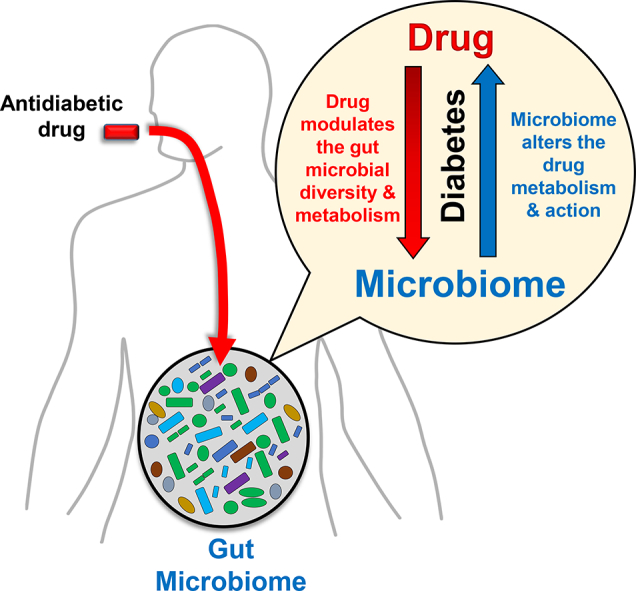
Abstract
Type 2 diabetes (T2D) has become a global epidemic. Although several drugs are available to manage T2D, problems associated with person-to-person variability in drug efficacy and potential side-effects remain unresolved. Owing to the emerging role of the gut microbiome in obesity and T2D, the interaction between gut microbes and anti-diabetic drugs and its influence on drugs' functions remains of immediate research interest. On one hand, drugs can manipulate gut microbiome composition and metabolic capacity. Conversely, the metabolic activities of the microbiome and its metabolites can also influence drug metabolism and effects. Hence, understanding this bi-directional drug-microbiome interaction and how it influences the clinical outcomes of antidiabetic drugs can pave the way to develop next-generation strategies to ameliorate diabetes. This review presents evidences demonstrating the putative interactions between anti-diabetic drugs and the gut microbiome, and discusses the potential of microbiome modulators to manipulate drug-microbiome interactions and the drug metabolism.
Keywords: Diabetes, Drugs, Metformin, Microbiome, Type-2 diabetes, Probiotics, Prebiotics
Abbreviations: AGIs, α-Glucosidase inhibitors; AMP, adenosine monophosphate; AMPK, AMP-kinases; BCCA, branched-chain amino acid; cAMP, cyclic AMP; DPP-4, Dipeptidyl peptidase-4; GLP-1, glucagon-like protein-1; HbA1c, hemoglobin A1c; HFD, high-fat diet; HFHSD, high-fat high-sucrose diet; IR, insulin resistance; LPS, lipopolysaccharide; MS, multiple sclerosis; PPAR, peroxisome proliferator-activated receptor; SCFAs, short-chain fatty acids; SGLT, sodium-glucose cotransporters; SGLT2, sodium-dependent glucose transport-2; T2D, type-2 diabetes; IR, insulin resistance; TLR, toll-like receptor; TZDs, thiazolidinediones
Graphical abstract
Highlights
-
•
Gut microbes interact with medications used to treat T2D, that at least partially mediates potential benefits of these drugs.
-
•
Anti-diabetic drugs impact gut microbiome and its metabolic activity and vice-versa.
-
•
Understanding the dynamics of drug-microbiome cross-talk would offer better therapeutic outcomes for diabetes.
1. Introduction
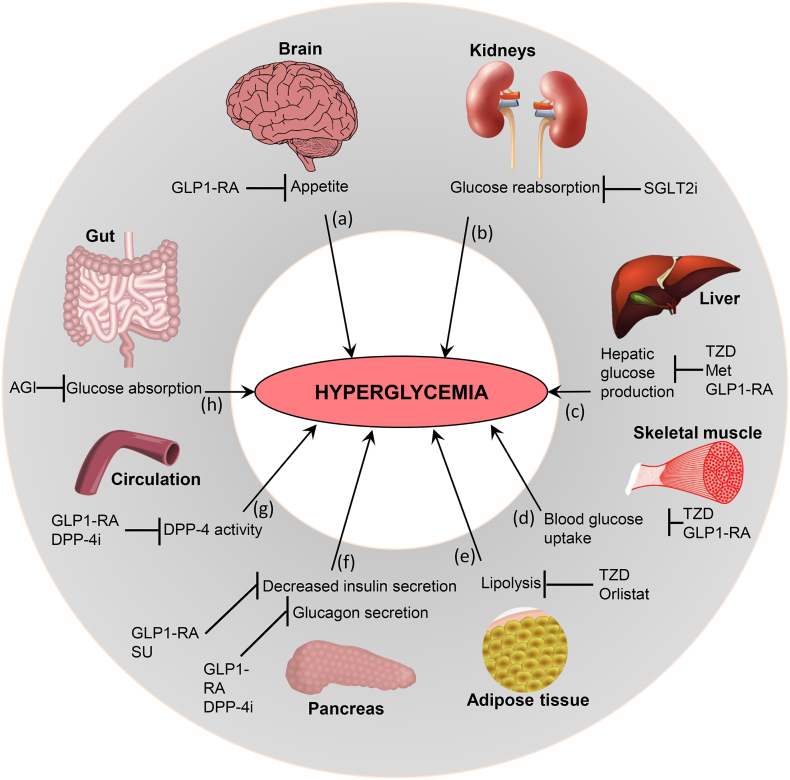
Type-2 diabetes (T2D) has recently become a global pandemic, largely due to increasing degrees of obesity and a sedentary lifestyle. T2D is a multifactorial syndrome characterized by carbohydrate/fat metabolism abnormalities and often includes hyperglycemia, hypertension, and abnormal cholesterol profiles. Different metabolic organs regulate/influence diabetic hyperglycemia differently, e.g., brain via appetite, kidney via glucose reabsorption, liver via gluconeogenesis, muscle/adipose tissue via glucose uptake, pancreas via insulin, and gut via sugar absorption and gut hormones (Fig. 1). Many anti-diabetic drugs normalize blood glucose by targeting these organs: e.g., gut hormones (e.g., glucagon-like protein-1; GLP-1) mimetic and dipeptide-4 (DPP-4; a GLP-1 degrading enzyme) inhibitors suppress appetite in brain; sodium-dependent glucose transport-2 (SGLT2) inhibitors block renal glucose reabsorption; metformin (a biguanide) reduce hepatic gluconeogenesis; thiazolidinediones (TZDs; PPAR-γ agonists) increase glucose uptake in skeletal muscles and adipose tissues and lipolysis; sulfonylureas (SU) increase pancreatic insulin secretion (Fig. 1). Emerging discoveries indicate that the gut microbiota (bacterial communities inhabiting our intestine) play an important role in the development of obesity, metabolic syndrome and T2D.
Fig. 1.
Metabolic organs contributing in diabetic hyperglycemia, and targeted mechanisms of antidiabetic medications. (a) Increased appetite by the brain can contribute to hyperglycemia, and is inhibited by glucagon-like peptide-1 receptor agonists (GLP1-RAs). (b) Elevated glucose reabsorption by the kidneys participates in hyperglycemia, and SGLT2i (Sodium-Glucose co-transporter 2 inhibitors) blocks glucose reabsorption in kidneys. (c) Enhanced endogenous hepatic glucose production leads to hyperglycemia, and TZDs (thiazolidinediones), Met (metformin), and GLP1-RA inhibit this process. (d) Decreased blood glucose uptake by the skeletal muscle contributes to hyperglycemia, and its inhibition is targeted by TZDs and GLP1-RAs. (e) Augmented lipolysis in adipose tissue increases hyperglycemia, and is inhibited by TZDs and Orlistat. (f) Abnormal insulin secretion from pancreas contributes in diabetic hyperglycemia, and is inhibited by SUs (sulfonylureas) and GLP1-RAs. Glucagon secretion by pancreas can also lead to hyperglycemia and is inhibited by GLP1-RAs and DPP-4i (dipeptidyl peptidase-4 inhibitors). (g) DPP-4 enzymatic activity in circulation participates in blood hyperglycemia, and is inhibited by GLP-1RAs. (h) Increased glucose absorption in the gut can contribute to hyperglycemia, and is inhibited by AGIs (alpha-glucosidase inhibitors).
Human gut microbiota is predominated by bacterial phyla Firmicutes and Bacteroidetes, followed by Actinobacteria, Proteobacteria and Verrucomicrobia [1]. These bacteria affect host metabolism, immunity, and brain function, manifesting their indispensableness to human health. One main function of gut microbiota is to metabolize non-digestible carbohydrates into short-chain fatty acids (SCFAs; e.g., acetate, propionate, butyrate) and regulate host metabolism. Hence, perturbations in microbiota (gut dysbiosis) can instigate diseases not only of gut but also of other organs including brain, heart, pancreas, liver, adipose tissues, muscles and kidneys [2]. Gut dysbiosis is known to instigate obesity and T2D [2]. For example, energy-rich diets and obesity involve increased intestinal Firmicutes-Bacteroidetes ratio in rodents and humans [1,3,4]. Likewise, T2D patients have altered gut microbiota with reduced butyrate-producing genera (e.g., Roseburia, Subdoligranulum, Clostridiales) wherein metformin-treatment reduces/reverses changes in Firmicutes population [5]. While T2D treatments focus primarily on achieving normoglycemia by restoring insulin availability/sensitivity, carbohydrate gastrointestinal absorption, or glucose urinary excretion, some T2D therapies might be functioning via gut dysbiosis amelioration. For example, metformin, alters gut microbiome in rodents and humans, in a way that contrasts with high-fat diet (HFD) effects [6]. Not only do anti-diabetic drugs influence the gut microbiome, but gut microbiome also influences the metabolism of drugs and xenobiotics, thereby affecting patients' responses. Several orally-administered drugs and xenobiotics, before being absorbed in bloodstream, are encountered/processed by intestinal microbial enzymes, and hence, the gut microbiome's metabolic capacity could influence the absorption and function of these drugs by making them pharmacologically active, inactive or even toxic [7]. Thus, this drug-microbiome interface could also influence host's overall health. The present review discusses such potential interactions between the anti-diabetic drugs and gut microbiome, and contemplates how gut microbiome can contribute to drugs' efficacy and variability in clinical practice. Indeed, the decipherment of this drug-microbiome interface can facilitate novel precision-medicine approaches against T2D.
2. Role of gut bacterial metabolites in the regulation of host metabolism
Gut microbiome affects host metabolism through several mechanisms, viz. catabolism of dietary toxins/carcinogens, fermentation of indigestible nutrients, synthesis of micronutrients, and facilitating the absorption of electrolytes and minerals [8]. SCFAs are one of the most studied bacterial products due to their suggested health benefits. For example, fiber-rich diet is associated lower risk of obesity [9], whereas gut microbiome of diabetic patients has fewer SCFA-producing bacteria [10]. In mice, the dietary supplementation of butyrate (one of the most-studied and beneficial SCFAs) prevents weight gain and improves insulin sensitivity by increasing energy expenditure and decreasing food intake [11], suggesting that SCFAs might prevent HFD-induced obesity and insulin resistance (IR) by causing a shift from lipogenesis to fatty-acid oxidation in liver and adipose tissue [12]. Furthermore, butyrate and propionate may also induce intestinal gluconeogenesis, which can act through an intestine–brain neural circuit to improve peripheral glucose production and insulin sensitivity [13]. Interestingly, acetate can also act on the parasympathetic nervous system to increase food intake and promote glucose-stimulated insulin secretion in rodents [14,15]. Whereas, in HFD-fed mice, intravenous and colonic administration of acetate reduces food intake and weight gain [16]. However, it remains undetermined if these contradictory effects of acetate in rats vs. mice are due to host species-specific responses to acetate. For instance, in humans, acetate is associated with increased short-term subjective satiety ratings [17] and reduced body-weight [18].
Besides increased energy harvest and bacterial metabolites produced during food metabolism, other potential mechanisms linking gut microbiome to obesity may include chronic low-grade inflammation and endotoxemia. Gut bacteria can instigate obesity- and IR-associated inflammatory state through lipopolysaccharide (LPS; a component of gram-negative bacterial cell-wall), which triggers inflammation by binding to toll-like receptor-4 (TLR-4) complex at the surface of innate immune cells. Deletion of TLR-4 prevents HFD-induced IR, suggesting that TLR-4 is implicated in metabolic diseases [19]. HFD-induced obese mice demonstrate fewer gut bifidobacteria and eubacteria and increased circulating LPS levels [20]. In humans, a similar-grade endotoxemia associated to IR, and a high-fat-high-carbohydrate meal induces significant elevations in postprandial plasma LPS [21], hinting that endotoxemia might play a pathological role in obesity-associated inflammatory state and that the food ingestion may affect plasma endotoxin levels. Studies have also demonstrated the association between fasting serum branched-chain amino acid (BCAA) levels and incident T2D [22], the normalization of BCAA levels in obese individuals after bariatric surgery [23], and the development of IR in rats after BCAA-diet supplementation [24], suggesting a potentially causative role of BCAAs or their metabolites in metabolic disorders. Gut microbiome might also independently contribute to the elevated serum BCAA levels in humans with IR [25]. Association between gut microbiome functions—including BCAA biosynthesis—and IR are largely driven by specific species including Prevotella copri and Bacteroides vulgatus, suggesting that these species may directly influence host metabolism [25]. Hence, gut dysbiosis may potentially affect serum metabolome and contribute to IR through pathways involving BCAAs.
3. Impact of antidiabetic drugs on the gut microbiome configuration
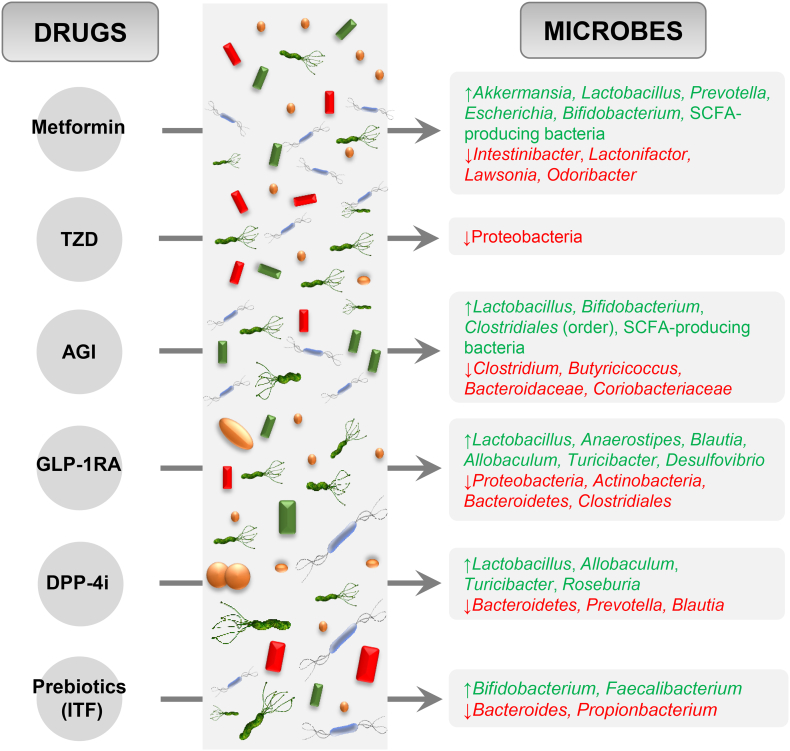
Given the importance of drug-microbiome interactions, we assembled evidences demonstrating the interactions of some of the most commonly used antidiabetic drugs with the gut microbiota while treating hyperglycemia or T2D (summarized in Fig. 2 and Table 1).
Fig. 2.
Effects of antidiabetic drugs on the relative abundance of gut microbes. ↑: increased relative abundance; ↓: decreased relative abundance; TZD: Thiazolidinedione; AGI: Alpha-glucosidase inhibitor; GLP-1RA: Glucagon-like peptide-1 receptor agonist; DPP-4i: Dipeptidyl peptidase-4 inhibitor; ITF: Inulin-type fructans; SCFA: short-chain fatty acids.
Table 1.
Summary of the effects of antidiabetic drugs on the gut microbiota.
| Drug | Model | Major findings | Reference |
|---|---|---|---|
Metformin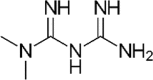
|
Rodent: C57BL/6 mice N = 24 |
|
7 |
| Rodent: C57BL/6 mice N = 41 |
|
33 | |
| Rodent: SPF Wistar rats N = 50 |
|
40 | |
| Rodent: Sprague-Dawley Rats N = 20 |
|
64 | |
| Rodent: C57BL/6 mice N = 40 |
|
62 | |
| Human: T2D Patients N = 40 |
|
67 | |
| Human: T2D patients N = 450 |
|
66 | |
| Human N = 784 |
|
8 | |
Thiazolidinediones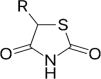
|
Rodent: Male SD rats n = 32 |
|
46 |
| Rodent: C57BL/6 mice N = 21 |
|
48 | |
α-Glucosidase inhibitors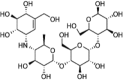
|
Rodent: C57BL/6 mice n = 30 |
|
51 |
| In vitro study |
|
52 | |
| Human: Chinese patients with prediabetes N = 52 |
|
53 | |
| Human: Chinese patients with T2D N = 95 |
|
58 | |
| Human: patients with primary hyperlipidemia N = 14 |
|
59 | |
GLP-1 Receptor Agonists
|
Rodent: ApoE −/− C57BL/6 mice N = 60 |
|
69 |
DPP-4 inhibitors
|
Rodent: ApoE −/− C57BL/6 mice N = 60 |
|
64 |
| Rodent: SD rats N = 15 |
|
70 | |
| Rodent: C57BL/6 J male mice N = 27 |
|
68 | |
SGLT2 Inhibitors
|
Rodent: C57BLKS male mice N = 24 |
|
82 |
Sulfonylurea
|
Rodent: Sprague-Dawley male rats N = 24 |
|
75 |
| Human: diabetic N = 43 |
|
74 | |
| Combination therapy (PGX+S/MET) | Rodent: male Zucker diabetic fatty rats N = 66 |
|
96 |
| Prebiotics (inulin-type fructans) | Human: obese women N = 30 |
|
108 |
HFD: high-fat diet; HFHSD: high-fat high-sucrose diet; ITF: Inulin-type fructans; LPS: lipopolysaccharide; SCFA: short-chain fatty acids; SD: standard diet; SPF: specific-pathogen free; T2D: type-2 diabetes; TG: triglycerides.
3.1. Biguanides
Among biguanides, metformin is used as first-line treatment in newly diagnosed T2D patients (Fig. 1c), and ameliorates glycemic control and cardiovascular mortality in overweight T2D patients and can even prevent T2D. Although, the precise mechanism(s) of action remains unclear, metformin's glucose-lowering effects are known by decreased hepatic gluconeogenesis, increased glycogenesis, reduced intestinal glucose absorption, and increased glucose uptake by muscle cells and adipocytes, via activation of AMP-kinase dependent and independent pathways [reviewed in [[26], [27], [28], [29]]]. In addition, accumulating evidences suggest that metformin also acts through pathways in the gut and its microbiome [30]. Multiple evidences suggest that metformin modulates gut microbiota [30] in humans and HFD-fed rodents and improves glycemia along with increased abundance of mucus-degrading gut bacteria, namely Akkermansia muciniphila [6]. Similarly, other studies have demonstrated that metformin increases the abundance of A. muciniphila and Clostridium cocleatum in HFD-fed mice [31]. Increased abundance of A. muciniphila by metformin may not only be correlational, since administration of A. muciniphila alone improves glucose tolerance in HFD-fed mice [6]. A recent study demonstrated that A. muciniphila's cell-wall consists of an active ingredient that mimics metformin's action to reduce obesity and diabetes in a rodent model [32].
Although, several studies have identified A. muciniphila as a potential mediator of the glucoregulatory effects of metformin, other studies report that the abundance of Akkermansia in the upper small intestinal luminal contents remain unchanged in response to 3-day HFD-feeding with or without metformin pretreatment [33]. The differences between these findings could be due to a lower abundance of this genus in the upper small intestine, as Akkermansia has been documented to colonize more efficiently in the cecum and colon compared to the small intestine [34]. In addition, a longer metformin treatment regimen and HFD-feeding may be required to induce significant changes in Akkermansia abundance, as previous studies detailing the effect of metformin on Akkermansia have used a chronic treatment protocol [5]. Still, another study has observed a decrease in Akkermansia in T2D patients after metformin treatment, and hence, further research is required to specify the role of Akkermansia with reference to metformin and T2D [35]. Other studies, instead, have found that HFD decreases the upper small intestinal abundance of Lactobacillus, while this effect is partially reversed with metformin pretreatment [33]. This is consistent with previous work showing that metformin increases the abundance of Lactobacillus in HFD-fed rodents [31]. Thus, in addition to increasing the abundance of A. muciniphila in the lower intestinal tract [36], metformin may increase the abundance of Lactobacillus in the upper small intestine, which could both contribute to the anti-diabetic effect of metformin.
Along with the modulation of microbiome composition, metformin treatment can also improve the microbiome metabolic function i.e., by increasing the population of SCFA-producing bacteria including Allobacum, Bacteriodes, Blautia, Butyricoccus, and Phascolarctobacterium in the gut [37]. These studies indicate that metformin can modify bacterial diversity, which contrasts the effects of a HFD, and selectively increases the abundance of specific bacteria in HFD-fed mice, while also altering multiple metabolic pathways in the gut microbiota, such as increasing those for sphingolipid and fatty acid metabolism [31]. One study involving treatment-naive T2D individuals showed that metformin administered over varying amounts of time results in altered abundance of Escherichia and Intestinibacter as well as SCFA-producing bacteria such as Bifidobacterium adolescentis [36]. This study involving newly diagnosed T2D individuals on a calorie-restricted diet showed that metformin, but not calorie restriction, had rapid effects on gut microbiota composition and function in association with reducted HbA1c and fasting blood glucose levels. Fecal analysis demonstrated dramatic shifts in the composition of the gut microbiota after 2 and 4 months of metformin treatment in individuals with newly diagnosed T2D, in particular, significant changes in Escherichia and Intestinibacter abundance across all sampling points in the metformin-treated group [36]. It was also shown that metformin promoted the growth of B. adolescentis both in-vivo and in-vitro using pure cultures which can be significant because previous findings have shown that supplementation with B. adolescentis in a rodent model of the metabolic syndrome can increase insulin sensitivity [38]. Interestingly, the study did not observe any significant correlations between HbA1c levels and A. muciniphila abundance. In addition, it was found that the transfer of the fecal microbiota from metformin-treated individuals to germ-free mice resulted in improved glucose tolerance in recipients. This indicates that metformin-adapted microbiota could contribute to the beneficial effects of metformin on glucose homeostasis. To examine the microbial mediation of the therapeutic effects of metformin, one study compared metformin-treated and -untreated T2D subjects to characterize the effect of the treatment in more detail. Univariate tests of the effects of metformin treatment showed a significant increase of Escherichia spp. and a reduced abundance of Intestinibacter spp. [5]. These significant microbiome alterations were consistent with well-known side effects of metformin treatment, including reduced intestinal lipid absorption, LPS-triggered local inflammation, and butyrate production, which has been shown to increase the intestinal gluconeogenesis gene expression [13]. In rodents, the net result of increased intestinal gluconeogenesis is a beneficial effect on glucose and energy homeostasis with reductions in hepatic glucose production, appetite and body-weight [5]. Another recent study examined the contributions that some intestinal microbes have on the host intestinal nutrient pool. The four intestinal microbes examined were Escherichia spp., A. muciniphila, and Subdoligranulum variabile, which were previously found to be increased after metformin, as well as Intestinibacter bartlettii, which was found to be decreased after metformin treatment [39]. Using Genome-scale metabolic models to elucidate bacterial metabolism and effect on the host intestinal nutrient pool, this study suggested that Escherichia sp. and S. variabile may contribute to the production of important SCFAs such as acetate and butyrate, respectively, involved in the host physiology. Hence, the commensal and competing behaviors in bacteria and its relation to the production of extracellular compounds such as SCFA and amino acids may play a role in the effects of metformin in the treatment of T2D.
Overall, these studies suggest that the glucose-lowering effect of metformin can be partly attributed to certain species within the gut microbiome that helps metformin function more efficiently. As the mechanisms by which metformin influences the gut microbiota becomes more elucidated, it is likely that the oral administration of metformin has both direct and indirect effects on the gut microbiota. Historically, biguanide, due to its anti-microbial activities, has been used to treat infections in natural healing practices, and such properties of metformin may be the main reason why it modulates the gut microbiome. One clinical trial has shown that the metformin treatment in humans significantly inhibits two functional groups containing potential pathogen-like genera, including Alistipes, Oscillibacter, and Bacteroides, and this inhibition is significantly correlated with improved glucose homeostasis by metformin [35], suggesting that the inhibition of potential pathogen-like bacteria may be involved in some of the beneficial effects of metformin. Interestingly, it was found that metformin impairs folate metabolism in bacteria such as E. coli, and hence, this may be one potential mechanism through which metformin exerts its effects on the gut microbiota [40].
3.2. Thiazolidinediones
Thiazolidinediones (TZDs), or glitazones, attenuate and improve IR in T2D. Because of their ability to restore peripheral insulin sensitivity, TZDs such as pioglitazone are commonly used clinically in the management of T2D. The effects of TZDs, which are attributed to their agonistic action on the peroxisome proliferator-activated receptor-(PPAR)γ, are detailed in other studies [[41], [42], [43]].
In addition to their antidiabetic properties, TZDs also possess antibacterial activities and may consequently affect gut bacteria. HFD feeding increases the abundance of Proteobacteria, Enterobacteriaceae and Desulfovibrionaceae in rodents [44], and pioglitazone treatment reduces the abundance of Proteobacteria in the HFD-fed rats, with no significant decline in Enterobacteriaceae and Desulfovibrionaceae [44], suggesting that pioglitazone only partially reverse the HFD-induced gut dysbiosis. Another study has demonstrated that the gut-associated butyrate-producing microbes can activate PPAR-γ signaling and prevent a dysbiotic expansion of potentially pathogenic bacteria belonging to the genera Escherichia and Salmonella [45], suggesting that antidiabetic drugs affecting the composition of butyrate-producing microbes in the gut may play an important role through PPAR-γ signaling pathway. Lastly, a study found that the HFD-induced gut microbial dysbiosis including increased Firmicutes, Proteobacteria and Verrucomicrobia and decreased Bacteroidetes abundance could be reversed by switching the mice back to the standard diet or treating them for one week with rosiglitazone, another TZD [46]. These studies indicate that there is an interaction between gut microbiota and TZDs, which may possibly be involved in the antidiabetic effects of TZDs.
3.3. α-Glucosidase inhibitor
α-Glucosidase inhibitors (AGIs) delay the intestinal absorption and digestion of non-absorbable complex carbohydrates into absorbable monosaccharides and reduce postprandial hyperglycemia (Fig. 1h) [47]. Acarbose, an AGI initially isolated from bacterial cultures, gives a selective advantage to their bacterial producers in a community where there is competition for similar nutrients [48].
Given that AGIs impact the nutrient sources of bacteria by altering the composition and length of time that carbohydrates remain in the intestines, it is unsurprising that AGIs can influence the gut microbiota composition as well. It has been shown that in mice that are fed a high-fat high-sucrose diet (HFHSD), treatment with Miglitol, an AGI, not only suppresses IR and liver fat accumulation and shortens intestinal transit time, but can also result in gut microbiota changes, thereby suppressing HFHSD-induced increase in the composition of Erysipelotrichaceae within the phylum Firmicutes and Coriobacteriaceae within the phylum Actinobacteria [49]. In addition, an in-vitro study demonstrated that the Acarbose can specifically inhibit the growth of E. coli (when grown on maltose) by blocking the maltose importer [50]. In a randomized-double-blind controlled study, Acarbose treatment in 52 Chinese prediabetic patients was shown to increase SCFA-producing bacteria such as Faecalibacterium, Prevotella, and Lactobacillus [51], and Lactobacillus treatments are known to ameliorate glycemia [[52], [53], [54], [55]]. An increase in the abundance of Dialister has also been found following the Acarbose treatment, which was negatively correlated with HbA1c [51]. Hence, some Dialister species may potentially play a role in the regulation of glucose metabolism. Another study suggested that the addition of Acarbose to antidiabetic treatment leads to increase in the abundance of Bifidobacterium longum [56]. A similar effect was also seen in the fecal microbiota of hyperlipidemic patients that are administered Acarbose, with a significant increase in Lactobacillus and Bifidobacterium and decrease in Enterobacteriaceae and Bacteroidaceae [57]. These studies indicate that Acarbose treatment plays a significant role in partially restoring the gut microbiota imbalance in T2D patients.
3.4. GLP-1 receptor agonist
Glucagon-like peptide-1 receptor agonists (GLP-1 RA) used in the treatment of T2D have demonstrated efficacy in improving glycemic control through numerous physiological effects (Fig. 1c, d, f, g); however, the precise molecular mechanisms remain largely unclear [reviewed in [[58], [59], [60], [61]]]. Studies have demonstrated that the GLP-1 RA can also modulate the gut microbiota. Liraglutide (an injectable GLP-1 RA) has been shown to produce substantial gut microbiota changes in mice compared to non-treated controls [62], with enrichment of genera Allobaculum, Turicibacter, Anaerostipes, Blautia, Lactobacillus, Butyricimonas, and Desulfovibrio accompanied by reduced abundance of phylotypes mainly within the order Clostridiales (phylum Firmicutes) and Bacteroidales (phylum Bacteroides) [62]. Interestingly, the relative abundance of all obesity-related phylotypes (viz. Erysipelotrichaceae Incertae Sedis, Marvinbryantia, Roseburia, Candidatus Arthomitus, and Parabacteroides) were substantially decreased under liraglutide administration [62]; whereas in terms of phylotypes associated with leanness, liraglutide seemed to induce enrichment of Lactobacillus and Turicibacter [62]. In addition, we have previously demonstrated that a multi-strain probiotic cocktail called VSL#3 (now Visbiome) can prevent and treat obesity and T2D in mice models via modulating gut microbiome and its metabolites viz. SCFAs (specifically butyrate) and increasing intrinsic GLP-1 production from L-cells [55].
3.5. DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4), is a proteolytic enzyme found in the cell membrane of most of the cells in human body with the primary function of inactivating GLP-1 [63]. Inhibition of DPP-4 with gliptins such as sitagliptin, vildagliptin, and saxagliptin, prolongs the circulating half-life of GLP-1, thus enhancing its insulinotropic and glucoregulatory abilities (Fig. 1f, g). The effects of gliptins are reviewed in previous studies [64,65]. It is also known that certain commensal gut bacteria exert DPP-4-like activity [66]. Saxagliptin-treatment in mice increased Firmicutes abundance, especially in the genus Lactobacillus along with the genera Allobaculum and Turicibacter [62]. A decrease in the phylum Bacteroidetes was also observed and was mainly from the genus Bacteroides and Prevotella [62]. In addition, when comparing liraglutide, a GLP-1 receptor agonist, saxagliptin treatments have milder effects on gut microbiome signature. The relative abundance of all obesity-related phylotypes significantly decreased with liraglutide, while only one phylotype, the genus Candidatus Arthromitus, was affected by saxagliptin [62]. In observing the relative abundance of phylotypes associated with a decrease in body-weight, both liraglutide and saxagliptin were shown to enrich Lactobacillus and Turicibacter [62]. Interestingly, Lactobacillus possesses inhibitory activity against DPP-4 [67]. Mice receiving a Western-diet (45% kilojoules from fat and 17% kilojoules from sucrose) with vildagliptin found that vildagliptin modulates the gut microbiota composition and its metabolic activities [66]. Vildagliptin decreased Oscillibacter spp., increased Lactobacillus spp., promoted antimicrobial peptide production in the ileum, and indirectly reduced hepatic expression of proinflammatory cytokines [66]. Thus, it is possible that the DPP-4 inhibitory activity of saxagliptin partially involves the enrichment of Lactobacilli and decrease in other species such as Oscillibacter. In another study, administration of sitagliptin in T2D rats decreased abundance of Firmicutes and Blautia, while increased Bacteroidetes,Proteobacteria and Roseburia [68]. These studies indicate that the DPP-4 inhibitor mediated changes in gut microbiome may interact with mechanism(s) that benefit the host in maintaining better glucose homeostasis [68].
3.6. Sulfonylureas
The sulfonylureas (SU) have been important in the treatment of T2D over the past 50 years for their ability to stimulate insulin release in a glucose-independent manner (Fig. 1f) [[69], [70], [71]]. To our knowledge, no study has hitherto examined the direct impact of sulfonylureas on the gut microbiota; however, indirect studies have demonstrated that patients treated with sulfonylureas have increased hippurate (a normal metabolite found in urine, mainly derived from the breakdown of plant phenolics and aromatic amino acids by the gut microflora) and aromatic amino acids in the urine, suggesting that the sulfonylureas may have some effect on the gut microbiota and their ability to metabolize plant phenolics and aromatic amino acids [72]. In addition, some recent studies indicate that SUs like Glebclamide and Glipizide have interactions with gut microbiome [73,74], wherein Glibenclamide showed minor impact on the rat gut microbiome [74] while Glipizide showed no effects on gut microbiome in diabetic human subjects [73]. However, these studies were not focused specifically to illustrate the effects of these sulfonylureas on gut microbiome and hence studies that are more direct are warranted to establish the impact of sulfonylureas on the gut microbiome and vice-versa.
3.7. SGLT2 inhibitors
The two major sodium-glucose cotransporters (SGLTs) i.e., SGLT-1 and -2, found in the kidney, are responsible for the reabsorption of glucose in the renal tubules [75]. Various SGLT2 inhibitors are used as a T2D therapy due to their ability to improve glycemic control by inhibiting reabsorption of glucose filtered through the renal glomerulus (Fig. 1b) [76]. The effects of SGLT2 inhibitors on the gut microbiota or specific gut microbial species remain to be elucidated. As of yet, one recent study found that the dapaglifozin treatment reduces microbiome diversity and richness in control mice, with no effects in diabetic mice. Firmicutes:Bacteroidetes ratio is reduced in dapaglifozin-treated diabetic mice [77]; notably, previous studies have associated reduction in this ratio with a lean phenotype. This study reported that the dapaglifozin-treated diabetic mice show significantly increased body-weight compared to the control mice. The increase in body-weight, however, was thought to be protective because diabetic mice tend to lose weight as the severity of diabetes progresses and medications that improve health outcomes in diabetic mice are known to preserve or even increase the body-weight [78,79]. At species level, A. muciniphila abundance was increased in dapaglifozin-treated diabetic mice, which may be associated with improved metabolic outcomes [77]. Hence, the impact of SGLT2 inhibitors on the gut microbiota is an area of active research.
3.8. Orlistat
Orlistat is a pancreatic and gastric lipase inhibitor that is used in the management of obesity to facilitate weight loss (Fig. 1e). Orlistat has been shown to reduce the progression of diabetes, especially in those with impaired glucose tolerance [[80], [81], [82]].
Considering the mechanism of Orlistat, there may be an indirect effect on the gut bacteria through a higher input of dietary fat into the colon. However, a study examining the effects of increased dietary fat reaching to the colon after Orlistat treatment reported only minor, non-significant changes in gut microbiome, suggesting that the increased dietary fat does not modify the microbiota composition [83]. However, this study remains inconclusive due to the limitation of short period of Orlistat intervention. Furthermore, while HFD administration has been reported to decrease Bifidobacterium spp. in mice, administration of Orlistat prevented this decline [83]. Another study employing batch-culture fermentation experiments demonstrated no effect of Orlistat in the presence or absence of olive oil on the composition of bacterial communities [84] Hence, the impact of Orlistat on gut microbiome is still uncertain and further studies are needed to establish these facts.
3.9. Combination therapy
Because T2D is a progressive disorder characterized by increasing degree of hyperglycemia and a need to gradually increase the dose to maintain glycemic control, combination therapy has been used to target multiple mechanisms. The most commonly used combinations are those of DPP-4 inhibitors and metformin, metformin-sulfonylureas, and metformin-thiazolidinediones [85]. At present, however, there is limited research on the effects of different combination therapies on the gut micrbiome. One study found that the administration of prebiotic polysaccharide PolyGlycopleX (PGX) with metformin alone (MET) or with a combination of sitagliptin and metformin (S/MET) in rodents increases the abundance of Bacteroides, compared to MET, PGX, and PGX + S/MET [86]. All treatments reduced the population of Clostridium coccoides compared to cellulose, with C. coccoides being the lowest in PGX + S/MET-treated rats, suggesting that combination therapy was dominant in decreasing C. coccoides abundance. Bifidobacterium were also very low across all groups [86]. Significantly higher levels of bifidobacteria were seen in MET and PGX compared with C, S/MET and PGX + MET [86]. Lastly, Enterobacteriaceae was significantly higher in PGX compared with all other groups, indicating that different combination therapies used to treat diabetes/hyperglycemia can impact the gut microbiota [86]. However, whether and how these changes help different combination therapies work better or delay resistance in their efficacy in the long term remains unclear.
4. Metabolism of anti-diabetic drugs by the gut microbiome
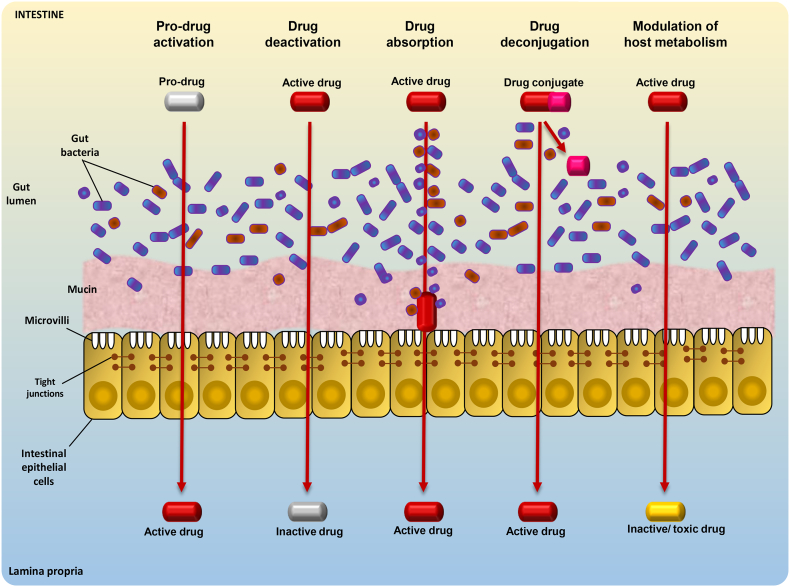
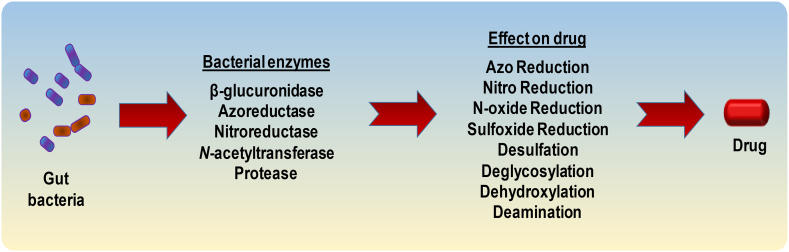
Orally-administered drugs are absorbed into the circulation through gut epithelia and the fate/efficacy of this course is influenced not only by drug characteristics (e.g., solubility, stability, permeability) but also by the drug metabolism capacity of host and microbiome enzymes [87]. Host enzymes influencing the drug metabolism are well studied but the dynamics of gut microbiome in drug metabolism and efficacy remain unclear. Gut microbiome might play a central role - perhaps even analogous to that of the liver or any other potential organ in the human body - in the efficacy and metabolism of drugs including xenobiotics and other endogenous/exogenous compounds [88,89]. For instance, gut microbes produce hydrophobic by-products via reductive and hydrolytic metabolisms, thus facilitating their absorption from gut into the circulation [88]. Further, the effect, if any, of gut microbiome on drug metabolism may also be influenced by drug- as well as host-related elements, viz. drug absorption in the upper gut, drug's quantity reaching the distal gut, and enzymes secreted by the gut microbiome [90]. Given that the drug-microbiome interactions could make a drug pharmacologically active, inactive or even toxic, it is imperative to decipher these drug-microbiome interactions pertaining to differences in the absorption and metabolic fates of different drugs and xenobiotics, as well as individual-specific gut microbiome disparities [7] (Figs. 3 and 4).
Fig. 3.
Different mechanisms by which gut microbes can positively or negatively influence drug metabolism and efficacy. Gut microbes may act on a pro-drug or drug conjugate in order to activate it. They may also deactivate an active drug, aid in the absorption of an active drug, or modulate the host metabolism to inactivate a drug or create a toxic compound.
Fig. 4.
Various gut microbial enzymes that have been found to influence the pharmacokinetics of different drugs.
Nevertheless, due to inadequate data on the metabolism of TZDs, GLP-1 RAs, DPP-4 inhibitors, sulfonylureas, and SLGT2 inhibitors by the gut microbiome, it remains unclear how anti-diabetics are metabolized by the gut microbiome. For example, Acarbose is metabolized exclusively within the gastrointestinal tract, principally by the intestinal bacteria and digestive enzymes [91]. Acarbose, in contact with α-amylases and cyclodextrin glucanotransferases (a bacterial enzyme commonly found in the genus Bacillus), is converted to longer-chain derivatives and can be regarded as a pro-drug since it can form more active inhibitors by the catalytic activity of its target site [92]. Voglibose, another α-glucosidase inhibitor, is poorly absorbed and is rapidly excreted in stools with no metabolites identified to-date [93]. In contrast, Miglitol is fully absorbed in the gut and cleared unchanged by kidneys [94]. Lastly, though no specific study has linked microbiome with Orlistat metabolism, it is speculated that Orlistat is metabolized primarily within the gastrointestinal wall, forming relatively inactive metabolites [95].
5. Probiotics and prebiotics for the modulation of gut microbiome and metabolism
Probiotics are live microorganisms that, when administered in sufficient amounts, can offer health benefits to the host, including improvements in immune system function. A meta-analysis assessing the efficacy of probiotics in glucose metabolism shows that probiotics can decrease fasting blood glucose and HbA1c levels and improve glucose metabolism [96]. However, how different probiotics and their combinations with different anti-diabetics interact with the gut microviome remain unclear. The term prebiotics, on the other hand, refers to non-digestible dietary ingredients such as fibers and related oligosaccharides (e.g., inulin, fructo-oligosaccharides, galacto-oligosaccharides) that selectively modulate the growth of beneficial bacteria in the large intestine. For instance, inulin has been associated with positive gut microbiome changes; wherein inulin-type fructans prebiotics, but not placebo, fostered Bifidobacterium and Faecalibacterium prausnitzii population and decreased Bacteroides intestinalis, Bacteroides vulgatus and Propionibacterium, an effect that associated with lower fat mass, plasma lactate, and phosphatidylcholine levels and suggested that prebiotics can induce subtle changes in the gut microbiome that may impact several key metabolites implicated in diabetes [97].
6. Conclusion and future prospects
Besides gut eubacterial community, various commensal archaea, viruses, and fungi, which otherwise remain understudied, might also influence human health. Archaea such as Methanobrevibacter genus and Methanobrevibacter smithii have been linked with obesity [98] and increased energy harvest [99], respectively. Thus, such groups may also be a therapeutic target to prevent obesity and T2D. Fecal DNA and RNA viral population has been correlated positively with Firmicutes and negatively with Bacteroidetes and bifidobacteria [100]. T2D patients also demonstrate higher carriage of specific gut phages [101]. These studies hint that direct or indirect interaction of viral population with host cells might modulate host metabolism. Intestinal fungi are also associated with gut inflammation and Crohn's disease [102]. HFD-feeding increased dysbiosis in bacterial and fungal taxa [103]. Although the data on gut archaea, fungi and eukaryotes are relatively limited, these studies suggest their possible role in obesity-gut microbiome association and indicate that these microbes together with eubacteria form a highly complex ecosystem in the gut and hence can also influence T2D and drug effects.
Drugs can change the gut microbiome and its metabolites, thus affecting the host metabolic functions (drug-microbiome-metabolism axis). However, the interactions of microbiome with host is bi-directional. For example, Bariatric surgery, a common treatment for morbid obesity, improves host metabolic functions that are tightly correlated with specific GM changes. Furthermore, certain anti-diabetic (e.g., insulin, GLP-1R agonists) may first impact host metabolic function than microbiome. Diabetic people who maintain better glycemia using insulin injections have better health outcomes like reduced cardiovascular diseases and may also have different gut microbiome; hence, such studies are highly warranted. However, Liraglutide primarily improves diabetes that are associated with microbiome changes too, suggesting that drug-metabolism-microbiome axis may also explain the drug-microbiome interactions in regulating diabetes and obesity. However, more studies are warranted to establish such facts that can help in deciphering these interactions in clinical practice.
Interestingly, supplementation with specific bacteria such as Bifidobacterium adolescentis and A. muciniphila has shown beneficial effects in T2D rodents. Hence, it would be interesting to examine whether and how such bacteria and probiotics/prebiotics can help increase drug efficacy, eliminate side-effects, and/or ameliorate diabetic complications in humans. Given that the microbiome dysbiosis is implicated in T2D and obesity, research on the characterization of gut bacteria and their metabolites involved in these pathologic states would provide important insights for the development of novel drugs and even personalized medicine. Thus, the modulation of gut microbiome by drugs can represent a target to improve, modify, or reverse the efficacy of current medications for T2D.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge funding support for this work from the Center for Diabetes, Obesity and Metabolism; the Center of Cardiovascular Sciences, the Claude D. Pepper Older Americans Center (funded by NIH P30AG12232); and the Clinical and Translational Science Center (funded by NIH UL1TR001420), all at Wake Forest School of Medicine. We also acknowledge support from Department of Defense funding (Grant number: PR170446).
Authors contributions
AW: conducted literature search, compiled and analyzed reports, and wrote parts of manuscript; RN: compiled and wrote part of manuscript and figures, language editing: HY: conceived the idea, helped to analyze and interpret reports, and conceptual and linguistic editing manuscript; with overall supervision of the paper. All the authors reviewed data, read the manuscript, and provided critical feedback to be included in the final version of the manuscript.
Competing interests
Authors declare that they have no competing interests.
References
- 1.Woting A., Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8(4):202. doi: 10.3390/nu8040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microbial Ecol Health Dis. 2015;26 doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Zhang H. Microbiota associated with type 2 diabetes and its related complications. Food Sci Human Wellness. 2013;2(3):167–172. [Google Scholar]
- 4.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 7.Kim D.-H. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab Dispos. 2015;43(10):1581. doi: 10.1124/dmd.115.063867. [DOI] [PubMed] [Google Scholar]
- 8.Devaraj S., Hemarajata P., Versalovic J. The human gut microbiome and body metabolism: Implications for obesity and diabetes. Clin Chem. 2013;59(4):617–628. doi: 10.1373/clinchem.2012.187617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho S.S., Qi L., Fahey J.G.C., Klurfeld D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr. 2013;98(2):594–619. doi: 10.3945/ajcn.113.067629. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson F.H., Tremaroli V., Nookaew I., Bergström G., Behre C.J., Fagerberg B. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 11.Gao Z., Yin J., Zhang J., Ward R.E., Martin R.J., Lefevre M. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Besten G., Bleeker A., Gerding A., van Eunen K., Havinga R., van Dijk T.H. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 13.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Perry R.J., Peng L., Barry N.A., Cline G.W., Zhang D., Cardone R.L. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ussar S., Griffin N.W., Bezy O., Fujisaka S., Vienberg S., Softic S. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22(3):516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost G., Sleeth M.L., Sahuri-Arisoylu M., Lizarbe B., Cerdan S., Brody L. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostman E., Granfeldt Y., Persson L., Bjorck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59(9):983–988. doi: 10.1038/sj.ejcn.1602197. [DOI] [PubMed] [Google Scholar]
- 18.Kondo T., Kishi M., Fushimi T., Ugajin S., Kaga T. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci Biotechnol Biochem. 2009;73(8):1837–1843. doi: 10.1271/bbb.90231. [DOI] [PubMed] [Google Scholar]
- 19.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J Clin Investig. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 21.Anderson P.D., Mehta N.N., Wolfe M.L., Hinkle C.C., Pruscino L., Comiskey L.L. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92(6):2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 22.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laferrere B., Reilly D., Arias S., Swerdlow N., Gorroochurn P., Bawa B. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3(80):80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen H.K., Gudmundsdottir V., Nielsen H.B., Hyotylainen T., Nielsen T., Jensen B.A.H. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 26.Cho S.S., Qi L., Fahey G.C., Jr., Klurfeld D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr. 2013;98(2):594–619. doi: 10.3945/ajcn.113.067629. [DOI] [PubMed] [Google Scholar]
- 27.Duca F.A., Côté C.D., Rasmussen B.A., Zadeh-Tahmasebi M., Rutter G.A., Filippi B.M. Metformin activates a duodenal Ampk–dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21(5):506. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller R.A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madiraju A.K., Erion D.M., Rahimi Y., Zhang X.-M., Braddock D.T., Albright R.A. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCreight L.J., Bailey C.J., Pearson E.R. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426–435. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H., Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. 2014;80(19):5935–5943. doi: 10.1128/AEM.01357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plovier H., Everard A., Druart C., Depommier C., Van Hul M., Geurts L. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2016;23:107. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 33.Bauer P.V., Duca F.A., Waise T.M.Z., Rasmussen B.A., Abraham M.A., Dranse H.J. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab. 2018;27(1):101–117. doi: 10.1016/j.cmet.2017.09.019. [e5] [DOI] [PubMed] [Google Scholar]
- 34.Derrien M., Van Baarlen P., Hooiveld G., Norin E., Muller M., de Vos W.M. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader akkermansia muciniphila. Front Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong X., Xu J., Lian F., Yu X., Zhao Y., Xu L. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional chinese herbal formula: A multicenter, randomized, open label clinical trial. MBio. 2018;9(3) doi: 10.1128/mBio.02392-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Manneras-Holm L. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Zhao Y., Xu J., Xue Z., Zhang M., Pang X. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5 doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Wang R., Li X.-F., Wang R.-L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr. 2012;107(10):1429–1434. doi: 10.1017/S0007114511004491. [DOI] [PubMed] [Google Scholar]
- 39.Rosario D., Benfeitas R., Bidkhori G., Zhang C., Uhlen M., Shoaie S. Understanding the representative gut microbiota dysbiosis in metformin-treated type 2 diabetes patients using genome-scale metabolic modeling. Front Physiol. 2018;9(775) doi: 10.3389/fphys.2018.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabreiro F., Au C., Leung K.Y., Vergara-Irigaray N., Cocheme H.M., Noori T. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braissant O., Foufelle F., Scotto C., Dauca M., Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, −beta, and -gamma in the adult rat. Endocrinology. 1996;137(1):354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 42.Braissant O., Foufelle F., Scotto C., Dauça M., Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha,-beta, and-gamma in the adult rat. Endocrinology. 1996;137(1):354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 43.Berger J.P., Akiyama T.E., Meinke P.T. PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Bai J., Zhu Y., Dong Y. Response of gut microbiota and inflammatory status to bitter melon (Momordica charantia L.) in high fat diet induced obese rats. J Ethnopharmacol. 2016;194:717–726. doi: 10.1016/j.jep.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Byndloss M.X., Olsan E.E., Rivera-Chavez F., Tiffany C.R., Cevallos S.A., Lokken K.L. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science (New York, NY) 2017;357(6351):570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomas J., Mulet C., Saffarian A., Cavin J.B., Ducroc R., Regnault B. High-fat diet modifies the PPAR-gamma pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci U S A. 2016;113(40):E5934–e43. doi: 10.1073/pnas.1612559113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan C.-W., Yu C.-L., Lin J.-C., Hsieh Y.-C., Lin C.-C., Hung C.-Y. Glitazones and alpha-glucosidase inhibitors as the second-line oral anti-diabetic agents added to metformin reduce cardiovascular risk in Type 2 diabetes patients: a nationwide cohort observational study. Cardiovasc Diabetol. 2018;17(1):20. doi: 10.1186/s12933-018-0663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wehmeier U.F., Piepersberg W. Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose. Appl Microbiol Biotechnol. 2004;63(6):613–625. doi: 10.1007/s00253-003-1477-2. [DOI] [PubMed] [Google Scholar]
- 49.Kishida Y., Okubo H., Ohno H., Oki K., Yoneda M. Effect of miglitol on the suppression of nonalcoholic steatohepatitis development and improvement of the gut environment in a rodent model. J Gastroenterol. 2017;52(11):1180–1191. doi: 10.1007/s00535-017-1331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunkhorst C., Andersen C., Schneider E. Acarbose, a pseudooligosaccharide, is transported but not metabolized by the maltose-maltodextrin system of Escherichia coli. J Bacteriol. 1999;181(8):2612–2619. doi: 10.1128/jb.181.8.2612-2619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X., Fang Z., Zhang C., Xia H., Jie Z., Han X. Effects of acarbose on the gut microbiota of prediabetic patients: A randomized, double-blind, controlled crossover trial. Diabetes Therapy. 2017;8(2):293–307. doi: 10.1007/s13300-017-0226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav H., Jain S., Sinha P.R. Effect of Dahi containing Lactococcus lactis on the progression of diabetes induced by a high-fructose diet in rats. Biosci Biotechnol Biochem. 2006;70(5):1255–1258. doi: 10.1271/bbb.70.1255. [DOI] [PubMed] [Google Scholar]
- 53.Yadav H., Jain S. Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition (Burbank, Los Angeles County, Calif) 2007;23(1):62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Yadav H., Jain S., Sinha P.R. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res. 2008;75(2):189–195. doi: 10.1017/S0022029908003129. [DOI] [PubMed] [Google Scholar]
- 55.Yadav H., Lee J.H., Lloyd J., Walter P., Rane S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288(35):25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su B., Liu H., Li J., Sunli Y., Liu B., Liu D. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7(5):729–739. doi: 10.1111/1753-0407.12232. [DOI] [PubMed] [Google Scholar]
- 57.Maruhama Y., Nagasaki A., Kanazawa Y., Hirakawa H., Goto Y., Nishiyama H. Effects of a glucoside-hydrolase inhibitor (Bay g 5421) on serum lipids, lipoproteins and bile acids, fecal fat and bacterial flora, and intestinal gas production in hyperlipidemic patients. Tohoku J Exp Med. 1980;132(4):453–462. doi: 10.1620/tjem.132.453. [DOI] [PubMed] [Google Scholar]
- 58.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 59.Buteau J., Foisy S., Joly E., Prentki M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52(1):124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 60.Farilla L., Hui H., Bertolotto C., Kang E., Bulotta A., Di Mario U. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143(11):4397–4408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- 61.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Wang L., Li P., Tang Z., Yan X., Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. 2016;6 doi: 10.1038/srep33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flatt P.R., Bailey C.J., Green B.D. Dipeptidyl peptidase IV (DPP IV) and related molecules in type 2 diabetes. Front Biosci. 2008;13:3648–3660. doi: 10.2741/2956. [DOI] [PubMed] [Google Scholar]
- 64.Deacon C.F., Holst J.J. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: comparison, efficacy and safety. Expert Opin Pharmacother. 2013;14(15):2047–2058. doi: 10.1517/14656566.2013.824966. [DOI] [PubMed] [Google Scholar]
- 65.Scheen A.J. Cardiovascular effects of gliptins. Nat Rev Cardiol. 2013;10(2):73. doi: 10.1038/nrcardio.2012.183. [DOI] [PubMed] [Google Scholar]
- 66.Olivares M., Neyrinck A.M., Potgens S.A., Beaumont M., Salazar N., Cani P.D. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia. 2018;61:1838–1848. doi: 10.1007/s00125-018-4647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panwar H., Santos D., Grant I., Grover S., Green B. 2015. Lactobacilli possess inhibitory activity against dipeptidyl peptidase-4 (DPP-4) [Google Scholar]
- 68.Yan X., Feng B., Li P., Tang Z., Wang L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: An animal study. J Diabetes Res. 2016 doi: 10.1155/2016/2093171. [2093171] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aquilante C.L. Sulfonylurea pharmacogenomics in Type 2 diabetes: The influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther. 2010;8(3):359–372. doi: 10.1586/erc.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aquilante C.L. Sulfonylurea pharmacogenomics in Type 2 diabetes: The influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther. 2010;8(3):359–372. doi: 10.1586/erc.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huo T., Xiong Z., Lu X., Cai S. Metabonomic study of biochemical changes in urinary of type 2 diabetes mellitus patients after the treatment of sulfonylurea antidiabetic drugs based on ultra-performance liquid chromatography/mass spectrometry. Biomed Chromatogr. 2015;29(1):115–122. doi: 10.1002/bmc.3247. [DOI] [PubMed] [Google Scholar]
- 73.Gu Y., Wang X., Li J., Zhang Y., Zhong H., Liu R. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8(1):1785. doi: 10.1038/s41467-017-01682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheng Y., Zheng S., Ma T., Zhang C., Ou X., He X. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci Rep. 2017;7(1):12041. doi: 10.1038/s41598-017-12245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahmoune H., Thompson P.W., Ward J.M., Smith C.D., Hong G., Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non–insulin-dependent diabetes. Diabetes. 2005;54(12):3427–3434. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 76.Rizzo M., Al-Busaidi N., Rizvi A.A. Dapagliflozin therapy in type-2 diabetes: Current knowledge and future perspectives. Taylor & Francis. 2005;16:281–284. doi: 10.1517/14656566.2015.981528. [DOI] [PubMed] [Google Scholar]
- 77.Lee D.M., Battson M.L., Jarrell D.K., Hou S., Ecton K.E., Weir T.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17(1):62. doi: 10.1186/s12933-018-0708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sedeek M., Gutsol A., Montezano A.C., Burger D., Nguyen Dinh Cat A., Kennedy C.R. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin Sci. 2013;124(3):191–202. doi: 10.1042/CS20120330. London, England: 1979. [DOI] [PubMed] [Google Scholar]
- 79.Lin B., Koibuchi N., Hasegawa Y., Sueta D., Toyama K., Uekawa K. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torgerson J.S., Hauptman J., Boldrin M.N., Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study. Diabetes Care. 2004;27(1):155. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 81.Zhi J., Melia A., Guerciolini R., Chung J., Kinberg J., Hauptman J. Retrospective population-based analysis of the dose-response (fecal fat excretion) relationship of orlistat in normal and obese volunteers. Clin Pharmacol Ther. 1994;56(1):82–85. doi: 10.1038/clpt.1994.104. [DOI] [PubMed] [Google Scholar]
- 82.Al-Suwailem A., Al-Tamimi A., Al-Omar M., Al-Suhibani M. Safety and mechanism of action of orlistat (tetrahydrolipstatin) as the first local antiobesity drug. J Appl Sci Res. 2006;2(4):205–208. [Google Scholar]
- 83.Morales P., Fujio S., Navarrete P., Ugalde J.A., Magne F., Carrasco-Pozo C. Impact of dietary lipids on colonic function and microbiota: An experimental approach involving orlistat-induced fat malabsorption in human volunteers. Clin Transl Gastroenterol. 2016;7:e161. doi: 10.1038/ctg.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoyles L. 2009. In vitro examination of the effect of orlistat on the ability of the faecal microbiota to utilize dietary lipids. [Google Scholar]
- 85.Hampp C., Borders-Hemphill V., Moeny D.G., Wysowski D.K. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care. 2014;37(5):1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 86.Reimer R.A., Grover G.J., Koetzner L., Gahler R.J., Lyon M.R., Wood S. Combining sitagliptin/metformin with a functional fiber delays diabetes progression in Zucker rats. J Endocrinol. 2014;220(3):361–373. doi: 10.1530/JOE-13-0484. [DOI] [PubMed] [Google Scholar]
- 87.Al-Hilal T.A., Alam F., Byun Y. Oral drug delivery systems using chemical conjugates or physical complexes. Adv Drug Deliv Rev. 2013;65(6):845–864. doi: 10.1016/j.addr.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Joh E.H., Kim D.H. A sensitive liquid chromatography-electrospray tandem mass spectrometric method for lancemaside A and its metabolites in plasma and a pharmacokinetic study in mice. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(21):1875–1880. doi: 10.1016/j.jchromb.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 89.Tralau T., Sowada J., Luch A. Insights on the human microbiome and its xenobiotic metabolism: what is known about its effects on human physiology? Expert Opin Drug Metab Toxicol. 2015;11(3):411–425. doi: 10.1517/17425255.2015.990437. [DOI] [PubMed] [Google Scholar]
- 90.Yoo D.H., Kim I.S., Van Le T.K., Jung I.H., Yoo H.H., Kim D.H. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014;42(9):1508–1513. doi: 10.1124/dmd.114.058354. [DOI] [PubMed] [Google Scholar]
- 91.DailyMed Acarbose. 2009. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6c2db888-775c-4baf-a1b4-1cfa63b83357 [updated 10/18/2017]. Available from.
- 92.Hemker M., Stratmann A., Goeke K., Schröder W., Lenz J., Piepersberg W. 2011. Identification, cloning, expression, and characterization of the extracellular acarbose-modifying glycosyltransferase, AcbD, from Actinoplanes sp. strain SE50; pp. 4484–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goke B., Fuder H., Wieckhorst G., Theiss U., Stridde E., Littke T. Voglibose (AO-128) is an efficient alpha-glucosidase inhibitor and mobilizes the endogenous GLP-1 reserve. Digestion. 1995;56(6):493–501. doi: 10.1159/000201282. [DOI] [PubMed] [Google Scholar]
- 94.Standl E., Schernthaner G., Rybka J., Hanefeld M., Raptis S.A., Naditch L. Improved glycaemic control with miglitol in inadequately-controlled type 2 diabetics. Diabetes Res Clin Pract. 2001;51(3):205–213. doi: 10.1016/s0168-8227(00)00231-x. [DOI] [PubMed] [Google Scholar]
- 95.Bank HSD. Orlistat 2007 [updated 4/25/2008]. Available from: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+96829-58-2.
- 96.Zhang Q., Wu Y., Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas) 2016;52(1):28–34. doi: 10.1016/j.medici.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 97.Dewulf E.M., Cani P.D., Claus S.P., Fuentes S., Puylaert P.G., Neyrinck A.M. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut. 2013;62(8):1112–1121. doi: 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Million M., Maraninchi M., Henry M., Armougom F., Richet H., Carrieri P. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obesity. 2012;36(6):817–825. doi: 10.1038/ijo.2011.153. (2005) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Bhute S.S., Suryavanshi M.V., Joshi S.M., Yajnik C.S., Shouche Y.S., Ghaskadbi S.S. Gut microbial diversity assessment of Indian type-2-diabetics reveals alterations in Eubacteria, Archaea, and Eukaryotes. Front Microbiol. 2017;8:214. doi: 10.3389/fmicb.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yadav H., Jain S., Nagpal R., Marotta F. Increased fecal viral content associated with obesity in mice. World J Diabetes. 2016;7(15):316–320. doi: 10.4239/wjd.v7.i15.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Y., You X., Mai G., Tokuyasu T., Liu C. A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome. 2018;6(1):24. doi: 10.1186/s40168-018-0410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Q., Wang C., Tang C., He Q., Li N., Li J. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn's disease. J Clin Gastroenterol. 2014;48(6):513–523. doi: 10.1097/MCG.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heisel T., Montassier E., Johnson A., Al-Ghalith G., Lin Y.W., Wei L.N. High-fat diet changes fungal microbiomes and interkingdom relationships in the murine gut. mSphere. 2017;2(5) doi: 10.1128/mSphere.00351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]