Abstract
Background
The activation of multiple signaling pathways jeopardizes the clinical efficacy of EGFR tyrosine kinase inhibitors (TKIs) in EGFR-mutation positive non-small cell lung cancer (NSCLC). Integrin-linked kinase (ILK) regulates the interactions between tumor cells and extracellular environment to activate signaling pathways and promote cell proliferation, migration, and epithelial-mesenchymal transition. Src homology 2 domain-containing phosphatase 2 (SHP2) is essential for receptor tyrosine kinase signaling and mitogen-activated protein kinase (MAPK) pathway activation.
Methods
We analyzed tumor ILK, β-receptor subunit glycoprotein 130 (gp130), SHP2, and stromal hepatocyte growth factor (HGF) and interleukin-6 (IL-6) mRNA expression in baseline tumor specimens of advanced EGFR-mutation positive NSCLC patients treated with EGFR TKIs.
Results
ILK, when highly expressed, was an independent poor prognostic factor for the progression-free survival of the patients, both in the univariate (hazard ratio [HR for disease progression, 2.49; 95% CI, 1.37–4.52; P = .0020]) and in the multivariate (HR 3.74; 95% CI, 1.33–10.56; P = .0126) Cox regression model. Patients with high SHP2 expression had an almost 13-month shorter progression-free survival (P = .0094) and an 18-month shorter overall survival (P = .0182) in comparison to those with low SHP2 mRNA expression.
Interpretation
The levels of ILK and SHP2 could be predictive for upfront combinatory therapy of EGFR TKIs plus SHP2 or ILK inhibitors.
Fund
A grant from La Caixa Foundation, an Instituto de Salud Carlos III grant (RESPONSE, PIE16/00011), an Instituto de Salud Carlos III grant (PI14/01678), a Marie Skłodowska-Curie Innovative Training Networks European Grant (ELBA No 765492) and a Spanish Association Against Cancer (AECC) grant (PROYE18012ROSE).
Keywords: Integrin-linked kinase, EGFR mutations, NSCLC, Src homology 2 domain-containing phosphatase 2, Signaling pathways
Research in context.
Evidence before this study
We did a systematic search of PubMed using the search terms “lung cancer,” and “ILK” in one search, and “lung cancer,” and “SHP2” in another search, each for articles published between Jan 1, 2010, and Oct 31, 2018. Several original studies were identified. In the case of ILK, most of the studies related ILK with a mesenchymal phenotype, loss of E-cadherin and worse prognosis. SHP2 was mostly related with the activation of the mitogen-activated protein kinase (MAPK) in different molecular subtypes of lung cancer, including EGFR-mutation positive NSCLC. The majority of the identified studies were preclinical or in moderately sized cohorts of lung cancer patients, not selected for EGFR mutations. To our knowledge, the expression analysis of ILK, SHP2 and three more biomarkers involved in the interaction between tumor cells and tumor microenvironment, in EGFR-mutation positive NSCLC patients treated with first-line EGFR tyrosine kinase inhibitors (TKIs), has not been previously published. The association of ILK, β-receptor subunit glycoprotein 130 (gp130), SHP2, and stromal hepatocyte growth factor (HGF) and interleukin-6 (IL-6) mRNA with the progression-free and overall survival of EGFR-mutation positive NSCLC patients has not yet been evaluated in the clinical setting.
Added value of this study
Our study shows that high expression of ILK is an independent factor for progression-free survival to EGFR TKIs. ILK was positively correlated with gp130 and stromal HGF mRNA expression. We also found that SHP2 expression can predict progression-free and overall survival for EGFR-mutation positive NSCLC patients receiving first-line therapy with EGFR TKIs.
Implications of all the available evidence
Our study reinforces the concept that single EGFR TKIs will never cure EGFR-mutation NSCLC and there will always be subtypes of patients with unfavorable prognosis and poor outcome to the treatment. These subsets of EGFR-mutation positive NSCLC can be targetable by combinatorial approaches of EGFR TKIs with inhibitors that target the parallelly activated signaling pathways. The assessment of ILK and SHP2 might be useful for the stratification of patients with EGFR mutations and future combinational therapies. Moreover, our findings suggest further research investigating the interaction of tumor cells with the tumor microenvironment in EGFR-mutation positive and other molecular subtypes of lung cancer.
Alt-text: Unlabelled Box
1. Introduction
Epidermal growth factor receptor (EGFR)-positive non-small-cell cancer (NSCLC) has reached a plateau in outcomes with EGFR tyrosine kinase inhibitors (TKIs) [1,2]. First- (gefitinib, erlotinib) second- (afatinib, dacomitinib), and the third-generation EGFR TKI, osimertinib, are currently the standard of care for the first-line management of EGFR-mutation positive NSCLC. Icotinib is used in China [3]. Although numerous clinical trials have shown that patients with activating mutations in the exons 18–21 of the EGFR gene respond to EGFR TKIs, there are still no commonly recognized genetic alterations that may serve as negative predictive biomarkers to EGFR inhibitors. Signal transducer and activator of transcription 3 (STAT3) activation is one of the first mechanism of relative resistance to EGFR TKIs, observed in EGFR-mutation positive NSCLC patients and cell lines. In cell culture, neither an EGFR TKI nor a Src family kinase (SFK) inhibitor inhibited STAT3 phosphorylation. Only a pan-Janus kinase (JAK) inhibitor (P6) ablated STAT3 activation and inhibited tumorigenesis [4]. We reported that both gefitinib and osimertinib activate STAT3, Src and YES-associated protein 1 (YAP1) in EGFR-mutation positive lung cancer cells [5]. Combined EGFR, STAT3 and Src inhibition abrogated tumor growth more efficiently than single EGFR inhibition, both in culture and in vivo [5]. EGFR-mutation positive cells secrete interleukin-6 (IL-6), and blocking the IL-6/β-receptor subunit glycoprotein 130 (gp130)/JAK pathway, decreases STAT3 phosphorylation levels [4]. The introduction of EGFR mutation in immortalized breast epithelial cells leads to oncogenesis, IL-6 expression, and STAT3 activation, that can be inhibited with STAT3 or gp130 blockade [4].
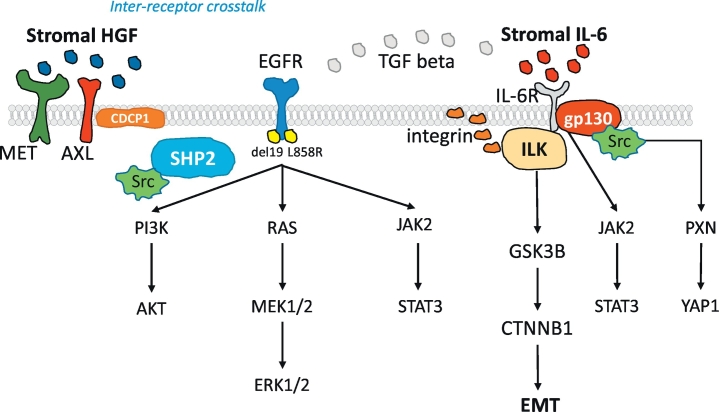
IL-6 is a pro-inflammatory cytokine that activates the JAK-STAT3, extracellular signal-regulated protein kinase (ERK) mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB, also called AKT) pathways, via the common co-receptor gp130 [6]. In erlotinib-sensitive cells, mutant EGFR regulates the expression of IL-6, however, in erlotinib-resistant cells, transforming growth factor-beta (TGF-β) drives the expression of IL-6, independently of EGFR activation [7] (Fig. 1). We have shown that the co-activation of receptor tyrosine kinases (RTKs) and non-RTKs, including AXL and CUB-domain-containing protein 1 (CDCP1), is a common trait in EGFR-mutation positive NSCLC cells and patient tumor samples. The tumor microenvironment-derived ligand hepatocyte growth factor (HGF) induces inter-receptor cross-talk of MET with CDCP1 or AXL. The combination of gefitinib or osimertinib with repotrectinib (formerly TPX-0005), a multi-kinase inhibitor that among others, inhibits Src, focal adhesion kinase (FAK) and JAK2, was synergistic in culture and in vivo [8]. Osimertinib activates integrins and downstream SFK and FAK signaling. Dasatinib enhances the effect of osimertinib by inhibiting SFK and FAK [9]. We obtained similar results with the combination of osimertinib and repotrectinib [8]. However, other authors have not found a relevant role of integrins in EGFR-mutation positive NSCLC [10]. Therefore, we posit that integrin-linked kinase (ILK) could have a function in EGFR-mutation positive NSCLC (Fig. 1). ILK couples integrins and growth factors to downstream signaling pathways [11]. The activity of ILK, modulated by integrin ligation in a PI3K-dependent manner, stimulates the phosphorylation of AKT on the serine 473. Activated AKT phosphorylates and inactivates glycogen synthase kinase 3β (GSK3β), resulting in the nuclear localization of β-catenin and activated transcription, cell cycle progression, and cell proliferation. Also, ILK directly phosphorylates GSK3β [12] (Fig. 1). Previous studies indicate that several types of tumors, including NSCLC, have a high ILK expression [[13], [14], [15], [16], [17]]. Hypoxia induces ILK expression through a hypoxia-inducible factor 1-alpha (HIF-1α)-dependent mechanism. ILK can de-repress HIF-1α signaling through the Y-box binding protein-1 (YB-1)-mediated inhibition of Foxo3a expression [18]. Both linear Foxo3 (Foxo3 mRNA) and circular Foxo3 (circ-Foxo3) are suppressors of tumor growth by regulating sponging endogenous miRNAs [19]. ILK and HIF-1α promote epithelial-mesenchymal transition (EMT) (Fig. 1). [18].
Fig. 1.
Overview of the role of the biomarkers explored in the study on EGFR signaling. EGFR activating mutations located in the EGFR tyrosine kinase domain enhance cell growth and invasion via tyrosine phosphorylation and lead to the activation of mitogen-activated protein kinase (MAPK), signal transducer and activator of transcription 3 (STAT3) and phosphatidylinositol 3-kinase (PI3K)-AKT pathways. Src homology 2 domain-containing phosphatase 2 (SHP2), a widely expressed cytoplasmic tyrosine phosphatase with two src homology (SH)2 domains, plays an essential role in most receptor-tyrosine kinase (RTK) signaling pathways. SHP2 function is required for MAPK pathway and activation of its downstream transcriptional targets. SHP2 activates several Src family kinases (SFKs), including Src as top hit. STAT3 can be activated not only by growth factor receptors, like EGFR, but also by interleukins, like IL-6. IL-6 is a glycoprotein which first binds to a-chain (IL-6R) and then recruits the b-chain (gp130) of the receptor. Subsequently, the IL-6/IL-6R complex initiates homodimerization of gp130, activates a cytoplasmic tyrosine kinase bound to gp130 and triggers signaling cascades through Janus-like kinase (JAK) and Src kinase. JAK2 mediates STAT3 phosphorylation and activation. The integrin-linked kinase (ILK) pathway activates beta-catenin (CTNNB1) signaling. ILK phosphorylates glycogen synthase kinase 3 (GSK3B) on serine 9 to inhibit its activity and induces the translocation of CTNNB1 into the nucleus. CTNNB1 regulates epithelial-mesenchymal transition (EMT). ILK expression is associated with IL-6 expression and can play an important role in mediating IL-6 function in EGFR-mutation positive lung cancer cells. Independently of STAT3, gp130 activates the Yes-Associated Protein (YAP1) oncoprotein through direct association with SFKs. Upon Src activation, several downstream Src binding partners are targeted for phosphorylation, including paxillin (PXN) on tyrosine 118. The tumor microenvironment-derived ligand hepatocyte growth factor (HGF) induces inter-receptor cross-talk of MET with (CUB) domain-containing protein-1 (CDCP1) or AXL.
We reported that the transmembrane glycoprotein, CDCP1, is overexpressed in EGFR-mutation positive tumors and cell lines, conferring shorter progression-free survival and overall survival in EGFR-mutation positive NSCLC patients [8]. CDCP1 triggers a cascade of tyrosine phosphorylation events, activates signaling networks, including SFKs, protein kinase C (PKC), integrins, catenins, and ephrins, and promotes cell growth and survival [20]. In addition to ILK, integrins also activate the tyrosine phosphatase, Src homology 2 domain-containing phosphatase 2 (SHP2), encoded by PTPN11. SHP2 activates Src by dephosphorylating the inhibitory site tyrosine 530 and activating Src substrates, such as paxillin [21]. We detected SHP2 phosphorylation on the tyrosine 542 in the EGFR mutation-positive PC9 cell line. Neither gefitinib, nor gefitinib plus TPCA-1 (STAT3 and NFKB inhibitor) were able to abrogate it [5].
In the current study, we are reporting the effect of tumor ILK and gp130 gene expression, and stromal IL-6 and HFG gene expression on the progression-free survival and overall survival of 64 EGFR mutation-positive NSCLC patients. We have previously demonstrated the negative effect of SHP2 in the outcome of these patients [8], and herein we provide additional information for progression-free survival and overall survival.
2. Methods
2.1. Sample collection
Pretreatment tumor specimens from advanced EGFR-mutation positive NSCLC patients were retrospectively collected from eight sites in Spain, France, Italy and Colombia [5,8] (Supplementary Fig. 1 in the Supplementary Material). The clinical data was assessed in accordance with the protocol approved by the institutional review board of Germans Trias i Pujol Hospital, Badalona, Spain and de-identified for patient confidentiality as previously described [5,8]. Detailed laboratory procedures (including the assessment of EGFR mutations) are presented in the Supplementary Material.
2.2. Real-time PCR analyses
Paraffin-embedded samples and slides were obtained by standard procedures. The discrimination between tumor and stromal cells was performed by a pathologist with the use of the laser microdissection (Zeiss-Palm, Oberlensheim, Germany) [22]. The present gene expression study was conducted in the ISO 15189-certified Pangaea Oncology laboratory located in Hospital Universitari Dexeus - Grupo Quirónsalud (Barcelona, Spain). We used remaining tissue material from our previous studies [5,8] for the mRNA analysis of ILK and gp130, by quantitative reverse transcription polymerase chain reaction (qRT-PCR). SHP2 mRNA expression was previously analyzed [8]. The mRNA expression of HGF and IL-6 were examined in the tumor stroma of a small number of patients (HGF, n = 26 and IL6, n = 25). The primer and probe sets were designed using Primer Express 3.0 Software (Applied Biosystems) according to their Ref Seq (http://www.ncbi.nlm.nih.gov/LocusLink). Gene-specific primers are as follows: ILK, forward 5′-GACGAAGCTCAACGAGAATCACT-3′ and reverse 5′-CTTCAGCACCTTCACGACAATG-3′; gp130, forward 5′-ATAGGACCAAAGATGCCTCAACTT-3′ and reverse 5′-GACACAGCATCCACCCGATC-3′; HGF, forward 5′ GCCATGAATTTGACCTCTATGAAAA-3′ and reverse 5′-AGCTGCGTCCTTTACCAATGA-3′; IL-6 Paxillin Hs00985639_m1, (Applied Biosystems). Gene expression quantification was performed as previously described [5,8] Details are provided in the Supplementary Material.
2.3. Statistical analyses
The primary endpoint of the study was to examine the potential effects of gene mRNA expression levels on survival. Progression-free survival and overall survival were estimated by means of the Kaplan–Meier method and compared with a nonparametric log-rank test. Biomarker expression was assessed as a dichotomous estimate (low versus high using the median as the cut-off). The median tumor ILK mRNA expression was 0.73 (interquartile range [IQR] 0.21, 1.81). The median tumor gp130 mRNA expression was 4.58 (IQR 0.80, 17.80). The median tumor SHP2 mRNA expression was 2.38 (IQR 0.25, 8.28) [8]. The median stromal IL-6 mRNA expression was 42.01 (IQR 6.93, 386.03). The median stromal HGF mRNA expression in the tumor stroma was 4.62 (IQR 0.55, 12.42) (Supplementary Table 1). A multivariate Cox proportional hazard model was applied with potential risk factors as covariates, obtaining Hazard Ratios (HR) and their 95% confidence intervals (CI). Each analysis was performed with the use of a two-sided 5% significance level and a 95%CI. Correlation analysis was performed using Pearson's or Spearman's correlation. The statistical analyses were performed using SAS version 9.3.
3. Results
3.1. Patients characteristics and correlation analysis
A total of 64 EGFR-mutation positive patients were included in this study, whose clinical information has been previously described (Supplementary Table 2) [8]. In summary, the median age was 67 years, most of the patients were women and never smokers, 69% of them had EGFR deletion 19 mutations, and 37% and 33% of them had bone and brain metastases, respectively. All patients received first-line therapy with first- (erlotinib and gefitinib), and second-generation (afatinib) EGFR TKIs. With a median follow-up of 26.7 months, the median progression-free survival was 14.1 months (95% CI, 8.8 to 16.3) and median overall survival was 26.7 months (95% CI, 17.9 to 37.1). We previously reported that the high mRNA expression of STAT3, YAP1 [5], AXL and CDCP1 [8] are related with worse outcome to EGFR TKIs in this cohort of 64 EGFR-mutation positive NSCLC patients. Herein, we evaluate whether the tumor expression of ILK, gp130 and SHP2 and the stromal expression of HGF and IL-6 may have an effect on therapy outcome.
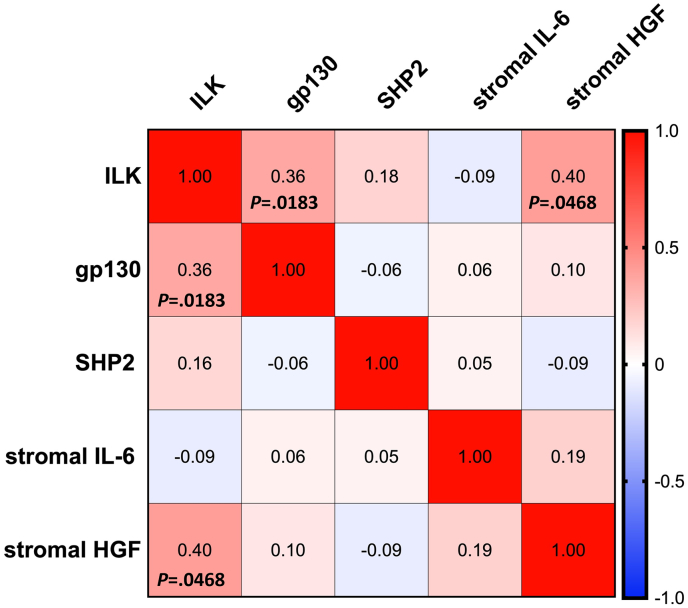
We first intended to see whether it exists a correlation among ILK, gp130, SHP2, stromal HGF, and stromal IL-6. (Fig. 2). The mRNA expression of ILK had a positive correlation with the mRNA expression of gp130 (Spearman coefficient = 0.36, P = .0183). A positive correlation between ILK and stromal HGF mRNA expression was also detected (Spearman coefficient = 0.40, P = .0468). No other statistically significant correlations were found (Fig. 2).
Fig. 2.
Heat map showing the Spearman correlation coefficient among the biomarkers. Negative correlations are in blue color and positive correlations in red.
3.2. Progression-free survival
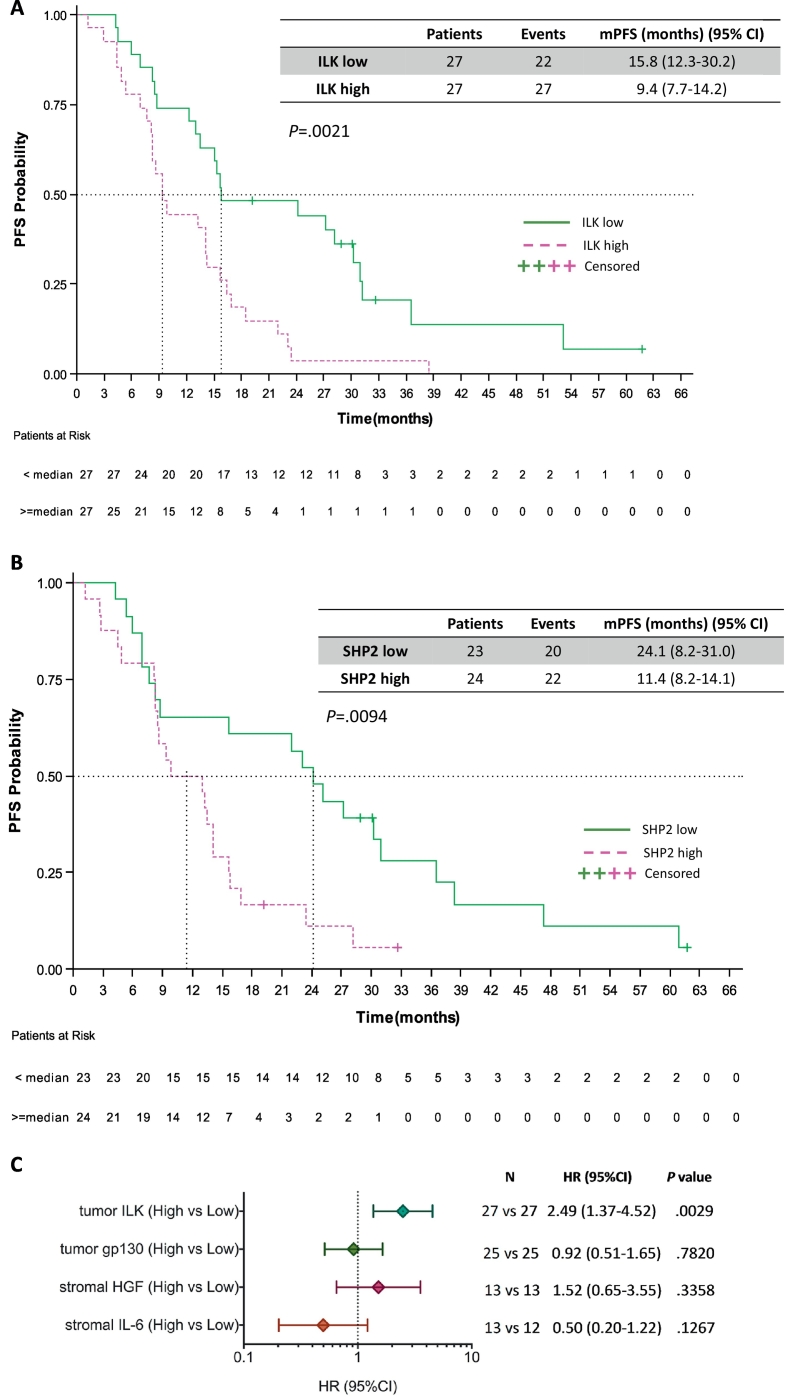
We then evaluated the effect of the tumor ILK, gp130, and SHP2, and the stromal HGF and IL-6 expression levels on progression-free survival. High tumor ILK mRNA expression was predictive of worse outcome in EGFR-mutation positive patients treated with EGFR TKIs. Median progression-free survival was 9.4 months (95% CI, 7.7 to 14.2) for the 27 patients with high ILK and 15.8 months (95% CI, 12.3 to 30.2) for the 27 patients with low ILK mRNA expression (P = .0021), hazard ratio (HR) of 2.49, 95% CI 1.37 to 4.52 (P = .0029) (Fig. 3A). As previously reported, patients with high tumor SHP2 mRNA expression had a higher risk for disease progression with a HR of 2.40 (95% CI 1.22 to 4.74), (P = .0115) compared to those with low SHP2 [8]. Kaplan Meier survival curves show that the patients with high SHP2 expression had a median progression-free survival of 11.4 months (95% CI, 8.2 to 14.1) compared to 24.1 months (95% CI, 8.2 to 31.1) for the patients with low SHP2 mRNA expression (P = .0094) (Fig. 3B). No significant differences in progression-free survival were observed according to the tumor expression levels of gp130. Median progression-free survival was 14.2 months (95% CI, 12.3 to 22.4) for the 25 patients with high tumor gp130 mRNA expression and 13.4 months (95% CI, 8.2 to 25.1) for the 25 patients with low expression of the biomarker (HR of 0.92, 95% CI 0.51 to 1.65, P = .7820) (Fig. 3C).
Fig. 3.
Progression-free survival by the expression of the biomarkers in EGFR-mutation positive NSCLC patients. A. Median progression-free survival was 9.4 months (95% CI, 7.7 to 14.2) for the 27 patients with high ILK and 15.8 months (95% CI, 12.3 to 30.2) for the 27 patients with low ILK mRNA expression; p = .0021. B. Median progression-free survival was 11.4 months (95% CI, 8.2 to 14.1) for the 24 patients with high SHP2 and 24.1 months (95% CI, 8.2 to 31.1) for the 23 patients with low SHP2 mRNA expression; p = .0094. C. Univariate analysis for progression-free survival. The bars correspond to 95% confidence intervals. mPFS, median progression-free survival; HR, hazard ratio.
HGF and IL-6 mRNA expression were examined in the tumor stroma and were evaluable for approximately one-third of the patients. In the case of HGF, the 13 EGFR-mutation positive patients with high stromal expression had a 4-month less progression-free survival compared to the 13 patients with low HGF stromal expression (9.3 months [95% CI, 5.4 to 15.8] versus 13.4 months [95% CI, 8.5 to 28.1], P = .3325). Surprisingly, for stromal IL-6 mRNA expression, we observed a longer progression-free survival of 23.1 months (95% CI, 8.2 to 60.9) for the 13 EGFR-mutation positive patients with high stromal IL-6 expression, compared to 11.2 months (95% CI, 6.9 to 38.3) for the 12 patients with low stromal IL-6 expression, although the difference was not statistically significant (P = .1186). The univariate Cox proportional hazard modeling of the five biomarkers for progression-free survival is summarized in Table 1 and Fig. 3C. In the multivariate Cox regression model, ILK mRNA expression remained highly significant for progression-free survival with a HR of 3.74 (95% CI 1.33 to 10.56), (P = .0126) (Table 1).
Table 1.
Cox regression model for the interaction between molecular parameters and progression-free survival.
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Parameter | N | HR (95%CI) | P | HR (95%CI) | P |
| Tumor ILK | |||||
| Low | 27 | 1 | 1 | ||
| High | 27 | 2.49 (1.37–4.52) | .0029 | 3.74 (1.33–10.56) | .0126 |
| Tumor gp130 | |||||
| Low | 25 | 1 | 1 | ||
| High | 25 | 0.92 (0.51–1.65) | .7820 | 0.68 (0.30–1.58) | .3764 |
| Tumor SHP2 [8] | |||||
| Low | 23 | 1 | 1 | ||
| High | 24 | 2.40 (1.22–4.74) | .0115 | 1.21 (0.48–3.04) | .6880 |
| Stromal IL-6⁎ | |||||
| Low | 13 | 1 | – | – | |
| High | 12 | 0.50 (0.20–1.22) | .1267 | – | – |
| Stromal HGF⁎ | |||||
| Low | 13 | 1 | – | – | |
| High | 13 | 1.52 (0.65–3.55) | .3358 | – | – |
As observed the biomarkers stromal IL-6 and stromal HGF have 61% and 59% of missing data. For this reason, a multivariate Cox regression model with the 5 biomarkers will be of no interest because only in 9 cases all the 5 biomarkers have a known value. When considering the three biomarkers ILK, gp130, and, SHP2, 31 observations were included in the multivariate Cox regression model.
3.3. Overall survival
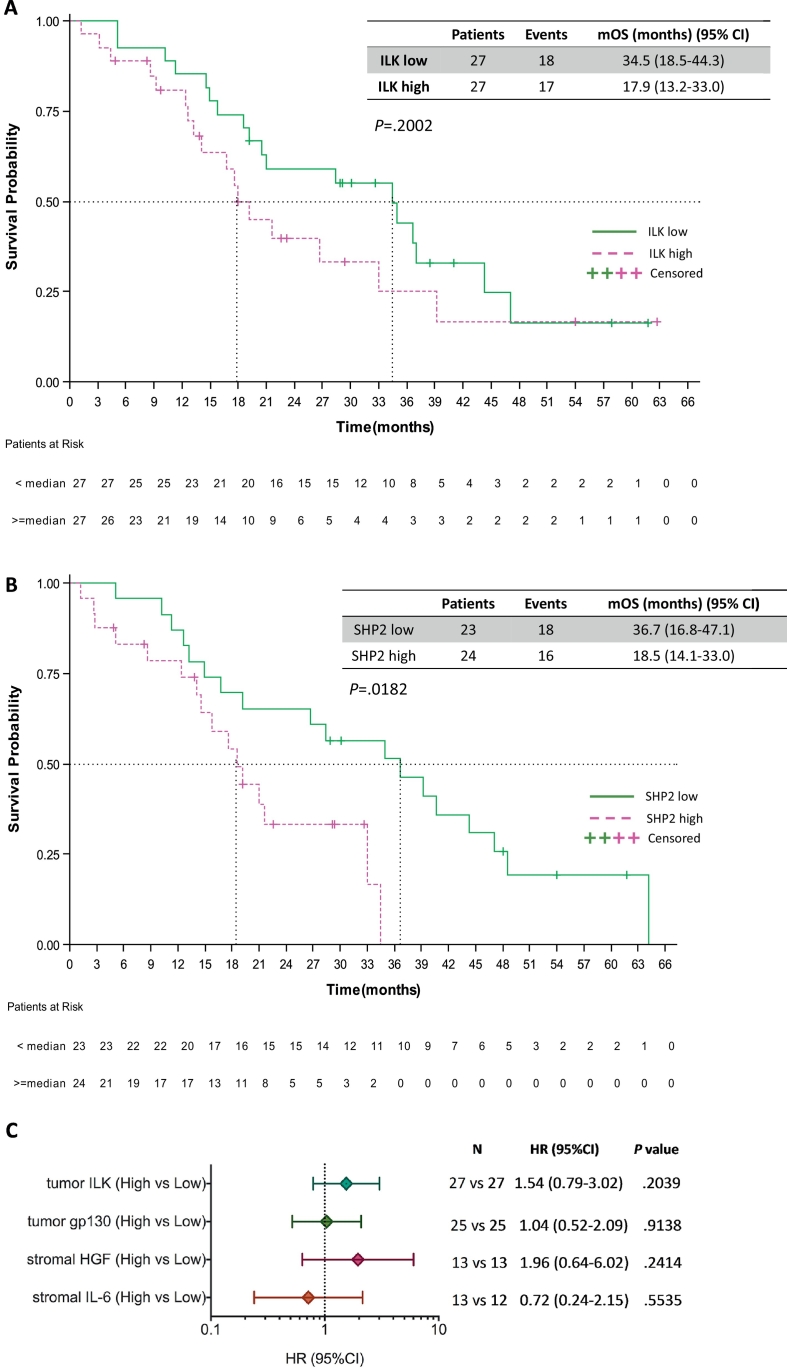
The effect of the five biomarkers on the survival of the 64 EGFR-mutation positive NSCLC patients included in our study, was also examined. In the case of ILK, patients with high tumor ILK mRNA expression trended towards shorter overall survival (HR of 1.54, 95% CI 0.79 to 3.02, P = .2039). Specifically, patients with high tumor ILK mRNA expression had a median progression-free survival of 17.9 months (95% CI 13.2 to 33.0) compared to 34.5 months (95% CI 18.5 to 44.3), (P = .2002) (Fig. 4A). We have previously reported the higher risk of death for patients with high tumor SHP2 mRNA expression [8]. The Kaplan-Meier curves in Fig. 4B illustrate now this risk, with a median overall survival of 18.5 months (95% CI, 14.1 to 33.0) for patients with high SHP2 mRNA and 36.7 months (95% CI, 16.8 to 47.1) for those with low SHP2 mRNA (P = .0182). No differences in overall survival were found between patients with high and low tumor gp130 mRNA expression. The median overall survival for patients with high gp130 mRNA was 33.0 months (95% CI, 18.5 to 39.2) and 34.9 months for those with low gp130 mRNA (95% CI, 9.3 to 47.1) (P = .9136). We also used R2 genomics visualization tool (http://r2.amc.nl) [8] in a TCGA (The Cancer Genome Atlas) dataset of 80 EGFR-mutation positive lung adenocarcinoma patients, to confirm the inverse relation between SHP2 mRNA levels and overall survival probability (P = .0043) (Supplementary Fig. 2 in the Supplementary Material).
Fig. 4.
Overall survival by the expression of the biomarkers in EGFR-mutation positive NSCLC patients. A. Median overall survival was 17.9 months (95% CI 13.2 to 33.0) for the 27 patients with high ILK and 34.5 months (95% CI 18.5 to 44.3) for the 27 patients with low ILK mRNA expression; p = .2002. B. Median overall survival was 18.5 months (95% CI, 14.1 to 33.0) for the 24 patients with high SHP2 and 36.7 months (95% CI, 16.8 to 47.1) for the 23 patients with low SHP2 mRNA expression; p = .0182. C. Univariate analysis for overall survival. The bars correspond to 95% confidence intervals. mOS, median overall survival; HR, hazard ratio.
As far as the stromal evaluated biomarkers concerns, the median overall survival was numerically shorter for patients with high compared to those with low stromal HGF mRNA expression (21.0 months [95% CI, 13.21 to 26.7] versus 34.5 months [95% CI, 18.5 to 44.3], P = .2329). Similar to progression-free survival, we noticed a longer survival of 40.7 months (95% CI, 15.8 to 64.1) for EGFR-mutation positive patients with high stromal IL-6 mRNA expression than those with low stromal IL-6 mRNA expression who experienced a shorter survival of 21.6 months (95% CI, 10.2 to not reached [NR], P = .5571). Fig. 4C and Table 2 summarize the univariate and multivariate Cox proportional hazard regressions for overall survival based on the expression of the five biomarkers. None of the biomarkers, including SHP2, remained significant for overall survival, in the multivariate Cox regression model.
Table 2.
Cox regression model for the interaction between molecular parameters and overall survival.
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| Parameter | N | HR (95%CI) | P | HR (95%CI) | P |
| Tumor ILK | |||||
| Low | 27 | 1 | 1 | ||
| High | 27 | 1.54 (0.79–3.02) | .2039 | 1.80 (0.61–5.12) | .2533 |
| Tumor gp130 | |||||
| Low | 25 | 1 | 1 | ||
| High | 25 | 1.04 (0.52–2.09) | .9138 | 0.80 (0.31–2.07) | .6455 |
| Tumor SHP2 [8] | |||||
| Low | 23 | 1 | 1 | ||
| High | 24 | 2.59 (1.14–5.86) | .0224 | 1.77 (0.61–5.12) | .2896 |
| Stromal IL-6⁎ | |||||
| Low | 13 | 1 | – | – | |
| High | 12 | 0.72 (0.24–2.15) | .5535 | – | – |
| Stromal HGF⁎ | |||||
| Low | 13 | 1 | – | – | |
| High | 13 | 1.96 (0.64–6.02) | .5535 | – | – |
As observed the biomarkers stromal IL-6 and stromal HGF have 61% and 59% of missing data. For this reason, a multivariate Cox regression model with the 5 biomarkers will be of no interest because only in 9 cases all the 5 biomarkers have a known value. When considering the three biomarkers ILK, gp130, and, SHP2, 31 observations were included in the multivariate Cox regression model.
4. Discussion
Treatment of EGFR-mutation positive NSCLC with single EGFR TKIs shows meaningful activity with progression-free survival, ranging from 10.2 months with gefitinib to 18.9 months with osimertinib [1]. Regardless of the acquisition of EGFR-resistant mutations, progression-free survival has not been improved, with the use of third-generation EGFR TKIs. We and others have shown that combinatory therapies of EGFR TKIs with STAT3 inhibitors [23], AKT inhibitors [24], Src inhibitors [9], STAT3 and Src inhibitors [5], or a Src/FAK/JAK2 inhibitor (repotrectinib) [8] can revert the emergence of resistance to single EGFR TKIs.
STAT3, SFKs, and YAP1 have been associated with resistance to gefitinib or osimertinib [5,8]. We showed the same when AXL and CDCP1 are overexpressed [8]. Now we have gained further insights into other therapeutic targets, such as ILK and SHP2. Our results indicate that increased tumor expression levels of ILK and SHP2 define a group of EGFR-mutation positive patients who derive less benefit from EGFR TKI therapy compared to those with low ILK and SHP2 expression. ILK was discovered in 1996 based on its interaction with the cytoplasmic domains of integrin-β1 and -β3 subunits. ILK inhibitors (QLT0267) were reported in preclinical studies [12]. In EGFR-mutation positive NSCLC, EGFR TKI (erlotinib) activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling by inhibiting microRNA 21, with subsequent transcription of tumor necrosis factor (TNF) [25]. In chronic lymphocytic leukemia, TNFα triggers the canonical NF-ĸB signaling and induces ILK transcription [26]. Also, it has been demonstrated that ILK and transforming growth factor beta 1 TGF-β1 are both involved in oral squamous cell carcinoma progression [27]. All these reports indicate that a complex crosstalk of various signaling pathways, involving ILK, subsists and could serve as hubs of accumulated resistance to EGFR TKIs. It is not completely known which is the best way to target ILK in cancer; until now, ILK-targeted therapy is a new therapeutic approach only in acute myeloid leukemia [28]. Interestingly, hesperetin, a flavonone glycoside found in citrus fruits, attenuates lipid peroxidation and has protective effects in diabetic nephropathy by possibly suppressing TGF-β1-ILK-AKT signaling [29], thus representing a potential therapeutic strategy.
SHP2 has a role in stimulating MAPK signaling, downstream of various growth factors [30,31]. MAPK/extracellular protein kinase kinase (MEK) which activates ERK, has been described as a mechanism of resistance to EGFR inhibitors [32]. Combined inhibition of EGFR and MEK has demonstrated synergistic activity in preclinical EGFR-mutation positive models [33,34]. Based on our results, combinatory therapy with SHP2 inhibitors could be the second approach for patients with EGFR-mutation positive NSCLC. SHP2 inhibitors (SHP099) have significant activity in KRAS-mutation positive cancers [35,36]. SHP099, in combination with ceritinib (ALK inhibitor), halted the growth of ALK resistant patient-derived cancer cells [37]. In melanoma, SHP2 is related to RTK-driven drug resistance in melanoma [38]. These data encourage further evaluation of the role of SHP2 inhibitors.
In summary, the current study further extends our previous findings on the mechanisms of resistance to EGFR-mutation positive NSCLC. We report potential new biomarkers and hypothesize combinatory therapies that could mitigate the current death toll of EGFR-mutation positive NSCLC patients.
Author contributions
Drs. Karachaliou and Rosell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Karachaliou and Rosell are joint senior authors.
Study concept and design: Karachaliou, Rosell.
Acquisition, analysis, or interpretation of data: All authors.
Laboratory experiments: Karachaliou, Bracht, Aldeguer, Rosell.
Drafting of the manuscript: Karachaliou, Rosell.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Karachaliou, Drozdowskyj.
Administrative, technical, or material support: Karachaliou, Cardona, Rosell.
Study supervision: Karachaliou, Rosell.
Conflict of interest disclosure
None Reported.
Funding/support
Work in Dr. Rosell's laboratory is partially supported by a grant from La Caixa Foundation, an Instituto de Salud Carlos III grant (RESPONSE, PIE16/00011), an Instituto de Salud Carlos III grant (PI14/01678), a Marie Skłodowska-Curie Innovative Training Networks European Grant (ELBA No 765492) and a Spanish Association Against Cancer (AECC) grant (PROYE18012ROSE). Work in Dr. Karachaliou's laboratory is partially supported by a Marie Skłodowska-Curie Innovative Training Networks European Grant (ELBA No 765492).
Role of the funder/sponsor
The Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.11.036.
Contributor Information
Niki Karachaliou, Email: dr.nikikarachaliou@gmail.com.
Rafael Rosell, Email: rrosell@iconcologia.net.
Appendix A. Supplementary data
Supplementary material
References
- 1.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 2.Mok T.S., Cheng Y., Zhou X., Lee K.H., Nakagawa K., Niho S. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 3.Karachaliou N., Fernandez-Bruno M., Paulina Bracht J.W., Rosell R. EGFR first- and second-generation TKIs—there is still place for them in EGFR-mutant NSCLC patients. Transl Cancer Res. 2018 doi: 10.21037/tcr.2018.10.06. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao S.P., Mark K.G., Leslie K., Pao W., Motoi N., Gerald W.L. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117(12):3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaib I., Karachaliou N., Pilotto S., Codony Servat J., Cai X., Li X. Co-activation of STAT3 and YES-associated protein 1 (YAP1) pathway in EGFR-mutant NSCLC. J Natl Cancer Inst. 2017;109(9) doi: 10.1093/jnci/djx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi K., Wu L.W., Grivennikov S.I., de Jong P.R., Lian I., Yu F.X. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Z., Fenoglio S., Gao D.C., Camiolo M., Stiles B., Lindsted T. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A. 2010;107(35):15535–15540. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karachaliou N., Chaib I., Cardona A.F., Berenguer J., Bracht J.W.P., Yang J. Common co-activation of AXL and CDCP1 in EGFR-mutation-positive non-small cell lung cancer associated with poor prognosis. EBioMedicine. 2018;29:112–127. doi: 10.1016/j.ebiom.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichihara E., Westover D., Meador C.B., Yan Y., Bauer J.A., Lu P. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res. 2017;77(11):2990–3000. doi: 10.1158/0008-5472.CAN-16-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami Y., Sonoda K., Abe H., Watari K., Kusakabe D., Azuma K. The activation of SRC family kinases and focal adhesion kinase with the loss of the amplified, mutated EGFR gene contributes to the resistance to afatinib, erlotinib and osimertinib in human lung cancer cells. Oncotarget. 2017;8(41):70736–70751. doi: 10.18632/oncotarget.19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipenko N.R., Attwell S., Roskelley C., Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005;24(38):5837–5849. doi: 10.1038/sj.onc.1208737. [DOI] [PubMed] [Google Scholar]
- 12.Tabe Y., Jin L., Tsutsumi-Ishii Y., Xu Y., McQueen T., Priebe W. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res. 2007;67(2):684–694. doi: 10.1158/0008-5472.CAN-06-3166. [DOI] [PubMed] [Google Scholar]
- 13.Graff J.R., Deddens J.A., Konicek B.W., Colligan B.M., Hurst B.M., Carter H.W. Integrin-linked kinase expression increases with prostate tumor grade. Clin Cancer Res. 2001;7(7):1987–1991. [PubMed] [Google Scholar]
- 14.Ahmed N., Riley C., Oliva K., Stutt E., Rice G.E., Quinn M.A. Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol. 2003;201(2):229–237. doi: 10.1002/path.1441. [DOI] [PubMed] [Google Scholar]
- 15.Dai D.L., Makretsov N., Campos E.I., Huang C., Zhou Y., Huntsman D. Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res. 2003;9(12):4409–4414. [PubMed] [Google Scholar]
- 16.Takanami I. Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer. 2005;5:1. doi: 10.1186/1471-2407-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui Y., Assi K., Ogawa O., Raven P.A., Dedhar S., Gleave M.E. The importance of integrin-linked kinase in the regulation of bladder cancer invasion. Int J Cancer. 2012;130(3):521–531. doi: 10.1002/ijc.26008. [DOI] [PubMed] [Google Scholar]
- 18.Chou C.C., Chuang H.C., Salunke S.B., Kulp S.K., Chen C.S. A novel HIF-1alpha-integrin-linked kinase regulatory loop that facilitates hypoxia-induced HIF-1alpha expression and epithelial-mesenchymal transition in cancer cells. Oncotarget. 2015;6(10):8271–8285. doi: 10.18632/oncotarget.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W., Du W.W., Li X., Yee A.J., Yang B.B. Foxo3 activity promoted by non-coding effects of circular RNA and Foxo3 pseudogene in the inhibition of tumor growth and angiogenesis. Oncogene. 2016;35(30):3919–3931. doi: 10.1038/onc.2015.460. [DOI] [PubMed] [Google Scholar]
- 20.Leroy C., Shen Q., Strande V., Meyer R., McLaughlin M.E., Lezan E. CUB-domain-containing protein 1 overexpression in solid cancers promotes cancer cell growth by activating Src family kinases. Oncogene. 2015;34(44):5593–5598. doi: 10.1038/onc.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sausgruber N., Coissieux M.M., Britschgi A., Wyckoff J., Aceto N., Leroy C. Tyrosine phosphatase SHP2 increases cell motility in triple-negative breast cancer through the activation of SRC-family kinases. Oncogene. 2015;34(17):2272–2278. doi: 10.1038/onc.2014.170. [DOI] [PubMed] [Google Scholar]
- 22.Bertos N.R., Park M. Laser capture microdissection as a tool to study tumor stroma. Methods Mol Biol. 2016;1458:13–25. doi: 10.1007/978-1-4939-3801-8_2. [DOI] [PubMed] [Google Scholar]
- 23.Codony-Servat C., Codony-Servat J., Karachaliou N., Molina M.A., Chaib I., Ramirez J.L. Activation of signal transducer and activator of transcription 3 (STAT3) signaling in EGFR mutant non-small-cell lung cancer (NSCLC) Oncotarget. 2017;8(29):47305–47316. doi: 10.18632/oncotarget.17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen K., Bertran-Alamillo J., Molina M.A., Teixido C., Karachaliou N., Pedersen M.H. Convergent Akt activation drives acquired EGFR inhibitor resistance in lung cancer. Nat Commun. 2017;8(1):410. doi: 10.1038/s41467-017-00450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong K., Guo G., Gerber D.E., Gao B., Peyton M., Huang C. TNF-driven adaptive response mediates resistance to EGFR inhibition in lung cancer. J Clin Invest. 2018;128(6):2500–2518. doi: 10.1172/JCI96148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krenn P.W., Hofbauer S.W., Pucher S., Hutterer E., Hinterseer E., Denk U. ILK Induction in lymphoid organs by a TNFalpha-NF-kappaB-regulated pathway promotes the development of chronic lymphocytic leukemia. Cancer Res. 2016;76(8):2186–2196. doi: 10.1158/0008-5472.CAN-15-3379. [DOI] [PubMed] [Google Scholar]
- 27.Qiao B., Cai J.H., King-Yin Lam A., He B.X. MicroRNA-542-3p inhibits oral squamous cell carcinoma progression by inhibiting ILK/TGF-beta1/Smad2/3 signaling. Oncotarget. 2017;8(41):70761–70776. doi: 10.18632/oncotarget.19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alasseiri M., Ahmed A.U., Williams B.R.G. Mechanisms and consequences of constitutive activation of integrin-linked kinase in acute myeloid leukemia. Cytokine Growth Factor Rev. 2018;43:1–7. doi: 10.1016/j.cytogfr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Wang B., Guo F., Li Z., Qin G. Involvement of the TGFbeta1- ILK-Akt signaling pathway in the effects of hesperidin in type 2 diabetic nephropathy. Biomed Pharmacother. 2018;105:766–772. doi: 10.1016/j.biopha.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Batth T.S., Papetti M., Pfeiffer A., Tollenaere M.A.X., Francavilla C., Olsen J.V. Large-scale phosphoproteomics reveals Shp-2 phosphatase-dependent regulators of Pdgf receptor signaling. Cell Rep. 2018;22(10):2784–2796. doi: 10.1016/j.celrep.2018.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedele C., Ran H., Diskin B., Wei W., Jen J., Geer M.J. SHP2 inhibition prevents adaptive resistance to MEK inhibitors in multiple cancer models. Cancer Discov. 2018;8(10):1237–1249. doi: 10.1158/2159-8290.CD-18-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinelli E., Morgillo F., Troiani T., Ciardiello F. Cancer resistance to therapies against the EGFR-RAS-RAF pathway: the role of MEK. Cancer Treat Rev. 2017;53:61–69. doi: 10.1016/j.ctrv.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Sato H., Yamamoto H., Sakaguchi M., Shien K., Tomida S., Shien T. Combined inhibition of MEK and PI3K pathways overcomes acquired resistance to EGFR-TKIs in non-small cell lung cancer. Cancer Sci. 2018;109(10):3183–3196. doi: 10.1111/cas.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Della Corte C.M., Ciaramella V., Cardone C., La Monica S., Alfieri R., Petronini P.G. Antitumor efficacy of dual blockade of EGFR signaling by osimertinib in combination with selumetinib or cetuximab in activated EGFR human NCLC tumor models. J Thorac Oncol. 2018;13(6):810–820. doi: 10.1016/j.jtho.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Ruess D.A., Heynen G.J., Ciecielski K.J., Ai J., Berninger A., Kabacaoglu D. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat Med. 2018;24(7):954–960. doi: 10.1038/s41591-018-0024-8. [DOI] [PubMed] [Google Scholar]
- 36.Mainardi S., Mulero-Sanchez A., Prahallad A., Germano G., Bosma A., Krimpenfort P. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat Med. 2018;24(7):961–967. doi: 10.1038/s41591-018-0023-9. [DOI] [PubMed] [Google Scholar]
- 37.Dardaei L., Wang H.Q., Singh M., Fordjour P., Shaw K.X., Yoda S. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018;24(4):512–517. doi: 10.1038/nm.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prahallad A., Heynen G.J., Germano G., Willems S.M., Evers B., Vecchione L. PTPN11 is a central node in intrinsic and acquired resistance to targeted cancer drugs. Cell Rep. 2015;12(12):1978–1985. doi: 10.1016/j.celrep.2015.08.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material