Abstract
Background
We evaluated the nephroprotective effect of diosmetin in streptozotocin (STZ)-induced diabetic nephropathy (DN) mice.
Material/Methods
Diabetes was induced by injecting STZ (50 mg/kg) i.p. for 5 days. Biochemical parameters, such as fasting blood glucose, creatinine, BUN in the serum, and albumin in the urine, were determined in STZ-induced DN mice after the 8th week of STZ administration. The level of inflammatory mediators in the serum and oxidative stress parameters in the tissue homogenate was estimated in STZ-induced DN mice. Expressions of Akt, NF-κB, and iNOS in the tissue homogenate were assessed by Western blot analysis.
Results
Our data reveal that treatment with diosmetin significantly reduces the fasting blood glucose (FBG), serum creatinine, and blood urea nitrogen (BUN) in the serum and albumin in urine compared to the negative control group. Treatment with diosmetin attenuated the altered level of oxidative stress parameters and inflammatory cytokines in the STZ-induced DN mice. Expression of Akt and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) was significantly reduced and inducible nitric oxide synthase (iNOS) was enhanced in the tissue homogenate of diosmetin-treated mice compared to the negative control group. Data from immunohistochemical analysis suggest that the expressions of NF-κB was significantly reduced in tissues of the diosmetin-treated group compared to the negative control group.
Conclusions
Our study shows that diosmetin protects against renal injury in STZ-induced diabetic nephropathy mice by modulating the Akt/NF-κB/iNOS signaling pathway.
MeSH Keywords: Apoptosis; Diabetes Mellitus, Experimental; Diabetic Nephropathies
Background
Diabetic nephropathy is a diabetic complication that causes end-stage renal disease [1]. DN is characterized by microalbuminuria, renal fibrosis, extracellular matrix deposition, glomerular basement membrane thickening, and decrease in the glomerular filtration rate (GFR) [2]. Fibrosis, inflammation, and oxidative stress play roles in the pathogenesis of DN [3]. Production of reactive oxygen species increases, which results in oxidative stress. In end-stage renal disease, expression of NADPH oxidase 4 is altered, which plays an important role in the production of reactive oxygen species (ROS) [4]. ROS activate many stress-related pathways and oxidative stress activates inflammation pathways. Inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interlukin-1β (IL-1β), and IL-6, are reported to occur at higher levels in patients with DN [5]. Activation of the NF-κB pathway is associated with expression of inflammatory cytokines and transcription of NF-κB is reported to be enhanced in the development of DN [6]. Moreover, in a STZ-induced diabetic animal model, the level of Akt was reported to be increased, which enhanced the progression of DN by increasing NF-κB [7]. In DN, fibrosis occurs due to accumulation of extracellular matrix (ECM) because the expression of transforming growth factor beta 1 (TGF-β1) is enhanced by the activation of NF-κB [8].
Moreover, production of nitric oxide is enhanced in the early stage of DN, and in the later stage, glomerular sclerosis occurs due to decreased production of nitric oxide (NO) [9]. Synthesis of NO is controlled by expression of inducible nitric oxide synthase (iNOS), and in the development of DN, synthesis of iNOS is promoted by inducing TLR2-dependent signaling pathways [10]. Thus, expression of iNOS and concentration of NO are important parameters for the development of DN.
Diosmetin is a flavonoid isolated from the leaves of the plant Olea europaea L [11]. Diosmetin has effects against several types of cancer by inhibiting the activity of CYP1B1 and CYP1A1 enzyme [12] and it attenuates the proliferation of human oral squamous carcinoma SCC-9 cells [13]. Other pharmacological properties of diosmetin are anti-inflammatory, anti-estrogenic, anti-oxidant, anti-microbial, and anti-cancer [14,15]. Moreover, on the basis of its strong anti-oxidant property, diosmetin attenuates the effects of diabetes in STZ-induced diabetic rats [16]. Thus, the present study evaluated the nephro-protective effect of diosmetin in STZ-induced diabetic nephropathic mice.
Material and Methods
Animal
Male albino mice (age 8–10 weeks, weight 18–25 g) were procured from Shanghai Medical College, Shanghai, China. Animals were kept in standard conditions as per guidelines. All mice were allowed to acclimatize to laboratory conditions for 7 days with free access to standard chow diet and tap water. The protocol of this study was approved by the Institutional Animal Ethics Committee of Chongqing General Hospital, China (IAEC/CGH/2017/02).
Induction of diabetic nephropathy
All mice were separated into 2 groups: the control group (n=10) received citrate buffer for 5 days and the diabetic group (n=40) received STZ (50 mg/kg, i.p.) for 5 days. Subsequently, blood glucose was estimated for the confirmation of diabetes. All mice with a fasting blood glucose level above 11.1 mmol/L were considered to be diabetic and were divided into 4 subgroups: the negative control group (which received saline solution), the diosmetin 25 mg/kg group, the 50 mg/kg group, and the diosmetin 100 mg/kg, all P.O. per day for 8 weeks. At the end of the protocol, blood was collected from the tail vein and urine was collected later by keeping all the animals in metabolic cages for 1 day. The collected blood and urine were used to estimate of biochemical parameters. Thereafter, all the animals were killed by decapitation and the kidneys were removed for further study.
Determination of biochemical parameters
Kidneys were weighed and the kidney-to-body weight ratio was calculated for each animal. Blood glucose, triglyceride, BUN, and serum creatinine levels in the serum were estimated by using kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The level of microalbumin was estimated using ELISA kits (R&D Systems, Minneapolis, MN, USA) as per the instructions of the manufacturer.
Determination of inflammatory mediators
Serum concentrations of the inflammatory mediators IL-6, IL-1β, and TNF-α were estimated using ELISA kits (CUSABIO, Wuhan, China) as per the instructions of the manufacturer.
Determination of oxidative stress parameters
Isolated kidney samples were washed with normal saline solution and a tissue homogenizer was used to homogenize the kidney tissues for 5 min at 10 000 rpm in saline solution. Thereafter, tissue homogenate was centrifuged to separate the supernatant for 10 min at 10 000 rpm. In the supernatant solution, the levels of NO, MDA, and MPO and activity of SOD were determined by using kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer’s instructions.
Western blotting assay
Kidney samples were homogenized with RIPA lysis buffer and protein was extracted from the sample by centrifuging at 10 000 rpm for 15 min at 4°C. BCA method was used for the estimation of total protein in the sample as per the instructions in the kit. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate protein from the supernatant solution equivalent to 50 μg of protein. Protein was poured on the membrane and then tris buffer was used to block binding to the membrane for 60 min. Rabbit polyclonal anti-NF-κB p65 (1: 1000), anti-inducible NO synthase (iNOS) [1: 1000], anti-Akt (1: 1000), and anti-pAkt (1: 1000) antibody (Proteintech Group, Inc, Wuhan, China) were incubated with the blots at 4°C for overnight. Later, secondary rabbit anti-mouse IgG horseradish peroxidase (HRP) antibody (Proteintech Group Inc, Wuhan, China) was used to incubate for 60 min at room temperature after washing the membrane with TBST. An ECL detection system was used for development and detection of blots.
Immunohistochemistry
Isolated kidney samples were fixed in a 10% formalin solution, dehydrated, and embedded in liquid paraffin. Then, 5-μm-thick tissue sections of were cut from the wax cubes using a microtome. Tissues were rehydrated in ethanol and boiled for 15 min with EDTA in a microwave oven for the retrieval of antigen. Hydrogen peroxide solution was used to block the activity of endogenous peroxidase enzyme. Anti-NF-κB p65 was incubated at 4°C overnight with tissue slides after washing it with PBS. The Envision Detection Kit was used to detect the antibody, and hematoxylin was used to counterstain the tissue sections. A trinocular microscope was used to examine the tissue sections.
Statistical analysis
All data are expressed as mean ±SEM (n=10). The statistical analysis was performed using one-way ANOVA. Post hoc comparison of means was carried out by Dunnett’s post hoc test (Gradpad prism 6.1., CA, USA) for multiple comparisons. The level of statistical significance was set at P<0.05.
Results
Effect of diosmetin on fasting blood glucose and kidney/body weight
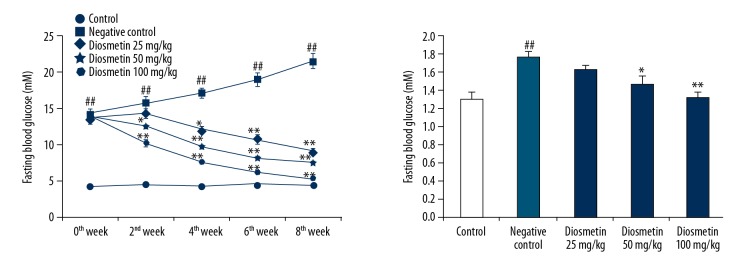
The effect of diosmetin on serum concentration of fasting blood glucose and kidney/body weight in STZ-induced DN mice was shown in Figure 1. We found that the fasting blood glucose levels were significantly higher in all groups treated with STZ on day 0 of the protocol compared to the control group. However, FBG was significantly higher at week 8 of the protocol in the negative control group compared to the control group. Treatment with diosmetin significantly reduced FBG in a dose-dependent manner compared to the negative control group. The kidney/body weight ratio was also significatly higher (p<0.01) in the negative control group compared to the control group. There was a significant decrease in kidney/body weight ratio in the diosmetin-treated group compared to the negative control group.
Figure 1.
Effect of diosmetin on the serum concentration of fasting blood glucose and kidney/body weight in STZ-induced DN mice. Mean ±SEM (n=10), ## p<0.01 compared to control group; * p<0.05, ** p<0.01 compared to negative control group.
Effect of diosmetin on the biochemical parameters
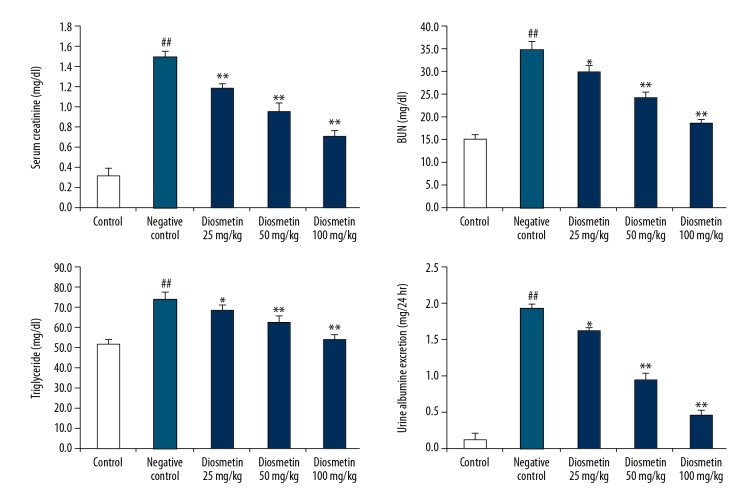
The effects of diosmetin on the concentrations of triglyceride, creatinine, and BUN in serum and albumin concentration in urine of STZ-induced DN mice are shown in Figure 2. There was a significant increase in the concentration of triglyceride, creatinine, and BUN in serum and albumin concentration in urine of the negative control group compared to the control group. However, treatment with diosmetin attenuated the altered level of triglyceride, creatinine, and BUN in serum and albumin concentration in urine of STZ-induced diabetic mice. Alteration of these biochemical parameter confirms that DN was induced in the negative control group and that treatment with diosmetin attenuated the DN in STZ-induced diabetic mice.
Figure 2.
Effect of diosmetin on the biochemical parameters in serum and urine of STZ-induced DN mice. Mean ±SEM (n=10), ## p<0.01 compared to control group; * p<0.05, ** p<0.01 compared to negative control group.
Effect of diosmetin on inflammatory mediators
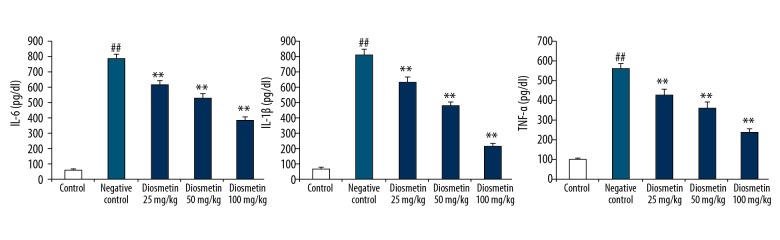
Effect of diosmetin on the concentration of inflammatory mediators in the serum of STZ-induced DN mice is shown in Figure 3. The levels inflammatory mediators TNF-α, IL-6, and IL-1β were significantly enhanced in the serum of the negative control group compared to the control group. There was a significant decrease in the levels of TNF-α, IL-6, and IL-1β in the serum of the diosmetin-treated group compared to the negative control group.
Figure 3.
Effect of diosmetin on the concentration of inflammatory mediators in the serum of STZ-induced DN mice. Mean ±SEM (n=10), ## p<0.01 compared to control group; * p<0.05, ** p<0.01 compared to negative control group.
Effect of diosmetin on the parameters of oxidative stress
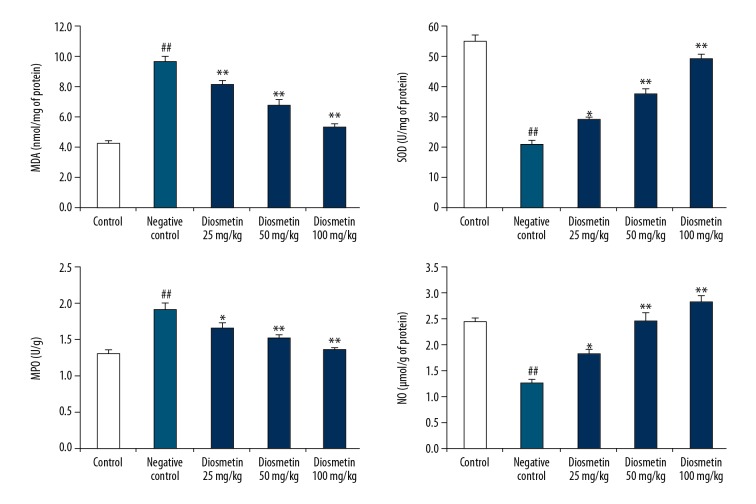
The effect of diosmetin on the oxidative stress parameters was estimated by determining the activity of SOD and MPO enzymes and the levels of NO and MDA in the kidney tissue homogenate of STZ-induced DN mice (Figure 4.). There was a significant increase in the level of MDA and a decrease in NO level in the tissue homogenate of the negative control group compared to the control group. Moreover, the activity of SOD was significantly reduced and the MPO enzyme activity was increased in the tissue homogenate of the diosmetin-treated group compared to the negative control group. However, treatment with diosmetin attenuated the altered levels of MDA and NO and the activity of SOD and MPO in the tissue homogenate of STZ-induced DN mice.
Figure 4.
Effect of diosmetin on the oxidative stress parameters in the kidney tissue homogenate of STZ-induced DN mice. Mean ±SEM (n=10), ## p<0.01 compared to control group; * p<0.05, ** p<0.01 compared to negative control group.
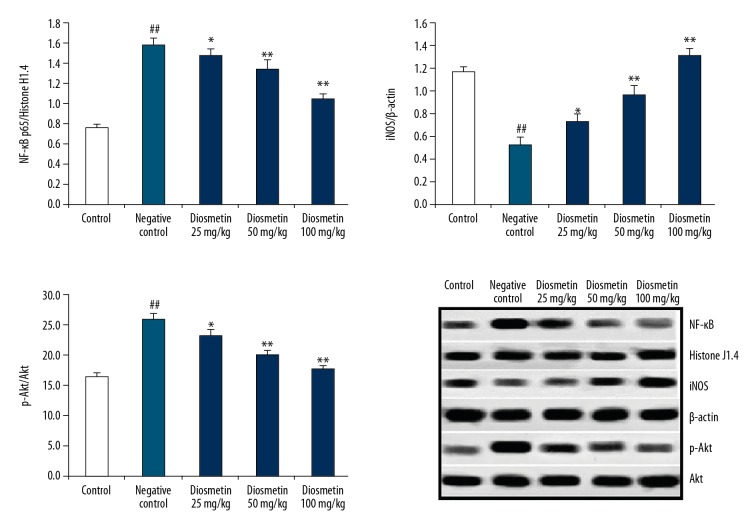
Effect of diosmetin on the expressions of p-Akt, NF-κB, and iNOS
Figure 5 shows the effects of diosmetin on the expressions of p-Akt, NF-κB, and iNOS in the kidney tissue homogenate of STZ-induced DN mice. The expression of p-Akt and NF-κB was significantly enhanced and expression of iNOS was decreased in the tissue homogenate of the negative control group compared to the control group. However, treatment with diosmetin significantly attenuated the altered expressions of p-Akt, NF-κB, and iNOS in the kidney tissue homogenate of STZ-induced DN mice in a dose-dependent manner.
Figure 5.
Effect of diosmetin on the expressions of p-Akt, NF-κB, and iNOS in the kidney tissue homogenate of STZ-induced DN mice. Mean ±SEM (n=10), ## p<0.01 compared to control group; * p<0.05, ** p<0.01 compared to negative control group.
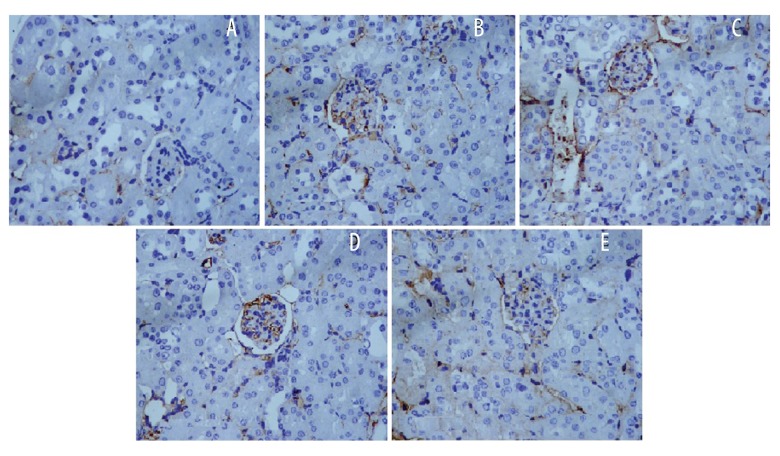
Effect of diosmetin on immunohistochemical analysis of NF-κB
The effect of diosmetin on the immunohistochemical analysis of NF-κB in the tissue homogenate of kidney of STZ-induced DN mice is shown in Figure 6. There was significant enhancement in the expression of NF-κB p65 in the cytoplasm and nucleus of tubular and glomerular cell of the negative control group compared to the control group. However, treatment with diosmetin attenuated the expression of NF-κB p65 in the renal tissues of STZ-induced DN mice.
Figure 6.
Effect of diosmetin on the immunohistochemical analysis of NF-κB in the tissue homogenate of kidney of STZ-induced DN mice (×400). (A) Control; (B) Negative control; (C) Diosmetin 25 mg/kg; (D) Diosmetin 50 mg/kg; (E) Diosmetin 100 mg/kg.
Discussion
In diabetes, chronic hyperglycemia leads to various complications, such as diabetic neuropathy, gastroparesis, retinopathy, and diabetic nephropathy [17]. DN is the major complication of diabetes and blood pressure is reported to be increased in these patients. The literature reveals that drugs used for the management of DN reduce the blood pressure [18]. Diosmetin is reported to possess anti-diabetic, anti-inflammatory, anti-oxidant, and anti-cancer properties [14–16]. Thus, the present study evaluated the nephroprotective effect of diosmetin in STZ-induced DN mice. Diabetes was induced by injecting STZ (50 mg/kg) i.p. for 5 days. Biochemical parameters, including fasting blood glucose, creatinine, BUN in the serum, and albumin in the urine, were determined in STZ-induced DN mice. Moreover, the level of inflammatory mediators in the serum and oxidative stress parameters in the tissue homogenate was assessed in STZ-induced DN mice. Expressions of Akt, NF-κB, and iNOS in the tissue homogenate were estimated by Western blot analysis.
Several biochemical parameters, including creatinine and urea level in the serum and microalbumin urea in urine, are markers of DN [19]. Our data also showed that the levels of these biochemical parameters were altered in the negative control group and that treatment with diosmetin attenuated the altered levels of in the serum and urine of STZ-induced DN mice. The literature reveals that in diabetes, the level of oxidative stress is enhanced, which affects the pathogenesis of DN [20]. Results of the present study reveal that treatment with diosmetin significantly attenuated the altered level of level of oxidative stress in the tissue homogenate of STZ-induced DN mice in a dose-dependent manner. Several cellular processes, including angiogenesis, metabolism, growth, survival, and proliferation, were reported to be regulated by Akt and PI3K/Akt pathways involved in DN [21]. A previous study reported that several molecules for the management renal dysfunction attenuate the Akt pathway [22]. Results of the present study reveal that diosmetin attenuated the altered expression of Akt protein in the renal tissue of DN mice.
The inflammatory cytokines IL-6, IL-1β, and TNF-α were reported to be involved in the development of DN by stimulating the NF-κB signaling pathway [23,24]. In DN, inflammation occurs by activating the NF-κB signaling pathway and by enhanced oxidative stress [25,26]. Data of the present study reveal that diosmetin attenuates the altered level of inflammatory cytokines in the serum of DN mice. Moreover, diosmetin reduced the expression of NF-κB protein the tissue homogenate of DN mice compared to the negative control group.
Development of renal injury occurs due to decrease in the level of NO and is involved in cardiovascular diseases. Several studies report that the production of NO is enhanced by iNOS and is thereby involved in renal injury [27]. We found that the expression of iNOS was significantly lower in the diosmetin-treated group compared to the negative control group of mice.
Conclusions
Our study shows that diosmetin protects against renal injury in STZ-induced diabetic nephropathy mice by modulating the Akt/NF-κB/iNOS signaling pathway. Our results reveal that oxidative stress parameters and inflammatory mediator levels were significantly reduced by diosmetin in STZ-induced DN mice.
Acknowledgement
We thank Chongqing General Hospital, China for providing the necessary facilities for our investigation.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Lim AK. Diabetic nephropathy – complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–81. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda-Díaz AG, Pazarín-Villaseñor L, Yanowsky-Escatell FG, Andrade-Sierra J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J Diabetes Res. 2016;2016 doi: 10.1155/2016/7047238. 7047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duran-Salgado MB, Rubio-Guerra AF. Diabetic nephropathy and inflammation. World J Diabetes. 2014;5(3):393–98. doi: 10.4239/wjd.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan C, Su H, Zhang C. Role of NADPH oxidase in metabolic disease-related renal injury: An update. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/7813072. 7813072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, et al. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015;2015:948417. doi: 10.1155/2015/948417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S, Zhang J, Wang S, et al. SOCS2 overexpression alleviates diabetic nephropathy in rats by inhibiting the TLR4/NF-κB pathway. Oncotarget. 2017;8(53):91185–98. doi: 10.18632/oncotarget.20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y, Zhang X, Wang Y, et al. Targeting JNK by a new curcumin analog to inhibit NF-κB-mediated expression of cell adhesion molecules attenuates renal macrophage infiltration and injury in diabetic mice. PLoS One. 2013;8(11):e79084. doi: 10.1371/journal.pone.0079084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Li D, Fan L, et al. CAPE-pNO2 ameliorated diabetic nephropathy through regulating the Akt/NF-κB/iNOS pathway in STZ-induced diabetic mice. Oncotarget. 2017;8(70):114506–25. doi: 10.18632/oncotarget.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellamea BS, Leitão CB, Friedman R, Canani LH. Nitric oxide system and diabetic nephropathy. Diabetol Metab Syndr. 2014;6:17. doi: 10.1186/1758-5996-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh M-Y, Chang MY, Chen Y-J, et al. The inducible nitric oxide synthase (iNOS)/Src axis mediates toll-like receptor 3 Tyrosine 759 phosphorylation and enhances its signal transduction, leading to interferon-β synthesis in macrophages. J Biol Chem. 2014;289(13):9208–20. doi: 10.1074/jbc.M113.508663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meirinhos J, Silva BM, Valentão P, et al. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat Prod Res. 2005;19(2):189–95. doi: 10.1080/14786410410001704886. [DOI] [PubMed] [Google Scholar]
- 12.Androutsopoulos VP, Spyrou I, Ploumidis A, et al. Expression profile of CYP1A1 and CYP1B1 enzymes in colon and bladder tumors. PLoS One. 2013;8(12):e82487. doi: 10.1371/journal.pone.0082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browning AM, Walle UK, Walle T. Flavonoid glycosides inhibit oral cancer cell proliferation – role of cellular uptake and hydrolysis to the aglycones. J Pharm Pharmacol. 2005;57(8):1037–42. doi: 10.1211/0022357056514. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Gong XB, Huang LG, et al. diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget. 2017;8(19):30723–33. doi: 10.18632/oncotarget.15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge A, Liu Y, Zeng X, et al. Effect of diosmetin on airway remodeling in a murine model of chronic asthma. Acta Biochim Biophys Sin (Shanghai) 2015;47(8):604–11. doi: 10.1093/abbs/gmv052. [DOI] [PubMed] [Google Scholar]
- 16.Juárez-Reyes K, Brindis F, Medina-Campos ON, et al. Hypoglycemic, antihyperglycemic, and antioxidant effects of the edible plant Anoda cristata. J Ethnopharmacol. 2015;161:36–45. doi: 10.1016/j.jep.2014.11.052. [DOI] [PubMed] [Google Scholar]
- 17.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546–51. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Xiong X. Evidence-based chinese medicine for hypertension. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/978398. 978398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110(1):32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 20.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg Oncol Clin N Am. 2013;22(4):641–44. doi: 10.1016/j.soc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma ZG, Xia HQ, Cui SL, Yu J. Attenuation of renal ischemic reperfusion injury by salvianolic acid B via suppressing oxidative stress and inflammation through PI3K/Akt signaling pathway. Braz J Med Biol Res. 2017;50(6):e5954. doi: 10.1590/1414-431X20175954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: A review. HSR Proc Intensive Care Cardiovasc Anesth. 2010;2(3):161–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Gala-Błądzinska A, Żyłka A, Dumnicka P, et al. Sterile leukocyturia affects urine neutrophil gelatinase-associated lipocalin concentration in type 2 diabetic patients. Arch Med Sci. 2017;2:321–27. doi: 10.5114/aoms.2016.64043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Lin R, Zhao B, et al. Correlation between oxidative stress and the NF-κB signaling pathway in the pulmonary tissues of obese asthmatic mice. Mol Med Rep. 2016;13(2):1127–34. doi: 10.3892/mmr.2015.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahraman C, Kahraman NK, Aras B, et al. The relationship between neutrophil-to-lymphocyte ratio and albuminuria in type 2 diabetic patients: A pilot study. Arch Med Sci. 2016;12(3):571–75. doi: 10.5114/aoms.2016.59931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JU, Bae EH, Ma SK, Kim SW. Altered nitric oxide system in cardiovascular and renal diseases. Chonnam Med J. 2016;52(2):81–90. doi: 10.4068/cmj.2016.52.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]