Abstract
The tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) has been shown to improve dendritic spine density and cognitive memory in the triple transgenic mouse model of Alzheimer disease (AD) when administered intraperitoneally. The present study was designed to investigate the therapeutic effects of SPG101 on dendritic spine density and morphology and sensorimotor and cognitive functional recovery in a rat model of traumatic brain injury (TBI) induced by controlled cortical impact (CCI). Young adult male Wistar rats with CCI were randomly divided into the following two groups (n = 7/group): (1) Vehicle, and (2) SPG101. SPG101 (30 mg/kg) dissolved in vehicle (1% dimethyl sulfoxide in phosphate buffered saline) or Vehicle were intraperitoneally administered starting at 1 h post-injury and once daily for the next 34 days. Sensorimotor deficits were assessed using a modified neurological severity score and adhesive removal and foot fault tests. Cognitive function was measured by Morris water maze, novel object recognition (NOR), and three-chamber social recognition tests. The animals were sacrificed 35 days after injury, and their brains were processed for measurement of dendritic spine density and morphology using ballistic dye labeling. Compared with the vehicle treatment, SPG101 treatment initiated 1 h post-injury significantly improved sensorimotor functional recovery (days 7–35, p < 0.0001), spatial learning (days 32–35, p < 0.0001), NOR (days 14 and 35, p < 0.0001), social recognition (days 14 and 35, p < 0.0001). Further, treatment significantly increased dendritic spine density in the injured cortex (p < 0.05), decreased heterogeneous distribution of spine lengths in the injured cortex and hippocampus (p < 0.0001), modifications that are associated with the promotion of spine maturation in these brain regions. In summary, treatment with SPG101 initiated 1 h post-injury and continued for an additional 34 days improves both sensorimotor and cognitive functional recovery, indicating that SPG101 acts as a spinogenic agent and may have potential as a novel treatment of TBI.
Keywords: : dendritic spine, functional outcome, rat, SPG101, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major public health problem worldwide, with approximately 2.8 million new cases occurring each year in the United States alone.1 Previous therapeutics targeting TBI have included anti-inflammatory drugs,2 hyperbaric oxygen,3 progesterone,4 erythropoietin,5 hypothermia,6 and many other treatments.7 To date, however, no effective agents other than supportive care have been identified from TBI clinical trials. Therefore, developing new therapeutic methods that can be used to treat those with TBI is a significant unmet medical need.
Dendritic spines are tiny protrusions along neuronal dendrites that receive excitatory input from a single synapse of an axon and play a critical role in integrating these synaptic inputs and in determining the extent to which action potentials are produced by the neurons.8 Mild TBI induces extensive dendritic degeneration of the cortex,9 and moderate TBI also causes acute dendrite degeneration and synaptic loss in spared neurons of the hippocampus.10 Extensive dendritic damage in the spared neurons disrupts neural circuits and likely contributes to functional impairment after TBI.11 Therefore, loss of synapses and dendritic spines (that are key to the formation of new synapses) in important brain areas may lead to persistent cognitive, sensory, and motor dysfunction.10,12–14
The tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101, formerly known as BTA-EG4), acts as an amyloid-binding small molecule that promotes dendritic spine density and improves cognitive memory in the triple transgenic mouse model of Alzheimer disease (AD) and in wild-type mice when administered intraperitoneally (IP),15–18 suggesting that SPG101 may have potential for treatment of those with TBI. The present randomized, placebo-controlled, and blinded study was designed to investigate the therapeutic effect of SPG101 on sensorimotor and cognitive function using a battery of functional tests as well as on dendritic spine density and morphology evaluated using a ballistic dye labeling technique in a rat model of TBI.
Methods
Animal model of TBI
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Health System. All functional tests and spine analyses were performed by investigators blinded to the treatment status.
Male Wistar rats weighing 305 ± 10 g (2–3 months old, Charles River Breeding Company, Wilmington, MA) were employed in our experiments. A controlled cortical impact (CCI) rat model of TBI was utilized to induce moderate focal brain injury (contusion). The moderate CCI-TBI we used is one of the most widely used TBI models with high reproducibility. It causes both cortical lesion and subcortical injury including the hippocampus that lead to sensorimotor and cognitive deficits, which can last up to one year after TBI compared with age-match sham rats as demonstrated by us and other investigators.19–21
Briefly, each rat was anesthetized with IP ketamine/xylazine (100/10 mg/kg). Rectal temperature was maintained at 37 ± 5°C using a feedback-regulated water-heating pad. Rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The second craniotomy allowed for lateral movement of cortical tissue. The dura mater was kept intact over the cortex. Cortical injury was delivered by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 6-mm–diameter tip at a rate of 4 m/sec and 2.5 mm of compression. CCI corresponding to moderate TBI was performed in male Wistar rats.22-–25
The moderate CCI-TBI model employed in this study is one of the most widely used clinically relevant animal models of focal TBI.25–27 This model causes not only cortical injury but also selective neuronal death in the hippocampus in rats, leading to sensorimotor dysfunction and spatial learning and memory deficits, respectively.19,28–34
SPG101 treatment (35-day study)
Animals with TBI were randomly divided into one of the two groups: (1) TBI + Vehicle at 1 h (n = 7), and (2) TBI + SPG101 (30 mg/kg) at 1 h (n = 7). SPG101 was administered IP to rats once daily for the next 34 days, starting at 1 h after TBI, at a dose of 30 mg/kg. To date, the optimal dose and time of SPG101 have not been identified for treatment of rats with TBI. This dosing protocol is based on previous studies in wild-type mice17 and in a mouse model of AD16 because SPG101 at 30 mg/kg daily IP for two weeks has been demonstrated to have pronounced effects on increasing dendritic spine density and improving cognitive performance in wild-type mice and AD mice.16,17 Animals treated with vehicle (1% dimethyl sulfoxide [DMSO] in phosphate buffered saline [PBS]) were used as a control group. SPG101 was supplied by Spinogenix, which provided funding for this study.
Evaluation of neurological outcome
Primary end-points include the following battery of behavioral and neurological tests: sensorimotor tests (foot fault, modified Neurological Severity Score-mNSS, and adhesive patch removal),14,35,36 and cognitive tests (the Morris water maze [MWM], Novel Object Recognition [NOR], and Three-Chamber Social Interaction).14,37,38 All personnel were blinded to the treatment groups for tests. Animal behavioral outcomes were compared between the two groups. Secondary end-points were the effects of SPG101 on dendritic spine density and morphology measured using ballistic dye labeling technique.39
Sensorimotor tests
Foot fault test
To evaluate sensorimotor function, the foot fault test was performed before TBI and at one, seven, 14, 21, and 35 days after TBI. The rats were allowed to walk on a grid. With each weight-bearing step, a paw might fall or slip between the wires and, if this occurred, it was recorded as a foot fault. A total of 50 steps were recorded for the right forelimb and hindlimb.14
mNSS test
To investigate neurological functional outcome, the mNSS test was performed. The test was carried out on rats pre-injury and at one, seven, 14, 21, and 35 days after TBI. mNSS is a composite of the motor (muscle status, abnormal movement), sensory (visual, tactile, and proprioceptive), and reflex tests and has been used in previous TBI studies.14 Neurological function was graded on a scale of 0 to 18 (normal score 0; maximal deficit score 18). One point is awarded for each abnormal behavior or for lack of a tested reflex; thus, the higher the score, the more severe the injury.14
Adhesive patch removal test
Briefly, two pieces of adhesive-backed paper (113.1 mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb.36 Each animal received three trials per testing day, and the mean time (seconds) required to remove stimuli from the right forelimb was recorded. The average of three trials was used for statistical analysis. The test was performed on the rat pre-injury and at one, seven, 14, 21, and 35 days after TBI.
Cognitive tests
MWM test
The modified MWM test was used for assessment of spatial learning function, as described previously.24,40 This test was performed daily for five days in all rats at one month (days 31–35 after injury). Briefly, the experimental apparatus consists of a circular water tank (180 cm in diameter) that is placed in a large test room with many external cues (e.g., pictures, lamps, camera, etc.) visible to the rats. A transparent platform (10 cm in diameter) is submerged 2 cm below the surface of the water at a random location within the northeast (correct) quadrant of the maze.
For each trial, the rat was placed randomly at one of four fixed starting points (north, south, east, and west) and allowed to swim for a maximum of 90 sec. If the animals did not locate the platform within 90 sec, they were guided gently to it by the experimenter. All animals were allowed to remain on the platform for 10 sec before being removed from the tank. The swim speed, latency to find the hidden platform, and the time spent within the correct quadrant were recorded. Data are presented as the percentage of time spent within the correct quadrant relative to the total amount of time spent to find the hidden platform.
NOR test
The NOR task is a well-characterized behavioral measure of hippocampally based working nonspatial visual recognition memory in rodents.41 NOR task has been used to evaluate cognition, particularly recognition memory, in rodent models of central nervous system disorders. This test is based on the spontaneous tendency of rodents to spend more time exploring a novel object than a familiar one. The choice to explore the novel object reflects the use of learning and recognition memory. Given the more subtle nature of the histological damage, it is likely that traditional behavioral tasks used for TBI models may lack the sensitivity to detect deficits after a mild injury. The hippocampus and the perirhinal cortex play different roles in object recognition memory.
The NOR test is a simple method that does not need external motivation reward or punishment; however, a little training or habituation is required. It can be completed in a short time so animals are not stressed, and it can assess the recognition memory after only one trial, which gives it an advantage over other methods.42 This test was performed on all rats at 14 days and 35 days after injury.
Briefly, rats were habituated individually to an open field Plexiglas arena (50 × 40 × 30 cm) for a period of 3 min. Twenty-four hours later, in the acquisition phase, two identical objects (A and B) were placed in a symmetrical position within the arena. The objects are sufficiently large to ensure that the rat could neither move nor climb over them. The memory recognition assessment was performed 4 hours later after the acquisition test. Thereafter, one of the objects (A or B) was replaced randomly by a novel one (C), and the rat exploratory behavior was analyzed over a 3 min period. Exploration of an object was defined as rearing on the object, sniffing it at a distance of less than 2 cm, and/or touching it with the nose.
Successful recognition was represented by preferential exploration of the novel object over the familiar object. The time spent by each rat exploring the novel object over the familiar object was recorded and used to generate a preference index. A discrimination preference index was calculated as the following: time spent near the new object minus time spent near the old object)/(time spent near the new object plus time spent near the old object).37 After each session, the objects and arena were cleaned thoroughly with 70% ethanol to prevent odor recognition.
Three-chamber social interaction test
This test is used to assess memory for interactions with novel conspecifics.43,44 Rats tend to spend more time interacting with a rat over an object (sociability) or a novel rat versus one they have encountered previously (social novelty). The three chamber test can help identify rodents with deficits in sociability and/or social novelty.43 Decreased duration and/or number of contacts may be associated with depressive- and/or anxiety-like behavior,45 which are common after TBI, especially after mild TBI or in post-traumatic stress disorder (PTSD).46 This test was performed on all rats at 14 days and 35 days after injury.
The test rat was placed in the central compartment (20 × 35 × 35 cm) and remained there for a 2-min habituation period. Thereafter, retractable doors were removed, and the rat explored the left and right compartment (30 × 35 × 35 cm) for 5 min. After 5-min habituation, the left and right compartments were equipped with a cylinder (15 cm in diameter, 35 cm high) that contains either an unfamiliar rat or an unfamiliar object (empty container). The cylinders are perforated with holes (0.5 cm) to allow olfactory and nose-to-nose contact. The time spent by the test rat sniffing either the rat (social target) or the novel object (inanimate target) was recorded manually from videotapes by an experimenter who is blinded to the treatment groups. Then, an unfamiliar rat was placed in the empty cylinder (social novelty). The time spent by the test rat sniffing either the new rat (social novel target) or the old rat (social familiar target) was recorded manually.
A discrimination preference index was calculated as the following: (time spent near the new rat minus time spent near the old rat)/(time spent near the new rat plus time spent near the rat object).37 After each session, the arena was cleaned thoroughly with 70% ethanol to prevent odor recognition.
Evaluations of dendritic spine morphology
Animals
Brains were fixed following methods developed by Afraxis.47 Briefly, rats were anesthetized with IP ketamine/xylazine (100/10 mg/kg) and transcardially perfused with 4% paraformaldehyde. Whole brains were removed and post-fixed in the same fixative. All brain tissue was stored in 0.1 M PBS at 4°C. Within three days, brains were sectioned using a tissue vibratome (Leica VT1000) to collect sections (300 μm thick) from the anterior to posterior extremes of each brain.
Ballistic dye labeling and microscopy
Ballistic dye labeling (DiI and DiO) was performed according to protocols developed by Afraxis (San Diego, CA) to label target neurons, as described previously.48 Sections were slide mounted and cover slipped using ProLong Gold Mountant (ThermoFisher Scientific). Super-resolution laser-scanning confocal microscopy (Zeiss LSM880, Airyscan)49 was performed using a 63X objective (1.42 NA) to individually scan labeled neurons at high resolution (scan resolution = 0.06 μm/pixel; axial resolution = 0.06 μm/focal step). Target neurons were identified in the brain region of interest by epifluorescence navigation using anatomical location and cell morphology. Microscopy was performed blind to experimental conditions. A minimum of five rats was tested in each experimental condition. A minimum of five samples per rat (range = 5–12) was measured for each segment.
Afraxis enhanced spine profiling (ESP) dendritic spine analysis and assessment of dendritic membrane integrity
Airyscan post-processing (ZEN 2.3, Zeiss) was applied to raw three-dimensional digital micrographs followed by analysis for spine density and morphology by trained analysts. Individual spines were measured manually for (a) head diameter, (b) length, and (c) neck diameter from image Z-stacks using custom-built Afraxis ESP software. Each dendrite was analyzed by three (on average) independent analysts. Automated image assignment software distributed images to analysts in a randomized manner and ensured that each analyst performed measurements of near equal numbers of dendrites per group. Analysts were blinded to all experimental conditions (including treatment, brain region, and cell type). Statistical analysis of interanalyst variability for each dendrite was examined on line and used to eliminate dendrites that did not meet interanalyst reliability criteria: for spine density and spine morphological classification, data across analysts were averaged to report data for each dendrite.
Statistical analysis
All data are presented as the means with standard error of the mean. Analysis of variance (ANOVA) followed by post hoc Bonferroni tests was used for repeated measurements of cognitive and sensorimotor function to compare the difference between the SPG101-treated and DMSO-treated groups. Independent samples t test was used for NOR and three chamber tests between two groups (DMSO versus SPG101). For evaluations of total spine density, statistical significance was determined using the ANOVA test (SPSS), and spine morphological classes were evaluated by multi-variate (M) ANOVA. Treatment groups expressed normally distributed values (Shapiro-Wilk test, p > 0.05), and homogeneity of variances was not significant for any comparison (Levene statistic, p > 0.4; Box test of equality of co-variances, p > 0.3). Non-parametric comparisons of individual measure population distributions were conducted using the two-sample Kolmogorov-Smirnov (KS) test.
Considering the sensitivity of pooled-population non-parametric statistics, we applied a conservative hypothesis test (α = 0.0001). All experimenters were fully blinded to treatment conditions during the collection, assembly, and interpretation of the data.
Results
Safety
Body weight and side effects
Weight gain was monitored as the only major safety index in addition to multiple functional tests. There were no significant differences in body weight between the two groups over the 35-day study (Fig. 1, p > 0.05). In general, in both groups, all animals gained weight over time (p < 0.01). No other side effects of SPG101 or DMSO (i.e., death, seizures, dehydration, etc.) were observed during the 35-day study period.
FIG. 1.
Tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) administration does not change body weight in rats after traumatic brain injury (TBI). There was no significant difference in body weight change between rats with TBI treated with dimethyl sulfoxide (DMSO) and SPG101. All animals gained body weight over time. Data represent mean ± standard error of the mean. N = 7 rats/group. Color image is available online at www.liebertpub.com/neu
Death
A total of 14 animals were included in the study. No animal died during the 35-day study period.
Sensorimotor tests
Compared with the DMSO treatment, SPG101 treatment IP administered 1 h post-TBI and once daily for the next 34 days significantly improved sensorimotor recovery measured by foot fault (Fig. 2A for forelimb, F(1,72) = 94.92, p < 0.0001; and Fig. 2B for hindlimb, F(1,72) = 42.04, p < 0.0001), adhesive removal (Fig. 2C, F(1,72) = 35.95, p < 0.0001), and mNSS (Fig. 2D, F(1,72) = 96.30, p < 0.0001) starting at day seven post-TBI and lasting up to 35 days (p < 0.05 vs. DMSO, corrected by post hoc Bonferroni test).
FIG. 2.
Tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) administration significantly improves sensorimotor function measured by right forelimb, right hindlimb foot fault test, adhesive removal, and modified Neurological Severity Score (mNSS) in rats after traumatic brain injury (TBI). The line graph shows that SPG101 treatment significantly reduces forelimb (A) and hindlimb (B) foot faults, and adhesive removal (C), and significantly lowers mNSS scores (D) at days 7–35 compared with the DMSO group (p < 0.05). Data represent mean ± standard error of the mean. N = 7/group. Color image is available online at www.liebertpub.com/neu
MWM test
The MWM test is probably the most widely used test of spatial learning and memory. The MWM test demonstrated that TBI caused spatial learning deficits in rats after TBI induced by CCI in this study, consistent with our previous studies.14,50–52 Compared with the DMSO group, SPG101 treatment significantly reduced the time (latency, Fig. 3A, F(1, 60) = 218.83, p < 0.0001) for animals to reach the hidden platform in the water maze, and increased the percent time spent in the correct quadrant (Fig. 3B, F(1,60) = 267.89, p < 0.0001), indicating that SPG101 significantly improved spatial learning and memory in rats after TBI. There was no significant difference on the swim speed between the two TBI groups (Fig. 3C, F(1,60) = 0.5545, p = 0.4494), indicating that the swim speed did not contribute to spatial learning and memory deficits in these rats.
FIG. 3.
Tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) treatment significantly improves spatial learning performance measured by using a Morris water maze test. (A) SPG101 treatment reduces the latency to reach the hidden platform compared with dimethyl sulfoxide (DMSO) treatment at days 32–35 after traumatic brain injury (TBI) (p < 0.05). (B) SPG101 administration significantly improves percentage of time spent in the correct quadrant compared with the DMSO group at days 32–35 (p < 0.05). (C) There was no significant difference on the swim speed between the two TBI groups (p > 0.05), Data represent mean ± standard error of the mean. N = 7 rats/group. Color image is available online at www.liebertpub.com/neu
NOR test
The NOR task is a well-characterized behavioral measure of hippocampally based working nonspatial memory in rodents.53 NOR task has been used to evaluate cognition, particularly recognition memory, in rodent models of brain disorders.53,54 This test is based on the spontaneous tendency of rodents to spend more time exploring a novel object than a familiar one. The choice to explore the novel object reflects the use of learning and recognition memory. Compared with the DMSO treatment, SPG101 treatment significantly improved the NOR task measured at 14 days (t(12) = -7.463, p < 0.0001) and 35 days (t(12) = -3.881, p = 0.002) in rats after TBI (Fig. 4).
FIG. 4.
Tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) administration significantly improved the novel object recognition task measured at 14 and 35 days (p < 0.05) in rats after traumatic brain injury compared with the dimethyl sulfoxide (DMSO) treatment. Data represent mean ± standard error of the mean. N = 7 rats/group. Color image is available online at www.liebertpub.com/neu
Three-chambered social interaction task
This test is used to assess memory for interactions with novel conspecifics.55 Rats tend to spend more time interacting with a novel rat versus one they have encountered previously. Decreased duration and/or number of contacts may be associated with depressive- and/or anxiety-like behavior, which are common after TBI, especially after mild TBI or in PTSD. Compared with the DMSO treatment, SPG101 treatment significantly improved the sociability and social novelty task measured at 14 (Fig. 5A, t(12) = -5.076, p < 0.0001) and 35 days (Fig. 5B, t(12) = -4.480, p < 0.0001) in rats after TBI.
FIG. 5.
Tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) treatment significantly improved the sociability and social novelty task measured at 14 (A, p < 0.05) and 35 days (B, p < 0.05) in rats after traumatic brain injury compared with the dimethyl sulfoxide (DMSO) treatment. Data represent mean ± standard error of the mean. N = 7 rats/group. Color image is available online at www.liebertpub.com/neu
Dendritic spine analysis
Dendritic spine morphology was analyzed from samples taken from the dorsal hippocampus or somatosensory cortex, regions that express rapid and dramatic perturbations in dendritic arbors and spines after CCI.10,56 In total, three brain regions were sampled including CA1 pyramidal neurons (stratum radiatum, two degree branches), dentate granule neurons (DG; middle molecular layer, inner blade, two degree branches), and Layer 3 pyramidal neurons (L3P; apical two degree branches) of somatosensory cortex (SS-CTX). Representative laser scanning confocal micrographs of dendritic segments and dendritic spines for neurons are shown in Figure 6 for CA1, DG, and L3P samples from DMSO treated and SPG101 treated rats. Examples of individual spine classes (e.g., mushroom, stubby) are identified.
FIG. 6.
Representative super-resolution micrographs of dendritic segments and dendritic spines from CA1, dentate granule (DG), and somatosensory cortex (SS-CTX) neurons. Representative dendritic segments from dimethyl sulfoxide (DMSO)- and tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101)-treated rats with traumatic brain injury are shown. Inset panels show magnified views of segments for (a) CA1, (b) DG, and (c) SS-CTX. Distinct morphological classes are indicated: mushroom (arrow) and stubby (arrowhead).
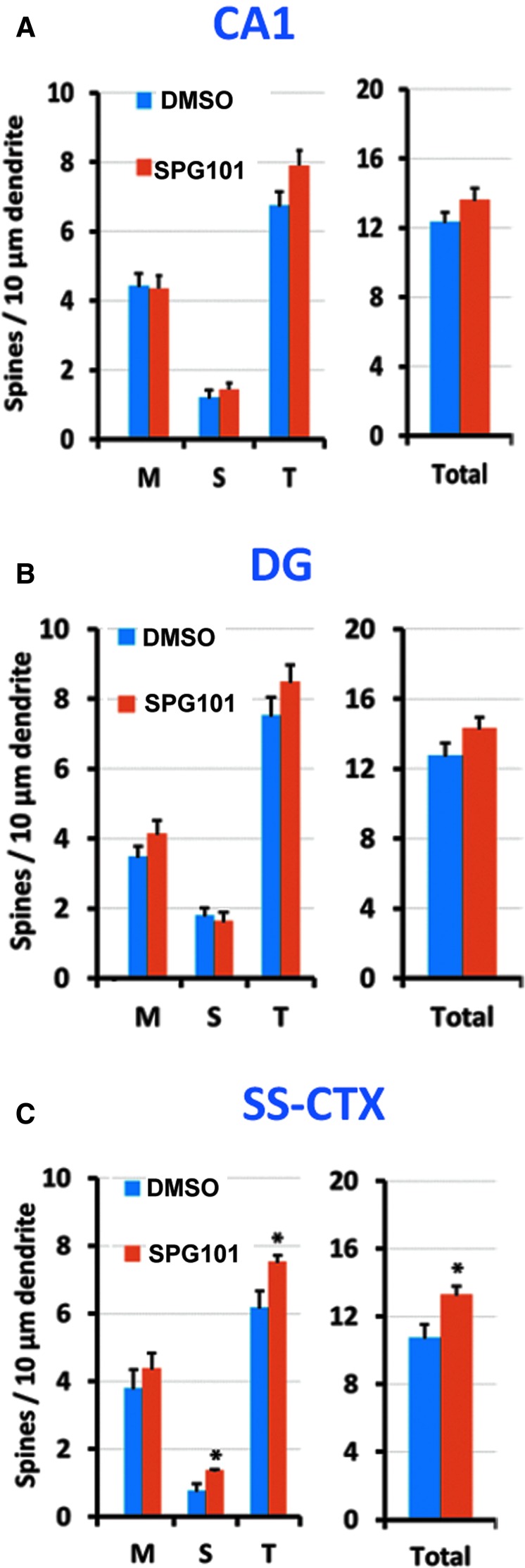
Total spine density
Total spine density values for each group are described in Fig. 7A–C. No significant differences in total spine density between DMSO and SPG101 treatment were detected in CA1, F(1,9) = 1.360, p = 0.28 or DG, F(1,9) = 0.844, p = 0.39. In contrast, SS-CTX neurons had significantly elevated densities in SPG101-treated (13.3 ± 0.97 spines/10 μm) versus DMSO-treated (10.77 ± 1.83) rats, F(1,9) = 6.298, p = 0.036.
FIG. 7.
Effect of tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) treatment on dendritic spine maturity and density in (A) CA1, (B) dentate granule (DG), and (C) somatosensory cortex (SS-CTX). Plots show group means (± standard error of the mean) spine morphologic classes (left plots) and total spine density in the same dendrites (right plots). M, mushroom, S, stubby, T, thin. Significance: * p < 0.05 compared with the dimethyl sulfoxide (DMSO) group. N = 7 rats/group. Color image is available online at www.liebertpub.com/neu
Dendritic spine maturity
Dendritic spine densities based on morphological class are described in Fig. 7A–C. There are no significant treatment differences in spine maturity (M, mushroom, S, stubby, T, thin) for either CA1, F(3,6) = 0.534, p = 0.68; Wilk Λ = 0.789; partial η = 0.211, or DG, F(3,6) = 1.048, p = 0.44; Wilk Λ = 0.656; partial η = 0.344, in TBI rats. Compared with DMSO treatment, however, SPG101 significantly increased the spine density of thin and stubby types (p < 0.05) in SS-CTX in TBI rats, F(3,6) = 0.444, p < 0.05; Wilk Λ = 2.505; partial η = 0.556.
Raw dendritic spine morphometric values
Frequency distributions were generated for: (a) spine lengths, (b) head diameters, and (c) neck diameters from pooled group samples. Cumulative frequency curves (Fig. 8–10) are shown for all segments. Compared with DMSO, SPG101 initiated 1 h post-injury produced a significant left-shift in spine lengths in the injured cortex, CA1, and DG (2-sample KS test, p < 0.0001), indicating shortening of population spine lengths. Additionally, DG selectively showed decreased heterogeneous distributions of spine head and spine neck diameters (p < 0.0001), indicating collective shrinkage or narrowing of these features. Although SPG101 did not increase the total number of mushroom-shaped spines, it produced a population-level shortening of spine length in CA1, DG, and cortex (Fig. 11).
FIG. 8.
Effect of tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) treatment on raw dendritic spine morphometric values in CA1. Plots show cumulative distributions for (A) spine length, (B) head diameter, and (C) neck diameter for pooled values from all five rats/group. *p < 0.0001 compared with the dimethyl sulfoxide (DMSO) group, two-sample Komolgorov-Smirnov test. Color image is available online at www.liebertpub.com/neu
FIG. 9.
Effect of tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) treatment on raw dendritic spine morphometric values in dentate granule neurons. Plots show cumulative distributions for (A) spine length, (B) head diameter, and (C) neck diameter for pooled values from all five rats/group. *p < 0.0001 compared with the dimethyl sulfoxide (DMSO) group, two-sample Komolgorov-Smirnov test. Color image is available online at www.liebertpub.com/neu
FIG. 10.
Effect of tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) treatment on raw dendritic spine morphometric values in somatosensory cortex neurons. Plots show cumulative distributions for (A) spine length, (B) head diameter, and (C) neck diameter for pooled values from all five rats/group. *p < 0.0001 compared with the dimethyl sulfoxide (DMSO) group, two-sample Komolgorov-Smirnov test. Color image is available online at www.liebertpub.com/neu
FIG. 11.
Effect of tetra (ethylene glycol) derivative of benzothiazole aniline (SPG101) treatment on multi-dimensional spine measurements (spine length × head diameter) in CA1, dentate granule (DG), and somatosensory cortex (SS-CTX). Plots show pseudocolor quantification of dual-dimension densities (top plots) and subtraction plots (target group minus paired control, bottom plots). (A) dimethyl sulfoxide (DMSO); (B) SPG101. N = five rats/group. Color image is available online at www.liebertpub.com/neu
Discussion
This randomized, placebo-controlled, and blinded pre-clinical study demonstrates that SPG101 at 30 mg/kg administered 1 h after TBI induced by CCI for a total of 34 consecutive days significantly improved sensorimotor and cognitive functional outcomes over time starting at seven days post-injury and lasted up to 35 days. SPG101 increases dendritic spine density in the injured cortex, decreases heterogeneous distribution of spine length in the injured cortex, CA1, and DG as well as decreases heterogeneous distribution of spine head diameter and neck diameter in the DG. SPG101 treatment promotes maturation (shortening) of spines on the apical dendrites of neurons in the injured cortex, CA1, and DG. These findings indicate that SPG101 acts as a novel spinogenic agent and may hold potential for treatment of those with TBI to improve both sensorimotor and cognitive functional recovery likely by modulating spine density, morphology, and maturation.
Post-synaptic activity is intimately linked with the dynamics of dendritic spines.57 The dendritic spine is the smallest neuronal compartment capable of performing a complete neurotransmission in a single synapse and is vital for higher brain functions, including learning and memory.57 Dendritic spines are tiny protrusions along neuronal dendrites that receive excitatory input from a single synapse of an axon.58 Changes in dendritic spine numbers and morphology have been implicated in a diverse set of neurological disorders including fragile X syndrome and Alzheimer disease.59,60
Many cognitive disorders are accompanied with loss of dendritic spines, yet there are few examples of molecules that promote the formation of new dendritic spines.61 A previous study demonstrates that SPG101 produces an age-specific improvement in synaptic density and cognitive function in a well-established AD mouse model.16 In particular, improvement in dendritic spine density is accompanied by changes in dendritic spine morphology in cortical layers II/III and the CA1 region of the hippocampus in 3xTg AD mice.16
Recently, a pre-clinical study has demonstrated that TBI induced by a lateral fluid percussion injury causes a transient reduction in the number of dendritic spines in the rat cortex.62 TBI-induced spine loss can be prevented with the calcineurin inhibitor FK506,62 demonstrating the involvement of this calcium-dependent pathway in the spine retraction process after injury. Previous studies show that a widespread reduction of dendritic spines occurs in layer II/III of the ipsilateral cortex and dentate gyrus in mice 24 h after CCI.56 A reduction in dendritic spines in multiple regions of the injured brain points to dendritic spines as potential substrates to explain, in part, the development of post-traumatic memory deficits. These data also suggest that dendritic spines may serve as a therapeutic target after TBI.
In the present study, we selected a dosage of 30 mg/kg of SPG101 daily for five weeks because of its pronounced effect on dendritic spine density in wild-type mice.17 Our data demonstrate that compared with the vehicle treatment, SPG101 initiated 1 h post-injury significantly improves sensorimotor functional recovery, spatial learning, novel object recognition, and social recognition in rats with TBI. Our present study demonstrates that SPG101 treatment increases dendritic spine density and decreases heterogeneous distribution of spine length in the injured cortex, which may partially contribute to functional recovery in rats with TBI.
We classified dendritic spines into filopodia, thin, stubby, or mushroom morphologies using ballistic dye labeling analysis combined with superresolution laser-scanning confocal microscopy. Mushroom-shaped spines are large and are the post-synaptic spines of mature synapses, whereas the filopodia- and stubby-shaped spines are small and are in the process of forming new synapses. To our knowledge, this is the first time such analysis has been performed in rats with TBI treated with a novel spinogenic agent.
In addition to dendritic spine density, dendritic spine morphology analyses can elucidate the effects of treatment on synapse formation. Each type of spine represents different stages during synaptogenesis. For example, long and thin dendritic spines are often classified as “immature learning” spines, whereas short and wide dendritic mushroom-shaped spines are classified as “mature memory” spines.63,64 Specifically, longer spines are thought of as substrates for conversion into mature spines via long-term potentiation (LTP)-type mechanisms, while wider spines typically mediate stronger synaptic transmission.65
The relation of spines of various morphologies to function remain a central enigma in the development of functional neuronal circuits.66 A filopodia can develop into various types of spines. A thin spine with a small head may transduce weak signals while a stubby spine with a large head and no neck may elicit strong signals diffusing through the surrounding dendrite.66 A relatively small mushroom spine may elicit a small amount of signal. A large mushroom spine with a short neck that elicits strong signal may diffuse through the surrounding dendrite.66 A large mushroom spine with a long and thin neck allows for strong compartmentalized signal within the spine head.66
Previously, it was reported that SPG101 treatment does not alter dendritic spine morphology in wild-type mice.17 It was observed, however, that SPG101 alters dendritic spine morphology in 3xTg AD mice at 6–10 months of age. In particular, dendritic spines were longer and wider after daily SPG101 application for two weeks, suggesting that SPG101 can regulate dendritic spine structure. In wild-type mice, SPG101 mainly improved memory with little improvement on learning while in 3xTg AD mice, both learning and memory were improved by SPG101.17
Our present study demonstrates that SPG101 treatment increases the number of stubby and thin spines in the injured cortex and decreases heterogeneous distribution of spine length in the injured cortex, CA1, and DG as well as decreases heterogeneous distribution of spine head diameter and neck diameter in the DG. Specifically, SPG101 increased the thin spine density in the CA1 and SS-CTX as well as the number of stubby spine density in the SS-CTX (Fig. 7). More importantly, although SPG101 did not increase the total number of mushroom-shaped spines, it produced a population-level shortening of spine length in CA1, DG, and cortex (Fig. 11). These data indicate that SPG101 promotes spine maturation, which may have functional significance.
The mechanisms underlying SPG101-induced dendritic spine plasticity have not been investigated fully. Administration of SPG101 to 3xTg AD mice shows improvement in learning and memory up to 6–10 months of age.16 At the cellular level, SPG101 treatment increased dendritic spine density in an AD mouse model with mild and moderate Aβ plaque deposition and synapse loss, and this effect was associated with increased Ras activity.17 Such an effect is supported by evidence that SPG101 promotes dendritic spine density through a full length amyloid precursor protein and Ras ERK-dependent mechanism in wild-type mice.17 These findings suggest that SPG101 may be a beneficial therapy for preventing and/or treating the synaptic loss accompanying AD, but also provide insights into how SPG101 can regulate synapses and spines generally. Ras GTPases are fundamental regulators of filamentous actin (F-actin), which is highly enriched in dendritic spines, and their capacity to change shape relies on the rapid rearrangement of the actin cytoskeleton within spines.67 Such plastic structural adjustments are indeed required for the stable formation of long-term potentiation and fear memory consolidation.68 Therefore, it is not surprising that promoting spine remodeling after TBI attenuates cognitive/motor functional deficits that may result, at least in part, from spine degeneration and loss.13
Collectively, our findings suggest that BTA-ET4 promotes spine maturation in these brain regions that is associated with improved behavioral recovery after TBI. Our results suggest that SPG101 might be a valuable drug for the treatment of those with TBI, particularly if used in combination therapy with other neuroprotective and/or anti-inflammatory drugs or hypothermia.
There are several limitations in this TBI study with a relatively early and long-term treatment with SPG101. First, it is unclear whether the early and short-term or later treatments or different doses have similar beneficial effects on functional recovery and spine remodeling. Second, it is warranted to determine whether delayed treatment with SPG101 is beneficial because early treatment within 1 h is not practical in clinical settings. Third, this study focused on spine remodeling, which is important for synaptic plasticity. There are many other important aspects of neurovascular remodeling including the effect of SPG101 on angiogenesis, neurogenesis, and remyelination involved in TBI recovery that have not been investigated in the present study. Fourth, the TBI model we employed in this study produces mainly focal contusion. TBI in the clinical setting is a heterogeneous injury with a combination of hematomas, contusion, diffuse axonal injury, subarachnoid hemorrhage, hypoxia, and ischemia and medication/substance use. To better mimic those clinical situations, it is warranted to test the effects of SPG101 in multiple animal TBI models. Fifth, SPG101 may have the beneficial effects in sham animals, as demonstrated in wild-type mice.17
The aim of the present study was to investigate whether SPG101 would improve functional recovery after TBI and have potential therapeutic effects on spine plasticity compared with the vehicle DMSO therapy in rats with TBI. To minimize the number of animals used, the effect of SPG101 on sham animals was not studied. To evaluate the efficacy of a therapeutic agent for treatment of those with TBI, investigation of sham-injured animals treated with or without the agent is not necessary if the TBI models like the CCI model we used in this study are well established and the safety of the drug is not a concern, as shown by us36 and other groups69–74 with the CCI model in rodents and swine. Inclusion of the sham group treated with SPG101 may provide some information on its effects on sham rats but will not change the conclusion of this study.
Conclusion
Our data support the idea that defects in changes in spine density and morphology may be an important contributor to TBI-induced sensorimotor and learning and memory dysfunction. Spinogenic agent SPG101 increases spine density and changes distribution of specific spine types as well as promotes spine maturation, reduces sensorimotor, learning, and memory deficits in rats after TBI. Further investigations of the optimal dose and therapeutic window of SPG101 treatment for TBI and the molecular mechanisms underlying beneficial effects on functional recovery and spine remodeling are warranted.
Acknowledgments
This work was supported by Spinogenix and NIH NOA R43AG056236. Super-resolution microscopy was supported by NINDS P30 NS047101. The authors would like to thank Susan MacPhee-Gray for editorial assistance and Janelle Ilagen, Kimberly St. Aubin, David Chau, and Chase Shankula for technical assistance.
Author Disclosure Statement
Vincent Simmon is an employee of Spinogenix, Inc. San Diego, California. Stella Sarraf is a founder of Spinogenix, Inc. Christopher Rex is a founder and employee of Afraxis, Inc. For the remaining authors, no competing financial interests exist.
References
- 1. Taylor C.A., Bell J.M., Breiding M.J., and Xu L. (2017). Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon D.W., McGeachy M.J., Bayir H., Clark R.S., Loane D.J., and Kochanek P.M. (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 572. [DOI] [PubMed] [Google Scholar]
- 3. Hu Q., Manaenko A., Xu T., Guo Z., Tang J., and Zhang J.H. (2016). Hyperbaric oxygen therapy for traumatic brain injury: bench-to-bedside. Med. Gas. Res. 6, 102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldstein F.C., Caveney A.F., Hertzberg V.S., Silbergleit R., Yeatts S.D., Palesch Y.Y., Levin H.S., and Wright D.W. (2017). Very early administration of progesterone does not improve neuropsychological outcomes in subjects with moderate to severe traumatic brain injury. J. Neurotrauma 34, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bramlett H.M., Dietrich W.D., Dixon C.E., Shear D.A., Schmid K.E., Mondello S., Wang K.K., Hayes R.L., Povlishock J.T., Tortella F.C., and Kochanek P.M. (2016). Erythropoietin treatment in traumatic brain injury: operation brain trauma therapy. J. Neurotrauma 33, 538–552 [DOI] [PubMed] [Google Scholar]
- 6. Lewis S.R., Evans D.J., Butler A.R., Schofield-Robinson O.J., and Alderson P. (2017). Hypothermia for traumatic brain injury. Cochrane Database Syst. Rev. 9, CD001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiong Y., Zhang Y., Mahmood A., and Chopp M. (2015). Investigational agents for treatment of traumatic brain injury. Expert. Opin. Investig. Drugs 24, 743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magee J.C. (2000). Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 1, 181–190 [DOI] [PubMed] [Google Scholar]
- 9. Gao X. and Chen J. (2011). Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 70, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao X., Deng P., Xu Z.C., and Chen J. (2011). Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS One 6, e24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao S., Gao X., Dong W., and Chen J. (2016). The role of 7,8-dihydroxyflavone in preventing dendrite degeneration in cortex after moderate traumatic brain injury. Mol. Neurobiol. 53, 1884–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell J.N., Low B., Kurz J.E., Patel S.S., Young M.T., and Churn S.B. (2012). Mechanisms of dendritic spine remodeling in a rat model of traumatic brain injury. J. Neurotrauma 29, 218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang C.F., Zhao C.C., Jiang G., Gu X., Feng J.F., and Jiang J.Y. (2016). The role of posttraumatic hypothermia in preventing dendrite degeneration and spine loss after severe traumatic brain injury. Sci. Rep. 6, 37063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y., Zhang Z.G., Chopp M., Meng Y., Zhang L., Mahmood A., and Xiong Y. (2017). Treatment of traumatic brain injury in rats with N-acetyl-seryl-aspartyl-lysyl-proline. J, Neurosurg, 126, 782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee N.J., Song J.M., Cho H.J., Sung Y.M., Lee T., Chung A., Hong S.H., Cifelli J.L., Rubinshtein M., Habib L.K., Capule C.C., Turner R.S., Pak D.T., Yang J., and Hoe H.S. (2016). Hexa (ethylene glycol) derivative of benzothiazole aniline promotes dendritic spine formation through the RasGRF1-Ras dependent pathway. Biochim. Biophys. Acta 1862, 284–295 [DOI] [PubMed] [Google Scholar]
- 16. Song J.M., DiBattista A.M., Sung Y.M., Ahn J.M., Turner R.S., Yang J., Pak D.T., Lee H.K., and Hoe H.S. (2014). A tetra(ethylene glycol) derivative of benzothiazole aniline ameliorates dendritic spine density and cognitive function in a mouse model of Alzheimer's disease. Exp. Neurol. 252, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Megill A., Lee T., DiBattista A.M., Song J.M., Spitzer M.H., Rubinshtein M., Habib L.K., Capule C.C., Mayer M., Turner R.S., Kirkwood A., Yang J., Pak D.T., Lee H.K,. and Hoe H.S. (2013). A tetra(ethylene glycol) derivative of benzothiazole aniline enhances Ras-mediated spinogenesis. J. Neurosci. 33, 9306–9318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prangkio P., Rao D.K., Lance K.D., Rubinshtein M., Yang J., and Mayer M. (2011). Self-assembled, cation-selective ion channels from an oligo(ethylene glycol) derivative of benzothiazole aniline. Biochim. Biophys. Acta 1808, 2877–2885 [DOI] [PubMed] [Google Scholar]
- 19. Dixon C.E., Kochanek P.M., Yan H.Q., Schiding J.K., Griffith R.G., Baum E., Marion D.W., and DeKosky S.T. (1999). One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J. Neurotrauma 16, 109–122 [DOI] [PubMed] [Google Scholar]
- 20. Mahmood A., Lu D., Qu C., Goussev A., and Chopp M. (2005). Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery 57, 1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiong Y., Mahmood A., Meng Y., Zhang Y., Qu C., Schallert T., and Chopp M. (2010). Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J. Neurosurg. 113, 598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiong Y., Gu Q., Peterson P.L., Muizelaar J.P., and Lee C.P. (1997). Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 14, 23–34 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y., Chopp M., Zhang Z.G., Katakowski M., Xin H., Qu C., Ali M., Mahmood A., and Xiong Y. (2017). Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 111, 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y., Zhang Z.G., Chopp M., Meng Y., Zhang L., Mahmood A., and Xiong Y. (2017). Treatment of traumatic brain injury in rats with N-acetyl-seryl-aspartyl-lysyl-proline. J. Neurosurg, 136, 782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiong Y., Mahmood A., and Chopp M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marklund N. and Hillered L. (2011). Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 164, 1207–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morganti-Kossmann M.C., Yan E., and Bye N. (2010). Animal models of traumatic brain injury: is there an optimal model to reproduce human brain injury in the laboratory? Injury 41, Suppl 1, S10–S13 [DOI] [PubMed] [Google Scholar]
- 28. Dixon C.E., Kraus M.F., Kline A.E., Ma X., Yan H.Q., Griffith R.G., Wolfson B.M., and Marion D.W. (1999). Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 14, 285–294 [PubMed] [Google Scholar]
- 29. Faden A.I., Movsesyan V.A., Knoblach S.M., Ahmed F., and Cernak I. (2005). Neuroprotective effects of novel small peptides in vitro and after brain injury. Neuropharmacology 49, 410–424 [DOI] [PubMed] [Google Scholar]
- 30. Hall E.D., Bryant Y.D., Cho W., and Sullivan P.G. (2008). Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 25, 235–247 [DOI] [PubMed] [Google Scholar]
- 31. Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 32. Kline A.E., Massucci J.L., Marion D.W., and Dixon C.E. (2002). Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma 19, 415–425 [DOI] [PubMed] [Google Scholar]
- 33. Kochanek P.M., Hendrich K.S., Dixon C.E., Schiding J.K., Williams D.S., and Ho C. (2002). Cerebral blood flow at one year after controlled cortical impact in rats: assessment by magnetic resonance imaging. J. Neurotrauma 19, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 34. Kochanek P.M., Marion D.W., Zhang W., Schiding J.K., White M., Palmer A.M., Clark R.S., O'Malley M.E., Styren S.D., Ho C., et al. (1995). Severe controlled cortical impact in rats: assessment of cerebral edema, blood flow, and contusion volume. J. Neurotrauma 12, 1015–1025 [DOI] [PubMed] [Google Scholar]
- 35. Chen J., Sanberg P.R., Li Y., Wang L., Lu M., Willing A.E., Sanchez-Ramos J., and Chopp M. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y., Chopp M., Meng Y., Zhang Z.G., Doppler E., Mahmood A., and Xiong Y. (2013). Improvement in functional recovery with administration of Cerebrolysin after experimental closed head injury. J. Neurosurg. 118, 1343–1355 [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y., Chopp M., Gang Zhang Z., Zhang Y., Zhang L., Lu M., Zhang T., Winter S., Brandstatter H., Mahmood A., and Xiong Y. (2018). Prospective, randomized, blinded, and placebo-controlled study of Cerebrolysin dose-response effects on long-term functional outcomes in a rat model of mild traumatic brain injury. J. Neurosurg. E-ub ahead of print [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y., Chopp M., Meng Y., Zhang Z.G., Doppler E., Winter S., Schallert T., Mahmood A., and Xiong Y. (2015). Cerebrolysin improves cognitive performance in rats after mild traumatic brain injury. J. Neurosurg. 122, 843–855 [DOI] [PubMed] [Google Scholar]
- 39. Staffend N.A. and Meisel R.L. (2011). DiOlistic labeling in fixed brain slices: phenotype, morphology, and dendritic spines. Curr. Protoc. Neurosci. Chapter 2, Unit 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi S.H., Woodlee M.T., Hong J.J., and Schallert T. (2006). A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J. Neurosci. Methods 156, 182–193 [DOI] [PubMed] [Google Scholar]
- 41. Stuart S.A., Robertson J.D., Marrion N.V., and Robinson E.S. (2013). Chronic pravastatin but not atorvastatin treatment impairs cognitive function in two rodent models of learning and memory. PLoS One 8, e75467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bengoetxea X., Rodriguez-Perdigon M., and Ramirez M.J. (2015). Object recognition test for studying cognitive impairments in animal models of Alzheimer's disease. Front. Biosci. (Schol Ed) 7, 10–29 [DOI] [PubMed] [Google Scholar]
- 43. Gonzales E.L., Yang S.M., Choi C.S., Mabunga D.F., Kim H.J., Cheong J.H., Ryu J.H., Koo B.N., and Shin C.Y. (2015). Repeated neonatal propofol administration induces sex-dependent long-term impairments on spatial and recognition memory in rats. Biomol. Ther. (Seoul) 23, 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crawley J.N. (2004). Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 10, 248–258 [DOI] [PubMed] [Google Scholar]
- 45. Semple B.D., Canchola S.A., and Noble-Haeusslein L.J. (2012). Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J. Neurotrauma 29, 2672–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elder G.A., Dorr N.P., De Gasperi R., Gama Sosa M.A., Shaughness M.C., Maudlin-Jeronimo E., Hall A.A., McCarron R.M., and Ahlers S.T. (2012). Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma 29, 2564–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waworuntu R.V., Hanania T., Boikess S.R., Rex C.S., and Berg B.M. (2016). Early life diet containing prebiotics and bioactive whey protein fractions increased dendritic spine density of rat hippocampal neurons. Int. J. Dev. Neurosci. 55, 28–33 [DOI] [PubMed] [Google Scholar]
- 48. Ota K.T., Liu R.J., Voleti B., Maldonado-Aviles J.G., Duric V., Iwata M., Dutheil S., Duman C., Boikess S., Lewis D.A., Stockmeier C.A., DiLeone R.J., Rex C., Aghajanian G.K., and Duman R.S. (2014). REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 20, 531–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sheppard C.J., Mehta S.B., and Heintzmann R. (2013). Superresolution by image scanning microscopy using pixel reassignment. Opt. Lett. 38, 2889–2892 [DOI] [PubMed] [Google Scholar]
- 50. Zhang Y., Chopp M., Meng Y., Katakowski M., Xin H., Mahmood A., and Xiong Y. (2015). Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 122, 856–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xiong Y., Mahmood A., Meng Y., Zhang Y., Zhang Z.G., Morris D.C., and Chopp M. (2011). Treatment of traumatic brain injury with thymosin beta(4) in rats. J. Neurosurg. 114, 102–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiong Y., Qu C., Mahmood A., Liu Z., Ning R., Li Y., Kaplan D.L., Schallert T., and Chopp M. (2009). Delayed transplantation of human marrow stromal cell-seeded scaffolds increases transcallosal neural fiber length, angiogenesis, and hippocampal neuronal survival and improves functional outcome after traumatic brain injury in rats. Brain Res. 1263, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cohen S.J. and Stackman R.W., Jr (2015). Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 285, 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grayson B., Leger M., Piercy C., Adamson L., Harte M., and Neill J.C. (2015). Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 285, 176–193 [DOI] [PubMed] [Google Scholar]
- 55. Reilly M.P., Weeks C.D., Topper V.Y., Thompson L.M., Crews D., and Gore A.C. (2015). The effects of prenatal PCBs on adult social behavior in rats. Horm. Behav. 73, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Winston C.N., Chellappa D., Wilkins T., Barton D.J., Washington P.M., Loane D.J., Zapple D.N., and Burns M.P. (2013). Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. J. Neurotrauma 30, 1966–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maiti P., Manna J., Ilavazhagan G., Rossignol J., and Dunbar G.L. (2015). Molecular regulation of dendritic spine dynamics and their potential impact on synaptic plasticity and neurological diseases. Neurosci. Biobehav. Rev. 59, 208–237 [DOI] [PubMed] [Google Scholar]
- 58. Rochefort N.L. and Konnerth A. (2012). Dendritic spines: from structure to in vivo function. EMBO Rep. 13, 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Catala I., Ferrer I., Galofre E., and Fabregues I. (1988). Decreased numbers of dendritic spines on cortical pyramidal neurons in dementia. A quantitative Golgi study on biopsy samples. Hum. Neurobiol. 6, 255–259 [PubMed] [Google Scholar]
- 60. Nagaoka A., Takehara H., Hayashi-Takagi A., Noguchi J., Ishii K., Shirai F., Yagishita S., Akagi T., Ichiki T., and Kasai H. (2016). Abnormal intrinsic dynamics of dendritic spines in a fragile X syndrome mouse model in vivo. Sci. Rep. 6, 26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cheung Z.H. and Ip N.Y. (2011). From understanding synaptic plasticity to the development of cognitive enhancers. Int. J. Neuropsychopharmacol. 14, 1247–1256 [DOI] [PubMed] [Google Scholar]
- 62. Campbell J.N., Register D., and Churn S.B. (2012). Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. J. Neurotrauma 29, 201–217 [DOI] [PubMed] [Google Scholar]
- 63. Kasai H., Matsuzaki M., Noguchi J., and Yasumatsu N. (2002). [Dendritic spine structures and functions]. Nihon Shinkei Seishin Yakurigaku Zasshi 22, 159–164 [PubMed] [Google Scholar]
- 64. Yasumatsu N., Matsuzaki M., Miyazaki T., Noguchi J., and Kasai H. (2008). Principles of long-term dynamics of dendritic spines. J. Neurosci. 28, 13592–13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matsuzaki M., Ellis-Davies G.C., Nemoto T., Miyashita Y., Iino M., and Kasai H. (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4, 1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ebrahimi S. and Okabe S. (2014). Structural dynamics of dendritic spines: molecular composition, geometry and functional regulation. Biochim. Biophys. Acta 1838, 2391–2398 [DOI] [PubMed] [Google Scholar]
- 67. Patterson M.A., Szatmari E.M., and Yasuda R. (2010). AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc. Natl. Acad. Sci. U. S. A. 107, 15951–15956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rex C.S., Gavin C.F., Rubio M.D., Kramar E.A., Chen L.Y., Jia Y., Huganir R.L., Muzyczka N., Gall C.M., Miller C.A., Lynch G., and Rumbaugh G. (2010). Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron 67, 603–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y., Liu Y., Lopez D., Lee M., Dayal S., Hurtado A., Bi X., and Baudry M. (2018). Protection against TBI-induced neuronal death with post-treatment with a selective calpain-2 inhibitor in mice. J. Neurotrauma 35, 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Talley Watts L., Long J.A., Boggs R.C., Manga H., Huang S., Shen Q., and Duong T.Q. (2016). Delayed methylene blue improves lesion volume, multi-parametric quantitative magnetic resonance imaging measurements, and behavioral outcome after traumatic brain injury. J. Neurotrauma 33, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Korley F.K., Nikolian V.C., Williams A.M., Dennahy I.S., Weykamp M., and Alam H.B. (2018). Valproic acid treatment decreases serum glial fibrillary acidic protein and neurofilament light chain levels in swine subjected to traumatic brain injury. J. Neurotrauma 35, 1185–1191 [DOI] [PubMed] [Google Scholar]
- 72. Talley Watts L., Long J.A., Chemello J., Van Koughnet S., Fernandez A., Huang S., Shen Q., and Duong T.Q. (2014). Methylene blue is neuroprotective against mild traumatic brain injury. J. Neurotrauma 31, 1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Williams A.M., Dennahy I.S., Bhatti U.F., Halaweish I., Xiong Y., Chang P., Nikolian V.C., Chtraklin K., Brown J., Zhang Y., Zhang Z.G., Chopp M., Buller B., and Alam H.B. (2018). Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J. Neurotrauma Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 74. Georgoff P.E., Nikolian V.C., Halaweish I., Chtraklin K., Bruhn P.J., Eidy H., Rasmussen M., Li Y., Srinivasan A., and Alam H.B. (2017). Resuscitation with lyophilized plasma is safe and improves neurological recovery in a long-term survival model of swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J. Neurotrauma 34, 2167–2175 [DOI] [PubMed] [Google Scholar]