Abstract
Objectives: Anxiety disorders (ADs) are commonly associated with high-functioning Autism Spectrum Disorder (HF-ASD) and often worsen with age. Buspirone is a commonly prescribed anxiolytic drug with a favorable tolerability profile that may offer potential benefits in anxiety management for patients with HF-ASD. This study examines inadequately explored tolerability and effectiveness of buspirone in treating ADs comorbid with high-functioning ASD.
Methods: A retrospective chart review of a 1-year period was conducted in psychiatrically referred population of HF-ASD youth with AD (age 8–17 years) who were treated with buspirone (N = 31). Information on the demographics and treatment history was recorded. Effectiveness was assessed through the Clinical Global Impressions Scale (CGI) severity (CGI-S) and improvement (CGI-I) scores noted by the treating clinician.
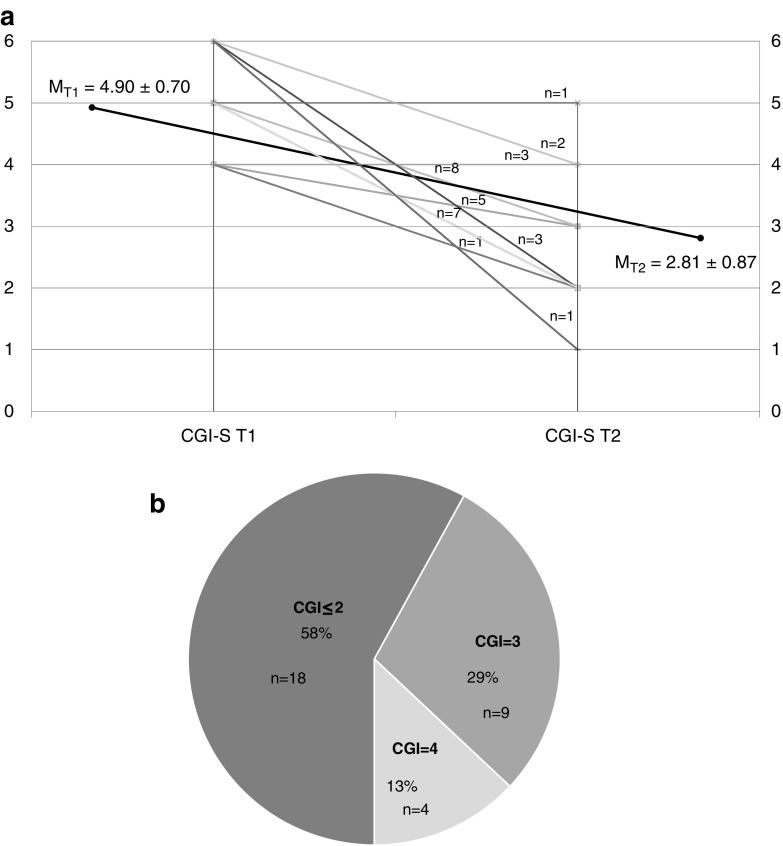
Results: A total of 31 patients were prescribed buspirone during the determined period, at a mean dose of 41.61 ± 24.10 mg for an average duration of 272 ± 125 days. Change in the CGI-S mean scores with treatment suggests an overall improvement in the severity of anxiety symptoms (MT1 = 4.9 ± 0.7; MT2 = 2.8 ± 0.87; p < 0.001). Significant improvement in anxiety symptoms (CGI-I ≤ 2) was observed in 58% and mild improvement (CGI-I = 3) in 29% of the HF-ASD patients who received buspirone treatment. Buspirone was well tolerated with no adverse events reported by the majority of participants, with the exception of two subjects who developed treatment emergent adverse events (activation and mood lability).
Conclusions: Findings from this retrospective chart review suggest a promising role of buspirone in managing anxiety among youth with HF-ASD. Further research with prospective and randomized-controlled trials is necessary.
Keywords: : autism spectrum disorder, anxiety, psychopharmacology, child and adolescent, clinical trial, buspirone
Introduction
Autism Spectrum Disorder (ASD) refers to a neurodevelopmental disorder distinguished by variable presentations of impairment in socialization, communication, and restricted, repetitive behaviors, which is now estimated to affect up to 2% of children and adolescents in the general population (Blumberg et al. 2013). In psychiatrically referred populations of youth, much higher rates of ASD ranging from 2% to 14% have been reported, thereby comprising a substantial subgroup of patients referred for psychiatric care (Sverd et al. 1995; Wozniak et al. 1997; Sverd 2003; Joshi et al. 2010). Psychiatrically referred populations of youth with ASD suffer from multiple anxiety disorders (ADs) at a much higher rate than that observed in the rest of the psychiatric referrals (Joshi et al. 2010).
ADs frequently present in youth with high-functioning ASD (HF-ASD), which is defined as the presence of intact language skills and absence of intellectual disability. While the prevalence rate for all ADs in the general population is estimated at 29% (Kessler et al. 2005), several uncontrolled studies have reported the prevalence of ADs in ASD youth ranging between 43% and 84% (Muris et al. 1998; Leyfer et al. 2006; de Bruin et al. 2007; Sukhodolsky et al. 2008; White et al. 2009; Shekunov et al. 2017). Higher rates of ADs may present in patients with ASD compared with typically developing children (Joshi et al. 2010).
Autistic traits can be a unique trigger for precipitating anxiety in individuals with ASD in two ways: first, the inability to meet needs for “insistence of sameness or inflexible adherence to routines” may result in heightened anxiety when opposed or challenged. Second, anxiety may worsen in the presence of stimuli that trigger sensory integration deficits (McDougle et al. 2000). In fact, anxiety-related difficulties are so frequently exhibited in children with ASD that the diagnostic nomenclature includes highlighted anxiety-like responses as an “associated feature” of autism; for example, “excessive fearfulness in response to harmless objects” (American Psychiatric Association 2000, 2013). Furthermore, awareness of social deficits and the legacy of failure in the social domain may amplify social anxiety in youth with higher functioning forms of ASD (Bachevalier and Loveland 2006).
Presence of disabling anxiety further worsens social performance in HF-ASD individuals with already compromised social competence, highlighting the importance of assessment and treatment of anxiety problems in addition to interventions targeting social communication impairment in children with ASD (van Steensel et al. 2011; Duvekot et al. 2017). Despite high prevalence of AD in ASD in general, there remains a lack of empirical evidence on the role of available treatments. Therefore, research on the tolerability and efficacy of treatments for anxiety comorbid with HF-ASD in youth is warranted.
Buspirone is an anxiolytic agent with a favorable tolerability profile and with no abuse liability (Garattini et al. 1982; Cohn et al. 1986). Buspirone displays high affinity and selectivity for 5-HT1A receptors present both presynaptically and postsynaptically, and acts as a serotonin partial agonist, with a low incidence of adverse events (Realmuto et al. 1989; Buitelaar et al. 1998; Ghanizadeh and Ayoobzadehshirazi 2015). Empirical efficacy and safety of buspirone are well established in adults with AD (Feighner et al. 1982; Rickels et al. 1982; Cohn et al. 1986). In children and adolescents, similar research on potential role of buspirone in treatment of anxiety alone is lacking. Available limited literature has mostly focused on the role of buspirone in treatment of irritability in patients with a broader phenotype of ASD (Pervasive Developmental Disorder, Not Otherwise Specified [PDD-NOS]) (Buitelaar et al. 1998; Ghanizadeh and Ayoobzadehshirazi 2015; Chugani et al. 2016). In these studies, buspirone was very well tolerated with no serious or treatment-limiting adverse events.
This study aims to assess whether buspirone treatment is effective in reducing anxiety symptoms in children and adolescents with HF-ASD and anxiety.
Methods
Ascertainment of study participants
A retrospective review of medical records of patients at the Massachusetts General Hospital (MGH) Outpatient Child Psychiatry Clinic diagnosed with ASD, aged 8–17 years, and treated with buspirone for at least 8 weeks during a 12-month period (October 12, 2014 to September 12, 2015) was carried out, including chart review and discussions with the treating clinician of each identified patient. All care providers were board-certified child and adolescent psychiatrists who established a diagnosis based on the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) criteria after a comprehensive psychiatric evaluation of each patient, and determined the diagnosis severity at the time of the evaluation of both ASD and AD separately. A treatment period of 8 weeks was chosen for the study duration, as most of the previous studies had utilized a 6- to 8-week duration of treatment, including dose titration (Realmuto et al. 1989; Pfeffer et al. 1997; Buitelaar et al. 1998; Ghanizadeh and Ayoobzadehshirazi 2015), with the exception of one study (Chugani et al. 2016) that measured the efficacy of a 24-week treatment with buspirone. Demographic data, diagnostic information including comorbidities, treatment records including concomitant medications and adjunct treatments (e.g., medical, educational, and counseling), and symptom changes were collected. The selected patients met the criteria for ASD of at least mild severity, as determined by a Clinical Global Impressions-Severity (CGI-S) score ≥3, while ADs were noted to be of at least moderate severity (CGI-S ≥ 4). Intellectual ability was clinically determined by history of intellectual functioning at school. Language ability was assessed per clinical evaluation. High-functioning ASD definition was based on clinically ruling out intellectual disability and impaired language skills.
Assessment procedures
Treatment response was based on the treating clinician's assessment noted in medical record, was confirmed with each clinician individually, and was coded on the CGI for both ASD and AD separately (National Institute of Mental Health 1985). The CGI is a clinician-rated scale, with scores ranging from 1 to 7. It includes subscales for global severity (CGI-S: 1 indicates not at all ill; 2, borderline mentally ill; 3, mildly ill; 4, moderately ill; 5, markedly ill; 6, severely ill; and 7, extremely ill) and global improvement (CGI-I: 1 indicates very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; and 7, very much worse).
Statistical analysis
CGI-S scores at the beginning of the review period, or at treatment (whichever is earlier) and at endpoint of review period or at time of treatment discontinuation (whichever is later) were compared using dependent t-test analysis. Two-tailed test was applied at the 0.05 alpha level. Descriptive statistics are reported as mean ± standard deviation or N (%).
Results
Clinical characterization
A total of 31 patients received at least one dose of buspirone during the determined period. Age at time of treatment and demographic parameters are listed in Table 1. The average age of the patient population was 12.71 ± 2.56, and majority were male (25 [81%]). Patients received an average dose of 41.6 ± 24.10 mg for an average duration of 271.9 ± 125.36 days. Thirteen (42%) patients were started on buspirone during this selected period, and the remaining 18 (58%) patients were started on buspirone prior. Twenty-nine (94%) of the 31 patients continued their medication at the time of last clinical contact. Seven patients (22%) presented with medical comorbidities, including seizure disorder and gastrointestinal problems. Finally, three (9%) patients had a change in their academic placement, and one patient was hospitalized for aggressive behavior, toward the end of the study period and long after anxiety response was established. Comparative analyses did not reveal any significant difference in outcome measures based on gender, age, medical comorbidities, and change in adjunct therapies.
Table 1.
Demographics and Clinical Characteristics
| ASD | |
|---|---|
| Demographics | |
| Total participants (n) | 31 |
| Age (years), mean ± SD (range) | 12.71 ± 2.56 (8–17) |
| Gender (male), n (%) | 25 (80.6) |
| Race (Caucasian), n (%) | 31 (100) |
| Clinical characteristics, n (%) | |
| ASD diagnostic subtypes (DSM-IV) | |
| Autistic disorder | 24 (77.4) |
| Asperger's disorder | 2 (6.5) |
| PDD-NOS | 5 (16.1) |
| ASD impairment (current) | |
| Mild | 8 (25.8) |
| Moderate | 20 (64.5) |
| Severe | 3 (9.7) |
| Medical comorbidities | |
| Seizure disorder | 3 (9.7) |
| Gastrointestinal disorders | 5 (16.1) |
| Other medical disorders | 4 (12.9) |
| Comorbid diagnoses (DSM-IV) | |
| ADHD | 28 (90.3) |
| Mood disordersa | 22 (71.0) |
| Learning disorders | 5 (16.1) |
| Otherb | 2 (6.5) |
| Adjunct psychotropic medications | |
| Antipsychotics | 15 (48.4) |
| Stimulants/psychostimulants | 15 (48.4) |
| Alpha-2-agonists | 11 (35.5) |
| SSRIs and bupropion | 10 (32.3) |
| Benzodiazepines | 8 (25.8) |
| Anticonvulsants and lithium | 4 (12.9) |
| SNRI | 1 (3.2) |
| Tricyclic antidepressants | 1 (3.2) |
Mood Disorders consisted of patients with Bipolar Disorder, Mood Disorder NOS, and Major Depressive Disorder.
Other consisted of patients with Obsessive Compulsive Disorder and those who were diagnosed with more than one disorder.
ADHD, attention-deficit/hyperactivity disorder; ASD, Autism Spectrum Disorder; PDD-NOS, Pervasive Developmental Disorder, Not Otherwise Specified; SD, standard deviation; SNRI, selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors.
Twenty-six (84%) patients were diagnosed with ASD, five (16%) with Asperger's Syndrome and two (7%) with PDD-NOS. AD, Not Otherwise Specified constituted most of the AD diagnoses (22 [71%]), followed by Generalized AD (9 [29%]) and Social AD (3 [10%]). Four patients (13%) were diagnosed with two or more comorbid ADs. At least one other comorbidity was noted for each patient who had AD and ASD. Attention-deficit/hyperactivity disorder (ADHD) was the most common comorbid condition (28 [90%]) in our sample, followed by mood disorders (both Major Depressive Disorder and Bipolar Spectrum Disorders) (22 [71%]) and Learning Disorders (5 [16%]). No patients were diagnosed with Intellectual Disability. Only one (3%) patient had AD alone comorbid with ASD, and eight (26%) other patients had no mood disorder comorbidity. Only two (7%) patients received buspirone monotreatment, while the remaining patients received multiple concomitant medications. Twelve (39%) patients had significant medical comorbidities; however, no clinically significant fluctuations associated with change in anxiety severity occurred during the reviewed period.
Comparative analyses applied at the 0.05 alpha level did not indicate any significant difference based on gender, age, medical comorbidities, and change in adjunct therapies. Two-tailed independent t-test analyses were utilized to compare the CGI-I scores of the different age (8–12 and 13–17) and gender (male and female) groups. These analyses were similarly applied to compare the CGI-ST1–T2 score difference of the groups. None of these analyses indicated a statistically significant difference. In addition, comparisons of the CGI-S scores for a subsample not including patients who reported a service change and for a subsample not including patients with comorbidities were conducted using two-tailed dependent t-test analyses. The CGI-S scores still differed significantly despite the removal of the two patient groups (patients with service change and patients with comorbidities). Finally, two-tailed independent t-test analyses, which were used to compare the CGI-I scores of these subsamples divided based on age and gender, did not reveal any significant differences.
Course of symptoms of ADs
Anxiety symptoms were rated to be markedly or severely ill (CGI-S = 5 or 6) among 22 (71%) patients, and the remaining 9 patients were noted to be moderately ill (CGI-S = 4). Eighteen (58%) patients were noted to have improved at least markedly during the last clinical contact (CGI-I ≤ 2), and nine (29%) patients were assessed to have moderately improved (CGI-I = 3). Four (13%) patients had no change in their symptom severity (CG-I = 4). Of the four patients who were nonresponders to buspirone, three had received this treatment for >162 days, and the fourth was discontinued after 38 days due to the side effect of mood lability. No patients had a worsening of their anxiety symptoms. Overall, there was a downward trend in anxiety symptom severity. Figure 1a and b show the trajectory of symptom severity and distribution of CGI-I scores of all reviewed patients. Two (7%) patients were discontinued from buspirone treatment due to side effects. One patient, aforementioned, experienced mood lability after 38 days with no change in clinical severity. Another patient developed hypomania-like symptoms after 687 days of treatment, during which marked improvement in anxiety symptoms was observed. No other severe side effects were reported. One patient experienced mild insomnia, and another experienced sedation; both patients recovered with no further recurrence following dosage adjustment. No other side effects were noted in the sample population who had received at least one buspirone dose during the study period. Slower titration is a standard practice at our study center and could have contributed to this improved tolerability of buspirone, even when higher doses were reached at the end.
FIG. 1.
(a) Change over time in Clinical Global Impressions Anxiety Severity (CGI-S) scores. (b) Clinical Global Impression Anxiety Improvement (CGI-I) score distribution.
Discussion
In this retrospective medical chart review, we investigated the treatment response of buspirone for the management of anxiety in patients with ASD. These results suggest that treatment with buspirone was overall well tolerated and was associated with clinically significant improvement in symptoms of anxiety in youth with ASD.
In this study, a majority (58%) of clinical patients had a robust response to the buspirone therapy (CGI-I of ≤3). Mild antianxiety improvement (CGI-I = 3) was achieved by 29% of youth with HF-ASD, while 13% of patients did not experience any improvement. This variation might have been due to two factors: first, fluctuations in anxiety symptoms over the course of treatment period are common, and may not have been detected. Second, duration of treatment at endpoint may not have been sufficient to effect a change in any direction. None of the patients treated with buspirone had experienced a worsening of their AD (CGI-I ≥ 5). The buspirone treatment was generally well tolerated. Treatment-limiting side effects reported in this study are similar to previous reports; however, more common side effects were considerably less frequent compared with previous reports on buspirone found in the literature, including sedation, dizziness, appetite changes, fatigue, headaches, and nausea (Buitelaar et al. 1998; Ghanizadeh and Ayoobzadehshirazi 2015; Chugani et al. 2016). Slower titration is a standard practice at the study site and could have contributed to this improved tolerability to buspirone, even when higher doses were reached at the end.
Findings from this study also suggest that ADs in HF-ASD patients are frequently comorbid with other psychopathologies. ADHD was the most common comorbid diagnosis, followed by mood disorders. Persistent and untreated anxiety symptoms may further interfere with attention problems as well as mood symptoms and intensify clinical burden among patients with HF-ASD.
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed medications for treatment of ADs among patients with HF-ASD (50%). Most recent naturalistic survey on the prescribing patterns of the psychopharmacological interventions for the management of psychopathology in youth with ASD attending an ambulatory care clinic noted that SSRIs are the most commonly prescribed medications for the management of anxiety in ∼50% of patients treated (Shekunov et al. 2017). In that study, anxiety symptoms were identified as the primary target in 12% of patients (N = 25). Most patients received antidepressants (SSRIs, selective norepinephrine reuptake inhibitors [SNRIs], tricyclic antidepressants, and bupropion), benzodiazepines, antidopaminergics, and anticonvulsants, and a rather small number of patients (N = 4) were prescribed buspirone. All patients who received buspirone did so as monotherapy, at lower doses than were reported in this study (10–30 mg total daily) and experienced no treatment-limiting side effects.
This study suggests that buspirone is a frequently overlooked option likely secondary to absence of sufficient data in treatment of ADs in patients with ASD. Despite its relatively benign safety profile and low cost, buspirone was considered less frequently than benzodiazepines and other classes of medications that equally lack empirical evidence on safety and efficacy for treating anxiety in this population (anticonvulsants and antidopaminergics). Buspirone was often prescribed in combination with other medications, most commonly with antipsychotics, stimulants, and antidepressants (SSRI and bupropion), except for two patients who received buspirone monotherapy.
The following limitations must be considered when interpreting findings from this study. First, the sample size was small. The low number of patients treated with buspirone is consistent with the lesser amount of attention this medication has received in clinical practice regarding treatment of ADs in patients with HF-ASD. Second, we used a convenience sample, derived from our patient population. While a convenience sample represents a practical method, it is commonly used when dealing with rarer cases or smaller sample sizes, which are characteristic of buspirone utilization in HF-ASD population. It should be noted that the general characteristics of our sample were consistent with the general population regarding the gender distribution among patients with ASD, with male-to-female ratio of 4:1, suggesting that our convenience sample still reflected some of the main features of the general clinical population. Third, combination with other medication classes was noted and might have had an impact on the outcome. Detailed information regarding additional services patients might have received during the study period was not available. Given that the anxiety symptoms may be triggered by sensory integration deficits, information regarding whether a patient received specific occupational therapy targeting sensory integration was not available. It is possible that co-occurring educational and therapeutic services might have had an impact on the outcome data as well. Fourth, common limitations regarding retrospective studies also apply to our findings. There is no placebo or control group; however, given its retrospective nature, clinician bias is also less relevant. Similarly, CGIs were the only measures used to determine the severity of anxiety symptoms. At our clinic, using CGIs to monitor effectiveness of treatment is the standard procedure, while other measures are not uniformly applied. We should also note that intellectual ability was not assessed by neuropsychological tools. Intellectual ability was clinically determined by history of intellectual functioning at school and language ability per clinical evaluation. Finally, findings from a study carried out at a tertiary care center may not be readily generalizable to other stages of care.
The current retrospective chart review suggests the relative safety of buspirone in HF-ASD. While the generalizability of our findings is limited by factors aforementioned, the data revealed may point to the potential effectiveness of buspirone in ADs comorbid with HF-ASD. Further research with prospective and randomized-controlled trials is necessary.
Conclusions
ADs are commonly present in individuals with HF-ASD. Buspirone is generally well tolerated and may be an effective treatment for ADs comorbid with HF-ASD. Further research with prospective and randomized-controlled trials is needed.
Clinical Significance
ADs are commonly present in individuals with ASD with intact intellectual functioning and confound social communication and interaction deficits. Buspirone may be a safe treatment for ADs comorbid with HF-ASD, and further research is required to assess its efficacy.
Disclosures
Dr. T. Atilla Ceranoglu is employed at MGH. He received training support from Massachusetts Department of Mental Health, and received research support from Department of Defense, Lundbeck A/S, Magceutics, National Institutes of Health (NIH), Pumlab, Pfizer and Sunovion Pharmaceuticals. In addition, he received honorarium from LEK Consulting. In the past, he served as a member on Mental Health Advisory for Jack Kent Cooke Foundation located in Lansdowne, VA. Dr. Gagan Joshi is supported by the National Institute of Mental Health (NIMH) of the NIH under Award number K23MH100450. He has received research support from Pfizer and the Simons Center for the Social Brain as a principal investigator (PI) for investigator-initiated studies. In addition, he has received research support from Duke University and Sunovion Pharmaceuticals as a site PI for multisite trials. He is a coinvestigator for a clinical trial sponsored by the U.S. Department of Defense. He received an honorarium from the Governor's Council for Medical Research and Treatment of Autism in New Jersey for grant review activities and speaker's honorariums from the American Academy of Child and Adolescent Psychiatry, MGH Psychiatry Academy, and the Medical Society of Delaware. In 2016–2017, Dr. Janet Wozniak received no outside research support. She is author of the book “Is Your Child Bipolar” published in May 2008, Bantam Books. In 2016–2017, her spouse received royalties from UpToDate and consultation fees from Advance Medical, FlexPharma, Merck, Otsuka, and Gerson Lehman Group. Dr. Ronna Fried is currently receiving research support from the Food and Drug Administration (FDA) as well as honoraria from the MGH Psychiatry Academy for tuition-funded continuing medical education (CME) courses. She has also been on an advisory board for Lundbeck. During previous years, Dr. Fried received research support from the NIH and Shire. Dr. Joseph Biederman is currently receiving research support from the following sources: AACAP, The Department of Defense, FDA, Headspace, Lundbeck, Neurocentria, Inc., NIDA, PamLab, Pfizer, Shire Pharmaceuticals, Inc., Sunovion, and NIH. Dr. Biederman has a financial interest in Avekshan, LLC, a company that develops treatments for ADHD. His interests were reviewed and are managed by MGH and Partners HealthCare in accordance with their conflict of interest policies. Dr. Biederman's program has received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2017, Dr. Biederman served as a consultant for Aevi Genomics, Akili, Guidepoint, Ironshore, Medgenics, and Piper Jaffray. He is on the scientific advisory board for Alcobra and Shire. He received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. Through MGH corporate licensing, he has a U.S. Patent (No. 14/027,676) for a non-stimulant treatment for ADHD, and a patent pending (No. 61/233,686) on a method to prevent stimulant abuse. In 2016, he received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses, and from Alcobra and APSARD. He was on the scientific advisory board for Arbor Pharmaceuticals. He was a consultant for Akili and Medgenics. He received research support from Merck and SPRITES. In 2015, he received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses and from Avekshan. He received research support from Ironshore, Magceutics, Inc., and Vaya Pharma/Enzymotec. In 2014, he received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals, Inc. In the previous years, he received research support, consultation fees, or speaker's fees for/from the following additional sources: Abbott, Alza, APSARD, AstraZeneca, Boston University, Bristol Myers Squibb, Cambridge University Press, Celltech, Cephalon, The Children's Hospital of Southwest Florida/Lee Memorial Health System, Cipher Pharmaceuticals, Inc., Eli Lilly and Co., Esai, ElMindA, Fundacion Areces (Spain), Forest, Fundación Dr. Manuel Camelo A.C., Glaxo, Gliatech, Hastings Center, Janssen, Juste Pharmaceutical Spain, McNeil, Medice Pharmaceuticals (Germany), Merck, MGH Psychiatry Academy, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shionogi Pharma, Inc, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma, Inc., Veritas, and Wyeth. All other authors have nothing to disclose.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Publishing; 2013 [Google Scholar]
- Bachevalier J, Loveland KA: The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev 30:97–117, 2006 [DOI] [PubMed] [Google Scholar]
- Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC: Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011–2012. Natl Health Stat Report 1–11, 1 p following 11, 2013 [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ, van der Hoeven J: Buspirone in the management of anxiety and irritability in children with pervasive developmental disorders: Results of an open-label study. J Clin Psychiatry 59:56–59, 1998 [DOI] [PubMed] [Google Scholar]
- Chugani DC, Chugani HT, Wiznitzer M, Parikh S, Evans PA, Hansen RL, Nass R, Janisse JJ, Dixon-Thomas P, Behen M, Rothermel R, Parker JS, Kumar A, Muzik O, Edwards DJ, Hirtz D; Autism Center of Excellence N: Efficacy of low-dose buspirone for restricted and repetitive behavior in young children with autism spectrum disorder: A randomized trial. J Pediatr 170:45.e1–e4–53.e1–e4, 2016 [DOI] [PubMed] [Google Scholar]
- Cohn JB, Bowden CL, Fisher JG, Rodos JJ: Double-blind comparison of buspirone and clorazepate in anxious outpatients. Am J Med 80:10–16, 1986 [DOI] [PubMed] [Google Scholar]
- de Bruin EI, Ferdinand RF, Meester S, de Nijs PF, Verheij F: High rates of psychiatric co-morbidity in PDD-NOS. J Autism Dev Disord 37:877–886, 2007 [DOI] [PubMed] [Google Scholar]
- Duvekot J, van der Ende J, Verhulst FC, Greaves-Lord K: Examining bidirectional effects between the autism spectrum disorder (ASD) core symptom domains and anxiety in children with ASD. J Child Psychol Psychiatry 59:277–284, 2018 [DOI] [PubMed] [Google Scholar]
- Feighner JP, Merideth CH, Hendrickson GA: A double-blind comparison of buspirone and diazepam in outpatients with generalized anxiety disorder. J Clin Psychiatry 43(Pt 2):103–108, 1982 [PubMed] [Google Scholar]
- Garattini S, Caccia S, Mennini T: Notes on buspirone's mechanisms of action. J Clin Psychiatry 43(Pt 2):19–24, 1982 [PubMed] [Google Scholar]
- Ghanizadeh A, Ayoobzadehshirazi A: A randomized double-blind placebo-controlled clinical trial of adjuvant buspirone for irritability in autism. Pediatr Neurol 52:77–81, 2015 [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, Kotarski M, Walls S, Biederman J: The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. J Autism Dev Disord 40:1361–1370, 2010 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE: Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602, 2005 [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE: Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J Autism Dev Disord 36:849–861, 2006 [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Kresch LE, Posey DJ: Repetitive thoughts and behavior in pervasive developmental disorders: Treatment with serotonin reuptake inhibitors. J Autism Dev Disord 30:427–435, 2000 [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C: Comorbid anxiety symptoms in children with pervasive developmental disorders. J Anxiety Disord 12:387–393, 1998 [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health: CGI (Clinical Global Impressions Scale)—NIMH. Psychopharmacol Bull 21:839–844, 1985 [Google Scholar]
- Pfeffer CR, Jiang H, Domeshek LJ: Buspirone treatment of psychiatrically hospitalized prepubertal children with symptoms of anxiety and moderately severe aggression. J Child Adolesc Psychopharmacol 7:145–155, 1997 [DOI] [PubMed] [Google Scholar]
- Realmuto GM, August GJ, Garfinkel BD: Clinical effect of buspirone in autistic children. J Clin Psychopharmacol 9:122–125, 1989 [DOI] [PubMed] [Google Scholar]
- Rickels K, Weisman K, Norstad N, Singer M, Stoltz D, Brown A, Danton J: Buspirone and diazepam in anxiety: A controlled study. J Clin Psychiatry 43(Pt 2):81–86, 1982 [PubMed] [Google Scholar]
- Shekunov J, Wozniak J, Conroy K, Friedman N, Pinsky E, Fitzgerald M, Joshi G: Prescribing patterns in a psychiatrically referred sample of youth with autism spectrum disorder. J Clin Psychiatry 78:e1276–e1283, 2017 [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, McCracken JT, Tierney E, Williams White S, Lecavalier L, Vitiello B: Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. J Abnorm Child Psychol 36:117–128, 2008 [DOI] [PubMed] [Google Scholar]
- Sverd J: Psychiatric disorders in individuals with pervasive developmental disorder. J Psychiatr Pract 9:111–127, 2003 [DOI] [PubMed] [Google Scholar]
- Sverd J, Sheth R, Fuss J, Levine J: Prevalence of pervasive developmental disorder in a sample of psychiatrically hospitalized children and adolescents. Child Psychiatry Hum Dev 25:221–240, 1995 [DOI] [PubMed] [Google Scholar]
- van Steensel FJ, Bogels SM, Perrin S: Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clin Child Fam Psychol Rev 14:302–317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, Scahill L: Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev 29:216–229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J, Biederman J, Faraone SV, Frazier J, Kim J, Millstein R, Gershon J, Thornell A, Cha K, Snyder JB: Mania in children with pervasive developmental disorder revisited. J Am Acad Child Adolesc Psychiatry 36:1552–1559; discussion 1559–1560, 1997 [DOI] [PubMed] [Google Scholar]