Abstract
Adult pancreatic regeneration is one of the most contentious topics in modern biology. The decades-long view that the islets of Langerhans can be replenished throughout adult life through the reactivation of ductal progenitor cells has been replaced over the last decade by the now prevailing notion that regeneration does not involve progenitors and occurs only through the duplication of pre-existing mature cells. Here we dissect the limitations of lineage tracing to draw categorical conclusions about pancreatic regeneration, especially in view of the emerging evidence that traditional lineages are far less homogeneous and cell fates much more dynamic than previously thought. This new evidence further suggests that the two competing hypotheses about regeneration may not be mutually exclusive.
Keywords: Human Pancreatic Progenitor Cells, Type 1 diabetes, Islet Regeneration, Dedifferentiation, Ductal Cells
The rise and fall of the pancreatic progenitor cell hypothesis
When, back in the 80s, an islet of Langerhans was photographed sprouting from an adult human pancreatic duct [1], the notion that the ductal epithelium could harbor progenitor cells capable of regenerating the endocrine compartment was only logical. After all, although islets are typically described as “interspersed” throughout the pancreatic parenchyma, their distribution is far from random. Historical observations that islets are nearly always in the vicinity of murine and human major ducts (recently confirmed by 3D-imaging of the whole murine organ [2]) are aligned with our understanding of ductal branching as a driving force of pancreatic morphogenesis and differentiation during development [3].
In the three decades that followed, dozens of reports further reinforced the idea that ducts were not merely passive conductors of digestive juices. When cultured and stimulated with a variety of growth factors, ductal cells from rodents and humans were shown to differentiate along all pancreatic lineages, including endocrine cells [4-7], By the mid 90s, these collective observations had taken shape into the “progenitor cell hypothesis”, by which adult pancreatic regeneration recapitulates embryonic development in its pattern of ductal proliferation/differentiation [8, 9].
This view was frontally challenged in 2004, when, using an elegant lineage-tracing (LT) design, Dor and colleagues established that post-natal β-cell replenishment in mice occurred through replication of pre-existing β-cells rather than by neogenesis [10]. Although the existence of facultative multipotent cells was not disproven, this impactful study delivered a lasting blow to the progenitor hypothesis. This and other similar reports have led to the dominant view today that pancreatic regeneration does not rely on progenitors and is greatly diminished after birth [11]. A recent high-impact review on this subject dismissed adult progenitors in one paragraph, stating that the lineage tracing data published thus far are inconsistent with any meaningful contribution of any such cells (if they existed) to other pancreatic tissues [12].
Does the pancreas regenerate, anyway?
Most of these studies refer to the endocrine compartment, whose damage results in severe metabolic conditions. There is a perinatal expansion period throughout which insulin-positive cells are commonly found within ducts or nearby in both rodents and humans [13]. In fact, it has been recently discovered that there are ductal networks within the islet originating from the main ductal tree, which can regenerate β-cells in young mice and humans [14]. Incidentally, the observation of endocrine markers in ductal niches has also been reported in adult human pancreata [15, 16]. However, it is now widely accepted that islet number remains largely unchanged throughout life. Once they coalesce in this early period, islet cells grow only by self-replication, with division rates gradually decreasing until adulthood [13]. It is important to frame the progenitor vs. replication debate under the clarification that both sides agree that islet regeneration is rare under normal conditions. Dissent arises from the different interpretations of the reaction of the pancreas to pathological/non-physiological insults. For instance, duct ligation in rodents (a catastrophic injury model) has been reported to cause islet regeneration by either ductal neogenesis [17, 18] or replication of pre-existing β-cells [19]; but also not to induce endocrine regeneration at all [20]. The same discrepancies can be observed in other settings, such as pregnancy or chemical islet ablation (where both progenitor-driven neogenesis and β-cell replication were observed at the same time [21]). Although many of these interpretations could be qualified in the context of the use of different mouse strains, as well as sex and age variables, these contradictions bring us directly to the root of the problem.
Lineage tracing in the mouse: an unreliable tool in an inadequate model
For all its perceived strength, most of the evidence cited to support the indictment of the progenitor hypothesis is based on the use of one single model (the mouse) and one technique (LT). Striking differences between islets of mice and humans are not simply a matter of scale: they have been hypothesized to explain why treatments that revert diabetes in the former have not been successfully translated to the latter [22]. Anatomic and functional differences between islets from both species include the histological architecture, the relative abundance and position of various endocrine cell types, and vascularization and innervation patterns [23-25]. Even from a developmental perspective, there are substantial disparities in the onset and rate of resolution of key differentiation markers, the number of endocrine differentiation waves (single in humans vs. dual in mice) and the embryonic degree of association of developing islets with the neurovascular milieu (reviewed in [26]). The normal turnover of β-cells is several orders of magnitude lower in humans than it is in mice [27]. and they adapt to stressors such as pregnancy or obesity in radically different ways [28, 29]. Taken together, these considerations question the validity of the mouse model to draw conclusions about pancreatic regeneration in humans.
The use of LT adds another layer of potential inaccuracy. This is a very powerful tool that allows for the tagging and tracking of specific cell lineages and their progeny (see Glossary). Over the last two decades, LT has become the method of choice for the study of stem cell fate during development and regeneration. However, its limitations are also well known: the tissue-specific promoters used to tag cells may not recapitulate exactly native patterns of expression, and are often dynamically and unevenly expressed. Promoter leakage (i.e., basal degree of expression of the tissue-specific promoter in non-desired cell types, leading to inaccurate tagging) also compromises frequently the specificity of the tagging. Finally, labeling efficiency is usually low, which results in absence of tag in most of the cells of interest [30].
As a result, LT in rodents has been known to yield contradictory results. That was the case with Sox9, which earlier this decade was reported to be [31] and not to be [32] a marker of adult progenitor cells. Similarly, LT has been wielded to support that acinar cells are [33] and are not [34] facultative endocrine progenitors; and that ductal cells do [17] and do not [35] contribute to postnatal β-cell formation. Authors from both camps do not shy away from listing the caveats of LT when the observations of others do not suit their own. The very choice of a marker for any particular lineage already introduces a bias. A case in point is precisely the report still cited as proof that progenitors do not contribute to β-cell regeneration [10]. There, Dor and colleagues tagged β-cells using Cre driven by the insulin promoter. The chase period showed that a stable percentage of the β-cells generated after that point were also tagged, thereby leading to the conclusion that they only arise from pre-existing β-cells. However, in 2011, using a very similar LT design, Smukler and colleagues demonstrated that islets harbored progenitor-like cells that also expressed insulin [36]. In view of this, those “pre-existing β-cells” that were proposed to be the only possible source of new β-cells in the earlier report may very well have been progenitors after all.
On-demand progenitor cells?
New findings on the plasticity of all pancreatic compartments have blurred traditional lineage barriers, calling into question the accuracy of the previous body of LT work. In response to physiological stress, murine β-cells have been shown to de-differentiate and revert to a progenitor-like state characterized by the expression of the pro-endocrine marker Ngn3 and the pluripotency genes Oct4 and Nanog [37, 38]. or arise from pre-existing α- [39] and δ- [28, 40] cells after near-total β-cell loss. In fact, mouse and human islets harbor “virgin” β-cells that represent a transitional stage from α-cells [41]. In this context, choosing any given lineage tracer may give us, at best, a snapshot of a cell that is in the middle of a dynamic fate flux. The duct offers once again a good example of this ultimate limitation of LT: In 2008, it was reported that cells expressing the archetypal mature ductal marker carbonic anhydrase II (CAII) were progenitors [42]. A few years later, using LT in vitro, Klein and colleagues specifically discarded CAII+ cells as a source of new β-cells [43], showing instead the multipotency of CAII− subpopulations [44], This apparent contradiction could be easily explained away by citing the usual LT shortcomings, including technical problems with CAII tagging, or, in this case, the fact that the first study was conducted in mice and the second with human cells. However, the CAII− cells described by Qadir and colleagues were found in major ducts and pancreatic duct glands (PDGs), interspersed between “regular” CAII+ cells. So, what are these ductal cells that are not “ductal” according to the CAII expression criterion? Are they part of a distinct, stable population of adult progenitors that remains there after childhood? Or could they be regular ductal cells that de-differentiate, lose CAII expression and acquire progenitor-like characteristics such as proliferative capacity and multipotency? If the latter, would it not be conceivable that both seemingly discordant LT interpretations may be correct, and simply reflect the tagging of the same cell type at two different stages (one where CAII is expressed and another when CAII expression has been lost)? New evidence indicates that specific compartments of the pancreas, long thought to be composed of equivalent cells, are in fact heterogeneous and present a broad palette of differentiation stages. Aligned with the conclusions of the above study, it was reported earlier this year that only specific subpopulations within human and murine ducts exhibit organoid-forming capacity [45]. Emergent high-resolution analytical tools, especially single-cell RNA sequencing (scRNAseq), further support this paradigm-shifting notion. These techniques are not devoid of their own caveats, including the a posteriori classification of cells using principal component analysis (or comparison of cellular signatures to pre-existing transcriptional profiles), which can lead to incorrect identity assignment; the use of insufficient sequencing depth, which may fail to detect the expression of genes expressed at low levels; or our current inability to sequence directly, and reproducibly, from freshly isolated pancreatic tissue, which demands pre-culture to allow for the stabilization of the highly proteolytic milieu of the pancreas. Notwithstanding these limitations, scRNAseq has unveiled an unexpected assortment of maturational lineages within the adult pancreas. This was the conclusion of several recent single-cell RNA-seq analyses of mouse [46] and human [46-50] islets –which, as they contain a representation of virtually all cell types of the organ, are considered a pancreatic microcosm of sorts. Ducts and β-cells were invariably the two populations with the highest heterogeneity. As also reported by Dorrell and colleagues [51], these studies described multiple β-cell subpopulations, suggesting different stages of maturation. In one of them [47], α- and β-cells from children (i.e., at a stage of active islet growth/remodeling) were found to have less defined gene signatures than those in adults (i.e., in α- and β-cells from young children, many “adult signature genes” were no longer expressed in the expected cell type-specific manner, with multiple α-cell signature genes preferentially observed in juvenile β-cells). This pattern was also observed when the donors had type 2 diabetes (i.e., subjected to pancreatic stress), indicating a partial dedifferentiation process.
The concept of β-cell dedifferentiation, first described in mouse, was originally thought to be merely a response aimed at protecting the cell from metabolic stress [37]. By shutting down insulin synthesis and secretion, the cell would keep the unfolded protein response (UPR) to a minimum, avert apoptosis and remain in “stand-by” until better days came and business could be resumed as usual. However, the concomitant acquisition of stem cell markers (see above) and the reported multipotent re-differentiation into other cell types, including α-, δ- and PP cells [37, 38] further suggested that the loss of β-cell identity may in fact entail a reversion to a progenitor-like state. Stress-mediated dedifferentiation mechanisms are also thought to be behind the reported increase in foci of chromogranin A-positive/hormone-negative endocrine cells during pancreatitis [52], type 2 diabetes [53] and type 1 diabetes [54] in humans, as well as the observation of multi-hormonal cells expressing PDX1 or NGN3 in samples from patients with glucose intolerance or newly diagnosed type 2 diabetes [55].
Beyond the islet, this “on-demand under duress” generation of progenitor cells via de-differentiation has been extensively reported in pancreatic ducts. Transition through a Ngn3+ “endocrine committed” stage and the fluid feedback between Notch-inactive Ngn3+ cells and Notch-active proliferating cells in the ductal tree have been common themes in an extensive body of research on duct-mediated morphogenesis [56, 57]. Using the duct ligation model, Bouwens and colleagues showed a decade ago that Ngn3 was reactivated in the ducts of adult mice. These cells proliferated in a progenitor-like fashion and gave rise to all endocrine cell types, both in vivo and in vitro [18]. This report was so compelling that even the original proponents of the self-replication hypothesis published a commentary later that year acknowledging the presence of “facultative endocrine progenitor cells” in the exocrine pancreas [58]. More recently, the use of proinflammatory cytokines was also shown to activate Ngn3 in mouse ducts [59]. Similarly, subtotal pancreatectomy and gastrin treatment were reported to induce ductal dedifferentiation and β-cell neogenesis in rats [60]. One of the single-cell human RNAseq studies cited above [50] unequivocally demonstrates the existence of an intermediate/transitional population between ductal and β-cells. These are only a few examples that suggest that “terminal differentiation” may be a misnomer when applied to the various compartments of the adult pancreas.
So, how do islets regenerate?
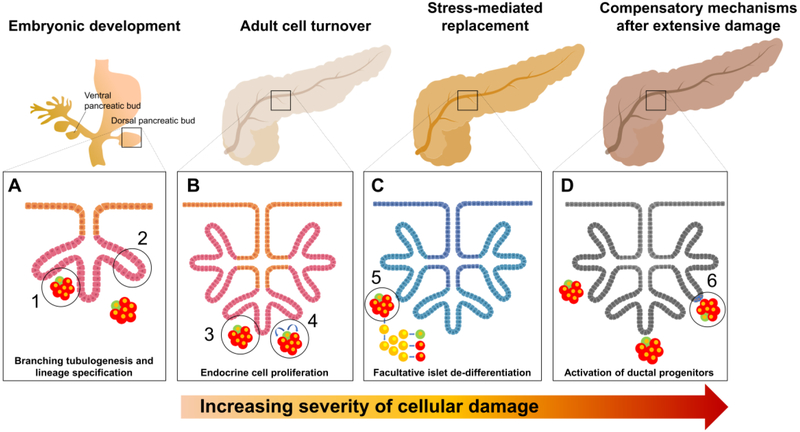
The integration of all these findings into a cohesive model of regeneration remains a challenge in the field. It is probably safe to assume that, since there is very little endocrine cell turnover in humans throughout adulthood [13], these facultative regeneration mechanisms normally remain dormant. Despite their high metabolic activity, islet cells are exceedingly long-lived under regular conditions [27]. Therefore, replication [13] or α-to-β cell transition in intraislet neogenic niches [41] may take care of the occasional β-cell demise. As suggested by studies in mice, islets under stress may further activate the β-cell dedifferentiation and redifferentiation pathway [37]. The consistent observation of this phenomenon during childhood islet remodeling [47], metabolic stress [38], pancreatitis [52] and type 2 diabetes [47] in humans suggests that dedifferentiation/redifferentiation may be an evolutionarily conserved first-line regenerative response during growth and when islets are subjected to relatively common stressors. More extensive damage to the pancreas, such as that induced by partial pancreatectomy [60], duct ligation [18], inflammation [59] or immune attack [16, 61] may, in addition, reactivate more primitive developmental pathways in specific ductal subpopulations, with the potential ability to address widespread islet loss and/or partial organ remodeling. A model where successive layers of regenerative safeguards (replication; intra-islet neogenesis; β-cell dedifferentiation-redifferentiation; ductal dedifferentiation-redifferentiation) are added one of top of the other depending on the severity of the damage fits the available evidence and probably reconciles decades of ostensibly disparate findings (see Key Figure).
KEY FIGURE LEGEND.
An inclusive model of pancreatic regeneration that compiles all the evidence generated thus far. This model proposes an additive, multi-layered response to progressive damage in the adult pancreas. (A) During pancreatic development, branching tubulogenesis from the ductal tree is now an accepted progenitor-dependent mechanism of tissue formation and subsequent lineage specification. (1) Multipotent progenitors form islets. (2) Multipotent progenitors expand the ductal tree and form acini. (B) In the adult organ, during normal tissue turnover, β-cells may give to other β-cells by replication (3), or, alternatively, arise from de-differentiated α-cells through a “virgin β-cell” intermediate stage (4). (C) When subjected to metabolic stress, adult endocrine cells may undergo de-differentiation, acquire progenitor-like multipotency and re-differentiate (facultative islet de-differentiation) (5). (D) After extensive damage, broad de novo islet regeneration may occur through ductal progenitor (resident or facultative) cell remodeling (6). This process may mimic, to some degree, the embryonic process of branching tubulogenesis (A). The models presented here are an amalgamation of evidence from murine and human data.
Can these regenerative pathways be harnessed?
Beyond its academic worth, an obvious reason to unravel the different pathways used by the pancreas to regenerate is the possibility of pharmaceutically inducing/enhancing them for therapeutic purposes. Type 1 diabetes, an autoimmune disease that results in near-total β-cell annihilation, is probably the foremost target of all the efforts conducted in this direction. Pancreatic samples from “medalists” (patients who have had the disease for five decades or longer) contain residual β-cells, probably reflecting the ongoing effort of the organ to replenish them [62]. Although this is a losing battle as long as autoimmunity persist, there is hope that an ever-growing arsenal of immunotherapies may allow for regeneration to occur. Still, given the extraordinarily low natural turnover of β-cells in humans, additional interventions may be necessary to boost regeneration. In this context, bone morphogenetic proteins (BMPs) have been proven to stimulate pancreatic progenitor cell proliferation in mice [63] and humans [43, 44] by activating the BMP signaling pathway and inducing Inhibition of Differentiation (ID) proteins. Resident pancreatic pericytes secrete high levels of BMP proteins [64], suggesting the potential existence of local BMP signaling loops in the murine organ. Since BMP-like agents have been safely tested in clinical settings [65], the possibility of using them to induce β-cell regeneration in situ is worth exploring. Similarly, a combination of epidermal growth factor (EGF) and ciliary neurotrophic factor (CNTF) was reported earlier this decade to induce β-cell regeneration in live diabetic mice from exocrine cells in a Stat3-dependent manner [66]. However, for human pancreatic exocrine cells to adopt a similar fate, lentiviral transduction of MAPK and STAT3 was required [67].
Concluding remarks and future perspectives
The above observation exposes once more the limitations of the mouse in its ability to predict the regenerative behavior of human pancreatic tissues. After decades of over-reliance on this model, we are currently at a juncture where the path to clinical therapies will likely require confirmation of the multi-layered regeneration model in human pancreatic cells (see Outstanding Questions). Recent advances in organotypic culture techniques have enabled the generation of 120-130 μm-thick live pancreatic slices that maintain the histological architecture of the organ [68], thus affording the study of pancreatic physiology and the interplay between exocrine, endocrine, vascular, neural and immune compartments in a unique ex vivo setting [69, 70]. Originally described in mice, refined conditions have now been applied to culture pancreatic slices from human donors [71, 72], opening a window to regeneration events in nearly undisturbed pancreatic niches. The possibility of xenotransplanting human pancreatic tissue into the anterior chamber of the eye of immunodeficient animals [73] offers also the possibility to longitudinally monitor potential regeneration by simply placing the anesthetized mouse’s eye under the microscope. Coupled with the continuous advances and increased affordability of single-cell resolution analytical tools, all the conditions seem to be in place to foster the onset of a new humancentric era in our quest to understand and exploit the natural regenerative abilities of the pancreas.
OUTSTANDING QUESTIONS.
New and compelling evidence points out at the developmental heterogeneity of pancreatic compartments long-thought to be made of terminally differentiated cells, and there is an increasingly large number of studies showing facultative dedifferentiation of multiple pancreatic cell types in response to stress. Should these factors be taken into account when using lineage tracing as the molecular tool of choice for the study of dynamic regeneration phenomena?
Can the self-duplication and progenitor cell-based replenishment models be reconciled under a multi-layered process entailing the sequential (and additive) activation of progressively more primitive regeneration processes? Such unified model would contemplate self-duplication and low-level local transdifferentiation as the primary mechanisms controlling natural turnover, with the activation of dedifferentiation and redifferentiation as well as ductal-based regeneration in response to more extensive damage.
Are the more developmentally competent cells within the ductal tree constitutive, or facultative? Are they residual progenitor cell types that remain in place after embryonic development, or do they arise from the dedifferentiation of ductal cells in response to stress? If the latter, why are these cells regularly detected in the pancreas of healthy donors (i.e., not under stress)? Does the natural function of the exocrine pancreas induce a basal/non-pathological level of stress that is sufficient to permanently maintain the progenitor-like dedifferentiation of a subset of ductal cells?
Are human pancreatic regeneration pathways similar to those described in the mouse model? Can we use organotypic cultures and novel xenotransplantation settings to fill this gap in our understanding? Could these regeneration pathways be harnessed for therapeutic purposes to replenish the endocrine cells that are lost in type 1 and type 2 diabetes?
Just as Bilbo made the journey to the Lonely Mountain and back again [74], so has the progenitor cell hypothesis made a figurative journey back into favor after a decade of relative discredit. In the same vein, we now see ductal progenitor cells as the protagonists of two biological journeys: from differentiated cells to facultative progenitors and back again as differentiated cells; and from multipotent embryonic progenitors to differentiated cells and back again as facultative progenitors.
HIGHLIGHTS.
Conventional views on pancreatic regeneration, long thought to involve the reactivation of a duct-based embryonic program, have been challenged over the past decade by lineage tracing studies, which suggest instead the self-duplication of mature cells.
However, single-cell resolution analyses have blurred lineage barriers and suggest a high degree of developmental heterogeneity within each pancreatic compartment. Evidence for dynamic fate plasticity and facultative dedifferentiation calls into question the validity of lineage tracing findings.
Conflicting views may be reconciled in a model where multiple layered regeneration mechanisms follow a specific activation sequence depending on the extent of the damage.
Novel organotypic culture and transplantation techniques herald a new era of humancentric studies on pancreatic regeneration, circumventing the limitations of the mouse model.
Acknowledgments
We thank Dr. Dagmar Klein and Ms. Silvia Alvarez-Cubela for their critical reading of our manuscript. The corresponding authors are funded by the Diabetes Research Institute Foundation (DRIF), the Inserra family, the Fred and Mabel R. Parks Foundation, the Foundation for Diabetes Research, the Tonkinson Foundation, the Michael J. and Katherine E. Franco Foundation, the Frank Strick Foundation, Mildred Graff, and NIH grants 1R43DK105655-01, 2R44DK105655-02 and 1U01DK120393-01 (Human Islet Research Consortium, HIRN). M.M.F.Q is funded by an International Fulbright pre-doctoral fellowship/grant administered by the Foreign Fulbright Scholarship Board and the International Institute of Education.
GLOSSARY BOX
- Acinar cells:
Exocrine cells of the pancreas (organized in acini) that secrete digestive enzymes. These are collected by ductal cells and transported to the duodenum, where they contribute to the digestion of nutrients.
- Carbonic anhydrase II (CAII):
Protein that catalyzes the reversible hydration of carbon dioxide, generating bicarbonate. Mature ductal cells express CAII and secrete bicarbonate, thus increasing luminal pH and avoiding the activation of acinar-secreted digestive enzymes prior to their arrival to the duodenum.
- Islets of Langerhans:
Clusters of endocrine cell types (α, β, δ, γ, ε), which collectively form the endocrine pancreas. Their main role is the regulation of glucose homeostasis. The β-cell is typically the most abundant endocrine cell type in islets, β-cells secrete insulin, which fosters glucose uptake by all the cells of the body. These cells are typically under metabolic stress in type 2 diabetes and destroyed in autoimmune (type 1) diabetes.
- Lineage tracing:
Molecular tagging technique used to identify and follow the progeny of any given cell type. A typical labeling system entails the Cre recombinase-mediated excision of a “floxed” stop cassette, upon which a fluorescent reporter gene is expressed. The recombinase is driven by a tissue-specific promoter and can be activated by a “pulse” of tamoxifen or doxycycline. As a result, the cells of interest are tagged in a permanent and inheritable way, which allows us to follow their fate (“chase”) over time.
- Neogenesis:
De novo formation (i.e., of β-cells from non-β-cells). When applied to the generation of a new differentiated cell, this concept is usually presented as an alternative to self-replication (e.g., a β-cell giving rise to two β-cells by proliferation)
- Pancreatic ducts:
Pancreatic-wide network of channels primarily in charge of collecting and conducting acinar-secreted enzymes to the duodenum. During embryonic development, pancreatic morphogenesis is driven by a process of ductal-mediated expansion, branching and differentiation. The ductal epithelium has been hypothesized to contain either resident or facultative progenitor cells beyond embryonic development.
- Progenitor cells:
Cells that exhibit a variable degree of potency and proliferation potential. The potency and role of adult progenitor cells is highly organ- and context-specific.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Swartz FJ and Carstens PH (1986) An islet of Langerhans located within the epithelium of a human pancreatic duct. Histol Histopathol 1 (2), 111–7. [PubMed] [Google Scholar]
- 2.Tainaka K et al. (2014) Whole-body imaging with single-cell resolution by tissue decolorization. Cell 159 (4), 911–24. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q et al. (2007) A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 13 (1), 103–14. [DOI] [PubMed] [Google Scholar]
- 4.Sarvetnick NE and Gu D (1992) Regeneration of pancreatic endocrine cells in interferon-gamma transgenic mice. Adv Exp Med Biol 321, 85–9; discussion 91-3. [DOI] [PubMed] [Google Scholar]
- 5.Gao R et al. (2003) Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 52 (8), 2007–15. [DOI] [PubMed] [Google Scholar]
- 6.Huch M et al. (2013) Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32 (20), 2708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomans CJM et al. (2018) Expansion of Adult Human Pancreatic Tissue Yields Organoids Harboring Progenitor Cells with Endocrine Differentiation Potential. Stem Cell Reports 10 (3), 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonner-Weir S et al. (1993) A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42 (12), 1715–20. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg L (1995) In vivo cell transformation: neogenesis of beta cells from pancreatic ductal cells. Cell Transplant 4 (4), 371–83. [DOI] [PubMed] [Google Scholar]
- 10.Dor Y et al. (2004) Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429 (6987), 41–6. [DOI] [PubMed] [Google Scholar]
- 11.Xiao X et al. (2013) No evidence for beta cell neogenesis in murine adult pancreas. J Clin Invest 123 (5), 2207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q and Melton DA (2018) Pancreas regeneration. Nature 557 (7705), 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier JJ et al. (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57 (6), 1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Gohary Y et al. (2015) Intra-Islet Pancreatic Ducts Can Give Rise to Insulin-Positive Cells. Endocrinology Published ahead of print 10.1210/en.2015-1175, en20151175. [Google Scholar]
- 15.Carpino G et al. (2016) Progenitor cell niches in the human pancreatic duct system and associated pancreatic duct glands: an anatomical and immunophenotyping study. J Anat 228 (3), 474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Pagola A et al. (2008) Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 51 (10), 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonner-Weir S et al. (2008) Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans 36 (Pt 3), 353–6. [DOI] [PubMed] [Google Scholar]
- 18.Xu X et al. (2008) Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132 (2), 197–207. [DOI] [PubMed] [Google Scholar]
- 19.Yuchi Y et al. (2015) Estrogen Receptor alpha Regulates beta-Cell Formation During Pancreas Development and Following Injury. Diabetes 64 (9), 3218–28. [DOI] [PubMed] [Google Scholar]
- 20.Rankin MM et al. (2013) beta-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes 62 (5), 1634–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song I et al. (2015) Beta Cell Mass Restoration in Alloxan-Diabetic Mice Treated with EGF and Gastrin. PLoS One 10 (10), e0140148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levetan CS and Pierce SM (2013) Distinctions between the islets of mice and men: implications for new therapies for type 1 and 2 diabetes. Endocr Pract 19 (2), 301–12. [DOI] [PubMed] [Google Scholar]
- 23.Dolensek J et al. (2015) Structural similarities and differences between the human and the mouse pancreas. Islets 7 (1), e1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabrera O et al. (2006) The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103 (7), 2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rorsman P and Ashcroft FM (2018) Pancreatic beta-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol Rev 98 (1), 117–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair G and Hebrok M (2015) Islet formation in mice and men: lessons for the generation of functional insulin-producing beta-cells from human pluripotent stem cells. Curr Opin Genet Dev 32, 171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cnop M et al. (2011) Longevity of human islet alpha- and beta-cells. Diabetes Obes Metab 13 Suppl 1, 39–46. [DOI] [PubMed] [Google Scholar]
- 28.Cigliola V et al. (2016) Stress-induced adaptive islet cell identity changes. Diabetes Obes Metab 18 Suppl 1, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee RR (2018) Piecing together the puzzle of pancreatic islet adaptation in pregnancy. Ann N Y Acad Sci 1411 (1), 120–139. [DOI] [PubMed] [Google Scholar]
- 30.Wuidart A et al. (2016) Quantitative lineage tracing strategies to resolve multipotency in tissue-specific stem cells. Genes Dev 30 (11), 1261–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuyama K et al. (2011) Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43 (1), 34–41. [DOI] [PubMed] [Google Scholar]
- 32.Kopp JL et al. (2011) Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138 (4), 653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan FC et al. (2013) Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140 (4), 751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai BM et al. (2007) Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 117 (4), 971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solar M et al. (2009) Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 17 (6), 849–60. [DOI] [PubMed] [Google Scholar]
- 36.Smukler Simon R. et al. (2011) The Adult Mouse and Human Pancreas Contain Rare Multipotent Stem Cells that Express Insulin. Cell Stem Cell 8 (3), 281–293. [DOI] [PubMed] [Google Scholar]
- 37.Talchai C et al. (2012) Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150 (6), 1223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim-Muller JY et al. (2016) Aldehyde dehydrogenase 1a3 defines a subset of failing pancreatic beta cells in diabetic mice. Nat Commun 7, 12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorel F et al. (2010) Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464 (7292), 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chera S et al. (2014) Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature 514 (7523), 503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Meulen T et al. (2017) Virgin Beta Cells Persist throughout Life at a Neogenic Niche within Pancreatic Islets. Cell Metab 25 (4), 911–926 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inada A et al. (2008) Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 105 (50), 19915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein D et al. (2015) BMP-7 induces adult human pancreatic exocrine-to-endocrine conversion. Diabetes 64, 4123–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qadir MMF et al. (2018) P2RY1/ALK3-Expressing Cells within the Adult Human Exocrine Pancreas Are BMP-7 Expandable and Exhibit Progenitor-like Characteristics. Cell Rep 22 (9), 2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezanejad H et al. (2018) Heterogeneity of SOX9 and HNF1beta in Pancreatic Ducts Is Dynamic. Stem Cell Reports 10 (3), 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baron M et al. (2016) A Single-Cell Transcriptomic Map of the Human and Mouse Pancreas Reveals Inter- and Intra-cell Population Structure. Cell Syst 3 (4), 346–360 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YJ et al. (2016) Single-Cell Transcriptomics of the Human Endocrine Pancreas. Diabetes 65 (10), 3028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muraro MJ et al. (2016) A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst 3 (4), 385–394 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Segerstolpe A et al. (2016) Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enge M et al. (2017) Single-Cell Analysis of Human Pancreas Reveals Transcriptional Signatures of Aging and Somatic Mutation Patterns. Cell 171 (2), 321–330 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorrell C et al. (2016) Human islets contain four distinct subtypes of beta cells. Nat Commun 7, 11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moin ASM et al. (2018) Increased Chromogranin A-Positive Hormone-Negative Cells in Chronic Pancreatitis. J Clin Endocrinol Metab 103 (6), 2126–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Md Moin AS et al. (2016) Increased Frequency of Hormone Negative and Polyhormonal Endocrine Cells in Lean Individuals With Type 2 Diabetes. J Clin Endocrinol Metab 101 (10), 3628–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Md Moin AS et al. (2016) Increased Hormone-Negative Endocrine Cells in the Pancreas in Type 1 Diabetes. J Clin Endocrinol Metab 101 (9), 3487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoneda S et al. (2013) Predominance of beta-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab 98 (5), 2053–61. [DOI] [PubMed] [Google Scholar]
- 56.Magenheim J et al. (2011) Ngn3(+) endocrine progenitor cells control the fate and morphogenesis of pancreatic ductal epithelium. Dev Biol 359 (1), 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qu X et al. (2013) Notch-mediated post-translational control of Ngn3 protein stability regulates pancreatic patterning and cell fate commitment. Dev Biol 376 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- 58.Dor Y and Melton DA (2008) Facultative endocrine progenitor cells in the adult pancreas. Cell 132 (2), 183–4. [DOI] [PubMed] [Google Scholar]
- 59.Valdez IA et al. (2016) Proinflammatory Cytokines Induce Endocrine Differentiation in Pancreatic Ductal Cells via STAT3-Dependent NGN3 Activation. Cell Rep 15 (3), 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tellez N and Montanya E (2014) Gastrin induces ductal cell dedifferentiation and beta-cell neogenesis after 90% pancreatectomy. J Endocrinol 223 (1), 67–78. [DOI] [PubMed] [Google Scholar]
- 61.Moin AS et al. (2017) Increased Proliferation of the Pancreatic Duct Gland Compartment in Type 1 Diabetes. J Clin Endocrinol Metab 102 (1), 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keenan HA et al. (2010) Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 59 (11), 2846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hua H et al. (2006) BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem 281 (19), 13574–80. [DOI] [PubMed] [Google Scholar]
- 64.Sakhneny L et al. (2018) Pancreatic Pericytes Support beta-Cell Function in a Tcf7l2- Dependent Manner. Diabetes 67 (3), 437–447. [DOI] [PubMed] [Google Scholar]
- 65.Himmelfarb J et al. (2018) Perioperative THR-184 and AKI after Cardiac Surgery. J Am Soc Nephrol 29 (2), 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baeyens L et al. (2013) Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol 32 (1), 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Lemper M et al. (2015) Reprogramming of human pancreatic exocrine cells to beta-like cells. Cell Death Differ 22 (7), 1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marciniak A et al. (2014) Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nat Protoc 9 (12), 2809–22. [DOI] [PubMed] [Google Scholar]
- 69.Almaca J et al. (2018) The Pericyte of the Pancreatic Islet Regulates Capillary Diameter and Local Blood Flow. Cell Metab 27 (3), 630–644 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weitz JR et al. (2018) Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 61 (1), 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohrs CM et al. (2017) Vessel Network Architecture of Adult Human Islets Promotes Distinct Cell-Cell Interactions In Situ and Is Altered After Transplantation. Endocrinology 158 (5), 1373–1385. [DOI] [PubMed] [Google Scholar]
- 72.Liang T et al. (2017) Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem 292 (14), 5957–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leibiger IB and Berggren PO (2017) Intraocular in vivo imaging of pancreatic islet cell physiology/pathology. Mol Metab 6 (9), 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tolkien JRR (1937) The Hobbit, or There and Back Again, George Allen & Unwin; (UK). [Google Scholar]