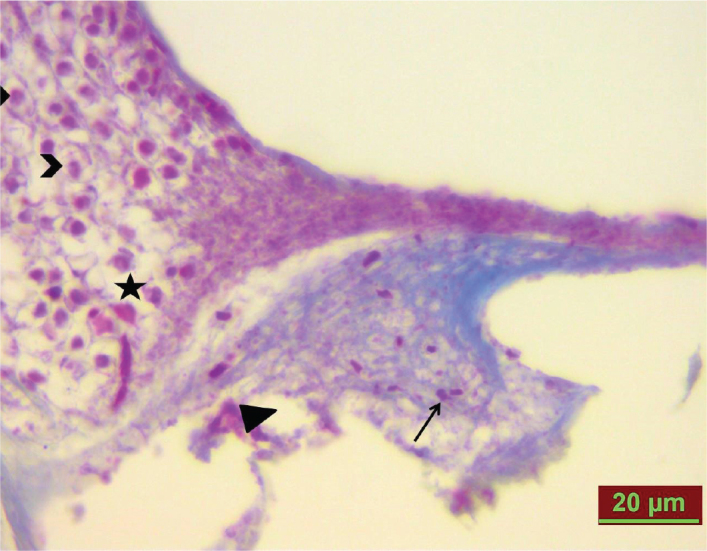
Cisplatin group. Congestion (arrow), tissue separation and breakdown (arrow head), and spiral ganglion cell edema (*), and vacuolization (>) on day 8.
Figure 4.
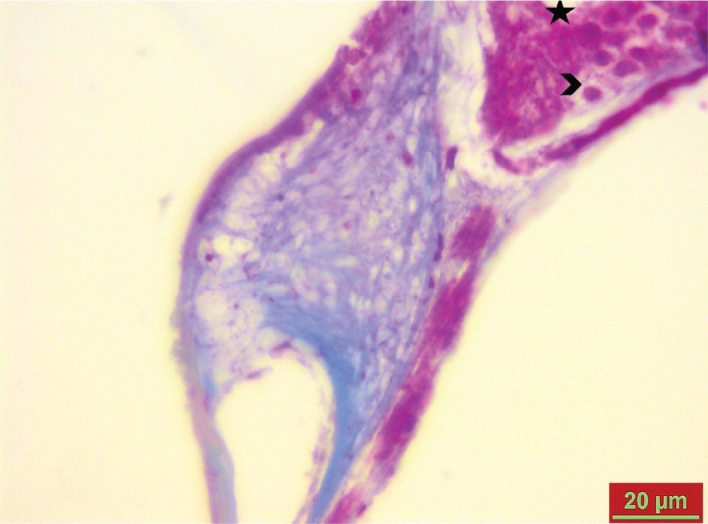
Mild decrease in spiral ganglion cell edema (*) and vacuolization (>) in the cisplatin+cortexin group on day 8.
Histo-scoring, performed on the basis of the histopathological changes observed in this study, showed that a significant level of edema and congestion developed in cisplatin and cisplatin+cortexin groups compared with the control group on the fourth day of study (p<0.05) (Table 4).
Table 4.
Distribution of histopathological changes according to study groups
| Edema | Congestion | Tissue separation and breakdown | Edema in spiral ganglion cells | Vacuolization in spiral ganglion cells | ||
|---|---|---|---|---|---|---|
| 0 1 2 3 | 0 1 2 3 | 0 1 2 3 | 0 1 2 3 | 0 1 2 3 | ||
| Group I (Control Group) | Day 4 | 4 1 - - | 5 - - - | 5 - - - | 5 - - - | 5 - - - |
| Day 8 | 4 1 - - | 5 - - - | 5 - - - | 5 - - - | 5 - - - | |
| Group II (Cisplatin group) | Day 4 | - 2 3 - | - 3 1 - | 4 1 - - | 5 - - - | 5 - - - |
| Day 8 | - - 2 3 | - - 2 3 | - 1 1 3 | - 1 2 2 | - - 2 3 | |
| Group III (Cisplatin + Cortexin group) | Day 4 | - 3 2 - | - 4 2 - | 4 1 - - | 5 - - - | 5 - - - |
| Day 8 | 3 2 - - | 3 2 - - | 4 1 - - | 3 2 - - | 4 1 - - |
Statistically significant increases were noted in the scores of edema, congestion, tissue separation and breakdown, spiral ganglion cell edema, and spiral ganglion cell vacuolization in the cisplatin group compared with the control group on the eighth day of study and compared with the scores recorded in cisplatin group on the fourth day of study (p<0.05). On the eighth day of study, cisplatin+cortexin group had a significantly higher decrease in edema, congestion, tissue separation and breakdown, spiral ganglion cell edema, and spiral ganglion cell vacuolization scores compared with cisplatin group (p<0.05) (Table 5).
Table 5.
Histo-scoring based on histopathological changes in each study group
| Edema | Congestion | Tissue separation and breakdown | Edema in spiral ganglion cells | Vacuolization in spiral ganglion cells | ||
|---|---|---|---|---|---|---|
| Group I (Control Group) | Day 4 | 0.2±0.44a | 0±00 | 0±00 | 0±00 | 0±00 |
| Day 8 | 0.2±0.44a | 0±00 | 0±00 | 0±00 | 0±00 | |
| Group II (Cisplatin group) | Day 4 | 1.60±0.54a | 1.00±0.70a | 0.20±0.44 | 0±00 | 0±00 |
| Day 8 | 2.60±0.54ab | 2.60±0.54ab | 2.40±0.89ab | 2.20±0.83ab | 2.60±0.54ab | |
| Group III (Cisplatin + Cortexin group) | Day 4 | 1.40±0.54a | 1.20±0.44a | 0.20±0.44 | 0±00 | 0±00 |
| Day 8 | 0.40±0.54c | 0.40±0.54c | 0.20±0.44c | 0.40±0.54c | 0.20±0.44c |
The variables were expressed as mean±standard deviation.
Histopathological changes were significantly different in Cisplatin and Cisplatin+Cortexin groups compared to the control group on the 8th day of study, and between Cisplatin and control groups on the 8th day of study (p<0.05).
When the values recorded in Cisplatin group on Days 4 and 8 were compared, Day 8 histo-scores were found to be statistically significantly higher (p<0.05).
Comparison of Cisplatin+Cortexin and Cisplatin groups on Day 8 indicated significant decrease in histo-scores of Cisplatin+Cortexin group(p<0.05).
DISCUSSION
Ototoxicity is a general term describing the damage that occurs in vestibular and cochlear organs as a result of exposure to chemical substances or certain therapeutic agents [1]. Several studies have been performed to define ototoxicity. In general, an agent is defined to be ototoxic if it causes at least 10 dB bilateral hearing loss between frequencies of 250 and 8000 Hz [9, 10].
Cisplatin-induced ototoxicity generally manifests as tinnitus and hearing loss starting at higher frequencies; however, these effects may extend to lower frequencies significant to understand speech [11, 12]. The effects are dose-dependent, cumulative, and generally permanent [13]. Ototoxic effects of cisplatin manifest with the damage on external hairy cells starting from the cochlear base [14, 15]. Studies performed later on demonstrated that, in addition to the external hairy cells, damage also occurs in the stria vascularis and spiral ganglion cells, and these events take place parallel to the directly targeted external hairy cell loss [16].
Several studies in the literature investigated methods to provide protection from cisplatin ototoxicity. Those studies demonstrated the protective effects of N-acetylcysteine [17], D-methionine [18], L-methionine [19], ebselen and allopurinol [20], tiopronin [21], vitamin E, [22] and steroids [23, 24] against cisplatin ototoxicity.
Cortexin is a polypeptide consisting of 82 amino acids [25]. Cortexin shows antiapoptotic activity through the caspase-3 pathway [26]. A previous study performed on rats reported that cortexin shows antioxidant activity by reducing lipid peroxidation [27]. Cortexin is a neurotropic agent, and it is available in the form of a lyophilized drug extracted from animal cortex consisting of neuropeptides, amino acids, and trace element. This nucleoprotein complex of the cerebral cortex may also include DNA fragments and chromatin elements. These elements of cortexin have specific target ranges to modulate several molecular and cellular steps of the pathologic process [28]. In a clinical study investigating cortexin, patients with brain ischemia showed favorable outcomes such as a decrease or complete regression in focal neurological symptoms, positive alterations in the markers of cognitive impairment, mood normalization, and decreased levels of depression after cortexin therapy [29]. In a study investigating the effects of cortexin on the recovery of traumatic peripheral facial nerve paralysis models experimentally induced in rabbits by conserving nerve integrity, favorable effects were observed on neural fibrotic degeneration, axonal degeneration, normal myelin construction, and edema in cortexin group compared to the control group [30]. Prospective placebo-controlled trials confirmed the neuroprotective efficacy and activity of cortexin as a stress-limiting agent in the presence of acute cerebral pathologies such as severe brain damage and encephalitis in infants. Those studies reported that addition of cortexin to therapy regimens at an early stage provides quite beneficial effects on recovery [31].
Several studies have been performed on cortexin, particularly in the recent years. Most of those studies investigated the neuroprotective and antioxidant activities of cortexin. These already known mechanism of action of cortexin was investigated in the present study considering it may be effective against the mechanisms associated with the reactive oxygen species that are generated because of cisplatin-induced ototoxicity. Considering cortexin has antioxidant activity, it affects the caspase-3 pathway in addition to increasing the neuroprotective modulators in the brain, and it plays a neuroprotective role, we supposed that it may be a potential agent to provide protection from cisplatin-induced ototoxicity. Moreover, there is currently no study in the literature investigating whether cortexin has a protective activity against ototoxicity, which was another reason encouraging us to carry out this research.
Auditory brainstem response tests performed in the scope of this study indicated that, compared with the control group, the groups given cisplatin developed hearing loss on the fourth and the eighth days of the study. On the other hand, the group given cortexin concomitant to cisplatin did not show a significant recovery on the fourth day but experienced statistically significant recovery in hearing loss on the eighth day of the study. Moreover, DPOAE tests showed that cisplatin and cisplatin+cortexin groups developed cisplatin ototoxicity on the fourth and the eighth days of the study, whereas cisplatin+cortexin group did not experience a significant emission loss at 2, 5, and 12 kHz frequencies on the same days. The fact that emission loss at these frequencies was lower suggests that cortexin may provide protective activity against cisplatin-induced ototoxicity.
Findings of the TUNEL staining, performed to identify the apoptotic cells, and histopathological examination results were also consistent with the results of the hearing tests. Yet, compared with the control group, the group given cisplatin had a significantly higher apoptotic cell count specifically on the eighth day, and Group III receiving concomitant cortexin had a marked reduction in apoptotic cell count particularly on the eighth day. The fact that cortexin significantly reduced apoptotic cell count may be related to its antioxidant activity. Likewise, previous studies showed that cortexin shows antioxidant activity through the caspase-3 pathway [26] and by reducing lipid peroxidation [27].
CONCLUSION
In conclusion, experimental cisplatin administration induced prominent hearing loss, cochlear apoptosis, and histopathological changes in rats on the fourth and the eighth of the study, and cortexin prophylaxis provided marked improvement in these parameters on the eighth day. This activity of cortexin can be associated with its antioxidant capacity, which is probably effective against the mechanisms induced by the reactive oxygen species produced during cisplatin ototoxicity. This study gives a different perspective on the treatment of ototoxicity, as it is the first study to demonstrate the protective activity of cortexin against cisplatin-induced ototoxicity. We believe that cortexin-based treatment strategies to prevent ototoxicity can be tested in more advanced and detailed studies in near future.
Acknowledgements
We thank to Fırat University Scientific Research Projects (FUSRP) for financial support.
Footnotes
This study was presented at the 38th Turkish Otolaryngology and Head and Neck Surgery Congress, 26–30 October 2016, Antalya, Turkey.
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Fırat University.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - O.E.,T.K.; Design - O.E., T.K., İ.K.; Supervision - T.K., İ.K., E.K.; Resources - O.E., T.Ku.; Materials - O.E., T.K., T.Ku.; Data Collection and/or Processing - E.K., İ.K., Ş.Y.; Analysis and/or Interpretation - O.E.,T.K., Ş.Y.; Literature Search - E.K., İ.K., T.Ku.; Writing Manuscript - O.E., T.K., T.Ku.; Critical Review - T.K., S.Y.,İ.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This study was funded by Fırat University Scientific Research Projects.
REFERENCES
- 1.Mutlu C. Ototoksisite. In: Celik O, editor. Kulak Burun Boğaz Hastalıkları ve Baş Boyun Cerrahisi. İstanbul: Turgut Yayıncılık; 2002. pp. 257–70. [Google Scholar]
- 2.Riggs LC, Matz GJ, Rybak RP. Ototoxicity. In: Bailey BJ, Calhoun KH, editors. Head and Neck Surgery- Otolaryngology. 2nd ed. Philadelphia: Lippincott-Raven; 1998. pp. 2165–70. [Google Scholar]
- 3.Wackym PA, Storper IS, Newman AN. Cochlear and vestibular ototoxicity. In: Canalis RP, Lampert PR, editors. The Ear Comprehensive Otology. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 571–85. [Google Scholar]
- 4.Sakamoto M, Kaga K, Kamio T. Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol Head Neck Surg. 2000;122:828–33. doi: 10.1016/S0194-5998(00)70009-X. [DOI] [PubMed] [Google Scholar]
- 5.Cooley ME, Davis L, Abrahm J. Cisplatin: a clinical review. Part II-Nursing assessment and management of side effects of cisplatin. Cancer Nurs. 1994;17:283–93. doi: 10.1097/00002820-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Rybak LP, Whitworth CA, Mukerjea D. Mechanisms of cisplatin- induced ototoxicity and prevention. Hear Res. 2007;226:157–67. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Lee JE, Nagakawa T, Kita T, Kim TS, Iguchi F, Endo T, et al. Mechanisms of apopitosis induced by cisplatin in marginal cells in Mouse stria vascularis. ORL J Otorhinolaryngol Relat Spec. 2004;66:111–8. doi: 10.1159/000079329. [DOI] [PubMed] [Google Scholar]
- 8.Skvortsova VI, Petrova EA, Meshkova KS. Development of the neuroprotectıve strategıes ın the treatment of acute ıschemıc stroke. Registry of pharmaceuticals (Doctor: neurology and psychiatry) 2008;11:528–9. [Google Scholar]
- 9.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–95. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 10.Brock PR, Knight KR, Freyer DR, Camphell KCM, Steyger PS, Blakely BW, et al. Platinum- induced ototoxicity in children: a consensus review on mechanisms, predispositions, and protection, Including a new international society of pediatric oncology Boston ototoxicity scale. J ClinOncol. 2012;30:2408–17. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–62. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biro K, Noszek L, Prekopp P. Characteristics and risk factors of cisplatin-induced ototoxicity in testicular cancer patients detected by distortion product otoacoustic emission. Oncology. 2006;70:177–84. doi: 10.1159/000093776. [DOI] [PubMed] [Google Scholar]
- 13.Laurell G. High-dose cisplatin treatment: hearing loss and plasma concentrations. Laryngoscope. 1990;100:724–34. doi: 10.1288/00005537-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Komune S, Asakuma S, Snow JBJ. Pathophysiology of the ototoxicity of cisdiaminedichloro platinum. Otolaryngol Head Neck Surg. 1981;89:275–82. doi: 10.1177/019459988108900226. [DOI] [PubMed] [Google Scholar]
- 15.Nakai Y, Konishi K, Chang KC. Ototoxicity of the anti cancer drug cisplatin. An experimental study. Acta Otolaryngol (Stockh) 1982;93:227–32. doi: 10.3109/00016488209130876. [DOI] [PubMed] [Google Scholar]
- 16.Van Ruijven MWM, de Groot JCMJ, Klis SFL, Smoorenburg G. Cochlear targets of cisplatin: an electro physiological and morphological time-sequence study. Hear Res. 2005;205:241–8. doi: 10.1016/j.heares.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Dickey DT, Muldoon LL, Kraemer DF, Neuwelt EA. Protection against cisplatinin duced ototoxicity by N-acetylcysteine in a rat model. Hear Res. 2004;193:25–30. doi: 10.1016/j.heares.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Korver KD, Rybak LP, Whitworth C, Campbell KM. Round window application of D methionine provides complete cisplatin otoprotection. Otolaryngol Head Neck Surg. 2002;126:683–9. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Frenz DA, Brahmblatt S. Round window membraned elivery of L-methionine provides protection from cisplatin ototoxicity without compromising chemotherapeutic efficacy. Neurotoxicology. 2001;22:163–76. doi: 10.1016/S0161-813X(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 20.Korver KD, Rybak LP, Whitworth C, Campbell KM. Effect of protective agentsagainst cisplatin ototoxicity. Am J Otol. 2000;21:513–20. [PubMed] [Google Scholar]
- 21.Fetoni AR, Quaranta N, Marchese R, Cadoni G, Paludetti G, Sergi B. The protective role of tiopronin in cisplatin ototoxicity in Wistarrats. Int J Audiol. 2004;43:465–70. doi: 10.1080/14992020400050059. [DOI] [PubMed] [Google Scholar]
- 22.Teranishi MA, Nakashima T. Effects of trolox, locally applied on round windows, on cisplatin-induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol. 2003;67:133–9. doi: 10.1016/S0165-5876(02)00353-1. [DOI] [PubMed] [Google Scholar]
- 23.Hill GW, Morest DK, Parham K. Cisplatin-induced ototoxicity: effect of intratympanic dexamethasone injections. Otol Neurotol. 2008;29:1005–11. doi: 10.1097/MAO.0b013e31818599d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waissbluth S, Salehi P, He X, Daniel SJ. Systemic dexamethasone for the prevention of cisplatin-induced ototoxicity. Eur Arch Otolarnyngol. 2013;270:1597–605. doi: 10.1007/s00405-012-2150-0. [DOI] [PubMed] [Google Scholar]
- 25.Coulter PM, Bautista EA, Margulies JE, Watson JB. Identification of cortexin: a novel, neuron-specific, 82-residue membrane protein enriched in rodent cerebral cortex. J Neurochem. 1993;61:756–9. doi: 10.1111/j.1471-4159.1993.tb02183.x. [DOI] [PubMed] [Google Scholar]
- 26.Mendzheritskiĭ AM, Karantysh GV, Ryzhak GA, Dem’ianenko SV. Regulation of content of cytokines in blood serum and of caspase-3 activity in brains of old rats in model of sharp hypoxichypoxia with Cortexin and Pinealon. Adv Gerontol. 2014;27:94–7. [PubMed] [Google Scholar]
- 27.Zarubina IV, Shabanov PD. Cortexin and cortagen as correcting agents in functional and metabolic disorders in the brain in chronic ischemia. Eksp Klin Farmakol. 2011;74:8–15. [PubMed] [Google Scholar]
- 28.Gomazkov OA. Cortexin. Molecular mechanisms and targets of neuroprotective activity. Zh Nevrol PsikhiatrIm SS Korsakova. 2015;115:99–104. doi: 10.17116/jnevro20151158199-104. [DOI] [PubMed] [Google Scholar]
- 29.Mashin VV, Belova LA, Chaplanova OI, Khusnullina AF, Manasian AM. An open clinical trial of cortexin in treatment of brain ischemia. Zh Nevrol Psikhiatr Im S S Korsakova. 2014;114:49–52. [PubMed] [Google Scholar]
- 30.Tunçcan T, Yalçın Ş, Demir CF, Akın MM, Karlıdağ T, Keleş E, et al. Efficacy of Cortexin and Methylprednisolone on Traumatic Facial Nerve Paralysis. J Int Adv Otol. 2016;12:303–9. doi: 10.5152/iao.2016.1166. [DOI] [PubMed] [Google Scholar]
- 31.Shmakov AN, Kasymov VA, Kokhno VN. Adjuvants to the treatment of acut ecerebral insufficiency in newborns. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111:60–3. [PubMed] [Google Scholar]


