Abstract
Background: Use of artificial pancreas (AP) requires seamless interaction of device components, such as continuous glucose monitor (CGM), insulin pump, and control algorithm. Mobile AP configurations also include a smartphone as computational hub and gateway to cloud applications (e.g., remote monitoring and data review and analysis). This International Diabetes Closed-Loop study was designed to demonstrate and evaluate the operation of the inControl AP using different CGMs and pump modalities without changes to the user interface, user experience, and underlying controller.
Methods: Forty-three patients with type 1 diabetes (T1D) were enrolled at 10 clinical centers (7 United States, 3 Europe) and 41 were included in the analyses (39% female, >95% non-Hispanic white, median T1D duration 16 years, median HbA1c 7.4%). Two CGMs and two insulin pumps were tested by different study participants/sites using the same system hub (a smartphone) during 2 weeks of in-home use.
Results: The major difference between the system components was the stability of their wireless connections with the smartphone. The two sensors achieved similar rates of connectivity as measured by percentage time in closed loop (75% and 75%); however, the two pumps had markedly different closed-loop adherence (66% vs. 87%). When connected, all system configurations achieved similar glycemic outcomes on AP control (73% [mean] time in range: 70–180 mg/dL, and 1.7% [median] time <70 mg/dL).
Conclusions: CGMs and insulin pumps can be interchangeable in the same Mobile AP system, as long as these devices achieve certain levels of reliability and wireless connection stability.
Keywords: International Diabetes Closed-Loop Study, Continuous glucose monitor use, Insulin pump use
Introduction
To be ultimately established and accepted as a viable treatment of diabetes, artificial pancreas (AP) systems need to prove their safety and efficacy in large rigorous clinical trials in the patient's natural environment. After the first pilot trials of Mobile AP—a portable AP system using a smartphone as a computational hub1—a number of relatively small-scale studies were completed, including studies testing AP systems during home use.2–11 The progress of the AP research was presented in two symposia in 201412 and in 2016,13 which were almost exclusively dedicated to outpatient closed-loop control studies done with Mobile AP systems.14–19 These studies extended the duration of closed-loop control home use to several weeks and even up to 6 months,20 and the applicability of closed-loop control to young children16 and to demanding winter sports conditions (5 h of skiing per day for 5 days) in children and adolescents with type 1 diabetes (T1D).21 The feasibility of adaptive Mobile AP was demonstrated by a 12-week multicenter trial of 24/7 adaptive closed-loop control. In this trial, each participant's insulin requirements (e.g., basal rate settings and carbohydrate ratio) were algorithmically optimized every week.22 Another adaptive AP system was tested by Messori et al.23 The use of Mobile AP started as an attractive option for research implementation due to the ability to easily interchange system components, including control strategy—a difficult prospect when the controller is embedded in the insulin pump. This interoperability study is the first step toward an AP that can be used on the patient's phone and control the closed-loop system.
As summarized in a recent review,24 in 2017 the AP transitioned from research to routine clinical use. To accelerate this transition, the National Institutes of Health invested over $35M in four pivotal trials of closed-loop control technologies intended to bring these systems to market.24 The first AP system with a control algorithm embedded in the insulin pump—the Medtronic 670G—was deployed in a pivotal trial enrolling 124 patients with T1D for 3 months of system use.25 This was a “hybrid” closed-loop controller with automatic basal rate modulation without bolus automation. A recent report detailed the glycemic control outcomes from the use of this system in adolescents (ages 14–21 years) and adults.26 This study allowed comprehensive testing of the safety of in-home use of hybrid AP and the subsequent regulatory approval of the system by the U.S. Food and Drug Administration, thereby opening the AP field to routine clinical use.
The International Diabetes Closed-Loop (iDCL) trial is a multicenter international study supported by the NIH initiative already noted.24 The first phase of the iDCL trial included testing of several components, continuous glucose monitors (CGMs), and insulin pumps, for inclusion in a Mobile AP system to be used in a subsequent larger clinical trial. The emphasis was on testing AP component interoperability and in particular, the wireless connections between the CGM, the insulin pump, and the system's smartphone hub. This was done with the premise that any contemporary AP system that includes subcutaneous glucose monitoring and subcutaneous insulin infusion is inherently multicomponent and requires wireless communication between the participating devices. At a minimum, the wearable AP network has two components—CGM and a pump—provided that the control algorithm is embedded in one of these components (typically in the pump). Mobile AP configurations include a smartphone or a similar device to provide a more elaborate user interface,27 act as a computational hub, and provide a gateway to cloud applications, as well as cloud components providing remote monitoring and more extensive data review and analytical capabilities.28 In this article, we report the results from this first iDCL interoperability study and include data reflecting component connectivity and interoperability, as well as glycemic outcomes from the functioning of the control algorithm.
Methods
The objective of this interoperability study was to assess 24/7 in-home component connectivity and system usability before initiating a larger randomized controlled trial. The study was conducted at 10 research centers in the United States and Europe: University of Virginia (Charlottesville, VA), Joslin Diabetes Center (Boston, MA), the Mayo Clinic (Rochester, MN), Icahn School of Medicine at Mt. Sinai (New York City), University of Colorado (Denver, CO), Stanford University (Stanford, CA), Sansum Diabetes Research Institute (Santa Barbara, CA), Academic Medical Center Amsterdam (The Netherlands), University of Montpellier (France), and University of Padua (Italy), coordinated by the Jaeb Center for Health Research (Tampa, FL).
Subjects
Forty-three subjects were enrolled in the study. Two dropped before any closed-loop system use, 1 dropped after providing ∼1 week of closed-loop system use, and 40 completed the entire 2-week home use. Major eligibility criteria included (1) clinical diagnosis of T1D, (2) treated with insulin for at least 1 year, (3) use of an insulin infusion pump for at least 6 months, (4) age 14 to <75 years old, (5) HbA1c level <10.5% at screening, and (6) willingness to establish network connectivity daily either through local WiFi network or through a study-provided cellular service to maintain cell phone or WiFi connectivity in subjects' usual environment (work, home, etc.). Subject characteristics for the 41 evaluable participants are presented in Table 1.
Table 1.
Participant Characteristics at Enrollment
| Characteristic | N = 41 |
|---|---|
| Age (years), median (IQR) | 33 (20, 49) |
| Range | 15–72 |
| Male, n (%) | 25 (61) |
| U.S. location, n (%) | 34 (83) |
| Race, n (%) | |
| White non-Hispanic | 39 (95) |
| Unknown/not reported | 2 (5) |
| Diabetes duration (years), median (IQR) | 16 (11, 35) |
| Body mass index (kg/m2), median (IQR) | 25 (23, 27) |
| HbA1c (%), median (IQR) | 7.4 (6.8, 7.9) |
| Daily total insulin (U/kg per day), median (IQR) | 0.55 (0.43, 0.63) |
IQR, interquartile range.
Procedure
The protocol was considered a significant risk device study, due to the fact that the closed-loop system was experimental. Thus, the study was approved by FDA Investigational Device Exemption #G160097 and was registered in clinicaltrials.gov under numbers NCT02844517 and NCT02892604. The Institutional Review Board of each participating site approved the trial as well. The study was conducted in compliance with the policies described in the study policies document, with the ethical principles that have their origin in the Declaration of Helsinki and with the standards of Good Clinical Practice. Data were directly collected in electronic case report forms, which were considered the source data. There was no restriction on the number of subjects enrolled by each site toward the overall recruitment goal. Subject participation lasted ∼2–4 weeks depending on the subject's comfort with the study CGM and insulin pump, and included (1) screening/enrollment, (2) study CGM training and initiation and 0–1 week of home use of the study CGM, (3) study pump training and initiation and 0–1 week of home use of the study pump, (4) 1 day training on the use of the Mobile AP system, (5) 2 weeks of home use of the Mobile AP system in 24-h closed-loop configuration with daily contact, and (6) debriefing visit with Technology Acceptance questionnaire.
Mobile AP system
Several combinations of system hardware were assessed, including CGM 1 and CGM 2 and insulin Pump 1 and Pump 2. The goal of the system was to connect multiple pumps and sensors with the Mobile AP application; as such, this was not an attempt to build a commercial device. One insulin pump was a modified version of an insulin pump with added bluetooth (BT) low energy capabilities specifically designed to be used in Investigation Device Exemption studies only. The other pump had Bluetooth capabilities not originally designed to communicate with a mobile device. The Mobile AP application ran on Android smartphones and used Bluetooth or BT low energy to wirelessly communicate with the study CGM and insulin pump. The control algorithm remained unchanged throughout the study and was the same in all system configurations. The algorithm adjusted insulin delivery every 5 min and used several modules to keep the patients' glucose level in safe range: (1) model-based metabolic state estimator that calculated the patient's current and predicted state, including current/predicted glucose and insulin-on-board; (2) a safety supervision module that reduces or discontinues insulin delivery if the system determines that there is a risk of hypoglycemia; this module has “veto power” over all other algorithmic components and over the actions of the system user; (3) basal rate modulator that adjusted basal insulin delivery in response to estimated glucose levels based on a circadian target profile, that is, adapt its mode of operation during the course of every night to initially mitigate after-dinner hyperglycemia and then “slide” the patient overnight to a target glucose of 120 mg/dL by the morning; and (4) a hyperglycemia mitigation system that delivers automated insulin boluses when estimated glucose is more than the target. A network service communicated with the cloud component of the system over a secure Secure Sockets Layer (SSL) link to transfer data automatically and allow for remote monitoring, notifications, alerts, and archiving.
Adverse event reporting included severe hypoglycemia, severe hyperglycemia or diabetic ketoacidosis, and any study or device-related event.
Statistical analyses
The sample size of N = 43 participants is a convenience sample and no formal statistical analyses were planned for this study. All sensor and pump connectivity, glycemic control, usability, and safety analyses are considered exploratory. Safety analyses included all 43 participants. Sensor and pump connectivity, glycemic control, and usability analyses included the 41 participants who used the closed-loop system.
Operational mode data, recorded by the system during 2 weeks of home use of the Mobile AP system in 24-h closed-loop configuration, were used to calculate sensor and pump connectivity metrics. CGM data associated with the closed-loop mode were used to calculate glycemic metrics. Data were excluded from a study-imposed 19-day closed-loop hiatus that occurred due to a system bug that was not associated with any adverse events.
Results
The study met its objectives of allowing determination of the best system configuration with sufficient usability to be used in a larger randomized controlled trial.
Sensor connectivity
As presented in the “By Sensor” column of Table 2, the connectivity with the mobile system hub was similar for the two sensors, with closed-loop mode being 75% of the overall time for both sensors.
Table 2.
System Use and Operational Mode Summary (N = 41 Subjects)
| By sensor | By pump | |||||
|---|---|---|---|---|---|---|
| Time of day | Mode of system operation | Pooled | CGM1 | CGM2 | Pump 1 | Pump 2 |
| Overall | Total hours use | 13,934 | 8563 | 5371 | 6102 | 7832 |
| Closed loop (%) | 75 | 75 | 75 | 87 | 66 | |
| Open loop (%) | 5 | 4 | 7 | 5 | 5 | |
| Stopped (%) | 14 | 16 | 12 | 3 | 23 | |
| System off (%) | 6 | 5 | 6 | 5 | 6 | |
| Day | Total hours use | 10,501 | 6454 | 4047 | 4583 | 5918 |
| Closed loop (%) | 77 | 77 | 76 | 87 | 69 | |
| Open loop (%) | 5 | 4 | 8 | 5 | 5 | |
| Stopped (%) | 12 | 13 | 11 | 3 | 20 | |
| System off (%) | 6 | 5 | 6 | 5 | 6 | |
| Night | Total hours use | 3433 | 2109 | 1324 | 1519 | 1914 |
| Closed loop (%) | 71 | 69 | 73 | 86 | 59 | |
| Open loop (%) | 4 | 3 | 6 | 5 | 3 | |
| Stopped (%) | 19 | 23 | 14 | 3 | 32 | |
| System off (%) | 6 | 5 | 6 | 5 | 6 | |
The system operates in the following three functional modes, with an implicit fourth mode, “System off,” that refers to periods of time when the system is not running at all (>10 min without any of the three functional modes active): Closed loop—system automatically increases or decreases basal insulin delivery and can deliver automated correction boluses depending on current/predicted glucose values. Open loop—system delivers basal insulin according to preset daily profile with no automated increases or decreases based on glucose values; most typically occurs when CGM signal is unavailable for >20 min. In user documentation, this mode was called pump mode. Stopped—system is running, but has either just been initialized or else has lost communication with the insulin pump for >20 min; pump automatically reverts to preset daily insulin profile.
CGM, continuous glucose monitor.
Pump connectivity
As presented in the “By Pump” column of Table 2, Pump 1 maintained good connectivity with the mobile system hub such that subjects were able to keep this system configuration in closed-loop mode 87% of the time. Pump 2 exhibited significant connectivity issues, which remained a persistent problem throughout the study. As a result, subjects were only able to keep this system configuration in closed-loop mode 66% of the time. Majority of the remaining time (23% with this system configuration) was spent in stopped mode due to lack of connectivity.
Glycemic control
Table 3 provides a summary of glycemic outcomes separately for Pump 1 (Table 3A), Pump 2 (Table 3B), and pooled across all subjects and hardware configurations (Table 3C). These outcomes show that the system achieved good glycemic control with very little time spent in hypoglycemia during closed-loop operation, regardless of which sensor or insulin pump was used.
Table 3.
Continuous Glucose Monitor Metrics During Closed-Loop Mode System Use—By Pump (N = 41 Subjects)
| Time of the day | Overall | Daytime | Nighttime | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pump | Pooled (N = 41) | Pump 1 (N = 17) | Pump 2 (N = 24) | Pooled (N = 41) | Pump 1 (N = 17) | Pump 2 (N = 24) | Pooled (N = 41) | Pump 1 (N = 17) | Pump 2 (N = 24) |
| Hours of sensor readings, median (IQR) | 258 (207, 314) | 317 (292, 338) | 215 (166, 255) | 185 (145, 218) | 220 (206, 240) | 162 (127, 184) | 72 (53, 90) | 99 (83, 108) | 63 (42, 78) |
| Nadir (mg/dL), median (IQR) | 44 (40, 54) | 43 (40, 53) | 45 (40, 55) | 44 (40, 54) | 44 (40, 53) | 45 (40, 55) | 63 (55, 70) | 59 (43, 70) | 66 (58, 69) |
| % <50 mg/dL, median (IQR) | 0.1% (0.0%, 0.3%) | 0.1% (0.0%, 0.5%) | 0.1% (0.0%, 0.3%) | 0.2% (0.0%, 0.4%) | 0.1% (0.0%, 0.4%) | 0.2% (0.0%, 0.4%) | 0.0% (0.0%, 0.0%) | 0.0% (0.0%, 0.1%) | 0.0% (0.0%, 0.0%) |
| % <60 mg/dL, median (IQR) | 0.6% (0.2%, 1.2%) | 0.5% (0.2%, 1.3%) | 0.6% (0.2%, 1.0%) | 0.6% (0.2%, 1.6%) | 0.6% (0.2%, 1.7%) | 0.6% (0.2%, 1.4%) | 0.0% (0.0%, 0.4%) | 0.1% (0.0%, 0.5%) | 0.0% (0.0%, 0.3%) |
| % <70 mg/dL, median (IQR) | 1.7% (0.8%, 2.5%) | 1.8% (0.6%, 2.5%) | 1.7% (1.0%, 2.5%) | 1.9% (0.8%, 3.2%) | 2.0% (0.7%, 2.9%) | 1.8% (1.0%, 3.4%) | 0.5% (0.0%, 1.5%) | 0.7% (0.0%, 1.5%) | 0.5% (0.1%, 1.5%) |
| Low BG index, median (IQR) | 0.5 (0.3, 0.8) | 0.5 (0.3, 0.8) | 0.5 (0.3, 0.8) | 0.6 (0.3, 0.9) | 0.6 (0.3, 0.9) | 0.6 (0.3, 0.9) | 0.3 (0.2, 0.6) | 0.3 (0.2, 0.6) | 0.4 (0.2, 0.6) |
| Mean glucose (mg/dL), mean ± SD | 152 ± 17 | 154 ± 16 | 151 ± 18 | 154 ± 19 | 156 ± 20 | 153 ± 18 | 150 ± 21 | 150 ± 13 | 149 ± 25 |
| Coefficient of variation, median (IQR) | 32% (30%, 36%) | 34% (31%, 36%) | 32% (30%, 37%) | 34% (30%, 37%) | 35% (30%, 37%) | 34% (31%, 37%) | 29% (26%, 32%) | 29% (26%, 31%) | 29% (25%, 34%) |
| Standard deviation (mg/dL), median (IQR) | 48 (42, 56) | 52 (45, 58) | 48 (42, 55) | 51 (44, 59) | 53 (45, 62) | 48 (44, 56) | 41 (37, 51) | 43 (38, 50) | 40 (35, 55) |
| % In range 70–180 mg/dL, mean ± SD | 73 ± 10% | 71 ± 10% | 73 ± 10% | 70 ± 11% | 69 ± 13% | 71 ± 10% | 76 ± 16% | 77 ± 9% | 76 ± 19% |
| % In range 70–140 mg/dL, mean ± SD | 47 ± 11% | 44 ± 10% | 48 ± 11% | 44 ± 11% | 43 ± 12% | 45 ± 11% | 51 ± 16% | 47 ± 13% | 53 ± 18% |
| High BG index, median (IQR) | 5.1 (3.8, 7.3) | 5.8 (4.4, 6.8) | 4.8 (3.6, 7.4) | 5.7 (4.1, 7.8) | 6.4 (4.9, 7.8) | 5.7 (3.9, 7.5) | 4.0 (2.9, 6.4) | 4.0 (3.8, 6.2) | 4.1 (2.5, 6.5) |
| % >180 mg/dL, median (IQR) | 26% (18%, 32%) | 28% (20%, 32%) | 23% (17%, 33%) | 27% (20%, 36%) | 29% (22%, 36%) | 27% (17%, 34%) | 18% (13%, 31%) | 20% (15%, 31%) | 17% (10%, 31%) |
| % >250 mg/dL, median (IQR) | 4% (2%, 7%) | 5% (3%, 8%) | 3% (2%, 7%) | 4% (1%, 10%) | 6% (3%, 11%) | 3% (1%, 7%) | 2% (0%, 6%) | 2% (1%, 5%) | 2% (0%, 7%) |
| % >300 mg/dL, median (IQR) | 0.6% (0.1%, 2.1%) | 1.2% (0.2%, 2.9%) | 0.5% (0.0%, 1.9%) | 0.8% (0.0%, 2.5%) | 1.6% (0.0%, 4.0%) | 0.7% (0.0%, 2.0%) | 0.0% (0.0%, 0.3%) | 0.1% (0.0%, 0.8%) | 0.0% (0.0%, 0.3%) |
BG, blood glucose.
Usability
Full responses to the Technology Acceptance questionnaire are presented in Supplementary Table S1 (Supplementary Data are available at https://www.liebertpub.com/suppl/doi/10.1089/dia.2018.0308). Several of the questions highlight that the frequent disconnections encountered with Pump 2 resulted in usability concerns. For example, (Pump 1 vs. Pump 2: Question 1 [“It caused too many hassles in my daily life.”]—30% vs. 84% somewhat agree/strongly agree, Question 9 [“I spent much less time thinking about my diabetes.”]—35% vs. 58% somewhat disagree/strongly disagree, Question 11 [“I was less worried about how my insulin was working.”]—58% vs. 25% somewhat/strongly agree, Question 19 [“I felt less burdened in managing diabetes while I was using it than I do when using my typical method of diabetes care”]—30% vs. 71% somewhat/strongly disagree, Question 25 [“It had too many “glitches” and ‘bugs’.”]—59% vs. 87% somewhat/strongly agree, Question 38a [“How easy to use was inControl?”]—71% vs. 34% extremely easy or between somewhat easy and extremely easy).
Based on the connectivity, glycemic control, and usability results mentioned, the study concluded that Pump 1 should be used in a subsequent larger trial lasting for a longer period.
Adverse events
There were six reported adverse events. These included one instance of severe hypoglycemia requiring third-party assistance to administer rescue carbohydrates, with closed-loop system usability a contributing factor. There was one other serious adverse event (ischemic cardiomyopathy), unrelated to closed-loop system use. The remaining events were not related to closed-loop system functionality.
Discussion
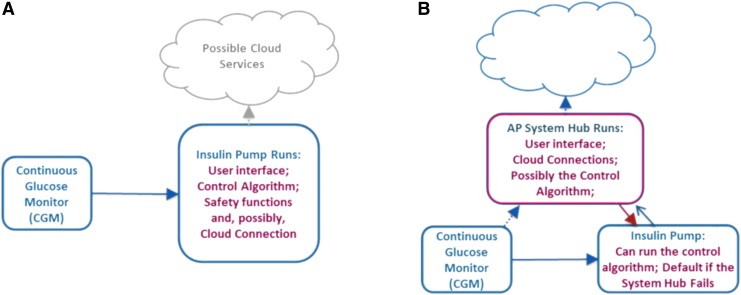
A user-friendly AP requires multiple devices to communicate and interact together while ensuring minimal disruption or burden to the patient to keep the system running. Although all research systems so far used Mobile AP solutions, it is traditionally assumed that commercial AP control systems should reside in the patient's insulin pump, which simplifies the system and adheres to current medical device regulatory approval process without the additional cybersecurity and device use risks of a mobile environment. We should not, however, completely ignore the potential for added capabilities a mobile solution could provide. For example, contemporary smartphones are readily available and inexpensive, suitable for ambulatory use and computationally capable of running closed-loop control algorithms, wirelessly connectable to CGM devices and insulin pumps, and capable of broadband communication with a central location for remote monitoring and safety supervision. No current insulin pump offers similar capabilities (Fig. 1). Furthermore, psychological studies show that many patients (particularly children and teenagers) are reluctant to use their insulin pump in public, missing boluses, and slipping into poor glycemic control when privacy is limited (e.g., during school days).13 However, no one is embarrassed to use a smartphone, and that may be a key to better patient engagement and better glucose control. However, this should be balanced with device security and resource utilization, which is still an open question that is discussed in the DTMost and DTSec security and assurance standards (https://www.diabetestechnology.org/dtsec.shtml).
FIG. 1.
(A) Embedded AP system configuration: all closed-loop control, user interface, and communication functions are run by the insulin pump. (B) Mobile AP system configuration: a consumer electronics device (e.g., a smartphone or a watch) runs certain AP functions. AP, artificial pancreas. Color images are available online.
This interoperability study showed that peripheral devices can be interchangeable within a Mobile AP system. The glycemic outcomes achieved by different CGMs and different insulin pumps were comparable, provided that the wireless communications were functioning properly. We can speculate that the use of remote monitoring and the daily contacts with study staff in this pilot study may have increased participant's adherence to the protocol. The systems pilot tested in this study included noncommercial software and pump hardware components used off-label under an Investigational Device Exemption. Because both software and hardware elements may have contributed to the pump connectivity problems observed during the study, we do not believe it is appropriate to disclose specific pump model names.
The major caveat with the mobile approach is that the use of consumer electronics as a medical device poses design challenges. A level of system security and reliability would need to be achieved, which is beyond the current consumer-grade software applications. Although still controversial, the good news is that off-the-shelf hardware appears similarly reliable to medical-grade devices: for example, in our studies, in hundreds of thousands of hours of use so far, the failure rate of the smartphones used as an AP hub was similar to, or lower than, the failure rate of approved medical devices.26 It follows that firming up the software of consumer electronics may be sufficient to turn ordinary smartphones and other portable devices into reliable AP platforms. A first attempt in this direction was made several years ago by reconfiguring the Android operating system of our research platform to meet certain clinical-use standards.27 This first attempt achieved regulatory approval and carried out 5 years of outpatient AP trials in the United States and in Europe successfully.1–3,5, 6,9,14–18,20–22,29 The lessons learned from these studies point to functionalities that need to be added, or upgraded, so such an approach can be successful beyond the research setting:
As confirmed by this interoperability study, connectivity between devices is paramount: indeed, provided that the wireless connection worked as intended, the degree of glycemic control did not differ between the system configurations tested in the trial. Thus, standardization and security of the communication protocols become essential for future Mobile AP systems.
Alerts need to be customizable and appropriate for outpatient long-term use, as interrupting sleep for a short-term overnight study is feasible but not for multiweek outpatient studies. The ability of the system to fall back to basal rate when connectivity is lost is a built-in mitigation to reduce the number of alerts needed and allow for customization by each participant.
Network–algorithm interactions need further refinement and rigorous testing in multiple scenarios. Particular attention needs to be paid to algorithm reaction to the absence of data, or to corrupt or inaccurate data, that is, in case of interlink failure or peripheral device malfunction. In silico experiments and computer simulation can be invaluable in this regard.
Data safety, privacy, and security of the control commands sent to the pump need to be addressed at a level suitable for devices that deliver a drug with a very narrow therapeutic index (insulin).
The limits of safe AP operation should be clearly defined and explained to the user, their family, and to health care professionals. Exceeding system capabilities carries risks with any device, but can be particularly harmful with medical equipment.
In summary, it is now evident that the AP is a feasible treatment for T1D; its potential system configurations are well defined, and studies are under way to ensure its widespread deployment and fulfill its promise to all.
Supplementary Material
Acknowledgments
Roche Diabetes Care supplied insulin pumps and blood glucose testing supplies for this study. The authors thank the study participants. A listing of the participants study sites, investigators, and coordinators is included as follows: IDCL Study Site and Personnel—Charlottesville, VA Center for Diabetes Technology, University of Virginia Health System: Stacey Anderson (PI), Boris Kovatchev (I), Sue Brown (I), Linda Gonder-Frederick (I), Emma Emory (C), Laura Kollar (C), and Charlotte Barnett (T); Boston, MA Joslin Diabetes Center: Lori Laffel (PI), Elvira Isganaitis (I), Sanjeev Mehta (I), and Louise Ambler-Osborn (C); Santa Barbara, CA Sansum Diabetes Research Institute: Jordan Pinsker (PI), Mei Mei Church (I), Tyler Jean (C), and Camille Andre (C); New York, NY Mt. Sinai School of Medicine: Carol Levy (PI), Camilla Levister (I), David Lam (I), Georgia Kulina (I), Hanna Lee (I), Anna Aluf (I), Robert Rapaport (I), Elizabeth Burtman (I), and Selassie Ogyaadu (C); Rochester, MN St. Mary's Hospital, Endocrine Unit, Mayo Clinic: Yogish Kudva (PI), Ananda Basu (I), Seema Kumar (I), Aida Lteif (I), Shelly McCrady-Spitzer (C), and V. Dadlani, (C); Aurora, CO Barbara Davis Center for Diabetes, University of Colorado: Paul Wadwa (PI), Gregory Forlenza (I), Todd Alonso (I), Robert Slover (I), and Emily Jost (C); Palo Alto, CA, Stanford University, Pediatric Endocrinology and Diabetes: Bruce Buckingham (PI), David Maahs (I), Paula Clinton (C), Tatiana Marcal (C), and Jasmine Doiev (C); Montpellier, France Montpellier University Hospital: Eric Renard (PI), Anne Farret (I), and Jerome Place (C); Padova, Italy University of Padova, Department of Engineering: Claudio Cobelli (PI), Simone Del Favero (I, C), Roberto Visentin (I), and Simone Faccioli; Padova, Italy University of Padova, Department of Medicine: Daniela Bruttomesso (I), Angelo Avogaro (I), Federico Boscari (I), Silvia Galasso (I), and Valeria Vallone (I); Amsterdam, Netherlands University of Amsterdam, Academic Medical Center: J Hans DeVries (PI), Jort Kropff (I), and Nathalie Masurel (C); Cambridge, MA John A. Paulson School of Engineering and Applied Sciences, Harvard University: Eyal Dassau (I) and Francis Doyle (I). Funding: The iDCL interoperability study was supported by NIH/NIDDK Grant UC4 DK 108483.
Author Disclosure Statement
S.M.A. reports grants from National Institutes of Health during the conduct of the study and grants from Medtronic and personal fees from Senseonics outside the submitted work. E.D., D.R., J.L., M.M.C., D.L., B.B., F.J.D., and F.B. have nothing to disclose. SAB reports non-financial support to the University of Virginia from Roche Diabetes, Dexcom, and Tandem Diabetes Care. J.E.P. reports personal fees, nonfinancial support, and other support from Tandem Diabetes Care, nonfinancial support and other support from Insulet Corporation, other support from Bigfoot Biomedical, nonfinancial support from Animas, Lifescan, Roche, Ascensia, and Dexcom outside the submitted work. C.L. reports grants and personal fees from NovoNordisk, personal fees from Medtronic, grants from Senseonics, and supplies for research from Dexcom and TypeZero during conduct of study. Y.C.K. reports nonfinancial support from Dexcom, Roche Diabetes, Tandem, and grants from Medtronic outside the submitted work. Y.C.K. has a patent Estimation of Insulin Sensitivity from CGM and Subcutaneous Insulin Delivery in T1D licensed. G.P.F. reports grants and personal fees from Medtronic, Dexcom, and Tandem; grants from Abbott, Insulet, TypeZero, and Beta Bionics during the conduct of the study. R.P.W. reports personal fees from Eli Lilly, grants, personal fees and nonfinancial support from Dexcom, grants and personal fees from Novo Nordisk, and grants from Bigfoot Biomedical outside the submitted work. L.L. reports grants from NIH during conduct of the study. J.H.D. reports nonfinancial support from Roche, Dexcom, and Tandem during the conduct of the study and nonfinancial support from Abbott, grants and nonfinancial support from Dexcom, nonfinancial support from Medtronic, personal fees from Roche Diabetes, personal fees from Senseonics, and personal fees from Zealand outside the submitted work. E.R. reports personal fees from Abbott, Eli-Lilly, Insulet, NovoNordisk, Roche, and Sanofi; nonfinancial support from Dexcom and Tandem during the conduct of study. C.C. reports nonfinancial support from Roche, Senseonics; personal fees from NovoNordisk outside the submitted work. C.C. also has a patent B9 PCT/US2008/082063 issued and a patent PCT/IT2012/000083 issued. S.D.F. reports nonfinancial support from Dexcom and Roche during the conduct of the study; grants from Dexcom outside the submitted work. B.P.K. reports research support handled by the University of Virginia: Dexcom, Roche, Sanofi, Tandem; patent royalties handled by the University of Virginia: Johnson & Johnson, Sanofi; Consultant: Sanofi, Tandem; Speaker's Bureau: Sanofi, Dexcom.
References
- 1. Cobelli C, Renard E, Kovatchev BP, et al. : Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chernavvsky DR, DeBoer MD, Keith-Hynes P, et al. : Use of an artificial pancreas among adolescents for a missed snack bolus and an underestimated meal bolus. Pediatr Diabetes 2016;17:28–35 [DOI] [PubMed] [Google Scholar]
- 3. Del Favero S, Bruttomesso D, Di Palma F, et al. : First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care 2014;37:1212–1215 [DOI] [PubMed] [Google Scholar]
- 4. Hovorka R, Elleri D, Thabit H, et al. : Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2014;37:1204–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kovatchev BP, Renard E, Cobelli C, et al. : Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care 2013;36:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kovatchev BP, Renard E, Cobelli C, et al. : Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care 2014;37:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumareswaran K, Thabit H, Leelarathna L, et al. : Feasibility of closed-loop insulin delivery in type 2 diabetes: a randomized controlled study. Diabetes Care 2014;37:1198–1203 [DOI] [PubMed] [Google Scholar]
- 8. Leelarathna L, Dellweg S, Mader JK, et al. : Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care 2014;37:1931–1937 [DOI] [PubMed] [Google Scholar]
- 9. Ly TT, Breton MD, Keith-Hynes P, et al. : Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care 2014;37:2310–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell SJ, El-Khatib FH, Sinha M, et al. : Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thabit H, Lubina-Solomon A, Stadler M, et al. : Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol 2014;2:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cefalu WT, Tamborlane WV: The artificial pancreas: are we there yet? Diabetes Care 2014;37:1182–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kovatchev B, Tamborlane WV, Cefalu WT, Cobelli C: The artificial pancreas in 2016: a digital treatment ecosystem for diabetes. Diabetes Care 2016;39:1123–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson SM, Raghinaru D, Pinsker JE, et al. : Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown SA, Kovatchev BP, Breton MD, et al. : Multinight “bedside” closed-loop control for patients with type 1 diabetes. Diabetes Technol Ther 2015;17:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Favero S, Boscari F, Messori M, et al. : Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care 2016;39:1180–1185 [DOI] [PubMed] [Google Scholar]
- 17. Del Favero S, Place J, Kropff J, et al. : Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab 2015;17:468–476 [DOI] [PubMed] [Google Scholar]
- 18. Renard E, Farret A, Kropff J, et al. : Day-and-night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: results of a single-arm 1-month experience compared with a previously reported feasibility study of evening and night at home. Diabetes Care 2016;39:1151–1160 [DOI] [PubMed] [Google Scholar]
- 19. Tauschmann M, Allen JM, Wilinska ME, et al. : Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, Randomized Clinical Trial. Diabetes Care 2016;39:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kovatchev B, Cheng P, Anderson SM, et al. : Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 21. Breton MD, Chernavvsky DR, Forlenza GP, et al. : Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the Artificial Pancreas Ski Study. Diabetes Care 2017;40:1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dassau E, Pinsker JE, Kudva YC, et al. : Twelve-week 24/7 ambulatory artificial pancreas with weekly adaptation of insulin delivery settings: effect on hemoglobin A1c and hypoglycemia. Diabetes Care 2017;40:1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Messori M, Kropff J, Del Favero S, et al. : Individually adaptive artificial pancreas in subjects with type 1 diabetes: a one-month proof-of-concept trial in free-living conditions. Diabetes Technol Ther 2017;19:560–571 [Google Scholar]
- 24. National Institute of Diabetes and Digestive and Kidney Disease: Four pivotal NIH-funded artificial pancreas research efforts begin. https://www.nih.gov/news-events/news-releases/four-pivotal-nih-funded-artificial-pancreas-research-efforts-begin (accessed August29, 2018)
- 25. Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 26. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keith-Hynes P, Guerlain S, Mize B, et al. : DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 2013;7:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Place J, Robert A, Ben Brahim N, et al. : DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol 2013;7:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kropff J, Del Favero S, Place J, et al. : 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol 2015;3:939–947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.