Abstract
From July 2006 through August 2017, a passive surveillance study of Ixodes ticks submitted from California, Oregon, and Washington was conducted by the TickReport program at the University of Massachusetts, Amherst. In total, 549 human-biting Ixodes ticks were submitted comprising both endemic and nonendemic species. We found that 430 endemic ticks were from 3 Ixodes species: Ixodes pacificus, Ixodes spinipalpis, and Ixodes angustus, whereas Ixodes scapularis (n = 111) was the most common species among the 119 nonendemic ticks. The submission peak for nymphal I. pacificus and I. spinipalpis was June, while submission peak for adult I. pacificus and nymphal I. angustus was April and September, respectively. Endemic ticks commonly attached to the lower extremities of their victims, and individuals younger than 9 years old were frequently bitten. The infection prevalence of Borrelia burgdorferi sensu lato, Borrelia miyamotoi, and Anaplasma phagocytophilum in I. pacificus ticks was 1.31%, 1.05%, and 0.52%, respectively, and the prevalence of B. burgdorferi s. l. and A. phagocytophilum in I. spinipalpis ticks was 14.29% and 10.71%, respectively. Furthermore, two species within the B. burgdorferi s. l. complex were detected in West Coast ticks: B. burgdorferi sensu stricto and Borrelia lanei. I. spinipalpis had the highest Borrelia prevalence among endemic ticks, and it was caused exclusively by B. lanei. Borrelia mayonii, Babesia microti, and Ehrlichia muris-like agent were not detected in these endemic ticks. In this study, we show that many nonendemic Ixodes ticks (119/549) are most likely acquired from travel to a different geographic region. We report cases of conventionally recognized nonhuman feeders (I. spinipalpis and I. angustus) parasitizing humans. The highest pathogen prevalence in I. spinipalpis may indicate a larger public health threat than previously thought, and the enzootic life cycle and pathogenicity of B. lanei warrant further study.
Keywords: human-biting Ixodes, pathogen, California, Oregon, Washington
Introduction
The single prostriate genus Ixodes comprises medically important ticks throughout the world (Keirans and Clifford 1978, Piesman and Eisen 2008). Several members within the Ixodes ricinus complex are the primary tick vectors that are involved in transmission of human pathogens (Xu et al. 2003). For example, in the eastern and north-central United States, Ixodes scapularis is the primary vector of several pathogens, including Borrelia burgdorferi (Lyme disease), Borrelia miyamotoi, Anaplasma phagocytophilum, and Babesia microti (Piesman and Eisen 2008). In the western United States, Ixodes pacificus is the primary vector of these pathogens (Burgdorfer et al. 1985, Lane et al. 1994, Eisen et al. 2016).
The prevalence and ecology of Lyme disease in the western United States differs significantly from that in the northeastern United States. The overall incidence of Lyme disease in the western United States is 0.2 cases compared to 30–80 cases (per 100,000 persons per year) in the northeastern United States (Schwartz et al. 2017). There is also greater vector and spirochete diversity in the western United States than in the northeastern United States (Brown and Lane 1992). In the Northeast, Lyme spirochetes are primarily maintained in white-footed mice (Peromyscus leucopus) and other small mammals by I. scapularis (Spielman et al. 1985). However, in the western United States, Peromyscus mice have low tick load and low prevalence of B. burgdorferi sensu lato (s. l.) (Brown and Lane 1992), whereas, the western gray squirrel (Sciurus griseus) and dusky-footed woodrat (Neotoma fuscipes) appear to be the predominant reservoir hosts of B. burgdorferi (Salkeld et al. 2008, 2014a, Salkeld and Lane 2010). I. pacificus mainly serves as a “bridge” vector of spirochetes to humans (Burgdorfer et al. 1985, Lane and Lavoie 1988, Clover and Lane 1995). In California, Borrelia species are also maintained among dusky-footed woodrats, California kangaroo rats (Dipodomys californicus), and other small mammals by Ixodes spinipalpis, Ixodes angustus, and Ixodes jellisoni, although they rarely bite humans (Burgdorfer et al. 1985, Brown and Lane 1992, Lane et al. 1994).
In addition to differences in hosts and tick vectors, the species and population of Lyme spirochetes are more diverse in California (Girard et al. 2009). Up to now, only four Borrelia species within B. burgdorferi s. l. have been found in the northeastern and upper midwestern United States: Borrelia andersonii, B. burgdorferi sensu stricto (s. s.), Borrelia kurtenbachii, and Borrelia mayonii, with B. burgdorferi s. s. representing the dominant species (Margos et al. 2010, 2014). Conversely, in addition to B. burgdorferi s. s., several species within the B. burgdorferi s. l. complex, including Borrelia americana (Rudenko et al. 2009), Borrelia bissettiae (Postic et al. 1998), Borrelia californensis (Postic et al. 2007), and Borrelia lanei (Margos et al. 2017) have been found in the western United States. B. burgdorferi s. s. is the only species associated with Lyme borreliosis: it contains two genetically distinct, but phylogenetically related populations in the Northeast and Midwest (Qiu et al. 2002, Hoen et al. 2009). However, the population structure of California strains of B. burgdorferi s. s. is more heterogeneous than the Northeast strains (Girard et al. 2009).
Several tick surveillance studies have been conducted to connect tick data with the risk of tick-borne disease in the western United States, including research by traditional tick flagging (Lane et al. 2004, Salkeld et al. 2014a, 2014b) and host trapping (Castro and Wright 2007). However, these studies generally do not link information about ticks and tick-borne diseases directly to human-tick encounters. A more detailed analysis relating the pathogen prevalence in different species of ticks parasitizing humans in the western United States is needed to better assess risk. To address this gap in knowledge, we report results of passive surveillance involving ticks that were submitted from the western United States and tested for pathogens by TickReport at the University of Massachusetts Amherst.
Materials and Methods
Ticks and morphological identification
TickReport is a public outreach service at the University of Massachusetts Amherst providing individuals with information about potential pathogen exposures associated with their tick bites and provides information about risk vis-a-vis the biting tick's species, its infection status with respect to several pathogen species, and an assessment of the tick's feeding status. Ticks analyzed in the present study were submitted to TickReport from July 2006 through August 2017. Orders were placed via an online form where submitters are asked to provide the location and date of tick collection; age, gender, and species of the host; and attachment site of the tick on the host's body (Xu et al. 2016).
The Ixodes genus-level identification of each tick was determined by morphological characterization of the anal groove, which is distinctively located anterior to the anus in all Ixodes ticks (Keirans and Clifford 1978).
DNA extraction and molecular identification
DNA was extracted from each tick using Epicenter Master Complete DNA and RNA Purification Kits (Epicenter Technologies, Madison, WI) following the manufacturer's protocols. Differentiation of I. scapularis and I. pacificus was performed by a species-specific TaqMan PCR assay (Table 1). Other Ixodes species were identified by amplifying and sequencing a fragment of the tick 16S rRNA gene (Table 1) (Krause et al. 2016, Xu et al. 2016).
Table 1.
Primers and Probes Used in This Study
| Target gene | Application | Type | Sequences (5′-3′) | Tm (C) | Reference |
|---|---|---|---|---|---|
| 16S | Tick species PCR and confirmation | Forward | TGCTGTAGTATTTTGACTATACAAAGG | 55 | This article |
| Reverse | ATCCTAATCCAACATCGAGGTC | ||||
| ITS | Ixodes scapularis identification | Forward | TGCGTTTTCTTTGAGCAAATGCACGAG | 60 | This article |
| Reverse | GTACGGGATTTTCCACAAACGGTATCCA | ||||
| Probe | TGCGCTTAACCAGTCCTCCTCCTCCTACGA | ||||
| ITS | Ixodes pacificus identification | Forward | CTCGGAGCAAGTACGGAGGTAG | 60 | This article |
| Reverse | TTTCCACAAAACGGTCGCCATC | ||||
| Probe | CTGAGCCAAGTCCTCTTCCTACCCGGTTTG | ||||
| P13 | EMLA detection | Forward | TACCTAATTCTTCTCAAGAGATTCAGTTG | 60 | This article |
| Reverse | ATGATGATACTGCGAACAACTATAAGAG | ||||
| Probe | ATATTGATAAAAGAGTCAGTGTTGATCCGTATGAGTTAGGGTT | ||||
| glpQ | Borrelia miyamotoi detection | Forward | GACATAGTTCTAACAAAGGACAATATTCC | 60 | Krause et al. (2016) |
| Reverse | TCCGTTTTCTCTAGCTCGATTGG | ||||
| Probe | TGCACGACCCAGAAATTGACACAACCACAA | ||||
| ospA | Borrelia burgdorferi Sensu Lato detection | Forward | ATAGGTCTAATATTAGCCTTAATAGCAT | 60 | This article |
| Reverse | AGATCGTACTTGCCGTCTT | ||||
| Probe | aagc+Aaa+Atgtt+Agc+Agccttga (LNA probe) | ||||
| Tubulin | Babesia detection | Forward | GATTTGGAACCTGGCACCATG | 60 | Xu et al. (2016) |
| Reverse | AATGACCCTTAGCCCAATTATTTCC | ||||
| Probe | ATCTGGCCCATACGGTGAATTGTTTCGC | ||||
| MSP-2 | Anaplasma detection | Forward | ATGGAAGGTAGTGTTGGTTATGGTATT | 60 | Xu et al. (2016) |
| Reverse | TTGGTCTTGAAGCGCTCGTA | ||||
| Probe | TGGTGCCAGGGTTGAGCTTGAGATTG |
EMLA, Ehrlichia muris-like agent.
B. burgdorferi s. l., B. miyamotoi, B. mayonii, B. microti, A. phagocytophilum, and Ehrlichia muris-like agent (EMLA) were detected by a multiplex TaqMan PCR assay targeting different genes (Table 1). Borrelia detection was performed by first applying a genus-specific detection assay for a conserved target, followed by specific qPCR assays for each of the three species (B. burgdorferi s. l., B. miyamotoi, B. mayonii). All B. burgdorferi s. l. isolates were further differentiated by multilocus sequence analysis (MLSA) using eight housekeeping loci (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA) (Margos et al. 2017).
Results
Tick species, geographical distributions, and seasonal activity
From July 2006 to August 2017, we received and identified 601 Ixodes ticks (58 from 2006 to 2013 and 543 from 2014 to 2017) from California, Oregon, and Washington, of which 549 (91.3%) had bitten humans, 43 were from dogs, 3 were from cats, and 6 were from lawns or unknown sources. The majority of submissions were received from 2014 to 2017. Analyses of ticks and associated pathogens were restricted to the 549 Ixodes ticks found on human subjects submitted from these three states (Table 2).
Table 2.
Pathogen Infection Rates Among Ixodes Ticks Submitted to TickReport Testing Service at UMass, Amherst
| No. of infected ticks/total (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tick species | State | N | Borrelia genus | BBSL | BOMI | BOMA | BAMI | ANPH | EMLA |
| Ixodes angustus (n = 21) | CA | 2 | — | — | — | — | — | — | — |
| OR | 1 | — | — | — | — | — | — | — | |
| WA | 18 | — | — | — | — | — | — | — | |
| Ixodes pacificus (n = 381) | CA | 302 | 6/302 (1.99) | 4/302 (1.32) | 3/302 (0.99) | — | — | 2/302 (0.66) | — |
| OR | 48 | 2/48 (4.17) | 1/48 (2.08) | 1/48 (2.08) | — | — | — | — | |
| WA | 31 | — | — | — | — | — | — | — | |
| Ixodes spinipalpis (n = 28) | CA | 13 | 1/13 (7.69) | 1/13 (7.69) | — | — | — | 2/13 (15.38) | — |
| OR | 3 | — | — | — | — | — | — | — | |
| WA | 12 | 3/12 (25.00) | 3/12 (25.00) | — | — | — | 1/12 (8.33) | — | |
| Endemic total | 430 | 12/430 (2.79) | 9/430 (2.09) | 4/430 (0.93) | — | — | 5/430 (1.16) | — | |
| Ixodes cookei (n = 1) | CA | 1 | — | — | — | — | — | — | — |
| Ixodes holocyclus (n = 1) | CA | 1 | — | — | — | — | — | — | — |
| Ixodes ricinus (n = 6) | CA | 6 | — | — | — | — | — | 1/6 (16.67) | — |
| Ixodes scapularis (n = 111) | CA | 71 | 18/71 (25.35) | 17/71 (23.94) | 1/71 (1.41) | — | 1/71 (1.41) | 3/71 (4.23) | — |
| OR | 18 | 6/18 (33.33) | 5/18 (27.78) | 1/18 (5.56) | — | 1/18 (5.56) | 2/18 (11.11) | — | |
| WA | 22 | 9/22 (40.91) | 8/22 (36.36) | 1/22 (4.55) | — | 2/22 (9.09) | 3/22 (13.64) | — | |
| Nonendemic total | 119 | 33/119 (27.73) | 30/119 (25.21) | 3/119 (2.52) | — | 4/119 (3.36) | 9/119 (7.56) | — | |
Tick species was determined unambiguously by DNA sequencing. One I. pacificus tick from CA was coinfected with Borrelia burgdorferi s. l. and Borrelia miyamotoi. For 119 nonendemic ticks, 107 tick bite victims had travel history to Midwest, east coast of the United States, or other countries.
“—”, Indicated no positive ticks were detected for the corresponding pathogen.
ANPH, Anaplasma phagocytophilum; BAMI, Babesia microti; BBSL, Borrelia burgdorferi sensu lato; BOMA, Borrelia mayonii; BOMI, Borrelia miyamotoi; EMLA, Ehrlichia muris-like agent.
Among the 549 human-biting Ixodes ticks, three species endemic to the western United States were identified: I. pacificus (N = 381: 2 larvae, 57 nymphs, 322 adults), I. spinipalpis (N = 28 nymphs), and I. angustus (N = 21: 17 nymphs, 4 adults). I. angustus and I. spinipalpis are considered occasional human biters (Merten and Durden 2000, Eisen, et al. 2006), however, 11.40% (49/430) of the 430 endemic ticks recovered from humans were I. angustus (4.88%, 21/430) and I. spinipalpis (6.52%, 28/430).
Surprisingly, we also found that 21.67% (119/549) of the ticks from California, Oregon, and Washington residents are not endemic to the western United States. These four tick species are as follows: I. scapularis (N = 111: 8 larvae, 47 nymphs, 56 adults), I. ricinus (N = 6: 5 nymphs, 1 adult), Ixodes cookei (N = 1 nymph), and Ixodes holocyclus (N = 1 adult). Among the 119 nonendemic ticks submitted, 107 (89.9%) bit people who reported in the TickReport order questionnaire, a travel history to the eastern or north-central United States or foreign countries. No travel history was volunteered for the remaining 12 individuals, but in all likelihood, these nonendemic ticks were also acquired during travel. Based on the travel history, it is clear that the 5 I. ricinus ticks and the single I. holocyclus tick were brought to the United States from Europe and Australia, respectively.
Over 94.2% of total ticks were submitted from Oregon, Northern California, and Washington. Only a small proportion, 5.8% (N = 25), of ticks were submitted from Southern California. For the three endemic tick species: 85.7% of I. angustus ticks were submitted from Washington; 79.3% of I. pacificus ticks were submitted from California; and 46.4% and 42.9% of I. spinipalpis ticks were submitted from California and Washington, respectively (Table 2).
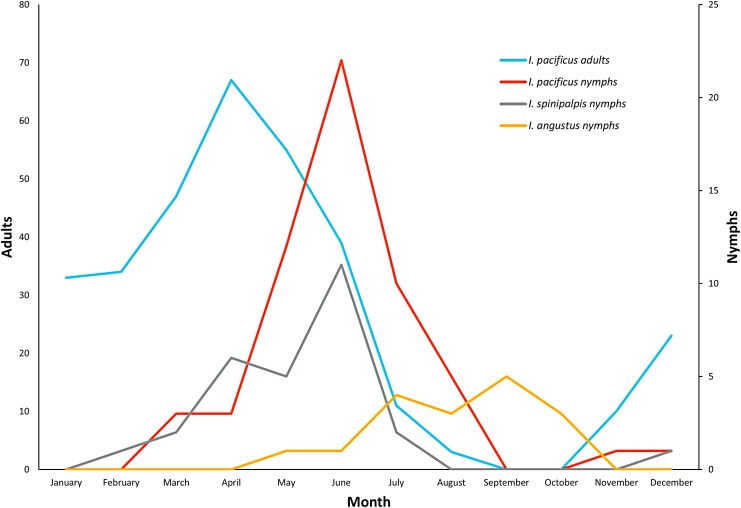
We received I. pacificus in every month of the year except September and October. The nymphal and adult I. pacificus exhibited different seasonal activity patterns (Fig. 1). We received 96.5% of nymphs between March and August, with a clear peak in June. Adult I. pacificus were abundant from November to July and then absent from September and October, with a peak in April (Fig. 1). We only found nymphal human-biting I. spinipalpis in this study. They were submitted between February and July, with a peak in June. Nymphal I. angustus were submitted from May to October, with a peak in September (Fig. 1).
FIG. 1.
Monthly submission of Ixodes pacificus (adults, nymphs), Ixodes spinipalpis (nymphs), and Ixodes angustus (nymphs) from July 2006 through August 2017.
Age distribution and tick attachment sites of tick bite victims
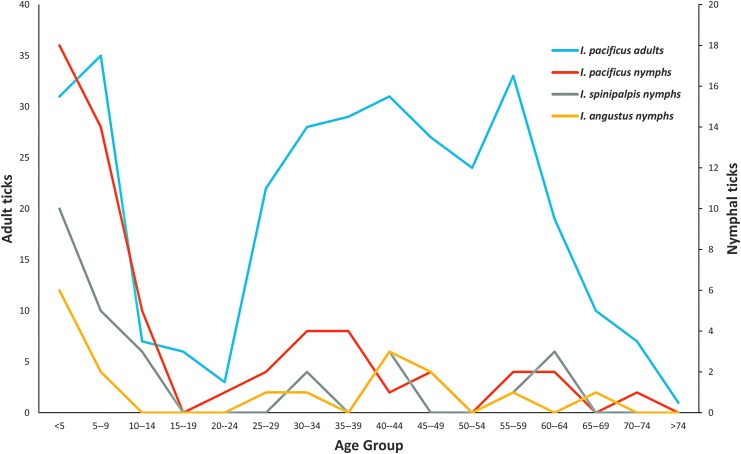
We received age data for 413 of the tick bite victims (Fig. 2). The age pattern was quite different for humans bit by I. pacificus nymphs compared to adults: 22% of attached adults and 57% of attached nymphs were found in children younger than 9 years. Adult ticks (70%) were commonly found among the age group of 25–64 years, however, only 30% of nymphs were found among this age. The individuals aged 0–9 years also had the largest proportion of I. spinipalpis and I. angustus submitted: 56% of I. spinipalpis nymphs and 47% of I. angustus nymphs (Fig. 2). The number of I. angustus adults was too small to compare age groups.
FIG. 2.
Age distribution of Ixodes pacificus (adults, nymphs), Ixodes spinipalpis (nymphs), and Ixodes angustus (nymphs) bite victims from July 2006 through August 2017.
The percentages of I. pacificus adults attached to the abdomen/groin, buttocks, chest, head, lower extremities, neck, shoulder/back, and upper extremities were 4% (A/G), 2% (B), 12% (C), 16% (H), 24% (LE), 4% (N), 24% (S/B), and 13% (UE), respectively. The percentages of I. pacificus nymphs attached to the same sites were 4% (A/G), 0% (B), 17% (C), 9% (H), 30% (LE), 11% (N), 6% (S/B), and 23% (UE), respectively. The percentages of I. spinipalpis nymphs attached to the same sites were 8% (A/G), 12% (B), 8% (C), 8% (H), 31% (LE), 15% (N), 12% (S/B), and 8% (UE), respectively. The percentages of I. angustus nymphs attached to the same sites were 8% (A/G), 0% (B), 8% (C), 23% (H), 38% (LE), 0% (N), 8% (S/B), and 15% (UE), respectively. It seems that all these three tick species prefer to attach to the lower extremities, including thigh, leg, ankle, and foot.
Prevalence of pathogens and MLSA of B. burgdorferi
B. burgdorferi s. l., B. miyamotoi, and A. phagocytophilum were detected in I. pacificus ticks: prevalence was 1.31% (5/381), 1.05% (4/381), and 0.52% (2/381), respectively. B. burgdorferi s. l. and A. phagocytophilum were detected in I. spinipalpis ticks: prevalence was 14.29% (4/28) and 10.71% (3/28), respectively (Table 2). The tick species, life stage, and location of these B. burgdorferi s. l. positive ticks can be found in Appendix Table A1. No pathogens were detected in I. angustus ticks. B. mayonii, B. microti, and EMLA were not detected in these three endemic tick species.
B. burgdorferi s. l., B. miyamotoi, B. microti, and A. phagocytophilum were detected in nonendemic I. scapularis ticks: prevalence was 27.03% (30/111), 2.70% (3/111), 3.60% (4/111), and 7.21% (8/111), respectively. A. phagocytophilum was also detected in the single I. ricinus tick. No pathogens were detected in the I. cookei and I. holocyclus ticks.
B. burgdorferi s. l. prevalence in I. pacificus nymphs was higher than in adults (3.5% vs. 0.9%). On the contrary, B. burgdorferi s. l. prevalence in I. scapularis nymphs was lower than that in adults (25.5% vs. 32.1%). B. burgdorferi s. l. prevalence was 14.29% (4/28) in I. spinipalpis nymphs, but no I. spinipalpis adults were submitted in this study.
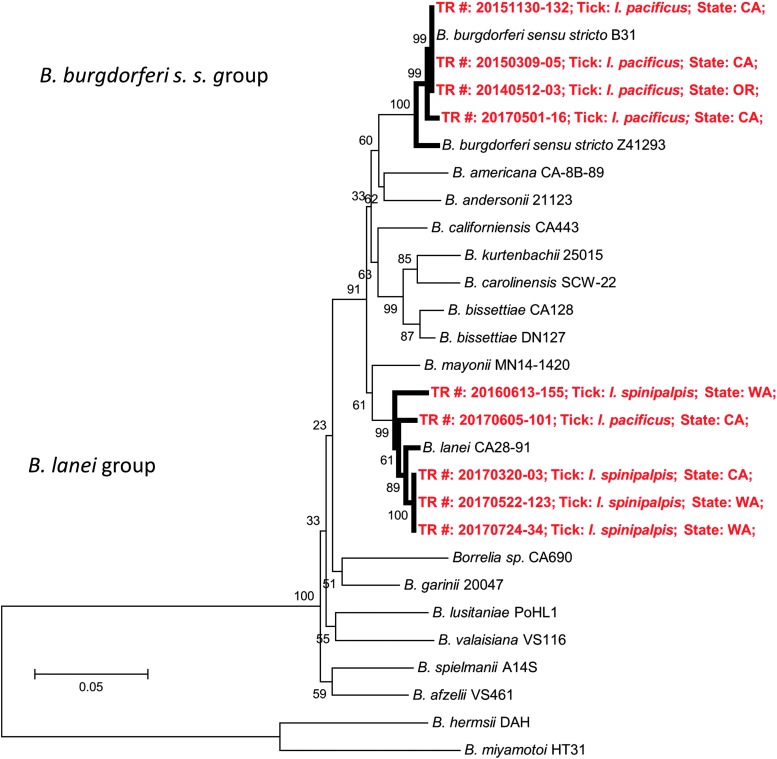
Based upon alignment from MLSA (GenBank accession nos. MH378169–MH378227), we concluded that two members of the B. burgdorferi s.l. complex were found in the present study: B. burgdorferi s. s. and the recently named B. lanei (genomospecies 2) (Schwan et al. 1993, Postic et al. 1994, Margos et al. 2017) (Fig. 3). Of the five Borrelia-infected I. pacificus ticks, four were infected with B. burgdorferi s. s., and one was infected with B. lanei. B. lanei was the only Borrelia species found in I. spinipalpis ticks.
FIG. 3.
Dendrogram showing only Borrelia burgdorferi s.s. and Borrelia lanei were detected within B. burgdorferi sensu lato in the present study. Reference Borrelia strains from GenBank are indicated by species (and strain where applicable). Isolates from the present study are represented by the tick species, in which they were detected (starting with TR#, red color). The dendrogram was constructed by minimum evolution method of MEGA 6 software with Borrelia relapsing fever group as outgroup. Numbers on the branches represent bootstrap support with 1000 bootstrap replicates.
Discussion
The levels of tick infestation, reservoir competence, and Borrelia infection prevalence vary widely among hosts in the western United States (Brown and Lane 1992, Eisen et al. 2004, Salkeld et al. 2008, Salkeld and Lane 2010). Passive tick surveillance studies have shown that the nymphal stage of I. pacificus is the primary vector of B. burgdorferi to humans in California (Clover and Lane 1995). Certain human behaviors, such as sitting on logs, can pose considerable risk of multiple exposures to nymphal ticks in this region (Lane et al. 2004). So too, travel history to the northeastern or upper midwestern United States impacts Lyme disease reporting from California patients diagnosed by an erythema migrans rash. Onset of the rash in patients exposed in California peaked in May–July, while the onset in patients (N = 219) with a travel history peaked in June–August (N = 177); reflecting differences in tick seasonal activity in each region (Salkeld et al. 2014a). Some tick species, such as I. spinipalpis and I. angustus are considered “rare” human biters (Damrow et al. 1989, Brown and Lane 1992, Merten and Durden 2000, Peavey et al. 2000), however, their encounter frequency and public health impact are yet to be determined.
In this study, we found that 21.67% of Ixodes ticks from California, Oregon, and Washington residents are not endemic to the western United States. These data indicate that tick species identification and travel history are critical factors for human-based tick surveillance in the western United States. Although I. pacificus and I. scapularis are closely related species, the acarologic risk of exposure to pathogens in these ticks is very different (Eisen et al. 2004, Hahn et al. 2018). Accuracy of species identification is challenging for several reasons. First, I. pacificus is very similar to I. scapularis morphologically. Second, most human-biting ticks are in poor condition, often with missing mouth parts, further complicating proper identification. Finally, the geographic distributions of these two species do not overlap (Eisen et al. 2016). Therefore, it is often assumed that a human-biting tick in a certain area is representative of the local tick species. In this study, we used real-time PCR and DNA sequencing to overcome these complications and accurately identify each sample to the species level. With proper species identification, we were able to conclude that nonendemic Ixodes ticks may influence local tick-borne disease due to human travel between nonoverlapping tick ranges.
Our data indicate that West Coast residents traveling to the northeast United States have a much higher risk of being bitten by an infected tick compared to nontraveling residents who are exposed to local tick populations. We found that 20.22% (111/549) of the total human-tick encounters were cases of I. scapularis ticks biting travelers. Second, B. burgdorferi s. l., B. miyamotoi, and A. phagocytophilum prevalence in I. scapularis is 20.6, 2.6, and 13.7 times higher, respectively, compared to I. pacificus (Table 2). We do not know how many tick-borne diseases were acquired from traveling for the West Coast residents. However, the 10-year data for humans with erythema migrans in California (2001–2011) showed two different frequency peaks: one is likely local exposure in May–July and another peak is likely travel-associated exposure in June–August (Salkeld et al. 2014). Assessing risk for tick-borne diseases in the western United States without accurate species identification and travel information would likely overestimate the number of local cases.
I. angustus and I. spinipalpis are normally considered nonhuman feeders but were experimentally demonstrated to be competent vectors of B. burgdorferi (Dolan et al. 1997, Merten and Durden 2000, Peavey et al. 2000). In this study, 11.40% (49/430) of the endemic ticks recovered from humans in the western United States were I. angustus and I. spinipalpis. We found 4.88% (21/430) of human-biting ticks were I. angustus. Both nymphal and adult I. angustus occasionally quest openly in moist and cool habitats, especially in coastal areas (Eisen et al. 2006). In this study, most I. angustus (14/21) were nymphs from Washington.
I. spinipalpis is more important than the human-biting “bridge” vector, I. pacificus, in maintaining the enzootic spirochete cycle in the western United States (Brown and Lane 1992, Oliver et al. 2003, Eisen et al. 2009). However, we found the human risk for exposure to I. spinipalpis and the pathogens it transmits may be underestimated. First, 6.52% (28/430) of the endemic ticks recovered from humans in the western United States were I. spinipalpis in this study. This species may use a primarily nidicolous host-seeking strategy in hot and dry climates, but commonly quest for hosts openly in moister habitats (Eisen et al. 2006). In some redwood-associated woodland sites in California, a larger number of openly host-seeking I. spinipalpis nymphs were collected by the dragging flag method, compared to I. pacificus (Eisen et al. 2006). All human-biting I. spinipalpis were nymphal ticks in this study. Second, the pathogen infection rate in I. spinipalpis was higher than that in I. pacificus: 14.29% (4/28) versus 1.31% (5/381) and 10.71% (3/28) versus 0.52% (2/381) for B. burgdorferi s. l. and A. phagocytophilum, respectively.
Although only B. burgdorferi s. s. and B. mayonii in B. burgdorferi s. l. complex have been culture confirmed as human pathogens in the United States (Steere et al. 1983, Pritt et al. 2016), several other species, including B. americana, B. bissettiae, B. burgdorferi s. s., Borrelia californiensis, and B. lanei, have been found in I. pacificus or I. spinipalpis ticks (Postic et al. 2007, Rudenko et al. 2009, Fedorova et al. 2014, Margos et al. 2017). Based upon results of MLSA in the present study, human-biting I. pacificus and I. spinipalpis are associated with only two species within the B. burgdorferi s. l. complex: B. burgdorferi s. s. and the recently named B. lanei (genomospecies 2) (Schwan et al. 1993, Postic et al. 1994, Margos et al. 2017) (Fig. 3). Although the Borrelia prevalence was 14.29% (4/28) in I. spinipalpis ticks, B. lanei was the only Borrelia species found in this tick species. B. lanei was recently found in I. spinipalpis ticks collected from rabbits in California and Canada, suggesting that rabbits may play an important role in the transmission cycle of B. lanei (Margos et al. 2017, Scott et al. 2017). It is well known that B. burgdorferi s. s. is pathogenic to humans in North America; however, human cases of B. lanei infection have not been found in the western United States (Margos et al. 2017). The vector role of I. pacificus and I. spinipalpis in the enzootic cycle of B. lanei and the pathogenicity of B. lanei to humans warrants further study.
The role of adult I. pacificus in disease transmission is not clear, however, we found adult ticks carry B. burgdorferi, B. miyamotoi, and A. phagocytophilum in California and Oregon. The nymphal stage of I. pacificus is the primary vector of Lyme disease in California (Clover and Lane 1995), but nymphs are difficult to detect and remove before repletion due to the tick's small body size (Xu et al. 2016). We found that a high rate (57%) of nymph bite-related encounters was in children aged 9 years and younger. Of these nymphal attacks, 96.5% of them were found between March and August, with a clear peak in June. We also received 56% of I. spinipalpis nymphs and 47% of I. angustus nymphs in victims aged 9 years and younger. In addition, we found that 48% of I. pacificus adults were attached to the lower extremities and back, whereas 30%, 31%, and 38% of I. pacificus, I. spinipalpis, and I. angustus nymphs were attached to the lower extremities. To help prevent tick-borne disease, it is critical to educate parents of young children to check for nymphal ticks during March–August in the West Coast, paying close attention to the lower extremities of young children.
Conclusions
We show that many nonendemic Ixodes ticks (119/549) submitted to the TickReport public testing program from the West Coast are most likely acquired from travel to a different geographic region. Traveling to an area where the tick population has a relatively higher pathogen infection rate increases the risk of exposure to infected ticks: as in the case of West Coast residents traveling to the northeast United States. For tick species that are endemic to the West Coast, we report cases of conventionally recognized nonhuman feeders (I. spinipalpis and I. angustus) parasitizing humans. Importantly, I. spinipalpis had the highest pathogen prevalence of the endemic species, indicating that it may pose a larger public health threat than previously thought. Furthermore, two species within the B. burgdorferi s. l. complex were detected in West Coast ticks; B. burgdorferi s. s. and B. lanei. B. lanei was detected in I. spinipalpis and I. pacificus ticks, corroborating previous reports. The vector role of I. pacificus and I. spinipalpis in the enzootic cycle of B. lanei and the pathogenicity of B. lanei to humans warrants further study. Seasonal activity, age distribution, and site of attachment data have yielded valuable public health information: for instance, young children are at an increased risk of tick bites, likely around their lower extremities, especially during the month of June when seasonal activity peaks for both nymphal I. pacificus and I. spinipalpis ticks. Public health professionals and physicians are encouraged to use this information to limit tick bites and tick-borne pathogens.
These results highlight the importance of passive surveillance of human-biting ticks and pathogens. Epidemiological case reports and/or entomological infection rate of questing ticks provide only proxies of human risk of tick-borne disease. Testing human-biting ticks allows assessment of the biological correlates of risk (tick species, degree of engorgement, and infection status) and the most reliable geographic distribution of that risk.
Acknowledgment
We thank Timothy Daly and Fumiko Ribbe for sample preparation and DNA extractions.
Appendix Table A1.
Borrelia Positive Samples from Human-Biting Endemic Ixodes Ticks in This Study
| TR ID no. | Tick species | Tick stage | Location | Tick removed date | Borrelia species |
|---|---|---|---|---|---|
| 20140512-03 | Ixodes pacificus | Adult female | Applegate, OR | May 5, 2014 | Borrelia burgdorferi sensu stricto |
| 20150309-05 | I. pacificus | Adult female | Santa Rosa, CA | March, 8, 2015 | B. burgdorferi sensu stricto |
| 20151130-132 | I. pacificus | Adult female | Santa Cruz Mountains, CA | November 29, 2015 | B. burgdorferi sensu stricto |
| 20170501-16 | I. pacificus | Nymph | Glen Ellen, CA | April 24, 2017 | B. burgdorferi sensu stricto and Borrelia miyamotoi |
| 20170605-101 | I. pacificus | Nymph | San Lorenzo, CA | May 29, 2017 | Borrelia lanei |
| 20160613-155 | Ixodes spinipalpis | Nymph | Gig Harbor, WA | June 11, 2016 | B. lanei |
| 20170320-03 | I. spinipalpis | Nymph | Half Moon Bay, CA | March 12, 2017 | B. lanei |
| 20170522-123 | I. spinipalpis | Nymph | Gig Harbor, WA | May 4, 2017 | B. lanei |
| 20170724-34 | I. spinipalpis | Nymph | Gig Harbor, WA | July 15, 2017 | B. lanei |
| 20150202-03 | I. pacificus | Adult female | Stanislaus County, CA | January 25, 2015 | Borrelia miyamotoi |
| 20170327-22 | I. pacificus | Adult female | Salinas, CA | March 26, 2017 | B. miyamotoi |
| 20170403-97 | I. pacificus | Adult female | Rogue River, OR | March 30, 2017 | B. miyamotoi |
The GenBank accession numbers of deposited MLSA sequences are MH378169–MH378227.
MLSA, multilocus sequence analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- Brown RN, Lane RS. Lyme disease in California: A novel enzootic transmission cycle of Borrelia burgdorferi. Science 1992; 256:1439–1442 [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Lane RS, Barbour AG, Gresbrink RA, et al. The western black-legged tick, Ixodes pacificus: A vector of Borrelia burgdorferi. Am J Trop Med Hyg 1985; 34:925–930 [DOI] [PubMed] [Google Scholar]
- Castro MB, Wright SA. Vertebrate hosts of Ixodes pacificus (Acari: Ixodidae) in California. J Vector Ecol 2007; 32:140–149 [DOI] [PubMed] [Google Scholar]
- Clover JR, Lane RS. Evidence Implicating nymphal Ixodes pacificus (Acari, Ixodidae) in the epidemiology of Lyme disease in California. Am J Trop Med Hyg 1995; 53:237–240 [DOI] [PubMed] [Google Scholar]
- Damrow T, Freedman H, Lane RS, Preston KL. Is Ixodes (Ixodiopsis) angustus a vector of Lyme disease in Washington State? West J Med 1989; 150:580–582 [PMC free article] [PubMed] [Google Scholar]
- Dolan MC, Maupin GO, Panella NA, Golde WT, et al. Vector competence of Ixodes scapularis, I. spinipalpis, and Dermacentor andersoni (Acari:Ixodidae) in transmitting Borrelia burgdorferi, the etiologic agent of Lyme disease. J Med Entomol 1997; 34:128–135 [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Chang CC, Mun J, et al. Acarologic risk of exposure to Borrelia burgdorferi spirochaetes: Long-term evaluations in north-western California, with implications for Lyme borreliosis risk-assessment models. Med Vet Entomol 2004; 18:38–49 [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Lane RS. Geographical distribution patterns and habitat suitability models for presence of host-seeking ixodid ticks in dense woodlands of Mendocino County, California. J Med Entomol 2006; 43:415–427 [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Mun J, Salkeld DJ, et al. Transmission cycles of Borrelia burgdorferi and B. bissettii in relation to habitat type in northwestern California. J Vector Ecol 2009; 34:81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol 2016; 53:349–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova N, Kleinjan JE, James D, Hui LT, et al. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick Borne Dis 2014; 5:951–961 [DOI] [PubMed] [Google Scholar]
- Girard YA, Travinsky B, Schotthoefer A, Fedorova N, et al. Population structure of the Lyme borreliosis spirochete Borrelia burgdorferi in the western black-legged tick (Ixodes pacificus) in northern California. Appl Environ Microbiol 2009; 75:7243–7252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Bjork JKH, Neitzel DF, Dorr FM, et al. Evaluating acarological risk for exposure to Ixodes scapularis and Ixodes scapularis-borne pathogens in recreational and residential settings in Washington County, Minnesota. Ticks Tick Borne Dis 2018; 9:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen AG, Margos G, Bent SJ, Diuk-Wasser MA, et al. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci U S A 2009; 106:15013–15018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Clifford CM. The genus Ixodes in the United States: A scanning electron microscope study and key to the adults. J Med Entomol Suppl 1978; 2:1–149 [DOI] [PubMed] [Google Scholar]
- Krause PJ, Schwab J, Narasimhan S, Brancato J, et al. Hard tick relapsing fever due to Borrelia miyamotoi in a child. Pediatr Infect Dis J 2016; 35:1352–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Brown RN, Piesman J, Peavey CA. Vector competence of Ixodes pacificus and Dermacentor occidentalis (Acari, Ixodidae) for various isolates of Lyme disease spirochetes. J Med Entomol 1994; 31:417–424 [DOI] [PubMed] [Google Scholar]
- Lane RS, Lavoie PE. Lyme borreliosis in California - acarological, clinical, and epidemiological studies. Ann N Y Acad Sci 1988;539:192–203 [DOI] [PubMed] [Google Scholar]
- Lane RS, Steinlein DB, Mun J. Human behaviors elevating exposure to Ixodes pacificus (Acari: Ixodidae) nymphs and their associated bacterial zoonotic agents in a hardwood forest. J Med Entomol 2004; 41:239–248 [DOI] [PubMed] [Google Scholar]
- Margos G, Fedorova N, Kleinjan JE, Hartberger C, et al. Borrelia lanei sp. nov. extends the diversity of Borrelia species in California. Int J Syst Evol Microbiol 2017; 67:3872–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Hojgaard A, Lane RS, Cornet M, et al. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis 2010; 1:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Piesman J, Lane RS, Ogden NH, et al. Borrelia kurtenbachii sp nov., a widely distributed member of the Borrelia burgdorferi sensu lato species complex in North America. Int J Syst Evol Microbiol 2014; 64:128–130 [DOI] [PubMed] [Google Scholar]
- Merten HA, Durden LA. A state-by-state survey of ticks recorded from humans in the United States. J Vector Ecol 2000; 25:102–113 [PubMed] [Google Scholar]
- Oliver JH, Jr., Lin T, Gao L, Clark KL, et al. An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc Natl Acad Sci U S A 2003; 100:11642–11645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavey CA, Lane RS, Damrow T. Vector competence of Ixodes angustus (Acari: Ixodidae) for Borrelia burgdorferi sensu stricto. Exp Appl Acarol 2000; 24:77–84 [DOI] [PubMed] [Google Scholar]
- Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol 2008; 53:323–343 [DOI] [PubMed] [Google Scholar]
- Postic D, Assous MV, Grimont PA, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol 1994; 44:743–752 [DOI] [PubMed] [Google Scholar]
- Postic D, Garnier M, Baranton G. Multilocus sequence analysis of atypical Borrelia burgdorferi sensu lato isolates - description of Borrelia californiensis sp nov., and genomospecies 1 and 2. Int J Med Microbiol 2007; 297:263–271 [DOI] [PubMed] [Google Scholar]
- Postic D, Ras NM, Lane RS, Hendson M, et al. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J Clin Microbiol 1998; 36:3497–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Mead PS, Johnson DKH, Neitzel DF, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect Dis 2016; 16:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics 2002; 160:833–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Lin T, Gao LH, et al. Delineation of a new species of the Borrelia burgdorferi sensu lato Complex, Borrelia americana sp nov. J Clin Microbiol 2009; 47:3875–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld DJ, Castro MB, Bonilla D, Kjemtrup A, et al. Seasonal activity patterns of the western black-legged tick, Ixodes pacificus, in relation to onset of human Lyme disease in northwestern California. Ticks Tick Borne Dis 2014a; 5:790–796 [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Cinkovich S, Nieto NC. Tick-borne pathogens in northwestern California, USA. Emerg Infect Dis 2014b; 20:493–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld DJ, Lane RS. Community ecology and disease risk: Lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology 2010; 91:293–298 [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Leonhard S, Girard YA, Hahn N, et al. Identifying the reservoir hosts of the Lyme disease spirochete Borrelia burgdorferi in California: The role of the western gray squirrel (Sciurus griseus). Am J Trop Med Hyg 2008; 79:535–540 [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Schrumpf ME, Karstens RH, Clover JR, et al. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J Clin Microbiol 1993; 31:3096–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, et al. Surveillance for Lyme Disease—United States, 2008–2015. MMWR Surveill Summ 2017; 66:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JD, Clark KL, Foley JE, Anderson JF, et al. Detection of Borrelia genomospecies 2 in Ixodes spinipalpis Ticks Collected from a Rabbit in Canada. J Parasitol 2017; 103:38–46 [DOI] [PubMed] [Google Scholar]
- Spielman A, Wilson ML, Levine JF, Piesman J. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol 1985; 30:439–460 [DOI] [PubMed] [Google Scholar]
- Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, et al. The spirochetal etiology of Lyme disease. N Engl J Med 1983; 308:733–740 [DOI] [PubMed] [Google Scholar]
- Xu G, Fang QQ, Keirans JE, Durden LA. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J Parasitol 2003; 89:452–457 [DOI] [PubMed] [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, Rich SM. Passive Surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vector Borne Zoonotic Dis 2016; 16:520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]