Abstract
Background
The Global Programme to Eliminate Lymphatic Filariasis recommends mass treatment of albendazole co‐administered with the microfilaricidal (antifilarial) drugs diethylcarbamazine (DEC) or ivermectin; and recommends albendazole alone in areas where loiasis is endemic.
Objectives
To assess the effects of albendazole alone, and the effects of adding albendazole to DEC or ivermectin, in people and communities with lymphatic filariasis.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials, MEDLINE (PubMed), Embase (OVID), LILACS (BIREME), and reference lists of included trials. We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform and ClinicalTrials.gov to identify ongoing trials. We performed all searches up to 15 January 2018.
Selection criteria
We included randomized controlled trials (RCTs) and cluster‐RCTs that compared albendazole to placebo or no placebo, or compared albendazole combined with a microfilaricidal drug to a microfilaricidal drug alone, given to people known to have lymphatic filariasis or communities where lymphatic filariasis was known to be endemic. We sought data on measures of transmission potential (microfilariae (mf) prevalence and density); markers of adult worm infection (antigenaemia prevalence and density, and adult worm prevalence detected by ultrasound); and data on clinical disease and adverse events.
Data collection and analysis
At least two review authors independently assessed the trials, evaluated the risks of bias, and extracted data. The main analysis examined albendazole overall, whether given alone or added to a microfilaricidal drug. We used data collected from all randomized individuals at time of longest follow‐up (up to 12 months) for meta‐analysis of outcomes. We evaluated mf density data up to six months and at 12 months follow‐up to ensure that we did not miss any subtle temporal effects. We conducted additional analyses for different follow‐up periods and whether trials reported on individuals known to be infected or both infected and uninfected. We analysed dichotomous data using the risk ratio (RR) with a 95% confidence interval (CI). We could not meta‐analyse data on parasite density outcomes and we summarized them in tables. Where data were missing, we contacted trial authors. We used GRADE to assess the certainty of evidence.
Main results
We included 13 trials (12 individually‐randomized and one small cluster‐randomized trial) with 8713 participants in total. No trials evaluated population‐level effects of albendazole in mass drug administration programmes. Seven trials enrolled people with a variety of inclusion criteria related to filarial infection, and six trials enrolled individuals from endemic areas. Outcomes were reported as end or change values. Mf and antigen density data were reported using the geometric mean, log mean and arithmetic mean, and reductions in density were variously calculated. Two trials discounted any increases in mf density in individuals at follow‐up by setting any density increase to zero.
For mf prevalence over two weeks to 12 months, albendazole alone or added to another microfilaricidal drug makes little or no difference (RR 0.95, 95% CI 0.85 to 1.07; 5027 participants, 12 trials, high‐certainty evidence). For mf density there is no trend, with some trials reporting a greater reduction in mf density with albendazole and others a greater reduction with the control group. For mf density up to six months and at 12 months, we do not know if albendazole has an effect (one to six months: 1216 participants, 10 trials, very low‐certainty evidence; at 12 months: 1052 participants, 9 trials, very low‐certainty evidence).
For antigenaemia prevalence between six to 12 months, albendazole alone or added to another microfilaricidal drug makes little or no difference (RR 1.04, 95% CI 0.97 to 1.12; 3774 participants, 7 trials, high‐certainty evidence). For antigen density over six to 12 months, the trend shows little or no effect of albendazole; but we do not know if albendazole has an effect on antigen density (1374 participants, 5 trials, very low‐certainty evidence). For adult worm prevalence detected by ultrasound at 12 months, albendazole added to a microfilaricidal drug may make little or no difference (RR 1.16, 95% CI 0.72 to 1.86; 165 participants, 3 trials, low‐certainty evidence).
For people reporting adverse events, albendazole makes little or no difference (RR 0.97, 95% CI 0.84 to 1.13; 2894 participants, 6 trials, high‐certainty evidence).
We also provide meta‐analyses and GRADE tables by drug, as operationally this may be of interest: for albendazole versus placebo (4 trials, 1870 participants); for albendazole with DEC compared to DEC alone (8 trials, 3405 participants); and albendazole with ivermectin compared to ivermectin alone (4 trials, 3438 participants).
Authors' conclusions
There is good evidence that albendazole makes little difference to clearing microfilaraemia or adult filarial worms in the 12 months post‐treatment. This finding is consistent in trials evaluating albendazole alone, or added to DEC or ivermectin. Trials reporting mf density included small numbers of participants, calculated density data variously, and gave inconsistent results.
The review raises questions over whether albendazole has any important contribution to the elimination of lymphatic filariasis. To inform policy for areas with loiasis where only albendazole can be used, it may be worth conducting placebo‐controlled trials of albendazole alone.
11 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (15 Jan, 2018) were included.
Plain language summary
Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis
In this Cochrane Review, Cochrane researchers examined the effects of using albendazole alone and albendazole added to antifilarial drugs to treat infected people and people who live in areas with lymphatic filariasis. After searching for relevant trials up to January 2018, we included 13 randomized controlled trials (RCTs), including one cluster‐RCT, with a total of 8713 participants.
Lymphatic filariasis
Lymphatic filariasis, a disease common in tropical and subtropical areas, is spread by mosquitoes and caused by infection with parasitic filarial worms. After a person is infected from a mosquito bite, the worms grow into adults and mate to produce microfilariae (mf). The mf circulate in the blood so they can be collected by mosquitoes, and the infection can be spread to another person. Infection can be diagnosed by checking for the presence of circulating mf (microfilaraemia) or parasite antigens (antigenaemia), or by ultrasound imaging to detect live adult worms.
The World Health Organization (WHO) recommends mass treatment of entire populations once a year for many years. Treatment is a two‐drug combination of albendazole and a microfilaricidal (antifilarial) drug, either diethycarbamazine (DEC) or ivermectin. Albendazole alone is recommended for people when DEC or ivermectin can not be used.
What the research says
Albendazole alone or added to a microfilaricidal drug makes little or no difference to mf prevalence over two weeks to 12 months after treatment (high‐certainty evidence), but we do not know if albendazole alone or in combination reduces mf density between one to six months (very low‐certainty evidence) or at 12 months (very low‐certainty evidence).
Treatment with albendazole alone or added to a microfilaricidal drug makes little or no difference to antigenaemia prevalence between six to 12 months (high‐certainty evidence). We do not know if albendazole alone or in combination reduces antigen density over six to 12 months (very low‐certainty evidence). Albendazole added to a microfilaricidal drug may make little or no difference to adult worm prevalence detected by ultrasound at 12 months (low‐certainty evidence).
When given alone or added to a microfilaricidal drug, albendazole makes little or no difference to the number of people reporting an adverse event (high‐certainty evidence).
Authors' conclusions
There is good evidence that albendazole, alone or added to DEC or ivermectin, delivers little or no benefit for totally clearing the mf or the adult worms up to 12 months after treatment. Evidence for an effect of albendazole in reducing the numbers of mf and adult worms is inconsistent. To inform policy for areas where ivermectin and DEC can not be given, further research could help determine whether there is any effect of albendazole alone.
Summary of findings
Summary of findings for the main comparison. Albendazole alone or added to a microfilaricidal drug for lymphatic filariasis.
| Albendazole alone or added to a microfilaricidal drug for lymphatic filariasis | ||||||
|
Patient or population: people with lymphatic filariasis or communities where lymphatic filariasis is endemic
Setting: Brazil, Ghana, Haiti, India, Papua New Guinea, Tanzania, and Zanzibar Intervention: albendazole alone or in combination with a microfilaricidal drug Comparison: placebo or a single microfilaricidal drug | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment or a single microfilaricidal drug | Risk with albendazole alone or in combination with a microfilaricidal drug | |||||
| Microfilaraemia (mf) prevalence follow‐up: range 2 weeks to 12 months | 179 per 1000 | 174 per 1000 (154 to 196) | RR 0.95 (0.85 to 1.07) | 5027 (12 RCTs) | ⊕⊕⊕⊕ HIGH | Albendazole makes little or no difference to mf prevalence. |
| Mf density follow‐up: range 1 month to 6 months | In the included studies the effects of treatment with albendazole varied. The difference between treatment groups ranged from a 81.7% greater reduction with albendazole to 13.6% greater reduction with a single microfilaricidal drug.a | ‐ | 1216 (10 RCTs) | ⊕⊝⊝⊝
VERY LOWb,c,d Due to risk of bias, inconsistency, and imprecision |
We do not know if albendazole has an effect on mf density. | |
| Mf density follow‐up: 12 months | In the included studies the effects of treatment with albendazole varied. The difference between treatment groups ranged from a 55.5% greater reduction with albendazole to a 15.8% greater reduction with a single microfilaricidal drug.e | ‐ | 1052 (9 RCTs) | ⊕⊝⊝⊝
VERY LOWc,f Due to inconsistency and imprecision |
We do not know if albendazole has an effect on mf density. | |
| Antigenaemia prevalence follow‐up: range 6 months to 12 months | 435 per 1000 | 452 per 1000 (422 to 487) | RR 1.04 (0.97 to 1.12) | 3774 (7 RCTs) | ⊕⊕⊕⊕ HIGHg | Albendazole makes little or no difference to antigenaemia prevalence. |
| Antigen density follow‐up: range 6 months to 12 months | In the included studies treatment with albendazole had little or no effect on antigen density. There was a 1.5% to 17.1% greater reduction with albendazole in all studies except one; this study reported a 64.4% greater reduction in antigen density due to a small reduction with albendazole (16.9%) but a large increase in the placebo group.h | ‐ | 1374 (5 RCTs) | ⊕⊝⊝⊝
VERY LOWi,j,k Due to risk of bias and imprecision |
We do not know if albendazole has an effect on antigen density. | |
| Adult worm prevalence detected by ultrasound follow‐up: 12 months | 268 per 1000 | 311 per 1000 (193 to 499) | RR 1.16 (0.72 to 1.86) | 165 (3 RCTs) | ⊕⊕⊝⊝
LOWl,m,n Due to indirectness and imprecision |
Albendazole may make little or no difference to adult worm prevalence detected by ultrasound. |
| Adverse events | 184 per 1000 | 178 per 1000 (155 to 208) | RR 0.97 (0.84 to 1.13) | 2894 (6 RCTs) | ⊕⊕⊕⊕ HIGHo | Albendazole makes little or no difference to adverse events. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aEight studies reported geometric means, one study reported log mean, and one study reported the arithmetic mean. An effect of albendazole (P < 0.05) on the geometric mean mf density was reported in three analyses in two studies. No effect of albendazole (P > 0.05) was reported in six studies that used the geometric mean. bDowngraded by one for risk of bias: we judged the analytical methods used by Beach 1999 and Fox 2005 to obtain the change in density from baseline to follow‐up to be at high risk of bias. cDowngraded by one for inconsistency: the direction and magnitude of effect reported varied in favour of both albendazole and a microfilaricidal drug alone. We judged the effects of albendazole to be inconsistent. dDowngraded by two for imprecision: the optimal information size was met. There was considerable variation in the effects of albendazole, ranging from a statistically significant effect of albendazole (P < 0.05) to little no effect. Authors reported mf density using geometric means, log means, and arithmetic means. We judged that the range of values that the effect estimate might take would likely include a meaningful effect and no effect. eSix studies reported geometric means, one study reported the log mean, and two studies reported the arithmetic mean. Five studies that assessed the geometric mean reported no effect was detected in six analyses (P > 0.05). fDowngraded by two for imprecision: the optimal information size was met. There was considerable variation in the effects of albendazole; ranging from estimates with apparently large but underpowered effects (P > 0.05) to estimates with little or no effect. Authors reported mf density using geometric means, log means, and arithmetic means. Given the differences in these measures, we are unable to judge the precision of the estimate of effect across the studies. gNot downgraded for risk of bias: most information is from studies at low or unclear risk of bias. Four studies had high risk of bias for attrition, but participant numbers at follow‐up were generally comparable between groups. We judge plausible bias unlikely to seriously alter the results. hThree studies reported geometric means, one study reported the log mean, and one study reported the arithmetic mean. Five analyses in three studies reported no effect of albendazole (P > 0.05). iDowngraded by one for risk of bias: we judged the analytical methods used by Fox 2005 to obtain the change in density from baseline to follow‐up to be at high risk of bias. jNot downgraded for inconsistency: little to no benefit of albendazole was seen consistently across the studies. We judged the direction and the magnitude of effect to be consistent across studies. kDowngraded by two for imprecision: the optimal information size was met. Little to no effect of albendazole was consistently reported across the studies. All studies that reported a test for differences reported no statistically significant effect on geometric mean antigen density (P > 0.05). We judged that the range of values would probably include little or no effect and exclude appreciable benefit or harm, but with no effect estimate or measure of precision we judged this to be seriously imprecise. lNot downgraded for risk of bias: all studies had unclear risk of bias for random sequence generation. The study contributing the most (68.7%) to the effect estimate had high risk of bias for attrition, but the number of participants followed up was comparable between groups. We judged plausible bias unlikely to seriously alter the results. mDowngraded by one for indirectness: this outcome was assessed only in men and boys (three studies). Two studies included adult men only, and one very small study included adults and children. We judged the evidence for this outcome to have serious indirectness due to the lack of applicability to the wider population of interest. nDowngraded by one for imprecision: there were insufficient events to meet optimal information size. The 95% CI around the pooled estimate of effect includes both no effect and appreciable benefit and harm, using a 25% relative risk reduction (RRR). oNot downgraded for risk of bias: for participant and personnel blinding, two studies had unclear risk of bias and one study was at high risk of bias. A large safety study contributing the most to the overall effect estimate (52.6%) had low risk of bias for blinding. We judged plausible bias unlikely to seriously alter the results.
Summary of findings 2. Albendazole alone for lymphatic filariasis.
| Albendazole alone for lymphatic filariasis | ||||||
| Patient or population: people with lymphatic filariasis or communities where lymphatic filariasis is endemic Setting: Ghana, Haiti and India Intervention: albendazole Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with albendazole | |||||
| Microfilaraemia (mf) prevalence follow‐up: range 4 months to 12 months | 207 per 1000 | 203 per 1000 (168 to 246) | RR 0.98 (0.81 to 1.19) | 1406 (4 RCTs) | ⊕⊕⊕⊕ HIGHa,b | Albendazole makes little or no difference to mf prevalence. |
| Mf density follow‐up: range 4 months to 6 months | Trend favoured albendazole to a variable extent. Albendazole reduced the geometric mean mf density by 28.7% to 61.1%. Placebo reduced the geometric mean mf density up to 17.2%, but the density also increased by 20.6%.c | ‐ | 285 (4 RCTs) | ⊕⊝⊝⊝
VERY LOWd,e,f Due to risk of bias, inconsistency, and imprecision |
We do not know if albendazole has an effect on mf density. | |
| Mf density follow‐up: 12 months | No trend. In one study that reported the geometric mean, albendazole reduced mf density by 68.5% and in the placebo group the reduction was 13%; however, the authors reported no significant difference with albendazole (P > 0.05).g | ‐ | 169 (2 RCTs) | ⊕⊝⊝⊝
VERY LOWh,i Due to inconsistency and imprecision |
We do not know if albendazole has an effect on mf density. | |
| Antigenaemia prevalence follow‐up: range 6 months to 12 months | 355 per 1000 | 380 per 1000 (323 to 444) | RR 1.07 (0.91 to 1.25) | 1054 (2 RCTs) | ⊕⊕⊕⊕ HIGH | Albendazole makes little or no difference to antigenaemia prevalence. |
| Antigen density follow‐up: range 6 months to 12 months | Trend showed little or no effect of albendazole. Albendazole reduced the geometric mean antigen density by 3.2% to 16.9%, and the placebo group antigen density was reduced by 1.7% and also increased by 47.5%.j | ‐ | 371 (2 RCTs) | ⊕⊝⊝⊝
VERY LOWk,l,m Due to risk of bias and imprecision |
We do not know if albendazole has an effect on antigen density. | |
| Adult worm prevalence detected by ultrasound ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Adult worm prevalence detected by ultrasound was not measured for this comparison. |
| Adverse events | 106 per 1000 | 101 per 1000 (65 to 157) | RR 0.95 (0.61 to 1.48) | 678 (2 RCTs) | ⊕⊕⊕⊝
MODERATEn,o Due to imprecision |
Albendazole probably makes little or no difference to adverse events. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
aNot downgraded for risk of bias: most information is from studies at low or unclear risk of bias. Three studies had unclear or high risk of bias for attrition, but numbers of participants followed up were comparable between groups in each study. We judged plausible bias unlikely to seriously alter the results. bNot downgraded for imprecision: borderline sufficient events to meet optimal information size (289 total events), and the 95% CI around the pooled estimate of effect includes little or no effect and excludes clinically appreciable benefit and harm. We used a relative risk reduction (RRR) of 25% as a cut‐off for imprecision. cOf the three studies that reported the geometric mean; one study reported an effect of albendazole (P < 0.05), one study reported no effect (P > 0.05), and one study did not statistically test this. One study reporting the arithmetic mean suggested a large benefit with albendazole, but we judged this to be an inappropriate measure for skewed data. dDowngraded by one for risk of bias: we judged the analytical methods used by Beach 1999 and Fox 2005 to obtain the change in density from baseline to follow‐up to be at high risk of bias. eDowngraded by one for inconsistency: the benefit of albendazole and the magnitude of effect was inconsistent. fDowngraded by two for imprecision: the optimal information size was not met. There was considerable variation in the effects of albendazole on geometric mean mf density; ranging from an effect in one study (P < 0.05), an apparently large effect in one study that was not statistically evaluated, and no effect in one study (P > 0.05). One study reported the arithmetic mean. We judged that the range of values could include a meaningful effect and no effect. gOne study reported the arithmetic mean and showed a large benefit with albendazole, but we judged it to be an inappropriate measure for skewed data. hDowngraded by one for inconsistency: two studies reported a greater reduction in mf density with albendazole, but the magnitude of effect was unclear. One study reported the geometric mean and reported no effect of albendazole (P >0.05), and one study reported the arithmetic mean and did not test for differences. iDowngraded by two for imprecision: the optimal information size was not met. One study reported the geometric mean mf density and an apparently large but underpowered effect (P > 0.05). One study suggested a large reduction in the arithmetic mean with albendazole and did not statistically evaluate the effect. We judged that the range of values could include a meaningful effect and no effect. jBoth studies reported that there was no effect using albendazole (P > 0.05). kDowngraded by one for risk of bias: we judged the analytical methods used by Fox 2005 to obtain the change in density from baseline to follow‐up to be at high risk of bias. lNot downgraded for inconsistency: we found little to no effect of albendazole consistently across the studies. We judged the direction and the magnitude of effect to be consistent across studies. mDowngraded by two for imprecision: the optimal information size was not met. Two studies reported geometric mean antigen density and no benefit of using albendazole (P > 0.05). We judged that the range of values would likely include little or no effect and exclude appreciable benefit or harm, but we can not be certain with no effect estimate or measure of precision. nNot downgraded for indirectness: albendazole regimens differed, one study provided single dose 400 mg albendazole and one study provided daily dose 400 mg albendazole for seven days. However, we judge this does not have serious indirectness. oDowngraded by one for imprecision: insufficient events to meet optimal information size. The 95% CI around the pooled estimate of effect includes both no effect and appreciable benefit and harm, using a relative risk reduction (RRR) of 25%.
Summary of findings 3. Albendazole added to DEC for lymphatic filariasis.
| Albendazole added to DEC for lymphatic filariasis | ||||||
| Patient or population: people with lymphatic filariasis or communities where lymphatic filariasis is endemic Setting: Brazil, Haiti, India and Papua New Guinea Intervention: albendazole plus DEC Comparison: DEC | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DEC | Risk with albendazole plus DEC | |||||
| Microfilaraemia (mf) prevalence follow‐up: range 6 months to 12 months | 262 per 1000 | 236 per 1000 (197 to 286) | RR 0.90 (0.75 to 1.09) | 1102 (7 RCTs) | ⊕⊕⊕⊝
MODERATEa,b Due to imprecision |
Albendazole probably makes little or no difference to mf prevalence. |
| Mf density follow‐up: range 1 months to 6 months | No trend. The difference between the albendazole plus DEC and the DEC groups percentage reductions from baseline ranged from a 30% greater reduction with albendazole plus DEC to a 13.6% greater reduction with DEC alone.c | ‐ | 559 (6 RCTs) | ⊕⊝⊝⊝
VERY LOWd,e,f Due to risk of bias, inconsistency, and imprecision |
We do not know if albendazole has an effect on mf density. | |
| Mf density follow‐up: 12 months | Trend showed little or no effect of albendazole. The difference between the albendazole plus DEC and the DEC groups percentage reductions from baseline ranged from a 5.6% greater reduction with albendazole plus DEC to a 15.8% greater reduction with DEC alone.g | ‐ | 535 (6 RCTs) | ⊕⊕⊝⊝
LOWh,i Due to imprecision |
Albendazole may make little or no difference to mf density. | |
| Antigenaemia prevalence follow‐up: range 6 months to 12 months | 503 per 1000 | 518 per 1000 (463 to 574) | RR 1.03 (0.92 to 1.14) | 954 (5 RCTs) | ⊕⊕⊕⊕ HIGHj | Albendazole makes little or no difference to antigenaemia prevalence. |
| Antigen density follow up: range 6 months to 12 months | Trend showed little or no effect of albendazole. The difference between the albendazole plus DEC and the DEC groups percentage reductions from baseline ranged from a 9.7% greater reduction in the geometric mean to a 10.7% greater reduction in the log mean with albendazole plus DEC.k | ‐ | 270 (3 RCTs) | ⊕⊝⊝⊝
VERY LOWh,l,m Due to risk of bias and imprecision |
We do not know if albendazole has an effect on antigen density. | |
| Adult worm prevalence detected by ultrasound follow up: 12 months | 268 per 1000 | 311 per 1000 (193 to 499) | RR 1.16 (0.72 to 1.86) | 165 (3 RCTs) | ⊕⊕⊝⊝
LOWn,o,p Due to indirectness and imprecision |
Albendazole may make little or no difference to adult worm prevalence detected by ultrasound. |
| Adverse events | 240 per 1000 | 225 per 1000 (189 to 266) | RR 0.94 (0.79 to 1.11) | 1589 (4 RCTs) | ⊕⊕⊕⊕ HIGHq | Albendazole makes little or no difference to adverse events. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aNot downgraded for inconsistency: I2 of 40% was explained through subgroup analysis. The heterogeneity was a result of one study which used a more intensive treatment regimen (daily dose for 12 days) compared to the other six studies (single dose). We therefore judged inconsistency does not seem to be a serious issue. bDowngraded by one for imprecision: insufficient events to meet optimal information size (276 total events). Using a relative risk reduction (RRR) of 25% as a cut‐off for imprecision, the 95% CI around the pooled estimate of effect includes no effect and no clinically appreciable harm, but the upper boundary of the CI represents a 25% RRR. We therefore judge that the 95% CI around the pooled estimate of effect could include clinically appreciable benefit if the optimal information size had been met. cOne study reported an effect of adding albendazole to DEC (P < 0.05) and four studies reported no effect (P > 0.05). Five studies reported geometric means and one study reported the log mean. dDowngraded by one for risk of bias: we judged the analytical methods used by Fox 2005 to obtain the change in density from baseline to follow‐up to be at high risk of bias. eDowngraded by one for inconsistency: the direction and magnitude of effect reported varied in favour of both albendazole plus DEC and DEC alone. We judged the effects of adding albendazole to DEC to be inconsistent. fDowngraded by two for imprecision: the optimal information size was met. The effect of adding albendazole to DEC varied considerably. One trial reported an effect of adding albendazole (P < 0.05) and no effect was reported in the others (P > 0.05). We judged that the range of values would likely include a meaningful effect and no effect. gFour studies reporting the geometric mean reported no effect of adding albendazole to DEC (P > 0.05). One study reported the log mean and one study reported the arithmetic mean, no effect was seen. hNot downgraded for inconsistency: the direction and magnitude of the effect was consistent; we found no benefit of adding albendazole to DEC consistently across the studies. iDowngraded by two for imprecision: the optimal information size was met. No effect of adding albendazole to DEC was consistently reported across the studies; all studies reported no effect on geometric mean mf density (P > 0.05). We judged that the range of values would likely include little or no effect and exclude appreciable benefit or harm, but we can not be certain as there is no estimate of effect or measure of precision. jNot downgraded for risk of bias: most information was at low or unclear risk of bias. Three studies had high risk of bias for attrition, but the number of participants followed up was comparable between groups in the studies. We judged plausible bias unlikely to alter the results. kOne study reported the geometric mean, one study reported the log mean and one study reported the arithmetic mean; two studies reported no effect of adding albendazole to DEC (P > 0.05). lDowngraded by one for risk of bias: we judged the analytical methods used by Fox 2005 to obtain the change in density from baseline to follow‐up to be at high risk of bias. mDowngraded by two for imprecision: the optimal information size was not met. Two studies reported no effect of albendazole added to DEC (P > 0.05). One study reported geometric mean, one study reported log mean and one study reported arithmetic mean. Given the differences in these measures and small number of participants, we are unable to judge the precision of the estimate of effect across the studies. nNot downgraded for risk of bias: all studies had unclear risk of bias for random sequence generation. The study contributing the most (68.7%) to the effect estimate had high risk of bias for attrition, but the number of participants followed up was comparable between groups. We judged plausible bias unlikely to seriously alter the results. oDowngraded by one for indirectness: this outcome was assessed only in men and boys (three studies). Two studies included adult men only, and one study included adults and children. We judged the evidence for this outcome to have serious indirectness due to the lack of applicability to the wider population of interest. pDowngraded by one for imprecision: there were insufficient events to meet optimal information size (47 total events). The 95% CI around the pooled estimate of effect includes both no effect and appreciable benefit and harm, using a relative risk reduction (RRR) of 25%. qNot downgraded for risk of bias: for participant and personnel blinding, one study had unclear risk of bias and one study was at high risk of bias; however, a large safety study contributing the most to the overall effect estimate (73.1%) was at low risk of bias. We judged plausible bias unlikely to seriously alter the results.
Summary of findings 4. Albendazole added to ivermectin for lymphatic filariasis.
| Albendazole added to ivermectin for lymphatic filariasis | ||||||
| Patient or population: people with lymphatic filariasis or communities where lymphatic filariasis is endemic Setting: Ghana, Haiti, Tanzania and Zanzibar Intervention: albendazole plus ivermectin Comparison: ivermectin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ivermectin | Risk with albendazole plus ivermectin | |||||
| Microfilaraemia (mf) prevalence follow‐up: range 2 weeks to 12 months | 129 per 1000 | 108 per 1000 (70 to 169) | RR 0.84 (0.54 to 1.31) | 2519 (4 RCTs) | ⊕⊕⊕⊝
MODERATEa,b,c Due to imprecision |
Albendazole probably makes little or no difference to mf prevalence. |
| Mf density follow‐up: range 4 months to 6 months | No trend. The difference between the albendazole plus ivermectin and the ivermectin groups percentage reductions from baseline ranged from a 3% to 22.8% greater reduction with albendazole plus ivermectin.d | ‐ | 372 (3 RCTs) | ⊕⊝⊝⊝
VERY LOWe,f,g Due to risk of bias, inconsistency, and imprecision |
We do not know if albendazole has an effect on mf density. | |
| Mf density follow‐up: 12 months | Trend showed little or no effect of albendazole. The difference between the albendazole plus ivermectin and the ivermectin groups percentage reductions from baseline ranged from a 6.7% to 9.1% greater reduction with albendazole plus ivermectin.h | ‐ | 348 (2 RCTs) | ⊕⊕⊝⊝
LOWi,j,k Due to imprecision |
Albendazole may make little or no difference to mf density. | |
| Antigenaemia prevalence follow up: 12 months | 444 per 1000 | 462 per 1000 (418 to 516) | RR 1.04 (0.94 to 1.16) | 1766 (2 RCTs) | ⊕⊕⊕⊕ HIGHi | Albendazole makes little or no difference to antigenaemia prevalence. |
| Antigen density follow‐up: 12 months | Trend showed little or no effect of albendazole. The difference between the albendazole plus ivermectin and the ivermectin groups percentage reductions from baseline ranged from a 10.9% to 17.1% greater reduction with albendazole plus ivermectin.h | ‐ | 733 (2 RCTs) | ⊕⊕⊝⊝
LOWi,j,l Due to imprecision |
Albendazole may make little or no difference to antigen density. | |
| Adult worm prevalence detected by ultrasound ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Adult prevalence detected by ultrasound was not measured for this comparison. |
| Adverse events | 122 per 1000 | 142 per 1000 (94 to 212) | RR 1.16 (0.77 to 1.74) | 627 (1 RCT) | ⊕⊕⊕⊝
MODERATEm,n Due to imprecision |
Albendazole probably makes little or no difference to adverse events. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
aNot downgraded for risk of bias: most information is from studies at low or unclear risk of bias. Two studies had high risk and one had unclear risk of bias for attrition, but the number of participants followed up were comparable between groups in most of the studies. We judged plausible bias unlikely to seriously alter the results. bNot downgraded for inconsistency: although we found heterogeneity between studies (I2 = 65%), a subgroup analysis for length of follow‐up showed no statistical variability when two studies with earlier follow‐up time points (two weeks and four months) and two studies with later follow‐up time points (12 months) were analysed as subgroups. Overall, we judged that the effect estimate is not inconsistent. cDowngraded by one for imprecision: the optimal information size was met. The 95% CI around the pooled estimate of effect includes both no effect and appreciable benefit and harm, using a relative risk reduction (RRR) of 25%. dOne small study reported an effect of adding albendazole to ivermectin (P < 0.05), one study reported no effect (P > 0.05), and one study did not clearly report the outcome of the statistical analyses. eDowngraded by one for risk of bias: we judged the analytical methods used by Beach 1999 to obtain the change in density from baseline to follow‐up to be at high risk of bias. fDowngraded by one for inconsistency: the magnitude of the effect of adding albendazole to ivermectin varied and we judged it to be inconsistent. gDowngraded by two for imprecision: the optimal information size was not met. The effect of adding albendazole to ivermectin showed considerable variability; ranging from an effect in one study (P < 0.05) and little or no effect (P > 0.05) in another. We judged that the range of values could include a meaningful effect and no effect. hOne study reported no effect of adding albendazole to ivermectin (P > 0.05), and one study did not clearly report the outcome of the statistical analyses. iNot downgraded for risk of bias: most information is from studies at low or unclear risk of bias. Two studies had high risk or unclear risk of bias for attrition, but losses between groups were generally comparable in the studies. We judged plausible bias unlikely to seriously alter the results. jNot downgraded for inconsistency: we judged the direction and magnitude of effect to be consistent across studies. kDowngraded by two for imprecision: the optimal information size was not met. Two studies reported little or no effect with albendazole; statistically evaluated in one study (P > 0.05). We judged that the range of values would likely include little or no effect and exclude appreciable benefit or harm, but we can not be certain as there is no estimate of effect or measure of precision. lDowngraded by two for imprecision: the optimal information size was met. Two studies reported little or no effect of albendazole; statistically evaluated in one study (P > 0.05). We judged that the range of values would likely include little or no effect and exclude appreciable benefit or harm, but we can not be certain as there is no estimate of effect or measure of precision. mNot downgraded for risk of bias: most information was at low and unclear risk of bias. The study had unclear risk of bias for participant and personnel blinding and unclear risk of bias for attrition. However, for this outcome 90% of individuals were followed up. We judged plausible bias unlikely to seriously alter the results. nDowngraded by one for imprecision: insufficient events to meet optimal information size (83 total events). The 95% CI around the pooled estimate of effect includes both no effect and appreciable harm, using a 25% relative risk reduction (RRR).
Background
Epidemiology
Lymphatic filariasis is a parasitic infection of threadlike filarial worms and is endemic in 72 countries. Globally, 856 million people in 52 countries require preventive chemotherapy to stop the spread of infection (WHO 2018a). Bancroftian filariasis, caused by Wuchereria bancrofti, is responsible for over 90% of infections, and occurs in tropical regions of Asia, Africa, the Pacific islands, and in parts of the Caribbean and South America (WHO 2016). Brugian filariasis is less common, with Brugia malayi occurring in parts of Asia, and Brugia timori in Indonesia (Taylor 2010). The implications of lymphatic filariasis for individuals and societies are manifold. Clinical severity and progression of the disease can lead to chronic health complications and disability, which may be accompanied by mental health issues and social stigma, while the resultant reduced productivity causes nearly USD 1.3 billion per year in economic losses (Conteh 2010).
Filariasis is transmitted by female mosquitoes from several genera, including Culex,Anopheles,Mansonia, and Aedes (Bockarie 2009). The mosquito vectors become infected when they take blood meals from people with early stage larvae, which are termed microfilariae (mf). The larvae develop for about 12 to 15 days in the mosquito to a third‐stage infective larvae (L3 larvae) (Scott 2000). When the mosquito takes a subsequent blood meal, the larvae enter the skin, migrate to the lymph vessels, and develop into adult worms (macrofilariae) in the lymph nodes, where male and female worms pair. Female worms then produce mf, which migrate to the blood causing microfilaraemia. The time between being infected and adult worms producing microfilaraemia is estimated to be about 12 months (Mahoney 1971).
Microfilariae move in and out of circulating peripheral blood according to a daily cycle. In most species, levels peak during the night, between 10 pm and 4 am (Simonsen 1997), a time when mosquito vectors are actively feeding. In the diurnal subperiodic strain of W bancrofti, found only in the South Pacific region, mf are continuously circulating but peak during the day (Bockarie 2009).
Diagnosis and clinical features
Historically, filarial infection has been diagnosed by examination of a blood smear for mf using microscopy. However, even if blood is taken at night when mf are in the peripheral blood, not all infections are detected because mf levels are very low in many people. Adult worms may also be present but not yet producing mf, or there may be only a single unmated worm in a lymph node. Antigen‐detection assays for W bancrofti circulating filarial antigen (CFA) became available for field use during the 1990s. The assays can be used for sensitive diagnosis of infection at any time of day (Weil 1997), as they indicate the presence of the adult worm and do not depend on the temporal presence of mf. A point‐of‐care rapid diagnostic test for bancroftian filariasis, the Filariasis Test Strip (FTS), is used by the Global Programme to Eliminate Lymphatic Filariasis (GPELF) to detect the presence of filarial antigens (WHO 2015). Parasite antigen levels can be measured using the Og4C3 Filariasis Ag ELISA, and the circulating antigen density is thought to be correlated with the numbers of adult W bancrofti worms (Harnett 1990; Weil 1990). Ultrasound imaging can demonstrate the presence of live adult worms (Dreyer 1995).
Many people with filariasis are asymptomatic, even when there are high parasite densities. However, even people without clinical symptoms often have lymphatic changes, including lymphangiectasia (widening of the lymphatic vessels) and thickening of the spermatic cord (Addiss 2000; Dreyer 2000), which can be detected using ultrasound.
People can experience acute inflammatory episodes, including acute filarial lymphangitis (AFL), believed to be triggered by the death of the adult worm, and acute dermatolymphangioadenitis (ADLA), linked with secondary bacterial infection (Dreyer 1999). An AFL episode presents with lymphangitis that spreads distally or in a ‘retrograde' manner along the lymphatic vessel, creating a palpable ‘cord' (Addiss 2007). ADLA episodes reportedly may last up to 16 days and cause malaise, fever, chills, pain, and swelling, with episodes typically recurring several times a year (Addiss 2007). Symptoms of ADLA are more severe and occur much more frequently compared to AFL (Dreyer 1999). Recurrent ADLA attacks are a major factor in the progression to chronic lymphoedema. Clinical symptoms and signs of chronic conditions include hydrocoele (excess fluid inside the scrotal sac), lymphoedema (swelling and enlargement of affected areas of the body), and elephantiasis (long‐standing enlargement and swelling of the limbs, scrota, or breasts associated with skin thickening).
How the filarial worm causes disease is not well understood. The following have been proposed: adult worms living in and damaging lymph vessels; immunological reactions to the presence and death of filarial worms; secondary infections of affected areas, which contribute significantly to both acute and chronic disease manifestations; and host genetics (Dreyer 2000; Cuenco 2009). A major contributor to inflammation is the release of lipoproteins from the bacterial endosymbiont Wolbachia, which is found within the cells of filarial worms (Taylor 2001; Turner 2009). Some or all of these processes may be important in pathogenesis and immunopathogenesis (Babu 2012).
Control and elimination
The main strategy used by the GPELF consists of community‐wide mass drug administration (MDA) to entire populations at risk in order to interrupt transmission of the disease and prevent morbidity due to infection. Preventive chemotherapy is considered necessary where the total population in an implementation unit (province, district, or smaller unit) of a given country has an infection prevalence of 1% or higher. Preventive chemotherapy aims to interrupt transmission by sustainably reducing community microfilaraemia below a critical threshold or by completely clearing the mf (Ottesen 2006).
The GPELF recommends yearly, single‐dose, two‐drug regimens (albendazole plus diethylcarbamazine (DEC) or albendazole plus ivermectin) for at least five years (corresponding to the reproductive lifespan of the adult worm), with coverage of at least 65% of the total at‐risk population to prevent transmission. More recently, for special settings the WHO has recommended the use of annual treatment with the triple‐drug therapy of ivermectin, DEC, and albendazole (termed IDA) rather than two‐drug therapy of albendazole and DEC (WHO 2017a). Overall mf prevalence rates are believed to be relatively stable over time in endemic communities in the absence of treatment because of reinfection and new adult worms producing mf (Meyrowitsch 1995).
The transmission assessment survey (TAS) is used to determine when infection prevalence (estimated from the number of CFA‐positive or antibody‐positive cases in children) is below critical cut‐off thresholds and MDA can stop, and also as a surveillance tool in order to validate elimination (WHO 2011). Palau, Vietnam, Wallis and Futuna, the Republic of the Marshall Islands, and Tonga eliminated lymphatic filariasis as a public health problem in 2018 and 2017 (WHO 2017b; WHO 2017c; WHO 2018b), along with Togo, the first country in sub‐Saharan Africa (WHO 2017d), and Egypt, the first country in the Eastern Mediterranean region (WHO 2018c). They join six countries validated as having achieved elimination in 2016 (WHO 2016), and China and the Republic of Korea in 2007 and 2008, respectively.
Transmission dynamics may show variable efficiency depending on the vector species in the locality; in processes referred to as limitation, facilitation, and proportionality (WHO 2013; Graves 2016). Higher treatment coverage for longer periods or other strategies such as vector control may be required in areas where vectors are responsible for a high proportion of transmission (Burkot 2002; Pichon 2002). Vector control for lymphatic filariasis can enhance the impact on transmission during and after MDA (WHO 2013), and elimination has also been achieved in some areas such as the Solomon Islands and Australia using vector control methods (Burkot 2002; Pichon 2002).
In addition to ‘microfilaricidal’ drugs DEC and ivermectin, ‘macrofilaricidal’ drugs that kill the adult worms have also been shown to be effective. Antibiotics, such as doxycycline, target the Wolbachia obligate endosymbiont in the parasite, leading to long‐term sterility and a gradual, sustained killing of adult worms (Taylor 2005; Debrah 2007). Doxycycline is not currently used in community‐based treatment programmes due to the logistics of longer treatment regimens and contraindications in pregnant women and children.
DEC and ivermectin
Both ivermectin and DEC rapidly clear mf from the blood and suppress their reappearance (Stolk 2005; Geary 2010). Reductions of 90% from pre‐treatment mf levels have been seen after a single dose of DEC or ivermectin, even one year after treatment (Ottesen 1999). Microfilaraemia can therefore be effectively reduced by DEC or ivermectin (Taylor 2010). However, the limited effects on adult worm viability cause new mf infections to replace those whose microfilaraemia subsides (Vanamail 1990; Weil 1999).
DEC has been in use for filariasis for more than 50 years. In the early years of control the recommended regimen for DEC was 6 mg/kg daily for 12 days (WHO 1984). Later, clinical and community trials determined that single doses given at various intervals − weekly, monthly, twice a year, and annually − were equally effective (Eberhard 1989; Mataika 1993; Andrade 1995; Simonsen 1995). There is reasonable evidence from ultrasound and clinical observations that DEC kills some adult worms after single doses (Figueredo‐Silva 1996; Norões 1997; Addiss 2000).
Ivermectin is used for the treatment and community control of onchocerciasis (caused by another filarial worm, Onchocerca volvulus). It has also been effective in community control programmes for lymphatic filariasis (Cartel 1990; Coutinho 1994; Cao 1997). Ivermectin is used in areas where both onchocerciasis and lymphatic filariasis coexist, as DEC can result in eye damage if given to individuals with onchocerciasis. Ivermectin is not known to have any macrofilaricidal activity, and ultrasound studies have shown that adult worms are not killed by ivermectin even at high doses over a period of six months (Dreyer 1996; Addiss 2000).
In areas of Central and West Africa co‐endemic for lymphatic filariasis and Loa loa, the filarial eye worm causing loiasis, treatment with ivermectin or DEC can cause serious adverse events (SAEs) when there are high L loa mf densities (more than 30,000 mf/mL) (Boussinesq 1997; Gardon 1997). In these areas, albendazole alone given twice a year with vector control is recommended if ivermectin has not already been distributed for either onchocerciasis or lymphatic filariasis (WHO 2012; WHO 2017a). Ivermectin can also cause SAEs in people with onchocerciasis and high L loa densities; however, treatment with ivermectin was recommended for onchocerciasis meso‐ and high‐endemic areas following one of three strategies to manage complications, should they occur (Mectizan Expert Committee 2004). See Table 5.
1. Mass drug administration (MDA) programmes for filariasis.
| Endemic for | Drug recommendation | |||||
| Lymphatic filariasis | Onchocerciasis | Loiasis | Albendazole | Ivermectin | Diethylcarbamazine | Regimen |
| + | + | +a | Yes | No | No | Twice per yearb,c |
| + | + | ‐ | Yes | Yes | No | Annualb |
| + | ‐ | +a | Yes | No | No | Twice per yearb,c |
| ‐ | + | + | No | Yes | No | Annualc |
| +d | ‐ | ‐ | Yes | No | Yes | Annualb |
| ‐ | + | ‐ | No | Yes | No | Annual |
| ‐ | ‐ | + | No | No | No | ‐ |
aIn areas where L loa is endemic, ivermectin must be used with caution as people with high L loa microfilaraemia are at greater risk of experiencing serious adverse effects (SAEs). Albendazole alone given twice per year is recommended when mass drug administration with ivermectin has not yet occurred. Where mass drug administration with ivermectin has already occurred for either lymphatic filariasis or onchocerciasis, ivermectin distribution can continue under current guidance on the use of ivermectin for onchocerciasis in areas co‐endemic for loiasis. For further information, see reference c. bWHO 2017a cMectizan Expert Committee 2004 dAnnual treatment with the triple‐drug therapy of ivermectin, DEC and albendazole is recommended in specified settings.
Adverse effects of antifilarial drugs can be serious (although rarely fatal) and prevent people from starting or completing treatment. The most serious appear to be due to a host immunologic reaction induced by the rapid killing of mf, and associated with the release of inflammatory Wolbachia lipoproteins (Cross 2001; Turner 2009). Adverse effects include fever, headache, malaise, muscle pain, and blood in urine. Local effects include localized pain, tender nodules, lymphadenitis (inflammation of the lymph nodes), and lymphangitis (inflammation of lymph vessels) (Addiss 2000).
Albendazole
Albendazole has been used widely to treat intestinal parasites since the late 1980s and may have a potential role in lymphatic filariasis control (Ottesen 1999). In an early study on albendazole for lymphatic filariasis, a high (400 mg) dose taken twice a day for 21 days was believed to be macrofilaricidal due to the serious adverse reactions the authors attributed to adult worm death (Jayakody 1993). A report from an informal consultation organized by the WHO went on to suggest that repeated high doses of albendazole have a killing or sterilizing effect on W bancrofti adult worms (CDS/FIL 1998). However, it was unclear whether adding albendazole to either DEC or ivermectin improves cure, prevents further transmission, or influences the occurrence of adverse events (Addiss 2005).
In 2000, a narrative review by Horton 2000 from GlaxoSmithKline, which manufactures albendazole, did not demonstrate that adding albendazole to either drug increased the frequency or severity of adverse events. GlaxoSmithKline stated that albendazole does not have a role in morbidity management − it will not treat the symptoms in people already affected by filariasis (GlaxoSmithKline 2002). A recent trial reported that a significant proportion of children with W bancrofti infection had their lymphatic pathology reversed when given the combination of albendazole and DEC annually (Kar 2017). We therefore include the effectiveness of albendazole for reducing disease progression and incidence of new symptoms as a secondary outcome.
The use of albendazole in MDA programmes for lymphatic filariasis is considered to have ‘beyond filariasis' benefits, as it additionally addresses ‘polyparasitism’ through treatment of intestinal helminth infections (Shenoy 2011). However, a narrative review by Horton 2009 stated "while there is no doubt about the efficacy of albendazole for the treatment of many helminth diseases, as a single agent it could never be recommended for filariasis". In 2005, a systematic review concluded "the addition of albendazole to DEC or ivermectin does not appear to improve the effectiveness of either drug alone, and therefore may not directly benefit the transmission elimination aspect of the lymphatic filariasis control programme" (Tisch 2005). The authors also commented on the insufficiency of existing data for comparing the efficacy of drug regimens against bancroftian filariasis, and highlighted the need for more evidence from comparative randomized controlled studies. Conversely, an expert opinion review that included meta‐analyses and observational data (also published in 2005) concluded that co‐administration of albendazole was more effective in reducing mf prevalence than one antifilarial drug alone (Gyapong 2005).
Why it is important to do this review
Since the GPELF's inception, interventions for lymphatic filariasis have prevented or cured an estimated 97 million cases and obviated over USD 100 billion in economic losses over the lifetimes of the beneficiaries (Ramaiah 2014; Turner 2016). The combined therapy (albendazole with either ivermectin or DEC) has been endorsed for nearly two decades by the WHO and GPELF, as well as the Global Alliance to Eliminate Lymphatic Filariasis (GAELF), who currently state that "the combination of albendazole with either Mectizan® or DEC has been proven to enhance the efficacy of the individual‐drug treatments in reducing the numbers of parasites in the blood" (GAELF 2018). More recently, researchers have been investigating higher or more frequent dosing with albendazole (De Britto 2015; Kar 2015), as well as the effectiveness of the single‐dose triple therapy IDA (Thomsen 2016; King 2018).
However, despite policy recommending the addition of albendazole to ivermectin or DEC, or albendazole monotherapy in L loa co‐endemic areas, it remains unclear whether its addition is of any benefit specifically for lymphatic filariasis.
The previous published version of this Cochrane Review concluded that there was not enough evidence on the effectiveness of the drug albendazole, either alone or in combination with antifilarial drugs, for killing or interrupting transmission of the worms that cause lymphatic filariasis (Addiss 2005). In light of this, we aimed to summarize the evidence for the effects of albendazole alone or combined with a microfilaricidal drug for both individual treatment and transmission control, updating the previous edition with new methods and including new trials.
Objectives
To assess the effects of albendazole alone, and the effects of adding albendazole to DEC or ivermectin, in people and communities with lymphatic filariasis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), including those randomized by cluster.
Types of participants
Adults or children with filarial infection defined by the presence of mf in the blood, filarial antigens in the blood, or ultrasound detection of adult worms in lymphatic vessels.
Populations normally resident in endemic communities and who are eligible for treatment, regardless of microfilaraemia status.
Types of interventions
Albendazole alone versus placebo or no placebo.
Albendazole plus DEC versus DEC alone (DEC dose and regimen same in both arms).
Albendazole plus ivermectin versus ivermectin alone (ivermectin dose and regimen same in both arms).
Types of outcome measures
Primary outcomes
Measures of transmission potential
Mf prevalence.
Mf density (individual or average community density in community trials).
Secondary outcomes
Markers of adult worm infection
Antigenaemia prevalence.
Antigen density.
Adult worm prevalence (macrofilariae viability detected by ultrasound).
Clinical disease
Acute filariasis (fever plus clinical evidence of inflammation of the lymphatic system, as defined by primary investigators).
Appearance or disappearance of hydrocoele or lymphoedema.
Reduction in size (or severity or grade) of hydrocoele or lymphoedema.
Adverse events
Adverse events that prevent daily activities or require hospitalization.
Systemic adverse events (e.g. fever, headache, malaise, myalgia, or haematuria).
Local adverse events (e.g. localized pain and inflammation, tender nodules, lymphadenitis, or lymphangitis).
Search methods for identification of studies
Electronic searches
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
We searched the following databases using the search terms and strategy described in Appendix 1.
Cochrane Infectious Diseases Group Specialized Register (up to 15 January 2018).
MEDLINE (PubMed, 1966 to 15 January 2018).
Embase (OVID, 1974 to 15 January 2018).
Cochrane Central Register of Controlled Trials (CENTRAL) published in the Cochrane Library (Issue 1, 2018).
Latin American and Caribbean Health Sciences Literature (LILACS) (BIREME, 1982 to 15 January 2018).
We also searched the WHO International Clinical Trials Registry Platform (www.who.int/ictrp/search/en/) and ClinicalTrials.gov, to identify ongoing trials using the terms: filariasis; albendazole; benzimidazole.
Searching other resources
We checked the reference lists of all included trials to identify relevant studies.
Data collection and analysis
Selection of studies
Two review authors, Cara Macfarlane (CM) and Shyam Budhathoki (SB), screened titles and abstracts identified from the search strategy, obtained full‐text copies of all potentially relevant trials and checked each trial report for evidence of multiple publications from the same data set. CM and SB independently assessed each trial for inclusion using an eligibility form based on the inclusion criteria and resolved any disagreements through discussion or, where necessary, by consulting a third review author, Paul Garner (PG). We contacted trial authors when we required further information. We planned to contact authors of unpublished trials. We listed excluded studies and the reasons for their exclusion in the ‘Characteristics of excluded studies’ table, and studies awaiting classification in the ‘Studies awaiting classification’ table along with any known details. We illustrated the study selection process in a PRISMA diagram.
Data extraction and management
Two review authors (CM and SB) independently extracted data on trial characteristics, including methods, participants, interventions (including dose and treatment frequency), and outcomes using a pretested data extraction form. We resolved any differences in data extraction through discussion or by consulting a third review author (PG). In the case of unclear or missing data, we attempted to contact the primary investigators for further information. We recorded the number of participants randomized in each treatment group and the number of participants that were analysed for each outcome of interest, and reported the loss to follow‐up in each group. When data were shown in figures but were not reported in the article text, we extracted data using WebPlotDigitizer software (Version 3.12) (Rohatgi 2017).
RCTs that randomized individuals
For dichotomous outcomes, we recorded the number of participants experiencing the event and the total number of participants in each treatment group. For continuous outcomes, we aimed to extract geometric means and confidence intervals (CIs), together with the numbers of participants in each group. Where these were not reported, we extracted the summary measure used (geometric mean, log mean, or arithmetic mean) and standard deviations (SDs) or CIs where possible, along with the numbers of participants in each group. Where change from baseline results were presented alongside results purely based on the end value, we only extracted the change from baseline results.
RCTs that randomized clusters
For cluster‐RCTs that met the inclusion criteria, we attempted to extract the cluster unit, the number of clusters in the trial, the average size of clusters, and the unit of randomization (such as household). We extracted the statistical methods used to analyse the trial along with details describing whether these methods adjusted for clustering or other covariates. We attempted to extract the intra‐cluster correlation coefficient (ICC) for the cluster‐RCT, as if this was reported we could adjust the analyses.
We aimed to extract the cluster‐adjusted results when a cluster‐RCT adjusted for clustering in their analysis. When the trial did not account for clustering in their analysis, we extracted the same data as for trials that randomize individuals.
Assessment of risk of bias in included studies
Two review authors (CM and SB) independently assessed the risks of bias for each included trial using the Cochrane ‘Risk of bias' tool (Higgins 2011), and resolved differences of opinion through discussion with Samuel Johnson (SJ) and PG. For RCTs that randomized individuals we assessed six components: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other potential biases. For the cluster‐RCT, we addressed additional components: recruitment bias; baseline imbalance; loss of clusters; incorrect analysis; and compatibility with RCTs randomized by individual.
For sequence generation and allocation concealment, we reported the methods used. For blinding, we described who was blinded and the blinding method. For incomplete outcome data, we reported the percentage and proportion of participants lost to follow‐up. For selective outcome reporting, we stated any discrepancies between the methods used and the results in terms of the outcomes measured or the outcomes reported. For other biases, we described any other trial features that could have affected the trial result (for example, if the trial was stopped early).
We categorized our ‘Risk of bias' judgements as either ‘low’, ‘high’, or ‘unclear’. We displayed the results in ‘Risk of bias' tables, a ‘Risk of bias' summary, and a ‘Risk of bias' graph.
Measures of treatment effect
We used the risk ratio (RR) to compare the treatment and control groups for dichotomous outcomes, and presented the treatment effects with 95% CIs.
For continuous data summarized using geometric means, we planned to report the geometric mean ratios. Due to the variability in the summary measures reported and the lack of reporting of CIs or measures of variance in the trials, we could not synthesize data to obtain pooled treatment effects. We report continuous outcomes in ‘Additional tables', and we compare the difference in the intervention and the control groups' percentage reductions in parasitaemia from baseline.
Unit of analysis issues
For a particular cluster‐RCT when the analyses had not been adjusted for clustering, we planned to try and adjust the results for clustering by estimating the design effect calculated as 1+(m‐1)*ICC, where m is the average cluster size and ICC is the ICC. When the true ICC was unknown, we planned to estimate it from other included cluster‐RCTs. As we were unable to estimate the ICC due to the inclusion of a single cluster‐RCT, we presented the trial authors' unadjusted data in Appendix 2.
Dealing with missing data
We aimed to conduct a complete‐case analysis in this review, such that all participants with a recorded outcome were included in the analysis. When necessary, we made extensive efforts to obtain clarification over aspects of the parasite density data and to obtain the original data from the trial authors.
Assessment of heterogeneity
We assessed statistical heterogeneity using Chi2 and I2 statistics (Higgins 2003), and judged any heterogeneity using values of I2 greater than 50% and a Chi2 P value of 0.10 or less to indicate moderate to substantial statistical heterogeneity (Deeks 2017).
Assessment of reporting biases
We planned to assess the possibility of publication bias by examining funnel plots for asymmetry, but there were too few trials.
Data synthesis
One review author (CM) analysed the data using Review Manager 5 (Review Manager 2014). The main analysis examined albendazole alone or added to a microfilaricidal drug. We sought to identify evidence of an overall effect of albendazole; in the presence of high heterogeneity of effects between albendazole alone or added to either of the microfilaricidal drugs, we would then proceed to analysis of individual comparisons to see if this explained the heterogeneity. However, no such inconsistency was apparent. Nevertheless, we included additional comparisons of albendazole alone or in combination versus the background drug, be that placebo, DEC, or ivermectin. We provide this to summarize the reliable evidence for policy‐makers interested in the effectiveness of albendazole regimens for global lymphatic filariasis programmes.
We directly compared treatments using pairwise comparisons. Some trials randomized infected and uninfected individuals, but only analysed subgroups of participants who were infected at baseline. The primary analysis for each outcome included the number of individuals randomized as the denominator, where possible. When a trial reported data at multiple time points we included data collected at the longest follow‐up time up to 12 months in the analysis. The exception to this was data for mf density, which we analysed by longest follow‐up time up to six months and at 12 months to seek evidence of any temporally‐dependent effects. Within the individual drug comparator groups (e.g. albendazole versus placebo), we also conducted meta‐analyses for different follow‐up time points, and included data from subgroups of individuals known to be infected or participants who were both infected and uninfected.
We planned to combine RCTs that randomized individuals and cluster‐RCTs that adjusted for clustering using meta‐analysis. When a cluster‐RCT did not adjust for clustering and could not be combined with RCTs, we reported the results of the cluster‐RCT in an appendix. We used a fixed‐effect meta‐analysis when the assessments of heterogeneity did not reveal heterogeneity. In the presence of statistical heterogeneity we used random‐effects meta‐analysis.
For continuous data, we presented data that could not be meta‐analysed in ‘Additional tables' and reported on these in each section under the relevant outcome heading. For the parasite density data, we examined the summary measure used (geometric, log, or arithmetic mean), the methods that were used to estimate this and the change in density post‐treatment, and whether the analysis included the whole population or only infected participants. We sought approaches to allow meta‐analysis of the density data, but this was not possible due to the variability in the summary measures reported and the lack of reporting of CIs or measures of variance. We were also unable to calculate measures of treatment effect for individual studies, due to the lack of reported measures of variances or CIs. We therefore reported on the trial authors' statistical tests of significance.
Where trial authors provided geometric or log estimates of percentage reduction for parasite density outcomes (as an average across participants), we took the estimated percentage reduction in the intervention and the estimated percentage reduction in the control and calculated the percentage difference in density reduction between intervention and control. Whilst we could not conduct meta‐analyses to assess the treatment effect, it gave a measure of the direction of the possible effect.
Certainty of the evidence
We assessed the certainty of the evidence for each important outcome using the GRADE approach (Schünemann 2013). All review authors participated in the GRADE assessment through several meetings. For the main outcomes in each comparison, we used GRADE profiler to assess five domains: risk of bias; inconsistency; indirectness; imprecision; and publication bias (GRADEpro 2015).
We assessed the overall certainty of the evidence using four categories (high, moderate, low, or very low). The baseline for each outcome was set as high‐certainty evidence, as all studies were RCTs. Each GRADE domain could be downgraded by one or two levels if we judged it to have serious or very serious concerns, and we detailed the justification for downgrading in footnotes.
We displayed the GRADE rating of the certainty of evidence and justification for downgrading in the ‘Summary of findings' tables.
‘Summary of findings' tables
We interpreted results using ‘Summary of findings' tables, which provide key information about the certainty of the evidence for the included trials in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. Using GRADE profiler (GRADEpro 2015), we imported data from Review Manager 5 (Review Manager 2014). We present the main outcomes for the review in the ‘Summary of findings' tables. When there was no pooled effect estimate for an outcome, we presented a narrative synthesis of quantitative data.
Subgroup analysis and investigation of heterogeneity
In the presence of statistically significant heterogeneity, we planned to explore the following potential sources of heterogeneity using subgroup analyses: drug dose (comparing regimens where there are significant variations in drug dose), participant age (children only, adults only, or whole populations), and length of follow‐up. We conducted subgroup analyses for drug dose and length of follow‐up only, as this appeared to explain the heterogeneity.
Sensitivity analysis
We carried out sensitivity analyses including only those trials with a low risk of bias for allocation concealment.
Results
Description of studies
Results of the search
See PRISMA flow diagram (Figure 1).
1.

Study flow diagram.
We included 13 trials (8713 participants), reported in 18 articles (see Characteristics of included studies). In this Cochrane Review update, we dropped two comparisons (albendazole versus DEC and albendazole versus ivermectin), so we re‐screened all included, excluded, and ongoing studies from the last published version (Addiss 2005), in addition to 149 records identified from the update search. We were unable to locate one record cited in the previous version of this review, which was a two‐year follow‐up to Pani 2002.
We excluded 15 studies (reported in 20 records) at full‐text screening stage (see Characteristics of excluded studies). One study we excluded that was listed in a trial register (NCT01975441) published the full‐text article after we conducted the search in 15 January 2018 (King 2018). One trial, Purkait 2017, is awaiting classification (see Characteristics of studies awaiting classification). We excluded one trial included in the previous published review, as it no longer meets the inclusion criteria due to the removal of a comparison (albendazole versus DEC) (Jayakody 1993).
Included studies
Location
The included trials were undertaken in eight different countries: India (Pani 2002; Kshirsagar 2004; Gayen 2013; De Britto 2015), Haiti (Beach 1999; Fox 2005), Brazil (Dreyer 2006; Rizzo 2007), Papua New Guinea (Bockarie 2007), Zanzibar (Dahoma 2000), Ghana (Dunyo 2000), Tanzania (Simonsen 2004), and Kenya (Wamae 2011). All trials were conducted in endemic regions.
Participants
Three trials were school‐based and recruited children and adolescents (5 to 18 years old) from school populations (Beach 1999; Simonsen 2004; Fox 2005); five trials were conducted in community settings and recruited adults and children (Dahoma 2000; Dunyo 2000; Kshirsagar 2004; Bockarie 2007; Wamae 2011). Three studies were hospital‐based and recruited only children and adolescents (9 to 19 years of age) (Rizzo 2007), only adult men (Dreyer 2006), or adults and children (Pani 2002). Two trials recruited mf‐positive adults from endemic villages (Gayen 2013; De Britto 2015).
Seven trials enrolled people with a variety of inclusion criteria related to filarial infection; four only enrolled individuals who were mf‐positive (Pani 2002; Rizzo 2007;Gayen 2013;De Britto 2015); Dreyer 2006 enrolled individuals with detectable filaria dance sign (FDS); Dahoma 2000 enrolled individuals who had either microfilaraemia or who were amicrofilaraemic with clinical disease; and Wamae 2011 enrolled individuals if one or more members of a household were microfilaraemic.
Six trials enrolled individuals irrespective of their infection status at baseline (Beach 1999; Dunyo 2000; Kshirsagar 2004; Simonsen 2004; Fox 2005; Bockarie 2007). Kshirsagar 2004 enrolled 1403 participants for a safety study and included 103 men in a separate analysis of efficacy at 3, 6, and 12 months follow‐up. Forty‐three of the 103 participants in the smaller efficacy analysis were mf‐positive, 30 had clinical disease, and 30 were mf‐negative and asymptomatic. For subsequent assessments at 12, 24, and 36 months follow‐up, men and women from the safety study who were mf‐positive at baseline were also included (155 participants).
Intervention
Four trials assessed albendazole alone versus placebo (Beach 1999; Dunyo 2000; Fox 2005; Gayen 2013), eight trials assessed albendazole plus DEC versus DEC alone (Pani 2002; Kshirsagar 2004; Fox 2005; Dreyer 2006, Bockarie 2007; Rizzo 2007; Wamae 2011; De Britto 2015) and four trials assessed albendazole plus ivermectin versus ivermectin alone (Beach 1999; Dahoma 2000; Dunyo 2000; Simonsen 2004).
Twelve trials used the same albendazole dose (400 mg) (Beach 1999; Dunyo 2000; Pani 2002; Kshirsagar 2004; Simonsen 2004; Fox 2005; Dreyer 2006; Bockarie 2007; Rizzo 2007; Wamae 2011; Gayen 2013; De Britto 2015), and Dahoma 2000 did not report the dose. Drug dose information for Dahoma 2000 appeared to be reported in the appendices, which were not included in our copy of the thesis. We contacted the author of Dahoma 2000 and the library where the thesis was deposited to obtain the appendices, but received no response. As albendazole is usually given as a standard 400 mg single dose and there was no indication that a non‐standard dose was used, we included this trial. In the four placebo‐controlled trials, Dunyo 2000 and Gayen 2013 described tablets as identical or matching albendazole‐placebo, while Beach 1999 and Fox 2005 provided 250 mg vitamin C tablets.
All trials used a 6 mg/kg dose of DEC except for De Britto 2015, where 300 mg DEC was given. De Britto 2015 also provided a placebo for 12 days following treatment with DEC and with albendazole plus DEC.
Of the four trials that included ivermectin, three trials used doses varying from 200 to 400 μg/kg (Beach 1999) and 150 to 200 μg/kg (Dunyo 2000; Simonsen 2004). Dahoma 2000 did not report the ivermectin dose, but the thesis discussion indicated the dosage was similar to 200 μg/kg.
In nine trials the drugs were given as a single‐dose treatment (Beach 1999; Dahoma 2000; Dunyo 2000; Pani 2002; Simonsen 2004; Fox 2005; Dreyer 2006; Bockarie 2007; Rizzo 2007); Kshirsagar 2004 and Wamae 2011 provided three annual single doses. Two trials used more intensive treatment regimens; Gayen 2013 provided albendazole daily for seven days, and De Britto 2015 provided albendazole plus DEC or DEC daily for 12 days.
Study design
Twelve trials were individually‐RCTs, and Wamae 2011 was a cluster‐RCT. The cluster‐RCT used households as the unit of randomization, and included 64 households containing 205 adults and children.
The length of follow‐up varied between trials. Dahoma 2000 followed up participants for two weeks; Beach 1999 for four months; Fox 2005 for six months; Dunyo 2000, Simonsen 2004, Dreyer 2006, Rizzo 2007, Gayen 2013, and De Britto 2015 for 12 months; Bockarie 2007 and Wamae 2011 for 24 months; and Pani 2002 and Kshirsagar 2004 for 36 months.
Outcomes
Measures of transmission potential
All trials reported on mf prevalence and density, but the methods of measurement varied. Beach 1999 and Fox 2005 assessed 20 μL of blood with thick smear microscopy. Dunyo 2000, Simonsen 2004, and Wamae 2011 assessed 100 μL of blood using a counting chamber, and Dahoma 2000 assessed 200 μL of blood using a counting chamber. Seven trials assessed 1 mL blood using membrane filtration (Pani 2002; Kshirsagar 2004; Dreyer 2006; Bockarie 2007; Rizzo 2007; Gayen 2013; De Britto 2015). Kshirsagar 2004 also assessed prevalence in 60 μL of blood with thick smear microscopy.
Markers of adult worm infection
Eight trials reported antigenaemia prevalence (Dunyo 2000; Pani 2002; Kshirsagar 2004; Simonsen 2004; Fox 2005; Bockarie 2007; Wamae 2011; De Britto 2015), of which all except Kshirsagar 2004 also reported on antigen density. Five trials assessed antigenaemia using the TropBio Og4C3 ELISA (Dunyo 2000; Simonsen 2004; Fox 2005; Bockarie 2007; Wamae 2011); Kshirsagar 2004 used the BinaxNOW Filariasis ICT; and Pani 2002 and De Britto 2015 used both the ELISA and the immunochromatographic card test (ICT). Three trials also assessed the effect of treatment on adult worm FDS by ultrasound scan in male participants (Pani 2002; Kshirsagar 2004; Dreyer 2006).
Clinical disease
Dunyo 2000 reported on the effect of treatment on clinical disease (lymphoedema or hydrocoele), including the reduction in grade or disappearance of clinical disease, the increase in clinical disease grade, and the appearance of new clinical disease at 12 months follow‐up.
Adverse events
Twelve trials reported on adverse events, but the reporting varied between trials. Some trials reported the proportion of participants experiencing adverse events (Dunyo 2000; Pani 2002; Kshirsagar 2004; Rizzo 2007; Wamae 2011; Gayen 2013; De Britto 2015), while some also reported the incidence of specific systemic adverse events (Beach 1999;Dahoma 2000; Dunyo 2000;Pani 2002; Simonsen 2004; Fox 2005; Rizzo 2007), tolerability (Kshirsagar 2004), or calculated scores based on severity and intensity (Beach 1999;Pani 2002; Fox 2005). Dreyer 2006 reported appearance of intrascrotal nodules in adult worm nests of male participants as a ‘sensitive reaction' to treatment. Bockarie 2007 did not mention adverse events post‐treatment.
Reported statistical analysis
Individually‐randomized trials
The statistical analyses used in the trials for density data are reported in Table 6, and detailed further here. The methods used to calculate mf density and antigen density and the percentage reductions from baseline to follow‐up were inconsistently reported across trials, and SDs or CIs for density data were absent in all but one study reporting the geometric mean (Dunyo 2000), and two studies reporting the arithmetic mean (Pani 2002; Kshirsagar 2004). We obtained CIs from the investigators of Rizzo 2007, and CIs for density data reported in Fox 2005 were obtained by the authors of the last published version of this review (Addiss 2005). As so few trials reported any measure of variance or CIs, and the summary measures presented differed between and within trials (such as arithmetic means, geometric means, and log means), we could not pool results for changes in parasite density. Results quoted in this review are the original trial author's calculations.
2. Parasitaemia density data: reported statistical analysis.
| Study details | Reported statistical analysis | ||||||
| Trial | Type of people enrolled | Mf density outcome denominator | CFA density outcome denominator | Mean reported | Explicit about method used to accommodate people with zero counts | Explicit about method used to calculate % reduction in density | If density increased post‐ treatment, authors set change to zero |
| Beach 1999 | Infected and uninfected | All mf‐positive | NA | GM | No | Yes | Yes |
| Bockarie 2007 | Infected and uninfected | All CFA‐positivea | All CFA‐positiveb | GM | Yes (“n+1”) |
Noa | NRa |
| De Britto 2015 | All mf‐positive | All mf‐positive | All CFA‐positive | LM | No | NR | NA |
| Dreyer 2006 | All FDS‐positive | All individuals | NA | GM | Noc | Noc | NRc |
| Dunyo 2000 | Infected and uninfected | All mf‐positived | All CFA‐positived | GM | Yes Calculation provided |
Noe | NRe |
| Fox 2005 | Infected and uninfected | All mf‐positive | All CFA‐positive | GM | Yes (“n+1”) |
Yes | Yes |
| Gayen 2013 | All mf‐positive | All mf‐positive | NA | AM | NA | Noe | NRe |
| Kshirsagar 2004 | Infected and uninfected | All mf‐positive | NA | AM | NAf | Noe | NRe |
| Pani 2002 | All mf‐positive | All mf‐positive | All individuals | GM and AM | Nog | No | NR |
| Rizzo 2007 | All mf‐positive | All mf‐positive | NA | GM | Yes (“n+1”) |
NR | NA |
| Simonsen 2004 | Infected and uninfected | All mf‐positive | All CFA‐positive | GM | Yes Calculation provided |
Noe | NRe |
Abbreviations: AM: arithmetic mean; CFA: circulating filarial antigen; FDS: filarial dance sign; GM: geometric mean; LM: log mean; Mf: microfilariae; NA: not applicable; NR: not reported. aMf density and percentage reduction in density were reported for all participants irrespective of their pre‐treatment infection status only at the 24‐month follow‐up. Details were not provided in the Methods, but the standard percentage change calculation was used. bAntigen density was reported as number of antigenaemic participants with high antigenaemia decreasing to low or to negative, and number with low antigenaemia converting to negative only at 24‐month follow‐up. cAuthors provided further details on request; for mf density the "n+1" formula before log transforming values was used, and % reduction was calculated using method reported in Addiss 1993. dAuthors also reported mf and CFA unit geometric mean densities for individuals who were negative for the markers at baseline and positive at 12 months; however, the change or reduction in population mf or CFA densities for all enrolled individuals was not reported. eDetails were not provided in the Methods, but the standard percentage change calculation was used. fAuthors used the arithmetic mean and only assessed participants who remained mf‐positive at follow‐ups; participants who had previously been mf‐positive but converted to negative were excluded from density calculations. gThe last version of this review, Addiss 2005, reported further details were provided by Pani 2002 on request; this trial calculated a William's mean (a modified geometric mean to take into account zero counts).
Six trials enrolled individuals irrespective of their infection status at baseline (Beach 1999; Dunyo 2000; Kshirsagar 2004; Simonsen 2004; Fox 2005; Bockarie 2007), and none reported the overall change in mf density or antigen density in the total population enrolled up to 12 months; only Bockarie 2007 provided a measure of the impact on community mf density at 24 months post‐treatment. Most trials reported geometric mean mf density (Beach 1999; Dunyo 2000; Pani 2002; Simonsen 2004; Fox 2005; Dreyer 2006; Bockarie 2007; Rizzo 2007), and geometric mean antigen density (Dunyo 2000; Simonsen 2004; Fox 2005); De Britto 2015 reported the log mean mf density and log mean antigen density; the arithmetic mean was also used for mf density (Pani 2002; Kshirsagar 2004; Gayen 2013), and for antigen density in Pani 2002. Dahoma 2000 reported mf density data by intensity categories ("1‐20mff, 21‐39 mff, 40‐59 mff, >60mff"), and Wamae 2011 reported that they calculated geometric mean mf intensity, but reported log mean mf densities that had not been adjusted for clustering. We did not include parasite density data from Dahoma 2000 and Wamae 2011 in our analyses.
Four studies were not explicit about the method used to accommodate zero counts (Beach 1999; Pani 2002; Dreyer 2006; De Britto 2015), but Pani 2002 and Dreyer 2006 provided further details on request; the authors calculated a William's mean (a modification of the geometric mean to accommodate zero values) (Willams 1937; Basáñez 1994). Five trials reported using the "n+1" formula before log transforming the data. Seven trials were not explicit about the method used to calculate the percentage reduction for density data (Dunyo 2000; Pani 2002; Kshirsagar 2004; Simonsen 2004; Dreyer 2006; Bockarie 2007; Gayen 2013), but five of these trials used the standard percentage change calculation (Dunyo 2000; Kshirsagar 2004; Simonsen 2004; Bockarie 2007; Gayen 2013). Dreyer 2006 provided further details on request; this trial used the method described by Addiss 1993. Beach 1999 and Fox 2005 calculated the geometric mean mf density and antigen density reduction by dividing the difference between densities before and after treatment by the pretreatment mf density and log transforming the results. If pretreatment mf density was less than the density after treatment, the reduction was deemed to be zero. The trialists performed this adjustment to eliminate the problem of log transforming a negative value, but this method may bias estimates of treatment effectiveness, as increases in mf density after treatment are set to zero.
Two trials reported inappropriate statistical methods for assessing differences in mf density or antigen density between treatment groups. Gayen 2013 reported use of a paired t‐test, which is an unsuitable test for comparing different groups. Simonsen 2004 estimated the combined effect on both mf density and antigen density over the one‐year follow‐up period using repeated measures ANOVA, and used pairwise contrast tests to examine differences between groups at specific time points; however, repeated measures ANOVA is unsuitable for comparing groups, and results of pairwise contrast tests were not reported.
Cluster‐randomized trials
One cluster‐RCT reported the use of a multilevel mixed‐effects regression model that adjusted for the cluster design (Wamae 2011); however, the primary and secondary outcomes of the review were not adjusted using this model and the authors reported on subgroups of microfilaraemic or antigenaemic individuals at follow‐up. It was not possible to adjust the results for clustering by estimating the design effect, as the average cluster size and ICC were not reported. We also could not estimate the ICC, as no other cluster‐RCTs were included. No outcomes from this trial were therefore suitable for meta‐analysis or comparative analysis, and we present the authors' unadjusted results in Appendix 2.
Dealing with missing data
We attempted to clarify aspects of the parasite density data and to obtain the original data from the authors, but we could not acquire most of the data that we required from the primary studies for our analysis. We contacted authors of Beach 1999, Fox 2005, Dreyer 2006, Bockarie 2007, Rizzo 2007, and De Britto 2015, and also attempted to contact Simonsen 2004, but the email addresses that we obtained from recently published articles were inactive. At our request, the authors of Rizzo 2007 provided us with CIs and SDs of log‐transformed density data and the number of participants reporting adverse events, and the authors of Dreyer 2006 gave us the raw data files. We contacted the authors of Beach 1999 and Fox 2005 to obtain the raw study data in order to recalculate the percentage reduction in density from baseline to follow‐up. We received no response from the authors of Fox 2005. The authors of Beach 1999 were unable to provide this at the time of preparing the review, due to issues with the file formats. We hope to incorporate new data analyses from Beach 1999 into any future updates of this Cochrane Review.
Excluded studies
We excluded 15 trials (reported in 20 records) at the full‐text screening stage, because they did not include the comparison groups or participant population sought for the review, the methods and results were not coherent or clearly expressed, the number of participants randomized for each group was very small with differential losses to follow‐up between treatment groups, or they were not an RCT. See Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2 and Figure 3 for ‘Risk of bias' summaries, and Characteristics of included studies section for details of the risks of bias and methods used in each trial.
2.

‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.

‘Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All trials described themselves as randomized. We judged the risk of bias to be low in six trials that described a method of randomization (Beach 1999; Dahoma 2000; Dunyo 2000; Simonsen 2004; Fox 2005; Rizzo 2007), and unclear in seven trials that did not provide further details (Pani 2002; Kshirsagar 2004; Dreyer 2006; Bockarie 2007; Wamae 2011; Gayen 2013; De Britto 2015).
We judged eight trials to be at low risk of bias for allocation concealment (Beach 1999; Dahoma 2000; Dunyo 2000; Pani 2002; Kshirsagar 2004; Simonsen 2004; Fox 2005; Gayen 2013). We judged Rizzo 2007 to be at high risk of bias, as allocation of participants was not concealed. We judged four trials to be at unclear risk, due to insufficient information (Dreyer 2006; Bockarie 2007; Wamae 2011; De Britto 2015).
Blinding
Nine trials described themselves as "double blind". For blinding of participants and personnel, five studies described blinding and we judged these to be at low risk of bias (Beach 1999; Pani 2002; Kshirsagar 2004; Fox 2005; Gayen 2013). We judged Rizzo 2007 to be at high risk of bias, as they did not use blinding. We judged details of blinding to be unclear in seven trials (Dahoma 2000; Dunyo 2000; Simonsen 2004; Dreyer 2006; Bockarie 2007; Wamae 2011; De Britto 2015).
For blinding of outcome assessors, seven trials described blinding of outcome assessment and we judged these to be at low risk of bias (Beach 1999; Dahoma 2000; Dunyo 2000; Pani 2002; Kshirsagar 2004; Fox 2005; Rizzo 2007). Six trials did not provide details of outcome assessor blinding and we judged risk of bias to be unclear (Simonsen 2004; Dreyer 2006; Bockarie 2007; Wamae 2011; Gayen 2013; De Britto 2015).
Incomplete outcome data
About half the included studies (6/13) reported that more than 85% of all randomized individuals had been followed up, and we judged these studies to be at low risk of bias (Dahoma 2000; Pani 2002; Dreyer 2006; Rizzo 2007; Gayen 2013; De Britto 2015). We judged six studies to be at high risk of bias due to attrition, as losses or exclusions of participants during the follow‐up period were considerable (Beach 1999; Kshirsagar 2004; Simonsen 2004; Fox 2005; Bockarie 2007; Wamae 2011). We judged Dunyo 2000 to be at unclear risk.
We judged Beach 1999, Simonsen 2004, Fox 2005, and Bockarie 2007 to be at high risk of bias, as they excluded randomized participants who did not have pre‐ and post‐treatment blood samples. We judged Kshirsagar 2004 to be at high risk of bias as the authors included a very small subset of randomized participants in a separate efficacy analysis. Wamae 2011 (cluster‐RCT) did not clearly report the number of individuals that were analysed among those randomized. Dunyo 2000 analysed 1181 of 1425 participants (17.1% lost) at 12 months, with losses attributed to participant absence during survey times and some being unwilling to have repeated finger pricks. Sixty‐seven of the 340 mf‐positive participants (20%) were also lost to follow‐up.
Selective reporting
Eight trials had no obvious evidence of selective reporting and we judged these to be at low risk of bias (Dahoma 2000; Dunyo 2000; Pani 2002; Simonsen 2004; Dreyer 2006; Rizzo 2007; Gayen 2013; De Britto 2015). Four trials had evidence of selective reporting and we judged them to be at high risk of bias (Kshirsagar 2004; Fox 2005; Bockarie 2007; Wamae 2011). We judged Beach 1999 to be at unclear risk, as not all the adverse events prespecified in the Methods were reported.
Other potential sources of bias
We judged three studies to be at high risk of bias due to other potential sources of bias (Simonsen 2004; Wamae 2011; Gayen 2013). Gayen 2013 reported an inappropriate statistical analysis (paired t‐test) for testing for differences between treatments, which could bias interpretation of the intervention effects. Simonsen 2004 did not report the findings of statistical tests for differences between groups at specific time points, but reported a significant effect for the intervention over time using repeated measures ANOVA. We rated one cluster‐RCT at high risk of bias due to incorrect analysis (some data were not adjusted for clustering) and the number of clusters and participants followed up or included in the analyses was not clearly reported (Wamae 2011).
We judged two studies to have unclear risk of bias (Beach 1999; Fox 2005). For parasite density data outcomes, the authors of Beach 1999 and Fox 2005 omitted increases in density prior to estimating the percentage reduction between baseline and follow‐up. This simply provides an assessment of the decrease in density only in people experiencing a decrease. Whilst this rule was applied to both intervention and control groups, we were uncertain of the effect of this on the estimate, or exactly what the estimate was measuring.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
The first set of analyses examine albendazole given alone or added to a microfilaricidal drug; and the subsequent analyses are grouped by the different background drugs (placebo, DEC, ivermectin).
For each comparison, we present the results at the longest follow‐up (up to 12 months) from each study, and include all individuals enrolled as the denominator where possible.
Within each different background drug analysis, we also analysed different follow‐up time points and stratified by the following.
People known to be infected
People both infected and uninfected in community studies
The data on mf density and antigen density are presented in ‘Additional tables'; this was expressed differently across studies, often with no measure of variance, and we therefore summarized it narratively in the text.
Overall effect
Albendazole alone or added to a microfilaricidal drug
One cluster‐randomized trial randomized households, and then only reported on people found to be infected and who gave blood at baseline (Wamae 2011). The authors reported the mean log density in a graph but this was complicated by interaction, and a logistic regression analysis was not clear as to who was included, and so further interpretation was not possible (see Description of studies above). The results are in Appendix 2.
Mf prevalence
Treatment with albendazole had no effect on mf prevalence at the longest follow‐up up to 12 months (5027 participants, 12 trials; Analysis 1.1).
1.1. Analysis.

Comparison 1 Albendazole alone or added to a microfilaricidal drug, Outcome 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months).
Mf density
Eleven trials reported the effects of albendazole on mf density. Pani 2002, Rizzo 2007, Gayen 2013, and De Britto 2015 only enrolled mf‐positive people at baseline; Dreyer 2006 only enrolled people with adult worms detected by ultrasound, irrespective of mf status; Beach 1999, Dunyo 2000, Kshirsagar 2004, Simonsen 2004, Fox 2005, and Bockarie 2007 recruited mf‐positive and ‐negative participants, but only reported density in people who were mf‐positive at baseline; none reported the overall change in mf density in the total population enrolled.
Overall, albendazole was associated with inconsistent reductions in mf density up to six months (1216 participants, 10 trials; Table 7) and at 12 months (1052 participants, 9 trials; Table 8).
3. Microfilarial density: up to 6 months follow‐up.
| Background drug | Risk of bias: analysis used | Trial (follow‐up) | Intervention (albendazole) | Control | Difference between groups post‐treatment | ||||
| Participants | Baseline to follow‐up (% reduction) | Participants | Baseline to follow‐up (% reduction) | % reduction | Significance testing (% reduction) | Significance testing: mf density | |||
| Placebo | Low or unclear risk | Dunyo 2000a,b,c,d (6 months) |
62 | 1783 (95% CI 1215 to 2617) to 693 (95% CI 335 to 1431) (61.1%) |
57 | 2277 (95% CI 1576 to 3289) to 2745 (95% CI 1505 to 5007) (20.6% increase) |
81.7% | NR | NR |
| High risk | Beach 1999a,d,e (4 months) |
29 | 14.1 to 5.1 (28.7%) |
29 | 9.3 to 5.3 (17.2%) |
11.5% | NS (P > 0.05) | NS (P > 0.05) | |
| Fox 2005a,d,e (6 months) |
42 | 12.1 (95% CI 10.3 to 14.2) to 4.4 (95% CI 3.7 to 5.3) (34.7%) |
34 | 17.3 (95% CI 14.5 to 20.6) to 11.2 (95% CI 9.2 to 13.7) (10.3%) |
24.4% | * (P < 0.05) | * (P < 0.05) | ||
| Gayen 2013a,f,g (4 months) |
17 | 3942.32 to 821.88 (79%) |
15 | 4460.7 to 4390.7 (1.6%) |
77.4% | NRh | NR | ||
| DEC | Low or unclear risk | Pani 2002a,d,f (6 months) |
18 | 79.4, post‐treatment NR (81%) |
17 | 81.3, post‐treatment NR (74.7%) |
6.3% | NR | NS (P > 0.05) |
| Dreyer 2006d,f (1 month) |
21 | 55.9 to 12.7 (53.5%) |
23 | 129.5 to 18.8 (67.1%) |
−13.6% | NS (P = 0.24) | NS (P = 0.83) | ||
| Rizzo 2007a,d,f (6 months) |
41 | 232.6 to 17.7 (92.4%) [2.36 (95% CI 2.16 to 2.57) to 1.27 (95% CI 0.94 to 1.60)]i |
43 | 182.6 to 10.5 (94.2%) [2.26 (95% CI 2.04 to 2.49) to 1.09 (95% CI 0.74 to 1.43)]i |
−1.8% | NR | NS (P > 0.05) | ||
| Bockarie 2007d,f (6 months) |
126 | 25.4 to 4.46 (82.4%) |
119 | 24.4 to 7.49 (69.3%) |
13.1% | NR | NS (P = 0.21) | ||
| De Britto 2015a,f,j (6 months) |
36 | 2.26 (± 0.57) to 0.15 (± NR) (99.2%) |
35 | 2.22 (± 0.52) to 0.83 (± NR) (96%) |
3.2% | NR | NR | ||
| High risk | Fox 2005a,d,e (6 months) |
41 | 13.4 (95% CI 11.4 to 15.8) to 0.76 (95% CI 0.7 to 0.9) (80.4%) |
39 | 12.9 (95% CI 11.0 to 15.2) to 2.8 (95% CI 2.3 to 3.4) (50.4%) |
30% | * (P = 0.02) | * (P <0.05) | |
| Ivermectin | Low or unclear risk | Dunyo 2000a,b,c,d (6 months) |
62 | 1585 (95% CI 1069 to 2350) to 110 (95% CI 50 to 239) (93.1%) |
55 | 2055 (95% CI 1389 to 3041) to 204 (95% CI 91 to 451) (90.1%) |
3% | NS (P = 0.71) | NR |
| Simonsen 2004a,b,d (6 months) |
105 | 812.6 to 29.8 (96.3%) |
98 | 763.5 to 150 (80.4%) |
15.9% | NRk | NRk | ||
| High risk | Beach 1999a,d,e (4 months) |
24 | 13.7 to 0.3 (98.9%) |
28 | 15.5 to 1.5 (76.1%) |
22.8% | *** (P < 0.001) | * (P < 0.05) | |
Microfilariae (mf) density data and significance testing for differences between groups at baseline and follow‐up, as reported by study authors. We calculated the percentage reduction when this was not reported by the authors (values are italicized), and also the difference between the percentage reductions in the intervention and control groups. We judged the risk of bias as high when studies used analytical methods that could affect the interpretation of the data, and low or unclear risk when there was no obvious analytical issues. Abbreviations: CI: confidence interval; mf: microfilariae; NR: not reported; NS: not significant; * (P < 0.05): significant; *** (P < 0.001): significant; ±: standard deviation. aOnly participants positive for mf at baseline. bMeasured in 100 µL blood using counting chamber, and expressed as mf/mL. cOnly in those individuals with over 100 mf/mL blood before treatment. dReported as geometric mean. eMeasured in 20 µL thick smear. fMeasured in 1 mL blood by membrane filtration, and expressed as mf/mL. gReported as arithmetic mean. hAuthors reported "a significant difference between the control and the treated groups (P < 0.05)" using paired t‐test for analysis; however, this statistical test is inappropriate for comparing different groups. iData within square brackets [ ] indicates log mean intensity data and CIs provided by authors of Rizzo 2007. jReported as log mean. kAuthors reported statistical analysis by paired t‐test and repeated‐measures ANOVA for correlated samples, and use of pairwise contrast tests to examine differences between groups at specific time points; results of pairwise tests for differences between groups do not appear to be reported.
4. Microfilarial density: 12 months follow‐up.
| Background drug | Risk of bias: analysis used | Trial | Intervention (albendazole) | Control | Difference between groups post‐treatment | ||||
| Participants | Baseline to follow‐up (% reduction) | Participants | Baseline to follow‐up (% reduction) | % reduction | Significance testing: % reduction | Significance testing: mf density | |||
| Placebo | Low or unclear risk | Dunyo 2000a,b,c | 71 | 798 to 251 (68.5%) |
66 | 971 to 845 (13%) |
55.5% | NR | NS (P = 0.10) |
| High risk | Gayen 2013a,d,e | 17 | 3942.32 to 432.64 (89%) |
15 | 4460.7 to 4245 (4.8%) |
84.2% | NRf | NR | |
| DEC | Low or unclear risk | Pani 2002a,c,d | 18 | 79.4, post‐treatment NR (95.4%) |
17 | 81.3, post‐treatment NR (89.6%) |
5.8% | NR | NS (P > 0.05) |
| Dreyer 2006c,d | 22 | 55.9 to 6.1 (69.5%) |
25 | 129.5 to 4.8 (85.3%) |
−15.8% | NS (P = 0.21) | NS (P = 0.87) | ||
| Rizzo 2007a,c,d | 41 | 232.6 to 5.2 (97.8%) [2.36 (95% CI 2.16 to 2.57) to 0.74 (95% CI 0.44 to 1.03)]g |
43 | 182.6 to 3.6 (98%) [2.26 (95% CI 2.04 to 2.49) to 0.65 (95% CI 0.35 to 0.95)]g |
−0.2% | NR | NS (P > 0.05) | ||
| Bockarie 2007c,d | 126 | 25.4 to 3.47 (86.3%) |
119 | 24.4 to 4.27 (82.5%) |
3.8% | NR | NS (P = 0.6) | ||
| De Britto 2015a,d,h | 36 | 2.26 (± 0.57) to 0.07 (± NR) (99.4%) |
35 | 2.22 (± 0.52) to 0.52 (± NR) (98%) |
1.4% | NR | NR | ||
| High risk | Kshirsagar 2004a,d,e | 29 | NR to 249.2 (± 276.1) (NR) |
24 | NR to 245.9 (± 314.8) (NR) |
NR | NR | NR | |
| Ivermectin | Low or unclear risk | Dunyo 2000a,b,c | 75 | 614 to 78 (87.3%) |
70 | 640 to 124 (80.6%) |
6.7% | NR | NS (P = 0.80) |
| Simonsen 2004a,b,c | 105 | 812.6 to 59.4 (92.7%) |
98 | 763.5 to 124.9 (83.6%) |
9.1% | NRi | NRi | ||
Microfilariae (mf) density data and significance testing for differences between groups at baseline and follow‐up, as reported by study authors. We calculated the percentage reduction when this was not reported by the authors (values are italicized), and also the difference between the percentage reductions in the intervention and control groups. We judged the risk of bias as high when studies used analytical methods that could affect the interpretation of the data, and low or unclear risk when there was no obvious analytical issues.
Abbreviations: mf: microfilariae; NR: not reported; NS: not significant; ±: standard deviation; CI: confidence interval. aOnly participants positive for mf at baseline. bMeasured in 100 µL blood using counting chamber, and expressed as mf/mL. cReported as geometric mean. dMeasured in 1 mL blood by membrane filtration, and expressed as mf/mL. eReported as arithmetic mean. fAuthors reported "a significant difference between the control and the treated groups (P < 0.05)" using paired t‐test for analysis; however, this statistical test is inappropriate for comparing different groups. gData within square brackets [ ] indicates log mean intensity data and CIs provided by authors of Rizzo 2007. hReported as log mean. iAuthors reported statistical analysis by paired t‐test and repeated‐measures ANOVA for correlated samples, and use of pairwise contrast tests to examine differences between groups at specific time points; results of pairwise tests for differences between groups do not appear to be reported.
Up to six months, there were four studies that gave albendazole alone, and we assessed three of these as being at high risk of bias (Gayen 2013 used the arithmetic mean, Beach 1999 and Fox 2005 excluded increases in mf density post‐treatment). One study (119 participants), assessed as low or unclear risk of bias, suggested an effect on density although this was not evaluated statistically (Dunyo 2000); and the other studies are difficult to interpret, given the risks of bias. When albendazole was used with other drugs, the results were similarly inconsistent or problematic to interpret.
At 12 months, a similar pattern emerged with albendazole alone, where we rated one study at high risk of bias (Gayen 2013 used the arithmetic mean), and an effect on density was suggested in Dunyo 2000, although this was not statistically significant (P = 0.10). When used with other drugs, the results showed little or no effect of albendazole.
Antigenaemia prevalence
Treatment with albendazole had no effect on antigen prevalence at the longest follow‐up (3774 participants, 7 trials; Analysis 1.2).
1.2. Analysis.

Comparison 1 Albendazole alone or added to a microfilaricidal drug, Outcome 2 Antigenaemia prevalence: longest follow‐up (up to 12 months).
Antigen density
Five trials reported the effects of albendazole on antigen density. Pani 2002 and De Britto 2015 only enrolled people mf‐positive at baseline; Dunyo 2000, Simonsen 2004, and Fox 2005 recruited infected and uninfected participants, but only reported density in people who were antigen‐positive at baseline; none reported the overall change in antigen density in the total population enrolled.
Overall, albendazole was not associated with greater reductions in antigen density between six and 12 months post‐treatment (1374 participants, 5 trials; Table 9).
5. Antigen density: longest follow‐up (up to 12 months).
| Background drug | Risk of bias: analysis used |
Trial (follow‐up) |
Intervention (albendazole) | Control | Difference between groups post‐treatment | ||||
| Participants | Baseline to follow‐up (% reduction) | Participants | Baseline to follow‐up (% reduction) | % reduction | Significance testing: % reduction | Significance testing: CFA density | |||
| Placebo | Low or unclear risk |
Dunyo 2000a,b (12 months) |
105 | 1370 to 1139 (16.9%) |
103 | 1869 to 2757 (47.5% increase) |
64.4% | NR | NS (P = 0.11) |
| High risk |
Fox 2005a,c (6 months) |
89 | 2640 (95% CI 2279 to 3058) to 2428 (95% CI 2071 to 2847) (3.2%) |
74 | 2298 (95% CI 1951 to 2706) to 2479 (95% CI 2105 to 2919) (1.7%) |
1.5% | NS (P > 0.05) | NS (P > 0.05) | |
| DEC | Low or unclear risk |
De Britto 2015a,d (12 months) |
36 | 3.88 (± 0.48) to 2.89 (± NR) (89.8%) | 35 | 3.58 (± 0.69) to 2.9 (± NR) (79.1%) | 10.7% | NR | NS (P = 0.750) |
| High risk |
Fox 2005a,c (6 months) |
85 | 2116 (95% CI 1798 to 2490) to 1350 (95% CI 1176 to 1549) (26.7%) |
79 | 2194 (95% CI 1842 to 2613) to 1597 (95% CI 1375 to 1855) (17%) |
9.7% | NS (P > 0.05) | NS (P > 0.05) | |
|
Pani 2002e (12 months) |
18 | 0.47 (± 0.18) to 0.08 (± 0.15) (83%) | 17 | 0.39 (± 0.21) to 0.07 (± 0.15) (82.1%) | 0.9% | NR | NR | ||
| Ivermectin | Low or unclear risk |
Dunyo 2000a,b (12 months) |
121 | 1404 to 834 (40.6%) |
99 | 1689 to 1187 (29.7%) |
10.9% | NR | NS (P = 0.80) |
|
Simonsen 2004a,b (12 months) |
247 | 1338.4 to 986.6 (26.3%) |
266 | 1026.3 to 931.6 (9.2%) |
17.1% | NRf | NRf | ||
Circulating filarial antigen (CFA) density data and significance testing for differences between groups at baseline and follow‐up, as reported by study authors. We calculated the percentage reduction when this was not reported by the authors (values are italicized), and also the difference between the percentage reductions in the intervention and control groups. We judged the risk of bias as high when studies used analytical methods that could affect the interpretation of the data, and low or unclear risk when there was no obvious analytical issues.
Abbreviations: CFA: circulating filarial antigen; CI: confidence interval; DEC: diethylcarbamazine; mf: microfilariae; NR: not reported; NS: not significant; ±: standard deviation. aOnly participants positive for CFA at baseline. bMeasured in fingerprick blood, expressed as CFA unit geometric mean intensity. cMeasured in fingerprick blood, expressed as geometric mean CFA units/mL. dVolume of blood not reported, expressed as log mean CFA units. eMeasured in 50 µL blood, expressed as arithmetic mean CFA optical density value. fAuthors reported statistical analysis by paired t‐test and repeated‐measures ANOVA for correlated samples, and use of pairwise contrast tests to examine differences between groups at specific time points; results of pairwise tests for differences between groups do not appear to be reported.
Two studies gave albendazole alone; Fox 2005 was assessed at high risk of bias (the authors excluded increases in antigen density post‐treatment) and Dunyo 2000 at low or unclear risk of bias. Dunyo 2000 included 208 participants and suggested a large difference in the antigen density percentage reductions between albendazole and placebo; however, albendazole alone reduced density by 16.9% while the placebo group increased by 47.5%, and the difference was not statistically significant (P = 0.11). The results showed little or no effect of albendazole when used with other drugs.
Adult worm prevalence detected by ultrasound
There was no difference associated with adding albendazole to DEC for reducing adult worm prevalence in men examined for FDS by ultrasonography at the longest follow‐up up to 12 months (165 participants, 3 trials; Analysis 1.3). However, the individual trials were all small and underpowered.
1.3. Analysis.

Comparison 1 Albendazole alone or added to a microfilaricidal drug, Outcome 3 Adult worm prevalence by ultrasound: longest follow‐up (up to 12 months).
Clinical disease: new and pre‐existing
Treatment with albendazole had no effect on new (535 participants, 1 trial; Analysis 1.4) or existing clinical disease (85 participants, 1 trial; Analysis 1.5); however, the trial was underpowered for clinical outcomes.
1.4. Analysis.

Comparison 1 Albendazole alone or added to a microfilaricidal drug, Outcome 4 New clinical disease (new cases hydrocoele).
1.5. Analysis.

Comparison 1 Albendazole alone or added to a microfilaricidal drug, Outcome 5 Pre‐existing clinical disease (net improvement).
Adverse events
Treatment with albendazole had no effect on the number of participants experiencing adverse events (2894 participants, 6 trials; Analysis 1.6).
1.6. Analysis.

Comparison 1 Albendazole alone or added to a microfilaricidal drug, Outcome 6 Adverse events.
Sensitivity analysis
In the sensitivity analyses including only trials where the risk of bias for allocation concealment was low, no difference between intervention and control groups in mf prevalence, antigenaemia prevalence, adult worm prevalence detected by ultrasound, or adverse events was evident. We do not present the sensitivity analyses here, as the results did not differ from those in the primary analyses.
Effects stratified by background drug
In the absence of any substantive evidence for an overall effect of albendazole, this became our main finding. However, we provide comparisons of albendazole grouped by background drug, as countries and policy‐makers may want to scrutinize the effectiveness of individual treatment regimens.
Albendazole versus placebo
No trials assessed adult worm prevalence (FDS) using ultrasound.
Mf prevalence
Treatment with albendazole had no effect on mf prevalence at the longest follow‐up (1406 participants, 4 trials; Analysis 2.1).
2.1. Analysis.

Comparison 2 Albendazole versus placebo, Outcome 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months).
Treatment with albendazole had no effect on mf prevalence up to six months (Analysis 2.2), or at 12 months (Analysis 2.3), irrespective of baseline infection status.
2.2. Analysis.

Comparison 2 Albendazole versus placebo, Outcome 2 Microfilaraemia (mf) prevalence: stratified by baseline infection (up to 6 months follow‐up).
2.3. Analysis.

Comparison 2 Albendazole versus placebo, Outcome 3 Microfilaraemia (mf) prevalence: stratified by baseline infection (12 months follow‐up).
Mf density
Four trials reported the effects of albendazole on mf density. Gayen 2013 only enrolled people mf‐positive at baseline; Beach 1999, Dunyo 2000, and Fox 2005 recruited mf‐positive and ‐negative participants, but only reported density in people mf‐positive at baseline; none reported the overall change in mf density in the total population enrolled.
Albendazole was associated with greater reductions in mf density up to six months (285 participants, 4 trials; Table 7) and 12 months (169 participants, 2 trials; Table 8).
Up to six months, there were four studies that assessed albendazole against placebo, but the magnitude of the effect of albendazole varied. One study (119 participants) suggested an effect on density (Dunyo 2000), but this was not statistically evaluated. Three studies were at high risk of bias: Beach 1999 and Fox 2005 excluded increases in mf density post‐treatment, and Gayen 2013 used the arithmetic mean; and could not be meaningfully interpreted.
At 12 months, there were two studies that gave albendazole; Dunyo 2000 included 143 participants and reported an effect of albendazole on density but this was not statistically significant (P = 0.10); the results of Gayen 2013 were difficult to interpret, as this study included 33 participants and was at high risk of bias.
Antigenaemia prevalence
Treatment with albendazole had no effect on antigen prevalence at the longest follow‐up (1054 participants, 2 trials; Analysis 2.4).
2.4. Analysis.

Comparison 2 Albendazole versus placebo, Outcome 4 Antigenaemia prevalence: longest follow‐up (up to 12 months).
Treatment with albendazole had no effect on antigen prevalence in people who were infected and uninfected at six months (Analysis 2.5) and 12 months (Analysis 2.6) post‐treatment; and no effect at 12 months follow‐up in participants who were antigenaemic at baseline (Analysis 2.6).
2.5. Analysis.

Comparison 2 Albendazole versus placebo, Outcome 5 Antigenaemia prevalence: stratified by baseline infection (6 months follow‐up).
2.6. Analysis.

Comparison 2 Albendazole versus placebo, Outcome 6 Antigenaemia prevalence: stratified by baseline infection (12 months follow‐up).
Antigen density
Two trials reported the effects of albendazole on antigen density (Dunyo 2000; Fox 2005). Both trials recruited antigen‐positive and ‐negative participants, but only reported density in people antigen‐positive at baseline; none reported the overall change in antigen density in the total population enrolled.
Albendazole was not associated with significantly greater reductions in antigen density between six and 12 months post‐treatment (371 participants, 2 trials; Table 9).
Dunyo 2000 included 208 participants and density was reduced by 16.9% with albendazole, while density increased by 47.5% with placebo, but the difference was not statistically significant (P = 0.11). Fox 2005 reported no difference with albendazole in a study including 163 participants (P > 0.05), but we judged it to be at high risk of bias (the authors excluded increases in mf density post‐treatment).
Clinical disease: new and pre‐existing
Treatment with albendazole had no effect on new (255 participants, 1 trial; Analysis 2.7: subgroup 1) or existing clinical disease (Analysis 2.7: subgroups 2 and 3); however, Dunyo 2000 was underpowered for clinical outcomes.
2.7. Analysis.

Comparison 2 Albendazole versus placebo, Outcome 7 Clinical disease.
Adverse events
Treatment with albendazole had no effect on the number of participants experiencing adverse events (678 participants, 2 trials; Analysis 2.8).
2.8. Analysis.
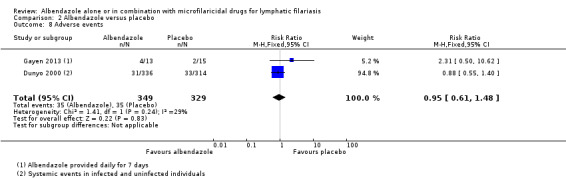
Comparison 2 Albendazole versus placebo, Outcome 8 Adverse events.
Beach 1999 and Fox 2005 did not provide data in a form that we could use in the meta‐analysis. Beach 1999 reported adverse reactions as generally mild and well tolerated, with no significant difference between participants receiving placebo or albendazole. Fox 2005 reported statistically significant reductions (P < 0.05) in myalgias and cough for albendazole compared with placebo, but no statistically significant differences in headache, fever, or mean treatment impact score.
Beach 1999, Dunyo 2000, and Fox 2005 reported that no localized inflammatory reactions were detected following treatment, and Gayen 2013 did not report this. No serious adverse events were reported in any trials.
Sensitivity analysis
In the sensitivity analyses including only trials where the risk of bias for allocation concealment was low, no difference between albendazole and placebo groups in mf prevalence, antigenaemia prevalence, or adverse events was evident. We do not present the sensitivity analyses here, as the results did not differ from those in the primary analyses.
Albendazole plus DEC versus DEC
No trials assessed new or pre‐existing clinical manifestations post‐treatment.
One cluster‐randomized trial randomized households, and then only reported on people found to be infected and who gave blood at baseline (Wamae 2011). The trial authors reported the mean log density in a graph but a logistic regression analysis was not clear as to who was included, and was complicated by interaction, so further interpretation was not possible (see Description of studies above). The results are in Appendix 2.
Mf prevalence
Adding albendazole to DEC had no effect on mf prevalence at the longest follow‐up (1102 participants, 7 trials; Analysis 3.1).
3.1. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months).
There was no benefit of adding albendazole to DEC up to six months (Analysis 3.2) or at 12 months post‐treatment (Analysis 3.3), irrespective of baseline infection status. There was moderate to substantial heterogeneity detected up to six months (Analysis 3.2; I2 = 79%) and at 12 months (Analysis 3.3; I2 = 61%) in the microfilaraemic participant subgroups, but subgroup analysis for dose seemed to explain this. There were not enough trials to formally investigate the source of heterogeneity.
3.2. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 2 Microfilaraemia (mf) prevalence: stratified by baseline infection (up to 6 months follow‐up).
3.3. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 3 Microfilaraemia (mf) prevalence: stratified by baseline infection (12 months follow‐up).
There was no difference in mf prevalence at 24 months follow‐up in participants who were all mf‐ or all antigen‐positive at baseline (Analysis 3.4). There was no benefit of adding albendazole to DEC for individuals infected and uninfected at baseline after a single dose or two annual doses; or at 36 months after three annual doses (Analysis 3.5).
3.4. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 4 Microfilaraemia (mf) prevalence: stratified by baseline infection (24 months follow‐up).
3.5. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 5 Microfilaraemia (mf) prevalence: stratified by baseline infection (36 months follow‐up).
Mf density
Seven trials reported the effects of adding albendazole to DEC on mf density. Pani 2002, Rizzo 2007, and De Britto 2015 only enrolled microfilaraemic individuals; Dreyer 2006 only enrolled individuals with FDS irrespective of mf status; and Kshirsagar 2004, Fox 2005, and Bockarie 2007 recruited mf‐positive and ‐negative participants, but only reported density in subsets of individuals enrolled at baseline; none reported the overall change in mf density in the total population enrolled up to 12 months follow‐up.
Overall, albendazole was associated with inconsistent effects on mf density up to six months (559 participants, 6 trials; Table 7), and was not associated with greater reductions in mf density at 12 months (535 participants, 6 trials; Table 8).
Up to six months, there were six studies that compared albendazole added to DEC to DEC alone. Five studies showed little or no effect with albendazole, and one study reported a slightly greater reduction with DEC alone (Dreyer 2006); there was no significant difference (P > 0.05) in the four trials that statistically evaluated this. One study assessed at high risk of bias (Fox 2005 excluded increases in mf density post‐treatment) reported a significant reduction (P = 0.02) with the addition of albendazole, but this is difficult to interpret given the risk of bias.
At 12 months, there were five studies at low or unclear risk of bias that showed no effect of adding albendazole, and Dreyer 2006 reported a slightly greater reduction with DEC alone; there was no statistically significant difference (P > 0.05) in four studies that tested this.
At 24 months, there was no effect of adding albendazole to DEC in two studies after one dose (Pani 2002; Bockarie 2007), and one study after two annual doses (Kshirsagar 2004); reported as not significant in two studies (P > 0.05) (795 participants, 3 trials; Table 10). At 36 months, two very small trials at high risk of bias reported no effect with albendazole after one annual dose (Pani 2002), or three annual doses (Kshirsagar 2004) (57 participants, 2 trials; Table 10).
6. Microfilarial density: 24 months and 36 months follow‐up.
| Background drug |
Risk of bias: Analysis used |
Trial (follow‐up) |
Intervention (albendazole) | Control | Difference between groups post‐treatment | ||||
| Participants | Baseline to follow‐up (% reduction) | Participants | Baseline to follow‐up (% reduction) | % reduction | Significance testing: % reduction | Significance testing: mf density | |||
| DEC | Low or unclear risk |
Bockarie 2007a,b (24 months)c |
348 | NR to 0.5 (83.7%) |
381 | NR to 0.7 (87.5%) |
−3.8% | NR | NS (P = 0.53) |
| High risk |
Pani 2002d,e (24 months)c |
18 | 98 (± 57) to 0.52 (± NR) (99.5%) | 17 | 133 (± 157) to 0.94 (± NR) (99.3%) |
0.2% | NR | NS (P > 0.05) | |
|
Kshirsagar 2004d,e (24 months)f |
16 | NR to 109.5 (± 143.3) (NR) | 15 | NR to 99.5 (± 119.3) (NR) |
NR | NR | NR | ||
|
Pani 2002d,e (36 months)c |
18 | 98 (± 57) to 0 (100%) |
17 | 133 (± 157) to 0 (100%) |
0% | NR | NR | ||
|
Kshirsagar 2004d,e (36 months)g |
4 | NR to 57.6 (± 56.0) (NR) |
8 | NR to 60.3 (± 61.5) (NR) |
NR | NR | NR | ||
Microfilariae (mf) density (mf/mL) data and significance testing for differences between groups at baseline and follow‐up, as reported by study authors. We calculated the percentage reduction when this was not reported by the authors (values are italicized), and also the difference between the percentage reductions in the intervention and control groups. We judged the risk of bias as high when studies used analytical methods that could affect the interpretation of the data, and low or unclear risk when there was no obvious analytical issues.
Abbreviations: DEC: diethylcarbamazine; mf: microfilariae; NR: not reported; NS: not significant; ±: standard deviation. aAll evaluable participants irrespective of baseline mf status. bReported as geometric mean. cAfter one annual dose albendazole plus DEC and DEC provided. dOnly participants positive for mf at baseline. eReported as arithmetic mean. fAfter two annual doses albendazole plus DEC and DEC provided. gAfter three annual doses albendazole plus DEC and DEC provided.
Antigenaemia prevalence
There was no effect of adding albendazole to DEC in reducing antigen prevalence at the longest follow‐up (954 participants, 5 trials; Analysis 3.6).
3.6. Analysis.
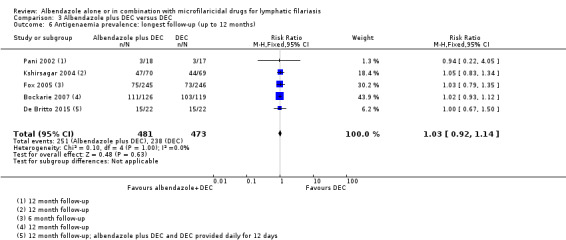
Comparison 3 Albendazole plus DEC versus DEC, Outcome 6 Antigenaemia prevalence: longest follow‐up (up to 12 months).
There was no benefit of albendazole plus DEC at six months (Analysis 3.7) or at 12 months (Analysis 3.8) post‐treatment, irrespective of baseline infection status. Treatment with albendazole plus DEC had no additive effect at 24 months follow‐up (Analysis 3.9) after either one annual dose or two annual doses; and no effect at 36 months (Analysis 3.10) after either one annual dose or three annual doses.
3.7. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 7 Antigenaemia prevalence: stratified by baseline infection (6 months follow‐up).
3.8. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 8 Antigenaemia prevalence: stratified by baseline infection (12 months follow‐up).
3.9. Analysis.
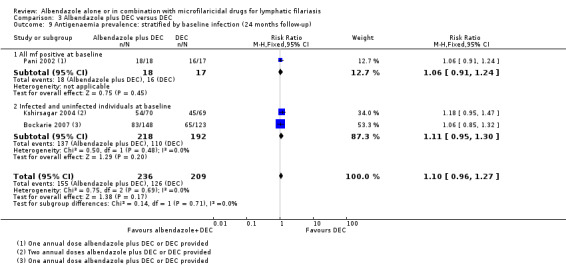
Comparison 3 Albendazole plus DEC versus DEC, Outcome 9 Antigenaemia prevalence: stratified by baseline infection (24 months follow‐up).
3.10. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 10 Antigenaemia prevalence: stratified by baseline infection (36 months follow‐up).
Antigen density
Three trials reported the effects of adding albendazole to DEC on antigen density. Pani 2002 and De Britto 2015 only recruited mf‐positive participants; Fox 2005 recruited antigen‐positive and ‐negative participants and reported density in people antigenaemic at baseline, not the overall change in antigen density in the total population enrolled.
Adding albendazole to DEC was not associated with greater reductions in antigen density between six and 12 months (270 participants, 3 trials; Table 9).
One study was at low or unclear risk of bias (De Britto 2015), and two studies were at high risk of bias (Fox 2005 excluded increases in mf density post‐treatment; Pani 2002 used the arithmetic mean). All three studies reported little or no effect of adding albendazole to DEC, reported as not significant (P > 0.05) in two studies that statistically evaluated this.
At 24 and 36 months after a single treatment, one small study at high risk of bias reported density was near pre‐treatment levels in both groups after 24 months (Pani 2002), and at 36 months density had increased in the albendazole plus DEC group but remained at pre‐treatment levels with DEC alone (35 participants, 1 trial; Table 11). At 24 months after a single treatment, Bockarie 2007 reported antigen concentration decreased from high to low in 16 (18.8%) participants with albendazole plus DEC, and 9 (14.7%) participants with DEC alone.
7. Antigen density: 24 months and 36 months follow‐up.
| Background drug | Risk of bias: analysis used | Trial (follow‐up) | Intervention (albendazole) | Control | Difference between groups post‐treatment | ||||
| Participants | Baseline to follow‐up (% reduction) | Participants | Baseline to follow‐up (% reduction) | % reduction | Significance testing: % reduction | Significance testing: CFA density | |||
| DEC | High risk |
Pani 2002a,b (24 months) |
18 | 0.5 to 0.48 (4%) |
17 | 0.39 to 0.44 (12.8% increase) |
16.8% | NR | NR |
|
Pani 2002a,b (36 months) |
18 | 0.5 to 1.2 (140% increase) |
17 | 0.39 to 0.79 (102.6% increase) |
−37.4% | NR | NR | ||
Circulating filarial antigen (CFA) density data and significance testing for differences between groups at baseline and follow‐up, as reported by study authors. Data was reported as the arithmetic mean and presented by the authors in graphs only; we extracted this information using WebPlotDigitizer software. We calculated the percentage reduction after treatment, and the difference between the percentage reductions in the intervention and control groups. We judged the risk of bias as high when studies used analytical methods that could affect the interpretation of the data, and low or unclear risk when there was no obvious analytical issues.
Abbreviations: CFA: circulating filarial antigen; DEC: diethylcarbamazine; mf: microfilariae; NR: not reported. aOnly participants positive for mf at baseline. bVolume of blood not reported, expressed as arithmetic mean CFA optical density value.
Adult worm prevalence detected by ultrasound
There was no difference with albendazole plus DEC for reducing adult worm prevalence in men examined for FDS by ultrasound at the longest follow‐up (165 participants, 3 trials; Analysis 3.11). However, the individual trials were all small and underpowered.
3.11. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 11 Adult worm prevalence by ultrasound: longest follow‐up (up to 12 months).
There was no benefit of adding albendazole to DEC at six months (Analysis 3.12) or at 12 months (Analysis 3.13) post‐treatment, or at 24 months (Analysis 3.14) after single dose or two annual doses, irrespective of baseline infection status.
3.12. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 12 Adult worm prevalence by ultrasound: stratified by baseline infection (6 month follow‐up).
3.13. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 13 Adult worm prevalence by ultrasound: stratified by baseline infection (12 month follow‐up).
3.14. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 14 Adult worm prevalence by ultrasound: stratified by baseline infection (24 month follow‐up).
Adverse events
Treatment with albendazole plus DEC had no effect on the number of participants experiencing adverse events (1589 participants, 4 trials; Analysis 3.15). Adverse events were systemic in three trials and De Britto 2015 did not provide details.
3.15. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 15 Adverse events.
There was no difference in adverse events that interfered with daily activity when albendazole was added to DEC (Analysis 3.16: subgroup 2). One small trial reported localized inflammatory reactions following treatment, but no difference between treatment groups (Analysis 3.16: subgroup 3). One small trial that enrolled only men with FDS reported intrascrotal nodules (a "sensitive reaction" to antifilarial drugs) at seven days post‐treatment; nodules were detected at the site of 21 (46.7%) adult worm nests with DEC alone compared to 2 (6.1%) with albendazole plus DEC (P = 0.002) (Dreyer 2006).
3.16. Analysis.

Comparison 3 Albendazole plus DEC versus DEC, Outcome 16 Adverse events: stratified by type.
Bockarie 2007 did not report adverse events, and Fox 2005 did not did not provide data in a form that we could use in meta‐analysis. Fox 2005 reported that adverse reactions were generally mild and well tolerated, with no statistically significant differences in specific symptoms or treatment impact scores between groups. Kshirsagar 2004 also assessed a smaller subset of individuals from the large safety study who were retreated at 12 months and 24 months, but differences between groups were not reported.
No life‐threatening adverse events or adverse events requiring hospitalization were reported in any trials.
Sensitivity analysis
In the sensitivity analyses including only trials where the risk of bias for allocation concealment was low, no difference between albendazole plus DEC and DEC groups in mf prevalence, antigenaemia prevalence, adult worm prevalence by ultrasound, or adverse events was evident. We do not present the sensitivity analyses here, as the results did not differ from those in the primary analyses.
Albendazole plus ivermectin versus ivermectin
No trials assessed adult worm prevalence (FDS) by ultrasound.
Mf prevalence
Treatment with albendazole plus ivermectin had no effect on mf prevalence at the longest follow‐up (2519 participants, 4 trials; Analysis 4.1). There was moderate heterogeneity detected (I2 = 65%) in this analysis, but subgroup analysis for length of follow‐up seemed to explain this. There were not enough trials to formally investigate the source of heterogeneity.
4.1. Analysis.
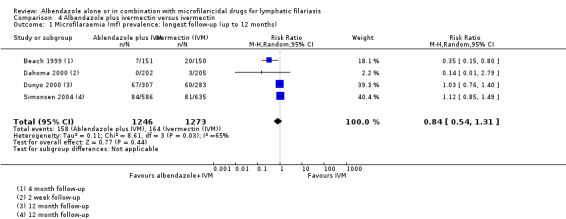
Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months).
Treatment with albendazole plus ivermectin did not have a statistically significant effect on mf prevalence up to six months (Analysis 4.2) or at 12 months (Analysis 4.3), irrespective of baseline infection status. Moderate to substantial heterogeneity was also detected within the subgroups of microfilaraemic participants (I2 = 75%) and infected and uninfected participants (I2 = 63%) at six months (Analysis 4.2). This also appeared to be explained by length of follow‐up, but could not be formally investigated.
4.2. Analysis.

Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 2 Microfilaraemia (mf) prevalence: stratified by baseline infection (up to 6 months follow‐up).
4.3. Analysis.

Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 3 Microfilaraemia (mf) prevalence: stratified by baseline infection (12 months follow‐up).
Mf density
Four trials reported the effects of adding albendazole to ivermectin on mf density. Beach 1999, Dunyo 2000, and Simonsen 2004 recruited mf‐positive and mf‐negative participants, but only reported density in people mf‐positive at baseline; none reported the overall change in the population mf density post‐treatment. Dahoma 2000 assessed mf density by density categories and we did not include these data in our analysis.
Adding albendazole to ivermectin was associated with inconsistent reductions in mf density up to six months (372 participants, 3 trials; Table 7), and was not associated with greater reductions at 12 months (348 participants, 2 trials; Table 8).
Up to six months, there were three studies that gave albendazole with ivermectin, and one of these was assessed at high risk of bias (Beach 1999 excluded increases in mf density post‐treatment). Two studies, Dunyo 2000 and Simonsen 2004, which we assessed as at low or unclear risk of bias, reported little or no effect on density with albendazole. Beach 1999 reported a significant effect (P < 0.001) but what this means is unclear, given the risk of bias.
At 12 months, there were two trials at low or unclear risk of bias (Dunyo 2000; Simonsen 2004). There was little or no difference in density with albendazole, reported as not significant (P = 0.80) in one study that statistically tested this.
Antigenaemia prevalence
There was no difference in antigen prevalence at the longest follow‐up up to 12 months (1766 participants, 2 trials; Analysis 4.4).
4.4. Analysis.

Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 4 Antigenaemia prevalence: longest follow‐up (up to 12 months).
There was no benefit of adding albendazole to ivermectin at six months (Analysis 4.5) or 12 months post‐treatment (Analysis 4.6), irrespective of baseline infection status.
4.5. Analysis.

Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 5 Antigenaemia prevalence: stratified by baseline infection (6 months follow‐up).
4.6. Analysis.

Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 6 Antigenaemia prevalence: stratified by baseline infection (12 months follow‐up).
Antigen density
Two trials reported the effects of adding albendazole to ivermectin on antigen density (Dunyo 2000; Simonsen 2004). Both trials recruited antigen‐positive and ‐negative participants, but only reported density in people antigen‐positive at baseline; none reported the overall change in the population antigen density post‐treatment.
Albendazole was associated with marginal reductions in antigen density at 12 months (733 participants, 2 trials; Table 9).
A slightly greater reduction in density with albendazole was reported in Dunyo 2000 (10.9% difference) and Simonsen 2004 (17.1% difference); the antigen density post‐treatment with albendazole was not significantly different (P > 0.80) in Dunyo 2000.
Clinical disease
At 12 months post‐treatment, adding albendazole to ivermectin had no effect on new (280 participants, 1 trial; Analysis 4.7: subgroup 1) or existing clinical disease (Analysis 4.7: subgroups 2 and 3); however, Dunyo 2000 was underpowered for clinical outcomes.
4.7. Analysis.

Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 7 Clinical disease.
Adverse events
Treatment with albendazole plus ivermectin had no effect on the number of participants experiencing adverse events (627 participants, 1 trial; Analysis 4.8).
4.8. Analysis.

Comparison 4 Albendazole plus ivermectin versus ivermectin, Outcome 8 Adverse events.
Beach 1999, Dahoma 2000, and Simonsen 2004 did not provide data in a form that we could use in meta‐analysis. Simonsen 2004 did not report the number of participants with adverse events in each group, but reported that all reactions were mild, and no significant relationship between headache or fever and the treatment given (P = 0.42 and P = 0.96). Beach 1999 reported that adverse reactions were generally mild, with no significant differences (P > 0.05) in the frequency or severity of symptoms between groups. Dahoma 2000 reported significant differences in fever (P = 0.045) and dizziness (P = 0.029) with ivermectin alone, and significant differences (P = 0.012) in headaches were reported with the combination treatment.
No serious or severe adverse reactions were reported in any of the trials. No localized inflammatory reactions were observed in Beach 1999 and Dunyo 2000, and Dahoma 2000 and Simonsen 2004 did not report this.
Sensitivity analysis
In the sensitivity analyses including only trials where the risk of bias for allocation concealment was low, no difference between albendazole plus ivermectin and ivermectin groups in mf prevalence, antigenaemia prevalence, or adverse events was evident. We do not present the sensitivity analyses here, as the results did not differ from those in the primary analyses.
Discussion
Summary of main results
Albendazole given alone or added to a microfilaricidal drug makes little or no difference to mf prevalence over two weeks to 12 months post‐treatment (high‐certainty evidence), but we do not know if there is an effect on mf density over one to six months (very‐low certainty evidence), or at 12 months follow‐up (very low‐certainty evidence). For antigenaemia prevalence between six to 12 months, albendazole alone or in combination makes little or no difference (high‐certainty evidence). For antigen density over six to 12 months, we do not know if albendazole has an effect (very low‐certainty evidence). For adult worm prevalence detected by ultrasound at 12 months, albendazole may make little or no difference (low‐certainty evidence). Albendazole alone or added to a microfilaricidal drug makes little or no difference to adverse events (high‐certainty evidence). See Table 1.
Albendazole given alone makes little or no difference to mf prevalence over four to 12 months post‐treatment (high‐certainty evidence), but we do not know if there is an effect on mf density after four to six months (very low‐certainty evidence), or at 12 months follow‐up (very low‐certainty evidence). For antigenaemia prevalence over six to 12 months post‐treatment, albendazole makes little or no difference (high‐certainty evidence). For antigen density over six to 12 months, we do not know if albendazole has an effect (very low‐certainty evidence). For adult worm prevalence detected by ultrasound, the effect of albendazole was not measured. Albendazole probably makes little or no difference to adverse events (moderate‐certainty evidence). See Table 2.
Albendazole added to DEC probably makes little or no difference to mf prevalence over six to 12 months post‐treatment (moderate‐certainty evidence). For mf density between one to six months, we do not know if there is an effect (very low‐certainty evidence), but albendazole co‐administered with DEC may make little or no difference to mf density at 12 months (low‐certainty evidence). For antigenaemia prevalence between six to 12 months post‐treatment, albendazole makes little or no difference (high‐certainty evidence). For antigen density over six to 12 months, we do not know if albendazole has an effect (very low‐certainty evidence). For adult worm prevalence detected by ultrasound at 12 months, albendazole plus DEC may make little or no difference (low‐certainty evidence). Albendazole added to DEC makes little or no difference to adverse events (high‐certainty evidence). See Table 3.
Albendazole added to ivermectin probably makes little or no difference to mf prevalence over two weeks to 12 months post‐treatment (moderate‐certainty evidence). For mf density between four to six months, we do not know if there is an effect (very low‐certainty evidence), but albendazole co‐administered with ivermectin may make little or no difference at 12 months (low‐certainty evidence). For antigenaemia prevalence at 12 months, albendazole makes little or no difference (high‐certainty evidence). For antigen density at 12 months, the albendazole plus ivermectin combination may make little or no difference (low‐certainty evidence). For adult worm prevalence detected by ultrasound, the effect of albendazole plus ivermectin versus ivermectin was not measured. Albendazole added to ivermectin probably makes little or no difference to adverse events (moderate‐certainty evidence). See Table 4.
Overall completeness and applicability of evidence
Measures of transmission potential
In people with lymphatic filariasis and people from lymphatic filariasis‐endemic communities, treatment with albendazole alone or albendazole added to antifilarial drugs, DEC or ivermectin, had little or no effect on mf prevalence. All trials included in the review assessed mf prevalence, and the evidence for the lack of effect comes from trials that were conducted in a variety of locations and settings, included both adults and children, and included both infected and uninfected individuals.
The trials used a range of methods to measure and calculate changes in mf density, and the reported efficacy of albendazole given alone or in combination with a microfilaricidal drug ranged from showing an effect to no effect, with greater inconsistency seen up to six months post‐treatment. All trials measured mf density, but trial authors mainly reported the results of small subgroups of microfilaraemic individuals at follow‐up, rather than all randomized individuals. The benefit of albendazole regimens when given to endemic communities could not be assessed.
No trials included in the review assessed treatment twice per year with albendazole, so we could not determine whether the WHO recommendation for albendazole alone twice per year to treat lymphatic filariasis in loiasis‐endemic areas is supported (WHO 2012). Other studies have reported a benefit of an increased dose or frequency of albendazole for individual treatment and community control, but these were either not placebo‐controlled trials (Pion 2015), or were not designed to assess the effects of albendazole alone (Kar 2015; Tafatatha 2015).
Markers of adult worm infection
Albendazole is thought to have some macrofilaricidal properties when given at high doses over several weeks (Jayakody 1993). However, a single 400 mg dose of albendazole (the dose used in MDA programmes), given either as monotherapy or as a combination therapy, had little or no effect on adult worm prevalence after six to 12 months.
Evidence for an overall effect of albendazole for reducing adult worm viability was limited to comparing the antigen density reductions and the trial authors' statistical interpretation, but no studies reported a significant effect (P < 0.05) of albendazole alone or when added to a microfilaricidal drug. The trials were individually‐randomized and primarily assessed subgroups of antigenaemic individuals, and so we could not evaluate the effect of albendazole on CFA density at the community level.
Three trials also assessed adult worm (filarial dance sign) prevalence using ultrasound with male participants treated with albendazole co‐administered with DEC or DEC alone. The limited current evidence suggests that albendazole may give little or no additional benefit over DEC alone. One trial included in this review reported that the addition of albendazole appeared to decrease the macrofilaricidal effect of DEC against W bancrofti (Dreyer 2006). However, these trials were small and so we can not completely rule out any macrofilaricidal effect.
Clinical disease
The effect of albendazole, either alone or when added to ivermectin for clinical disease, was not remarkable. This is not surprising as effect sizes for clinical outcomes were small and the one trial that assessed this was not powered to detect small clinical benefits (Dunyo 2000).
Adverse events
Nearly all trials reported on adverse events, with treatment with albendazole alone or combined with ivermectin or DEC making little difference to adverse events in people with lymphatic filariasis or in people in endemic communities. Adverse events were generally mild and systemic. Local adverse events were reported in two small trials that compared albendazole co‐administered with DEC to DEC alone (Dreyer 2006; Rizzo 2007). Rizzo 2007 observed no difference between groups, but Dreyer 2006 detected a higher proportion of "sensitive reactions" in men in the DEC group compared to men given the albendazole and DEC combination. There do not appear to be safety concerns for albendazole when given at the dose or in the drug combinations recommended for lymphatic filariasis MDA programmes (WHO 2006).
Long‐term effects
Multiple rounds of annual treatment with albendazole and either DEC or ivermectin are recommended in lymphatic filariasis elimination programmes in order to sustainably interrupt transmission. There is insufficient evidence to draw any meaningful conclusions on the long‐term impact of albendazole for lymphatic filariasis. The impact of albendazole on outcomes in the long term (at 24 or 36 months post‐treatment) was evaluated in four trials that compared albendazole added to DEC with DEC alone.
In a small subgroup of randomized participants, Kshirsagar 2004 reported that there was no effect of adding albendazole for any of the parasitological outcomes measured after three annual rounds of treatment. Pani 2002 and Bockarie 2007 showed little or no effect of adding albendazole for parasitological outcomes at 24 or 36 months after a single dose of the treatments; and Pani 2002, a very small trial, reported a greater increase in antigen density at 36 months post‐treatment with the albendazole combination therapy.
Certainty of the evidence
Thirteen trials, including one cluster‐RCT, with 8713 participants met the inclusion criteria. We assessed the certainty of the evidence for mf prevalence and antigenaemia prevalence outcomes as high for our main analysis, albendazole alone or added to a microfilaricidal drug. In individual comparisons, we graded the certainty of the evidence for mf prevalence as high for albendazole alone, and moderate for albendazole added to DEC and albendazole added to ivermectin. The other parasitological outcomes, mf density, antigen density, and adult worm prevalence detected by ultrasound, had low or very low certainty evidence for an effect of albendazole.
All trials were described as randomized, but they had important limitations. Most included studies were designed primarily to assess the effectiveness of albendazole for treatment of individuals, and did not explicitly consider the effects on transmission in whole communities. The numbers of participants lost to or excluded from the follow‐up were also very high (above 20%) in almost half of the trials, which could lead to imbalances in the comparison groups. However, the numbers lost were generally comparable between treatment groups within the trials.
Differences in design (mf‐positive participants only compared to positive and negative participants, variable outcome measurement and reporting, and follow‐up times) made it difficult to compare the trials. Most trials reported outcomes mainly for those who were mf‐positive or antigen‐positive at baseline. Selectively analysing subgroups of randomized participants may bias the conclusions of the study, and result in an overestimation or dilution of potential treatment effects.
For parasite density data, the difference in outcome summary measure reported (i.e. geometric mean, arithmetic mean, log mean), the analysis methods used, and the lack of reporting of SDs or CIs in most trials made it impossible to include these results in a meta‐analysis. Studies should report measures of variance or CIs so that the amount of uncertainty in the point estimate is clear. We judged the analytical methods used by some trials to be at high risk of bias due to the method used to calculate the change from baseline (Beach 1999; Fox 2005), or use of the arithmetic mean as the average estimate. For studies that reported no transformation onto the log scale for skewness in the data, using the arithmetic mean to measure skewed data is not appropriate. Tests of statistical significance were also not always carried out or reported. For these reasons, we downgraded the certainty of the evidence for density outcomes by two levels for imprecision; by one for risk of bias when data from Beach 1999 or Fox 2005 were included; and by one when there was also inconsistency between trials.
Potential biases in the review process
Statistical errors in analysis
We included one cluster‐RCT in the review (Wamae 2011), but the trial authors did not take adequate account of cluster randomization. The analyses for primary and secondary outcomes were not adjusted for clustering, and the trial authors reported results from subgroups of microfilaraemic and antigenaemic individuals. This could impact the interpretation of the trial, and we did not use these data in our analyses. However, we have reported all relevant outcomes not included in our analyses in Appendix 2.
Parasite density outcomes
Due to the poor reporting of parasite density outcomes we could not combine trials in a meta‐analysis. We attempted to contact several trial authors to clarify their methods or request CIs for the data (Beach 1999; Simonsen 2004; Fox 2005; Dreyer 2006; Bockarie 2007; Rizzo 2007; De Britto 2015). We received a response from Dreyer 2006 and Rizzo 2007, and are awaiting data from Beach 1999. We could not find an active email address for Simonsen 2004.
We therefore analysed density data by comparing the difference in percentage reduction between the intervention and control groups, with less weighting given to trials that reported the mean (as this does not account for potentially skewed data). We also considered the results of the statistical analyses reported by the authors. This could introduce bias, as authors assessed subgroups of the total randomized individuals and calculated the geometric mean and percentage reduction in geometric mean using different methods. Tests of statistical significance were not always carried out or reported. However, we judged the evidence to be low to very low certainty.
Subgroup analyses
Many of the included trials had several dissimilar follow‐up intervals and reported on subgroups of participants for the outcomes. We analysed the longest follow‐up up to 12 months from each trial, and used the number randomized as the denominator where possible. This meant combining trials that analysed individuals who were all microfilaraemic or positive for adult worms with trials that analysed infected and uninfected individuals. We believed this would not bias the findings of our review.
We did detect moderate heterogeneity when comparing albendazole plus ivermectin to ivermectin alone for mf prevalence, but this appeared to be explained by trial follow‐up periods, which ranged from two weeks to 12 months.
We also conducted additional meta‐analyses to assess different follow‐up times (up to six, and at 12, 24 and 36 months), and stratified the analyses by the participants' baseline infection status to rule out any potential time‐dependent effects or other specific effects of albendazole. The number of participants in the subgroup analyses were generally small, but the results of these additional meta‐analyses were in broad agreement with our primary analyses assessing the longest follow‐up data.
Agreements and disagreements with other studies or reviews
The findings from our review are in agreement with the findings from a literature review published in 2005, Tisch 2005, which conducted a systematic evaluation of data from publicly available drug trials to determine estimates of drug effect against W bancrofti mf in individuals and populations. Tisch 2005 concluded that the use of albendazole with a microfilaricidal drug does not appear to augment the effectiveness of a single microfilaricidal drug, and the authors also emphasized the need for further research and clearer reporting of trials. However, the methods of this literature review differed from our Cochrane Review: it was not a protocol‐driven systematic review; effect estimates and precision around the effect estimate for outcomes were not determined using meta‐analyses; and the study quality was not assessed for included studies.
The findings of our review are at odds with the original documents that led to the introduction of albendazole to filarial control programmes, including a WHO consultation on albendazole research findings in lymphatic filariasis (WHO 1998) and a narrative review (Ottesen 1999). The narrative review conducted by the WHO concluded that "single dose 2‐drug combinations of albendazole plus either ivermectin or DEC are superior in efficacy to single drug treatment for decreasing microfilaraemia in lymphatic filariasis", and that "Albendazole alone has a killing or sterilizing activity on lymphatic filarial adult worms" (WHO 1998).
An expert opinion review and meta‐analysis by Gyapong 2005 favoured the two‐drug regimens over single microfilaricidal drugs for treating and preventing lymphatic filariasis. Their analyses differ from our analyses in a number of ways: it was not a protocol‐driven systematic review; the authors included scientific literature supplemented by reports and studies, and did not assess the quality of the studies; the authors only included studies where the participants were microfilaraemic; the statistical significance may also have been overstated in some analyses, since data from several studies were incorporated twice (by counting results at six and 12 months and combining them in the same meta‐analysis), which artificially narrows the 95% CIs.
A narrative literature review by Olsen 2007 presented evidence reported by individual studies, and concluded: “Results with ALB added to single‐drug therapy with IVM or DEC against lymphatic filariasis were inconclusive, but DEC and IVM in combination appeared to be superior to DEC or IVM alone.” Their analyses differ from ours, in that: it was not a protocol‐driven systematic review; it was a narrative summary of studies rather than a meta‐analysis of data; and the study quality was not assessed for included studies.
Authors' conclusions
Implications for practice.
There is good evidence from individually‐randomized trials that albendazole has little or no effect on completely clearing the mf or adult worms up to 12 months after treatment, and no convincing data across studies of an effect on mf density or adult worm viability. This finding is consistent in studies evaluating albendazole alone, or studies where albendazole is added to DEC or ivermectin‐ two drugs known to be effective in community treatment programmes.
If there is a true but as yet unproven effect on parasite density, then it is possible that albendazole could have an effect on transmission in mass treatment programmes. There are no large cluster‐randomized studies to determine whether there is a population‐level effect, although these were called for in the initial WHO informal consultation in 1998 (WHO 1998).
This review, and the earlier editions, raise fundamental questions around the evidence base of the effectiveness of albendazole and thus its inclusion in the global lymphatic filariasis elimination programme. Given that the drug is part of mainstream policy, and the WHO now recommend the triple‐drug regimen IDA (ivermectin, DEC, and albendazole), we are unlikely to see new research evaluating albendazole in combination with DEC or ivermectin.
However, albendazole alone is recommended in areas endemic for L loa. In our view, this remains a priority for research through placebo‐controlled trials to know whether the drug is effective in these communities.
Implications for research.
The key area that needs elucidation is whether albendazole has an independent effect on mf density, to guide treatment decisions for lymphatic filariasis in L loa‐endemic areas.
Re‐analysis of the existing parasite density data as part of an individual patient data meta‐analysis would be theoretically helpful, but we have sought the data without success, and this does not look feasible. Future study authors should consider depositing their data and analyses in community‐recognized repositories, to make it possible to reproduce results and facilitate meta‐analysis.
In further research, it would help if there were better standardization in field and analytical methods. Techniques for assessing mf in blood and outcome measures for mf densities should also be standardized, with complete reporting of all randomized individuals. The synthesis of data for mf density in this review proved to be challenging. In many studies, the authors applied log transformations to the data to be able to calculate geometric means, since data were skewed. It was not possible to meta‐analyse data for this outcome due to poor reporting of methods of analysis and results in the individual study reports. Firstly, many studies described methods to accommodate zero values (such as adding 1 to each value before taking the log of each value), but these methods were often not sufficiently detailed and referenced. Study authors should describe exactly how the method was applied (i.e. to all values or to zero values only), and exactly what summary measures are presented (i.e. geometric means, log means) and how these were calculated.
For example, Simonsen 2004 reports that “geometric mean intensities (mf GMIs) were calculated as antilog[(∑ log x + 1))/n] – 1”; this is perfectly sufficient detail, but many studies’ methods were not so clear. Secondly, several studies reported only the point estimates of the geometric mean, or the log mean, without any measure of variance or CIs. Studies should report measures of variance or CIs so that the amount of uncertainty in the estimate is clear; this would also enable study results to be included in meta‐analyses. Finally, some studies reported no transformation onto the log scale for skewness in the data; if data were skewed then summarizing using arithmetic means is not appropriate, and it then becomes impossible to combine studies which report arithmetic means with studies that report geometric or log means.
What's new
| Date | Event | Description |
|---|---|---|
| 8 January 2019 | New citation required and conclusions have changed | We performed a search update and included 13 trials in total. We assessed the certainty of the evidence using the GRADE approach. |
| 8 January 2019 | New search has been performed | New author team; search update; all data re‐extracted; density data summarized more comprehensively; ‘Summary of findings' tables constructed. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 5 August 2008 | Amended | Converted to new review format with minor editing. |
| 14 August 2005 | New search has been performed | The first review update, published in Issue 4, 2005, includes three new trials, Fox 2005, Kshirsagar 2004, and Simonsen 2004, and a two‐year update of results from the Pani 2002 trial. |
Acknowledgements
The Academic Editor of this review update was Dr Patricia Graves.
This document is an output from the COUNTDOWN project funded by the UK Department for International Development (DFID). CM received salary support from the COUNTDOWN Research Consortium. COUNTDOWN is committed to trials and development of mass treatment programmes related to NTDs (Grant: 6407).
SJ, MR, PG, and the editorial base of the Cochrane Infectious Diseases Group are supported by the Research, Evidence and Development Initiative (READ‐It) project. READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
We thank Jose Angelo Rizzo and Gerusa Dreyer of the Rizzo 2007 trial, and Gerusa Dreyer and David Addiss of the Dreyer 2006 study, for providing us with additional data and analyses.
We also thank David Addiss for his assistance in updating this review.
Appendices
Appendix 1. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | filaria* | filaria* ti, ab | "Filariasis"[Mesh] ti, ab | Filariasis [Emtree, ti, ab] | filaria* |
| 2 | albendazole | elephantiasis ti, ab | "lymphatic filariasis" ti, ab | "lymphatic filariasis" ti, ab | elephantiasis |
| 3 | benzimidazole | lymphedema ti, ab | "Elephantiasis, Filarial"[Mesh] | Elephantiasis [Emtree, ti, ab | lymphedema |
| 4 | 2 or 3 | wuchereria ti, ab | lymphedema ti, ab | lymphedema ti, ab | wuchereria |
| 5 | 1 and 4 | brugia ti, ab | "Wuchereria bancrofti"[Mesh] | "Wuchereria bancrofti" [Emtree, ti, ab] | brugia |
| 6 | — | 1 or 2 or 3 or 4 or 5 | "Brugia"[Mesh] | Brugia [Emtree, ti, ab] | 1 or 2 or 3 or 4 or 5 |
| 7 | — | diethylcarbamazine ti, ab | 1 or 2 or 3 or 4 or 5 or 6 | 1 or 2 or 3 or 4 or 5 or 6 | diethylcarbamazine |
| 8 | — | ivermectin ti, ab | "Filaricides"[Mesh] | antifilarial agent [Emtree] | ivermectin |
| 9 | — | benzimidazole ti, ab | diethylcarbamazine ti, ab | diethylcarbamazine ti, ab | benzimidazole |
| 10 | — | albendazole ti, ab | ivermectin ti, ab | ivermectin ti, ab | albendazole |
| 11 | — | carbamazine ti, ab | benzimidazole ti, ab | benzimidazole ti, ab | carbamazine |
| 12 | — | hetrazan ti, ab | "Albendazole"[Mesh] ti, ab | albendazole ti, ab | hetrazan |
| 13 | — | luxuran ti, ab | carbamazine ti, ab | carbamazine ti, ab | luxuran |
| 14 | — | mectizan ti, ab | hetrazan ti, ab | hetrazan ti, ab | mectizan |
| 15 | — | metiazol ti, ab | luxuran ti, ab | luxuran ti, ab | metiazol |
| 16 | — | valbazen ti, ab | mectizan ti, ab | mectizan ti, ab | valbazen |
| 17 | — | 7‐16/OR | metiazol ti, ab | metiazol ti, ab | 7‐16/OR |
| 18 | — | 6 and 17 | valbazen ti, ab | valbazen ti, ab | 6 and 17 |
| 19 | — | Limit 18 to human | 8‐18/OR | 8‐18/OR | — |
| 20 | — | — | 7 and 19 | 7 and 19 | — |
| 21 | — | — | Limit 20 to human | Limit 20 to human | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by the Cochrane Collaboration (Lefebvre 2011).
Appendix 2. Cluster‐RCT not included in analyses
| Cluster‐RCT | Outcome | Albendazole plus DEC (follow‐up) | DEC (follow‐up) | Trial authors' comments | Reported multilevel mixed‐effects regression model analysis |
| Wamae 2011 | Mf prevalence | Number of participants: NR Prevalence: NR |
Number of participants: NR Prevalence: NR |
“none of the persons receiving DEC/ALB combination had detectable microfilaraemia at 24 months follow‐up” |
NR |
| Mf density (mean log mf/mL)a | Number of participants: 25/54 Pre‐treatment: 5.84 1 week: 2.92 6 months: 2.81 12 months: 0.76 24 monthsb: 0.01 |
Number of participants: 26/54 Pre‐treatment: 5.73 1 week: 4.10 6 months: 3.22 12 months: 2.05 24 monthsb: 1.03 |
“… at two years of follow‐up the decrease in geometric mean MF count was very high for all the 3 treatment groups, 98%, 99% and 100% for ALB, DEC and DEC/ALB groups, respectively” | Showed no effect of albendazole plus DEC: geometric mean difference 2.9, 95% CI 1.5 to 12.9, P = 0.146 | |
| Antigenaemia prevalence | Number of participants: NR Prevalence: NR |
Number of participants: NR Prevalence: NR |
NR | NR | |
| Antigen density (mean log CFA units/mL)a | Number of participants: 21/54 Pretreatment: 6.82 1 week: 7.13 6 months: 6.89 12 months: 6.34 24 monthsb: 5.02 |
Number of participants: 26/54 Pretreatment: 7.03 1 week: 7.59 6 months: 7.24 12 months: 6.94 24 monthsb: 6.12 |
“… compared to pre‐treatment levels, the overall reduction in mean CFA levels at 2 years was 34%, 60% and 85% for ALB, DEC and DEC/ALB groups, respectively” | Suggested an effect of albendazole plus DEC: geometric mean difference 4.4, 95% CI 0.6 to 9.67, P = 0.049 |
Abbreviations: CFA: circulating filarial antigen; CI: confidence interval; DEC: diethylcarbamazine; mf: microfilariae; NR: not reported; RCT: randomized controlled trial.
aData were presented by the authors in graphs only, and were extracted using WebPlotDigitizer software (Rohatgi 2017). bAfter two annual doses, albendazole plus DEC and DEC were provided.
Data and analyses
Comparison 1. Albendazole alone or added to a microfilaricidal drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months) | 12 | 5027 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 1.1 Albendazole versus placebo | 4 | 1406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 1.2 Albendazole plus DEC versus DEC | 7 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.09] |
| 1.3 Albendazole plus ivermectin versus ivermectin | 4 | 2519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.19] |
| 2 Antigenaemia prevalence: longest follow‐up (up to 12 months) | 7 | 3774 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.12] |
| 2.1 Albendazole versus placebo | 2 | 1054 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.25] |
| 2.2 Albendazole plus DEC versus DEC | 5 | 954 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.14] |
| 2.3 Albendazole plus ivermectin versus ivermectin | 2 | 1766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 3 Adult worm prevalence by ultrasound: longest follow‐up (up to 12 months) | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.72, 1.86] |
| 3.1 Albendazole plus DEC versus DEC | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.72, 1.86] |
| 4 New clinical disease (new cases hydrocoele) | 1 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.23, 8.36] |
| 4.1 Albendazole versus placebo | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.45] |
| 4.2 Albendazole plus ivermectin versus ivermectin | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.17, 19.73] |
| 5 Pre‐existing clinical disease (net improvement) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Albendazole versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Albendazole plus ivermectin versus ivermectin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse events | 6 | 2894 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.13] |
| 6.1 Albendazole versus placebo | 2 | 678 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.48] |
| 6.2 Albendazole plus DEC versus DEC | 4 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 6.3 Albendazole plus ivermectin versus ivermectin | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.77, 1.74] |
Comparison 2. Albendazole versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months) | 4 | 1406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.19] |
| 2 Microfilaraemia (mf) prevalence: stratified by baseline infection (up to 6 months follow‐up) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 All mf positive at baseline | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.10] |
| 2.2 Infected and uninfected individuals at baseline | 2 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.43] |
| 3 Microfilaraemia (mf) prevalence: stratified by baseline infection (12 months follow‐up) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 All mf positive at baseline | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.86, 1.03] |
| 3.2 Infected and uninfected individuals at baseline | 1 | 591 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.26] |
| 4 Antigenaemia prevalence: longest follow‐up (up to 12 months) | 2 | 1054 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.25] |
| 5 Antigenaemia prevalence: stratified by baseline infection (6 months follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Infected and uninfected individuals at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Antigenaemia prevalence: stratified by baseline infection (12 months follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 All adult worm positive (CFA) at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Infected and uninfected individuals at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Clinical disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 New cases hydrocoele | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Net improvement (lymphoedema) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Total improvement (hydrocoele) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events | 2 | 678 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.48] |
Comparison 3. Albendazole plus DEC versus DEC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months) | 7 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.09] |
| 2 Microfilaraemia (mf) prevalence: stratified by baseline infection (up to 6 months follow‐up) | 7 | 1004 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.08] |
| 2.1 All mf positive at baseline | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.21] |
| 2.2 All adult worm positive (CFA or ultrasound) at baseline | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.74, 1.18] |
| 2.3 Infected and uninfected individuals at baseline | 1 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.32, 1.21] |
| 3 Microfilaraemia (mf) prevalence: stratified by baseline infection (12 months follow‐up) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 All mf positive at baseline | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.54, 1.45] |
| 3.2 All adult worm positive (CFA or ultrasound) at baseline | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.27] |
| 3.3 Infected and uninfected individuals at baseline | 1 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.78, 1.82] |
| 4 Microfilaraemia (mf) prevalence: stratified by baseline infection (24 months follow‐up) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 All mf positive at baseline | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 13.93] |
| 4.2 All adult worm positive (CFA) at baseline | 1 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.17] |
| 4.3 Infected and uninfected individuals at baseline | 2 | 868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.71, 1.27] |
| 5 Microfilaraemia (mf) prevalence: stratified by baseline infection (36 months follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Infected and uninfected individuals at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Antigenaemia prevalence: longest follow‐up (up to 12 months) | 5 | 954 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.14] |
| 7 Antigenaemia prevalence: stratified by baseline infection (6 months follow‐up) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 All mf positive at baseline | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.11] |
| 7.2 All adult worm positive (CFA) at baseline | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.04] |
| 7.3 Infected and uninfected individuals at baseline | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.27] |
| 8 Antigenaemia prevalence: stratified by baseline infection (12 months follow‐up) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 All mf positive at baseline | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.22, 4.05] |
| 8.2 All adult worm positive (CFA) at baseline | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.10] |
| 8.3 Infected and uninfected individuals at baseline | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.83, 1.34] |
| 9 Antigenaemia prevalence: stratified by baseline infection (24 months follow‐up) | 3 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.27] |
| 9.1 All mf positive at baseline | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.24] |
| 9.2 Infected and uninfected individuals at baseline | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.30] |
| 10 Antigenaemia prevalence: stratified by baseline infection (36 months follow‐up) | 2 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.42] |
| 10.1 All mf positive at baseline | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.95, 1.53] |
| 10.2 Infected and uninfected individuals at baseline | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.84, 1.50] |
| 11 Adult worm prevalence by ultrasound: longest follow‐up (up to 12 months) | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.72, 1.86] |
| 12 Adult worm prevalence by ultrasound: stratified by baseline infection (6 month follow‐up) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 All mf positive at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 All adult worm positive (ultrasound) at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Infected and uninfected individuals at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Adult worm prevalence by ultrasound: stratified by baseline infection (12 month follow‐up) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 All mf positive at baseline | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.37, 1.66] |
| 13.2 All adult worm positive (ultrasound) at baseline | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.69, 3.40] |
| 13.3 Infected and uninfected individuals at baseline | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.53, 1.75] |
| 14 Adult worm prevalence by ultrasound: stratified by baseline infection (24 month follow‐up) | 2 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.62, 2.79] |
| 14.1 All mf positive at baseline | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.09, 40.60] |
| 14.2 Infected and uninfected individuals at baseline | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.59, 2.77] |
| 15 Adverse events | 4 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 16 Adverse events: stratified by type | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Any | 4 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.11] |
| 16.2 Interferred with daily activity | 2 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.67, 1.77] |
| 16.3 Localized | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.43] |
Comparison 4. Albendazole plus ivermectin versus ivermectin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microfilaraemia (mf) prevalence: longest follow‐up (up to 12 months) | 4 | 2519 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 2 Microfilaraemia (mf) prevalence: stratified by baseline infection (up to 6 months follow‐up) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 All mf positive at baseline | 4 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.50, 1.02] |
| 2.2 Infected and uninfected individuals at baseline | 3 | 1929 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.23, 1.25] |
| 3 Microfilaraemia (mf) prevalence: stratified by baseline infection (12 months follow‐up) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 All mf positive at baseline | 2 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3.2 Infected and uninfected individuals at baseline | 2 | 1811 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 4 Antigenaemia prevalence: longest follow‐up (up to 12 months) | 2 | 1766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 5 Antigenaemia prevalence: stratified by baseline infection (6 months follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 All adult worm positive (CFA) at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Infected and uninfected individuals at baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Antigenaemia prevalence: stratified by baseline infection (12 months follow‐up) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 All adult worm positive (CFA) at baseline | 2 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.08] |
| 6.2 Infected and uninfected individuals at baseline | 2 | 1766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 7 Clinical disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 New cases hydrocoele | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Net improvement (lymphoedema) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Net improvement (hydrocoele) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beach 1999.
| Methods | RCT Study dates: January 1996 to May 1996 Length of follow‐up: 4 months Method of microfilariae (mf) assessment/volume of blood: thick smear, 20 µL of finger‐prick blood collected between 7pm and 9.30pm Method of adverse event assessment: schools were revisited for 3 – 5 days after treatment to systematically measure adverse reactions in the microfilaraemic children and to provide medical consultation to other children. Adverse event severity was graded and a total‐peak intensity score calculated |
|
| Participants | All children attending 5 selected primary schools Number analysed for primary outcome: 585 participants of 965 participants randomized Mean age (years): 7.4 Inclusion criteria: 1) age 5 to 11 years; 2) anthropometric measurements before and 4 months after treatment; 3) stool specimens before and 5 weeks after treatment; 4) random assignment to a treatment group; 5) height, weight, and age within limits of the anthropometric database |
|
| Interventions | Single dose
|
|
| Outcomes | For all children
For mf‐positive children only
Not included in review: Intestinal helminth prevalence and intensity; reduction in intensity of geohelminth infections reported as geometric means, as defined by egg count (eggs/gram of stool [epg]); anthropometric measurements of height and weight measurements; a stool examination for intestinal helminths; hematocrit measurements |
|
| Notes | Study type: school‐based Location: Leogane, Haiti Medication supervised: children took the medication under direct investigator observation Source of funding: USAID Endemicity level: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “For each school, all eligible students were assigned, using a random number table, to four treatment groups” |
| Allocation concealment (selection bias) | Low risk | Quote: “Treatment was given... by one of the investigators from Centers for Disease Control and Prevention, where the code for allocation was kept. The code was broken at the end of the second follow‐up.” |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Low risk | Quote: “personnel evaluating students for adverse reactions were blinded to the treatment status of the children” Quote: "double blind". Comment: although drugs were not identical, patients had no way of identifying them |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Quote: “Laboratory personnel, measurement teams… were blinded to the treatment status of the children.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 585/965 (61%) of randomized participants were evaluated for primary outcome. Reason for losses to follow‐up were reported as exclusion of children without both pre‐ and post‐treatment blood samples from analyses. Inclusion of all randomized participants (number evaluable/number randomized): 61% (585/965) |
| Selective reporting (reporting bias) | Unclear risk | Comment: authors stated in the methods: "Adverse reactions included headache, fever, myalgias, abdominal pain, passage of worms in the stool, vomiting, diarrhoea, cough, and dyspnoea". Author did not report on dizziness, weakness, or abdominal pain |
| Other bias | Unclear risk | Comment: risk of bias for mf density is unclear, as before estimating the percentage reduction between baseline and follow‐up, the authors omitted increases in density. This study simply provides an assessment of the decrease in density only in people experiencing a decrease. Whilst this rule was applied to both intervention and control groups, we were uncertain of the effect of this on the estimate, or exactly what the estimate was measuring |
Bockarie 2007.
| Methods | RCT Study dates: September 1999 to September 2001 Length of follow‐up: 24 months Method of mf assessment/volume of blood: light microscopy after Nuclepore® filtration, 1 mL venous blood collected between 10pm and 2am Antigen testing: Og4C3 antigen ELISA |
|
| Participants | All adults and children living in an endemic area Number analysed for primary outcome: 729 participants of 1007 participants randomized (at 24 month final follow‐up only) Mean age (years): 23.4 (DEC) and 24.7 (DEC plus albendazole) Inclusion criteria: all residents > 2 years of age Exclusion criteria: pregnant women |
|
| Interventions | Single dose
|
|
| Outcomes | For all individuals and the subset of individuals antigen‐positive at baseline Measured:
Reported: Outcomes were analysed for different subsets of participants based on availability of samples at different time points or pre‐treatment parasitological status; however, outcomes were not fully reported for some subsets of individuals or for the time points surveyed |
|
| Notes | Study type: community‐based Location: all 3 villages on Bagabag Island, northeast of Madang in Madang Province, Papua New Guinea Source of funding: WHO/CTD grant and WHO grant Medication supervised: witnessed drug administration Endemicity level: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “assigned randomly” Comment: Not clear how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 729/1007 (72.4%) of randomized participants were evaluated for primary outcome. Losses to follow‐up were attributed to the availability of participant samples at different time points. Inclusion of all randomized participants (number evaluable/number randomized): 72.4% (729/1007). There were high losses to follow‐up for other outcome analyses: 245/527 (46.5%) of randomized antigen‐positive participants were evaluated at 6, 12 and 24 months for mf outcomes, and months 6 and 12 for antigenaemia outcomes; 271/1007 (26.9%) of randomized participants were evaluated (different individuals from other analysis) for antigenaemia outcomes at 24 months |
| Selective reporting (reporting bias) | High risk | Authors stated in the methods: "The MF and Og4C3 levels intensities were compared between treatment groups and across follow‐up periods..." Comment: Antigen density data were measured at 6 and 12 months, but only reported at 24 months follow‐up in a small subset of participants; the intervention was favoured at this time |
| Other bias | Low risk | No other obvious source of bias |
Dahoma 2000.
| Methods | RCT Study dates: November 1999 to February 2000 Length of follow‐up: 2 weeks Method of mf assessment/volume of blood: counting chamber technique, 200 μL fingerprick blood collected between 10pm and 3am, and between 10pm and 12pm at follow‐up Method of adverse event assessment: Side effects and their types were determined by follow‐up and close monitoring for development of adverse signs and symptoms up to 96 hours post‐treatment |
|
| Participants | All individuals living in 2 endemic areas Number analysed for primary outcome: 407 participants of 418 participants randomized (97.4%) Age range/mean age: not reported Inclusion criteria: Individuals > 2 years of age with microfilaraemia or clinically active disease Exclusion criteria: Sick, pregnant, history of allergy to treatment drugs |
|
| Interventions | Single dose
|
|
| Outcomes |
Not included in review: Community screening data; reduction (%) in mf post‐treatment by age and sex; percentage reduction in mf intensity post‐treatment stratified by 3 intensity categories; symptoms reported post‐treatment with a prevalence 1 ‐ 3.9%; significance of change in proportion of reported symptoms with values greater than 4% prevalence; measurement of pulse, respiratory, systolic and diastolic blood pressure in individuals over 12; prevalence, intensity and reduction in geohelminth infection post‐treatment (by age and sex); prevalence of co‐infection of LF with geohelminths |
|
| Notes | Study type: community‐based Location: Unguja Island, Zanzibar Source of funding: author sponsored by MOH‐Zanzibar and WHO Tanzania office Medication supervised: not reported Endemicity level: 13.7% in the south district |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants allocation to treatment arms was done by tossing a coin." |
| Allocation concealment (selection bias) | Low risk | Quote: "Since the drugs were received unrandomized, drug randomisation had to be done locally basing on patient weight.... This procedure was done by an experienced clinical officer and drugs were coded." |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Unclear risk | Quote: “Double blind” Comment: unclear if the placebo and albendazole were identical, but participants likely had no way of identifying them Unclear how personnel were blinded |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Quote: "Drug codes were broken when post‐treatment when parasitological examination was completed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 407/418 (97.4%) of randomized participants were evaluated for primary outcome. Reasons for losses to follow‐up were reported. Inclusion of all randomized participants (number evaluable/number randomized): 97.4% (407/418) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No obvious other source of bias |
De Britto 2015.
| Methods | RCT Study dates: not reported Length of follow‐up: 12 months Method of mf assessment/volume of blood: membrane filtration (with 5 micron membrane filter, Millipore, type TMTP) and examination of stained filters by microscopy, 1 mL venous blood collected between 8pm and 10pm Antigen testing: Og4C3 ELISA and Immunochromatographic card test (ICT) Method of adverse event assessment: clinical nurse visited the study participants every day to record the symptoms of adverse reactions |
|
| Participants | Microfilaraemic individuals identified by screening Number analysed for primary outcome: 64 participants of 75 participants randomized in the DEC treatment group and the DEC plus albendazole treatment group Mean age (years): 36.1 (DEC) and 35.8 (DEC plus albendazole) Inclusion criteria: adults with night blood microfilaria counts > 10 mf/mL Exclusion criteria: body weight < 30 kg, filariasis treatment in previous 2 years or de‐worming treatment in previous year, concurrent illness, psychiatric disorders and patients under rifampicin, minocycline or doxycycline therapy Pregnant women and lactating mothers |
|
| Interventions | Multiple doses
|
|
| Outcomes |
Note: SD reported only for baseline mean (log) mf count and mean (log) antigen units, but not at follow‐up |
|
| Notes | Study type: community‐based Location: 35 endemic villages of Vector Control Research Centre (VCRC) field practice areas in Pondicherry and Tamil Nadu regions, South India Source of funding: Indian Council of Medical Research (ICMR), Department of Health Research, Government of India Medication supervised: not reported Endemicity level: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “All eligible participants were divided into blocks of size four and within each block, individual randomization irrespective of the gender and blood microfilaria count was done to have almost equal number of participants in each regimen.” Comment: unclear how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Unclear risk | Quote: "double‐blind". Comment: placebo used for 2nd treatment pulse in 3 of 4 treatment groups, unlikely participants knew which treatment they were given Unclear how personnel were blinded |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: inclusion of all randomized participants (number evaluable/number randomized): 88.4% (129/146). 85.3% (64/75) of randomized participants in the DEC treatment group and DEC plus albendazole treatment group were evaluated. Reasons for loss to follow‐up reported, and there was similar attrition between 2 treatment groups. Inclusion of all randomized participants (number evaluable/number randomized): 85.3% (64/75) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported Comment: mf clearance at 26 weeks and 52 weeks reported graphically |
| Other bias | Low risk | No obvious other source of bias |
Dreyer 2006.
| Methods | RCT Study dates: not reported Length of follow‐up: 12 months Method of mf assessment/volume of blood: membrane filtration (3 μm Nucleopore filter, Nuclepore Corporation, Pleasanton, CA, USA) and microscopy of stained filter, 1 mL venous blood collected at night Method of macrofilariae viability assessment: physical and ultrasound examinations of the scrotal area to identify intrascrotal nodules and filaria dance sign (FDS). Ultrasound examinations involved a portable ALOKA SSD‐500 (Japan) or a portable Pie Medical 200 (The Netherlands) ultrasound machine, both equipped with a 7.5 mHz probe. Physical and ultrasound examinations of the lymphatic vessels and lymph nodes elsewhere in the body were also performed |
|
| Participants | Adult men with FDS identified by screening Number analysed for primary outcome: 46 participants of 47 participants randomized Mean age (years): 21.5 (DEC) and 29.4 (DEC plus albendazole) Inclusion criteria: (1) over 18 years of age; (2) reproducible FDS confirmed by 2 independent investigators on 3 separate occasions pre‐treatment; (3) no hydrocoele or genital lymphoedema; (4) no history of DEC or ivermectin treatment; (5) no anthelminthic drugs post‐treatment; (6) adhered to follow‐up schedule |
|
| Interventions | Single dose
|
|
| Outcomes |
(Note: the raw data files were obtained from the authors on request) |
|
| Notes | Study type: hospital‐based Location: outpatient clinic of NEPAF, Hospital das Clínicas, Federal University of Pernambuco, Recife, Brazil Source of funding: Amaury Coutinho Non‐Governmental Organization, Recife, Brazil Medication supervised: treated under direct observation Endemicity level: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The men were randomly assigned to a treatment group” Comment: unclear how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Unclear risk | Comment: unclear whether assessors counting mf were blinded. Physical examinations were blinded, but there may be insufficient blinding of ultrasound examinations: Quote: “The physician performing the physical examinations (J.N.) was unaware of the subject’s treatment status or ultrasound findings.” Quote: “Two sonographers independently performed ultrasound examinations; one of these examiners remained blinded both to treatment status and physical examination results throughout the study.” Quote: “The two sonographers agreed on ultrasound findings for all study subjects.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 97.9% (46/47) of randomized participants were evaluated. Reasons for losses to follow‐up were reported. Inclusion of all randomized participants (number evaluable/number randomized): 97.9% (46/47) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Comment: men who were treated with DEC alone were significantly younger (mean age, 21.5 years) than those who received both drugs (mean, 29.4 years) |
Dunyo 2000.
| Methods | RCT Sudy dates: October 1996 to July 1998 Length of follow‐up: 12 months Method of mf assessment/volume of blood: counting chamber technique, 100 µL of fingerprick blood collected at night from 9pm Antigen testing: ELISA testing using fingerprick blood specimens Method of clinical disease assessment: Individuals were clinically examined during the day for evidence of elephantiasis and hydrocoele. Limb lymphoedema and hydrocoele were graded Method of adverse event assessment: treated individuals were monitored for 5 days to record self‐reported adverse reactions using a check‐list. Reaction severity was graded as 0 = none; 1 = mild (noticeable to the participant but not interfering with daily activities); 2 = moderate (some interference with daily activities); and 3 = severe (complete interruption of daily activities), and for 1 year to report any long‐term untoward events |
|
| Participants | All individuals living in 4 endemic areas Number analysed for primary outcome: 1181 participants of 1425 participants randomized Mean age: 26.4 Exclusion criteria: children aged < 6 years and pregnant women |
|
| Interventions | Single dose
|
|
| Outcomes |
Not included in review: mortality during follow‐up (Note: standard deviation (SD) for geometric mean density data was not reported. 95% CIs for geometric mean mf intensity were reported only for individuals who had ≥ 100 mf/mL before treatment and who were also examined at 12 months after treatment) |
|
| Notes | Study type: community‐based Location: south‐western Ghana (Butre, Achowa, Adjan, and Miamia villages) Source of funding: Danish Bilharziasis Laboratory, Denmark Medication supervised: treatment administered under direct observation of the study team Endemicity level: 18% to 25% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The treatment group assignment was performed by random allocation of numbers 1‐4 to the study individuals using a dBASE IV computer software programme.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The consignments of drugs were received at the Danish Bilharziasis Laboratory (DBL), Charlottenlund, Denmark, where they were coded by a scientist who was not part of the study team. Coding was carried out independently for each village.” |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Unclear risk | Quote: “double‐blind placebo‐controlled field trial” Quote: “Ivermectin in 3‐mg tablets and identical placebo were supplied by Merck & Co., Inc., USA while albendazole in 200‐mg tablets and identical placebo were supplied by SmithKline Beecham. UK.” Comment: unclear how personnel were blinded |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Quote: "sealed copies of the codes were kept at DBL until the end of the trial when they were opened.” Comment: unclear if codes were revealed before or after completion of parasitological analyses, but we judge assessment of objective outcomes to be at low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 82.9% (1181/1425) of randomized participants were evaluated for primary outcome. Reasons for losses to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No other obvious sources of bias |
Fox 2005.
| Methods | RCT Study dates: October 1998 to May 1999 Length of follow‐up: 6 months Method of mf assessment/volume of blood: 20 µL–thick smear, fingerprick blood collected between 7.30pm and 9.30pm Antigen testing: fingerprick blood assessed with Og4C3 ELISA Method of adverse event assessment: children were questioned and examined at school for adverse reactions for 7 days. Information was collected on adverse reactions that included headache, fever, myalgias, abdominal pain, passage of worms in the stool, vomiting, diarrhoea, cough, and dyspnoea. A treatment impact score was determined for each child Treatment impact score grading: 1) symptoms were noticed but did not interfere with daily activities: 2) symptoms caused some interference with daily activities; 3) symptoms prevented usual daily activities |
|
| Participants | All children attending any of 12 selected primary schools Number analysed for primary outcome: 990 participants of 1292 participants randomized Mean age (years): 7.6 Inclusion criteria: 1) an age of 5 – 11 years; 2) anthropometric measurements collected before and 6 months after treatment; 3) stool specimens collected before and 5 weeks after treatment; 4) mf smears prepared before and 6 months after treatment; 5) random assignment to a treatment group |
|
| Interventions | Single dose
|
|
| Outcomes |
Not reported: mean percentage reduction in mf density and CFA density post‐treatment Not included in review: height and weight (anthropometric indices reported as Z‐scores), stool examination for intestinal helminths (Note: SDs for geometric mean density changes reported on request by previous review authors (Addiss 2005) |
|
| Notes | Study type: school‐based Location: Leogane commune, Haiti Source of funding: Emerging Infections Program of the Centers for Disease Control and Prevention and by an Institutional Strengthening Grant from the World Health Organization to the Hôpital Sainte Croix Medication supervised: children took the medication under direct investigator observation Endemicity level: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “For each school, all eligible students were assigned using a random number table” |
| Allocation concealment (selection bias) | Low risk | Quote: “All laboratory specimens were collected and coded before treatment group assignment and the code, kept by CDC researchers, was only broken after completion of sample testing” |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Low risk | Quote: "double blind, placebo controlled".
Comment: although drugs were not identical, patients likely had no way of identifying them. Quote: “a clinician who was blinded as to treatment group questioned and examined the children at school for adverse reactions.” |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Quote: “Laboratory personnel, measurement teams, and personnel evaluating students for adverse reactions were blinded to the treatment status of the children.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 76.6% (990/1292) of randomized participants were evaluated. Reasons for losses to follow‐up were reported as due to absence of pretreatment or post‐treatment mf smears required for analysis. Inclusion of all randomized participants (number evaluable/number randomized): 76.6% (990/1292) |
| Selective reporting (reporting bias) | High risk | Comment: prespecified adverse events were not fully reported; abdominal pain, vomiting, diarrhoea and dyspnoea were measured but not reported Mean percentage reduction in mf or CFA density 3 and 6 months after treatment (efficacy outcome measure 2) were not reported |
| Other bias | Unclear risk | Comment: risk of bias for mf density and antigen density is unclear, as prior to estimating the percentage reduction between baseline and follow‐up, the authors omitted increases in density. This study simply provides an assessment of the decrease in density only in people experiencing a decrease. Whilst this rule was applied to both intervention and control groups, we were uncertain of the effect of this on the estimate, or exactly what the estimate was measuring |
Gayen 2013.
| Methods | RCT Study dates: 2006 to 2008 Length of follow‐up: 12 months Method of mf assessment/volume of blood: At pretreatment the method was not stated, fingerprick blood was collected at night; during treatment and post‐treatment it was membrane filtration, 2 to 3 mL or 8mL venous blood Method of adverse event (AE) assessment: assessed before and 48 hours after drug administration by medical questionnaire. AEs were quantified using a scorecard based on temperature, blood pressure measurements and questionnaire responses that focused on rash, fatigue, diarrhoea, appetite changes, vomiting, scrotal pain, headache, myalgias, cough, and dyspnoea. Scoring was based on a WHO system: mild AE (1); moderate AE (2); severe AE (3); and life‐threatening or disabling AE (4). Scores assigned for all parameters over all time points for individual participants were added up |
|
| Participants | Microfilaraemic individuals identified by screening Number analysed for primary outcome: 32 participants of 32 participants randomized in the placebo treatment group and albendazole treatment group Age range/mean age: not reported Inclusion criteria: asymptomatic mf carriers, aged 18 – 65, > 40 kg, not pregnant or breastfeeding, and in good health Exclusion criteria: abnormal hepatic and renal function (SGPT > 60 I.U./L, SGOT > 40 I.U./L, creatinine > 1.4 mg/100 ml), intolerance to treatment drugs, and alcohol abuse |
|
| Interventions | Multiple doses
|
|
| Outcomes |
Not included in review: change in Wolbachia density post‐treatment |
|
| Notes | Study type: community‐based Location: 2 rural areas in 2 districts of Bankura and Birbhum, West Bengal, India Source of funding: Department of Biotechnology and the Council of Scientific and Industrial Research, Ministry of Science and Technology, Government of India Medication supervised: not reported Endemicity level: 10.9% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomly assigned.. by a trial monitor who was not associated in the study” Sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | Quote: “Blinding and coding of drugs was done by an independent monitor (a scientist who was not an investigator)” |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Low risk | Quote: “double‐blind: neither the patient nor the evaluating physician was aware of the kind of medication that was given.” Quote: “repacking (drugs) in identical capsules provided by a pharmaceutical company.” |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 100% (32/32) of randomized participants in the placebo group and albendazole treatment group were evaluated. Inclusion of all randomized participants (number evaluable/number randomized): 100% (32/32) For adverse reactions, 23.5% (4/17) participants in the albendazole group refused to be evaluated for this outcome, and 100% (15/15) were evaluated in the placebo group |
| Selective reporting (reporting bias) | Low risk | Comment: prespecified outcomes reported Mean mf count and percentage reduction in mean mf count reported graphically for some time points post‐treatment |
| Other bias | High risk | Authors reported: "Differences between treatments were assessed by paired t test using MS Excel software." Comment: This method of analysis is inappropriate for comparing differences between groups, and differences between treatment groups may be inappropriately reported |
Kshirsagar 2004.
| Methods | RCT Study dates: October 2000 to November 2003 Length of follow‐up: 36 months Method of mf assessment/volume of blood: thick smear with 60 µL fingerprick or venepuncture blood, and membrane filtration with 1 mL venepuncture blood, collected between 9pm and 1am. Antigen testing: ICT Method of macrofilariae viability assessment: detection of adult filarial worm by ultrasound machine; all regions of scrotum and spermatic cord systematically studied, and FDS identified. Number and location of sites in the scrotal sac were recorded in first year follow‐up. In the second and third year follow‐up, individuals were classed ar FDS‐positive or ‐negative. Method of adverse event assessment: AEs were recorded during the trial, including their description, frequency, duration, severity and relationship to trial drug i.e. causality (defined as likely, unlikely, not assessable), and whether it interfered with daily activity. Safety and tolerability were graded by assessing clinically significant presentation (using NCI CTC grades) and AEs evaluation based on the description, incidence, severity and relationship of adverse drug events (using NCI CTC grades) to the drug administration |
|
| Participants | All individuals living in 2 endemic areas Number analysed for primary outcome: 139 participants of 1403 participants randomized Mean age (years): 35.5 (DEC); 34.9 (DEC plus albendazole) Inclusion criteria: The safety study included males and females over 5 years old. The efficacy study initially included men aged 18 ‐ 50 years old classed as microfilaraemic, amicrofilaraemic with clinical disease and amicrofilaraemic, asymptomatic. Criteria for clinical disease were the presence of hydrocoele, lymphoedema and/or lymphadenopathy. Criteria for inclusion for 12, 24 and 36 month follow‐up were participation in the first efficacy study, and individuals who were microfilaraemic at baseline in the safety study Exclusion criteria: pregnancy or breast‐feeding, history of allergy to DEC or albendazole (or drugs of that class), treatment with antifilarial drugs in the past year, participation in a new drug study in the past 6 months, seriously ill, conditions likely to hamper compliance of the person in the study, inability to take medication orally |
|
| Interventions | Single dose, given once every year (3 annual treatments in total)
|
|
| Outcomes | For participants in the efficacy group:
Note: At the 3‐, 6‐ and 12‐month follow‐up, results were stratified by male patients mf‐positive at baseline (43 participants), with clinical disease (30 participants), and mf‐negative and asymptomatic (30 participants), and some outcomes were not fully reported at all follow‐up time points. At 12, 24 and 36 months follow‐up, additional mf‐positive individuals were analysed, and all individuals were assessed together for each outcome (excluding ultrasound examination, which included only male participants). Measured but not reported: number of sites of FDS in each participant pre‐ and post‐treatment, and the reduction in number of sites of FDS at each time point up to 12 months. For participants in the safety group:
Not included in review: mean and SD plasma concentration of treatment drugs |
|
| Notes | Study type: community‐based Location: 2 endemic villages in Wardha, Maharashtra (Western India) Source of funding: UNDP/World bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) Medication supervised: "The drug from the assigned bottle... was then given under supervision" Endemicity level: 7.27% in 1995 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: states randomized, but random sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Each envelope (independently packaged by Cipla Limited) contained 10 tablets of DEC and 1 tablet of ALB (or placebo) according to the randomization code and was labelled with study allocation numbers." Quote: "The randomization code for each subject was sealed and kept with TDR, PI, and clinical monitor." |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Low risk | Quote: "double blind" Quote: "Tablets of Banocide brand of DEC (50 mg, GSK, India), ALB (400 mg, SmithKline Beecham,UK) and matching placebo were provided through product development team of WHO/TDR" Quote: "The investigating team and participants were blinded to the code." |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Quote: "Following completion of both the safety and efficacy study... the data was locked and sent to the statistician, who then broke the sealed code and analysed the data independently." Blinding for ultrasound outcome was specifically reported: “Detection of adult filarial worm was assessed by USG... which was carried out by trained personnel blinded to Mf result, the group to which patient belonged.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 99.4% (1395/1403) of randomized participants in the safety study group were evaluated. Reasons for losses to follow‐up were reported. Inclusion of all randomized participants (number evaluable/number randomized): 99.4% (1395/1403) For efficacy study group, 7.3% (103/1403) were included in assessments up to 12 months, and 10% (139/1403) were included in assessments at 12 months and later follow‐ups. Incomplete outcome data were reported at some follow‐up time points up to 12 months, and reasons for incomplete outcome data were not reported. Inclusion of all randomized participants (number evaluable/number randomized): 10% (139/1403) |
| Selective reporting (reporting bias) | High risk | Comment: Data were collected for efficacy outcomes every 3, 6 and 12 months for 3 years, but only the first 3 and 6 months were reported; annual follow‐up data was presented after 12 months due to "negligible results". After the second and third annual dose, measures of safety and tolerability were not reported as outlined in the Methods. In addition, the Methods state the number of sites of FDS in each participant and calculated reduction in number of sites of FDS was measured, but this was not reported Quote: "The secondary efficacy variables were the time to clear CFA and FDS, and number of sites of FDS in each patient at pre‐treatment, 6 months and 1 year.. reduction in number of sites of FDS at each time point were also calculated." |
| Other bias | Low risk | No other obvious sources of bias |
Pani 2002.
| Methods | RCT Study dates: not reported Length of follow‐up: 36 months Method of mf assessment/volume of blood: membrane filtration, 1 mL venous blood. Blood samples (2 mL) were collected from mf carriers at different time points during the night Antigen testing: ICT and Og4C3 ELISA test kit on 50 µL serum Method of macrofilariae viability assessment: FDS was assessed in male mf carriers by ultrasound examination. Both sides of the scrotum were examined serially, and inguinal lymphatic vessels and lymph nodes and thighs, and the lymphatic vessels and nodes of axillae and upper arms were also examined. Method of adverse event assessment: participants were monitored for adverse reactions at 8‐hourly intervals for 24 hours, and thereafter every 24 hours for 3 days. All systemic adverse reactions were recorded by assigning them a score of either 0 (none) or 1 (mild) or 2 (moderate) or 3 (severe) |
|
| Participants | Microfilariaemic individuals identified by screening Number analysed for primary outcome: 54 participants of 54 participants randomized Mean age (years): 24.67 Inclusion criteria: healthy asymptomatic volunteers (male and female) between 10 and 57 years old who were mf‐positive Exclusion criteria: patients with a history of any drug intolerance, reaction or allergy, presence of intestinal helminth cysts or ova in stool, history of consuming either albendazole or DEC in the preceding year |
|
| Interventions | Single dose
|
|
| Outcomes |
Not included in review: haematological and biochemical parameters |
|
| Notes | Study type: hospital‐based Location: Pondicherry, India Source of funding: Indian Council of Medical Research, New Delhi Medication supervised: “under the direct supervision of the medical team.” Endemicity level: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Generation of allocation sequence unclear |
| Allocation concealment (selection bias) | Low risk | Quote: "Blinding and coding of the drugs was done by an independent monitor (a senior scientist who was not an investigator) after repacking in look‐alike capsules by a pharmaceutical company" |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Low risk | Described as "double blind" Quote: "patients, clinicians evaluating the adverse effects... were unaware of the individual therapy schedules." |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Quote: "laboratory staff carrying out the laboratory tests and measuring mf and antigen levels, were unaware of the individual therapy schedules." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 100% (54/54) of randomized participants were evaluated. No losses to follow‐up were reported. Inclusion of all randomized participants (number evaluable/number randomized): 100% (54/54) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | The authors reported: "Student's t‐test was carried out for comparison of mean counts of mf and mean optical density values of Og4C3 test results between the drug groups." Comment: Mean optical density values of Og4C3 test results between groups were not compared and no statistical output reported; but outcome data were clearly reported |
Rizzo 2007.
| Methods | RCT Study dates: not reported Length of follow‐up: 12 months Method of mf assessment/volume of blood: membrane filtration of 1mL venous blood using Nucleopore filter (3 mm pore size). 5 mL venous blood was collected between 11pm and 1am, and if analysis of 1 mL blood appeared negative for mf, the remaining blood sample (4 mL) was also checked for mf by membrane filtration |
|
| Participants | Microfilaraemic individuals identified by screening and stratified by mf density Number analysed for primary outcome: 82 participants of 84 participants randomized Age range (years): 9 to 19 Inclusion criteria: aged 9 to 19 years and microfilaraemic Exclusion criteria: 1) antifilarial treatment in previous 6 months; 2) history of health conditions for which antifilarial drugs might be contraindicated; 3) pregnant women; 4) personal or parental alcohol or drug abuse; 5) frequently moved within or outside the Greater Recife area |
|
| Interventions | Single dose
|
|
| Outcomes |
Also reported adverse events: overall incidence of systemic AEs, incidence of localized AEs, duration of events, proportion experiencing mild and severe events. List of most common systemic AEs and proportion of participants experiencing them. (Note: CIs and SDs for log mean mf density, and proportion of participants with AEs in each treatment group were obtained from the authors on request) |
|
| Notes | Study type: hospital‐based Location: Jaboata˜o dos Guararapes, Greater Recife, Brazil Source of funding: The Amaury Coutinho Non‐governmental Organization Medication supervised: treated under direct supervision Endemicity level: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A restricted block‐randomization list for each stratum was then generated (by an individual who was not otherwise connected with the research).” |
| Allocation concealment (selection bias) | High risk | Quote: “Patients were allocated, as they were recruited, to one of the two treatment arms (by G.D.), according to their baseline levels of microfilaraemia.” Allocation was not concealed, participants were allocated according to a characteristic |
| Blinding of participants and personnel (performance bias) Laboratory outcome | High risk | Open study, no placebo used |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Low risk | Quote: “blinded primary evaluation of outcome (microfilaraemia prevalence and intensity)” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 82/84 (97.6%) of randomized participants were evaluated. Reasons for losses to follow‐up were reported. Inclusion of all randomized participants (number evaluable/number randomized): 97.6% (82/84) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No other obvious sources of bias |
Simonsen 2004.
| Methods | RCT Study dates: June 2001 to July 2002 Length of follow‐up: 12 months Method of mf assessment/volume of blood: counting chamber technique, 100 µL fingerprick blood Antigen testing: CFA quantified by Og4C3 TropBio ELISA kit using fingerprick blood; blood sampling for mf and CFA started at 9pm Method of adverse event assessment: children were followed for 5 days post‐treatment by passive observation Adverse reactions and their severity were recorded |
|
| Participants | All children attending any of 6 selected primary schools Number analysed for primary outcome: 1221 participants of 1829 participants randomized Age range (years): 6 to 18 Inclusion criteria: standard 1 ‐ 6 pupils Exclusion criteria: pupils from the highest class as they would not be attending the schools at the 1‐year follow‐up surveys |
|
| Interventions | Single dose 1. Albendazole plus ivermectin: 400 mg plus 150 to 200 µg/kg, 586 participants 2. Ivermectin: 150 to 200 µg/kg plus albendazole‐placebo, 635 participants |
|
| Outcomes | For mf‐positive individuals only:
For CFA‐positive individuals only: 1. CFA prevalence 2. Change in CFA prevalence 3. Geometric mean CFA intensity 4. Change in geometric mean CFA intensity For individuals mf/CFA‐negative at baseline: 5. New cases of mf positivity 6. New cases of CFA positivity Not included in review: specific adverse reactions, such as headache, fever, joint pain, diarrhoea, dizziness, vomiting and itching and the total number of cases were reported, but number of events in each treatment group was not reported |
|
| Notes | Study type: school‐based Location: Tanga and Pangani Districts, Tanzania Source of funding: Partnership for Child Development and the Danish Bilharziasis Laboratory Medication supervised: The tablets were swallowed under direct observation of a member of the project team Endemicity level: The school's catchment area was known to have high endemicity of lymphatic filariasis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The children were randomized into two treatment groups by using computer generated random numbers.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Before shipment to Tanzania, the albendazole and albendazole‐placebo tablets were coded (separately for each school) at the Danish Bilharziasis Laboratory by a scientist who was not part of the study team.” |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Unclear risk | Quote: “A randomized double‐blind field trial” Matching‐ albendazole placebo was used. Unclear how personnel blinded |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 1221/1829 (66.8%) of randomized participants were evaluated. Reasons for losses to follow‐up were reported as due to exclusion of participants from analyses if they were not present for subsequent follow‐up examinations. Inclusion of all randomized participants (number evaluable/number randomized): 66.8% (1221/1829) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | High risk | Comment: For mf density, authors reported: "overall reductions being slightly but statistically significantly higher for the combination than for ivermectin alone". However, authors reported statistical analysis by paired t‐test and repeated‐measures ANOVA for correlated samples, and use of pairwise contrast tests to examine differences between groups at specific time points. The results of pairwise tests for differences between groups have not been reported, and use of repeated measures ANOVA is unsuitable for between‐group comparisons |
Wamae 2011.
| Methods | Cluster‐RCT Unit of cluster: household Method to adjust for clustering: multilevel mixed‐effects regression models for some analyses Average cluster size: not reported ICCs: not reported Study dates: 1998 to 2000 Length of follow‐up: 24 months Method of mf assessment/volume of blood: counting chamber technique, 100 µL fingerprick blood collected between 8.30pm and 12am. Also reported venous samples were collected Antigen testing: Og4C3 antigen ELISA |
|
| Participants | Microfilaraemic households identified by screening Number analysed for primary outcome: 51 microfilaraemic participants of 108 participants randomized in the DEC treatment group and the DEC plus albendazole treatment were analysed for mf density. Unclear how many individuals were included in regression models Age range (low and upper quartiles): 12, 40 Inclusion criteria: over 5 years of age and a member of a household where at least 1 member was microfilaraemic Exclusion criteria: severely ill or pregnant |
|
| Interventions | Single dose, given once every year (3 annual treatments in total)
|
|
| Outcomes | Reported:
Also commented on adverse events. Not included in review: No data were useable for review. Also reported analyses of antifilarial IgG1 and IgG4 levels post‐treatment |
|
| Notes | Study type: community‐based Location: Muhaka area in Msambweni district, south coastal Kenya Source of funding: UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) Medication supervised: not reported Endemicity level: 15 – 25% mf prevalence and > 35% antigenaemia prevalence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “64 households were randomly assigned to three treatment groups” Unclear how they were randomized |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) Laboratory outcome | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Laboratory outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 82.9% (170/205) of randomized participants in selected households were treated at baseline. Reasons for exclusions due to absence of blood specimen, reasons for absence were not reported. Unclear if 170 participants treated were followed up. Methods state ITT analysis was done, but unclear if data were imputed for 35 participants that did not receive treatment. 64.7% (110/170) of participant samples were randomly assessed for antigenaemia at baseline, and 53.5% (91/170) samples were assessed post‐treatment |
| Selective reporting (reporting bias) | High risk | The authors state "Multilevel mixed‐effects regression models were used to compare changes in log MF... and log CFA with time between the three treatments". The effect of treatment over time (1 week, 6 months, 12 months, 24 months) was reported for mf density only. Effect of treatment on changes in CFA density were reported for 24 month follow‐up only (with statistically significant difference reported between treatment groups): Quote: "The model revealed significant reduction of MF count with treatment over time (p < 0.001) in all treatment groups and at all time points... there was greater reduction in MF count in the DEC/ALB group compared to the DEC group although the difference was not statistically significant (geometric mean difference 2.9, 95% confidence interval 1.5 to 12.9, p = 0.146)." Quote: "The model revealed significant reduction of CFA (p < 0.001) in all treatment groups at 2 years of follow‐up... DEC/ALB combination treatment was also significantly more effective than DEC alone (geometric mean difference 4.4, 95% confidence interval 0.6–9.67, p = 0.049)." |
| Other bias | High risk | Comment:
Data were not analysed in this review |
Abbreviations: (S)AE: (serious) adverse event; ALB: albendazole; CFA: circulating filarial antigen; DEC: diethylcarbamazine; FDS: filarial dance sign; ITT: intention‐to‐treat; mf: microfilariae; RCT: randomized controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Debrah 2006 | All participants received ivermectin and albendazole 4 months after treatment with either doxycycline or placebo. This trial did not compare albendazole co‐administered with ivermectin to ivermectin for lymphatic filariasis |
| Dembele 2010 | The comparison groups – albendazole plus ivermectin given together at increased dose and frequency versus the standard dose of albendazole plus ivermectin – do not provide answers to question of whether adding albendazole to ivermectin improves treatment outcomes |
| Ismail 1998 | The comparison groups – albendazole versus albendazole plus ivermectin versus albendazole plus DEC versus DEC plus ivermectin – do not match those in the review; these comparisons do not provide answers to the question of whether adding albendazole to ivermectin or DEC improves outcomes compared to ivermectin or DEC alone |
| Jayakody 1993 | The comparison groups ‐ albendazole versus DEC ‐ did not match those in the review; this does not provide answers to the question as to whether adding albendazole to DEC improves outcomes compared to DEC alone. |
| Kar 2015 | The comparison groups – albendazole plus DEC given together at increased dose and frequency versus the standard dose of albendazole plus DEC – do not provide answers to the question of whether adding albendazole to DEC improves treatment outcomes |
| King 2018 | The comparison groups – albendazole plus DEC given annually versus albendazole plus DEC given once versus albendazole plus DEC plus ivermectin given once – do not provide answers to the question of whether adding albendazole to DEC improves treatment outcomes |
| Makunde 2003 | Comparison groups do not match those in review; for single infections with W bancrofti these were albendazole plus ivermectin versus albendazole alone; for co‐infections of W bancrofti and Onchocerca volvulus these were ivermectin plus albendazole versus placebo |
| Namwanje 2011 | The comparison groups for people with lymphatic filariasis – albendazole plus ivermectin plus praziquantel versus albendazole plus ivermectin with no praziquantel or praziquantel given after 1 week – do not match those of the review; this does not provide answers to the question of whether adding albendazole to ivermectin improves treatment outcomes |
| Nash 2017 | Although the comparison groups ‐ albendazole versus placebo ‐ match those sought by the review, the study did not include the patient population relevant to the review (participants were not infected by W bancrofti) |
| Pion 2015 | Not an RCT; all individuals were given albendazole in a community study |
| Shenoy 1999 | The comparison groups – albendazole versus albendazole plus ivermectin versus albendazole plus DEC versus DEC plus ivermectin – do not match those in the review. |
| Shenoy 2002 | Study of safety and tolerability of adding albendazole to DEC; carried out only in people without microfilaraemia (i.e. presumably uninfected) |
| Tafatatha 2015 | The comparison groups – albendazole plus ivermectin given together at increased dose and frequency versus the standard dose of albendazole plus ivermectin – do not provide answers to the question of whether adding albendazole to ivermectin improves treatment outcomes |
| Thomsen 2016 | The comparison groups ‐ albendazole plus DEC versus albendazole plus DEC plus ivermectin ‐ do not match those in the review; this does not provide answers to the question of whether adding albendazole to DEC or ivermectin improves outcomes compared to DEC or ivermectin alone |
| Yongyuth 2006 | Although the comparison groups ‐ albendazole plus DEC versus DEC ‐ match those sought by the review, the trial reports were not clear or consistent. In one report the number of participants randomized to each group was very small, and differential losses to follow‐up between treatment groups were reported |
Abbreviations: DEC: diethylcarbamazine; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Purkait 2017.
| Methods | RCT Length of follow‐up: 12 months Method of microfilariae (mf) assessment/volume of blood: not reported Method of adverse event assessment: not reported |
| Participants | Number analysed: 164 participants Inclusion criteria: patients with filarial chyluria |
| Interventions |
|
| Outcomes |
|
| Notes | Conference abstract Corresponding authors contacted: purkaitbimalesh1@gmail.com; drashokkumarsokhal@gmail.com Study type: not reported Location: not reported Sources of funding: not reported Medication supervised: not reported Endemicity level: not reported |
Abbreviations: DEC: diethylcarbamazine; mf: microfilariae; RCT: randomized controlled trial.
Differences between protocol and review
Not applicable.
Differences between review and review update
2018 update: author team changed; we modified the review title from the original title of ‘Albendazole for lymphatic filariasis' and updated the entire review.
Following our prespecified protocol update modifications approved by the editorial team, we removed two comparisons (albendazole versus ivermectin and albendazole versus DEC). We added a new comparison as our main analysis, albendazole alone or added to a microfilaricidal drug versus placebo or a single microfilaricidal drug. We conducted a new search and added new trials; we excluded one trial (Jayakody 1993) as it no longer met the inclusion criteria due to the removal of a comparison (albendazole versus DEC).
We could not locate a record that was linked to the Pani 2002 study in the last review version, or the Dahoma record included in the previous edition's Characteristics of ongoing studies. After consulting the original review team, Mark Bradley (listed under contact information) and other researchers, we obtained the Dahoma 2000 record included in this update through David Addiss.
We adopted the latest synthesis methods, including the Cochrane ‘Risk of bias’ tool (Higgins 2011), used GRADE profiler (GRADEpro 2015) to grade the certainty of the evidence, and included ‘Summary of findings' tables. As we still could not meta‐analyse the parasite density data in this update, we produced additional tables for density outcomes in order to conduct an analysis.
We included a table detailing the reported statistical analysis of density data by trial authors. We changed the structure of the meta‐analyses, where previously the data were analysed by infected participants or all participants (infected and uninfected) separately, our main analyses assessed all randomized individuals by the longest follow‐up up to 12 months. We provided additional analyses by time point, stratified by whether individuals were infected or both uninfected and infected. We removed the Appendices containing information that could not be meta‐analysed; these remain available in the previous edition (Addiss 2005). We added an Appendix 2 including primary and secondary outcomes from a new cluster‐RCT (Wamae 2011) that could not be combined with RCTs.
Contributions of authors
CM updated the protocol, assessed studies for inclusion, extracted data, assessed the risk of bias in included trials, assessed the certainty of the evidence, conducted data analysis, and wrote the first draft of the review. SB assessed studies for inclusion, extracted data, and assessed the risk of bias in included trials. SJ assessed risk of bias and the certainty of the evidence, and contributed to the data analysis and drafting the final review. MR helped with the analyses and provided statistical input. PG provided advice at all stages of the review production, helped with the analyses, and edited the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Project number 300342‐104
-
DFID, UK.
Grant: 6407
Declarations of interest
CM received salary support from the COUNTDOWN Research Consortium.
SB has no known conflict of interest.
SJ has no known conflict of interest.
MR has no known conflict of interest.
Paul Garner is the Director of READ‐It, a UK AID development programme to help ensure evidence synthesis contributes to decision making, particularly relevant to low‐ and middle‐income countries for the benefit of the poor in these countries. The Department for International Development (DFID) had no part in preparing this review. Paul Garner is also a named investigator on COUNTDOWN, which is funded by a grant from DFID to promote community mass drug distribution to control neglected tropical diseases in endemic areas.
Unchanged
References
References to studies included in this review
Beach 1999 {published data only}
- Addiss DG, Beach MJ, Streit TG, Lutwick S, LeConte FH, Lafontant JG, et al. Randomised placebo‐controlled comparison of ivermectin and albendazole alone and in combination for Wuchereria bancrofti microfilaraemia in Haitian children. Lancet 1997;350(9076):480‐4. [DOI] [PubMed] [Google Scholar]
- Beach MJ, Streit TG, Addiss DG, Prospere R, Roberts JM, Lammie PJ. Assessment of combined ivermectin and albendazole for treatment of intestinal helminth and Wuchereria bancrofti infections in Haitian school children. American Journal of Tropical Medicine and Hygiene 1999;60(3):479‐86. [DOI] [PubMed] [Google Scholar]
Bockarie 2007 {published data only}
- Bockarie MJ, Tavul L, Ibam I, Kastens W, Hazlett F, Tisch DJ, et al. Efficacy of single‐dose diethylcarbamazine compared with diethylcarbamazine combined with albendazole against Wuchereria bancrofti infection in Papua New Guinea. American Journal of Tropical Medicine and Hygiene 2007;76(1):62‐6. [PubMed] [Google Scholar]
Dahoma 2000 {published data only}
- Dahoma MJ. A randomized community trial on safety and efficacy of co‐administration of albendazole and ivermectin on lymphatic filariasis and its secondary effects on geohelminths in Zanzibar. http://dspace.muhas.ac.tz:8080/xmlui/handle/123456789/1569 2000.
De Britto 2015 {published data only}
- Britto RL, Vanamail P, Sankari T, Vijayalakshmi G, Das LK, Pani SP. Enhanced efficacy of sequential administration of albendazole for the clearance of Wuchereria bancrofti infection: Double blind RCT. Tropical Biomedicine 2015;32(2):198‐209. [PubMed] [Google Scholar]
Dreyer 2006 {published and unpublished data}
- Dreyer G, Addiss D, Williamson J, Norões J. Efficacy of co‐administered diethylcarbamazine and albendazole against adult Wuchereria bancrofti. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006;100(12):1118‐25. [DOI] [PubMed] [Google Scholar]
Dunyo 2000 {published data only}
- Dunyo SK, Nkrumah FK, Simonsen PE. A randomized double‐blind placebo‐controlled field trial of ivermectin and albendazole alone and in combination for the treatment of lymphatic filariasis in Ghana. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(2):205‐11. [DOI] [PubMed] [Google Scholar]
- Dunyo SK, Nkrumah FK, Simonsen PE. Single‐dose treatment of Wuchereria bancrofti infections with ivermectin and albendazole alone or in combination: evaluation of the potential for control at 12 months after treatment. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(4):437‐43. [DOI] [PubMed] [Google Scholar]
- Dunyo SK, Simonsen PE. Ivermectin and albendazole alone and in combination for the treatment of lymphatic filariasis in Ghana: follow‐up after re‐treatment with the combination. Transactions of the Royal Society for Tropical Medicine and Hygiene 2002;96(2):189‐92. [DOI] [PubMed] [Google Scholar]
Fox 2005 {published and unpublished data}
- Fox LM, Furness BW, Haser JK, Desire D, Brissau JM, Milord MD, et al. Tolerance and efficacy of combined diethylcarbamazine and albendazole for treatment of Wuchereria bancrofti and intestinal helminth infections in Haitian children. American Journal of Tropical Medicine and Hygiene 2005;73(1):115‐21. [PubMed] [Google Scholar]
Gayen 2013 {published data only}
- Gayen P, Nayak A, Saini P, Mukherjee N, Maitra S, Sarkar P, et al. A double‐blind controlled field trial of doxycycline and albendazole in combination for the treatment of bancroftian filariasis in India. Acta Tropica 2013;125(2):150‐6. [DOI] [PubMed] [Google Scholar]
Kshirsagar 2004 {published data only}
- Kshirsagar NA, Gogtay NJ, Garg BS, Deshmukh PR, Rajgor DD, Kadam VS, et al. Efficacy and tolerability of treatment with single doses of diethylcarbamazine (DEC) and DEC plus albendazole (ABZ) for three consecutive years in lymphatic filariasis: a field study in India. Parasitology Research 2017;116(10):2683–94. [DOI] [PubMed] [Google Scholar]
- Kshirsagar NA, Gogtay NJ, Garg BS, Deshmukh PR, Rajgor DD, Kadam VS, et al. Safety, tolerability, efficacy and plasma concentrations of diethylcarbamazine and albendazole co‐administration in a field study in an area endemic for lymphatic filariasis in India. Transactions of the Royal Society of Tropical Medicine and Hygiene 2004;98(4):205‐17. [DOI] [PubMed] [Google Scholar]
Pani 2002 {published data only}
- Hoti SL, Pani SP, Vanamail P, Athisaya MK, Das LK, Das PK. Effect of a single dose of diethylcarbamazine, albendazole or both on the clearance of Wuchereria bancrofti microfilariae and antigenaemia among microfilaria carriers: a randomized trial. National Medical Journal of India 2010;23(2):72‐6. [PubMed] [Google Scholar]
- Pani S, Subramanyam Reddy G, Das L, Vanamail P, Hoti S, Ramesh J, et al. Tolerability and efficacy of single dose albendazole, diethylcarbamazine citrate (DEC) or co‐administration of albendazole with DEC in the clearance of Wuchereria bancrofti in asymptomatic microfilaraemic volunteers in Pondicherry, South India: a hospital‐based study. Filaria Journal 2002;1(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rizzo 2007 {published and unpublished data}
- Rizzo JA, Belo C, Lins R, Dreyer G. Children and adolescents infected with Wuchereria bancrofti in Greater Recife, Brazil: a randomized, year‐long clinical trial of single treatments with diethylcarbamazine or diethylcarbamazine‐albendazole. Annals of Tropical Medicine and Parasitology 2007;101(5):423‐33. [DOI] [PubMed] [Google Scholar]
Simonsen 2004 {published data only}
- Simonsen PE, Magesa SM, Dunyo SK, Malecela‐Lazaro MN, Michael E. The effect of single dose ivermectin alone or in combination with albendazole on Wuchereria bancrofti infection in primary school children in Tanzania. Transactions of the Royal Soceity of Tropical Medicine and Hygiene 2004;98(8):462‐72. [DOI] [PubMed] [Google Scholar]
Wamae 2011 {published data only}
- Wamae CN, Njenga SM, Ngugi BM, Mbui J, Njaanake HK. Evaluation of effectiveness of diethylcarbamazine/albendazole combination in reduction of Wuchereria bancrofti infection using multiple infection parameters. Acta Tropica 2011;120(Supplement 1):S33‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Debrah 2006 {published data only}
- Debrah AY, Mand S, Specht S, Marfo‐Debrekyei Y, Batsa L, Pfarr K, et al. Doxycycline reduces plasma VEGF‐C/sVEGFR‐3 and improves pathology in lymphatic filariasis. PLoS Pathogens 2006;2(9):e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dembele 2010 {published data only}
- Dembele B, Coulibaly YI, Dolo H, Konate S, Coulibaly SY, Sanogo D, et al. Use of high‐dose, twice‐yearly albendazole and ivermectin to suppress Wuchereria bancrofti microfilarial levels. Clinical Infectious Diseases 2010;51(11):1229‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 1998 {published data only}
- Ismail MM, Jayakody RL, Weil GJ, Fernando D, Silva MS, Silva GA, et al. Long‐term efficacy of single‐dose combinations of albendazole, ivermectin and diethylcarbamazine for the treatment of bancroftian filariasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(3):332‐5. [DOI] [PubMed] [Google Scholar]
- Ismail MM, Jayakody RL, Weil GJ, Nirmalan N, Jayasinghe KS, Abeyewickrema W, et al. Efficacy of single dose combinations of albendazole, ivermectin and diethylcarbamazine for the treatment of bancroftian filariasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(1):94‐7. [DOI] [PubMed] [Google Scholar]
Jayakody 1993 {published data only}
- Jayakody RL, Silva CS, Weerasinghe WM. Treatment of bancroftian filariasis with albendazole: evaluation of efficacy and adverse reactions. Tropical Biomedicine 1993;10:19‐24. [Google Scholar]
Kar 2015 {published data only}
- Kar SK, Dwibedi B, Kerketa AS, Maharana A, Panda SS, Mohanty PC, et al. A randomized controlled trial of increased dose and frequency of albendazole with standard dose DEC for treatment of Wuchereria bancrofti microfilaremics in Odisha, India. PLoS Neglected Tropical Diseases 2015;9(3):e0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2018 {published data only}
- King CL, Suamani J, Sanuku N, Cheng YC, Satofan S, Mancuso B. A trial of a triple‐drug treatment for lymphatic filariasis. New England Journal of Medicine 2018;379(19):1801‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01975441. Eval 3‐drug therapy diethylcarbamize, albendazole and ivermectin that could accelerate LF elimination outside of Africa. clinicaltrials.gov/ct2/show/NCT01975441 (first received 4 November 2013).
Makunde 2003 {published data only}
- Makunde WH, Kamugisha LM, Massaga JJ, Makunde RW, Savael ZX, Akida J, et al. Treatment of co‐infection with bancroftian filariasis and onchocerciasis: a safety and efficacy study of albendazole with ivermectin compared to treatment of single infection with bancroftian filariasis. Filaria Journal 2003;2(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Namwanje 2011 {published data only}
- Namwanje H, Kabatereine N, Olsen A. A randomised controlled clinical trial on the safety of co‐administration of albendazole, ivermectin and praziquantel in infected schoolchildren in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 2011;105(4):181‐8. [DOI] [PubMed] [Google Scholar]
Nash 2017 {published data only}
- Nash S, Mentzer AJ, Lule SA, Kizito D, Smits G, Klis FR. The impact of prenatal exposure to parasitic infections and to anthelminthic treatment on antibody responses to routine immunisations given in infancy: Secondary analysis of a randomised controlled trial. PLoS Neglected Tropical Diseases 2017;11(2):e0005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pion 2015 {published data only}
- Pion SD, Chesnais CB, Bopda J, Louya F, Fischer PU, Majewski AC. The impact of two semiannual treatments with albendazole alone on lymphatic filariasis and soil‐transmitted helminth infections: a community‐based study in the Republic of Congo. American Journal of Tropical Medicine and Hygiene 2015;92(5):959‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion SD, Chesnais CB, Weil GJ, Fischer PU, Missamou F, Boussinesq M. Effect of 3 years of biannual mass drug administration with albendazole on lymphatic filariasis and soil‐transmitted helminth infections: a community‐based study in Republic of the Congo. Lancet Infectious Diseases 2017;17(7):763‐9. [DOI] [PubMed] [Google Scholar]
Shenoy 1999 {published data only}
- Shenoy RK, Dalia S, John A, Suma TK, Kumaraswami V. Treatment of the microfilaraemia of asymptomatic brugian filariasis with single doses of ivermectin, diethylcarbamazine or albendazole, in various combinations. Annals of Tropical Medicine and Parasitology 1999;93(6):643‐51. [DOI] [PubMed] [Google Scholar]
- Shenoy RK, John A, Babu BS, Suma TK, Kumaraswami V. Two‐year follow‐up of the microfilaraemia of asymptomatic brugian filariasis, after treatment with two, annual, single doses of ivermectin, diethylcarbamazine and albendazole, in various combinations. Annals of Tropical Medicine and Parasitology 2000;94(6):607‐14. [DOI] [PubMed] [Google Scholar]
Shenoy 2002 {published data only}
- Shenoy RK, Suma TK, John A, Arun SR, Kumaraswami V, Fleckenstein LL, et al. The pharmacokinetics, safety and tolerability of the co‐administration of diethylcarbamine and albendazole. Annals of Tropical Medicine and Parasitology 2002;96(6):603‐14. [DOI] [PubMed] [Google Scholar]
Tafatatha 2015 {published data only}
- Tafatatha TT, Ngwira BM, Taegtmeyer M, Phiri AJ, Wilson TP, Banda LG. Randomised controlled clinical trial of increased dose and frequency of albendazole and ivermectin on Wuchereria bancrofti microfilarial clearance in northern Malawi. Transactions of the Royal Society of Tropical Medicine and Hygiene 2015;109(6):393‐9. [DOI] [PubMed] [Google Scholar]
Thomsen 2016 {published data only}
- Thomsen EK, Kumar JA, Sanuku N, Baea M, Satofan S, Maki E, et al. Efficacy, safety and pharmacokinetics of coadministered diethylcarbamazine, albendazole and ivermectin for the treatment of Wuchereria bancrofti. American Journal of Tropical Medicine and Hygiene 2014;91(5 Suppl. 1):368. [Google Scholar]
- Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E, Lombore B, et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clinical Infectious Diseases 2016;62(3):334‐41. [DOI] [PubMed] [Google Scholar]
Yongyuth 2006 {published data only}
- Yongyuth P, Koyadun S, Jaturabundit N, Jariyahuttakij W, Bhumiratana A. Adverse reactions of 300 MG diethylcarbamazine, and in a combination of 400 MG albendazole, for a mass annual single dose treatment, in migrant workers in Phang Nga province. Journal of the Medical Association of Thailand 2007;90(3):552‐63. [PubMed] [Google Scholar]
- Yongyuth P, Koyadun S, Jaturabundit N, Sampuch A, Bhumiratana A. Efficacy of a single‐dose treatment with 300 mg diethylcarbamazine and a combination of 400 mg albendazole in reduction of Wuchereria bancrofti antigenemia and concomitant geohelminths in Myanmar migrants in Southern Thailand. Journal of the Medical Association of Thailand 2006;89(8):1237‐48. [PubMed] [Google Scholar]
References to studies awaiting assessment
Purkait 2017 {unpublished data only}
- Purkait B, Singh V, Sankhwar SN, Sinha RJ, Kumar M, Bhaskar V, et al. Efficacy and safety of multi drugs combination therapy in Filarial Chyluria; a prospective randomized controlled trial. Indian Journal of Urology 2017;33(Suppl 1):S43‐178. [Google Scholar]
Additional references
Addiss 1993
- Addiss DG, Eberhard ML, Lammie PJ, McNeeley MB, Lee SH, McNeeley DF, et al. Comparative efficacy of clearing‐dose and single high‐dose ivermectin and diethylcarbamazine against Wuchereria bancrofti microfilaremia. American Society of Tropical Medicine and Hygiene 1993;48(2):178‐85. [DOI] [PubMed] [Google Scholar]
Addiss 2000
- Addiss D, Dreyer G. Treatment of lymphatic filariasis. In: Nutman BT editor(s). Lymphatic Filariasis. London: Imperial College Press, 2000:151‐99. [Google Scholar]
Addiss 2007
- Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria Journal 2007;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrade 1995
- Andrade LD, Medeiros Z, Pires ML, Pimentel A, Rocha A, Figueredo‐Silva J, et al. Comparative efficacy of three different diethylcarbamazine regimens in lymphatic filariasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(3):319‐21. [DOI] [PubMed] [Google Scholar]
Babu 2012
- Babu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Seminars in Immunopathology 2012;34(6):847‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basáñez 1994
- Basáñez MG, Boussinesq M, Prod'hon J, Frontado H, Villamizar NJ, Medley GF, et al. Density‐dependent processes in the transmission of human onchocerciasis: intensity of microfilariae in the skin and their uptake by the simuliid host. Parasitology 1994;108(Pt 1):115‐27. [DOI] [PubMed] [Google Scholar]
Bockarie 2009
- Bockarie MJ, Pedersen EM, White GB, Michael E. Role of vector control in the global program to eliminate lymphatic filariasis. Annual Review of Entomology 2009;54:469‐87. [DOI] [PubMed] [Google Scholar]
Boussinesq 1997
- Boussinesq M, Gardon J. Prevalences of Loa loa microfilaraemia throughout the area endemic for the infection. Annals of Tropical Medicine and Parasitology 1997;91(6):573‐89. [DOI] [PubMed] [Google Scholar]
Burkot 2002
- Burkot TR, Taleo G, Toeaso V, Ichimori K. Progress towards, and challenges for, the elimination of filariasis from Pacific‐island communities. Annals of Tropical Medicine and Parasitology 2002;96 Suppl 2:S61‐9. [DOI] [PubMed] [Google Scholar]
Cao 1997
- Cao WC, Ploeg CP, Plaisier AP, Sluijs IJ, Habbema JD. Ivermectin for the chemotherapy of bancroftian filariasis: a meta‐analysis of the effect of single treatment. Tropical Medicine & International Health 1997;2(4):393‐403. [DOI] [PubMed] [Google Scholar]
Cartel 1990
- Cartel JL, Sechan Y, Boutin JP, Celerier P, Plichart R, Roux JF. Ivermectin for treatment of bancroftian filariasis French Polynesia: efficacy in man, effect on transmission by vector Aedes polynesiensis. Tropical Medicine and Parasitology 1990;41(3):241‐4. [PubMed] [Google Scholar]
CDS/FIL 1998
- Filariasis Elimination Programme (CDS/FIL). Division of Control of Tropical Diseases, Communicable Diseases. Report from Informal Consultation on Albendazole Research Findings in Lymphatic Filariasis; WHO/FIL/98.194; (closed document). Geneva: World Health Organization, 1998. [Google Scholar]
Conteh 2010
- Conteh L, Engels T, Molyneux DH. Socioeconomic aspects of neglected tropical diseases. Lancet 2010;375(9710):239–47. [DOI] [PubMed] [Google Scholar]
Coutinho 1994
- Coutinho AD, Dreyer G, Medeiros Z, Lopes E, Machado G, Galdino E, et al. Ivermectin treatment of bancroftian filariasis in Recife, Brazil. American Journal of Tropical Medicine and Hygiene 1994;50(3):339‐48. [DOI] [PubMed] [Google Scholar]
Cross 2001
- Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M, Taylor MJ. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet 2001;358(9296):1873‐5. [DOI] [PubMed] [Google Scholar]
Cuenco 2009
- Cuenco KT, Ottesen EA, Williams SA, Nutman TB, Steel C. Heritable factors play a major role in determining host responses to Wuchereria bancrofti infection in an isolated South Pacific island population. Journal of Infectious Diseases 2009;200(8):1271‐8. [DOI] [PubMed] [Google Scholar]
Debrah 2007
- Debrah AY, Mand S, Marfo‐Debrekyei Y, Batsa L, Pfarr K, Buttner M, et al. Macrofilaricidal effect of 4 weeks of treatment with doxycycline on Wuchereria bancrofti. Tropical Medicine & International Health 2007;12(12):1433‐41. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG, (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook.
Dreyer 1995
- Dreyer G, Amaral F, Norões J, Medeiros Z, Addiss D. A new tool to assess in vivo the adulticidal efficacy of antifilarial drugs for bancroftian filariasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(2):225‐6. [DOI] [PubMed] [Google Scholar]
Dreyer 1996
- Dreyer G, Addiss D, Noroes J, Amaral F, Rocha A, Coutinho A. Ultrasonographic assessment of the adulticidal efficacy of repeat high‐dose ivermectin in bancroftian filariasis. Tropical Medicine & International Health 1996;1(4):427‐32. [DOI] [PubMed] [Google Scholar]
Dreyer 1999
- Dreyer G, Medeiros Z, Netto MJ, Leal NC, Castro LG, Piessens WF. Acute attacks in the extremities of persons living in an area endemic for bancroftian filariasis: differentiation of two syndromes. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(4):413‐7. [DOI] [PubMed] [Google Scholar]
Dreyer 2000
- Dreyer G, Norões J, Figueredo‐Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitology Today 2000;16(12):544‐8. [DOI] [PubMed] [Google Scholar]
Eberhard 1989
- Eberhard ML, Lammie PJ, Roberts JM, Lowrie RC Jr. Effectiveness of spaced doses of diethylcarbamazine citrate for the control of bancroftian filariasis. Tropical Medicine and Parasitology 1989;40(2):111‐3. [PubMed] [Google Scholar]
Figueredo‐Silva 1996
- Figueredo‐Silva J, Jungmann P, Norões J, Piessens WF, Coutinho A, Brito C, et al. Histological evidence for adulticidal effect of low doses of diethylcarbamazine in bancroftian filariasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(2):192‐4. [DOI] [PubMed] [Google Scholar]
GAELF 2018
- Global Alliance to Eliminate Lymphatic Filariasis. Community management. Planning an implementation programme. www.filariasis.org/about‐lf/community‐management/planning‐implementation‐programme (accessed 1 October 2018).
Gardon 1997
- Gardon J, Gardon‐Wendel N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997;350(9070):18‐22. [DOI] [PubMed] [Google Scholar]
Geary 2010
- Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, et al. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. International Journal for Parasitology 2010;40(1):1‐13. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2002
- GlaxoSmithKline. Lymphatic filariasis programme: eliminating lymphatic filariasis. web.archive.org/web/20020414025235/www.gsk.com/filariasis/eliminating.htm (accessed 1 May 2017).
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 28 September 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Graves 2016
- Graves PM, Wood P, Bossin HC. Lymphatic filariasis in Oceania. In: Loukas A editor(s). Neglected Tropical Diseases ‐ Oceania. Switzerland: Springer, 2016:101‐42. [Google Scholar]
Gyapong 2005
- Gyapong JO, Kumaraswami V, Biswas G, Ottesen EA. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opinion on Pharmacotherapy 2005;6(2):179‐200. [DOI] [PubMed] [Google Scholar]
Harnett 1990
- Harnett W, Worms MJ, Grainger M, Pyke SD, Parkhouse RM. Association between circulating antigen and parasite load in a model filarial system, Acanthocheilonema viteae in jirds. Parasitology 1990;101(Pt 3):435‐44. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horton 2000
- Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 2000;121 Suppl:S113‐32. [DOI] [PubMed] [Google Scholar]
Horton 2009
- Horton J. The development of albendazole for lymphatic filariasis. Annals of Tropical Medicine and Parasitology 2009;103(Supp 1):33‐40. [DOI] [PubMed] [Google Scholar]
Kar 2017
- Kar SK, Dwibedi B, Das BK, Agrawala BK, Ramachandran CP, Horton J. Lymphatic pathology in asymptomatic and symptomatic children with Wuchereria bancrofti infection in children from Odisha, India and its reversal with DEC and albendazole treatment. PLoS Neglected Tropical Diseases 2017;11(10):e0005631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Mahoney 1971
- Mahoney LE, Kessel JF. Treatment failure in filariasis mass treatment programmes. Bulletin of the World Health Organization 1971;45(1):35‐42. [PMC free article] [PubMed] [Google Scholar]
Mataika 1993
- Mataika JU, Kimura E, Koroivueta J, Kaisuva JN, Brown M, Tuivaga J, et al. Comparison of the efficacy of diethylcarbamazine between 5 rounds of annual single‐dose treatment and an intensive 28‐dose treatment spread over 2 years against diurnally subperiodic Wuchereria bancrofti in Fiji. Fiji Medical Journal 1993;19:2‐6. [Google Scholar]
Mectizan Expert Committee 2004
- Mectizan® Expert Committee, The Technical Consultative Committee. Recommendations for the Treatment of Onchocerciasis with Mectizan® in Areas Co‐endemic for Onchocerciasis and Loiasis. Georgia (USA): Mectizan® Expert Committee/The Mectizan® Donation Program, 2004. [Google Scholar]
Meyrowitsch 1995
- Meyrowitsch DW, Simonsen PE, Makunde WH. A 16‐year follow‐up study on bancroftian filariasis in three communities of north‐eastern Tanzania. Annals of Tropical Medicine and Parasitology 1995;89(6):665‐75. [DOI] [PubMed] [Google Scholar]
NCI 1999
- National Cancer Institute. Common toxicity criteria (CTC) manual. Version 2.0. Publish date: 30 April 1999. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4‐30‐992.pdf (accessed 28 September 2018).
Norões 1997
- Norões J, Dreyer G, Santos A, Mendes VG, Medeiros Z, Addiss D. Assessment of the efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Transactions of the Royal Society Tropical Medicine and Hygiene 1997;9(1):78‐81. [DOI] [PubMed] [Google Scholar]
Olsen 2007
- Olsen A. Efficacy and safety of drug combinations in the treatment of schistosomiasis, soil‐transmitted helminthiasis, lymphatic filariasis and onchocerciasis. Transactions of The Royal Society of Tropical Medicine and Hygiene 2007;101(8):747‐58. [DOI] [PubMed] [Google Scholar]
Ottesen 1999
- Ottesen EA, Ismail MM, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitology Today 1999;15(9):382‐6. [DOI] [PubMed] [Google Scholar]
Ottesen 2006
- Ottesen EA. Lymphatic filariasis: treatment, control and elimination. Advances in Parasitology 2006;61:395‐441. [DOI] [PubMed] [Google Scholar]
Pichon 2002
- Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles‐transmitted filariasis. Annals of Tropical Medicine and Parasitology 2002;96 Suppl 2:S143‐52. [DOI] [PubMed] [Google Scholar]
Ramaiah 2014
- Ramaiah KD, Ottesen EA. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLOS Neglected Tropical Diseases 2014;8(11):e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rohatgi 2017 [Computer program]
- Rohatgi A. WebPlotDigitizer. Version 3.12. Austin: Ankit Rohatgi, 2017.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group, 2013. Hamilton (ON): McMaster University, Available from: gdt.gradepro.org/app/handbook/handbook.html (accessed 1 June 2018). [Google Scholar]
Scott 2000
- Scott AL. Lymphatic‐dwelling filariae. In: Nutman BT editor(s). Lymphatic Filariasis. London: Imperial College Press, 2000:5‐39. [Google Scholar]
Shenoy 2011
- Shenoy RK, Bockarie MJ. Lymphatic filariasis in children: clinical features, infection burdens and future prospects for elimination. Parasitology 2011;138(12):1559‐68. [DOI] [PubMed] [Google Scholar]
Simonsen 1995
- Simonsen PE, Meyrowitsch DW, Makunde WH, Magnussen P. Selective diethylcarbamazine chemotherapy for control of Bancroftian filariasis in two communities of Tanzania: compared efficacy of a standard dose treatment and two semi‐annual single dose treatments. American Journal of Tropical Medicine and Hygiene 1995;53(3):267‐72. [DOI] [PubMed] [Google Scholar]
Simonsen 1997
- Simonsen PE, Niemann L, Meyrowitsch DW. Wuchereria bancrofti in Tanzania: microfilarial periodicity and effect of blood sampling time on microfilarial intensities. Tropical Medicine & International Health 1997;2(2):153‐8. [DOI] [PubMed] [Google Scholar]
Stolk 2005
- Stolk WA, Oortmarssen GJ, Pani SP, Vlas SJ, Subramanian S, Das PK, et al. Effects of ivermectin and diethylcarbamazine on microfilariae and overall microfilaria production in bancroftian filariasis. American Journal of Tropical Medicine and Hygiene 2005;73(5):881‐7. [PubMed] [Google Scholar]
Taylor 2001
- Taylor MJ, Cross HF, Ford L, Makunde WH, Prasad GB, Bilo K. Wolbachia bacteria in filarial immunity and disease. Parasite Immunology 2001;23(7):401‐9. [DOI] [PubMed] [Google Scholar]
Taylor 2005
- Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double‐blind, randomised placebo‐controlled trial. Lancet 2005;365(9477):2116‐21. [DOI] [PubMed] [Google Scholar]
Taylor 2010
- Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet 2010;376(9747):1175‐85. [DOI] [PubMed] [Google Scholar]
Tisch 2005
- Tisch DJ, Michael E, Kazura JW. Mass chemotherapy options to control lymphatic filariasis: a systematic review. Lancet Infectious Diseases 2005;5(8):514‐23. [DOI] [PubMed] [Google Scholar]
Turner 2009
- Turner JD, Langley RS, Johnston KL, Gentil K, Ford L, Wu B, et al. Wolbachia lipoprotein stimulates innate and adaptive immunity through toll‐like receptors 2 and 6 to induce disease manifestations of filariasis. Journal of Biological Chemistry 2009;284(33):22364‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2016
- Turner HC, Bettis AA, Chu BK, McFarland DA, Hooper PJ, Ottesen EA, et al. The health and economic benefits of the global programme to eliminate lymphatic filariasis (2000–2014). Infectious Diseases of Poverty 2016;5(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanamail 1990
- Vanamail P, Subramanian S, Das PK, Pani SP, Rajagopalan PK. Estimation of fecundic life span of Wuchereria bancrofti from longitudinal study of human infection in an endemic area of Pondicherry (south India). Indian Journal of Medical Research 1990;91:293‐7. [PubMed] [Google Scholar]
Weil 1990
- Weil GJ, Chandrashekar R, Liftis F, McVay CS, Bosshardt SC, Klei TR. Circulating parasite antigen in Brugia pahangi‐infected jirds. Journal of Parasitology 1990;76(1):78‐84. [PubMed] [Google Scholar]
Weil 1997
- Weil GJ, Lammie PJ, Weiss N. The ICT Filariasis Test: A rapid‐format antigen test for diagnosis of bancroftian filariasis. Parasitology Today 1997;13(10):401‐4. [DOI] [PubMed] [Google Scholar]
Weil 1999
- Weil GJ, Ramzy RM, Setouhy M, Kandil AM, Ahmed ES, Faris R. A longitudinal study of Bancroftian filariasis in the Nile Delta of Egypt: baseline data and one‐year follow‐up. American Journal of Tropical Medicine and Hygiene 1999;61(1):53‐8. [DOI] [PubMed] [Google Scholar]
WHO 1984
- World Health Organization Expert Committee on Filariasis. Lymphatic Filariasis: Fourth Report of the WHO Expert Committee on Filariasis [meeting held in Geneva from 31 October to 8 November 1983]; Technical Report Series no. 702. Geneva: World Health Organization, 1984. [PubMed] [Google Scholar]
WHO 1998
- Filariasis Elimination Programme (CDS/FIL). Report from informal consultation on albendazole research findings in lymphatic filariasis 13‐14 October 1998. Geneva: World Health Organization WHO/FIL/98.194 1998.
WHO 2006
- World Health Organization. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization, 2006. [Google Scholar]
WHO 2011
- World Health Organization. Lymphatic filariasis: monitoring and epidemiological assessment of mass drug administration: A manual for national elimination programmes. Lymphatic Filariasis: Monitoring and Epidemiological Assessment of Mass Drug Administration: A Manual for National Elimination Programmes. Geneva: World Health Organization, 2011. [WHO/HTM/NTD/PCT/2011.4] [Google Scholar]
WHO 2012
- World Health Organization. Provisional Strategy for Interrupting Lymphatic Filariasis Transmission in Loiasis‐Endemic Countries: Report of the Meeting on Lymphatic Filariasis, Malaria and Integrated Vector Management [Accra, Ghana, 5 – 9 March 2012]. Geneva: World Health Organization, 2012. [WHO/HTM/NTD/PCT/2012.6] [Google Scholar]
WHO 2013
- World Health Organization. Lymphatic Filariasis: A Handbook of Practical Entomology for National Lymphatic Filariasis Elimination Programmes. Geneva: World Health Organization, 2013. [WHO/HTM/NTD/PCT/2013.10] [Google Scholar]
WHO 2015
- World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2014. Weekly Epidemiological Record 2015;90(38):489–504. [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2015. Weekly Epidemiological Record 2016;91(39):441‐60. [Google Scholar]
WHO 2017a
- World Health Organization. Guideline: alternative mass drug administration regimens to eliminate lymphatic filariasis. Guideline: Alternative Mass Drug Administration Regimens to Eliminate Lymphatic Filariasis. Geneva: World Health Organization, 2017. [PubMed] [Google Scholar]
WHO 2017b
- World Health Organization. Republic of the Marshall Islands eliminates lymphatic filariasis as a public health problem. www.wpro.who.int/mediacentre/releases/2017/20170330/en/ (accessed 1 April 2017).
WHO 2017c
- World Health Organization. Tonga eliminates lymphatic filariasis as a public health problem. www.who.int/neglected_diseases/news/Tonga_eliminates_lymphatic_filariasis/en/ (accessed 1 September 2017).
WHO 2017d
- World Health Organization. Togo: first country in sub‐Saharan Africa to eliminate lymphatic filariasis. www.who.int/neglected_diseases/news/Togo_saying_goodbye_lymphatic_filariasis/en/ (accessed 1 May 2017).
WHO 2018a
- World Health Organization. Lymphatic filariasis. www.who.int/news‐room/fact‐sheets/detail/lymphatic‐filariasis (accessed 11 October 2018).
WHO 2018b
- World Health Organization. Three more countries eliminate lymphatic filariasis. www.who.int/westernpacific/news/detail/08‐10‐2018‐three‐more‐countries‐eliminate‐lymphatic‐filariasis (accessed 12 October 2018).
WHO 2018c
- World Health Organization. Egypt: first country in Eastern Mediterranean region to eliminate lymphatic filariasis. www.who.int/neglected_diseases/news/Egypt_first_EMRO_country_eliminate_LF/en/ (accessed 14 March 2018).
Willams 1937
- Williams CB. The use of logarithms in the interpretation of certain entomological problems. Annals of Applied Biology 1937;24(2):404‐14. [Google Scholar]
References to other published versions of this review
Addiss 2005
- Addiss D, Gamble CL, Garner P, Gelband H, Ejere HOD, Critchley JA. Albendazole for lymphatic filariasis. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003753.pub3] [DOI] [PubMed] [Google Scholar]
Critchley 2005
- Critchley J, Addiss D, Ejere H, Gamble C, Garner P, Gelband H. Albendazole for the control and elimination of lymphatic filariasis: systematic review. Tropical Medicine & International Health 2005;10(9):818‐25. [DOI] [PubMed] [Google Scholar]
IFRG 2002
- International Filariasis Review Group (David AD, Henry EJ, Paul GA, Hellen GE, Carrol PR, Ejere HOD, Addiss D, Gelband H, Garner P.). Albendazole for lymphatic filariasis. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003753] [DOI] [PubMed] [Google Scholar]
IFRG 2004
- International Filariasis Review Group (David Addiss, Julia Critchley, Henry Ejere, Paul Garner, Hellen Gelband, Carrol Gamble). Albendazole for lymphatic filariasis. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003753.pub2] [DOI] [PubMed] [Google Scholar]


