Abstract
Significance: NADPH oxidases (Noxs), of which there are seven isoforms (Nox1–5, Duox1/Duox2), are professional oxidases functioning as reactive oxygen species (ROS)-generating enzymes. ROS are signaling molecules important in physiological processes. Increased ROS production and altered redox signaling in the vascular system have been implicated in the pathophysiology of cardiovascular diseases, including hypertension, and have been attributed, in part, to increased Nox activity.
Recent Advances: Nox1, Nox2, Nox4, and Nox5 are expressed and functionally active in human vascular cells. While Nox1, Nox2, and Nox4 have been well characterized in models of cardiovascular disease, little is known about Nox5. This may relate to the lack of experimental models because rodents lack NOX5. However, recent studies have advanced the field by (i) elucidating mechanisms of Nox5 regulation, (ii) identifying Nox5 variants, (iii) characterizing Nox5 expression, and (iv) discovering the Nox5 crystal structure. Moreover, studies in human Nox5-expressing mice have highlighted a putative role for Nox5 in cardiovascular disease.
Critical Issues: Although growing evidence indicates a role for Nox-derived ROS in cardiovascular (patho)physiology, the exact function of each isoform remains unclear. This is especially true for Nox5.
Future Directions: Future directions should focus on clinically relevant studies to discover the functional significance of Noxs, and Nox5 in particular, in human health and disease. Two important recent studies will impact future directions. First, Nox5 is the first Nox to be crystallized. Second, a genome-wide association study identified Nox5 as a novel blood pressure-associated gene. These discoveries, together with advancements in Nox5 biology and biochemistry, will facilitate discovery of drugs that selectively target Noxs to interfere in uncontrolled ROS generation.
Keywords: Nox isoforms, vascular smooth muscle, endothelium, reactive oxygen species, oxidative stress, atherosclerosis
An Introduction to Reactive Oxygen Species and Cell Biology
Reactive oxygen species (ROS) are signaling molecules that influence gene expression, oxygen sensing, cell growth, and cell death. They define the cellular phenotype by regulating functions such as migration, contraction, differentiation, and secretion (109). ROS have been implicated in many biological processes, including immune cell activation, bone reabsorption, sperm–oocyte fusion, aging, and regulation of vascular tone (87, 129).
The most well-known function of ROS relates to host defense responses, where superoxide production by phagocytes plays a key role in killing of microorganisms (118). The phagocyte respiratory burst oxidase, NADPH oxidase (Nox), catalyzes NADPH-dependent reduction of molecular O2 to produce superoxide (NADPH +2O2 → NADP+ + H+ + 2O2−), which can dismute to generate secondary species, including hydrogen peroxide (H2O2) and hypochlorous acid, which together play a role in host defense by killing or damaging microbes (9, 111). Phagocytic Nox comprises two plasma membrane-associated subunits (gp91phox [also called Nox2] and p22phox), three cytosolic subunits (p47phox, p67phox, and p40phox), and a low-molecular-weight G protein (Rac1 or Rac2), which are disassociated in the resting state (77, 97). Upon stimulation, assembly of the plasma membrane subunits with cytosolic subunits and Rac1/2 leads to superoxide generation. The catalytic subunit is Nox2, which forms a transmembrane heterodimer with p22phox and functions as an electron transport chain. Phagocytic Nox has been very well described and its critical role in clinical medicine is highlighted in patients with chronic granulomatous disease (CGD) (14, 22), which is caused by a deficiency in one of the phox subunits. Patients with CGD are unable to generate superoxide and the disease is characterized by severe, and often fatal, infections that require bone marrow transplant to reduce clinical symptoms (43).
Nox, in particular Nox2, the Nox prototype (23, 25), was discovered in the 1960s, with studies demonstrating its functional activity in phagocytic cells such as neutrophils and monocytes/macrophages. Interestingly, some of the earliest studies actually identified Nox in nonphagocytic cells, including the liver, heart, and brain (9, 91, 98). However, it has only been in the past 20 years that the importance of nonphagocytic Nox-derived ROS in physiological and pathological processes has become evident. It is now clear that various cell types, including kidney cells, fibroblasts, adipocytes, spermatozoa, osteoclasts, thyroid, brain, colon, cardiomyocytes, and hepatocytes, as well as vascular cells, generate ROS (60, 69, 79, 89, 116, 122). Although many enzymatic sources, such as mitochondria, peroxisomes, and cytochrome P450, can produce ROS in these cells, Nox is especially important as its primary function is ROS generation, whereas other enzymes produce ROS as by-products of enzymatic activity (21).
Since the discovery of Nox2, six other Nox isoforms have been identified, including Nox1, Nox3, Nox4, Nox5, Duox1, and Duox2 (83, 115). The existence of multiple homologs of Nox2 in nonphagocytic cells/tissues suggests that production of ROS in these sites is distinct and different from host defense functions (6).
Although there has been enormous advancement in the field of Nox biology, the specific biological significance and mechanisms of regulation of the different Noxs still remain elusive (6). Rodent vascular, cardiac, and renal cells express Nox1, Nox2, and Nox4, and in humans, Nox5 is also present (64, 85, 107). These Noxs have been implicated in many redox-sensitive pathophysiological processes in the heart and vascular and renal systems (64, 85, 107) and have been suggested to be a cause of altered redox status in cardiovascular disease, including hypertension and atherosclerosis.
ROS and Hypertension
Mechanisms contributing to hypertension are complex, involving many pathological processes, including altered renal Na+ regulation, endothelial dysfunction, vascular remodeling, vascular hypercontractility, arterial calcification, immune cell activation, and inflammation (106, 114). Common to these processes is oxidative stress due to excessive Nox-derived ROS generation, reduced nitric oxide (NO) bioavailability, and decreased antioxidant capacity (31). Oxidative stress is associated with increased redox signaling, activation of transcription factors, stimulation of proinflammatory and profibrotic signaling pathways, induction of stress-related kinases and DNA damage, molecular processes that lead to cell damage, and vascular dysfunction (49).
Abnormal redox signaling and oxidative stress have been shown to play a role in development of hypertension in experimental models. In angiotensin II (Ang II)-induced hypertension in rodents, production of O2− and H2O2 is increased in vascular, renal, cardiac, and neural cells and antioxidant enzymatic activity is reduced (13, 26, 71, 95, 96). Oxidative stress has also been demonstrated in salt-sensitive forms of hypertension (29). Rats with deoxycorticosterone acetate salt-induced mineralocorticoid hypertension exhibit elevated vascular O2− production due to activation of Noxs and mitochondrial oxidases and uncoupling of endothelial nitric oxide synthase (110, 133). Nox1-dependent ROS production has also been demonstrated in experimental models of pulmonary hypertension (56) and seems to be especially important in arterial fibrosis and vascular aging in stroke-prone spontaneously hypertensive rats (52).
While extensive experimental data support a causative role for oxidative stress in hypertension, there is a paucity of evidence for a direct role of ROS in the pathophysiology of human hypertension (82, 84, 101). However, many clinical studies have demonstrated a positive association between blood pressure and plasma levels of biomarkers of oxidative stress, including malondialdehyde, F2-isoprostanes, and advanced oxidation protein products, and an inverse association between blood pressure and plasma antioxidants, including total antioxidant capacity, superoxide dismutase (SOD), and antioxidant vitamins (vitamins E and C) (35, 101, 120). Despite these strong suggestions linking oxidative stress and human hypertension, further studies are still required to unambiguously demonstrate a causal relationship. Moreover, to date, there are still no convincing data supporting a role for antioxidants in the prevention or treatment of essential hypertension (47, 104).
Of the many ROS-generating enzymes in the cardiovascular system, Noxs appear to be especially important in the pathophysiology of hypertension. A direct causal role for Noxs in the development of hypertension has been demonstrated in mice lacking Nox1, Nox2, Nox4, or p47phox where Ang II-induced endothelial dysfunction and hypertension are blunted (100, 124). While acute models of Ang II-mediated hypertension involve Nox activation, chronic models of Ang II-dependent hypertension seem to be independent of Nox1 and Nox2 (119, 131). Transgenic mice overexpressing Noxs in the vascular wall have exaggerated vascular and blood pressure responses to Ang II (100). In Ang II-infused mice overexpressing p22phox in a vascular smooth muscle cell-specific manner, vascular hypertrophy and pressor effects were exacerbated compared with control mice (125a). These mice also exhibited an increase in NO and H2O2 generation (65). Treatment of hypertensive mice and rats with various antioxidants, including vitamins C and E, SOD, and tempol, reduced vascular and systemic oxidative stress and decreased blood pressure (11, 127). Strategies to increase NO, including treatment with BH4, have been shown to have antihypertensive and vasoprotective effects in models of experimental and pulmonary hypertension (15, 105). Treatment of hypertensive mice with Nox inhibitors, such as apocynin, diphenyleneiodonium, gp91ds-tat, GKT137831, or antioxidants, to reduce ROS bioavailability improved vascular function and normalized blood pressure, further supporting an important link between Nox activation, oxidative stress, and blood pressure elevation (19, 123). With increasing interest in development of Nox isoform-specific inhibitors, the need to fully understand the differential role of Noxs in vascular oxidative stress is growing. We provide an overview on current knowledge regarding the role of p22phox-dependent Noxs (Nox1, Nox2, and Nox4) in the vascular system and discuss in detail the importance of p22phox-dependent Noxs, specifically Nox5, which is emerging as an important Nox isoform in human disease.
p22phox-Dependent Nox Isoforms in the Vascular System
p22phox, a nonglycosylated membrane-associated protein of molecular mass 22 kDa, associates with Nox in a 1:1 ratio (114). p22phox expression increases in response to Ang II and is upregulated in experimental diabetes and hypertension. Its major functions are to stabilize Nox proteins (Nox1, Nox2, Nox3, and Nox4) and to interact with cytosolic organizer subunits for Nox1, Nox2, and Nox3 (106). While Noxs 1–4 have an obligatory need for p22phox for activation, Nox5 does not require p22phox for activity (5).
Details about vascular Nox1, Nox2, and Nox4 have been extensively reviewed (8, 40, 68, 70, 90) and only an overview is given here for completeness. The message and protein for Nox1, Nox2, and Nox4 have been identified in arteries in rodents and humans. All Noxs are expressed to varying degrees in endothelial cells, vascular smooth muscle cells, perivascular adipocytes, and fibroblasts (36, 103, 112). Nox4 is abundant in endothelial cells. Nox1 and Nox2 are expressed in vascular smooth muscle cells, with Nox1 being predominant in large conduit arteries and Nox2 in peripheral arteries (68). Constitutively active Nox4 seems to play a role in basal generation of ROS.
The function of Nox-derived ROS in the vascular system is complex and depends on the target cell type, predominant Nox isoform, type of species generated, stimulating factor, and redox state. Ang II and other prohypertensive factors cause upregulation of vascular Nox1 and Nox2, important in redox-mediated hypertension in various experimental models. Vascular Nox1/2-derived ROS has also been suggested in atherosclerosis, diabetic vasculopathy, vascular remodeling, and aortic aneurysm (46, 113, 125, 126). The role of Nox4 is less clear because it has been shown to be both vasoinjurious and vasoprotective (8, 78), depending on experimental conditions. Endothelial Nox4 influences angiogenesis and vascular aging (67), and perivascular Nox4 is important in vascular remodeling in pulmonary hypertension (4). In ischemic or hypoxic conditions, endothelial Nox4 plays a role in blood–brain barrier breakdown and neural autotoxicity. Nox4−/− mice were protected from oxidative stress, blood–brain barrier leakage, and neuronal apoptosis in a stroke model (12), indicating a pathological role of Nox4 in this model. Nox4 is also important in fibrosis and cardiovascular remodeling (63).
On the other hand, emerging evidence indicates vasoprotective and antiatherosclerotic functions for endothelial and cardiomyocyte Nox4 because deletion of Nox4 in these cell types is associated with worse cardiovascular injury and endothelial dysfunction in experimental models of atherosclerosis, cardiac ischemia, and hypertension (86, 134). Reasons for the discrepant results likely relate to the site of Nox4 activation, type and site of ROS production, and experimental models studied.
The exact role of Nox4 in humans is still unclear, although clinical studies imply that Nox4 is important in fibrosis. Various drugs and small-molecule inhibitors targeting Nox4 in pathological fibrosis are under development (117). A Nox1/4 inhibitor, GKT136901, is currently in Phase 2 clinical trials for treating hepatic fibrosis (71). Whether such approaches will be beneficial in cardiovascular fibrosis and remodeling is unclear.
p22phox-Independent Nox Isoforms: Focus on Nox5
In addition to Noxs 1–4, three other Nox isoforms have been identified, including Nox5, Duox1, and Duox2, which share ≈50% homology with Nox2 (2, 27). These Noxs are unique, in that they possess a long NH2 terminus containing a Ca2+-binding EF-hand domain and do not require p22phox or cytosolic Nox subunits for their activation. Nox5 has a molecular mass of ≈85 kDa and does not seem to be glycosylated, whereas Duox1 and Duox2 have N-glycosylation sites and a molecular mass of 160–190 kDa (2). These Noxs are directly activated by Ca2+, which binds to the EF-hand Ca2+-binding domains, causing conformational change and activation of Nox to generate O2 (2, 3, 27).
Duox1/2
Duox1 and Duox2 were originally identified in the thyroid gland and are critically involved in redox-dependent synthesis of thyroid hormone (10). Duox1/2 are highly expressed in the thyroid gland, but Duox1 has also been identified in low levels in respiratory epithelium, while Duox2 has been identified in gastrointestinal glandular epithelium, salivary glands, and tumors (32, 128). Little is known about Duoxs in the cardiovascular system. A single study reported that aortic smooth muscle cells express low levels of Duox1 mRNA (59); however, the function is unclear. In the heart, Duox-mediated H2O2 generation has been observed in zebrafish (51), but to our knowledge, nothing is known about Duox in the mammalian heart.
Nox5
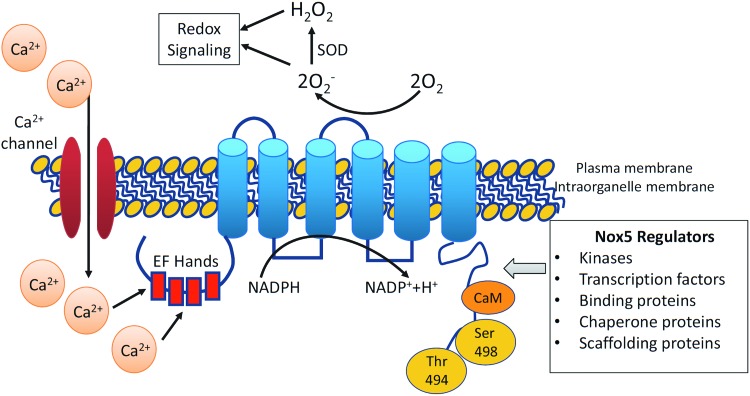
Nox5, of which there are multiple isoforms (Nox5α, -β, -γ, and -ɛ [also called Nox5S]), is the most recently discovered Nox and is unique (Fig. 1) (20). Nox5ɛ is a short form of Nox5 and lacks Ca2+-binding domains. Unlike other isoforms, the Nox5 gene is absent in rodents, it generates ROS from a single gene product, and does not require Nox subunits for its activation. Its long, intracellular N-terminal domain with EF-hands undergoes conformational changes upon Ca2+ binding. Increased cytoplasmic Ca2+ concentration ([Ca2+]i) is essential for Nox5 activation, as evidenced in cell studies where ROS are not generated in Ca2+-free buffer (20, 71). Because of its close association with Ca2+, Nox5 is likely to be involved in Ca2+-activated redox-dependent processes. Nox5 regulation also depends on its gene expression, subcellular localization, and post-translational modifications. Of all the Nox isoforms, only Nox5 has recently been crystallized (72).
FIG. 1.
Diagram demonstrating the structure and regulation of Nox5. Nox5 possesses a six-transmembrane domain, N-terminal domain with EF-hands, and C-terminal domain with phosphorylation sites. Nox5 is regulated by changes in intracellular Ca2+ levels, regulated, in part, by Ca2+ influx through Ca2+ channels. Nox5 is regulated by various kinases, transcription factors, and binding proteins, which may influence phosphorylation of Nox5. Nox5 activation generates O2−, which is dismutated by SOD to H2O2. O2− and H2O2 act as signaling molecules influencing redox-sensitive signaling pathways. H2O2, hydrogen peroxide; Nox, NADPH oxidase; O2−, superoxide; SOD, superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Nox5 Compartmentalization and Regulation
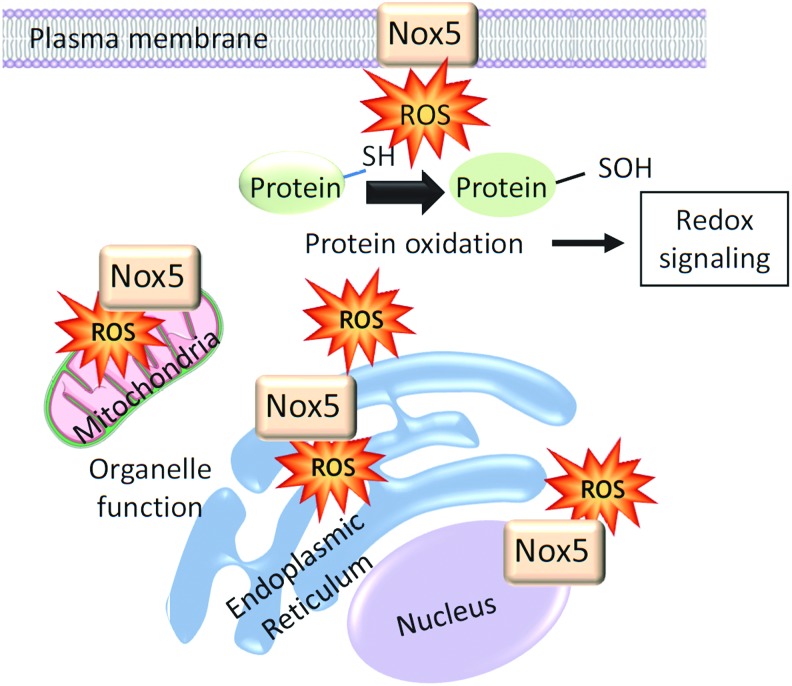
Unlike p22phox-regulated vascular Noxs (Nox1, Nox2, and Nox4), Nox5 is expressed primarily in intracellular compartments localized mainly in the perinuclear area and endoplasmic reticulum (ER) (Fig. 2) (66). In COS7-Nox5-transfected cells, Nox5 is also found in the Golgi and mitochondria, and in Nox5-overexpressing endothelial cells, Nox5 localizes in caveolae (66, 121). The significance of Nox5 in ER is unclear, but this is a site of Nox synthesis and post-translational modification. In addition, ER function is redox dependent and ER stress influences Nox5-ROS regulation (92). Hence, there may be bidirectional dependency. Nox5 seems to traffic from intracellular membranes to the plasma membrane where it may associate with cholesterol-rich microdomains, including caveolae and lipid rafts. The distinct intracellular distribution of Nox5 not only regulates ROS generation in a site-specific manner but also influences the function of redox-sensitive signaling molecules in close proximity, thereby controlling cell function.
FIG. 2.
Subcellular localization of Nox5. Nox5 has been identified in the plasma membrane as well as various intracellular locations. It is highly expressed in the perinuclear area and is also detected in mitochondria and ER. The site-specific location of Nox5 may influence the function of this isoform through close association with specific signaling molecules. Increased Nox5-induced generation of ROS leads to oxidation of signaling molecules that influence redox signaling. ER, endoplasmic reticulum; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In addition to its distinct subcellular localization, Nox5 activity is regulated, in part, by phosphorylation of serine/threonine (Ser475, Ser490, Ser494, and Ser498). Nox5 phosphorylation increases sensitivity to Ca2+, thereby facilitating ROS generation at lower Ca2+ levels (33). Numerous kinases, including protein kinase C (PKC)α, c-Abl, Ca2+/calmodulin-dependent protein kinase II (CAM kinase II), extracellular signal-regulated kinase 1/2 (ERK 1/2), and tyrosine, influence phosphorylation of Nox5 (Table 1) (16, 33, 108). Nox5 protein-binding partners, including Hsp90 and calmodulin, increase Nox5 activity, whereas binding to Hsp70 and caveolin-1 decreases Nox5 activity (16). Proinflammatory transcription factors, nuclear factor κB (NF-κB), AP-1 (activator protein 1), and signal transducer and activator of transcription 1/3 (STAT1/STAT3), which increase [Ca2+]i, also regulate Nox5 expression in human aortic smooth muscle cells (74).
Table 1. Regulators of Nox5.
| Stimulators | Inhibitors |
|---|---|
| Nox5-binding proteins/chaperone proteins | |
| Calmodulin | Cav-1 |
| Hsp90 | Hsp70 |
| CHIP | |
| Protein kinases | |
| PKCα | PKCɛ |
| CAM kinase II | |
| ERK1/2 | |
| c-Abl | |
| c-Src | |
| Transcription factors | |
| NF-κB | |
| AP-1 | |
| STAT1/STAT3 | |
AP-1, activator protein 1; CAM kinase II, Ca2+/calmodulin-dependent protein kinase II; ERK1/2, extracellular signal-regulated kinase1/2; NF-κB, nuclear factor κB; Nox, NADPH oxidase; PKC, protein kinase C; STAT, signal transducer and activator of transcription.
Nox5 is also modulated by other forms of post-translational modifications besides phosphorylation, including S-nitrosylation, SUMOylation (SUMO, small ubiquitin-related modifier), and palmitoylation (17). However, the significance of these different states of modification has yet to be elucidated.
Tissue Distribution and Cell Expression of Nox5
The Nox5 gene is expressed in a variety of fetal tissues and in adult testes, spleen, lymph nodes, pancreas, placenta, bone marrow, uterus, kidney, stomach, and cancer cells. It is also present in cells of the cardiovascular system, including cardiomyocytes, and endothelial and vascular smooth muscle cells (18, 73). In the spleen, Nox5 is abundant in regions of mature B cells and T lymphocytes. The presence of Nox5 in circulating lymphocytes is controversial because original studies failed to identify Nox5 in circulating cells (76), whereas recent studies suggest that Nox5 regulates human monocyte differentiation into dendritic cells (75). Human monocytes and macrophages were also found to express Nox5; however, studies were performed mainly in human leukemia cell line cells (leukemia cell line) (75). The function of Nox5 is still unclear and what is known is indirect and based on Nox5 expression patterns. Nevertheless, growing evidence suggests an important role for Nox5 in cardiovascular and renal (patho)physiology, as highlighted below.
Nox5 and Contraction
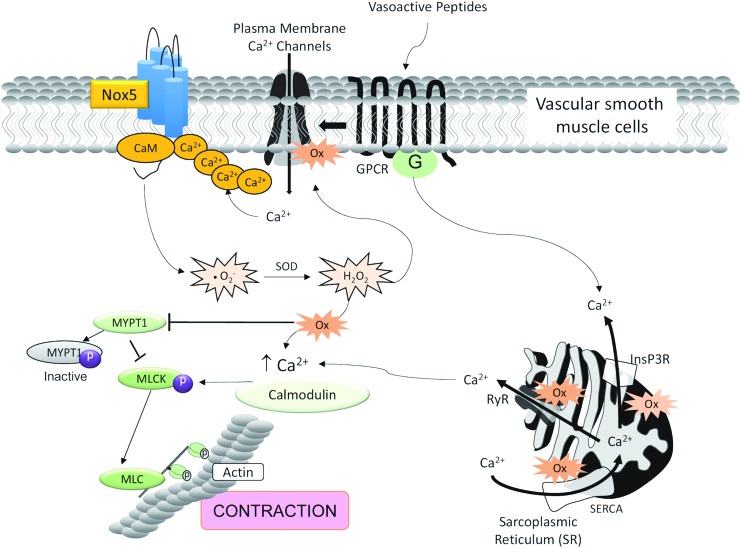
Considering the importance of [Ca2+]i in Nox5 regulation, and the critical role of Ca2+ in controlling the contractile machinery in muscle, we questioned whether Nox5 plays a role in vascular contraction. Our recent data show that in human vascular smooth muscle cells, Nox5 regulates procontractile signaling pathways, effects that are attenuated in siRNA Nox5-silenced human vascular smooth muscle cells (Fig. 3) (8, 81).
FIG. 3.
Possible role of Nox5 in vascular contraction. Nox5 may be a point of crosstalk between calcium and ROS, placing Nox5 as an important regulator of vascular contraction and development of vascular dysfunction in cardiovascular disease. Vasoactive factors, such as Ang II and ET-1, activate Nox5-induced ROS generation. Once ROS levels are increased in VSMCs, oxidation of calcium channels in the cell membrane or intracellular compartments, for example, ER, leads to dysregulated calcium influx and mobilization and activation of the contractile machinery—inhibition of MYPT1 and activation of MLCK and MLC, leading to contraction. Ang II, angiotensin II; ET-1, endothelin-1; MLC, myosin light chain; MLCK, myosin light chain kinase; MYPT1, myosin light chain phosphatase 1; VSMCs, vascular smooth muscle cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The possible role of Nox5 in contraction was previously highlighted in arthropod models, which express an ortholog of Nox5, called dNox (38, 99). Depletion of dNox in the musculature of Drosophila resulted in retention of mature eggs within ovaries, leading to female sterility (99). These effects were due to failure of ovarian muscles to contract in an agonist-dependent manner (99). Findings from this study highlighted the potentially important role of Nox5 in the regulation of smooth muscle contraction.
Function of Nox5 in Vascular and Renal Systems
Vascular Nox5
The expression and functional relevance of Nox5 and its isoforms in vessels remain unclear. In an attempt to address this, Pandey et al. (94) comprehensively characterized the expression and ROS-generating function of Nox5 and its splice variants in human arteries and veins. They found that mRNAs encoding Nox5α and Nox5β were present in isolated human internal mammary arteries and saphenous veins. However, unlike studies in cultured vascular cells, Nox5δ and Nox5γ were not detected in intact vessels and may reflect the absence of these Nox isoforms in blood vessels or possibly very low expression levels. Vascular Nox5α and Nox5β variants are catalytically active and generate ROS in both the endothelium and vascular media of arteries and veins.
In cultured human aortic endothelial cells, all Nox5 variants have been identified (107). However, only Nox5α and Nox5β seem to produce ROS (107). While Nox5δ, Nox5γ, and Nox5ɛ are expressed in cultured vascular cells, they are catalytically inactive, but associate with active Nox5 and function as dominant negatives by inhibiting ROS generation. In human microvascular endothelial cells, Nox5β and Nox5ɛ increased basal ROS levels, but in ionomycin-stimulated cells, only Nox5β was activated to generate O2− (80). Differential expression of Nox5 variants in human endothelial cells may reflect cellular heterogeneity between the aorta and microvessels.
In cultured human endothelial cells, Nox5 is regulated by Ca2+ and calmodulin, but not by Rac1 (109). Nox5 inactivates NO signaling and promotes phosphorylation of ERK1/2, c-Jun N-terminal kinases, P38 mitogen-activated protein kinase, and Janus kinase 2, inducing apoptosis, proliferation, migration, and angiogenesis (80). Nox5 also plays a role in thrombin-induced actin cytoskeleton derangement, monocyte adhesion, and migration in endothelial cells, effects that are inhibited by Ang-(1–7) through downregulation of Nox5-induced ROS generation (93). In cultured human vascular smooth muscle cells, Nox5 stimulates MAP kinase signaling and Ca2+-activated K+ channels and induces cell proliferation and migration (37).
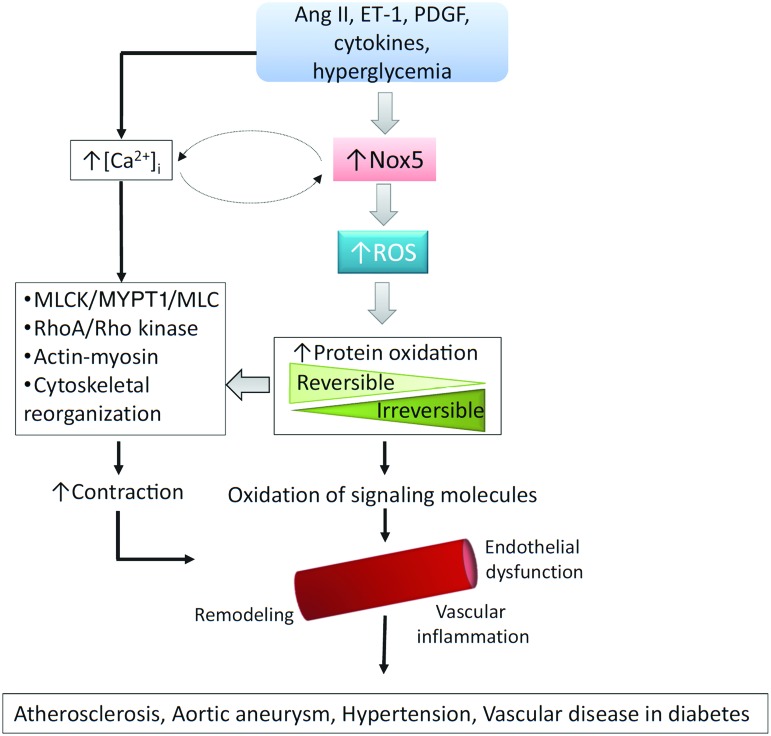
Of the Nox isoforms present in human vessels, Nox5 seems to be the major ROS-generating oxidase (58). In human vascular cells, Nox5 is activated by Ang II, endothelin-1 (ET-1), tumor necrosis factor-α, and platelet-derived growth factor (PDGF) and it plays an important role in agonist-stimulated O2− generation and redox signaling (80, 58) and has been implicated in vascular smooth muscle cell migration, proliferation, angiogenesis, inflammation, and contraction (Fig. 4). Human studies demonstrated increased vascular Nox5 expression in atherosclerosis, hypertension, myocardial infarction, and aortic aneurysm (58).
FIG. 4.
Schematic demonstrating vascular signaling effects of Nox5. Schematic demonstrating putative mechanisms whereby activation of Nox5 leads to vascular dysfunction, contraction, and injury in cardiovascular disease. Vasoactive peptides (Ang II and ET-1), growth factors, cytokines, and hyperglycemia induce Nox5 activation and increased levels of intracellular free Ca2+ ([Ca2+]i), which influence redox-sensitive and Ca2+-dependent signaling molecules associated with contraction, inflammation, growth, and endothelial function. Increased Nox5-mediated oxidative stress leads to increased protein oxidation (reversible and irreversible forms) and activation of signaling pathways that influence vascular function and structure in cardiovascular disease. PDGF, platelet-derived growth factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Renal Nox5
Nox5 is expressed in adult human kidneys and is upregulated in chronic kidney disease, including diabetic nephropathy (53). Nox5 has been identified in renal endothelial cells, mesangial cells, podocytes, tubular epithelial cells, and interstitial fibroblasts (44). In human diabetic glomeruli, Nox5 expression was increased compared with nondiabetic glomeruli. In human podocyte cultures, Ang II increased Nox5-induced ROS production, effects that were attenuated in siRNA-mediated Nox5 knockdown (42). Nox5 silencing in podocytes was associated with altered cytoskeletal dynamics and a Rac-mediated motile phenotype, with impaired podocyte function (54). Nox5 is also expressed in human tubule cells. Nox5 expression and Nox activity were increased in renal proximal tubule cells from hypertensive patients compared with cells from normotensive counterparts (132). This differential Nox5 expression in hypertension was attributed to an abnormal renal dopaminergic system (57, 132). Nox5 may also be important in sepsis-induced acute kidney injury, where its expression is markedly increased (41). This seems to be regulated by miR-4321 (41).
Insights from Mice Expressing the Human Nox5 Gene
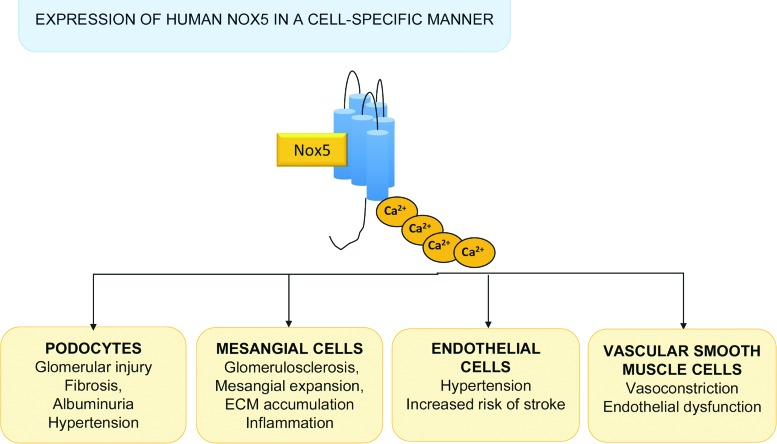
Genes encoding isoforms of Nox5 have been identified in invertebrates and vertebrates (38, 98). However, for unknown reasons, the Nox5 gene was lost in rodents during evolution. Hence, experimental data from mice or rats cannot be extrapolated to human physiology or disease, posing major challenges in Nox5 research. To address this, we generated transgenic mice expressing human Nox5 in a cell-specific manner. Nox5 transgenic mice with podocyte-specific expression of human Nox5 exhibited increased renal ROS production, glomerular injury and tubulointerstitial fibrosis, albuminuria, and elevated blood pressure, phenomena that are exacerbated in the presence of diabetes (Fig. 5) (44, 53). These mice also had podocyte foot process effacement, suggesting a deleterious role of Nox5 in podocyte damage and development of albuminuria in diabetic nephropathy. In mice expressing human Nox5 in vascular smooth muscle cells/renal mesangial cells and made diabetic with streptozotocin, glomerular ROS production was increased and this was associated with accelerated glomerulosclerosis, mesangial expansion, and extracellular matrix protein (collagen IV and fibronectin) accumulation (57). In addition, these mice exhibited renal inflammation as evidenced by increased macrophage infiltration and expression of the proinflammatory chemokine monocyte chemoattractant protein 1. Together, these studies in humanized Nox5 mice suggest an important role for Nox5 in vascular injury and progression of nephropathy in diabetes.
FIG. 5.
Insights from animals expressing Nox5. Studies in animals with inducible expression of human Nox5 in mice in a cell-specific manner demonstrated possible roles of Nox5 in the pathophysiology of cardiovascular diseases. Glomerular damage, kidney dysfunction, and hypertension were observed in mice expressing Nox5 specifically in podocytes. Nox5 expression in mesangial cells influenced processes related to fibrosis and inflammation. In endothelial cells, expression of Nox5 induced changes in blood pressure and increased risk of stroke. Nox5 in vascular smooth muscle cells is associated with vascular hypercontractility and endothelial dysfunction. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mice expressing human Nox5 in an endothelial-specific manner exhibited increased blood pressure and increased risk of stroke (Fig. 5) (61). This seemed to be especially evident in female mice. These studies suggested that targeting Nox5 may be vaso- and neuroprotective in ischemic stroke.
Nox5, Coronary Artery Disease, and Atherosclerosis
One of the earliest studies suggesting a link between vascular NOX5 and human cardiovascular disease was performed in coronary arteries in patients undergoing heart transplantation (48). In patients with coronary artery disease, expression of Nox5, both at the mRNA and protein levels, was markedly increased compared with those patients without coronary artery disease. Moreover, the magnitude of Nox5 expression and ROS generation correlated with the severity of atherosclerosis (48). Nox5 has also been identified in the heart and is expressed in endothelial and vascular smooth muscle cells in intramyocardial vessels and in cardiomyocytes. In infarcted hearts, Nox5 expression is increased, especially in infarctions older than 12 h (50).
The close association between Nox5 and Ca2+ may be especially important in the heart and coronary circulation where cardiovascular contractility is highly regulated. Studies in porcine coronary arteries demonstrated that Nox5 plays an important role in regulating the potassium intermediate/small-conductance calcium-activated channel, subfamily N member 4 (KCNN4), which seems to be important for coronary artery smooth muscle cell phenotypic modulation, contraction, and progression of atherosclerosis (45). AP-1 seems to be the link between Nox5 and KCNN4 in coronary arteries.
Nox5 and Hypertension
Although the exact role of Nox5 in the pathophysiology of human hypertension is unclear, findings show that mice expressing human Nox5 in the kidney and endothelium have elevated blood pressure, suggesting a potential role for Nox5 in blood pressure regulation (54, 57, 61, 132). The clinical relevance of this has recently been highlighted in a study searching for novel blood pressure-associated genes. In a genome-wide association study (GWAS) of 475,000 individuals, Nox5 was identified as an important blood pressure-associated gene, especially linked to systolic blood pressure (62). Comprehensive Nox5 phenotyping in patients with hypertension is needed to better clarify the potential importance of Nox5 as a pathophysiological factor in cardiovascular disease.
Nox5 Beyond the Cardiovascular System
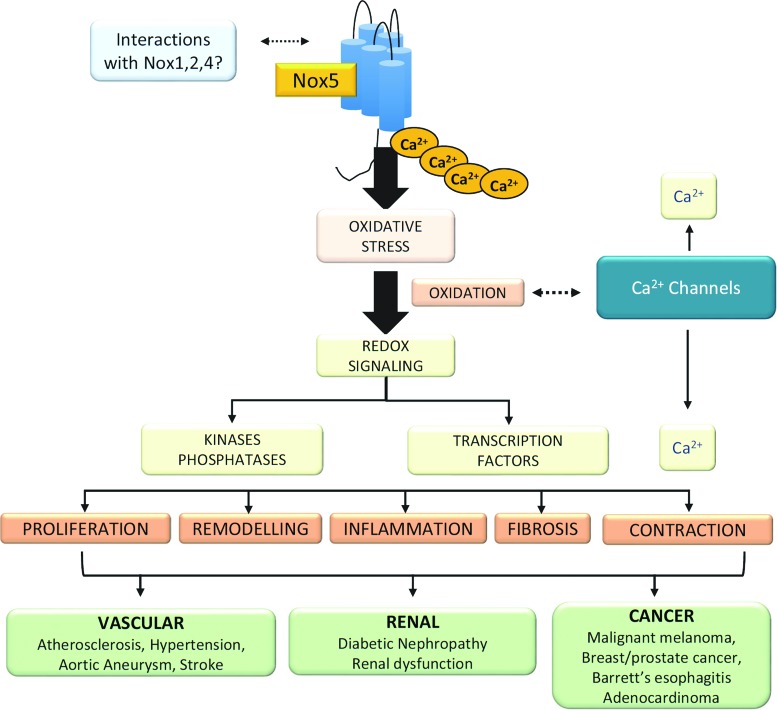
In addition to its potential role in cardiovascular (patho)physiology, Nox5 has been implicated to play an important role in various physiological and pathological processes (Fig. 6).
FIG. 6.
Nox5 and human disease. Nox5 is demonstrated to be a key regulator of calcium influx and signaling in many human cells. Nox5 regulates physiological signaling in cells from the cardiovascular, renal, and reproductive systems. Through ROS generation and oxidation, Nox5 influences signaling kinases, phosphatases, and transcription factors, resulting in contraction, inflammation, proliferation, fibrosis, and remodeling. These effects contribute to development of cardiovascular diseases, renal pathologies, and cancer. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Nox5 and regulation of sperm function
Nox5 seems to be critically involved in normal sperm function and motility, which are regulated by ROS (88). Nox5 is expressed in human spermatozoa and localizes in the flagella/neck region and acrosome. Stimulation of spermatozoa with a calcium ionophore, phorbol ester, or H2O2 increased sperm motility, effects that were dependent on Nox5-induced ROS generation (124, 125). These processes are regulated by the tyrosine kinase c-Abl and the Hv1 proton channel, which interact physically with Nox5 (2, 88).
Nox5 and cancer
Growing evidence indicates that Nox-derived ROS generation, either constitutively or as a result of chronic inflammation or cell stress, plays a role in proliferation of cancer cells and tumor growth (1, 24, 102). Nox1, Nox4, and Nox5 have been identified in various types of cancers. Increased Nox activity promotes redox-mediated DNA damage, tissue injury, dysregulated cell proliferation, adhesion, angiogenesis, and tumor growth (102). Nox5 is highly expressed in malignant melanomas, prostate cancer, breast cancer, and Barrett's esophagus-associated adenocarcinomas (28, 34, 130). Signaling pathways implicated in Nox-ROS-associated cancer involve protein kinases of the MAPK cascade, phosphatidylinositol-3-kinase, and PKC and transcription factors, including apurinic/apyrimidinic endonuclease 1/redox effector factor 1, hypoxia-inducible factor 1α, AP-1, nuclear factor erythroid 2-related factor 2, tumor protein 53, forkhead box protein, STAT, and β-catenin (39, 55). Similar redox-sensitive pathways have been identified in vascular hypertrophy associated with hypertension and atherosclerosis (11, 65, 120).
Conclusions
The importance of Noxs in ROS generation in the cardiovascular system and other systems is now clear. Nox isoforms are differentially regulated. Whereas Nox1, Nox2, and Nox4 have an obligatory need for p22phox for their activation to generate ROS, Nox5 is p22phox independent and produces O2− in a constitutive and inducible manner. Experimental models have identified a role for Nox1, Nox2, and Nox4 in various cardiovascular pathologies, including hypertension, atherosclerosis, cardiovascular and renal complications of diabetes, and pulmonary hypertension. While there have been advancements in the molecular biology and biochemistry of Nox5, there is a paucity of information on its pathophysiological role in humans. This is reflected by the relatively few publications on Nox5 (164 original articles with “Nox5” as keyword since its discovery in 2001) and may relate, in part, to the lack of rodent experimental models that express Nox5. However, studies in transgenic mice that express human Nox5 and investigations in larger mammals that contain Nox5 endogenously, including rabbits and primates, will shed light on the physiological and pathological significance of Nox5. Moreover, studies in humans in health and disease will unravel the clinical role of Nox5. The recent GWAS highlighting the association between the Nox5 gene and hypertension is certainly interesting and provides a credible foundation for further investigation of Nox5 in human cardiovascular disease.
Acknowledgments
The authors are funded by grants from the British Heart Foundation (BHF) (RG/13/7/30099 and RE/13/5/30177) and the MRC (MC-PC-15076). R.M.T. is supported through a BHF Chair Award (CH/12/29762).
Abbreviations Used
- Ang II
angiotensin II
- AP-1
activator protein 1
- CAM kinase II
Ca2+/calmodulin-dependent protein kinase II
- CGD
chronic granulomatous disease
- ER
endoplasmic reticulum
- ERK1/2
extracellular signal-regulated kinase1/2
- ET-1
endothelin-1
- GWAS
genome-wide association study
- H2O2
hydrogen peroxide
- KCNN4
potassium intermediate/small-conductance calcium-activated channel, subfamily N, member 4
- NF-κB
nuclear factor κB
- NO
nitric oxide
- Nox
NADPH oxidase
- PDGF
platelet-derived growth factor
- PKC
protein kinase C
- ROS
reactive oxygen species
- STAT
signal transducer and activator of transcription
References
- 1.Antony S, Jiang G, Wu Y, Meitzler JL, Makhlouf HR, Haines DC, Butcher D, Hoon DS, Ji J, Zhang Y, Juhasz A, Lu J, Liu H, Dahan I, Konate M, Roy KK, and Doroshow JH. NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates normoxic HIF-1α and p27Kip1 expression in malignant melanoma and other human tumors. Mol Carcinog 56: 2643–2662, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bánfi B, Molnár G, Maturana A, Steger K, Hegedûs B, Demaurex N, and Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bánfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnár GZ, Krause KH, and Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279: 18583–18591, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Barman SA. and Fulton D. Adventitial fibroblast Nox4 expression and ROS signaling in pulmonary arterial hypertension. Adv Exp Med Biol 967: 1–11, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Bedard K, Jaquet V, and Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52: 725–734, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bedard K, Lardy B, and Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie 89: 1107–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 7.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, and Görlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Burtenshaw D, Hakimjavadi R, Redmond EM, and Cahill PA. Nox, reactive oxygen species and regulation of vascular cell fate. Antioxidants (Basel) 6: E90, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cachat J, Deffert C, Hugues S, and Krause KH. Phagocyte NADPH oxidase and specific immunity. Clin Sci (Lond) 128: 635–648, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Caillou B, Dupuy C, Lacroix L, Nocera M, Talbot M, Ohayon R, Dème D, Bidart JM, Schlumberger M, and Virion A. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab 86: 3351–3358, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Carlström M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, and Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension 56: 907–913, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casas AI, Geuss E, Kleikers PWM, Mencl S, Herrmann AM, Buendia I, Egea J, Meuth SG, Lopez MG, Kleinschnitz C, and Schmidt HHHW. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc Natl Acad Sci U S A 114: 12315–12320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Case AJ, Tian J, and Zimmerman MC. Increased mitochondrial superoxide in the brain, but not periphery, sensitizes mice to angiotensin II-mediated hypertension. Redox Biol 11: 82–90, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casimir C, Chetty M, Bohler MC, Garcia R, Fischer A, Griscelli C, Johnson B, and Segal AW. Identification of the defective NADPH-oxidase component in chronic granulomatous disease: a study of 57 European families. Eur J Clin Invest 22: 403–406, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Chang P, Wang Q, Xu H, Yang M, Lin X, Li X, Zhang Z, Zhang X, Zhao F, Zhao X, Bai F, and Yu J. Tetrahydrobiopterin reverse left ventricular hypertrophy and diastolic dysfunction through the PI3K/p-Akt pathway in spontaneously hypertensive rats. Biochem Biophys Res Commun 463: 1012–1020, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Haigh S, Yu Y, Benson T, Wang Y, Li X, Dou H, Bagi Z, Verin AD, Stepp DW, Csanyi G, Chadli A, Weintraub NL, Smith SM, and Fulton DJ. Nox5 stability and superoxide production is regulated by C-terminal binding of sp90 and CO-chaperones. Free Radic Biol Med 89: 793–805, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen F, Wang Y, Barman S, and Fulton DJ. Enzymatic regulation and functional relevance of NOX5. Curr Pharm Des 21: 5999–6008, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Yin C, Dimitropoulou C, and Fulton DJ. Cloning, characteristics, and functional analysis of rabbit NADPH oxidase 5. Front Physiol 7: 284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Touyz RM, Park JB, and Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 38(Pt 2): 606–611, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Cross AR. and Segal AW. The NADPH oxidase of professional phagocytes-prototype of the NOX electron transport chain systems. Biochim Biophys Acta 1657: 1–22, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curnutte JT, Whitten DM, and Babior BM. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med 290: 593–597, 1974 [DOI] [PubMed] [Google Scholar]
- 23.Dahan I, Smith SM, and Pick E. A Cys–Gly–Cys triad in the dehydrogenase region of Nox2 plays a key role in the interaction with p67phox. J Leukoc Biol 98: 859–874, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, and Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 174: 1670–1689, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang PM, Cross AR, and Babior BM. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558. Proc Natl Acad Sci U S A 98: 3001–3005, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, and Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25: 2106–2113, 2005 [DOI] [PubMed] [Google Scholar]
- 27.De Deken X, Corvilain B, Dumont JE, and Miot F. Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid Redox Signal 20: 2776–2793, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Dho SH, Kim JY, Lee KP, Kwon ES, Lim JC, Kim CJ, Jeong D, and Kwon KS. STAT5A-mediated NOX5-L expression promotes the proliferation and metastasis of breast cancer cells. Exp Cell Res 351: 51–58, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Diao Z, Asico LD, Villar VAM, Zheng X, Cuevas S, Armando I, Jose PA, and Wang X. Increased renal oxidative stress in salt-sensitive human GRK4γ486V transgenic mice. Free Radic Biol Med 106: 80–90, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.This reference has been deleted. [Google Scholar]
- 31.Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertrán E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, My-Chan Dang P, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Förstermann U, Giniatullin R, Giricz Z, Görbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustín P, Hillion M, Huang J, Ilikay S, Jansen-Dürr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz LO, Korac B, Korkmaz KS, Koziel R, Kračun D, Krause KH, Křen V, Krieg T, Laranjinha J, Lazou A, Li H, Martínez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milković L, Miranda-Vizuete A, Mojović M, Monsalve M, Mouthuy PA, Mulvey J, Münzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavićević A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Antón N, Rodríguez-Mañas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schröder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanić V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentová K, Van Breusegem F, Varisli L, Veal EA, Yalçın AS, Yelisyeyeva O, Žarković N, Zatloukalová M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt HHHW, Di Lisa F, and Daiber A. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol 13: 94–162, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noël-Hudson MS, Bidart JM, Schlumberger M, Virion A, and Dupuy C. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol 288: G933–G942, 2005 [DOI] [PubMed] [Google Scholar]
- 33.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, and Clark RA. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med 44: 868–881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eun HS, Cho SY, Joo JS, Kang SH, Moon HS, Lee ES, Kim SH, and Lee BS. Gene expression of NOX family members and their clinical significance in hepatocellular carcinoma. Sci Rep 7: 11060, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Förstermann U, Xia N, and Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 120: 713–735, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Friederich-Persson M, Nguyen Dinh Cat A, Persson P, Montezano AC, and Touyz RM. Brown adipose tissue regulates small artery function through NADPH oxidase 4-derived hydrogen peroxide and redox-sensitive protein kinase G-1α. Arterioscler Thromb Vasc Biol 37: 455–465, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandara ACP, Torres A, Bahia AC, Oliveira PL, and Schama R. Evolutionary origin and function of NOX4-art, an arthropod specific NADPH oxidase. BMC Evol Biol 17: 92, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gào X. and Schöttker B. Reduction-oxidation pathways involved in cancer development: a systematic review of literature reviews. Oncotarget 8: 51888–51906, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Redondo AB, Aguado A, Briones AM, and Salaices M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol Res 114: 110–120, 2016. 27773825 [Google Scholar]
- 41.Ge QM, Huang CM, Zhu XY, Bian F, and Pan SM. Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS One 12: e0173292, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghanbari H, Keshtgar S, and Kazeroni M. Inhibition of the CatSper channel and NOX5 enzyme activity affects the functions of the progesterone-stimulated human sperm. Iran J Med Sci 43: 18–25, 2018 [PMC free article] [PubMed] [Google Scholar]
- 43.Giardino G, Cicalese MP, Delmonte O, Migliavacca M, Palterer B, Loffredo L, Cirillo E, Gallo V, Violi F, and Pignata C. NADPH oxidase deficiency: a multisystem approach. Oxid Med Cell Longev 2017: 4590127, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill PS. and Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Gole HK, Tharp DL, and Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5). PLoS One 9: e105337, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray SP. and Jandeleit-Dahm KA. The role of NADPH oxidase in vascular disease-hypertension, atherosclerosis and stroke. Curr Pharm Des 21: 5933–5944, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen YR, Harrison DG, Bhatnagar A; American Heart Association Council on Basic Cardiovascular Sciences. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res 119: e39–e75, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase ontributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzik TJ. and Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 70: 660–667, 2017 [DOI] [PubMed] [Google Scholar]
- 50.Hahn NE, Meischl C, Kawahara T, Musters RJ, Verhoef VM, van der Velden J, Vonk AB, Paulus WJ, van Rossum AC, Niessen HW, and Krijnen PA. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am J Pathol 180: 2222–2229, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Han P, Zhou XH, Chang N, Xiao CL, Yan S, Ren H, Yang XZ, Zhang ML, Wu Q, Tang B, Diao JP, Zhu X, Zhang C, Li CY, Cheng H, and Xiong JW. Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res 24: 1091–1107, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey AP, Montezano AC, Hood KY, Lopes RA, Rios F, Ceravolo G, Graham D, and Touyz RM. Vascular dysfunction and fibrosis in stroke-prone spontaneously hypertensive rats: the aldosterone-mineralocorticoid receptor-Nox1 axis. Life Sci 179: 110–119, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holterman CE, Thibodeau JF, and Kennedy CR. NADPH oxidase 5 and renal disease. Curr Opin Nephrol Hypertens 24: 81–87, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, and Kennedy CR. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol 25: 784–797, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong J, Li D, and Cao W. Rho kinase ROCK2 mediates acid-induced NADPH oxidase NOX5-S expression in human esophageal adenocarcinoma cells. PLoS One 11: e0149735, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hood KY, Montezano AC, Harvey AP, Nilsen M, MacLean MR, and Touyz RM. Nicotinamide adenine dinucleotide phosphate oxidase-mediated redox signaling and vascular remodeling by 16α-hydroxyestrone in human pulmonary artery cells: implications in pulmonary arterial hypertension. Hypertension 68: 796–808, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, Thallas-Bonke V, De Vos L, Holterman CE, Coughlan MT, Power DA, Skene A, Ekinci EI, Cooper ME, Touyz RM, Kennedy CR, and Jandeleit-Dahm K. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes 66: 2691–2703, 2017 [DOI] [PubMed] [Google Scholar]
- 58.Jha JC, Watson AMD, Mathew G, de Vos LC, and Jandeleit-Dahm K. The emerging role of NADPH oxidase NOX5 in vascular disease. Clin Sci (Lond) 131:981–990, 2017 [DOI] [PubMed] [Google Scholar]
- 59.Kalinina N, Agrotis A, Tararak E, Antropova Y, Kanellakis P, Ilyinskaya O, Quinn MT, Smirnov V, and Bobik A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler Thromb Vasc Biol 22:2037–2043, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Kang IS. and Kim C. NADPH oxidase gp91phox contributes to RANKL-induced osteoclast differentiation by upregulating NFATc1. Sci Rep 6: 38014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleikers PW, Dao VT, Göb E, Hooijmans C, Debets J, van Essen H, Kleinschnitz C, and Schmidt HH. SFRR-E Young Investigator Awardee. NOXing out stroke: identification of NOX4 and 5as targets in blood–brain-barrier stabilisation and neuroprotection. Free Radic Biol Med 75(Suppl 1): S16, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Kraja AT, Cook JP, Warren HR, Surendran P, Liu C, Evangelou E, Manning AK, Grarup N, Drenos F, Sim X, Smith AV, Amin N, Blakemore AIF, Bork-Jensen J, Brandslund I, Farmaki AE, Fava C, Ferreira T, Herzig KH, Giri A, Giulianini F, Grove ML, Guo X, Harris SE, Have CT, Havulinna AS, Zhang H, Jørgensen ME, Käräjämäki A, Kooperberg C, Linneberg A, Little L, Liu Y, Bonnycastle LL, Lu Y, Mägi R, Mahajan A, Malerba G, Marioni RE, Mei H, Menni C, Morrison AC, Padmanabhan S, Palmas W, Poveda A, Rauramaa R, Rayner NW, Riaz M, Rice K, Richard MA, Smith JA, Southam L, Stančáková A, Stirrups KE, Tragante V, Tuomi T, Tzoulaki I, Varga TV, Weiss S, Yiorkas AM, Young R, Zhang W, Barnes MR, Cabrera CP, Gao H, Boehnke M, Boerwinkle E, Chambers JC, Connell JM, Christensen CK, de Boer RA, Deary IJ, Dedoussis G, Deloukas P, Dominiczak AF, Dörr M, Joehanes R, Edwards TL, Esko T, Fornage M, Franceschini N, Franks PW, Gambaro G, Groop L, Hallmans G, Hansen T, Hayward C, Heikki O, Ingelsson E, Tuomilehto J, Jarvelin MR, Kardia SLR, Karpe F, Kooner JS, Lakka TA, Langenberg C, Lind L, Loos RJF, Laakso M, McCarthy MI, Melander O, Mohlke KL, Morris AP, Palmer CNA, Pedersen O, Polasek O, Poulter NR, Province MA, Psaty BM, Ridker PM, Rotter JI, Rudan I, Salomaa V, Samani NJ, Sever PJ, Skaaby T, Stafford JM, Starr JM, van der Harst P, van der Meer P, Understanding Society Scientific Group, van Duijn CM, Vergnaud AC, Gudnason V, Wareham NJ, Wilson JG, Willer CJ, Witte DR, Zeggini E, Saleheen D, Butterworth AS, Danesh J, Asselbergs FW, Wain LV, Ehret GB, Chasman DI, Caulfield MJ, Elliott P, Lindgren CM, Levy D, Newton-Cheh C, Munroe PB, Howson JMM; CHARGE EXOME BP, CHD Exome+, Exome BP, GoT2D:T2DGenes Consortia, The UK Biobank Cardio-Metabolic Traits Consortium Blood Pressure Working Group. New blood pressure-associated loci identified in meta-analyses of 475 000 individuals. Circ Cardiovasc Genet 10: e001778, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lan T, Kisseleva T. and Brenner DA. Deficiency of NOX1 or NOX4 prevents liver inflammation and fibrosis in mice through inhibition of hepatic stellate cell activation. PLoS One 10: e0129743, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lassègue B, San Martín A, and Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, and Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol 288: H7–H12, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Laurindo FR, Araujo TL, and Abrahão TB. Nox NADPH oxidases and the endoplasmic reticulum. Antioxid Redox Signal 20: 2755–2775, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HY, Zeeshan HMA, Kim HR, and Chae HJ. Nox4 regulates the eNOS uncoupling process in aging endothelial cells. Free Radic Biol Med 113: 26–35, 2017 [DOI] [PubMed] [Google Scholar]
- 68.Li Y. and Pagano PJ. Microvascular NADPH oxidase in health and disease. Free Radic Biol Med 109: 33–47, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang S, Kisseleva T, and Brenner DA. The role of NADPH oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front Physiol 7: 17, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liao Y, Gou L, Chen L, Zhong X, Zhang D, Zhu H, Lu X, Zeng T, Deng X, and Li Y. NADPH oxidase 4 and endothelial nitric oxide synthase contribute to endothelial dysfunction mediated by histone methylations in metabolic mory. Free Radic Biol Med 115: 383–394, 2018 [DOI] [PubMed] [Google Scholar]
- 71.Lopes RA, Neves KB, Tostes RC, Montezano AC, and Touyz RM. Downregulation of nuclear factor erythroid 2-related factor and associated antioxidant genes contributes to redox-sensitive vascular dysfunction in hypertension. Hypertension 66: 1240–1250, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Magnani F, Nenci S, Millana Fananas E, Ceccon M, Romero E, Fraaije MW, and Mattevi A. Crystal structures and atomic model of NADPH oxidase. Proc Natl Acad Sci U S A 114: 6764–6769, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahbouli S, Der Vartanian A, Ortega S, Rougé S, Vasson MP, and Rossary A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol Rep 38:3254–3264, 2017 [DOI] [PubMed] [Google Scholar]
- 74.Manea A, Manea SA, Florea IC, Luca CM, and Raicu M. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic Biol Med 52: 1497–1507, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Manea A, Manea SA, Gan AM, Constantin A, Fenyo IM, Raicu M, Muresian H, and Simionescu M. Human monocytes and macrophages express NADPH oxidase 5; a potential source of reactive oxygen species in atherosclerosis. Biochem Biophys Res Commun 461: 172–179, 2015 [DOI] [PubMed] [Google Scholar]
- 76.Marzaioli V, Hurtado-Nedelec M, Pintard C, Tlili A, Marie JC, Monteiro RC, Gougerot-Pocidalo MA, Dang PM, and El-Benna J. NOX5 and p22phox are 2 novel regulators of human monocytic differentiation into dendritic cells. Blood 130: 1734–1745, 2017 [DOI] [PubMed] [Google Scholar]
- 77.Mizrahi A, Berdichevsky Y, Ugolev Y, Molshanski-Mor S, Nakash Y, Dahan I, Alloul N, Gorzalczany Y, Sarfstein R, Hirshberg M, and Pick E. Assembly of the phagocyte NADPH oxidase complex: chimeric constructs derived from the cytosolic components as tools for exploring structure-function relationships. J Leukoc Biol 79: 881–895, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Mongue-Din H, Patel AS, Looi YH, Grieve DJ, Anilkumar N, Sirker A, Dong X, Brewer AC, Zhang M, Smith A, and Shah AM. NADPH oxidase-4 driven cardiac macrophage polarization protects against myocardial infarction-induced remodeling. JACC Basic Transl Sci 2: 688–698, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, and Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 120: 131–141, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, and Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106: 1363–1373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montezano AC, De Lucca Camargo L. Persson P. Rios FJ. Harvey AP. Anagnostopoulou A. Palacios R. Gandara ACP. Alves-Lopes R. Neves KB. Dulak-Lis M. Holterman CE. de Oliveira PL. Graham D. Kennedy C, and Touyz RM. NADPH oxidase 5 is a pro-contractile Nox isoform and a point of cross-talk for calcium and redox signaling-implications in vascular function. J Am Heart Assoc 7: e009388, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, and Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 31: 631–641, 2015 [DOI] [PubMed] [Google Scholar]
- 83.Montezano AC. and Touyz RM. Reactive oxygen species and endothelial function–role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol 110: 87–94, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Montezano AC. and Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal 20: 164–182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montezano AC, Tsiropoulou S, Dulak-Lis M, Harvey A, Camargo Lde L, and Touyz RM. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens 24: 425–433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morawietz H. Cardiovascular protection by Nox4. Cardiovasc Res 114: 353–355, 2018 [DOI] [PubMed] [Google Scholar]
- 87.Moris D, Spartalis M, Tzatzaki E, Spartalis E, Karachaliou GS, Triantafyllis AS, Karaolanis GI, Tsilimigras DI, and Theocharis S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann Transl Med 5: 324, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, and El Jamali A. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem 287: 9376–9388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neves KB, Nguyen Dinh Cat A, Lopes RA, Rios FJ, Anagnostopoulou A, Lobato NS, de Oliveira AM, Tostes RC, Montezano AC, and Touyz RM. Chemerin regulates crosstalk between adipocytes and vascular cells through Nox. Hypertension 66: 657–666, 2015 [DOI] [PubMed] [Google Scholar]
- 90.Nguyen Dinh Cat A, Montezano AC, Burger D, and Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal 19: 1110–1120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nishibayashi H, Omura T, and Sato R. A flavoprotein oxidizing NADPH isolated from liver microsomes. Biochim Biophys Acta 67: 520–522, 1963 [DOI] [PubMed] [Google Scholar]
- 92.Ochoa CD, Wu RF, and Terada LS. ROS signaling and ER stress in cardiovascular disease. Mol Aspects Med 63: 18–29, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pai WY, Lo WY, Hsu T, Peng CT, and Wang HJ. Angiotensin-(1–7) inhibits thrombin-induced endothelial phenotypic changes and reactive oxygen species production via NADPH oxidase 5 downregulation. Front Physiol 8: 994, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, and Fulton DJ. Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol 302: H1919–H1928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park YM, Lim BH, Touyz RM, and Park JB. Expression of NAD(P)H oxidase subunits and their contribution to cardiovascular damage in aldosterone/salt-induced hypertensive rat. J Korean Med Sci 23: 1039–1045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, and Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rastogi R, Geng X, Li F, and Ding Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci 10: 301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Redfearn ER. and King TE. Mitochondrial NADH2 dehydrogenase and NADH2 oxidase from heart muscle: possible existence of a ferredoxin-like component in the respiratory chain. Nature 202: 1313–1316, 1964 [DOI] [PubMed] [Google Scholar]
- 99.Ritsick DR, Edens WA, Finnerty V, and Lambeth JD. Nox regulation of smooth muscle contraction. Free Radic Biol Med 43: 31–38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rivera J, Sobey CG, Walduck AK, and Drummond GR. Nox isoforms in vascular pathophysiology: insights from transgenic and knockout mouse models. Redox Rep 15: 50–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodrigo R, Libuy M, Feliú F, and Hasson D. Oxidative stress-related biomarkers in essential hypertension and ischemia-reperfusion myocardial damage. Dis Markers 35: 773–790, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H, Jiang G, Lu J, Antony S, and Doroshow JH. NADPH oxidases and cancer. Clin Sci (Lond) 128: 863–875, 2015 [DOI] [PubMed] [Google Scholar]
- 103.Sahoo S, Meijles DN, and Pagano PJ. NADPH oxidases: key modulators in aging and age-related cardiovascular diseases? Clin Sci (Lond) 130: 317–335, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Mol Interv 10: 354–362, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Schreiber C, Eilenberg MS, Panzenboeck A, Winter MP, Bergmeister H, Herzog R, Mascherbauer J, Lang IM, and Bonderman D. Combined oral administration of L-arginine and tetrahydrobiopterin in a rat model of pulmonary arterial hypertension. Pulm Circ 7: 89–97, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schröder K, Weissmann N, and Brandes RP. Organizers and activators: cytosolic Nox proteins impacting on vascular function. Free Radic Biol Med 109: 22–32, 2017 [DOI] [PubMed] [Google Scholar]
- 107.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, and Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 108.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, and Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89: 1159–1167, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Sies H, Berndt C, and Jones DP. Oxidative stress. Annu Rev Biochem 86: 715–748, 2017 [DOI] [PubMed] [Google Scholar]
- 110.Silva GC, Silva JF, Diniz TF, Lemos VS, and Cortes SF. Endothelial dysfunction in DOCA-salt-hypertensive mice: role of neuronal nitric oxide synthase-derived hydrogen peroxide. Clin Sci (Lond) 130: 895–906, 2016 [DOI] [PubMed] [Google Scholar]
- 111.Singel KL. and Segal BH. NOX2-dependent regulation of inflammation. Clin Sci (Lond) 130: 479–490, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sirokmány G, Donkó Á, and Geiszt M. Nox/Duox family of NADPH oxidases: lessons from knockout mouse models. Trends Pharmacol Sci 37: 318–327, 2016 [DOI] [PubMed] [Google Scholar]
- 113.Siu KL, Li Q, Zhang Y, Guo J, Youn JY, Du J, and Cai H. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol 11: 118–125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stasia MJ. CYBA encoding p22(phox), the cytochrome b558 alpha polypeptide: gene structure, expression, role and physiopathology. Gene 586: 27–35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takac I, Schröder K, and Brandes RP. The Nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep 14: 70–78, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Tang CT, Lin XL, Wu S, Liang Q, Yang L, Gao YJ, and Ge ZZ. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell Signal 46: 52–63, 2018 [DOI] [PubMed] [Google Scholar]
- 117.Teixeira G, Szyndralewiez C, Molango S, Carnesecchi S, Heitz F, Wiesel P, and Wood JM. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br J Pharmacol 174: 1647–1669, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas DC. The phagocyte respiratory burst: historical perspectives and recent advances. Immunol Lett 192: 88–96, 2017 [DOI] [PubMed] [Google Scholar]
- 119.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, and Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 45: 530–537, 2005 [DOI] [PubMed] [Google Scholar]
- 120.Touyz RM, Montezano AC, Rios F, Widlansky ME, and Liang M. Redox stress defines the small artery vasculopathy of hypertension: how do we bridge the bench-to-bedside gap? Circ Res 120: 1721–1723, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE 2006: re8, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Varga ZV, Pipicz M, Baán JA, Baranyai T, Koncsos G, Leszek P, Kuśmierczyk M, Sánchez-Cabo F, García-Pavía P, Brenner GJ, Giricz Z, Csont T, Mendler L, Lara-Pezzi E, Pacher P, and Ferdinandy P. Alternative splicing of NOX4 in the failing human heart. Front Physiol 8: 935, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Virdis A, Gesi M, and Taddei S. Impact of apocynin on vascular disease in hypertension. Vascul Pharmacol 87: 1–5, 2016 [DOI] [PubMed] [Google Scholar]
- 124.Virdis A, Neves MF, Amiri F, Touyz RM, and Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 22: 535–542, 2004 [DOI] [PubMed] [Google Scholar]
- 125.Wang H. and Hartnett ME. Roles of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in angiogenesis: isoform-specific effects. Antioxidants (Basel) 6: E40, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125a.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, and Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol 288: H37–H42, 2005 [DOI] [PubMed] [Google Scholar]
- 126.Wendt MC, Daiber A, Kleschyov AL, Mülsch A, Sydow K, Schulz E, Chen K, Keaney JF, Jr., Lassègue B, Walter U, Griendling KK, and Münzel T. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med 39: 381–391, 2005 [DOI] [PubMed] [Google Scholar]
- 127.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126: 119–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu Y, Meitzler JL, Antony S, Juhasz A, Lu J, Jiang G, Liu H, Hollingshead M, Haines DC, Butcher D, Panter MS, Roy K, and Doroshow JH. Dual oxidase 2 and pancreatic adenocarcinoma: IFN-γ-mediated dual oxidase 2 overexpression results in H2O2-induced, ERK-associated up-regulation of HIF-1α and VEGF-A. Oncotarget 7: 68412–68433, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu Q, Huff LP, Fujii M, and Griendling KK. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic Biol Med 109: 84–107, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamamoto T, Nakano H, Shiomi K, Wanibuchi K, Masui H, Takahashi T, Urano Y, and Kamata T. Identification and characterization of a novel NADPH oxidase 1 (Nox1) inhibitor that suppresses proliferation of colon and stomach cancer cells. Biol Pharm Bull 41: 419–426, 2018 [DOI] [PubMed] [Google Scholar]
- 131.Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, and Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension 51: 500–506, 2008 [DOI] [PubMed] [Google Scholar]
- 132.Yu P, Han W, Villar VA, Yang Y, Lu Q, Lee H, Li F, Quinn MT, Gildea JJ, Felder RA, and Jose PA. Unique role of NADPH oxidase 5 in oxidative stress in human renal proximal tubule cells. Redox Biol 2: 570–579, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang A, Jia Z, Wang N, Tidwell TJ, and Yang T. Relative contributions of mitochondria and NADPH oxidase to deoxycorticosterone acetate salt hypertension in mice. Kidney Int 80: 51–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang M, Mongue-Din H, Martin D, Catibog N, Smyrnias I, Zhang X, Yu B, Wang M, Brandes RP, Schröder K, and Shah AM. Both cardiomyocyte and endothelial cell Nox4 mediate protection against hemodynamic overload-induced remodelling. Cardiovasc Res 114: 401–408, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]