Abstract
The developing pituitary is a rapidly changing environment that is constantly meeting the physiological demands of the growing organism. During early postnatal development, the anterior pituitary is refining patterns of anterior hormone secretion in response to numerous genetic factors. Our lab previously developed a somatotrope leptin receptor (LEPR) deletion mouse model that had decreased lean body mass, disrupted metabolism, decreased GH stores and was GH deficient as an adult. To understand how deletion of LEPR in somatotropes altered GH, we turned our attention to postnatal development. The current study examines GH, PRL, TSH, ACTH, LH, and FSH secretion during postnatal days 4,5,8,10, and 15 and compares age and sex differences. The LEPR mutants have dysregulation of GH (p<0.03) and a reduced developmental prolactin peak in males (p<0.04) and females (p<0.002). There were no differences in weight between groups, and the postnatal leptin surge appeared to be normal. Percentages of immunolabeled GH cells were reduced in mutants compared with controls in all age groups by 35–61% in males and 41–44% in females. In addition, we measured pituitary expression of pituitary transcription factors, Pou1f1 and PROP1. Pou1f1 was reduced in mutant females at PND 10 (p<0.009) and PND 15 (p<0.02) but increased in males at PND 10 (p<0.01). PROP1 was unchanged in female mutants but showed developmental increases at PND 5 (p<0.02) and PND 15 (p<0.01). These studies show that the dysfunction caused by LEPR deletion in somatotropes begins as early as neonatal development and involves developing GH and prolactin cells (somatolactotropes).
Keywords: Pou1f1, GH, PRL, Neonatal/Postnatal Development
Introduction
The morphogenesis of the murine pituitary is initiated by neurulation and continues throughout embryonic development where it is under the influence of numerous critically timed signaling pathways that lead to the differentiation and proliferation of the individual endocrine cell populations (Rizzoti 2015). The earliest exclusive pituitary marker is PROP1, a paired homeodomain transcription factor, which is expressed at embryonic day 10 (E10) in the mouse. SOX2 expressing stem cells maintain PROP1 expression through development, but PROP1 is down regulated postnatally (Chen, Gremeaux et al. 2009, Davis, Keisler et al. 2016). On E13.5, PROP1 interacts with B-catenin to form a complex that activates the transcription factor Pou1f1 (PIT1), which is responsible for the differentiation of somatotropes, lactotropes, and thyrotropes (Olson, Tollkuhn et al. 2006, Rizzoti 2015). Postnatal expression of Pou1f1 is maintained for the expansion and survival of these cell types and to regulate the expression of hormones and hypothalamic peptide receptors (Olson, Tollkuhn et al. 2006, Ward, Stone et al. 2006). Mutations in PROP1 and/or Pou1f1 regulation lead to deficiencies in the proliferation and maintenance of somatotropes, lactotropes, and thyrotropes (Bottner, Keller et al. 2004, Ward, Stone et al. 2006, Davis, Keisler et al. 2016).
Of these Pou1f1 lineage cell types, somatotropes are the most abundant and are vital for optimized growth and body composition throughout life (Childs 2000, Akhter, Odle et al. 2012, Allensworth-James, Odle et al. 2015). Most somatotropes express leptin receptors (LEPR), and the importance of leptin regulation in these cells was emphasized recently when the signaling component of LEPR (Childs, Akhter et al. 2011) or the entire LEPR (Allensworth-James, Odle et al. 2015) was selectively deleted using Cre-Lox technology.
In the somatotrope LEPR-null mouse model, deletion of all isoforms of LEPR in somatotropes resulted in severe reductions in serum GH, especially in males. It also resulted in decreased GH stores (detected by EIA) in purified somatotropes (Odle, Allensworth-James et al. 2016), a decrease in percentages of GH-bearing cells and expression of growth hormone releasing hormone receptors (GHRHR), loss of lean body mass, and significant increases in abdominal adiposity in 2.5–3 month old mice (Childs, Akhter et al. 2011, Akhter, Odle et al. 2012, Allensworth-James, Odle et al. 2015). Indirect calorimetry showed that these mice predominantly oxidized carbohydrates and had disrupted sleep and decreased activity (Akhter, Odle et al. 2012, Allensworth-James, Odle et al. 2015). In spite of the reduced percentages of GH-protein bearing cells, the percentages of cells with Gh mRNA were normal in adult males (Syed, Cozart et al. 2013), which suggest that somatotropes may have proliferated normally during early development. This also suggests that at least some of leptin actions on somatotropes may be post-transcriptional. This opens the question: at what point during the development of the pituitary in the mouse does leptin’s signal to somatotropes become important?
The neonatal developmental phenotype of the somatotrope LEPR-null mouse needs to be explored to determine the time of onset of growth hormone deficiency caused by the deletion of somatotrope LEPR and also to determine the effect of somatotrope LEPR ablation on the development of other anterior pituitary hormones.
The maximal response of somatotropes to GHRH is seen on postnatal day 1. During this time, high amplitude GH pulses can be seen largely because the somatostatin inhibitory influences on somatotropes are diminished due to limited somatostatin receptor (SSTR) expression. This phenomenon can also be explained by the higher concentrations of pituitary GHRH receptors at birth and increased numbers of somatotropes just before birth. In addition, thyrotropin-releasing hormone (TRH) also stimulates GH in neonates (Fink, Pfaff et al. 2012). Thus, as inhibitors of GH and their receptors are expressed differentially throughout development, GH is gradually regulated to reach levels and secretory patterns seen in mature mice. The GH secretory pattern is not sexually dimorphic in neonates, and sex differences in GH secretory patterns are not seen until just before puberty (Giustina and Veldhuis 1998, Veldhuis, Roemmich et al. 2005, Veldhuis, Roemmich et al. 2006, Farhy, Bowers et al. 2007).
Lactotropes appear in the pituitary around the same time as somatotropes. However, lactotropes are scarce in the fetal pituitary and begin to expand around postnatal day 3. These early lactotropes also express GH in rats and cows (Hoeffler, Boockfor et al. 1985). They are believed to come from a common progenitor, somatolactotropes. This is further deduced by studies that depleted lactotropes in transgenic mice by using the rat GH promoter to target the destruction of GH expressing cells (Behringer, Mathews et al. 1988, Borrelli, Heyman et al. 1989). More recently it was found that somatolactotropes undergo robust proliferation from PND 7-PND14, which accounts for much of the lactotrope expansion (Cao, Ma et al. 2016).
Leptin plays an important role in brain development and is detectable as early as E12.5 despite low levels of white adipose tissue (Bouret 2010). Leptin receptors are detected in the developing brain as early as embryonic day 10 (Cottrell, Cripps et al. 2009) (Bouret 2010). Morash et al. reported that Leprb mRNA is expressed in the rat pituitary as early as PND 4 but did not test younger animals (Morash, Wilkinson et al. 2001, Morash, Imran et al. 2003). The rodent leptin surge occurs between PND7–10 and plays a role in organogenesis as well as the development and maturation of neurons and glial cells in the fetus and neonate (L, Solomon et al., Ahima, Prabakaran et al. 1998, Attig, Solomon et al. 2008, Attig, Larcher et al. 2011).
Because the somatotrope population expands during postnatal development, we hypothesized that leptin optimization of the somatotrope population might occur during the neonatal period coinciding with the leptin surge. This study aimed to look at all anterior pituitary hormone storage and secretion during the developmental period in the somatotrope Lepr exon 1-null mouse and evaluate possible paracrine effects of the deletion. This report also details normal anterior pituitary hormone levels throughout development in normal mice, providing a valuable roadmap for future studies. The rationale for studying all anterior pituitary hormones came from our recent studies, in which we reported significant expression of prolactin and some expression of TSH by populations of purified somatotropes along with a reduction in expression of these hormones in somatotrope LEPR-null mice (Odle, Allensworth-James et al. 2016). These data pointed to the possibility that leptin may also regulate multi-hormonal expression by a subset of somatotropes. We also reported that Pou1f1 was reduced in the purified mutant population lacking LEPR in somatotropes, which suggests that leptin may be needed for the expression of TSH and prolactin in this subpopulation. These findings thus led us to study the potential impact of loss of neonatal leptin signals on the expression of Pou1f1-dependent hormones and Pou1f1 content. This report will show evidence that leptin receptors in somatotropes are important in the neonatal optimization of Pou1f1 and prolactin in the female mouse as well as full expression of stores of GH.
Methods
Animals
Development of our somatotrope (CreGH) Lepr Exon 1-null line has been described previously (Allensworth-James, Odle et al. 2015). For the studies described in this report, deletion mutant males bearing CreGH and Leprexon 1 fl/fl were bred to littermate control female bearing Leprexon 1 fl/fl only. Breeding cages were setup with one sire and one dam to control for timing of birth and collection. Experimental animals carried either one allele of CreGH and two floxed alleles of floxed Lepr exon 1 (deletion mutant) or no Cre alleles and two alleles of floxed Lepr exon 1 (controls). Pups were collected at PND 1, 4, 5, 8, 10, 15, and 21 around 09:00. Just before collection, approximately 50% to 75% of pups from a given litter were gently removed from their mother and placed in a small container with other littermates and bedding to reduce stress. Leaving some pups in the breeding cage not only decreased the stress to the mother but also allowed us to sample multiple ages from the same litter (and multiple litters for each age group). No stress was evident in mother’s care for surviving pups. On average, litter sizes were lower than seen in this strain (5–6 pups), and the numbers of pups/litter remained consistent.
To verify that excision of Lepr in somatotropes occurs early in development, we collected and genotyped PND 1 pituitaries and tail snips (n=3). We confirmed Cre recombinase activity was present at PND 1 since the pituitaries of animals expressing Cre had excision of Lepr exon 1 (data not shown).
Pups were weighed, anesthetized with isoflurane, and decapitated using sharp surgical scissors (PND 1 to PND 10) or a guillotine (PND 15 pups). Trunk blood was collected for assays, and a tail snip was collected for genotyping (as described previously) and sex determination (SRY: Reverse: 5’ CCA CTC CTC TGT GAC ACT TTA GCC CTC CGA, Forward: 5’ TTG TCT AGA GAG CAT GGA GGG CCA TGT CAA) (Allensworth-James, Odle et al. 2015). The whole pituitary was removed and placed in PBS on ice. The pituitaries were frozen at −20°C until they were thawed, homogenized in 100uL RIPA buffer with RNase inhibitor. Additional pituitaries from PND1 pups were genotyped to verify the excision of Lepr exon 1 and presence of Cre recombinase. The protocol was approved annually by the UAMS Animal Use and Care Committee.
Serum and Pituitary Hormone Measurements
Serum anterior pituitary hormone levels and pituitary hormone protein content were detected using Millipore Milliplex Magnetic Bead Assays (St. Louis, MO) with a dilution of 1:2.5, (Allensworth-James, Odle et al. 2015). Pou1f1 and PROP1 assays were obtained from MyBioSource (San Diego, CA) and were setup as directed by the protocol with 1:4 dilution of protein. Values were normalized to total pituitary BCA protein content. Serum Leptin Assays (R&D Systems, Minneapolis, MN) followed the included protocol and serum was diluted 1:10.
Cell Culture for GH Immunocytochemistry
Pituitaries from PND 5–21 animals were dispersed and cultured for a longitudinal study of GH. Our pituitary dispersion protocol was used as previously described with this exception: the amount of DME+ ITS added before counting and plating was modified to 125µL due to the smaller number of cells (Childs, Akhter et al. 2011). Fixed cells were immunolabeled with anti-GH (1:80,000 dilution, rabbit) as described previously (Childs, Unabia et al. 1994).
Statistical Analysis
Though Mendelian genetics were expected based on the breeding setup, there was a non-proportional reduction in Cre+ male mice animals. Because the sex and genotype of the pups was not determined before sample collection, and due to the small quantities of serum and protein products available from each pup, we needed a greater number mice/age group than our original power analysis predicted. For example, a post-hoc power analysis applied to the assays of content of Pou1f1 in 8 day female pituitaries allowed us to predict that a 38% reduction in POU1F1 (from 70 pg/ug protein to 44 pg/ug protein) with an SD of 16 and an n of 6 will achieve a 0.8 statistical power in a two-tailed, 0.05-level Student’s t test. (https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html). Most of our groups required a higher number of mice, however. Therefore, approximately 792 animals were collected for all components of this study. Mann-Whitney test was used to compare mutant and control differences and one-way ANOVA to compare differences in age groups. ANOVA was followed by Tukey’s or Fisher’s LSD post-hoc test to identify the groups that were different.
Results
Somatotrope Lepr Deletion Does Not Affect Normal Growth
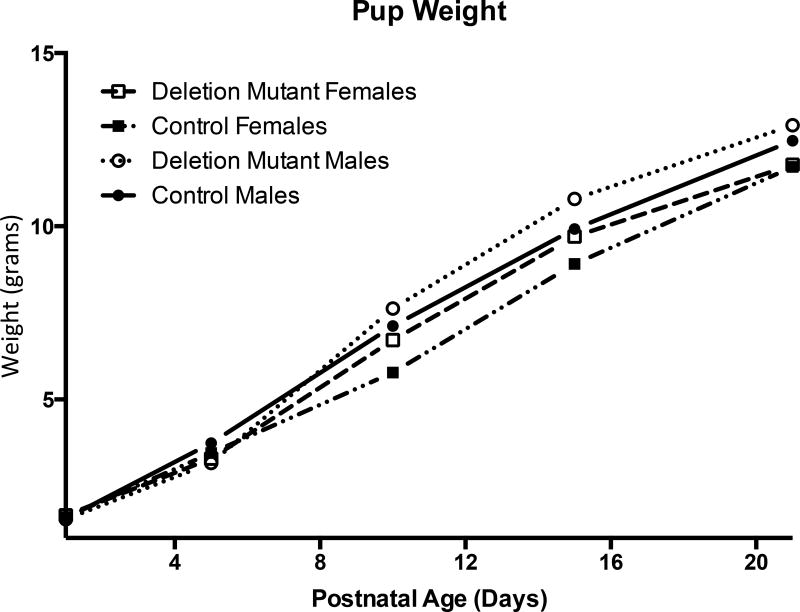
Pups weights were recorded before sacrifice. Weights from each experimental group were averaged and plotted based on age and sex (Figure 1). There were no significant differences between males and females or controls and deletion mutants. Early experiments on the F2 generation show growth in length rates were normal among somatotrope LEPR-null mice as indicated by tail-nose measurements with the use of calipers (data not shown). In addition, F2 generation mutants displayed a normal onset of puberty, as indicated by vaginal opening in females and testicular descent and preputial separation in males.
Figure 1.
Averaged weights from each experimental group showed no significant difference in weight between deletion mutants (DM) and controls. (PND1: n=4; PND5–21: n=10)
Deletion Mutant Somatotropes Store Less GH
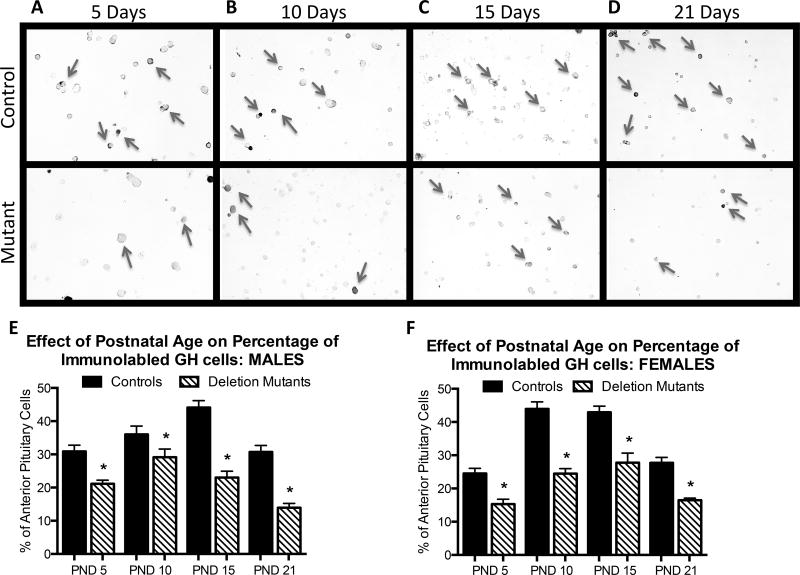
Immunolabeling of somatotropes with an anti-GH antibody revealed significant decreases overall in deletion mutant GH stores, which resulted in reduced numbers of immunolabeled somatotropes (photographs of labeling can be found in Figure 2A–D). Deletion mutants showed decreased percentages of immunolabeled somatotropes beginning at PND 5 and throughout development (p<0.001) (Figure 2E,F). In control males, the percentages of GH cells rose from 31± 2% at 5 days to a peak of 44± 2% by 15 days, followed by a decline to 31 ± 2% at 21 days, which was similar to percentages in the adult (Allensworth-James, Odle et al. 2015). In contrast, mutant males show significantly lower numbers of immunolabeled GH cells at each age point and no significant rise, although there is a significant drop from 23 ± 2% to 14 ± 2% by day 21 (p<0.001). This low level was similar to that seen in the mutant adult males (Allensworth-James, Odle et al. 2015). Control females show an earlier rise in percentages of GH cells from 24± 1% at 5 days to 43± 2%, or 44 ± 2.2% at 10 days and 15 days followed by a decline to adult levels (27± 1.6%) by 21 days. In contrast, mutant females show a blunted rise from 14± 1% at 5 days to 24 ± 2% and 26± 2% at 10–15 days of age followed by a significant decline to 16± 1% by 21 days, which is also similar to that seen in the mutant adult (Allensworth-James, Odle et al. 2015).
Figure 2.
These photographs of GH immunolabeling show obvious differences in number of GH-bearing cells between male littermate controls (top row) and deletion mutants (bottom row) at 5 days (A), 10 days (B), 15 days (C) and 21 days (D) of age. E Counts of GH-positive cells revealed a significant decrease in the percentage of overall anterior pituitary cells labeled for GH as early as 5 days (p<0.001) in both males and females. Deletion mutant males were reduced as follows (5d 20% ± 1%; 10d 23% ± 2%; 15d 23% ± 2%; 21d 14% ± 2%) in comparison to controls (5d 31% ± 2%; 10d 36% ± 2.5%; 15d 44% ± 2%; 21d 31% ± 2%). Deletion mutant females were reduced as follows (5d 14% ± 1%; 10d 24% ± 2%; 15d 26% ± 2%; 21d 16% ± 1% p<0.001) in comparison to control females (5d 24% ± 1.4%; 10d 43% ± 2%; 15d 44% ± 2.2%; 21d 27% ± 1.6% p<0.001). Values shown are mean ± SEM, Student’s t-test. At least two coverslips were analyzed per animal, and 3–8 animals were analyzed per experimental group. PND1: n=4, PND5: n=4–5, PND10: n=4–5, PND15: n=4–8, PND21: n=4.
Somatotrope LEPR deletion does not alter serum leptin surge
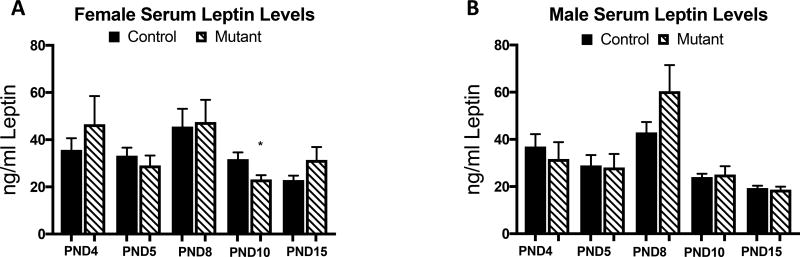
By PND 4, serum leptin has already began to surge and reaches its peak between PND 8 and PND 10 before decreasing again. Thus we bracketed this surge by looking at leptin levels in PND 4 to PND 15 animals. There was no blunting of the leptin surge in somatotrope LEPR-null mutants, though PND10 female mutants did have significantly lower leptin levels than those of littermate controls (p<0.01). The overall leptin surge in these animals is similar to previously published leptin surges (Ahima, Prabakaran et al. 1998). Leptin is surging between PND5 and PND 10 for both groups of males and females (Figure 3).
Figure 3.
Serum leptin levels were measured in female (A) and male (B) controls and somatotrope LEPR null mutants to identify potential changes in the leptin surge. There are no significant differences in the leptin peak at PND 8, but we do see a modest but significant decrease mutant serum leptin at PND 10. Values shown are mean ± SEM, Student t-test, PND 4: n=4–7, PND 5: n=12–18, PND 8: 4–7, PND 10: n=13–18, PND 15: 13–21.
Developmental Age-Related Changes in Anterior Pituitary Hormones in Control mice
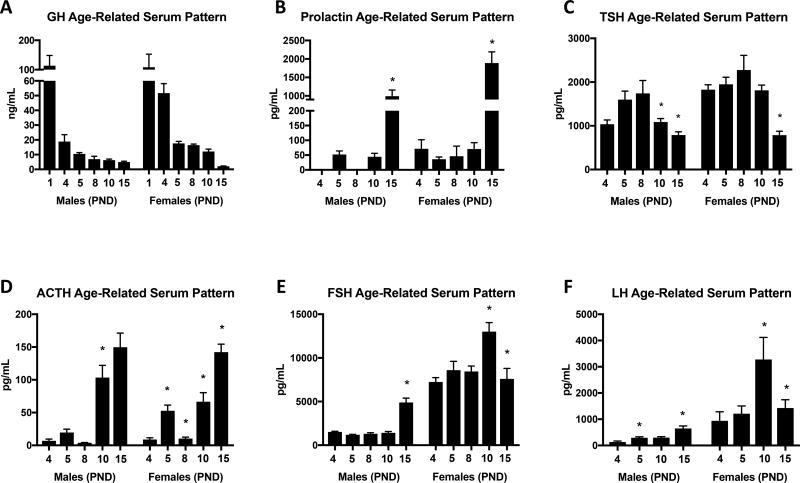
Growth Hormone (GH) serum levels peak at PND 1 in control mice and show a similar pattern of reduction as shown by previous studies (Veldhuis, Roemmich et al. 2006, Fink, Pfaff et al. 2012) (Figure 4A). Instead of significant pattern changes between time points, GH is reduced to adult levels gradually through development in both males and females.
Figure 4.
Anterior pituitary EIAs were used to analyze serum samples to compare hormone patterns in littermate controls during postnatal development starting at postnatal day 4 and ending on PND 15. A There is a gradual decline in GH from PND1 through PND 15, at which point GH levels are comparable to those in the adult animal. B Prolactin levels are undetectable or very low until PND 15. C TSH peaks at PND 8. D ACTH shows a similar pattern of ACTH secretion during the stress non-response period. FSH (E) and LH (F) show sexually dimorphic patterns of secretion with both being higher in females that males. Values shown are mean ± SEM, ANOVA, A: PND 1: n=4, PND 4: n=5–9, PND 5: n=14–22, PND 8: n=6–8, PND 10: n=16–23, PND 15: n=15–25. B PND 4: n=4–6, PND 5: n=4–6, PND 8: n=4, PND 10: n=6–7, PND 15: n=10–15. C PND 4: n=10, PND 5: n=16–18, PND 8: n=8, PND 10: n=12–16, PND 15: n=13–17. D PND 4: n=4, PND 5: n=8, PND 8: n=4–6, PND 10: n=9–12, PND 15: n=10–14. E PND 4: n=8–11, PND 5: n=11–12, PND 8: n=7–8, PND 10: n=10–11, PND 15: n=9–14. F PND 4: n=4, PND 5: n=4–9, PND 10: n=6, PND 15: n=5–6.
Prolactin (PRL) is undetectable (PND 4 and PND 8 males) or nearly undetectable before PND 15 (Figure 4B). Prolactin surges at PND15 in both males and females (p< 0.027 and p<0.0021 respectively).
Thyroid Stimulating Hormone (TSH) peaks around PND8 in both males and females before gradually dropping off (Figure 4C). PND10 and PND15 males exhibit significant TSH reductions (p<0.011). Females show a significant reduction at PND 15 (p<0.0001).
Adrenocorticotropin hormone (ACTH) serum levels increase significantly from PND 4 to PND 5 (p<0.006) followed by a reduction at PND 8 (p<0.001) in females (Figure 4D). By PND15, ACTH has risen again and is significantly higher than PND10 (p<0.001) and PND 8 (p<0.003). Males show a very similar pattern, but the only significant change was an increase from PND 8 to PND 10 (p<0.02).
Follicle Stimulating Hormone (FSH) is secreted in a sexually dimorphic pattern, with female FSH serum levels being 4 times higher than males until PND 15 (Figure 4E). Males appear to have a significant FSH surge at PND15 (p<0.0001) while females appear to have their peak at PND10 (p<0.0032) followed by a decrease at PND 15 (p<0.0029).
Luteinizing Hormone (LH) is also secreted in sexually dimorphic manner (Figure 4F). As with FSH, males have a significant surge at PND 15 (p<0.007), and females peak at PND10 (p<0.018) followed by a steep 56% decrease at PND 15 (p<0.089).
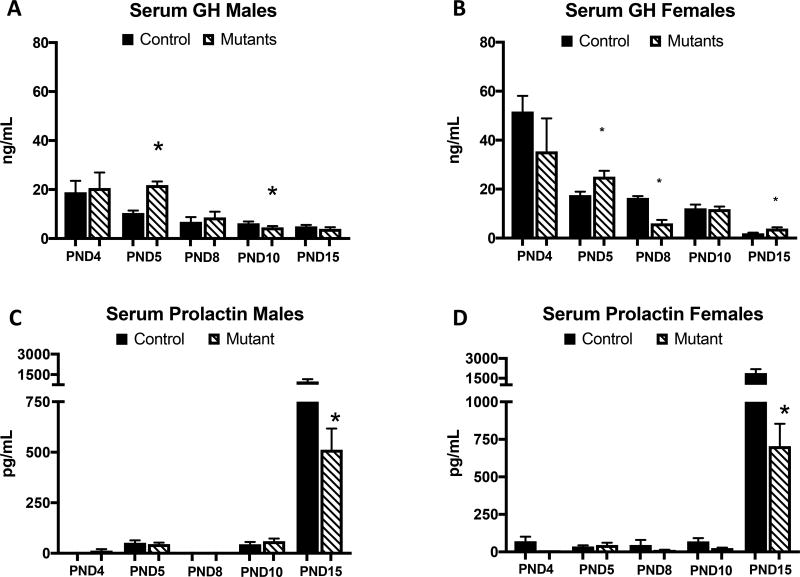
Somatotrope Deletion of LEPR alters GH and PRL Serum levels
Deletion of somatotrope LEPRs resulted in significant reduction in the expected prolactin peak (Figure 5 A,B) in both male (p<0.043) and female (p<0.002) mutants at PND15. At PND 5, male (p<0.0001) and female (p<0.015) mutants show increased GH (Figure 5 C,D). However, GH is significantly lower than controls at PND 8 in female mutants (p<0.0003) and PND 10 in males (p<0.051). Female mutant GH serum levels appear to rebound and are higher than controls at PND 15 (p<0.016).
Figure 5.
Somatotrope LEPR null males (A) and females (B) appear to have some dysregulation of the GH secretion pattern across ages. Instead of having a continuous pattern of GH decrease, mutants actually have some increases such as at PND 5 in males (p<0.0001) and females (p<0.05) and PND 15 in females (p<0.016). The prolactin peak is significantly reduced at PND 15 in males (C) (p<0.043) and females (p<0.001) (D). Values shown are mean ± SEM, ANOVA. A,B PND 4: n=5–9, PND 5: n=14–22, PND 8: n=6–8, PND 10: n=16–23, PND 15: n=15–25. C,D PND 4: n=4–6, PND 5: n=4–6, PND 8: n=4, PND 10: n=6–8, PND 15: n=7–15.
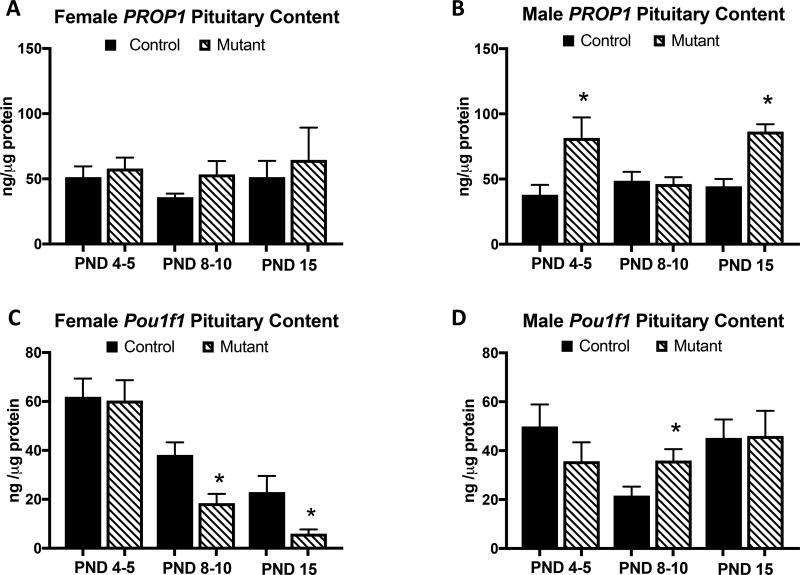
Female mutants have reduced Pou1f1
Whole pituitaries were collected to determine if the changes in GH and PRL in somatotrope LEPR-null animals were coming from changes in an upstream regulator. Since PRL and GH share a common lineage, we looked at Pou1f1 and PROP1. There were no differences between controls and mutants in female PROP1 (Figure 6A) protein levels. However, PROP1 was elevated in male mutants (Figure 6B) at PND 5 (p<0.024) and PND 15 (p<0.001). Pou1f1 (Figure 6 C,D) is reduced in female mutants on PND 8–10 (p<0.009) and PND 15 (p<0.024) but elevated in males at PND 8–10 (p<0.013).
Figure 6.
Protein EIAs were used to determine whole-pituitary levels of the transcriptional factors PROP1 and Pou1f1 in control and Somatotrope LEPR null mice from 3 age groups. A Female mutants do not show any differences in PROP1 expression. B Male mutants show increased PROP1 expression at PND 4–5 (p<0.024) and PND 15 (p<0.001). C Females mutants have reduced expression of Pou1f1 at PND 8–10 (p<0.009) and PND 15 (p<0.024). D Pou1f1 is elevated in male mutants on PND8–10 (p<0.013). Values shown are mean ± SEM. Students t-test; PND 4–5: n=11–16, PND 8–10: n=10–18, PND 15: n= 6–11.
Discussion
Our studies of adult murine somatotropes in which the signaling component (Childs, Akhter et al. 2011, Akhter, Odle et al. 2012, Syed, Cozart et al. 2013) or the entire molecule (Allensworth-James, Odle et al. 2015) of LEPR was ablated showed that GH stores in somatotropes were reduced to the point that the immunolabeling could no longer detect a subset of the cells. We reasoned that this was not due to a loss in somatotropes, because we could detect normal percentages of GH cells by detection of Gh mRNA (Syed, Cozart et al. 2013). We next needed to determine the point in development when the somatotropes become dependent on leptin for the maintenance and secretion of GH stores. Our initial hypothesis was that this time point might fall during neonatal development at the time of the leptin surge (Ahima, Prabakaran et al. 1998). Knowing that leptin optimizes somatotropes in the adult, we reasoned that if neonatal somatotropes did not receive a leptin signal, the GH cells may show low stores of hormone, which could affect their secretion during development. This in turn could impair growth and the timing of puberty.
To address these questions, we used our somatotrope Lepr exon 1-null mouse model (Allensworth-James, Odle et al. 2015) to study GH storage and secretion during neonatal and prepubertal development (bracketing the time of the leptin surge). We looked for the excision of exon 1 and detected it as early as PND1, ensuring deletion had occurred prior to the timepoints used in this study. This agreed with the original characterization of the CreGH line by Luque et al. showing Cre activity driven by the GH promoter was present at embryonic day 17 (Luque, Amargo et al. 2007).
Our assays of serum GH levels in control animals show an expected decrease in GH serum levels from PND 1-PND 15 in males and females. Curiously, somatotrope LEPR null mutant males and females also showed a pattern of GH secretion that was similar to each other as well as to that of control littermates. This discovery explains why adult mutant animals in our previous studies showed evidence of normal growth and pubertal development. There was no sign of growth hormone deficiency in the developing pre-weanling mice on the basis of GH serum levels. Serum GH in male and female mutants remained normal at 15 days, in spite of the significant decline in immunolabeled GH cells.
In mutants, there were sex differences in serum GH. Surprisingly mutant neonatal females showed higher serum GH levels than males as if they were not responding to factors that normally regulate the postnatal reduction in serum GH levels. This sex difference is difficult to explain, but it could be due to a change in expression of receptors for other hormones that are known to regulate GH secretion during the postnatal period (TRH, somatostatin, ghrelin). Future studies are needed to identify candidate receptors that might show sex-specific sensitivity to leptin.
We also discovered that leptin is needed to optimize GH protein stores in postnatal somatotropes. We detected loss of immunolabeled somatotropes as early as 5 days of postnatal age in somatotrope LEPR-null male and female mutants, and the normal neonatal rise in immunolabeled GH cells was blunted in females. There was also a significant reduction in GH cells in both males and females by 21 days of age to levels that were similar to those observed in mutant adults (Allensworth-James, Odle et al. 2015). Clearly, leptin signaling is not needed to optimize GH secretion during neonatal or postnatal development, but it is needed to optimize GH stores, with the most severe impact seen at weaning. We show here that serum leptin levels are not affected by loss of somatotrope leptin receptors, and the leptin surge appears to be normal in both mutants and littermate controls. Our previous studies of LEPR-null somatotropes indicate that the somatotrope population itself was not reduced, because normal numbers of cells could be detected by Gh mRNA (Syed, Cozart et al. 2013). These studies showing normal Gh mRNA but low GH proteins suggested that leptin may act at post-transcriptional levels to regulate GH protein translation.
It is interesting that the mutant neonatal GH cells are able to secrete normally, in spite of their low stores of GH. This is not the case for the adults, which show clear GH deficiency due possibly to the reduction in GH releasing hormone receptors (Childs, Akhter et al. 2011, Akhter, Odle et al. 2012, Syed, Cozart et al. 2013, Allensworth-James, Odle et al. 2015, Odle, Allensworth-James et al. 2016). Our studies of mice in which Lepr exon 17 or Lepr exon 1 was deleted in somatotropes showed that although the cells were deficient in GHRH receptors, they do appear to have ghrelin receptors as they retained responses to ghrelin. Thus, it is possible that the neonatal mutant somatotropes lacking LEPR can respond to the combined effect of low somatostatin signals (lack of SSTR2) and high ghrelin, TRH and corticosteroid signals during postnatal development. They may be able to produce GH proteins rapidly enough to maintain normal serum GH levels during this period of time, or there may be reduced degradation. In essence, we know from studies of adults that male and female somatotrope Lepr null mice (exon 1 and exon 17) become growth hormone deficient as adults and suffer numerous metabolic effects including decreased activity and energy, increased respiratory quotient and obesity (Akhter, Odle et al. 2012, Allensworth-James, Odle et al. 2015). From the current study, we can now see that leptin may be needed early in postnatal development to increase the translation of GH proteins in preparation for the adult patterns of stimulation.
In the process of collecting data on somatotrope LEPR-null animals, we also amassed a significant amount of data on normal control animals. We show serum secretion patterns from control mice of all major anterior pituitary hormones at several days during postnatal development. This provides a comprehensive view of the age-related changes in patterns of the anterior pituitary hormones secreted during the first 15 days of normal postnatal life. The brain becomes sexually dimorphic during the critical first few days of neonatal life as a result of steroid priming and may be partially responsible for the varying patterns of secretion seen in these anterior pituitary hormones (Cooke, Tabibnia et al. 1999) (Harris 1964). These data suggest that these steroids and/or brain may also stimulate different developmental patterns in neonatal pituitary cells to support the sex-specific changes in their function with postnatal age (i.e. gonadotropins). It is not within the scope of this paper to explain peak expressions and changes in each hormone, but we hope our data will serve as a reference for future studies.
Lactotropes and somatotropes share a common pituitary lineage, but activation, storage, and secretion of these 2 cell types varies greatly. GH is reported to be at peak expression on PND1 (Fink, Pfaff et al. 2012) and then taper as development progresses due to the many points of regulation coming into play. The rise in serum prolactin at PND 15 in our control mice correlates well with studies that show late prolactin secretion in neonatal rodents detected by reverse hemolytic plaque assays (Frawley and Miller 1989). It also suggests that a number of factors, such as expression of the LH alpha subunit, are needed for full expansion of lactotropes after birth.
When we analyzed GH and prolactin secretion in the somatotrope LEPR-null mutants, this study made unexpected discoveries about the apparent broad impact of somatotrope development on lactotropes. Somatotrope LEPR-deletion leads to dysregulation of GH throughout development as well as a reduced peak in serum prolactin in the mutants. We looked at changes in pituitary Pou1f1 expression since somatotropes and lactotropes are products of the Pou1f1 lineage. Pou1f1 is exclusively expressed in the pituitary starting on embryonic day 13.5 and is maintained in terminally differentiated somatotropes, lactotropes, and thyrotropes (Simmons, Voss et al. 1990). Pou1f1 is required for the hypomethylation of the Gh promoter, is associated with active transcription, and influences the accessibility of other regulators (Massah, Hollebakken et al. 2014). The reduction in female mutant Pou1f1 at PND 8–10 corresponds with the reduction in GH at PND 8 in those females. Since Pou1f1 is also reduced at PND 15, we need to investigate whether or not Pou1f1 remains down throughout development in this model. We do see sexual dimorphism of Pou1f1 expression in this model, as males show increased levels of Pou1f1 at PND 10 while having reduced serum GH. Pou1f1 is downstream of PROP1 so we also assayed pituitary PROP1 content. PROP1 was unchanged in female but was elevated in males at PND 5 and 15. These sexually dimorphic changes suggest that males and females are regulating pituitary hormones via different mechanisms. Previously we have shown that Pou1f1 protein levels are down but not Pou1f1 mRNA in purified somatotropes of mutant females (Odle, Allensworth-James et al. 2016). This indicates a dependency on leptin for the translation of Pou1f1 proteins in females. Thus, the lower serum prolactin levels might reflect low Pou1f1 levels in progenitor cells that must proliferate to support the expansion of the lactotrope population. This same dependency is not evident in neonatal males. At this point, we do not have an explanation for the increased Pou1f1 or Prop1 in mutant males.
Our studies of somatotrope LEPR-null mutants showed clear reductions in percentages of GH cells, which indicates a defect in GH protein stores in the mutant somatotropes and dysregulation of GH and PRL during early development. The reduced prolactin secretion may reflect important paracrine factors secreted by somatotropes (Denef 2008) or a dependency on leptin for the expression of somatolactotropes in females (Childs, Unabia et al. 1999). Clearly, more work is needed to investigate the mechanisms modulating Pou1f1 expression and to understand how leptin regulates these pathways upstream. In conclusion, the deletion of somatotrope LEPR leads to sex-specific neonatal dysregulation of GH, prolactin, Pou1f1, and PROP1. These changes may involve mechanisms underlying somatolactotrope and somatotrope differentiation that are important for normal adult metabolic functions.
Acknowledgments
Funding
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD059056), National Institute of Digestive and Diabetic Diseases (RO1 DK113776), National Institute of General Medical Sciences (P20 GM103425 and P30GM11070), and a Sturgis Charitable Trust award to support diabetes research.
Footnotes
We have nothing to disclose.
Bibliography
- Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101(5):1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter N, Odle AK, Allensworth-James ML, Haney AC, Syed MM, Cozart MA, Chua S, Kineman R, Childs GV. Ablation of Leptin Signaling to Somatotropes: Changes in Metabolic Factors that Cause Obesity. Endocrinology. 2012;153(10):4705–4715. doi: 10.1210/en.2012-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allensworth-James M, Odle A, Haney A, Childs G. Sex Differences in Somatotrope Dependency on Leptin Receptors in Young Mice: Ablation of LEPR Causes Severe Growth Hormone Deficiency and Abdominal Obesity in Males. Endocrinology. 2015 doi: 10.1210/EN.2015-1198. EN20151198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attig L, Larcher T, Gertler A, Abdennebi-Najar L, Djiane J. Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis. 2011;7(2):88–94. doi: 10.4161/org.7.2.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 2008;32(7):1153–1160. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- Behringer RR, Mathews LS, Palmiter RD, Brinster RL. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Dev. 1988;2(4):453–461. doi: 10.1101/gad.2.4.453. [DOI] [PubMed] [Google Scholar]
- Borrelli E, Heyman RA, Arias C, Sawchenko PE, Evans RM. Transgenic mice with inducible dwarfism. Nature. 1989;339(6225):538–541. doi: 10.1038/339538a0. [DOI] [PubMed] [Google Scholar]
- Bottner A, Keller E, Kratzsch J, Stobbe H, Weigel JF, Keller A, Hirsch W, Kiess W, Blum WF, Pfaffle RW. PROP1 mutations cause progressive deterioration of anterior pituitary function including adrenal insufficiency: a longitudinal analysis. J Clin Endocrinol Metab. 2004;89(10):5256–5265. doi: 10.1210/jc.2004-0661. [DOI] [PubMed] [Google Scholar]
- Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Ma X, Cai J, Luan J, Liu AJ, Yang R, Cao Y, Zhu X, Zhang H, Chen YX, et al. ZBTB20 is required for anterior pituitary development and lactotrope specification. Nat Commun. 2016;7 doi: 10.1038/ncomms11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 2009;27(5):1182–1195. doi: 10.1002/stem.51. [DOI] [PubMed] [Google Scholar]
- Childs GV. Growth hormone cells as co-gonadotropes: partners in the regulation of the reproductive system. Trends Endocrinol Metab. 2000;11(5):168–175. doi: 10.1016/s1043-2760(00)00252-6. [DOI] [PubMed] [Google Scholar]
- Childs GV, Akhter N, Haney A, Syed M, Odle A, Cozart M, Brodrick Z, Gaddy D, Suva LJ, Akel N, et al. The somatotrope as a metabolic sensor: deletion of leptin receptors causes obesity. Endocrinology. 2011;152(1):69–81. doi: 10.1210/en.2010-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Miller BT. Cytochemical detection of gonadotropin-releasing hormone-binding sites on rat pituitary cells with luteinizing hormone, follicle-stimulating hormone, and growth hormone antigens during diestrous up-regulation. Endocrinology. 1994;134(4):1943–1951. doi: 10.1210/endo.134.4.8137763. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Miller BT, Collins TJ. Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J Endocrinol. 1999;162(2):177–187. doi: 10.1677/joe.0.1620177. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96(13):7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Cripps RL, Duncan JS, Barrett P, Mercer JG, Herwig A, Ozanne SE. Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R631–639. doi: 10.1152/ajpregu.90690.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Keisler JL, Pérez-Millán MI, Schade V, Camper SA. All Hormone-Producing Cell Types of the Pituitary Intermediate and Anterior Lobes Derive From Prop1-Expressing Progenitors. Endocrinology. 2016;157(4):1385–1396. doi: 10.1210/en.2015-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20(1):1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy LS, Bowers CY, Veldhuis JD. Model-projected mechanistic bases for sex differences in growth hormone regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1577–1593. doi: 10.1152/ajpregu.00584.2006. [DOI] [PubMed] [Google Scholar]
- Fink G, Pfaff D, Levine J, editors. Handbook of Neuroendocrinology. San Diego, CA: Academic Press as an imprint of Elsevier; 2012. [Google Scholar]
- Frawley LS, Miller HA., 3rd Ontogeny of prolactin secretion in the neonatal rat is regulated posttranscriptionally. Endocrinology. 1989;124(1):3–6. doi: 10.1210/endo-124-1-3. [DOI] [PubMed] [Google Scholar]
- Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19(6):717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- Harris GW. SEX HORMONES, BRAIN DEVELOPMENT AND BRAIN FUNCTION. Endocrinology. 1964;75:627–648. doi: 10.1210/endo-75-4-627. [DOI] [PubMed] [Google Scholar]
- Hoeffler JP, Boockfor FR, Frawley LS. Ontogeny of prolactin cells in neonatal rats: initial prolactin secretors also release growth hormone. Endocrinology. 1985;117(1):187–195. doi: 10.1210/endo-117-1-187. [DOI] [PubMed] [Google Scholar]
- L A, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. doi: 10.1038/ijo.2008.39. (1476–5497 (Electronic)). [DOI] [PubMed] [Google Scholar]
- Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148(5):1946–1953. doi: 10.1210/en.2006-1542. [DOI] [PubMed] [Google Scholar]
- Massah S, Hollebakken R, Labrecque MP, Kolybaba AM, Beischlag TV, Prefontaine GG. Epigenetic characterization of the growth hormone gene identifies SmcHD1 as a regulator of autosomal gene clusters. PLoS One. 2014;9(5):e97535. doi: 10.1371/journal.pone.0097535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morash B, Wilkinson D, Murphy P, Ur E, Wilkinson M. Developmental regulation of leptin gene expression in rat brain and pituitary. Mol Cell Endocrinol. 2001;185(1–2):151–159. doi: 10.1016/s0303-7207(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Morash BA, Imran A, Wilkinson D, Ur E, Wilkinson M. Leptin receptors are developmentally regulated in rat pituitary and hypothalamus. Mol Cell Endocrinol. 2003;210(1–2):1–8. doi: 10.1016/j.mce.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Odle AK, Allensworth-James ML, Akhter N, Syed M, Haney AC, MacNicol M, MacNicol AM, Childs GV. A Sex-Dependent, Tropic Role for Leptin in the Somatotrope as a Regulator of POU1F1 and POU1F1-Dependent Hormones. Endocrinology. 2016;157(10):3958–3971. doi: 10.1210/en.2016-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl D R, et al. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125(3):593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Rizzoti K. Genetic regulation of murine pituitary development. J Mol Endocrinol. 2015;54(2):R55–73. doi: 10.1530/JME-14-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DM, Voss JW, Ingraham HA, Holloway JM, Broide RS, Rosenfeld MG, Swanson LW. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990;4(5):695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- Syed M, Cozart M, Haney AC, Akhter N, Odle AK, Allensworth-James M, Crane C, Syed FM, Childs GV. Ghrelin restoration of function in vitro in somatotropes from male mice lacking the Janus kinase (JAK)-binding site of the leptin receptor. Endocrinology. 2013;154(4):1565–1576. doi: 10.1210/en.2012-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27(2):101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26(1):114–146. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20(6):1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]