Abstract
Polyenes and azoles constitute 2 major drug classes in the antifungal armamentarium used to treat fungal infections of the eye such as fungal keratitis, endophthalmitis, conjunctivitis, and blepharitis. These classes of drugs have come to occupy an important niche in ophthalmic antifungal therapy due to their broad spectrum of activity against a variety of filamentous and yeast-like fungi. Natamycin suspension (Natacyn®), a polyene antifungal drug, is currently the only US FDA-approved formulation for treating ophthalmic fungal infections, whereas the other polyene and azole antifungals such as amphotericin B, fluconazole, itraconazole, ketoconazole, miconazole, voriconazole, and posaconazole are routinely used off-label in the clinical setting. Despite potent antifungal activity, the clinical utility of these agents in ophthalmic infections has been challenged by their physicochemical properties, the unique ocular anatomy and physiology, selective antifungal activity, ocular and systemic toxicity, emergence of resistance and cross-resistance, and absence of reliable techniques for developing a robust in vitro-in vivo correlation. This review discusses the aforementioned challenges and the common approaches undertaken to circumnavigate the difficulties associated with the polyene- and azole-based pharmacotherapy of ophthalmic fungal infections.
Keywords: ocular fungal infections, anti-infectives, polyene and azole antifungals, keratitis, ocular pharmacotherapy
Introduction
Ocular fungal infections such as fungal and bacterial keratitis affect a population of nearly 1 million annually in the United States.1–4 The incidences of keratitis account for an estimated 930,000 visits to the doctor's office and outpatient clinics and ∼58,000 emergency department visits with ∼76.5% of the keratitis visits requiring drug prescriptions.2–5 Episodes of keratitis and other ocular corneal infections led to an estimated $175 million in direct health care expenditures in the United States annually, including $58 million for Medicare patients and $12 million for Medicaid patients, according to an analysis by Collier et al. in Morbidity and Mortality Weekly Report for the Centers for Disease Control and Prevention.2,3,5
The polyene and azole antifungals have been the mainstay in the pharmacotherapy of invasive systemic fungal infections due to their broad antifungal spectrum and potent biological activity.6,7 The polyene class, comprising amphotericin B, nystatin, and natamycin, has been widely used in therapy owing to the antifungal activity against Candida spp., Aspergillus spp., Fusarium spp., Scedosporium spp., and Zygomycetes classes of fungi, which are the common causative species for fungal infections.8,9 The polyene antifungals also report few cases of emergence of resistance and cross-resistance, and the use of lipid-based polyene formulations (especially amphotericin B) has provided alternatives with greater safety and lower toxicity profiles.10,11 Azoles, such as the polyenes, exhibit a broad spectrum of activity. Azoles show biological activity against Candida spp., Fusarium spp., Aspergillus spp., and the Zygomycetes.12,13 Fluconazole is considered to be a more cost-effective and safe antifungal agent with low toxicity profile among the azole antifungals, whereas the other 2 clinically important azoles, itraconazole and voriconazole, even though displaying a broad spectrum of potent antifungal activity, exhibit concentration-dependent toxic and adverse side effects, in ocular and invasive systemic fungal infections.14–17
These polyene and azole antifungal agents have been used as the front-line therapeutic agents in invasive fungal systemic infections, onychomycosis, ophthalmic fungal infections, fungal dermatitis, and meningitis.18–22 However, their use in ocular fungal therapy has been a challenge due to the unique ocular anatomy and the physicochemical properties of these agents. This challenge is evident because only natamycin (Natacyn®) has been available commercially for the treatment of ophthalmic fungal infections; the other antifungals are used off-label.7
Ocular fungal infections, like fungal infections in general, are on a steady upsurge, necessitating the introduction of other polyene and azole antifungal drugs as optimized commercial ophthalmic formulations that can provide a safe and cost-effective therapy.3,23 This review delves into the challenges associated with the delivery of polyene and azole antifungal agents in ophthalmic infections, along with a literature review of the studies that have concentrated on circumnavigating the barriers to ocular delivery.
Chemistry, Mechanism of Action, and Antifungal Activity of the Polyene and Azole Antifungals
Chemistry
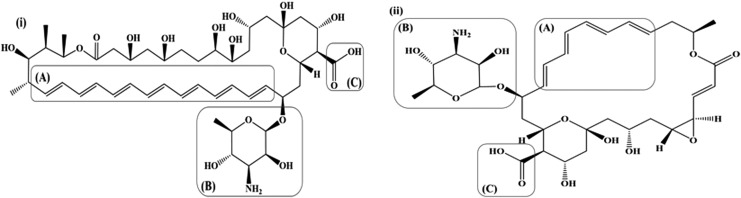
Polyenes are characterized by the presence of multiple conjugated double bonds [Fig. 1: i(A) and ii(A)] in a hydroxylated chromophore. Based on the No. of the conjugated double bonds, amphotericin B and natamycin are classified as heptaene and tetraene polyene antifungal drugs.24 The conjugated double bond lactone chromophore possesses an all-trans conformation and is essential to the antifungal activity and stability of amphotericin B and natamycin.25,26 The conjugated double bonds impart lipophilicity, while hydroxylation on the chromophore imparts hydrophilicity to the 2 polyene antifungals. The chromophores of both the polyene antifungal drugs (amphotericin B and natamycin) are also characterized by the attachment of a mycosamine (basic) moiety [Fig. 1: i(B) and ii(B)] through an ether linkage and carboxylic (acidic) group [Fig. 1: i(C) and ii(C)]. These groups impart an amphoteric/amphipathic character to both the polyenes. The amino group in the mycosamine moiety exhibits a pKa of ∼8.6, whereas the pKa value for the carboxyl group is reported to be ∼4–4.5. Thus, amphotericin B and natamycin are zwitterionic species with an isoelectric point at a pH range of ∼5–7.24,27–29 The mycosamine and the carboxyl terminals impart a polar character (contributes to the relative insolubility in organic solvents), whereas the opposite unsaturated terminal imparts a nonpolar character (contributes to the poor aqueous solubility) to amphotericin B and natamycin.24,29
FIG. 1.
(i) Amphotericin B, (ii) Natamycin; [(A): Conjugated multiple bonds, (B): Mycosamine moiety, (C): Carboxylic group].
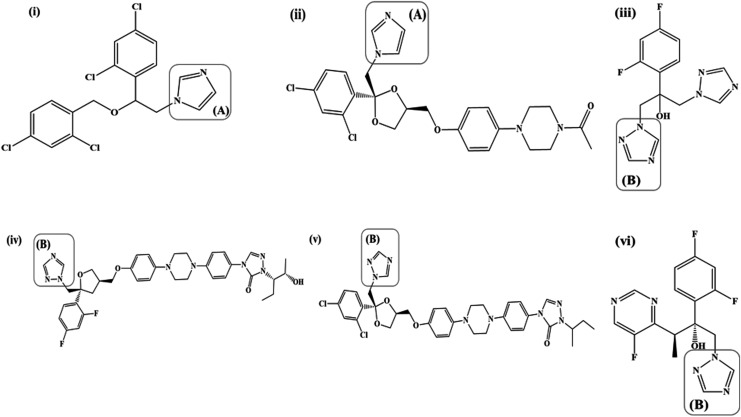
Azole antifungals are characterized by the presence of imidazole [Fig. 2: i(A) and ii(A)] or 1,2,4-triazole [Fig. 2: iii(B), iv(B), v(B), and vi(B)] rings bound to the rest of the structure through a nitrogen-carbon bond. The imidazole and 1,2,4-triazole rings impart a weak basic character to the azole antifungals with the nitrogen atoms having a pKa in the range of 6.5–6.8.30 The nitrogen atom (N-3 in the imidazoles and N-4 in the triazoles) is believed to bind to the heme portion of cytochrome P-450 resulting in inhibition of demethylation of lanosterol, an essential step in the biosynthesis of ergosterol.31 Azoles possess 2 or 3 aromatic rings with an attached halogen substituent (Cl: miconazole, itraconazole, ketoconazole; F: fluconazole, voriconazole, posaconazole); the aromatic rings and the halogen substituents are essential to the antifungal potency and activity.30,31 The No. of aromatic rings and halogen substituents determine the hydrophilic/lipophilic balance of the azole antifungals with the former imparting a lipophilic character and the latter a hydrophilic character.30 Most of the azole antifungals (except fluconazole) possess a high degree of lipophilicity, which renders them poorly soluble in an aqueous medium.30,31
FIG. 2.
(i) Miconazole, (ii) Ketoconazole, (iii) Fluconazole, (iv) Posaconazole, (v) Itraconazole, (vi) Voriconazole; [(A): Imidazole, (B): 1,2,4-triazole].
Mechanism of action
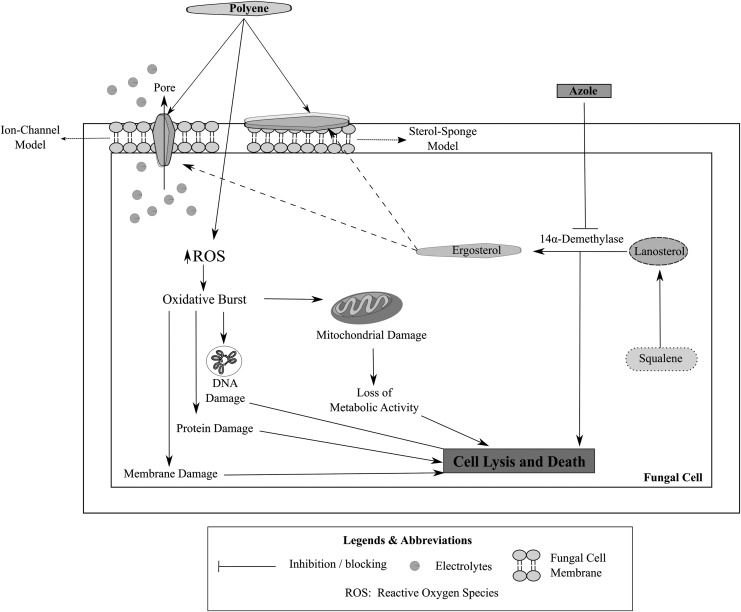
Fungi contain sterols that are responsible for mediating their cellular and physiological functions. Most of these sterols are similar to those found in humans; hence, a unique and specific fungal target (that is absent in the human host) is essential for the selective action of an antifungal agent.32 Ergosterol, a sterol that is specifically present in the fungal species and absent in the human hosts, is one of the most common targets for an antifungal drug.32,33 Disruption of the ergosterol biosynthesis pathway, depriving the fungal species of ergosterol (an essential structural and signaling sterol), constitutes the predominant mechanism of action of most of the polyene and azole antifungal agents.34,35
Ergosterol is an essential fungal sterol that is responsible for maintaining membrane fluidity and integrity and relaying cellular signals in fungal cells.36 Polyene antifungal drugs are known to exhibit antifungal activity by binding to ergosterol and inhibiting its cellular functions with (amphotericin B) or without (natamycin) permeabilizing the fungal membrane.23,33 Amphotericin B and natamycin are known to bind to ergosterol and to inhibit its cellular activity, leading to a fungicidal action.33,37,38 The binding of polyene antifungals to ergosterol results in the inhibition of ergosterol-dependent membrane fusion and fission processes, endocytosis, and plasma protein complexes, leading to the death of the fungal species.39–45 In addition, amphotericin B-ergosterol binding also results in the formation of aqueous pores leading to a permeabilization of the fungal cell membrane causing efflux of potassium and other cellular components and eliciting antifungal activity.46,47 The binding of amphotericin B to ergosterol is considered a predominant mode of action for amphotericin B, while the permeabilizing effect is considered a secondary mode of action.47,48
Azoles, in contrast to the polyene antifungals, act by inhibiting the biosynthesis of ergosterol. Azoles bind to the heme protein, which cocatalyzes cytochrome P-450-dependent 14α-demethylation of lanosterol, an essential step in the biosynthesis of ergosterol, leading to a depletion in the ergosterol reserves in the fungal cells,49 resulting in the accumulation of ergosterol precursors (lanosterol, 4,14-dimethylzymosterol, and 24-methylenedihydrolanosterol) and leading to an alteration in the structure and integrity of the fungal cell membrane that causes fungal cell death. In addition to the inhibition of 14α-demethylase enzyme, the newer azoles such as fluconazole, itraconazole, and voriconazole also exhibit supplementary mechanisms of action that lead to the buildup of other ergosterol precursors, apart from the abovementioned sterols, such as squalene, obtusifolione, and zymosterol.33
Figure 3 illustrates the mechanism of action of polyene and azole antifungal drugs in fungal cells.
FIG. 3.
Mechanism of action of polyene and azole antifungal drugs in fungal cells.
Antifungal activity
Polyene antifungal drugs—amphotericin B and natamycin—possess a broad spectrum of activity against filamentous and yeast-like fungi (natamycin exhibits weak-to-moderate action against the yeast-like fungal forms).22,23,31 The broad spectrum of activity of the polyene drugs is owing to the conjugated double bonds (affinity to ergosterol found in most fungal species) in the cyclic polyene structure and the mycosamine moiety.26 These structural features along with the hydroxyl groups in the cyclic backbone provide desired conformation for the formation of hydrogen bonds and ion channels.26 Table 1 elaborates on the antifungal activity of the 2 polyene drugs against various fungal species with the minimum inhibitory concentration (MIC) corresponding to each species.23,39,47,50–63
Table 1.
Antifungal Activity of Amphotericin B and Natamycin with Their Minimum Inhibitory Concentration Against Various Clinically Relevant Fungal Species
| Polyene drugs (MIC: μg/mL) | |||
|---|---|---|---|
| Species/strains | Amphotericin B | Natamycin | Reference |
| Candida | 0.25–2 | 1–2 | 44,47–50 |
| Aspergillus | 1–4 | 5–40 | 51–53 |
| Fusarium | 1 to >4 | 4–8 | 51,52,54 |
| Penicillium | 1 | 1–3 | 36,55 |
| Paecilomyces | 0.25 to >16 | 2–6 | 55 |
| Rhizopus | 0.5–2 | 2–6 | 52,56 |
| Cunninghamella | 0.5–2 | <4 | 55,57 |
| Others (Cryptococcus neoformans, Histoplasma capsulatum, Blastomyces dermatitidis, Sporothrix schenckii, Coccidioides immitis) | 0.25–4 | 1–25 | 33,36,57–60 |
MIC, minimum inhibitory concentration.
Among the polyene class of antifungal drugs, amphotericin B shows the most potent activity with low MIC values (<4 μg/mL) for all the clinically relevant fungal species (with a few exceptions from Paecilomyces species) that are responsible for the fungal infections in humans (Table 1). In comparison to amphotericin B, natamycin shows less potent activity with a broad MIC range for the fungal species (Table 1).
The azole class of antifungals, similar to the polyene group, is broad spectrum of antifungal agents. However, due to the presence of a variety of azole antifungals belonging to different generations, this class shows differential antifungal activity and potency against a variety of fungal species that are known to cause invasive and superficial fungal infections. Table 2 illustrates the fungal species and MIC of the various azole drugs against these fungal species.64–76
Table 2.
Antifungal Activity of Azoles with Their Minimum Inhibitory Concentration Against Various Clinically Relevant Fungal Species
| Azole drugs (MIC: μg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| Species/strains | FLU | ITR | KET | MICO | VOR | POS | Reference |
| Candida | 0.5–32 | 0.03–4 | 0.03 to >32 | 0.02–8 | 0.03–4 | ≤1 | 61–63 |
| Aspergillus | >64 | 0.5–2.0 | 0.06–8 | 1.5–3.5 | 0.5–2.0 | 0.25–0.5 | 64–67 |
| Fusarium | >64 | 32 | 0.1–50 | 0.07–40 | 0.5–8 | 0.25–16 | 64,68,69 |
| Rhizopus | >64 | 0.03–8 | 1–16 | 1 to >4 | 2 to >8 | 1–8 | 64,70 |
| Cunninghamella | >64 | 0.125–2 | >16 | 2 to >4 | 8 to >32 | 0.03–1 | 64,70,71 |
| Dimorphic fungi (Histoplasma, Blastomyces, Coccidioides, Paracoccidioides, Sporothrix species). | 0.06–32 | 0.06–0.5 | 0.03–8 | 0.1 to 0.001 | 0.03–1 | 0.01–1 | 64,72,73 |
FLU, fluconazole; ITR, itraconazole; KET, ketoconazole; MICO, miconazole; VOR, voriconazole; POS, posaconazole.
Posaconazole and voriconazole, belonging to the latest generation of azoles, exhibit the highest potency and broadest spectrum of activity (in vitro, preclinical, and clinical studies) compared to all the earlier generations of azole antifungals. Accordingly, posaconazole and voriconazole have exhibited clinical efficacy and outcomes superior to the other azole antifungals.73,75
Resistance
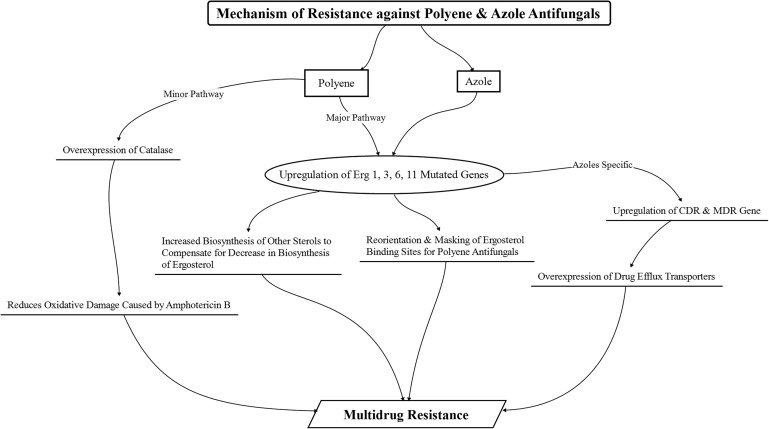
The polyene and azole class of antifungals exhibit susceptibility toward the emergence of fungal resistance similar to the antibacterial agents. The degree of susceptibility, however, differs between the 2 classes, with azole antifungals having greater predisposition to the development of fungal resistance than the polyene class of antifungals.33 In addition, the mechanism of resistance in fungal species against the different classes of antifungal drugs also differs.69
The occurrence of resistance against polyene antifungals is less prevalent than the occurrence of resistance against azole antifungals; however, resistance toward amphotericin B is observed in fungal species such as Candida lusitaniae, Candida glabrata, Candida guilliermondii, Aspergillus terreus, and Trichosporon beigelii and against natamycin in Aspergillus parasiticus, Aspergillus flavus, and Candida parapsilosis.23,33,77 An increase in the biosynthesis and accumulation of other sterols as a replacement for ergosterol, with a concomitant reduction in biosynthesis of ergosterol (due to a modified and mutated ERG3 gene that causes a reduction in ergosterol synthesis) in fungal cell membranes, is considered to be a major mechanism for the development of polyene resistance.78 The polyene resistance has led to the emergence of resistant fungal species with low ergosterol content. This emergence of resistant species, coupled with decreased susceptibility to oxidative damage due to enhanced catalase activity, has been attributed to the development of resistance against amphotericin B.77,79 Apart from these mechanisms, replacement, reorientation, and/or masking of some or all the polyene-binding sterols (ergosterol, cholesterol, or stigmasterol) with sterols having lower affinity for polyenes (hindering their binding) has been noted to be another major factor in the emergence of resistance.24 This mechanism has been particularly associated with the emergence of innate resistance against natamycin in the fungal species.23,77,79
In comparison to the polyenes, resistance to azoles has been known to occur through multiple mechanisms. One of the major underlying mechanisms contributing to the emergence of resistance80,81 has been the overexpression of active efflux pumps due to the upregulation of the confluence-dependent resistance and multidrug resistance genes that lead to a decreased concentration of the azole antifungals within the fungal cells (due to their efflux). Moreover, mutations in the ERG11 gene that encodes the target enzyme (lanosterol C14α-demethylase) result in the alteration in the structure and chemistry of the enzyme that leads to a hindrance in the efficient binding of the azole drugs to the enzyme, thus causing the emergence of resistance against these azole drugs.77 In some cases, similar to the polyene antifungals, mutation of ERG3 genes, leading to the inhibition of ergosterol biosynthesis and increase in the biosynthesis and accumulation of other sterols in the fungi, has also been implicated as a predominant mode of resistance development against azole drugs.82 Apart from these cases, upregulation of the ERG11 gene responsible for the coding of the target enzyme also occurs, leading to an increase in the target enzyme concentration in the fungal cells, thereby providing resistance against the azole drugs, which has also been suggested.83 Figure 4 provides an overview of the mechanisms of resistance against polyene and azole antifungals that occur in fungal cells.
FIG. 4.
Schematic representation of the development of resistance against polyene and azole antifungals manifested in fungal species. CDR, confluence-dependent resistance; MDR, multidrug resistance.
Challenges to the Ocular Delivery of Polyene and Azole Antifungals
Anatomical and physiological limitations
The topical route is the most preferred route for the administration of drugs in the treatment of ophthalmic infections and diseases. However, in the case of ocular fungal infections, the severity and localization of the fungal infection in the ocular tissues dictate the route of drug administration.84 For example, most of the fungal infections of the anterior chamber are treated by administering the drugs topically, whereas in case of endophthalmitis or deep-seated mycoses, parenteral or intraocular injections are routinely used.84
Delivering antifungal drugs, especially polyene and azole antifungals, to the different infected ocular tissues, is still one of the formidable challenges associated with antifungal pharmacotherapy,85 primarily because of the complex anatomy of the eye and the physicochemical properties of the various classes of antifungal drugs.
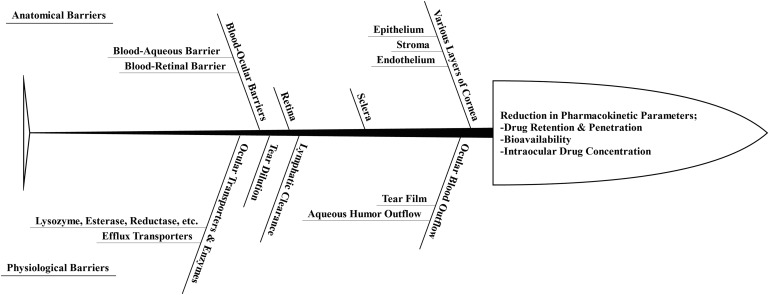
Anatomical and physiological barriers pose significant challenges to the topically administered formulations of polyene and azole antifungals.86–88 The anatomical barriers are composed of the different layers of cornea, sclera, retina, and blood-retinal and blood-aqueous barriers, whereas ocular blood flow, tear dilution, and ocular enzymes and transporters constitute the physiological barriers.89–92 These barriers collectively affect the ocular pharmacokinetics of all administered drugs.93 The cornea, conjunctiva, iris-ciliary, retina-choroid, and sclera act as barriers to the permeation of topically and parenterally administered drugs, including amphotericin B, natamycin, miconazole, ketoconazole, and fluconazole.85,87,94 The blood-aqueous barrier and the blood-retinal barrier collectively form the blood-ocular barriers.85 These blood-ocular barriers are composed of tight endothelial junctions that inhibit the movement of high molecular weight antifungal drugs such as amphotericin B, natamycin, ketoconazole, posaconazole, and itraconazole.95–98 Thus, the anatomical barriers are significantly responsible for altering the drug penetration, bioavailability, and intraocular concentration upon their topical and parenteral administration.95,99–101
The physiological barriers also act as a hindrance to the ocular delivery of drugs. Topical application of polyene and azole drugs as solution/suspension leads to considerable losses due to the nasolacrimal drainage.102–104 In addition, the tear film is also responsible for reducing the topical residence time of the antifungal drugs.95,105 The tear film, composed of mucin, salts, enzymes, and proteins, has been shown to bind anti-infective drug classes such as antifungals and antibacterials, thereby posing another challenge to effective ocular delivery of these agents.95,106–110
Figure 5 provides a brief overview of the anatomical and physiological ocular barriers that compromise the ocular pharmacokinetics of the drugs.
FIG. 5.
Brief overview on the anatomical and physiological ocular barriers that compromise the ocular pharmacokinetics of the drugs.
Physicochemical limitations
The physicochemical properties of drugs affect their ability to penetrate the ocular barriers and reach the site of activity in the ocular milieu. One of the major challenges associated with the antifungal agents lies in their molecular weight and aqueous solubility.85,86 The ocular delivery of amphotericin B is challenging due, in part, to its high molecular weight and low aqueous solubility, which hinders its penetration across the cornea and blood-retinal barrier and severely limits its ocular bioavailability.85 The reduced bioavailability of itraconazole and natamycin, due to poor corneal penetration, has also been attributed to their high molecular weights.84 In addition, binding of itraconazole to the proteins in the tear-lipid film and high hydrophobicity have contributed to its poor corneal penetration upon topical administration.84,85 Ketoconazole and miconazole have also exhibited poor penetration across the cornea and blood-ocular barriers due to their high molecular weights, hydrophobic characteristics, and protein binding.85 Voriconazole, even though having a broad and potent spectrum of activity against ocular fungal infections and effectiveness when administered systemically and intraocularly (intrastromal, intracameral, and intravitreous), has elicited side effects such as visual disturbances and increased sensitivity to light in more than 30% of patients in clinical trials, as reported by Kaur et al. in their review report.85 Thus, high molecular weight, high hydrophobic characteristics, and poor aqueous solubility have been the major physicochemical challenges reported with respect to the ocular delivery of polyene and azole antifungal drugs.
Selective antifungal activity
Selective activity of the polyene and azole antifungals is one of the major challenges associated with their monotherapy. This selective activity is apparent from the MIC values for these drugs that have been presented in Tables 1 and 2. The selectivity in their antifungal activity has been abundantly evidenced and discussed in the literature. Among the polyene class of antifungals, natamycin is known to have a potent activity against filamentous fungi such as Aspergillus and Fusarium but only a weak-to-moderate activity against the yeast-like fungi (Candida species).7,23,87 This selective activity has led to the use of natamycin as a front-line therapy drug in treating superficial ocular fungal keratitis caused by Fusarium and Aspergillus species.23 In treating fungal keratitis complicated by both filamentous and yeast-like fungal species, a combination antifungal drug regimen is initiated using natamycin concomitantly with miconazole, itraconazole, ketoconazole, or fluconazole (used off-label).111 Similarly, amphotericin B has shown differential activity against the filamentous and yeast-like fungal species, with a weak-to-moderate activity against Fusarium species and a potent activity against the Candida species.112 This selective activity has led to its frequent use as an off-label antifungal agent in treating the cases of severe Candida keratitis but not in cases of Fusarium keratitis in which natamycin is preferred.113 Fluconazole is also known to have weak-to-moderate action against the filamentous fungi, but has excellent activity against Candida species making it a suitable candidate in the treatment of deep-seated Candida keratitis but not a preferred candidate for filamentous fungal keratitis.112,114 Itraconazole and miconazole show variable activity against the filamentous fungal species with potent activity against Aspergillus species and a weak activity against Fusarium species, making them suitable candidates for treating deep-seated Aspergillus infection but not Fusarium infections.112
Ocular and systemic drug toxicity
The polyene and azole antifungals are known to exhibit ocular and systemic toxicities, which manifest as one of the foremost challenges associated with their therapeutic use. In a toxicity evaluation by Foster et al., the ocular toxicity of the polyene and azole antifungals (amphotericin B, flucytosine, miconazole, and ketoconazole) was studied on debrided rabbit cornea, in vivo, following topical application of these antifungals as 1% solutions or suspensions.115 The evaluations were based on rate of closure of the debrided epithelial corneal wounds, quality of regenerating epithelium, stromal edema and haze, and iritis (visual and microscopic evaluations). Amphotericin B was found to severely retard the rate of debrided corneal closure and manifested dramatic pathologic changes (evaluated on the bases of scores for quality of regenerating epithelium, stromal edema and haze, and iritis) that worsened each day with the continuation of therapy. However, ketoconazole, flucytosine, and miconazole produced histologically undetectable changes. Although toxicity manifestations were reported with the 1% dose, in ocular infections, the therapeutic dose is significantly lower.116 Thus, the toxicity evaluation of amphotericin B in the abovementioned study by Foster et al., at 1% w/v concentration (vs. its concentration in marketed formulations), may not accurately manifest its clinical ocular toxicity profile.
The ocular toxicity of amphotericin B was also observed in in vivo studies, in which it was shown to cause retinal toxicity and loss of retinal ganglion cells, at doses higher than 10 μg of amphotericin B, upon intravitreal injection in vitrectomized rabbit eyes. Interestingly, increased vitreal inflammation, corneal edema, neovascularization, and inflammation were observed upon the intrastromal and subconjunctival administration of amphotericin B and its deoxycholate form in comparison to the liposomal amphotericin B (L-AMB) complex.117–121 Evidently, from the above studies, the formulation of amphotericin B as a liposomal complex reduces the toxicity associated with the amphotericin B molecule, leading to the use of amphotericin B as a liposomal complex in cases of severe ocular fungal infections.
Similarly, itraconazole (doses above 10 μg injected intravitreally in rabbits) and voriconazole (400 mg b.i.d, orally in fungal keratitis patients) are known to cause retinal necrosis and visual disturbances, respectively.122–124 Systemic side effects/toxicities such as nephrotoxicity (amphotericin B), hepatotoxicity (ketoconazole and voriconazole), and gastrointestinal upsets (itraconazole) are also commonly encountered upon their oral and/or intravenous administration in ocular fungal infections.87,124–126 Hence, from the abovementioned discussion, ocular and systemic toxicities evidently present an important aspect of the challenge in the ocular delivery of these agents.
Emergence of clinical resistance and cross-resistance
Resistance has been one of the major challenges associated with the antifungal drug consortium; particularly with the azole antifungals.77 Clinical instances of primary and secondary resistances are observed for the different antifungal drugs.77 Polyene antifungals are generally associated with primary resistance, meaning that some of the fungal species are inherently and naturally resistant to them. Such a kind of primary or intrinsic resistance is observed against amphotericin B in C. lusitaniae, A. terreus, and T. beigelii and against natamycin in A. parasiticus, A. flavus, and C. parapsilosis.23,67,127,128 Azoles, in contrast to the polyene drugs, exhibit both primary and secondary resistance (resistance due to a prolonged exposure of the fungal species to the azole antifungals).22 For example, Candida krusei is known to be resistant toward fluconazole, and secondary resistance is emerging in Candida albicans and C. glabrata against fluconazole due to the continuous clinical use of fluconazole.22,129 In addition, cross-resistance has also been one of the challenges plaguing the use of azole drugs. Widespread and continuous use of itraconazole and fluconazole has resulted in the development of resistant species against them, as well as against other antifungals.22,33,77 The emergence of resistance and cross-resistance against the newer azoles is demonstrated by reports of voriconazole resistant Fusarium and Aspergillus ocular infections being reported. Use of posaconazole instead of voriconazole in the former case and a surgical method in the latter help overcome the challenge of resistance associated with voriconazole.130,131 Currently, posaconazole (off-label use) is considered one of the most effective therapies in cases of resistant fungal keratitis and other refractory ocular fungal infections.130–132
Lack of robust in vitro susceptibility testing of clinical samples
One of the challenges associated with antifungal agents, especially azole drugs, is the lack of in vitro susceptibility testing of clinical samples that provide a robust in vitro-in vivo correlation because the in vitro susceptibility testing with fungal species is not yet standardized, and the results of in vitro tests do not always correlate with the results obtained in vivo.22 The absence of the standardized tests for in vitro susceptibility testing in mycotic keratitis is attributed to small sample sizes, nonuniformity of MIC data due to the use of a variety of in vitro susceptibility testing methods, and focus on 1 particular species.133 One such challenge is faced in the evaluation of the most suitable polyene and/or azole antifungals for treating Fusarium keratitis due to the MIC variability of the different polyene/azole antifungals between and within the different Fusarium strains that cause keratitis.71 Similar situations have frequently been observed for fluconazole, which shows a weak in vitro activity against Candida and Cryptococcus neoformans isolates but exhibits potent in vivo activity against them,75 attributed to the lack of standardized in vitro susceptibility testing methods for the antifungal agents. Sensitive, specific, reliable, reproducible, and standardized in vitro susceptibility testing methods are warranted for the accurate and timely diagnosis of fungal infections, particularly fungal keratitis, to avoid treatment failures and relapses with the polyene and azole drug-based ocular pharmacotherapy.71
Approaches at Overcoming the Challenges to the Ocular Delivery of Polyene and Azole Antifungal Drugs
Formulation strategies have been one of the most preferred options for overcoming the anatomical and physiological ocular barriers, physicochemical challenges, and ocular toxicities/side effects associated with the polyene and azole drugs.
To improve the ocular penetration and safety of amphotericin B, lipoidal formulations containing amphotericin B have been formulated.126 Different lipoidal formulations [amphotericin B lipid complex (ABLC), L-AMB, and amphotericin B deoxycholate (D-AMB)] of amphotericin B were evaluated for their ocular penetration, biodistribution, and safety in rabbit eyes, in vivo, after intravenous administration at doses of 5 mg/kg/day for ABLC and L-AMB and 1 mg/kg/day for D-AMB. All the amphotericin B lipoidal formulations were observed to penetrate the blood-retinal barrier in the inflamed eyes (induced unilateral uveitis), and the concentration of amphotericin B in the aqueous humor following L-AMB administration was ∼8 times more than the concentration of the ABLC and D-AMB formulations after single dose administration. Upon repeated intravenous daily doses for 7 days, the amphotericin B concentration in the aqueous humor from L-AMB was ∼10 times higher than the concentration of the ABLC and D-AMB formulations.126 At the end of 7 days, amphotericin B was also detected in the vitreous, with the concentration from L-AMB being approximately twice the concentration from ABLC and D-AMB.126 This study demonstrated the utility of intravenous L-AMB formulations in deep-seated fungal infections and/or fungal inflammations, as an alternative to the topical administration of amphotericin B.
In another study, amphotericin B lipid emulsion (0.5%) was found to penetrate across the corneal barrier upon topical administration (one instillation every hour for 6 h) and was shown to be safer than the marketed amphotericin B formulation (Fungizone®, 0.5%) in rabbits.134 The corneal and aqueous concentrations of amphotericin B lipid emulsion were observed to be ∼2-fold higher than Fungizone. The plasma concentration following the administration of amphotericin B lipid emulsion was 20 ng/mL, which was deemed to be safe by the authors.134
The reduction in toxicity associated with liposomal formulations of amphotericin B in comparison to amphotericin B was also corroborated by Barza et al. and Tremblay et al., in in vivo studies carried out on rhesus monkeys and rabbits, respectively.135,136 Barza et al. observed a significantly lower presence of infiltrates in the anterior chamber of rhesus monkeys, on histopathological examination, upon intravitreal injection of L-AMB formulation in comparison to the marketed amphotericin B formulation. The reduction in toxicity was reported to be ∼4-fold, with 30 mg of commercial amphotericin B being tolerated versus 120 mg of the L-AMB formulation.135 In rabbits, the safety was evaluated using histopathological methods, in which significantly higher retinal necrosis and/or atrophy was observed with the marketed amphotericin B formulation at a lower dose (5 μg dose) compared to the higher dose of L-AMB formulation (20 μg dose) upon intravitreal administration.136
In 2 independent studies using the rabbit model, positively charged amphotericin B loaded Eudragit® RL100 nanoparticles and positively charged chitosan/lecithin nanoparticles exhibited higher corneal penetration in comparison to Fungizone due to an improvement in the precorneal residence time because of the mucoadhesive characteristics of the nanoparticles.137–139 The nanoparticles provided significantly higher precorneal retention (∼3.36-fold) compared to the marketed amphotericin B formulation (Fungizone). The nanoparticles also exhibited sustained release and antifungal activity against Fusarium solani, C. albicans, and Aspergillus fumigatus, upon in vitro evaluation.
In a study by Serrano et al., amphotericin B/γ-cyclodextrin aqueous solution was prepared and evaluated for its physicochemical stability and antifungal activity.140 The formulation was found to have a statistically higher physical (particle size, sterility, pH, and osmolarity) and chemical (% amphotericin B content) stability, along with an improvement in its in vitro antifungal activity against C. albicans (∼35%) in comparison to the reference formulation (Fungizone in dextrose solution).140 In vitro and/or in vivo data on permeability flux or bioavailability from these cyclodextrin-based formulations have not yet been studied.
Natamycin-loaded nanoparticles have also been formulated for their potential use in fungal keratitis. In the study by Bhatta et al. and Chandasana et al., natamycin-loaded lecithin/chitosan nanoparticles and poly-d-glucosamine (PDG) functionalized polycaprolactone (PCL) nanoparticles (PDG-PCL-NPs) were developed with the aim of improving the ocular residence time and providing a sustained release of natamycin.141,142 Upon in vitro evaluation, the natamycin release was found to be sustained up to 7 and 8 h, respectively, by the nanoparticulate carriers in comparison to the marketed formulation (natamycin suspension), which showed complete natamycin release within 2 h in the release medium used. In vitro antifungal activity of the nanoparticles and the marketed natamycin suspension were comparable. Ocular pharmacokinetic studies demonstrated statistically higher bioavailability of natamycin from the nanoparticles in comparison to the marketed suspension (AUC0-∞ for nanoparticles was 1.47 times higher than the marketed suspension) and a significantly lower clearance from the precorneal sites (clearance for nanoparticles was 7.9 times lower than the marketed suspension). In addition, a natamycin-loaded in situ gelling niosome formulation demonstrated a safe (absence of any signs of ocular irritation and/or inflammation in rabbit eyes, in vivo) and sustained release of natamycin, 24 h in comparison to the marketed natamycin suspension (nearly 100% release within 5 h), upon in vitro evaluation.143 All of these formulations exhibited significantly higher safety in comparison to the marketed natamycin suspension in rabbits, in vivo. Apart from these formulations, several other approaches such as improving the aqueous solubility of natamycin by complexing with cyclodextrins and formulating them in different lipid formulations are also being actively investigated as potential ways to circumvent the challenges associated with the ocular delivery.23,144
To improve the corneal penetration of fluconazole, Silva et al. developed fluconazole-loaded poly-lactide-co-glycolide implants (FL-PLGA implants). The FL-PLGA implants (25% w/w) in comparison to fluconazole suspension (1.8 mg/mL, control) injected into the vitreous humor of rabbits demonstrated significantly higher sustained and prolonged release (6 weeks for implants vs. 2 h for the suspension), ocular tolerance (clinical examination of the rabbit eyes using ophthalmoscopy), and ocular biocompatibility (using the ARPE-19 cell line).145 In addition, in an in vitro study by Fetih, fluconazole-loaded niosomal chitosan and poloxamer gels were found to show higher transcorneal fluconazole permeation and flux (∼2.5 times higher) in comparison to the free fluconazole gel (control). The drug could permeate the corneal barrier effectively from the niosomal gel formulation over a prolonged duration of time due to the permeation-enhancing properties of niosomes and an increase in the precorneal residence time (owing to the formulation being a gel).146
Ketoconazole loaded into a niosomal gel was formulated and evaluated by Abdelbary et al., and the gel showed a significantly higher bioavailability (∼20 times higher) in the aqueous humor than the control (ketoconazole suspension).147 Moreover, ketoconazole-loaded solid lipid nanoparticles showed higher bioavailability in aqueous and vitreous humor (∼2.5- and 1.6-fold higher than the control) in a study by Kakkar et al. In addition, in the same study, the ketoconazole-loaded solid lipid nanoparticles were found to be noncytotoxic on corneal and retinal cell lines (in vitro) and in rabbits (in vivo) in comparison to ketoconazole suspension (control).148 Grossman and Lee improved the ocular delivery of ketoconazole using trans-scleral and transcorneal iontophoresis.149 Higher amounts of ketoconazole were found in the aqueous humor (∼10-fold higher with trans-scleral iontophoresis and ∼5-fold higher with transcorneal iontophoresis) and cornea (∼5-fold higher with transcorneal iontophoresis) in comparison to the ketoconazole administered as a subconjunctival injection.149
To facilitate ocular delivery of itraconazole by surpassing the corneal barrier, itraconazole-loaded chitosan suspension, solid-lipid nanoparticles, and polymeric micelles entrapped in an in situ gel were evaluated. All formulations exhibited significantly higher corneal permeation and flux (greater than 2-fold in all the formulations) in comparison to the marketed itraconazole suspension (Itral®); the itraconazole-loaded polymeric micelles and solid lipid nanoparticles were found to have in vitro activity against C. albicans and A. flavus, respectively.150–152
Lipoidal formulations of voriconazole microemulsion, solid-lipid nanoparticles, and liposomes demonstrated corneal penetration of voriconazole at concentrations above its MIC value, both ex vivo and in vivo.153–155 In addition, the solid-lipid nanoparticles also exhibited ∼2-fold higher Cmax and AUC in the aqueous humor in comparison to the voriconazole suspension (control).154 To overcome the poor aqueous solubility of voriconazole, Pahuja et al. formulated voriconazole complexed with 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) for ocular delivery as aqueous drops.156 The HP-β-CD-complexed voriconazole demonstrated improved aqueous solubility when chitosan and EDTA (mucoadhesive agent and preservative) were added to the HP-β-CD-complexed voriconazole eye drops, which was ∼4 and 5 times higher in comparison to the controls (control 1: devoid of chitosan; control 2: devoid of chitosan and EDTA).
To overcome the challenges associated with the development of resistance and potential therapeutic relapse associated with monotherapy, a combination of antifungal therapies is usually initiated.157 The data, however, remain scarce in this regard due to quick replacement and/or substitution of 1 antifungal drug for the other in cases of resistance development and/or relapse in ocular fungal infections.87 In multilaboratory antifungal combination testing (in vitro), a posaconazole and caspofungin combination was found to be effective against Candida species, whereas posaconazole and voriconazole and anidulafungin and posaconazole combinations were found to be effective against A. fumigatus.158 In a study by Li et al., Fusarium species were isolated from ocular tissues of keratomycosis patients, and the effectiveness of combination antifungal therapy was evaluated in vitro.159 In the study, a greater effectiveness was observed against Fusarium species when amphotericin B was used in combination with terbinafine (synthetic allylamine antifungal) and itraconazole in comparison to its use alone, indicating that amphotericin B concomitant therapy with terbinafine and itraconazole could be more efficacious clinically due to their synergistic activity.159 Itraconazole and micafungin (echinocandin antifungal) combination and 5-flucytosine and amphotericin B and voriconazole and anidulafungin combinations have also shown better clinical activity and efficacy in comparison to itraconazole, 5-flucytosine, and voriconazole monotherapies, in systemic fungal infections, indicating that these combinations could also be utilized in ocular fungal infections, due to their synergistic activities.23,160
Amphotericin B and fluconazole combination has shown efficacy in the treatment of keratomycosis and in reducing the clinical cases of relapse in comparison to the monotherapies with the antifungals (83% success rate with the combination vs. 67% success rate with the monotherapies).161
Table 3 summarizes the challenges that are associated with the ocular delivery of polyene and azole antifungals and enlists the possible approaches/alternatives to overcome the challenges.
Table 3.
Summary of the Challenges and Possible Approaches at Overcoming the Challenges Associated with the Ocular Delivery of Polyene and Azole Antifungal Drugs
| Challenges | Approaches | References |
|---|---|---|
| 1. Anatomy and physiological limitations. Reduced precorneal residence time. Frequent dosing leading to a reduced patient compliance. Drug losses due to nasolachrymal drainage. Systemic toxicity. Ocular toxicity. |
Delivery of drugs using novel drug delivery systems, such as nanoparticles, films, liposomes, mucoadhesive formulations, ocular implants, and so on, for targeting of drugs to the ocular site. Use of excipients such as chitosan, poloxamer, EDTA to improve the precorneal residence times and enhance the drug penetration. To deliver the drugs as gels and viscous suspensions and emulsions to improve the precorneal residence time and reduce the nasolachrymal drainage. |
121,129–134,136–138,140–150,157 |
| 2. Physicochemical limitations. Reduce transcorneal flux. Poor drug solubility. Drug storage instability leading to reduced efficacy and increased cost. |
Use of abovementioned novel drug delivery systems for the delivery of polyene and azole antifungals. Use of salt forms of drugs, their complexation with cyclodextrins, etc., to improve their aqueous solubility. Encapsulation of drugs in surfactant and lipid core (in case of niosomal drug delivery) for improving its transcorneal permeation and/or flux. |
135,138,139,141,142,151 |
| 3. Ocular and systemic toxicities. Damage to the ocular tissues (retinal necrosis, loss of retinal ganglion cells, vitreal inflammation, corneal edema, neovascularization, and inflammation) and occurrence of systemic toxicities (hepatotoxicity and nephrotoxicity) and side effects (gastrointestinal disturbances) observed. |
Ocular targeting of drugs for treating of the ocular fungal infections using novel drug delivery systems such as lipoidal drug delivery systems, gels, nanoparticles, and so on. | 84, 112–121,134,136–138,145,147–150,158 |
| 4. Selectivity in antifungal activity. Efficacy of pharmacotherapy is reduced and chances of relapse increase. Long-term treatment required. |
Combination therapy is initiated. Newer generation of antifungals, such as posaconazole, ravuconazole, echinocandins, and so on, is used. |
153–156 |
| 5. Emergence of clinical resistance and cross-resistance. Limited activity of a drug against a given fungal species/strain. A class/category of drug rendered inactive against fungal species/strains. Increase in severity of the infection over time. |
Combination therapy is initiated. Newer generation of antifungals such as posaconazole, ravuconazole, echinocandins, and so on is used. |
153–156 |
Future Directions
Despite research in the formulation of novel dosage forms, US FDA-approved ophthalmic formulations for the polyene and azole antifungals, except for natamycin, for the pharmacotherapy of ocular fungal infections are lacking, leading to the off-label, nonoptimized use of the polyene and azole antifungals, which also increases the chances of resistance and cross-resistance. The absence of marketed formulations, exclusive to ocular fungal infections, leads to the lack of valid comparators (controls) for evaluating the efficacy, activity, and potency of the newly developed ocular dosage forms for the polyene and azole antifungals intended for ocular antifungal pharmacotherapy. Hence, concentrated effort is necessary to bring about the transition of novel dosage forms from the bench to the bedside.
The approaches to overcome the challenges of emergence of resistance and cross-resistance and lack of robust in vitro-in vivo correlation techniques still must be developed to improve the polyene- and azole-associated antifungal therapy. Combination drug therapy has provided some means of overcoming a few of these challenges. However, further research and studies are warranted to effectively tackle these 2 predominant clinical issues in improving the antifungal pharmacotherapy associated with polyene and azole antifungals. In addition, to combat the challenges of future emergence of resistance and cross-resistance and to improve treatment outcomes, it is essential to continue with the development of newer generation antifungals, with superior physicochemical properties and a broad antifungal spectrum, and/or potentiating agents and synergistic combinations to enhance activity against resistant strains and/or to lower the dose and associated toxicity of the currently available agents. Development of robust in vitro-in vivo correlation models is also necessary to optimize and improve the success rates of ocular antifungal therapy.
Summary
Polyene and azole antifungals have provided effective ocular antifungal therapy against fungal keratitis, endophthalmitis, blepharitis, and conjunctivitis.23,87 However, these classes of antifungals have faced some challenges, most of which have been countered using formulation approaches, whereas some preclinical (robust in vitro-in vivo correlation) and clinical (resistance, cross-resistance, and relapse) aspects still must be resolved for improved outcomes. Some challenges have been overcome by echinocandin antifungals. Since the polyene and azole class of antifungals constitutes such an important locus in the consortium of antifungal drugs, it is imperative to understand and overcome the preclinical and clinical challenges associated with these drugs to further improve effectiveness of ocular antifungal therapy.
Acknowledgments
This work was supported by National Institute of General Medical Sciences (Grant: P20 GM104932), National Institutes of Health (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health), and Graduate Student Council Research Grants Program, undertaken by the University of Mississippi.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. CDC. Fungal Diseases. 2014. www.cdc.gov/fungal/diseases/index.html Accessed September9, 2018
- 2. Collier S., Gronostaj M., MacGurn A., Cope J., Yoder J., and Beach M. Estimated Burden of Keratitis—United States, 2010. MMWR. 63:1027–1030, 2014 [PMC free article] [PubMed] [Google Scholar]
- 3. Patil A., Lakhani P., Taskar P., et al. . Formulation development, optimization, and in vitro-in vivo characterization of natamycin-loaded PEGylated nano-lipid carriers for ocular applications. J. Pharm. Sci. 107:2160–2171, 2018 [DOI] [PubMed] [Google Scholar]
- 4. Noor S.S.M., Michael K., Marshall S., and Ren J. Spatial and spectral analysis of corneal epithelium injury using hyperspectral images. In: Second International Conference on Robotics and Machine Vision: Bellingham, WA: SPIE; 2017; p. 11 [Google Scholar]
- 5. Acharya Y., Acharya B., and Karki P. Fungal keratitis: study of increasing trend and common determinants. Nepal J. Epidemiol. 7:685–693, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paramythiotou E., Frantzeskaki F., Flevari A., Armaganidis A., and Dimopoulos G. Invasive fungal infections in the ICU: how to approach, how to treat. Molecules. 19:1085, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patil A., and Majumdar S. Echinocandins in ocular therapeutics. J. Ocul. Pharmacol. Ther. 33:340–352, 2017 [DOI] [PubMed] [Google Scholar]
- 8. Cornely O.A. Aspergillus to Zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infections. Infection. 36:296–313, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cornely O.A., Vehreschild J.J., and Ullmann A.J. Is there a role for polyenes in treating invasive mycoses? Curr. Opin. Infect. Dis. 19:565–570, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Chandrasekar P. Amphotericin B lipid complex: treatment of invasive fungal infections in patients refractory to or intolerant of amphotericin B deoxycholate. Ther. Clin. Risk Manag. 4:1285–1294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chandrasekar P. Management of invasive fungal infections: a role for polyenes. J. Antimicrob. Chemother. 66:457–465, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Mohr J., Johnson M., Cooper T., Lewis J.S., and Ostrosky-Zeichner L. Current options in antifungal pharmacotherapy. Pharmacotherapy. 28:614–645, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Ashley E.S.D., Lewis R., Lewis J.S., Martin C., and Andes D. Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 43:S28–S39, 2006 [Google Scholar]
- 14. Zonios D.I., and Bennett J.E. Update on azole antifungals. Semin. Respir. Crit. Care Med. 29:198–210, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Akler M.E., Vellend H., McNeely D.M., Walmsley S.L., and Gold W.L. Use of fluconazole in the treatment of candidal endophthalmitis. Clin. Infect. Dis. 20:657–664, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Soliman O.A.E., Mohamed E.A., and Khatera N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: formulation, optimization, and in vitro-in vivo evaluation. Pharm. Dev. Technol. 1–15, 2017 [DOI] [PubMed] [Google Scholar]
- 17. Charlier C., Hart E., Lefort A., et al. . Fluconazole for the management of invasive candidiasis: where do we stand after 15 years? J. Antimicrob. Chemother. 57:384–410, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Del Rosso J.Q. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J. Clin. Aesthet. Dermatol. 7:10–18, 2014 [PMC free article] [PubMed] [Google Scholar]
- 19. Pound M.W., Townsend M.L., Dimondi V., Wilson D., and Drew R.H. Overview of treatment options for invasive fungal infections. Med. Mycol. 49:561–580, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Sheehan D.J., Hitchcock C.A., and Sibley C.M. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40–79, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta A.K., Nicol K., and Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. Am. J. Clin. Dermatol. 5:417–422, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Dixon D.M., and Walsh T.J. Antifungal agents. In: Baron S., eds. Medical Microbiology. Galveston (TX): University of Texas Medical Branch, Department of Microbiology; 1996 [PubMed] [Google Scholar]
- 23. Patil A., Lakhani P., and Majumdar S. Current perspectives on natamycin in ocular fungal infections. J. Drug Deliv. Sci. Technol. 41:206–212, 2017 [Google Scholar]
- 24. Hamilton-Miller J. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol. Rev. 37:166–196, 1973 [PMC free article] [PubMed] [Google Scholar]
- 25. Cheron M., Cybulska B., Mazerski J., Grzybowska J., Czerwinski A., and Borowski E. Quantitative structure-activity relationships in amphotericin B derivatives. Biochem. Pharmacol. 37:827–836, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Tevyashova A.N., Olsufyeva E.N., Solovieva S.E., et al. . Structure-antifungal activity relationships of polyene antibiotics of the amphotericin B group. Antimicrob. Agents Chemother. 57:3815–3822, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gagos M., Herec M., Arczewska M., Czernel G., Dalla Serra M., and Gruszecki W.I. Anomalously high aggregation level of the polyene antibiotic amphotericin B in acidic medium: implications for the biological action. Biophys. Chem. 136:44–49, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Connors K., Amidon G., and Stella V. and Amphotericin B. Chemical Stability of Pharmaceuticals: A Handbook for Pharmacists. New York: Wiley; 1986; p. 193–196 [Google Scholar]
- 29. Thomas A. Analysis and assay of polyene antifungal antibiotics. A review. Analyst. 101:321–340, 1976 [DOI] [PubMed] [Google Scholar]
- 30. Beale J. Anti-infective agents. In: Beale J., Block J., eds. Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical Chemistry. The People's Republic of China: Lippincott Williams & Wilkins; 2011; p. 190–205 [Google Scholar]
- 31. Griffith R. Antifungal agents. In: Lemke T., Williams D., Roche V., Zito W., eds. Foye's Principles of Medicinal Chemistry. China: Lippincott Williams & Wilkins; 2013; p. 1158–1174 [Google Scholar]
- 32. Onyewu C., Blankenship J., Del Poeta M., and Heitman J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47:956–964, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghannoum M.A., and Rice L.B. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501–517, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dupont S., Lemetais G., Ferreira T., Cayot P., Gervais P., and Beney L. Ergosterol biosynthesis: a fungal pathway for life on land? Evolution. 66:2961–2968, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Alcazar-Fuoli L., Mellado E., Garcia-Effron G., et al. . Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids. 73:339–347, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Borelli C., Schaller M., Niewerth M., et al. . Modes of action of the new arylguanidine abafungin beyond interference with ergosterol biosynthesis and in vitro activity against medically important fungi. Chemotherapy. 54:245–259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsuoka S., and Murata M. Membrane permeabilizing activity of amphotericin B is affected by chain length of phosphatidylcholine added as minor constituent. Biochim. Biophys. Acta. 1617:109–115, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Prasad R., Shah A., and Rawal M. Antifungals: mechanism of action and drug resistance. In: Ramos J., Sychrová H., Kschischo M., eds. Yeast Membrane Transport. Cham: Springer International Publishing; 2016; p. 327–349 [Google Scholar]
- 39. Van Leeuwen M., Golovina E., and Dijksterhuis J. The polyene antimycotics nystatin and filipin disrupt the plasma membrane, whereas natamycin inhibits endocytosis in germinating conidia of Penicillium discolor. J. Appl. Microbiol. 106:1908–1918, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Wickner W., and Haas A. Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem. 69:247–275, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Heese-Peck A., Pichler H., Zanolari B., Watanabe R., Daum G., and Riezman H. Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell. 13:2664–2680, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mayer A. Membrane fusion in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 18:289–314, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Baars T., Petri S., Peters C., and Mayer A. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol. Biol. Cell. 18:3873–3882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Munn A. Molecular requirements for the internalisation step of endocytosis: insights from yeast. Biochim. Biophys. Acta. 1535:236–257, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Banta L., Robinson J., Klionsky D., and Emr S. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 107:1369–1383, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hammond S., Lambert P., and Kliger B. The mode of action of polyene antibiotics; induced potassium leakage in Candida albicans. J. Gen. Microbiol. 81:325–333, 1974 [DOI] [PubMed] [Google Scholar]
- 47. Gray K., Palacios D., Dailey I., et al. . Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U. S. A. 109:2234–2239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Romani L. Amphotericin B still in the headlines. Pathog. Glob. Health. 106:80–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hitchcock C.A., Dickinson K., Brown S.B., Evans E.G., and Adams D.J. Interaction of azole antifungal antibiotics with cytochrome P-450-dependent 14 alpha-sterol demethylase purified from Candida albicans. Biochem. J. 266:475–480, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arthington-Skaggs B.A., Motley M., Warnock D.W., and Morrison C.J. Comparative evaluation of PASCO and national committee for clinical laboratory standards M27-A broth microdilution methods for antifungal drug susceptibility testing of yeasts. J. Clin. Microbiol. 38:2254–2260, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pfaller M.A., Arikan S., Lozano-Chiu M., et al. . Clinical evaluation of the ASTY colorimetric microdilution panel for antifungal susceptibility testing. J. Clin. Microbiol. 36:2609–2612, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pfaller M.A., Bale M., Buschelman B., et al. . Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J. Clin. Microbiol. 33:1104–1107, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Salvosa F., Cubillan L., and Nievera L. In vitro evaluation of natamycin 5% suspension against Aspergillus flavus, Fusarium solani, and Candida parasilopsis. Philipp. J. Ophthalmol. 29:26–28, 2004 [Google Scholar]
- 54. Arikan S., Lozano-Chiu M., Paetznick V., Nangia S., and Rex J.H. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946–3951, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Espinel-Ingroff A., Bartlett M., Bowden R., et al. . Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139–143, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brothers A., and Wyatt R. The antifungal activity of natamycin toward molds isolated from commercially manufactured poultry feed. Avian Dis. 44:490–497, 2000 [PubMed] [Google Scholar]
- 57. Al-Hatmi A., Meletiadis J., Curfs-Breuker I., Bonifaz A., Meis J., and Hoog S. In vitro combinations of natamycin with voriconazole, itraconazole and micafungin against clinical Fusarium strains causing keratitis. J. Antimicrob. Chemother. 71:953–955, 2015 [DOI] [PubMed] [Google Scholar]
- 58. Aguilar C., Pujol I., Sala J., and Guarro J. Antifungal susceptibilities of Paecilomyces species. Antimicrob. Agents Chemother. 42:1601–1604, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Espinel-Ingroff A., Dawson K., Pfaller M., et al. . Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob. Agents Chemother. 39:314–319, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wingard J. Natamycin (Pimaricin). In: Grayson M., ed. Kucers' the Use of Antibiotics Sixth Edition: A Clinical Review of Antibacterial, Antifungal and Antiviral Drugs. Florida: CRC Press; 2010; p. 1733–1738 [Google Scholar]
- 61. Li R.K., Ciblak M.A., Nordoff N., Pasarell L., Warnock D.W., and McGinnis M.R. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob. Agents Chemother. 44:1734–1736, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ellis D. Amphotericin B: spectrum and resistance. J. Antimicrob. Chemother. 49 Suppl 1:7–10, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Mauger T., and Craig E. Antimicrobials. Havener's Ocular Pharmacology. St. Louis, Missouri: Mosby; 1994 [Google Scholar]
- 64. Moon C.J., Shin J.H., Kim D.W., et al. . [Species-specific differences in Rhodamine 6G accumulation of Candida isolates detected by flow cytometric analysis]. Korean J. Lab. Med. 29:127–134, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Badiee P., and Alborzi A. Susceptibility of clinical Candida species isolates to antifungal agents by E-test, Southern Iran: a five year study. Iran. J. Microbiol. 3:183–188, 2011 [PMC free article] [PubMed] [Google Scholar]
- 66. Pfaller M.A., Messer S.A., Hollis R.J., and Jones R.N. In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans. Antimicrob. Agents Chemother. 45:2862–2864, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sabatelli F., Patel R., Mann P.A., et al. . In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009–2015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hsueh P.R., Lau Y.J., Chuang Y.C., et al. . Antifungal susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus species from Taiwan: surveillance of multicenter antimicrobial resistance in Taiwan program data from 2003. Antimicrob. Agents Chemother. 49:512–517, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lewis R.E. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 86:805–817, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Myoken Y., Sugata T., Myoken Y., Kyo T., Fujihara M., and Mikami Y. Antifungal susceptibility of Aspergillus species isolated from invasive oral infection in neutropenic patients with hematologic malignancies. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 87:174–179, 1999 [DOI] [PubMed] [Google Scholar]
- 71. Al-Hatmi A.M.S., Curfs-Breuker I., de Hoog G.S., Meis J.F., and Verweij P.E. Antifungal susceptibility testing of Fusarium: a practical approach. J. Fungi (Basel). 3:19, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pujol I., Guarro J., Gene J., and Sala J. In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J. Antimicrob. Chemother. 39:163–167, 1997 [DOI] [PubMed] [Google Scholar]
- 73. Almyroudis N.G., Sutton D.A., Fothergill A.W., Rinaldi M.G., and Kusne S. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 51:2587–2590, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eng R.H., Person A., Mangura C., Chmel H., and Corrado M. Susceptibility of zygomycetes to amphotericin B, miconazole, and ketoconazole. Antimicrob. Agents Chemother. 20:688–690, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Heeres J., Meerpoel L., and Lewi P. Conazoles. Molecules. 15:4129–4188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McGinnis M. Dimorphic fungi, antifungal agents and phylogenetics. In: 6th Congress of the European Confederation of Medical Mycology Societies Conference 2000. www.aspergillus.org.uk/content/dimorphic-fungi-antifungal-agents-and-phylogenetics Accessed September10, 2018 [Google Scholar]
- 77. Kanafani Z.A., and Perfect J.R. Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin. Infect. Dis. 46:120–128, 2008 [DOI] [PubMed] [Google Scholar]
- 78. Dick J.D., Merz W.G., and Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob. Agents Chemother. 18:158–163, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sokol-Anderson M.L., Brajtburg J., and Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 154:76–83, 1986 [DOI] [PubMed] [Google Scholar]
- 80. Albertson G.D., Niimi M., Cannon R.D., and Jenkinson H.F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40:2835–2841, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sanglard D., Ischer F., Monod M., and Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology (Reading, England). 143:405–416, 1997 [DOI] [PubMed] [Google Scholar]
- 82. Kelly S., Lamb D., Kelly D., et al. . Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 400:80–82, 1997 [DOI] [PubMed] [Google Scholar]
- 83. Lopez-Ribot J.L., McAtee R.K., Lee L.N., et al. . Distinct patterns of gene expression associated with development of fluconazole resistance in serial candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932–2937, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thomas P. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16:730–797, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kaur I.P., Rana C., and Singh H. Development of effective ocular preparations of antifungal agents. J. Ocul. Pharmacol. Ther. 24:481–493, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Kaur I.P., and Kakkar S. Topical delivery of antifungal agents. Expert Opin. Drug Deliv. 7:1303–1327, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Müller G., Kara-José N., and de Castro R. Antifungals in eye infections: drugs and routes of administration. Rev. Bras. Oftalmol. 72:132–141, 2013 [Google Scholar]
- 88. Lakhani P., Patil A., and Majumdar S. Recent advances in topical nano drug-delivery systems for the anterior ocular segment. Ther. Deliv. 9:137–153, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cholkar K., Patel A., Vadlapudi A.D., and Mitra A.K. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat. Nanomed. 2:82–95, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cholkar K., Patel S.P., Vadlapudi A.D., and Mitra A.K. Novel strategies for anterior segment ocular drug delivery. J. Ocul. Pharmacol. Therap. 29:106–123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kaur I.P., and Kakkar S. Nanotherapy for posterior eye diseases. J. Control. Release. 193:100–112, 2014 [DOI] [PubMed] [Google Scholar]
- 92. Duvvuri S., Majumdar S., and Mitra A.K. Role of metabolism in ocular drug delivery. Curr. Drug Metab. 5:507–515, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Behrens-Baumann W. Topical antimycotics in ophthalmology. Ophthalmologica. 211 Suppl 1:33–38, 1997 [DOI] [PubMed] [Google Scholar]
- 94. Kim Y.C., Chiang B., Wu X., and Prausnitz M.R. Ocular delivery of macromolecules. J. Control. Release. 190:172–181, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gunda S., Hariharan S., Mandava N., and Mitra A. Barriers in ocular drug delivery. In: Tombran-Tink J., Barnstable C.J., eds. Ocular Transporters in Ophthalmic Diseases and Drug Delivery: Ophthalmology Research. Totowa, NJ: Humana Press; 2008; p. 399–413 [Google Scholar]
- 96. Nettey H., Darko Y., Bamiro O., and Addo R. Ocular barriers. In: Addo R., ed. Ocular Drug Delivery: Advances, Challenges and Applications. Switzerland: Springer International Publishing; 2016; p. 27–36 [Google Scholar]
- 97. Salem H.F., Ahmed S.M., and Omar M.M. Liposomal flucytosine capped with gold nanoparticle formulations for improved ocular delivery. Drug Des. Devel. Ther. 10:277–295, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lim W.M., Rajinikanth P.S., Mallikarjun C., and Kang Y.B. Formulation and delivery of itraconazole to the brain using a nanolipid carrier system. Int. J. Nanomed. 9:2117–2126, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bill A. The blood-aqueous barrier. Trans. Ophthalmol. Soc. U. K. 105:149–155, 1986 [PubMed] [Google Scholar]
- 100. Cunha-Vaz J. The blood-ocular barriers. Surv. Ophthalmol. 23:279–296, 1979 [DOI] [PubMed] [Google Scholar]
- 101. Ghate D., and Edelhauser H.F. Ocular drug delivery. Expert Opin. Drug Deliv. 3:275–287, 2006 [DOI] [PubMed] [Google Scholar]
- 102. Schoenwald R.D. Ocular drug delivery. Pharmacokinetic considerations. Clin. Pharmacokinet. 18:255–269, 1990 [DOI] [PubMed] [Google Scholar]
- 103. Schoenwald R.D., Deshpande G.S., Rethwisch D.G., and Barfknecht C.F. Penetration into the anterior chamber via the conjunctival/scleral pathway. J. Ocul. Pharmacol. Ther. 13:41–59, 1997 [DOI] [PubMed] [Google Scholar]
- 104. de Juan E., Boyd S., Reich C., Rapacki A., Gifford H., and Deem M. Nasolacrimal drainage system implants for drug therapy. Google Patents; 2014. https://patents.google.com/patent/US8747884 Accessed October23, 2018
- 105. Ahsan S.M., and Rao C.M. Condition responsive nanoparticles for managing infection and inflammation in keratitis. Nanoscale. 9:9946–9959, 2017 [DOI] [PubMed] [Google Scholar]
- 106. Dawson D., Ubels J., and Edelhauser H. C.ornea and Sclera. In: Kaufman P., Adler F., Levin L., Alm A., eds. Adler's Physiology of the Eye. New York, USA: Saunders/Elsevier; 2011 [Google Scholar]
- 107. Dartt D.A. Tear lipocalin: structure and function. Ocul. Surf. 9:126–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McDermott A.M. Antimicrobial compounds in tears. Exp. Eye Res. 117:53–61, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 58:1131–1135, 2006 [DOI] [PubMed] [Google Scholar]
- 110. Weiner A.L. Drug delivery systems in ophthalmic applications. In: Yorio T., Clark A., Wax M., eds. Ocular Therapeutics. London: Academic Press; 2008; p. 7–43 [Google Scholar]
- 111. Tanure M.A., Cohen E.J., Sudesh S., Rapuano C.J., and Laibson P.R. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 19:307–312, 2000 [DOI] [PubMed] [Google Scholar]
- 112. Ansari Z., Miller D., and Galor A. Current thoughts in fungal keratitis: diagnosis and treatment. Curr. Fungal Infect. Rep. 7:209–218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Thomas P.A. Fungal infections of the cornea. Eye (London, England). 17:852–862, 2003 [DOI] [PubMed] [Google Scholar]
- 114. Rao S.K., Madhavan H.N., Rao G., and Padmanabhan P. Fluconazole in filamentous fungal keratitis. Cornea. 16:700, 1997 [PubMed] [Google Scholar]
- 115. Foster C.S., Lass J.H., Moran-Wallace K., and Giovanoni R. Ocular toxicity of topical antifungal agents. Arch. Ophthalmol (Chicago, Ill: 1960). 99:1081–1084, 1981 [DOI] [PubMed] [Google Scholar]
- 116. [USFDA] United States Food and Drug Administration. AmBisome Product Information hwac. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050740s021lbl.pdf Accessed October23, 2018
- 117. Baldinger J., Doft B., Burns S., and Johnson B. Retinal toxicity of amphotericin B in vitrectomised versus non-vitrectomised eyes. Br. J. Ophthalmol. 70:657–661, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kaji Y., Yamamoto E., Hiraoka T., and Oshika T. Toxicities and pharmacokinetics of subconjunctival injection of liposomal amphotericin B. Graefe's Arch. Clin. Exp. Ophthalmol. 247:549–553, 2008 [DOI] [PubMed] [Google Scholar]
- 119. Qu L., Li L., and Xie H. Toxicity and pharmacokinetics of intrastromal injection of amphotericin B in a rabbit model. Curr. Eye Res. 39:340–347, 2013 [DOI] [PubMed] [Google Scholar]
- 120. Axelrod A., Peyman G., and Apple D. Toxicity of intravitreal injection of amphotericin B. Am. J. Ophthalmol. 76:578–583, 1973 [DOI] [PubMed] [Google Scholar]
- 121. Cannon J., Fiscella R., Pattharachayakul S., et al. . Comparative toxicity and concentrations of intravitreal amphotericin B formulations in a rabbit model. Invest. Ophthalmol. Vis. Sci. 44:2112–2117, 2003 [DOI] [PubMed] [Google Scholar]
- 122. Neoh C., Daniell M., Chen S., Stewart K., and Kong D. Clinical utility of caspofungin eye drops in fungal keratitis. Int. J. Antimicrob. Agents. 44:96–104, 2014 [DOI] [PubMed] [Google Scholar]
- 123. Schulman J., Peyman G., Dietlein J., and Fiscella R. Ocular toxicity of experimental intravitreal itraconazole. Int. Ophthalmol. 15:21–24, 1991 [DOI] [PubMed] [Google Scholar]
- 124. Thiel M., Zinkernagel A., Burhenne J., Kaufmann C., and Haefeli W. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob. Agents Chemother. 51:239–244, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gaudana R., Ananthula H., Parenky A., and Mitra A. Ocular drug delivery. AAPS J. 12:348–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Goldblum D., Rohrer K., Frueh B., Theurillat R., Thormann W., and Zimmerli S. Ocular distribution of intravenously administered lipid formulations of amphotericin B in a rabbit model. Antimicrob. Agents Chemother. 46:3719–3723, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pfaller M.A., Messer S.A., and Hollis R.J. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn. Microbiol. Infect. Dis. 20:127–133, 1994 [DOI] [PubMed] [Google Scholar]
- 128. Walsh T.J., Melcher G.P., Rinaldi M.G., et al. . Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28:1616–1622, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pancholi P., Park S., Perlin D., Kubin C., and Della-Latta P. Molecular characterization of fluconazole resistance in a case of Candida albicans ocular infection. J. Clin. Microbiol. 42:5938–5939, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mittal V., Mittal R., and Sharma P.C. Voriconazole-refractory fungal infection of phacoemulsification tunnel. Ind. J. Ophthalmol. 58:434–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Altun A., Kurna S.A., Sengor T., et al. . Effectiveness of posaconazole in recalcitrant fungal keratitis resistant to conventional antifungal drugs. Case Rep. Ophthalmol. Med. 2014:701653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tu E.Y., McCartney D.L., Beatty R.F., Springer K.L., Levy J., and Edward D. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592). Am. J. Ophthalmol. 143:222–227, 2007 [DOI] [PubMed] [Google Scholar]
- 133. Maharana P.K., Sharma N., Nagpal R., Jhanji V., Das S., and Vajpayee R.B. Recent advances in diagnosis and management of Mycotic Keratitis. Ind. J. Ophthalmol. 64:346–357, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cohen T., Sauvageon-Martre H., Brossard D., et al. . Amphotericin B eye drops as a lipidic emulsion. Int. J. Pharm. 137:249–254, 1996 [Google Scholar]
- 135. Barza M., Baum J., Tremblay C., Szoka F., and D'Amico D.J. Ocular toxicity of intravitreally injected liposomal amphotericin B in rhesus monkeys. Am. J. Ophthalmol. 100:259–263, 1985 [DOI] [PubMed] [Google Scholar]
- 136. Tremblay C., Barza M., Szoka F., Lahav M., and Baum J. Reduced toxicity of liposome-associated amphotericin B injected intravitreally in rabbits. Invest. Ophthalmol. Vis. Sci. 26:711–718, 1985 [PubMed] [Google Scholar]
- 137. Das S., Suresh P., and Desmukh R. Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomed. Nanotechnol. Biol. Med. 6:318–323, 2010 [DOI] [PubMed] [Google Scholar]
- 138. Das S., and Suresh P.K. Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomed. Nanotechnol. Biol. Med. 7:242–247, 2011 [DOI] [PubMed] [Google Scholar]
- 139. Chhonker Y.S., Prasad Y.D., Chandasana H., et al. . Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int. J. Biol. Macromol. 72:1451–1458, 2015 [DOI] [PubMed] [Google Scholar]
- 140. Serrano D.R., Ruiz-Saldana H.K., Molero G., Ballesteros M.P., and Torrado J.J. A novel formulation of solubilised amphotericin B designed for ophthalmic use. Int. J. Pharm. 437:80–82, 2012 [DOI] [PubMed] [Google Scholar]
- 141. Bhatta R., Chandasana H., Chhonker Y., et al. . Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: in vitro and pharmacokinetics studies. Int. J. Pharm. 432:105–112, 2012 [DOI] [PubMed] [Google Scholar]
- 142. Chandasana H., Prasad Y., Chhonker Y., et al. . Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: an approach to reduce dose and dosing frequency. Int. J. Pharm. 477:317–325, 2014 [DOI] [PubMed] [Google Scholar]
- 143. Paradkar M., and Parmar M. Formulation development and evaluation of Natamycin niosomal in-situ gel for ophthalmic drug delivery. J. Drug Deliv. Sci. Technol. 39:113–122, 2017 [Google Scholar]
- 144. Koontz J., and Marcy J. Formation of natamycin: cyclodextrin inclusion complexes and their characterization. J. Agric. Food Chem. 51:7106–7110, 2003 [DOI] [PubMed] [Google Scholar]
- 145. Silva G.R., Almeida A.P.R., Fernandes-Cunha G.M., et al. . Safety and in vivo release of fluconazole-loaded implants in rabbits' eyes. J. Drug Deliv. Sci. Technol. 35:323–326, 2016 [Google Scholar]
- 146. Fetih G. Fluconazole-loaded niosomal gels as a topical ocular drug delivery system for corneal fungal infections. J. Drug Deliv. Sci. Technol. 35:8–15, 2016 [Google Scholar]
- 147. Abdelbary G.A., Amin M.M., and Zakaria M.Y. Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 24:309–319, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kakkar S., Karuppayil S.M., Raut J.S., et al. . Lipid-polyethylene glycol based nano-ocular formulation of ketoconazole. Int. J. Pharm. 495:276–289, 2015 [DOI] [PubMed] [Google Scholar]
- 149. Grossman R., and Lee D.A. Transscleral and transcorneal iontophoresis of ketoconazole in the rabbit eye. Ophthalmology. 96:724–729, 1989 [DOI] [PubMed] [Google Scholar]
- 150. Jaiswal M., Kumar M., and Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf. B Biointerfaces. 130:23–30, 2015 [DOI] [PubMed] [Google Scholar]
- 151. Ahuja M., Verma P., and Bhatia M. Preparation and evaluation of chitosan–itraconazole co-precipitated nanosuspension for ocular delivery. J. Exp. Nanosci. 10:209–221, 2013 [Google Scholar]
- 152. Mohanty B., Majumdar D.K., Mishra S.K., Panda A.K., and Patnaik S. Development and characterization of itraconazole-loaded solid lipid nanoparticles for ocular delivery. Pharm. Dev. Technol. 20:458–464, 2015 [DOI] [PubMed] [Google Scholar]
- 153. Kumar R., and Sinha V.R. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf. B Biointerfaces 117:82–88, 2014 [DOI] [PubMed] [Google Scholar]
- 154. Kumar R., and Sinha V.R. Solid lipid nanoparticle: an efficient carrier for improved ocular permeation of voriconazole. Drug Dev. Ind. Pharm. 42:1956–1967, 2016 [DOI] [PubMed] [Google Scholar]
- 155. de Sa F.A., Taveira S.F., Gelfuso G.M., Lima E.M., and Gratieri T. Liposomal voriconazole (VOR) formulation for improved ocular delivery. Colloids Surf. B Biointerfaces. 133:331–338, 2015 [DOI] [PubMed] [Google Scholar]
- 156. Pahuja P., Kashyap H., and Pawar P. Design and evaluation of HP-beta-CD based voriconazole formulations for ocular drug delivery. Curr. Drug Deliv. 11:223–232, 2014 [DOI] [PubMed] [Google Scholar]
- 157. Patil A., and Majumdar S. Echinocandins in antifungal pharmacotherapy. J. Pharm. Pharmacol. 69:1635–1660, 2017 [DOI] [PubMed] [Google Scholar]
- 158. Ren P., Luo M., Lin S., et al. . Multilaboratory testing of antifungal drug combinations against Candida species and Aspergillus fumigatus: utility of 100 percent inhibition as the endpoint. Antimicrob. Agents Chemother. 59:1759–1766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li L., Wang Z., Li R., Luo S., and Sun X. In vitro evaluation of combination antifungal activity against Fusarium species isolated from ocular tissues of keratomycosis patients. Am. J. Ophthalmol. 146:724–728, 2008 [DOI] [PubMed] [Google Scholar]
- 160. Titsworth E., and Grunberg E. Chemotherapeutic activity of 5-fluorocytosine and amphotericin B against Candida albicans in mice. Antimicrob. Agents Chemother. 4:306–308, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mahdy R., Nada W., and Wageh M. Topical amphotericin B and subconjunctival injection of fluconazole (combination therapy) versus topical amphotericin B (monotherapy) in treatment of keratomycosis. J. Ocul. Pharmacol. Ther. 26:281–285, 2010 [DOI] [PubMed] [Google Scholar]