Abstract
A practical synthesis of the novel highly fluorinated amino acid Fmoc-perfluoro-tert-butyl tyrosine was developed. The sequence proceeds in 2 steps from commercially available Fmoc-4-NH2-phenylalanine, via diazotization followed by diazonium coupling reaction with perfluoro-tert-butanol. In peptides, perfluoro-tert-butyl tyrosine was detected in 30 seconds by NMR spectroscopy at 500 nM peptide concentration, due to 9 chemically equivalent fluorines that are a sharp singlet by 19F NMR. Perfluoro-tert-butyl ether has an estimated σp Hammett substituent constant of +0.30.
Graphical Abstract
Sensitivity in NMR spectroscopy is limited by the concentration of the analyte (typically requiring mid-micromolar concentration for rapid analysis) and by challenges in resolution due to spectral overlap (due to competing signals obscuring the signals of interest). These problems are particularly acute for the study of proteins and for investigations in complex solutions. The problem of spectral overlap in proteins can be partially overcome via site-specific isotopic labeling, such as using 15N-, 13C- and/or 2H-labeled amino acids and selective pulse sequences to spectroscopically isolate these amino acids. These approaches have allowed the investigation of protein function in media as complex as living E. coli and human cells and Xenopus oocytes, albeit at a substantial cost in sample preparation, experimental complexity, and instrument time.1
Alternatively, the introduction of NMR-active nuclei that are not typically present in biological systems provides an approach that results in NMR signals that are only associated with the molecules of interest, eliminating the challenges associated with background signals and spectral assignment. 19F has been the most widely used non-native NMR-active nucleus applied for interpretation of biological structure and function.2 Fluorinated molecules have been used for analysis of events as diverse as protein folding in cells, screening of pharmaceutical inhibitors of protein function, and MRI.3 19F has 100% natural abundance and sensitivity similar to 1H. Moreover, due to the absence of background signals, including from water, experiments can, compared to 1H NMR, be typically conducted with increased gain and simpler pulse sequences, further increasing sensitivity. However, despite these substantial advantages, the inherent sensitivity limits of NMR spectroscopy result in an inability to probe many processes at physiologically relevant concentrations.
Increasing the concentration of equivalent signals in a molecule can substantially increase sensitivity and decrease the concentrations accessible for analysis. Thus, highly fluorinated molecules employing multiple chemically equivalent fluorines have found applications of small molecules, polymers, and proteins, in NMR spectroscopy and in MRI.3d, 4
We recently described the synthesis of two conformationally biased amino acids containing perfluoro-tert-butyl groups, 4R- and 4S-perfluoro-tert-butyl hydroxyproline.3c, 5 These amino acids were synthesized either in solution phase or within peptides on solid phase via Mitsunobu reaction of perfluoro-tert-butanol with the diastereomeric hydroxyprolines. Peptides containing perfluoro-tert-butyl hydroxyprolines have distinct conformational preferences and, owing to the presence of 9 chemically equivalent fluorines, can be rapidly detected at high nanomolar concentrations (5 minutes at 200 nM peptide concentration).
Given this high sensitivity achievable by 19F NMR, we sought to extend the applications of perfluoro-tert-butyl groups to applications with aromatic amino acids. In particular, we were inspired by recent work showing that tert-butyl tyrosine (Tby) (Figure 1) can be incorporated in expressed proteins via amber codon suppression using an evolved aminoacyl tRNA synthetase or pyrrolysyl tRNA synthetase.4g, 6 Tby was observed by 1H NMR in expressed proteins as a sharp singlet, due to 9 equivalent hydrogens.7 Moreover, this sharp singlet is present even when this amino acid is in proteins as large as 320 kDa: multiple bond rotations of the side chain result in isotropic averaging despite the slow rotational correlation times that typically prevent standard NMR spectroscopy of proteins over 30 kDa. Similarly, isotropic averaging of trifluoromethylphenylalanine allowed the detection of a 98 kDa protein in living E. coli cells.3d These data suggest significant advantages of tyrosine-tert-butyl ethers in the NMR spectroscopy of large proteins. Therefore, we sought to develop an approach to the synthesis of the amino acid perfluoro-tert-butyl tyrosine, which could combine high signal sensitivity (9 equivalent fluorines) with the potential for future incorporation in expressed proteins and detection in high molecular weight complexes due to isotropic signal averaging.
Figure 1.
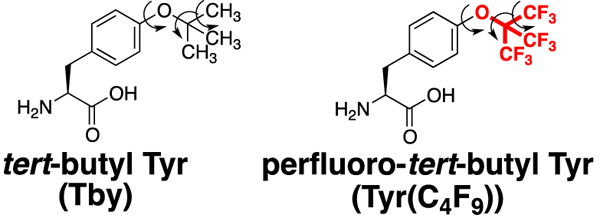
tert-Butyl tyrosine (Tby) and its fluorinated analogue. Arrows indicate bond rotations that result in isotropic averaging of the NMR-active 1H nuclei in Tby, even in large proteins.
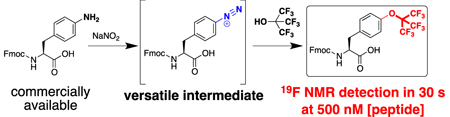
There is only one previous synthesis of a molecule with a perfluoro-tert-butyl aryl ether, which was prepared via a diazonium coupling reaction with perfluoro-tert-butanol.8 Therefore, we examined the synthesis of Fmoc-perfluoro-tert-butyl tyrosine using the commercially available amino acid Fmoc-4-NH2-phenylalanine (1) as the starting material (Scheme 1). Diazotization of 1 using sodium nitrite in tetrafluoroboric acid generated the intermediate diazonium 2, which has not previously been described and which was used in the subsequent step without purification. Diazonium coupling was achieved via heating 2 in perfluoro-tert-butanol at reflux, generating Fmoc-perfluoro-tert-butyl tyrosine 3 in good yield in two steps from commercially available starting material, with only a single purification step. This highly practical synthesis, which can be completed in 24 hours, should encourage broad application of this amino acid. In addition, removal of the Fmoc group generated the free amino acid, for potential applications in protein expression and protein engineering. Warning: while tetrafluoroborate salts of diazoniums exhibit greater stability than other diazonium salts, these reactions inherently generate nitrogen gas, yielding potential hazard from explosion. Reactions were conducted in glassware open to air, and the diazonium salt was used immediately after generation, without storage of this intermediate.
Scheme 1.
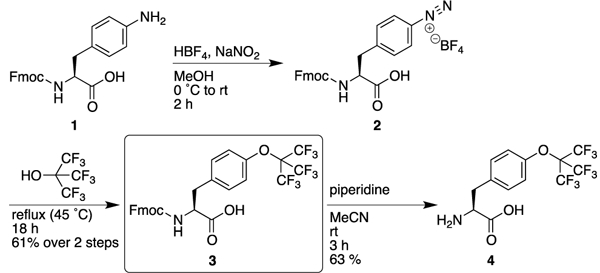
Synthesis of perfluoro-tert-butyl tyrosine.
Diazonium coupling is a versatile approach to the modification of aromatic rings, via direct coupling or via metal-mediated reactions, including Sandmeyer-type reactions and palladium-catalyzed (Heck, Suzuki, Stille, carbonylation) reactions.9 Diazotization of the free amino acid 4-amino-phenylalanine (synthesized from 4-nitro-phenylalanine) has previously been applied to the synthesis of 4-chloro, 4-azido, 4-tetrazole, 4-thiol-, and 4-selenol-phenylalanine derivatives, as well as phenylalanine derivatives containing nitrogen and sulfur mustards.10 Diazonium salts may also be converted to azo compounds or may be directly reduced to hydrazines. The facile generation of the diazonium of Fmoc-4-NH2-phenylalanine suggests that this approach may be highly practical and broadly applicable to the synthesis of diverse and functionally useful 4-substituted phenylalanines, for direct application in Fmoc solid-phase peptide synthesis.
To examine the structural and electronic effects of the perfluoro-tert-butyl aryl ether, Fmoc-perfluoro-tert-butyl tyrosine 3 was incorporated via solid-phase peptide synthesis in the model peptide context Ac-TXPN-NH2, where X = an aromatic amino acid.11 These peptides exhibit cis-trans isomerism about the aromatic-proline amide bond due to a proline/aromatic C–H/π interaction, with the extent of cis amide bond (greater cis amide bond = reduced Ktrans/cis) correlating with the electronics of the aromatic π face. This peptide was synthesized via standard solid-phase peptide synthesis, with optimization in yield achieved via PEG-polystyrene graft resin, 24 hour amide coupling with 3, and HATU as a coupling reagent. Subjection of the peptide to TFA cleavage/deprotection conditions using thioanisole and water as scavengers cleanly generated the peptide Ac-TTyr(C4F9)PN-NH2 (Scheme 2a). In contrast, the use of triisopropylsilane (TIS) as a scavenger resulted in product decomposition, generating the peptide with phenylalanine (elimination of the perfluoro-tert-butyl alcohol, Scheme 2b), presumably via an SNAr mechanism with the silyl hydride under acidic conditions. These results were reproduced using the purified peptide Ac-TTyr(C4F9)PN-NH2, indicating the compatibility of perfluoro-tert-butyl tyrosine with TFA cleavage/deprotection conditions as long as hydride sources are avoided. In addition, the purified peptide was exposed to both 20% piperidine in DMF and 8% DIPEA in DMF for 24 hours (Scheme 2cd). No significant decomposition was observed under either condition, confirming the compatibility of perfluoro-tert-butyl tyrosine with extended exposure to reagents employed in peptide synthesis.12
Scheme 2.
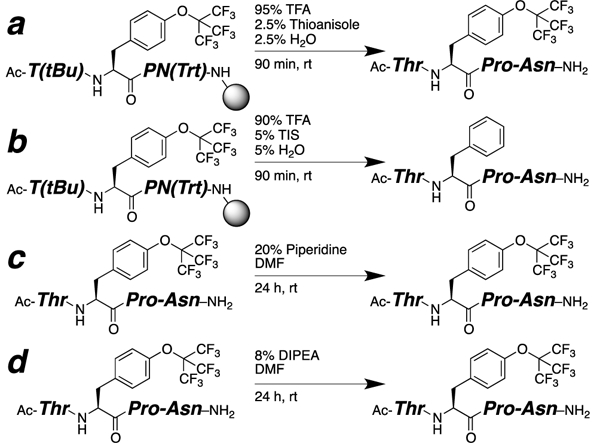
Stability of Tyr(C4F9) in a peptide to standard conditions of (a, b) TFA cleavage/deprotection, (c) Fmoc deprotection, and (d) amide coupling.
An aryl perfluoro-tert-butyl ether has only been described once previously,8 and thus no data exist on the aromatic electronic effects of a perfluoro-tert-butyl ether. Peptides with 4-substituted phenylalanines in the Ac-TXPN-NH2 context (X = 4-Z-Phe) exhibit a Hammett correlation between log Ktrans/cis and the Hammett σ constant of the 4-substituent (ρ = 0.295 ± 0.017 across 13 neutral 4-substituents).11c, 11e Therefore, the Ktrans/cis of Ac-TTyr(C4F9)PN-NH2 could be employed to provide an estimate of σ for the –OC4F9 group. The Ktrans/cis = 4.1 measured for this peptide (Figure 2) correlates to a σ of approximately +0.30, indicating a modestly electron-withdrawing effect for this group, despite 9 fluorines. These results are consistent with other fluorinated ethers, which are electron-withdrawing in contrast to the analogous hydrocarbon ethers, but less electron-withdrawing than the equivalent fluorocarbons (Table 1).13 Thus, the electronic properties of the perfluoro-tert-butyl aryl ether are affected by a balance of the electron-donating character of the ether and the electron-withdrawing character of the 9 fluorines, whose effect is reduced because they are located 4 atoms away from the aromatic ring.
Figure 2.
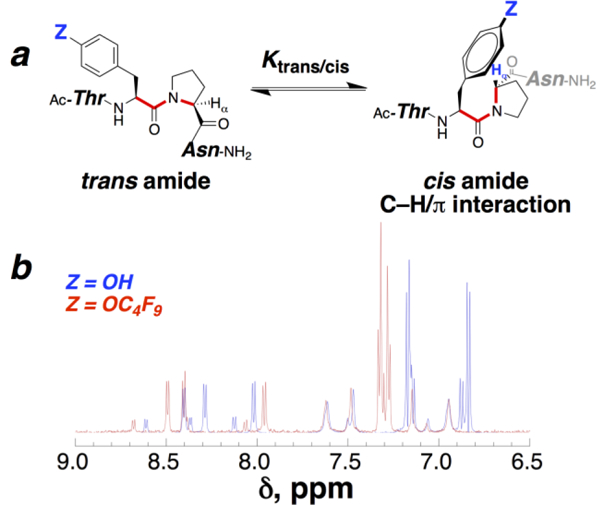
(a) cis-trans isomerism of the aromatic-proline amide bond (red) in Ac-TXPN-NH2 peptides (X = aromatic amino acid) can be used to quantify aromatic electronic effects, via Ktrans/cis and via the δ of proline Hα when in the cis conformation (with smaller Ktrans/cis and more upfield Procis Hα δ indicating a more electron-rich aromatic and a stronger C–H/π interaction). (b) 1H NMR spectra (amide-aromatic region, 90% H2O/10% D2O, 5 mM phosphate pH 4, 25 mM NaCl) of peptides with X = Tyr (Z = OH) (blue) and X = Tyr(C4F9) (Z = OC4F9) (red). Pro Hα for X = Tyr, δtrans = 4.42 ppm, δcis = 3.83 ppm; Pro Hα for X = Tyr(C4F9), δtrans = 4.40 ppm, δcis = 4.00 ppm.
Table 1.
Hammett σp substituent constants for alkyl and alkyl ether groups.a
| substituent | σp | substituent | σp |
|---|---|---|---|
| −CH3 | −0.07 | −OCH3 | −0.27 |
| −CHF2 | +0.32 | −OCHF2 | +0.31 |
| −CF3 | +0.54 | −OCF3 | +0.35 |
| −CF2CF3 | +0.52 | −OCF2CF3 | +0.28 |
| −C(CH3)3 | −0.20 | −OC(CH3)3 | −0.29 |
| −C(CF3)3 | +0.55 | −OC(CF3)3 | +0.30b |
Values from ref 13, except as indicated.
Estimated from results herein.
We also examined the solution-phase synthesis of perfluoro-tert-butyl tyrosine within peptides containing 4-NH2-phenylalanine (Scheme 3). Diazotization of Ac-TPhe(4-NH2)PN-NH2 followed by heating with perfluoro-tert-butanol generated the peptide Ac-TTyr(C4F9)PN-NH2, which was identical by NMR to the peptide synthesized using 3. While this approach will not be compatible with all amino acids (e.g. potential side reactions with Tyr, Cys, Met)14, it provides an alternative approach to the synthesis of peptides containing Tyr(C4F9), and is also suggestive of the general synthesis of peptides containing diverse unnatural substituted phenylalanines via diazonium coupling reactions.
Scheme 3.
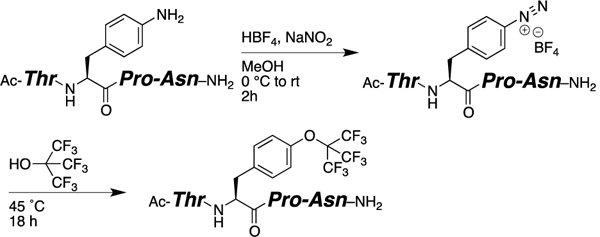
Solution-phase diazotization and synthesis of Tyr(C4F9) within peptides.
In order to examine potential magnetic resonance applications of perfluoro-tert-butyl tyrosine, Ac-TTyr(C4F9)PN-NH2, was examined by 19F NMR spectroscopy. Ac-TTyr(C4F9)PN-NH2 exhibited a sharp singlet peak (δ = –69.1 ppm) in water at 298 K (Figure 3a). Interestingly, the 19F δ of the cis and trans rotamers were identical, consistent with the isolation of the fluorines from the backbone conformation of the peptide. To determine the sensitivity of perfluoro-tert-butyl tyrosine, the 19F NMR spectrum of this peptide was examined at 500 nM and 200 nM peptide concentrations. Perfluoro-tert-butyl tyrosine was detected in 30 seconds (8 scans) at 500 nM [Ac-TTyr(C4F9)PN-NH2], and in 5 minutes (128 scans) at 200 nM [Ac-TTyr(C4F9)PN-NH2] (Figure 3b, Figures S10-S12), suggesting broad potential applications of this amino acid to sensitively probe peptide and protein structure and function.
Figure 3.
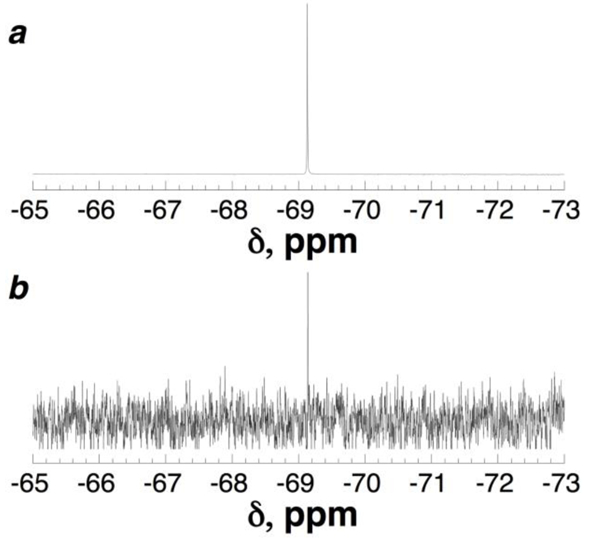
19F NMR spectra of the peptide Ac-TTyr(C4F9)PN-NH2 in 90% H2O/10% D2O, 5 mM phosphate pH 4, 25 mM NaCl. (a) As in Figure 2b. (b) Peptide concentration = 500 nM, signal/noise = 10.1. The experiment was conducted with 8 scans, 2 dummy scans, acquisition time = 0.8 s, relaxation delay = 2.0 s (28 seconds total experiment time).
We have described the highly practical synthesis of Fmoc-perfluoro-tert-butyl tyrosine in two steps from the commercially available amino acid Fmoc-4-NH2-phenylalanine. The synthesis proceeds via a diazonium coupling reaction. Ready access to this Fmoc-protected phenylalanine diazonium intermediate could provide highly practical access to a wide range of unnatural phenylalanine derivatives for applications in peptide synthesis. Perfluoro-tert-butyl tyrosine was detected at 500 nanomolar peptide concentration in 30 seconds by 19F NMR, promoting applications of this amino acid in the detection of protein function and protein interactions at physiologically relevant concentrations. Combined with the previously demonstrated ability of tert-butyl tyrosine to be encoded within proteins and to be a useful NMR probe even in extremely large proteins beyond the typical size limit of NMR spectroscopy, these results suggest broad potential future applications of perfluoro-tert-butyl tyrosine in NMR spectroscopy and MRI imaging.
Supplementary Material
ACKNOWLEDGMENT
We thank NSF (CHE-1412978) for funding this work. Instrumentation support was provided by NIH (GM110758) and NSF (CHE-1229234).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Synthetic procedures and 1H, 13C, and 19F NMR spectra for small molecules, synthesis and characterization data for peptides, and additional NMR data for peptides. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1) (a).Bodart JF; Wieruszeski JM.; Amniai L; Leroy A; Landrieu I; Rousseau-Lescuyer A; Vilain JP; Lippens G J. Magn. Reson. 2008, 192, 252–257; [DOI] [PubMed] [Google Scholar]; (b) Lippens G; Landrieu I; Hanoulle X Chem. Biol. 2008, 15, 311–312; [DOI] [PubMed] [Google Scholar]; (c) Inomata K; Ohno A; Tochio H; Isogai S; Tenno T; Nakase I; Takeuchi T; Futaki S; Ito Y; Hiroaki H; Shirakawa M Nature 2009, 458, 106–U111; [DOI] [PubMed] [Google Scholar]; (d) Maldonado AY; Burz DS; Shekhtman A Prog. Nucl. Magn. Reson. Spectrosc. 2011, 59, 197–212; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Theillet FX; Binolfi A; Frembgen-Kesner T; Hingorani K; Sarkar M; Kyne C; Li CG; Crowley PB; Gierasch L; Pielak GJ; Elcock AH; Gershenson A; Selenko P Chem. Rev. 2014, 114, 6661–6714; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Luchinat E; Banci LJ Biol. Chem. 2016, 291, 3776–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2) (a).Cobb SL; Murphy CD J. Fluorine Chem. 2009, 130, 132–143; [Google Scholar]; (b) Ruiz-Cabello J; Barnett BP; Bottomley PA; Bulte JWM. NMR Biomed. 2011, 24, 114–129; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen H; Viel S; Ziarelli F; Peng L Chem. Soc. Rev. 2013, 42, 7971–7982; [DOI] [PubMed] [Google Scholar]; (d) Marsh ENG; Suzuki Y ACS Chem. Biol. 2014, 9, 1242–1250; [DOI] [PubMed] [Google Scholar]; (e) Arntson KE; Pomerantz WC K. J. Med. Chem. 2016, 59, 5158–5171. [DOI] [PubMed] [Google Scholar]
- (3) (a).Higuchi M; Iwata N; Matsuba Y.; Sato K.; Sasamoto K.; Saido TC Nat. Neurosci. 2005, 8, 527–533; [DOI] [PubMed] [Google Scholar]; (b) Janjic JM; Srinivas M; Kadayakkara DKK; Ahrens ET J. Am. Chem. Soc. 2008, 130, 2832–2841; [DOI] [PubMed] [Google Scholar]; (c) Jiang ZX; Liu X; Jeong EK; Yu YB Angew. Chem., Int. Ed. 2009, 48, 4755–4758; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li C; Wang G-F; Wang Y; Creager-Allen R; Lutz EA; Scronce H; Slade KM; Ruf RAS; Mehl RA; Pielak GJ J. Am. Chem. Soc. 2010, 132, 321–327; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kitevski-LeBlanc JL; Prosser RS Prog. Nucl. Magn. Reson. Spectrosc. 2012, 62, 1–33; [DOI] [PubMed] [Google Scholar]; (f) Ye YS; Liu XL; Zhang ZT; Wu Q; Jiang B; Jiang L; Zhang X; Liu ML; Pielak GJ; Li CG Chem. Eur. J. 2013, 19, 12705–12710; [DOI] [PubMed] [Google Scholar]; (g) Tirotta I; Dichiarante V; Pigliacelli C; Cavallo G; Terraneo G; Bombelli FB; Metrangolo P; Resnati G Chem. Rev. 2015, 115, 1106–1129. [DOI] [PubMed] [Google Scholar]
- (4) (a).Dalvit C; Ardini E; Flocco M; Fogliatto GP; Mongelli N; Veronesi MJ Am. Chem. Soc. 2003, 125, 14620–14625; [DOI] [PubMed] [Google Scholar]; (b) Dalvit C; Fagerness PE; Hadden DTA; Sarver RW; Stockman BJ J. Am. Chem. Soc. 2003, 125, 7696–7703; [DOI] [PubMed] [Google Scholar]; (c) Dalvit C; Mongelli N; Papeo G; Giordano P; Veronesi M; Moskau D; Kummerle RJ Am. Chem. Soc. 2005, 127, 13380–13385; [DOI] [PubMed] [Google Scholar]; (d) Jäckel C; Koksch B Eur. J. Org. Chem 2005, 4483–4503; [Google Scholar]; (e) Papeo G; Giordano P; Brasca MG; Buzzo F; Caronni D; Ciprandi F; Mongelli N; Veronesi M; Vulpetti A; Dalvit CJ Am. Chem. Soc. 2007, 129, 5665–5672; [DOI] [PubMed] [Google Scholar]; (f) Jackson JC; Hammill JT; Mehl RA J. Am. Chem. Soc. 2007, 129, 1160–1166; [DOI] [PubMed] [Google Scholar]; (g) Cellitti SE; Jones DH; Lagpacan L; Hao XS; Zhang Q; Hu HY; Brittain SM; Brinker A; Caldwell J; Bursulaya B; Spraggon G; Brock A; Ryu Y; Uno T; Schultz PG; Geierstanger BH J. Am. Chem. Soc. 2008, 130, 9268–9281; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Durr UHN; Grage SL; Witter R; Ulrich AS J. Magn. Reson. 2008, 191, 7–15; [DOI] [PubMed] [Google Scholar]; (i) Grage SL; Durr UHN; Afonin S; Mikhailiuk PK; Komarov IV; Ulrich AS J. Magn. Reson. 2008, 191, 16–23; [DOI] [PubMed] [Google Scholar]; (j) Takaoka Y; Kiminami K; Mizusawa K; Matsuo K; Narazaki M; Matsuda T; Hamachi IJ Am. Chem. Soc. 2011, 133, 11725–11731; [DOI] [PubMed] [Google Scholar]; (k) Tkachenko AN; Mykhailiuk PK; Afonin S; Radchenko DS; Kubyshkin VS; Ulrich AS; Komarov IV Angew. Chem., Int. Ed. 2013, 52, 1486–1489. [DOI] [PubMed] [Google Scholar]
- (5) (a).Pandey AK; Naduthambi D; Thomas KM; Zondlo NJ J. Am. Chem. Soc. 2013, 135, 4333–4363; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tressler CM; Zondlo NJ J. Org. Chem. 2014, 79, 5880–5886; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jiang ZX; Yu YB J. Org. Chem. 2007, 72, 1464–1467; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yue X; Taraban MB; Hyland LL; Yu YB J. Org. Chem. 2012, 77, 8879–8887; perfluoro-tert-butyl homoserine: [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Buer BC; Levin BJ; Marsh EN G. J. Pept. Sci. 2013, 19, 308–314. [DOI] [PubMed] [Google Scholar]
- (6) (a).Young DD; Young TS; Jahnz M; Ahmad I; Spraggon G; Schultz PG Biochemistry 2011, 50, 1894–1900; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang YS; Fang XQ; Wallace AL; Wu B; Liu WS R. J. Am. Chem. Soc. 2012, 134, 2950–2953; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dumas A; Lercher L; Spicer CD; Davis BG Chem. Sci. 2015, 6, 50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chen W-N; Kuppan KV; Lee MD; Jaudzems K; Huber T; Otting GJ Am. Chem. Soc. 2015, 137, 4581–4586. [DOI] [PubMed] [Google Scholar]
- (8).Nabuurs RJA; Kapoerchan VV; Metaxas A; de Jongh S; de Backer M; Welling MM; Jiskoot W; Windhorst AD; Overkleeft HS; van Buchem MA; Overhand M; van der Weerd L Bioorg. Med. Chem. 2014, 22, 2469–2481. [DOI] [PubMed] [Google Scholar]
- (9).Roglans A; Pla-Quintana A; Moreno-Manas M Chem. Rev. 2006, 106, 4622–4643. [DOI] [PubMed] [Google Scholar]
- (10) (a).Colescott RL; Herr; Dailey JP J. Am. Chem. Soc. 1957, 79, 4232–4235; [Google Scholar]; (b) Bergel F; Stock JA J. Am. Chem. Soc. 1959, 90–97; [Google Scholar]; (c) Gram HF; Mosher CW; Baker BR J. Am. Chem. Soc. 1959, 81, 3103–3108; [Google Scholar]; (d) Schwyzer R; Caviezel M Helv. Chim. Acta 1971, 54, 1395-&; [DOI] [PubMed] [Google Scholar]; (e) Houghten RA; Rapoport HJ Med. Chem. 1974, 17, 556–558; [DOI] [PubMed] [Google Scholar]; (f) Hobbs DW; Still WC Tetrahedron Lett. 1987, 28, 2805–2808; [Google Scholar]; (g) Hobbs DW; Still WC Tetrahedron Lett. 1989, 30, 5405–5408; [Google Scholar]; (h) Sengupta S; Bhattacharyya S Tetrahedron Lett. 1995, 36, 4475–4478; [Google Scholar]; (i) Ganther HE Bioorg. Med. Chem. 2001, 9, 1459–1466; [DOI] [PubMed] [Google Scholar]; (j) Wang YZ; Lin Q Org. Lett. 2009, 11, 3570–3573; [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Zhu BW; Ge JY; Yao SQ Bioorg. Med. Chem. 2015, 23, 2917–2927. [DOI] [PubMed] [Google Scholar]
- (11) (a).Thomas KM; Naduthambi D; Tririya G; Zondlo N J. Org. Lett. 2005, 7, 2397–2400; [DOI] [PubMed] [Google Scholar]; (b) Meng HY; Thomas KM; Lee AE; Zondlo NJ Biopolymers 2006, 84, 192–204; [DOI] [PubMed] [Google Scholar]; (c) Thomas KM; Naduthambi D; Zondlo NJ J. Am. Chem. Soc. 2006, 128, 2216–2217; [DOI] [PubMed] [Google Scholar]; (d) Forbes CR; Zondlo NJ Org. Lett. 2012, 14, 464–467; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zondlo NJ Acc. Chem. Res. 2013, 46, 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).See the Supporting Information for details.
- (13).Hansch C; Leo A; Taft RW Chem. Rev. 1991, 91, 165–195. [Google Scholar]
- (14).Hooker JM; Kovacs EW; Francis MB J. Am. Chem. Soc. 2004, 126, 3718–3719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


