Abstract
Background
Small fiber neuropathy (SFN) is a severe and disabling chronic pain syndrome with no causal and limited symptomatic treatment options. Mechanistically based individual treatment is not available. We report an in-vitro predicted individualized treatment success in one therapy-refractory Caucasian patient suffering from SFN for over ten years.
Methods
Intrinsic excitability of human induced pluripotent stem cell (iPSC) derived nociceptors from this patient and respective controls were recorded on multi-electrode (MEA) arrays, in the presence and absence of lacosamide. The patient's pain ratings were assessed by a visual analogue scale (10: worst pain, 0: no pain) and treatment effect was objectified by microneurography recordings of the patient's single nerve C-fibers.
Findings
We identified patient-specific changes in iPSC-derived nociceptor excitability in MEA recordings, which were reverted by the FDA-approved compound lacosamide in vitro. Using this drug for individualized treatment of this patient, the patient's pain ratings decreased from 7.5 to 1.5. Consistent with the pain relief reported by the patient, microneurography recordings of the patient's single nerve fibers mirrored a reduced spontaneous nociceptor (C-fiber) activity in the patient during lacosamide treatment. Microneurography recordings yielded an objective measurement of altered peripheral nociceptor activity following treatment.
Interpretation
Thus, we are here presenting one example of successful patient specific precision medicine using iPSC technology and individualized therapeutic treatment based on patient-derived sensory neurons.
Keywords: Personalized therapy, Human nociceptors, Small fiber neuropathy, Microneurography, Patch-clamp, Multi-electrode-array
Graphical abstract
1. Introduction
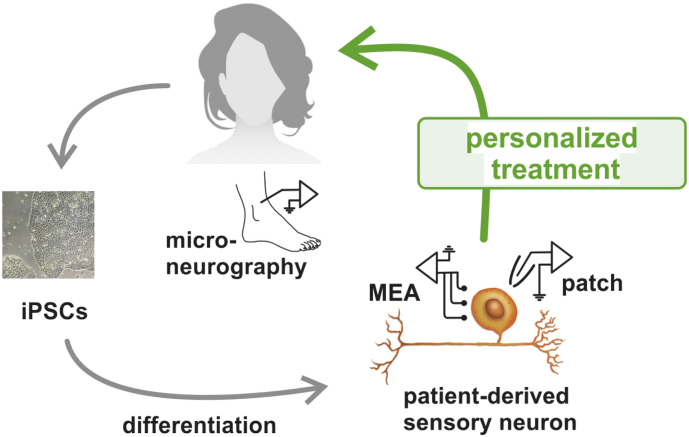
Chronic neuropathic pain associated with small fiber neuropathy (SFN) often manifests with intense burning pain in the peripheral limbs, and is accompanied by elevated temperature detection thresholds and often by a decrease of epidermal nerve fiber density as seen in histological stainings of skin biopsies. It was shown by microneurography that a significantly larger proportion of nociceptive C-fibers in the peripheral nerves of patients with high ongoing pain levels are spontaneously active compared to neuropathy patients without continuous pain. This spontaneous activity was suggested to be a major contributor to neuropathic pain [1], [2]. In recent years SFN was associated with mutations and variants of genes coding for peripheral ion channels, but the vast majority of cases are sporadic [3], [4]. It is almost impossible to gain access to sensory neurons of the patients during lifetime to perform in-depth electrophysiology. Thus, we and others apply the method of fibroblast reprogramming to generate induced pluripotent stem cells (iPSCs) and to obtain patients' sensory neurons derived thereof (Fig. 1, [5]). This human in vitro model is suited both for the analysis of monogenic and multifactorial disorders.
Fig. 1.
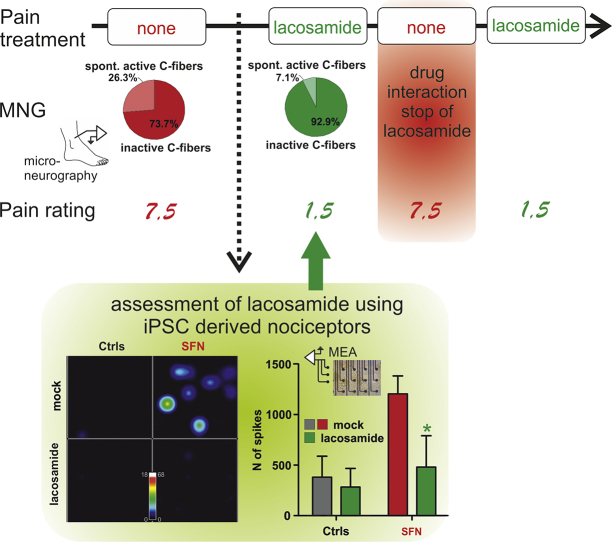
Personalized treatment for a patient suffering from severe neuropathic pain for >10 years.
An SFN patient was clinically characterized, pain ratings were assessed and her C-fiber spontaneous activity was evaluated by microneurography recordings. Patient's fibroblasts were reprogrammed into induced pluripotent stem cells (iPSCs). Applying a small molecule approach, iPSCs were differentiated into patient-derived sensory neurons, which were subjected to patch clamp and multielectrode array recordings. In multielectrode array the compound lacosamide was tested on its effect of suppressing spontaneous activity. After successful reduction of spontaneous activity in vitro, lacosamide treatment was started in the patient. Its remarkable effect in the patient was shown by lowered pain ratings and its peripheral site of action by reduced spontaneous activity of peripheral nociceptors via microneurography.
Despite major efforts, pain management of these patients is challenging, and pharmacological treatment algorithms frequently fail. Treatment of pain in SFN is still unsatisfactory due to limited effectivity and side effects, e.g. dizziness of centrally acting drugs such as antiepileptics. Lacosamide is such an FDA approved antiepileptic drug acting on sodium channels. It was tested in clinical trials for treatment of diabetic neuropathic pain but was not approved for this indication. However, lacosamide was shown to specifically interfere with the function of the peripheral sodium channels, such as Nav1.7 [6,7], thus it is considered to be a good candidate for treatment of neuropathic pain and is currently studied in a clinical trial on SFN patients with mutations in Nav 1.7 [8]. Whether it may be a candidate for treatment of sporadic SFN has not been tested yet.
2. Methods
2.1. Genetic evaluation
Fibroblasts of the patient suffering from SFN were cultured from a skin biopsy of the patient and two age matched controls (Ctrl1 and Ctrl2) after written informed consent (Review Board approvals Nr. 4120 UKER, Germany and Nr. 2012/2297 South East, Norway). Whole-exome Sequencing was performed with DNA from fibroblast cells. Enrichment was done with an Illumina Enrichment Kit (Nextera Rapid Capture Exome v1.2) and the respective libraries were sequenced on a NextSeq500 sequencer (Illumina, San Diego, USA). Alignment and variant calling was performed with SeqMule (v1.2), (FastQC (version: 0.11.2), BWA-MEM (version: 0.7.8-r455), SAMtools (rmdup; version: 0.1.19-44428cd), SAMtools (filter; version: 0.1.19-44428cd), SAMtools (index; version: 0.1.19-44428cd, and GATKLite (realign; version: 2.3-9-gdcdccbb). Genome version hg19 was used for the alignment. Three variant callers were applied for variant detection (GATKLite UnifiedGenotyper (variant; version: 2.3-9-gdcdccbb), SAMtools (mpileup; version: 0.1.19-44428cd), FreeBayes (version: 0.9.14-14-gb00b735)). Variants called by at least two programs were considered for further analysis. The resulting variant files were combined (GATK, v3.6, CombineVariants) and processed with KGGSeq (v1.0, 14/Apr./2017).
In the SFN patient we identified a variant in the sodium channel Nav1.9 (p.N1169S) which is also frequently found in control cohorts and a variant in Nav1.8 (p.R923H), but none in Nav1.7. We show that the Nav1.8 variant does not affect channel function in a heterologous expression system (Supplemental Fig. S1).
2.2. iPSC-derived nociceptors
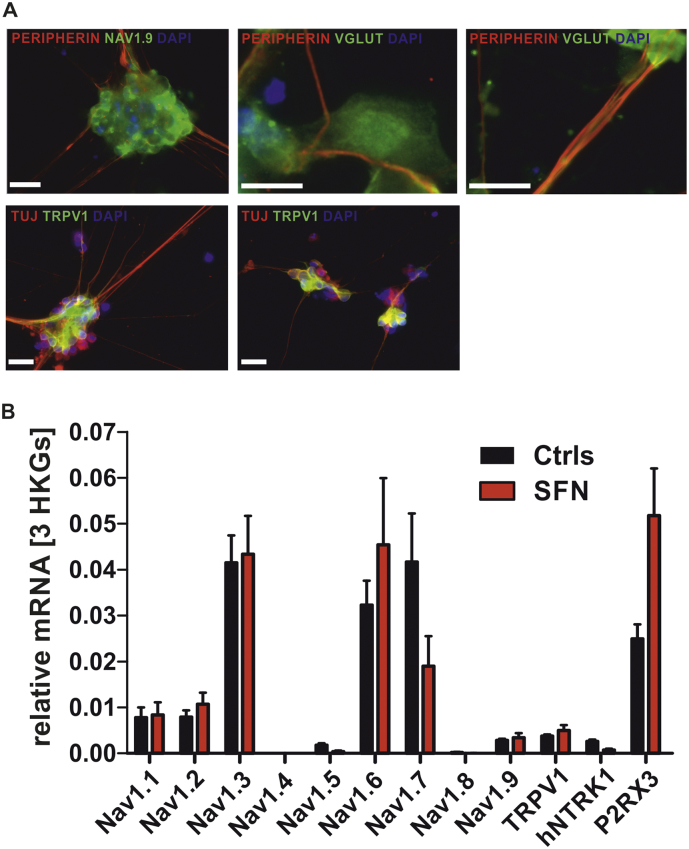
Fibroblasts were reprogrammed retrovirally using the Yamanaka factors [9], differentiated into nociceptors using a small molecule protocol [5,10] and matured for at least 30 days. iPSC-derived neurons were tested for peripheral neuron markers (peripherin, TRPV1 and Tuj1, see Fig. 2). Unique identifier for iPSC lines are for the patient: UKERi313-R1; and controls: UKERiO3H-R1-001 and UKERi82A-R1-001.
Fig. 2.
IPSC-derived human nociceptors of patients and controls show expression of peripheral markers.
A: Representative immunofluorescence images of SFN- and Ctrl-iPSC-derived nociceptors stained for (upper row) neuron- and nociceptor-specific markers Peripherin (red), Nav 1.9 (green), V-glut (green) DAPI (blue), and (lower row) for TRPV1 (red), Tuj1 (green) and DAPI (blue). Scale bars represent 20 μm. B: mRNA expression levels of genes coding for the sodium channel subtypes NAV1.1 – NAV1.9 and TRPV1, P2X3 and TRK1 as nociceptor typical receptors.
List of Primers:
| Genes | Primer |
|---|---|
| SCN1A (hNAV1.1) | for: GAAGAACAGCCCGTAGTGGAA |
| rev: TTCAAATGCCAGAGCACCA | |
| SCN2A (hNAV1.2) | for: GAAGGCAAAGGGAAACTCTGG |
| rev: CAGTGAGACATCAACAATCAGGAAG | |
| SCN3A (hNAV1.3) | for: TGCTTCTCAAATGGGTTGC |
| rev: GCATTGGCTACCAGGCTAAC | |
| SCN4A (hNAV1.4) | for: CAACAACCCCTACCTGACCATAC |
| rev: GCAGAGTCCACCACTTCTTCC | |
| SCN5A (hNAV1.5) | for: CGTGTGTAGATGGCTTCGAG |
| rev: GACACTTGTGGCGAGACTCC | |
| SCN6A (hNAV1.8) | for: AAGGTTGTGTCCAGCGGTTC |
| rev: GGATGGTGCGGATGGTCTT | |
| SCN9A (hNAV1.7) | for: 5′-ACCTATCTCTGCTTCAAGTTGC-3′ |
| rev: 5′-TGGGCTGCTTGTCTACATTAAC-3′ | |
| SCN10A (hNAV1.8) | for: 5′-CTGTCGATGTCTCGGCATTC-3′ |
| rev: 5′-TGGGCACTTCTGTTCAGACTC-3′ | |
| SCN11A (hNAV1.9) | for: 5′-GAAATGCTTACCTCGCTCTG-3′ |
| rev: 5′-GCTCTCAAACTCTGGCTGTTG-3′ | |
| hNTRK1 | for: CAGGACTTCCAGCGTGAGG |
| rev: GCAGCTTGGCATCAGGTC | |
| TRPV1 | for: 5′-GCACAGGAGAGCAAGAACATC-3′ |
| rev: 5′-GTCCAGTTCACCTCGTCCAC-3′ | |
| GAPDH | for: 5′-GTCGGAGTCAACGGATTTG-3′ |
| rev: 5′-TGGGTGGAATCATATTGGAAC-3′ | |
| HPRT1 | for: 5′-CCTGGCGTCGTGATTAGTG-3′ |
| rev: 5′-TCCCATCTCCTTCATCACATC-3′ | |
| B2M | for: 5′-GAGGCTATCCAGCGTACTCC-3′ |
| rev: 5′-AATGTCGGATGGATGAAACC-3′ |
2.3. Patch-clamp electrophysiology
Whole-cell recordings were performed with a HEKA EPC-10USB amplifier (HEKA electronics). Pipette potential was zeroed prior to seal formation and capacitive transients were compensated using C-fast for pipette-capacitance correction and subsequently C-slow for cell-capacitance compensation (PatchMaster, HEKA electronics). The series resistance was compensated by about 50%. Sampling rate and filter frequency were 20–100 kHz and 10 kHz, respectively.
The external solution contained (in mM): 140 NaCl, 3 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES and 20 d-Glucose (pH 7.4) and glass pipettes (1.5–3.0 MΩ) were loaded with internal solution (in mM): 4 NaCl, 135 K-gluconate, 3 MgCl2, 5 EGTA, 5 HEPES, 2 Na2-ATP and 0.3 Na3-GTP (pH 7.25).
The resting membrane potential and spontaneous action potentials (APs) were determined directly after gaining access to the cell after seal formation and after achieving the whole-cell configuration without current injection. Electrical activity was recorded, and firing neurons were classified as spontaneously active. Afterwards the membrane potential was set to − 70 ± 2 mV. APs were elicited either by 500 ms-current injections, increasing in 10 pA increments, or by ramp current injections, continuously increasing from 0 pA to 500 pA within 400 ms. Silent neurons were classified as inactive, whereas firing neurons were classified as active. The AP threshold was defined by the membrane potential turning point of the current trace before the AP (minimum of derivative). Upon stimulation >90% of the neurons showed APs.
2.4. Multielectrode array recordings
Experiments were performed using a Maestro, 768 channel, multi-well MEA system (Axion BioSystems). Cells were plated and recorded on 12- or 48-well plates, with each well containing an array of 64 or 16 embedded platinum or gold electrodes, respectively (12-well: 30 μm diameter, 200 μm center-to-center spacing; 48-well: 40–50 μm diameter, 350 μm center-to-center spacing) with integrated ground electrodes for a total of 768 channels (Axion BioSystems). The day before plating, MEA wells were coated with a 0.75 mg/ml solution of poly-d-lysine (Sigma-Aldrich). 60,000 cells per well were plated and a consistent cell density among all wells during the nociceptor culture was followed under the microscope.
Data were acquired using Axion BioSystems' Integrated Studio (AxIS, version 2.3.1.11) software, and 2 min raw data files were taken for each recording. Channels were sampled simultaneously with a sampling rate of 12.5 kHz/channel, voltage scale of 5.51E-08 V/sample, and digital high pass IIR-filter of 5 Hz. All recordings were conducted at 37 °C, unless otherwise stated. For recordings, a Butterworth band-pass filter (with a high-pass cutoff of 200 Hz and a low-pass cutoff of 3000 Hz) was applied, along with an adaptive threshold spike detector set to detect any amplitude greater than or equal to a multiple of six standard deviation (6xSD) of the estimated noise on each channel. Data from Ctrl1 and Ctrl2 were pooled. The weighted mean firing rate was calculated as the mean firing rate, averaged across only the active electrodes (minimum spike rate for active electrode was set to 5 spikes/min). Number of active electrodes and number of spikes were counted in a period of 2 min respectively. Bursts were detected using the Inter-Spike Interval (ISI). Threshold algorithm in AxIS software, with minimum 5 spikes per burst and maximum inter-spike interval set at 100 ms. Lacosamide (500 μM and 50 μM, AdooQ BioScience) was added to nociceptor cultures and recordings were performed following 1 min of incubation.
2.5. Microneurography recordings
C-fiber recordings were performed on the peroneal nerve at the level of the fibular head or the ankle [11], and the data gained before lacosamide treatment were published previously [12]. The method of microneurography has been described in detail elsewhere [13]. When the needle was inserted into a fascicle inside the nerve containing C-fibers, neuronal activity characteristic for unmyelinated C-fibers could be induced by scratching the skin at the dorsum of the foot. Innervation territories of individual C-fibers were then located with transcutaneous electrical stimulation with a pointed electrode (1–20 mA, 0.5 ms). C-fibers were identified by their low conduction velocity (<2 m/s), which was assessed from the latency of an electrically evoked action potential after a rest period of at least 2 min. A pair of thin needles (0.15 mm diameter) was intracutaneously inserted into the innervation territory in a spot with low electrical threshold. Through these needles the C-fibers under observation were continuously stimulated at a low repetition rate (0.125 to 0.5 Hz) via a constant current stimulator (Digitimer DS7, Digitimer Ltd. Hertfordshire UK). Following repetitive electrical stimulation at a fixed frequency from the skin, action potentials of individual C-fibers can be registered with the recording electrode at stable conduction latencies. An increase in the usually stable conduction latency of peripheral C-fibers is observed (“marking”) when the stimulation frequency at the skin is increased or after the afferent fiber has been additionally activated, e.g., by natural stimuli or by spontaneous activity [13]. Marking is due to activity-dependent slowing (ADS) of conduction in C-fibers, e.g. conduction of an action potential renders the axonal membrane of afferent C-fibers less excitable for tens of seconds and thus slows down conduction velocity of subsequent action potentials.
Custom-written Spike2 software and a micro1401 DAC (CED, Cambridge, UK) was used for data acquisition and analyses.
3. Results
3.1. Clinical data
Here, we report on a 69-year-old female Norwegian Caucasian patient suffering from SFN for >10 years. She presents with severe continuous burning pain with varying intensity partially relieved by cooling. Temperature thresholds, as assessed by quantitative sensory testing (QST), are elevated: the heat pain threshold is out of range of the measuring device (>50 °C). Her pain is most severe in the late evenings resulting in insomnia: ratings on a visual analog scale VAS (range 1–10) were around 7.5, with a major impact on quality of life. Sequential oral pharmacological treatment using gabapentin (2100 mg/day), pregabalin (600 mg/day) to the maximum dose had limited effects, which typically faded within months, and were then discontinued due to severe side effects. Amitriptyline (10 mg/day) was discontinued due to side effects and acetylsalicylic acid (160 mg/day) was ineffective. Microneurographic assessment of C-fiber activity of the patient revealed 26.3% of spontaneously active fibers [14]. Healthy age matched controls only display 11.8% [1].
3.2. Genetic analysis
By whole-exome sequencing of the patient's iPSCs, we investigated the molecular signature of genetic factors underlying the pain phenotype. The here reported patient carries two rare variants in pain-associated voltage-gated sodium channels. The first variant in Nav1.8 (R923H) is classified as “benign” accordingly to bioinformatics prediction. However, because of its low prevalence (20/ 277.130 alleles, gnomAD browser), we performed patch-clamp experiments and, as expected, heterologous expression of this variant did not alter channel properties (Suppl. Fig. 1). The second variant in Nav1.9 (N1169S) is predicted as “damaging”, however the overall prevalence (83/263.720 alleles, gnomAD browser) suggests that it may not be solely responsible for the SFN phenotype. Moreover, the effect of lacosamide on heterologously expressed Nav1.9 is minor (Suppl. Fig. 2), suggesting that the Nav1.9 variant may not interfere with the response to therapy. This may mean either that the variant is not pathogenic or that it only displays its pathological effect in human, or even the patient's specific cellular background.
In summary, we have no indication that the pain phenotype results from a monogenic disorder.
3.3. iPSC derived nociceptors
In order to identify potential treatment options for the patient, we reprogrammed the patient‘s fibroblasts into iPSC and differentiated these into peripheral sensory neurons using a small molecule approach (Fig. 1, [5]). iPSC derived sensory neurons expressed markers for nociceptors, such as TRPV1, and the sodium channels Nav1.7, Nav1.8 and Nav1.9 shown in mRNA expression levels and protein immune-stainings ([5], Fig. 2). Cell morphology and expression of nociceptor markers were unchanged compared to two age matched controls (Fig. 2): The amount of TRPV1+ cells out of TUJ1+ cells was 92.6% for Ctrls and 90,2% for SFN, amount of Nav1.9+ cells out of Peripherin+ cells in %: Ctrls: 99.6, SFN: 100.0.
3.4. Patch-Clamp and microelectrode arrays
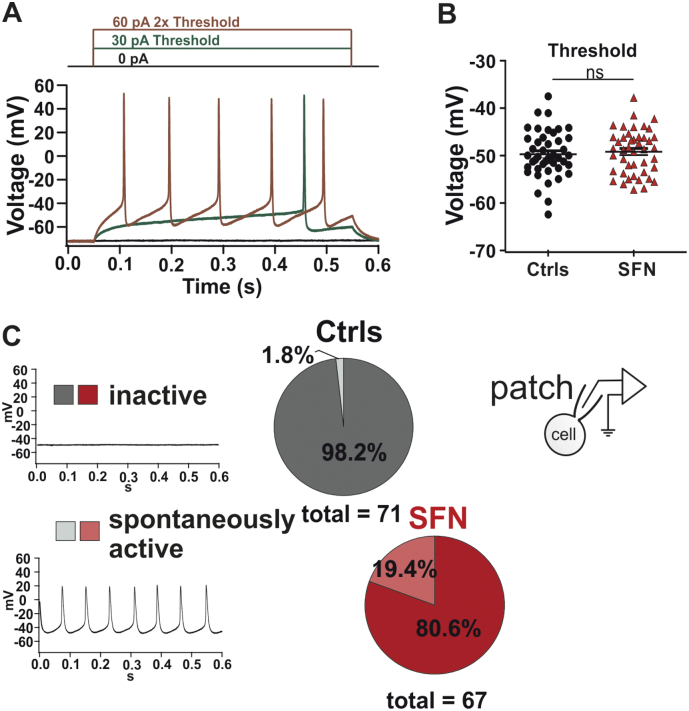
To assess the intrinsic excitability of iPSC derived sensory neurons we performed whole-cell current clamp analysis. Whereas the action potential threshold remained unaltered in SFN derived nociceptors (Fig. 3, P > 0.05; one-way ANOVA), we detected a larger number of spontaneously active SFN sensory neurons (19.4%) compared to control (1.8%) (Fig. 3, P = 0.0002; logistic regression model), mirroring the findings of microneurography [12].
Fig. 3.
SFN1-nociceptors exhibit enhanced spontaneous activity and increased AP firing in whole-cell current clamp recordings. A: Example traces of elicited action potentials (APs) at threshold and double threshold in SFN-nociceptors. B: AP threshold was not significantly different between controls and SFN derived nociceptors (−49.7 ± 0.7 mV (Ctrls); −49.2 ± 0.7 mV (SFN1); P > 0.05 one-way ANOVA); C: Quantification of the number of spontaneously firing IPSC-derived nociceptors of controls (grey) and the SFN patient (red) during whole cell patch clamping. 19.4% of the SFN derived nociceptors exhibit spontaneous activity (lower panels) whereas only 1.8% of the control derived nociceptors are spontaneously active (P = 0.0002; logistic regression model).
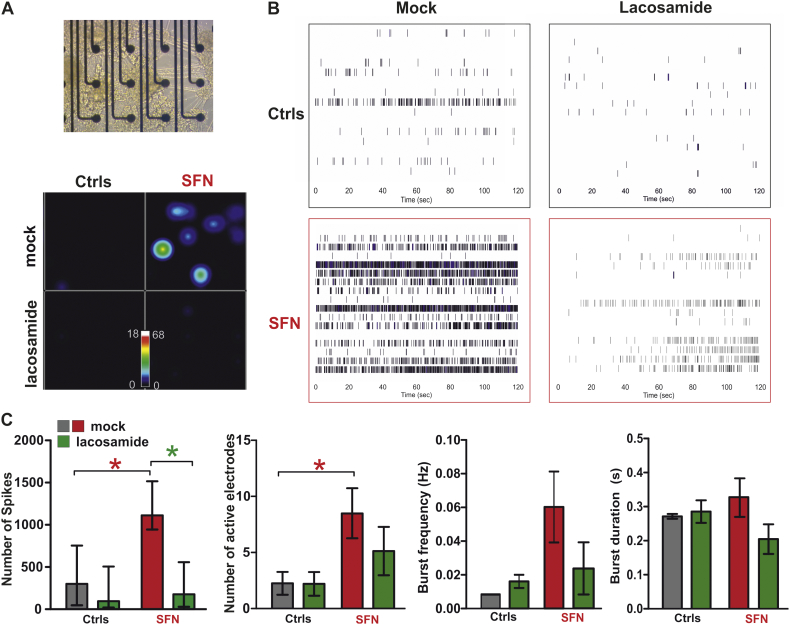
To assess the overall cellular excitability in larger cell populations, we seeded patient- and control-derived nociceptors onto multielectrode array (MEA) chips and assessed their spontaneous activity (Fig. 4A, B). Patient-derived nociceptors displayed a significantly increased excitability: more spikes (Ctrl: 380.6 ± 207.9, SFN: 1206.0 ± 177.4, P < 0.05; one-way ANOVA) and more active electrodes (Ctrl: 2.25 ± 1.01, SFN: 8.5 ± 2.23, P < 0.05, students t-test) were detected (Fig. 4C). The burst frequency (in Hz: Ctrl: 0.008 ± 0.000, SFN: 0.060 ± 0.021, P > 0.05, students t-test) and burst duration (in s: Ctrl: 0.271 ± 0.007, SFN: 0.327 ± 0.0571, P > 0.05, students t-test) were unchanged between the groups, suggesting that more patient neurons fired spontaneously and more frequently, but with a similar bursting behavior as neurons from controls. The FDA approved drug lacosamide was never used as a treatment by the patient reported here in contrast to other more frequently used medications for neuropathic pain. In MEA recordings of patient-derived nociceptors, lacosamide strongly reduced the number of spikes, but did not have an effect on controls (Fig. 4C, Ctrl: 282.8 ± 184.2, SFN: 480.4 ± 310.2, P < 0.05, students t-test), indicating that pathological hyperactivity was impaired, but the general action potential generation was not inhibited.
Fig. 4.
Spontaneous activity of SFN iPSC derived nociceptors in multi electrode array is reduced by lacosamide application.
A: Upper: nociceptors on MEA chip. Lower: Snapshot of a real time spike firing rate heat map of spontaneously active SFN- (right side) and Ctrl-(left side) nociceptors either mock treated (upper part) or with 500 μM lacosamide (lower part). The colour-coded scale bar shows spikes/s. B: Raster plot examples of spontaneous activity of Control (upper row) and SFN (lower row) iPSC derived nociceptors in multi electrode array with either mock treatment (left side) or with 500 μM lacosamide (right side) over 120 s. Each plot represents one well with 16 electrodes (rows). Each small vertical line indicates activity. Blue means high frequency bursting. Spontaneous activity is more pronounced in SFN iPSC derived nociceptors (upper row) in comparison to control nociceptors. Spontaneous activity is reduced by lacosamide application (left). C: Quantification of number of spikes, active electrodes, burst frequency and burst duration per well during 2 min recordings. First panel: Total number of spikes of all active electrodes during mock and lacosamide treatment are shown. Lacosamide significantly reduced number of spikes in SFN iPSC derived nociceptors (mock: Ctrl: 380.6 ± 207.9, SFN: 1206.0 ± 177.4, Lacosamide: Ctrl: 282.8 ± 184.2, SFN: 480.4 ± 310.2, P < 0.05, students t-test). Lacosamide did not significantly alter other parameters: Second panel: number of active electrodes (mock: Ctrl: 2.25 ± 1.01, SFN: 8.5 ± 2.23; Lacosamide: Ctrl: 2.20 ± 1.06, SFN: 5.13 ± 2.15; P > 0.05, students t-test.) Third panel: Burst frequency (in Hz: Mock: Ctrl: 0.008 ± 0.000, SFN: 0.060 ± 0.021; Lacosamide: Ctrl: 0.016 ± 0.004, SFN: 0.024 ± 0.016, P > 0.05, students t-test). Fourth panel: Burst duration (in s: Mock: Ctrl: 0.271 ± 0.007, SFN: 0.327 ± 0.0571, Lacosamide: Ctrl: 0.285 ± 0.033, SFN: 0.205 ± 0.043, P > 0.05, students t-test).
3.5. Treatment of patient
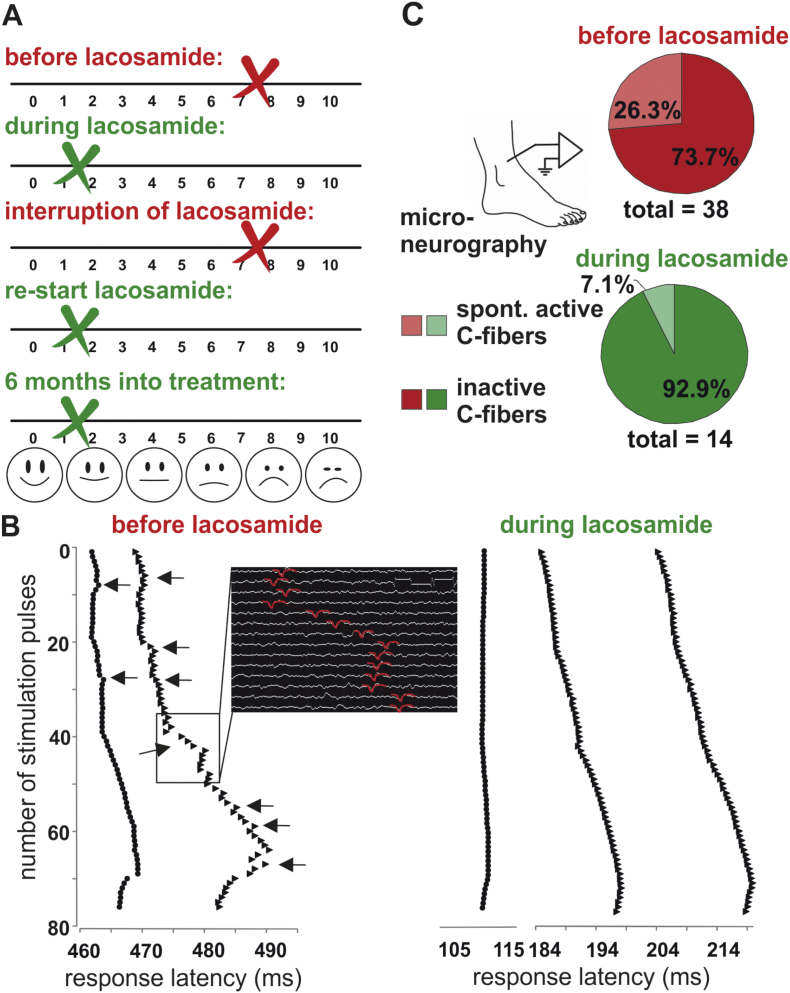
Based on this preclinical prediction derived from in-vitro MEA testing, the patient started off-label treatment with lacosamide (50 mg orally in the evenings, when pain was strongest). Within five days the patient's pain was tremendously reduced, and peak pain ratings in the evenings dropped from VAS 7.5 to 1.5 (Fig. 5A). For the first time in over ten years, the patient was able to fall asleep undisturbed by her pain. Lacosamide treatment interfered with the patient's antiallergic medication, causing extreme sleepiness during daytime. Therefore, she interrupted the therapy during the summer for 2.5 months and the pain sensations reoccurred (VAS 7.5). Re-start of lacosamide treatment in fall again dramatically decreased the patient's pain to VAS 1.5 (Fig. 5A). The symptoms were still controlled after 6 months of re-start of treatment, which suggests that the treatment had effects, which outlast a potential placebo effect.
Fig. 5.
Microneurography reveals effective, individualized treatment of the patient.
A: Pain ratings of the SFN patient with and without lacosamide treatment. Pain ratings were considerably reduced during lacosamide treatment. Lacosamide treatment was interrupted due to side effects in combination with anti-allergic medication during summer and the same pain level was reported as before lacosamide treatment. After the end of allergic season lacosamide treatment again reduced the pain rating substantially.
B: Example traces of C-fiber activity during microneurography recordings. Depicted are response latencies to electrical test pulses (20 pulses every 8 s, 20 pulses every 4 s, 30 pulses every 2 s and then pulses every 4 s): each black point and triangle represents the latency of one electrically induced action potential, symbols arranged in a vertical line (means same response latency) indicate that the respective action potentials belong to one single C-fiber; left panel: irregular latency responses of two spontaneously active C-fibers before lacosamide, black arrows point to latency shifts indicating spontaneous activity between the electrically induced action potentials, the black inset shows the original recording traces from the C-fiber responses depicted in the black square besides. Action potentials are marked in red. Right panel: regular C-fibers responses to electrical test pulses of three C-fibers during lacosamide treatment without spontaneous activity.
C: Microneurography recordings show a large proportion of spontaneously active C-fibers (26%) before lacosamide treatment, which considerably decreased during treatment to 7% which is in the upper range of age matched healthy controls.
3.6. Microneurography
To assess if the clinical response was based on central inhibition or resulted from treating the hyperexcitability of the patient's C-fibers, we used microneurography while the patient was under lacosamide treatment with low pain levels. The proportion of spontaneously active C-fibers was significantly reduced to 7% (from 26.3% reported previously [1,14],Fig. 5B, C), which represents a range comparable to healthy age-matched volunteers [15]. Not only did lacosamide treatment reduce pain, it also improved the patient's sensitivity to noxious heat: she now had a heat pain threshold of 48.8 °C, which was well measurable in QST. This shows that lacosamide, which was individually identified as a treatment option on iPSC-derived nociceptors in-vitro, specifically modified the patient's peripheral neurons to revert pathology and to improve their physiological function.
4. Discussion
We identified a potent pain treatment for a patient with SFN using patient-derived sensory neurons in an in-vitro testing system. Translating our findings from stem cell-derived sensory neurons in the dish directly to the patient tremendously alleviated her pain. Remarkably, lacosamide also normalized the firing pattern of the patient's C-fibers and reduced her heat pain thresholds, strongly suggesting that the treatment effect was not due to impaired central pain processing or a placebo effect, but rather a specific modification of the function of the peripheral f nociceptors.
In addition to the subjective pain ratings of the patient, the objective findings in microneurographic recordings of peripheral neurons support the assumption that the treatment success is not merely based on placebo effects. The number of spontaneously active nociceptors was reduced during lacosamide treatment to a level which is found in healthy age matched controls [15] proving reduced pathological neuronal activity in the peripheral nerve innervating the painful area. However, an additional placebo effect on the tremendously reduced pain ratings of the patient during lacosamide treatment cannot be excluded and changes in central inhibition might also occur and influence pain perception.
SFN causes high morbidity with disabling symptoms and impact on quality of life [16]. Treatment of the chronic burning pain is a tremendous challenge for patients and clinicians. Specifically, patients easily loose motivation after trying various compounds until eventually one pain reducing medication can be accepted when pain relief and side effects are in a bearable balance. Using patient's iPSCs and thereof derived sensory neurons, we show the novel possibility to investigate the individual, patient-specific neuronal response and to test FDA approved compounds for their efficacy. This approach allows identification of a medication that is precisely tailored for the patient and is likely to work best for this specific patient, even if in off-label use.
Lacosamide is used as an antiepileptic drug, which is tested in ongoing clinical trials with SFN patients [8], and it has also come into consideration as treatment for general neuropathic pain [17]. It enhances slow inactivation of voltage-gated sodium channels and interacts with the intracellular protein collapsin-response mediator protein-2 (CRMP-2, [18,19]). It was shown to be effective on the peripheral sodium channels Nav1.7 and Nav1.3, which may be the mechanism underlying its efficient pain treatment in the here presented patient. Lacosamide also inhibits Nav1.6, which is expressed in the central nervous system, and which may account for the observed side effects [19] [7], [20], [6].
A meta-analysis of antiepileptic drugs for the treatment of neuropathic pain showed no convincing results in general, and even identified evidence for a lack of effect of lacosamide in the treatment of neuropathic pain [21]. Thus, lacosamide is rarely used off label against neuropathic pain and the patient of this study would not have been treated with this drug without the observed effects in MEA recordings of patient iPSC-derived nociceptors. Side effects of lacosamide are typical for antiepileptic drugs, such as dizziness and in higher doses vestibule-cerebellar dysfunction [22]. For the here reported patient these effects added to those of her anti-allergic treatment and she had to discontinue lacosamide. In addition, during treatment with lacosamide on its own, she suffered from side effects (sleepiness) which she tolerated due to the impressive effect on her pain ratings. To save patients from continuous trial and error with drugs with limited pain alleviation but severe side effects, an individual patient-specific in vitro test system is highly desirable, which can be used to guide identification of efficient off-target drugs.
Oral doses of lacosamide used for the treatment of seizures and clinical trials evaluating this drug for neuropathic pain range between 200 and 600 mg per day. This drug is administered twice daily after titration. Reported plasma levels are in the range of 5–12.5 μg/ml [23,24] and the recommended therapeutic range is 5–15 μg/ml [25] corresponding to a concentration of about 20–60 μM. In this case report we focused on a potential therapeutic that mechanistically differed from the drugs that had no benefit for the patient. We therefore tested patient derived-sensory neurons for their response to lacosamide, an FDA approved compound known to block voltage-gated sodium channels. We used 500 μM, as this concentration was previously used in in vitro studies [26], and 50 μM, which corresponds to the plasma concentration in patients [25]. The concentration at the side of action is unknown and beyond the reach of most experiments. It is likely to differ from plasma levels and for lacosamide as functionalized aminoacid uptake via transporters could be possible.
In a previous attempt to use the stem-cell system to identify treatment, a drug was tested in sensory neurons derived from patients suffering from the inherited pain syndrome erythromelalgia [27]. Using patch-clamp the study revealed that the tested drug leads to an increase in heat-induced rheobase in the investigated single cells. Administered to five different patients with different mutations, the drug revealed a small, but significant alleviation of pain after 4-5 h. Our approach fundamentally differs from the reported one, as we investigate a patient without a clearly disease causing genetic mutation, and we tested and identified a drug, which is currently in clinical use with well-known effects and side effects. Additionally, using the MEA approach we assess the activity of a whole group of neurons, and need not rely on selecting representative single cells for their assessment in whole-cell patch-clamp.
Patient derived nociceptors fired more frequently and exhibited more active electrodes, but the burst frequency and burst duration were comparable between patient and controls. Thus, the overall bursting behavior may not have changed, but the conditions leading to the bursting may be affected by the patient's genetics. Although we did not identify a single point mutation or variant, which is causative for the patient's pain, the patient derived nociceptors mirror the spontaneous activity of the nerve fibers observed in microneurography recordings. Thus, we suggest, that iPSC derived nociceptors, which contain the complete genetic composition of the patient, are sufficient in this case to mimic the patient's phenotype in the dish.
When adding lacosamide to the MEA recordings we observed a reduction of number of spikes only in the patient derived sensory neurons. Control neurons seemed not to be affected (Fig. 4). This suggests that lacosamide mainly acts on pathological neuronal activity in the patient's hyperactive neurons and does not hamper the normal nociceptive function.
Our findings show that our approach is not only useful in monogenic pain disorders but can also be transferred to patients who are suffering from a polygenic, sporadic, or more complex pain syndrome. This is of great relevance because a large part of SFN patients cannot be diagnosed with a genetic cause for their pain disease and thus it is more difficult to find a suitable treatment option based on mechanistic insight in the disease-causing factors .
While large population-based randomized trials render important information on the average treatment effect, the individual benefit may often be very different. Large randomized studies tell only little about individual treatment effects. Thus, to date, we only have the means of an n-of-1 trial to identify whether an existing treatment is effective in a specific patient or not.
Treatment of pain is often very complex and needs specifically tailored treatment for the patients. Reaction to drugs varies largely even within one type of pain, such as neuropathies (e.g. SFN). During the progress of their chronic pain state, patients often receive three or more compounds with limited or none effect and/or considerable side effects. The patient of this study is a good example for such a “patient career”. This strains the patient-caretaker relationship, which is crucial for successful treatment of chronic pain states. Thus, finding a means to preclinically identify drugs that have a higher likelihood to work in specific patients, is likely to increase the overall effectiveness and outcome of treating chronic pain patients.
Thus, although it may seem unconventional at first sight, in patients with chronic pain conditions, n-of-1 trials are currently the best way we have to identify effective personalized treatment. Here we presented a promising iPSC-based tool to enhance and support single-patient-trials for a more effective precision treatment of chronic neuropathic pain.
Acknowledgments
Acknowledgements
We thank Holger Wend, Sonja Plötz, Iwona Izydorczyk, Daniela Gräf and Michaela Farrell for excellent technical support.
Funding sources
None of the mentioned funding sources were involved in the study design, data collection and analysis, interpretation of the data, writing the paper or decision to submit the paper for publication.
This work was supported by the Interdisciplinary Center for Clinical Research (University Hospital Erlangen; IZKF-Projects E25 and N3 and rotation fellowship and J66 to EE), the Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University (jonior research group to BN) the German Federal Ministry of Education and Research (BMBF, 01GQ113, 01GM1520A, and 01EK1609B), the German Research Foundation (DFG) funded research training group GRK2162, the Bavarian Ministry of Education and Culture, Science and the Arts in the framework of the Bavarian Molecular Biosystems Research Network BioSysNet and ForIPS, the German-Israeli-Foundation (GIF, 1091-27.1/2010 to AL), the Sonderforschungsbereich SFB 1158, the DFG grant NA 970 3-1 and LA 2740/3-1 (to BN and AL).
IZKF University Hospital Erlangen; IZKF University Hospital Aachen; BMBF, DFG, BioSysNet, ForIPS, GIF
Declaration of interests
None of the authors has to state any conflict of interest.
Author contributions
B.N.: conceived the study, planned and performed microneurography experiments, interpreted the data and wrote parts of the manuscript and designed parts of the figures
D.S.: planed experiments, generated IPSC, differentiated nociceptors, performed electrophysiological experiments and qRT-PCR experiments, performed immunohistochemistry and FACS experiments, analyzed and interpreted data, wrote parts of the manuscript.
E.E.: performed patch clamp and MEA experiments, interpreted and discussed the data, reviewed the manuscript.
M.M.: performed patch clamp experiments.
E.Do.: differentiated nociceptors, performed qRT-PCR experiments and immunohistochemistry.
I.P.K.: took skin biopsies, performed microneurography experiments, analyzed, interpreted and discussed the data.
L.K.: performed patch clamp experiments.
J.M.: analyzed and interpreted the data, reviewed the manuscript.
A.G. performed experiments on the Nav1.9 cell line, reviewed the manuscript.
Z. L. performed experiments on the Nav1.9 cell line.
A.W.: performed gene sequencing and analysis, reviewed the manuscript.
E.Dr.: performed MEA experiments and analysis, reviewed the manuscript.
Z.K.: took skin biopsies, reviewed the manuscript.
J.S.: discussed the data, reviewed the manuscript.
I.K.: performed gene sequencing and analysis, reviewed the manuscript.
T.W.: discussed the data, reviewed the manuscript.
E.J.: took skin biopsies, recruited the patients and characterized them clinically, participated in microneurography recordings, discussed the data, reviewed the manuscript.
B.W.: conceived the study, planned experiments, interpreted the data, wrote the manuscript.
A.L.: conceived the study, planned experiments, analyzed and interpreted the data, wrote the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.11.042.
Contributor Information
Beate Winner, Email: beate.winner@fau.de.
Angelika Lampert, Email: alampert@ukaachen.de.
Appendix A. Supplementary data
Supplementary material
References
- 1.Kleggetveit I.P., Namer B., Schmidt R., Helas T., Ruckel M., Orstavik K. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 2012;153(10):2040–2047. doi: 10.1016/j.pain.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Serra J., Bostock H., Sola R., Aleu J., Garcia E., Cokic B. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153(1):42–55. doi: 10.1016/j.pain.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Waxman S.G., Merkies I.S.J., Gerrits M.M., Dib-Hajj S.D., Lauria G., Cox J.J. Sodium channel genes in pain-related disorders: phenotype-genotype associations and recommendations for clinical use. Lancet Neurol. 2014;13(11):1152–1160. doi: 10.1016/S1474-4422(14)70150-4. [DOI] [PubMed] [Google Scholar]
- 4.Lampert A., Eberhardt M., Waxman S.G. Altered sodium channel gating as molecular basis for pain: contribution of activation, inactivation, and resurgent currents. Handb Exp Pharmacol. 2014;221:91–110. doi: 10.1007/978-3-642-41588-3_5. [DOI] [PubMed] [Google Scholar]
- 5.Eberhardt E., Havlicek S., Schmidt D., Link A.S., Neacsu C., Kohl Z. Pattern of functional TTX-resistant sodium channels reveals a developmental stage of human iPSC- and ESC-derived nociceptors. Stem Cell Rep. 2015;5(3):305–313. doi: 10.1016/j.stemcr.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo S., Bean B.P. Lacosamide inhibition of Nav1.7 voltage-gated sodium channels: slow binding to fast-inactivated states. Mol Pharmacol. 2017;91(4):277–286. doi: 10.1124/mol.116.106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheets P.L., Heers C., Stoehr T., Cummins T.R. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J Pharmacol Exp Ther. 2008;326(1):89–99. doi: 10.1124/jpet.107.133413. [DOI] [PubMed] [Google Scholar]
- 8.de Greef B.T., Merkies I.S., Geerts M., Faber C.G., Hoeijmakers J.G. Efficacy, safety, and tolerability of lacosamide in patients with gain-of-function Nav1.7 mutation-related small fiber neuropathy: study protocol of a randomized controlled trial-the LENSS study. Trials. 2016;17(1):306. doi: 10.1186/s13063-016-1430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Chambers S.M., Qi Y., Mica Y., Lee G., Zhang X.J., Niu L. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol. 2012;30(7):715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmelz M., Forster C., Schmidt R., Ringkamp M., Handwerker H.O., Torebjork H.E. Delayed responses to electrical stimuli reflect C-fiber responsiveness in human microneurography. Exp Brain Res. 1995;104(2):331–336. doi: 10.1007/BF00242018. [DOI] [PubMed] [Google Scholar]
- 12.Kleggetveit I.P., Schmidt R., Namer B., Salter H., Helås T., Schmelz M. Pathological nociceptors in two patients with erythromelalgia-like symptoms and rare genetic Nav 1.9 variants. Brain Behav. 2016;(6) doi: 10.1002/brb3.528. e00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmelz M., Forster C., Schmidt R., Ringkamp M., Handwerker H.O., Torebjörk H.E. Delayed responses to electrical stimuli reflect C-fiber responsiveness in human microneurography. Exp. Brain Res. 1995;104(2):331–336. doi: 10.1007/BF00242018. [DOI] [PubMed] [Google Scholar]
- 14.Kleggetveit I.P., Schmidt R., Namer B., Salter H., Helas T., Schmelz M. Pathological nociceptors in two patients with erythromelalgia-like symptoms and rare genetic Nav 1.9 variants. Brain Behav. 2016;6(10) doi: 10.1002/brb3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Namer B., Barta B., Orstavik K., Schmidt R., Carr R., Schmelz M. Microneurographic assessment of C-fibre function in aged healthy subjects. J Physiol. 2009;587(2):419–428. doi: 10.1113/jphysiol.2008.162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voortman M., Fritz D., Vogels O.J.M., Eftimov F., van de Beek D., Brouwer M.C. Small fiber neuropathy: a disabling and underrecognized syndrome. Curr Opin Pulm Med. 2017;23(5):447–457. doi: 10.1097/MCP.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 17.Hearn L., Derry S., Moore R.A. Lacosamide for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;2 doi: 10.1002/14651858.CD009318.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moutal A., Yang X., Li W., Gilbraith K.B., Luo S., Cai S. CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain. 2017;158(12):2301–2319. doi: 10.1097/j.pain.0000000000001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyreuther B.K., Freitag J., Heers C., Krebsfanger N., Scharfenecker U., Stohr T. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2007;13(1):21–42. doi: 10.1111/j.1527-3458.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obreja O., Hirth M., Turnquist B., Rukwied R., Ringkamp M., Schmelz M. The differential effects of two sodium channel modulators on the conductive properties of C-fibers in pig skin in vivo. Anesth Analg. 2012;115(3):560–571. doi: 10.1213/ANE.0b013e3182542843. [DOI] [PubMed] [Google Scholar]
- 21.Wiffen P.J., Derry S., Moore R.A., Aldington D., Cole P., Rice A.S. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11 doi: 10.1002/14651858.CD010567.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaccara G., Perucca P., Loiacono G., Giovannelli F., Verrotti A. The adverse event profile of lacosamide: a systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2013;54(1):66–74. doi: 10.1111/j.1528-1167.2012.03589.x. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Menachem E. Lacosamide: an investigational drug for adjunctive treatment of partial-onset seizures. Drugs Today. 2008;44(1):35–40. doi: 10.1358/dot.2008.44.1.1178468. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Menachem E., Biton V., Jatuzis D., Abou-Khalil B., Doty P., Rudd G.D. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48(7):1308–1317. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 25.McMillin G.A., Krasowski M.D. In: Therapeutic Drug Monitoring of Newer Antiepileptic Drugs. Clarke W., Amitava D., editors. Elsevier; 2016. pp. 101–134. [Google Scholar]
- 26.Hampl M., Eberhardt E., O'Reilly A.O., Lampert A. Sodium channel slow inactivation interferes with open channel block. Sci Rep. 2016;6:25974. doi: 10.1038/srep25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao L., McDonnell A., Nitzsche A., Alexandrou A., Saintot P.P., Loucif A.J. Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci. Transl. Med. 2016;8(335) doi: 10.1126/scitranslmed.aad7653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material