Abstract
Background
The status of p53 is critical to the chemoradiosensitivity of cervical cancer cells. Wild-type p53 is essential to orchestrate the cellular response to cytotoxic stimuli. Our previous data illustrated that cervical cancer patients whose specimens overexpressed microR-492 (miR-492) were highly sensitive to concurrent chemoradiation. Although p53 activation has been reported to upregulate miR-492 by a miRNA profiling assay in lung cancer cells, the transcriptional regulation of miR-492 in cervical cancer cells remains poorly understood. Therefore, we aimed to decipher the relationship between p53 and miR-492 in cervical cancer cells.
Material/Methods
The expression of p53 and miR-492 in cervical cancer cell lines was measured by western blot and real-time PCR. After cells were transfected with wild-type p53 plasmid or were treated by irradiation and 5-fluorouracil (5-FU), the expression changes of p53 as well as miR-492 were examined by western blot and real-time PCR. The putative p53 binding site of miR-492 was first analyzed by bioinformatics tools, then validated by chromatin immunoprecipitation and dual-luciferase reporter assays.
Results
We found that miR-492 was upregulated in cells with wild-type p53 compared to cells with mutant p53. Transfection of wild-type p53 plasmid or treatments with cytotoxic reagents including irradiation and 5-FU all induced miR-492 overexpression. Bioinformatics analysis and experimental validations further proved p53 interacted with miR-492 promoter directly.
Conclusions
In cervical cancer cells, p53 activated miR-492 expression transcriptionally.
MeSH Keywords: Genes, p53; MicroRNAs; Regulatory Elements, Transcriptional; Uterine Cervical Neoplasms
Background
Cervical cancer ranks the fourth most commonly diagnosed malignancy in females and is still the leading cause of death from gynecological cancer worldwide [1,2]. Because of persistent human papillomavirus (HPV) infection and the shortage of HPV vaccines, the incidence of cervical cancer is growing every year in China [3]. Since cervical cancer usually occurs at a younger age, leading to proportionally more life-years lost [4], such cancer burden requires urgent attention and efficient management nationwide.
The standard treatment of choice for stage IB2 to IVA cervical cancer patients is concurrent chemoradiation, which is composed of pelvic external beam radiation therapy (EBRT) and intracavitary brachytherapy in addition with single-agent cisplatin or cisplatin plus 5-fluorouracil (5-FU) chemotherapy [5,6]. The sensitivity of cervical cancer cells to DNA-damaging agents originating from chemotherapy or radiotherapy is partly dependent on the status of p53 [7,8]. Activated wild-type p53 is essential to induce growth arrest or apoptosis in response to various cytotoxic stimuli [9,10], while inactivation of p53 results in resistance to anticancer agents in cervical cancer [11–13]. Invalid p53 is mainly attributed to infection of HPV and p53 mutations in cervical cancer [14–16].
MicroRNA (miRNA) is a member of noncoding RNAs which modulates gene expressions post-transcriptionally and plays essential roles in the initiation and progression of malignant diseases including cervical cancer [17]. Mounting evidence has demonstrated that aberrantly expressed miRNAs were involved in multiple pathological processes of cervical cancer, and some differentially expressed miRNAs may have substantial diagnostic and prognostic values [18–22]. For instance, ectopically overexpressed miR-21 in HPV-positive cervical cancer promoted tumorigenesis through downregulating the expression of programmed cell death-4 (PCD4) [20,22] and mediated resistance to radiotherapy through targeting large tumor suppressor 1 (LATS1) [23]. In addition, expression of tumor suppressive miRNAs, such as miR-143, and miR-126 were significantly downregulated in HPV-positive cervical cancer [20,22,24,25].
As a potent transcriptional factor, increased expression of p53 due to genotoxic stimuli could also transactivate miRNA expression to inhibit cell proliferation and accelerate cell apoptosis or senescence [26]. MiR-34 family members, including miR-34a, miR-34b, and miR-34c, have been proven as potent p53 effectors to exert tumor suppressive functions in various cancers [26–28]. Furthermore, overexpressed miR-34a could not only increase chemosensitivity of prostate and bladder cancer cells to paclitaxel, cisplatin, and camptothecin [29–31], but also enhance radiosensitivity of non-small cell lung cancer cells [32].
In our previous study, we have compared miRNA expression profiles of pre-therapeutic tumor biopsy samples from cervical cancer patients who were sensitive or resistant to concurrent chemoradiation. And we found that patients whose specimens overexpressed miR-492 prior to treatment were highly sensitive to chemoradiation [33]. As reported by Raver-Shapira et al. previously, apart from miR-34a, miR-492 expression was also noticeably increased in the presence of activated p53 through miRNA array profiling experiment in non-small lung cancer cells [34]. Until now, the transcriptional regulation of miR-492 was poorly understood. Considering that wild-type p53 was necessary for effective chemoradiotherapy and that cervical cancer patients with upregulated miR-492 were highly sensitive to concurrent chemoradiation, we designed the present study to validate whether miR-492 was transactivated by p53 or p53 inducible treatment (UV and 5-FU) in cervical cancer.
In this study, we validated that miR-492 was transcriptionally activated by p53 either via wild-type p53 transfection or via irradiation and 5-FU treatment. The p53 response element of miR-492 was confirmed by ChIP and dual-luciferase reporter assay. The findings gave us some hints to the underlying mechanism of the correlation between miR-492 upregulation and good responsiveness to concurrent chemoradiation, and also implied that miR-492 may have some clinical implications to predict chemoradiosensitivity of cervical cancer patients.
Material and Methods
Cell culture and treatment
Three human cervical carcinoma cell lines (C-33A, SiHa, and HeLa) were purchased from National Cell Resource Center (Beijing, China). Cells were maintained in Dulbecco’s modified Eagle’s medium (C-33A and SiHa) (Hyclone, Marlborough, MA, USA) or RPMI-1640 (HeLa) (Hyclone, Marlborough, MA, USA) supplemented with 10% fetal bovine serum as well as 100 IU/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37°C. SiHa cells received irradiation (8 Gy with 6 MV x-ray) for 24, 48, or 72 hours, respectively. For 5-FU treatment, SiHa cells were cultured in complete medium with 0, 300, or 500 μM 5-FU for 20 hours.
Plasmid and transfection
The pCMV-Neo-Bam p53 wt, which expressed wild-type p53 protein, as well as the vector plasmid pCMV-Neo-Bam, were gifts from professor Bert Vogelstein (Addgene plasmid #16434 and #16440) [35]. To validate the transcriptional activity of p53, a 176 bp promoter fragment of pre-miR-492 containing putative p53 binding site (p53 BS) was amplified from human genomic DNA and cloned into the KpnI and HindIII restriction sites of the luciferase reporter pGL3-basic vector, which was promoterless and enhancerless (Promega Corp., Madison, WI, USA). The primer sets for p53 BS of pre-miR-492 was: forward: 5′-GGGGTACCCCCTGGCTGGAACAGAAGAT-3′, reverse: 5′-CCCAAGCTTCCCTGGTCTTGGCTGGGATC -3′. And the recombinant plasmid was named as pGL3-p53 BS WT. The mutant promoter fragment of pre-miR-492 with a complete deletion of consensus p53 binding motifs was synthesized and also cloned into pGL3-basic vector by Sinogenomax Company (Sinogenomax, Beijing, China), donated as pGL3-p53 BS MUT. After cells were seeded into appropriate culture plates and grown to 70–80% confluence, recommended amount of plasmid was transiently delivered into cells by using Lipofactamine™ 3000 following the manufacturer’s instructions (Invitrogen).
MiRNA-specific quantitative real-time RT-PCR (qRT-PCR)
Total RNA from cultured cells was extracted by using mirVana™ MicroRNA Isolation Kit (Ambion, Austin, TX). The concentration of RNA was determined by NanoDrop spectrophotometer ND-2000 (Thermo Scientific, Waltham, MA, USA). Then miRNA reverse transcription and real-time PCR were performed as described previously [36]. Briefly, 10 ng of total RNA was reverse transcribed to cDNA by TaqMan MicroRNA Reverse Transcription Kit (Life Technologies, Applied Biosystems). Thereafter, TaqMan MicroRNA Assays (Life Technologies, Applied Biosystems) together with TaqMan Universal PCR Master Mix (Life Technologies, Applied Biosystems) were employed to examine miR-492 expression by using a StepOne Plus Real-Time PCR System (Life Technologies, Applied Biosystems). U6 was used as an internal control. All reactions were performed for at least 3 times and the fold change of gene expression was determined by using the 2−ΔΔCt method [37].
Western blot
Indicated cells were harvested and lysed in RIPA buffer (Sigma, Saint Louis, MO, USA) containing protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). After extraction, the concentration of proteins was measured by Pierce BCA Protein Assay (Pierce, Thermo Fisher Scientific, USA). An equal amount of proteins was loaded and separated by 10% SDS-PAGE gel before transferred to nitrocellulose membranes. The membranes were first blocked with 5% nonfat milk for 1 hour at room temperature then immunoblotted with a primary antibody against p53 (DO-1, 1: 1000, Santa Cruz) or β-actin (AC-15, 1: 3000, Sigma) at 4°C overnight. After extensive washing by T-BST (0.05% Tween-20 in Tris-buffered saline, TBS), the membranes were incubated with the horseradish peroxidase-conjugated goat anti-mouse secondary antibody (ZB-2305, 1: 1000, ZSGB-BIO). A Luminol Detection System (Santa Cruz) was applied to detect the signal. Protein expression was quantified by using a Gel EDAS 290 Analysis System (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA) and Gel-Pro Analyzer 3.1 software (Media Cybernetics, Silver Spring, MD, USA).
Chromatin immunoprecipitation (ChIP) assay
Chromatin immunoprecipitation (ChIP) assay was performed by using ChIP-IT Express Chromatin Immunoprecipitation Kits (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s protocol. After cell fixation and chromatin shearing, 2 μg of anti-p53 (DO-1, 1: 1000, Santa Cruz) antibody or a monoclonal mouse IgG (Active Motif, Carlsbad, CA, USA) was mixed with chromatin solutions and incubated at 4°C overnight on an end-to-end rotator. Crosslinks were reversed, and protein was removed by digestion with proteinase K. Purified ChIP DNA or inputs were further detected by semi-quantitative PCR. Primers specific for putative p53-BS upstream of the pre-miR-492 were: forward: 5′-CGACATCAGCAGTCCCTA-3′, reverse: 5′-AGATACGTGCCGAGAAAG-3′. The PCR was conducted by using Taq DNA polymerase (TaKaRa, Japan) with a reaction condition as 36 cycles of 30 seconds at 95°C, 30 seconds at 52°C, and 1 minute at 72°C. The PCR products were separated by electrophoresis on 2% agarose gels and visualized under ultraviolet light.
Firefly dual-luciferase reporter assay
Cells reaching 70–80% confluence in 24-well plates were transfected with corresponding reporter plasmids (300 ng) together with the internal control plasmid pRL-SV40 (2 ng) with or without the presence of 300 μM 5-FU. Twenty-four hours after transfection, luciferase activity was determined by using the Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI, USA). Firefly luciferase activity was normalized to Renilla luciferase activity for each transfected well.
Statistical analysis
All experiments were carried out for at least 3 times with each sample tested in triplicate. The results were reported as means ± standard deviation (SD) and analyzed by SPSS 20.0 software (SPSS, Chicago, IL, USA). Bilateral Student’s t-test was employed to evaluate the difference between 2 groups where appropriate. All graphs were processed with GraphPad 6.0 software (GraphPad, San Diego, USA). P<0.05 was considered as statistically significant.
Results
MiR-492 was upregulated in wild-type p53 cervical cancer cells
To find the internal correlation between p53 and miR-492 in cervical cancer cell lines, we examined their expression in C-33A, SiHa, and HeLa cells by western blot and qRT-PCR, respectively. A trace amount of p53 was detected in SiHa and HeLa cells which carried wild-type p53 and were all HPV-positive [38,39]. While an excessive amount of p53 was found in C-33A cells which carried a C to T mutation at nucleotide 817 of p53 and was HPV-negative [38] (Figure 1A). Correspondingly, the expression of miR-492 was lower in p53 mutant C-33A cells than in p53 wild-type SiHa and HeLa cells (Figure 1B).
Figure 1.
Expression of p53 and miR-492 in cervical cancer cell lines. (A) The protein expression of p53 in C-33A, SiHa, and HeLa cells was detected by western blot; β-actin was used as an internal control. Quantification of p53 expression was performed by using Gel-Pro Analyzer 3.1 software. (B) miR-492 expression was examined by qRT-PCR with U6 as an internal control. All values were represented as means ±SD from at least 3 individual experiments.
MiR-492 expression was activated by p53 or p53 inducible treatments
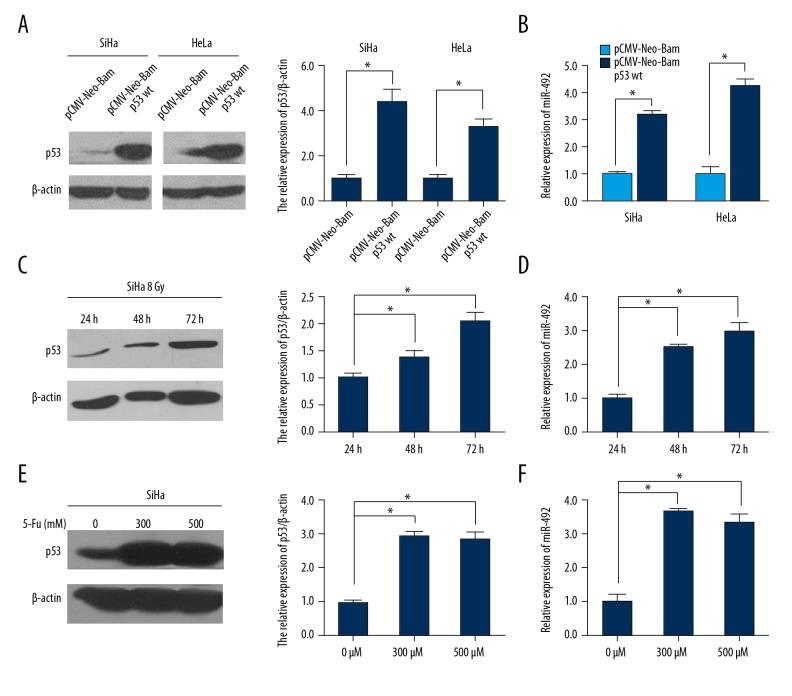
To validate whether p53 activation could result in upregulated miR-492 expression, 1.5 μg pCMV-Neo-Bam p53 wt or its control plasmid was transfected into HeLa and SiHa cells (Figure 2A). miR-492 was significantly increased when wild-type p53 was overexpressed (Figure 2B). Since irradiation led to DNA double-strands breaks thus elevated physiologic expression of p53, we treated SiHa cells with a dose of 8 Gy with 6 MV x-ray for 24, 48, or 72 hours, respectively. As shown in Figure 2C, along with the extension of exposure time, p53 was increased gradually. Meanwhile, miR-492 was elevated accompanied with the induction of p53, as well (Figure 2D). 5-FU is a thymidylate synthase inhibitor, administration of 5-FU can interrupt the synthesis of thymidine which is necessary for DNA replication and lead to p53 activation [40]. SiHa cells were harvested after exposed to 0, 300, or 500 μM 5-FU for 20 hours. The elevated expression of p53 came up to the platform period after the concentration of 5-FU reached 300 μM (Figure 2E). In like manner as irradiation, enrichment of miR-492 was observed when p53 was activated by the chemotherapeutic reagent (Figure 2F).
Figure 2.
MiR-492 expression was induced by p53. (A, B) HeLa and SiHa cells were transiently transfected with wild-type p53 expression plasmid pCMV-Neo-Bam p53 wt or its control vector, and the levels of p53 and miR-492 were examined by western blot and qRT-PCR. Expression changes of p53 and miR-492 were also detected when SiHa cells were treated with 8 Gy with 6 MV x-ray irradiation for 24, 48, or 72 hours (C, D), or exposed to different concentrations of 5-FU for 20 hours (E, F) by using western blot and qRT-PCR. The densitometric measurement was conducted with each western blot analysis. All data were shown as means ±SD from at least 3 individual experiments. * P<0.05.
Identification of a p53 responsive element upstream of the miR-492 locus
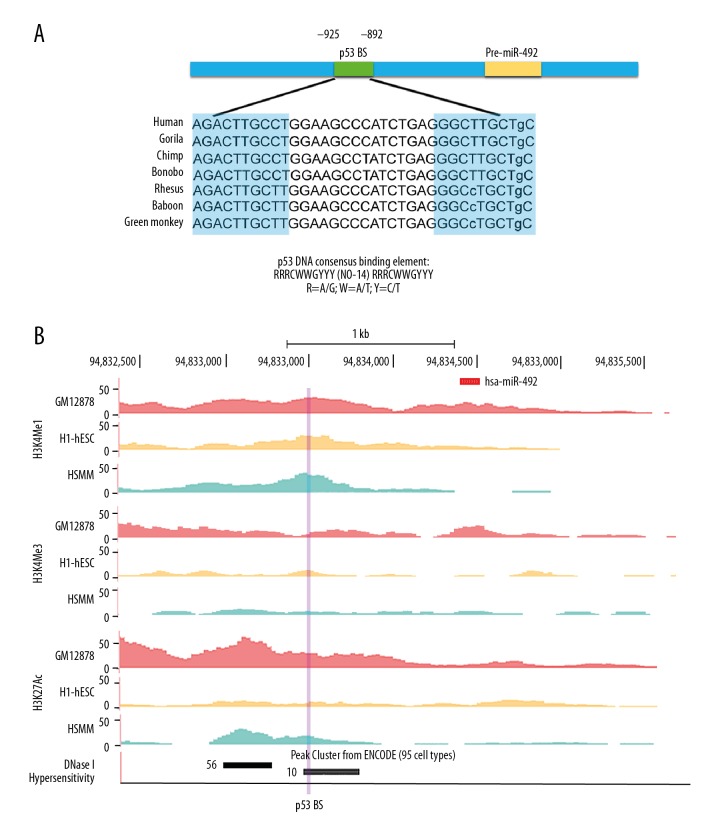
Because of the induction of miR-492 was highly dependent on p53, a search for p53 BS was conducted 2 kb upstream and 1 kb downstream of the miR-492 locus by using PROMO, an online program to identify putative transcription factor binding sites (TFBS) [41]. The submitted sequence of miR-492 was retrieved from the University of California, Santa Cruz (UCSC) Genome Browser. According to the classic nucleotide composition pattern of p53 response elements [42], two 10 bp monomers homologous to the consensus p53 binding motif 5′-PuPuPuC(A/T)(A/T)GPyPyPy-3′ were found from -925 to -916 (5′-AGACTTGCCT-3′) and -901 to -892 (5′-GGGCTTGCTg-3′) relative to the miR-492 locus, and were separated by a 14 bp spacer (Figure 3A). The p53 binding sites (BS) identified within the promoter region of miR-492 was a canonical p53 whole-site response element with only one mismatch at the last base. Moreover, the p53 BS was evolutionary conserved in primates (Figure 3A).
Figure 3.
Features of the miR-492 promoter region. (A) Schematic representation of p53 BS position in the miR-492 promoter region. The sequence of putative p53 response element of miR-492 was compared with the consensus p53 BS in detail. Besides, p53 BS of miR-492 was conserved among 7 primate species. The sequences were derived from the UCSC Genome Browser, and the tandem repeats of consensus p53 binding motifs in miR-492 promoter were highlighted with blue. (B) Promoter sequence 2 kb upstream and 1 kb downstream of the pre-miR-492 was visualized on the UCSC Genome Browser. Upper panel: The histone post-transcriptional modifications including H3K4me1, H3K4me3, and H3K27Ac of miR-492 promoter were presented from GM12878, H1-hESC, and HSMM cell lines, as determined by the ChIP-seq assays from ENCODE data. Lower panel: DNase cluster tracks showed DNase I hypersensitivity areas. The black or gray box indicated a higher degree of DNase I hypersensitivity. The number of cell lines hypersensitive to DNase I in the putative p53 BS was shown, as well. The locus of p53 BS was highlighted with purple. BS – binding site; ChIP – chromatin immunoprecipitation.
We further analyzed the putative p53 BS by the functional annotations in the ENCODE consortium [43]. Mono- and tri-methylation of histone H3 lysine 4 (H3K4me1, H3K4me3), as well as acetylation of histone H3 lysine 27 (H3K27Ac), are 3 histone posttranslational modifications associated with active promoters or enhancers [44]. Moderate enrichment of H3K4me1 and H3K27Ac marks were observed in GM12878, H1-hESC, and HSMM cell lines at putative p53 BS upstream to the miR-492 locus (Figure 3B). In addition, this sequence was also hypersensitive to DNase I (Figure 3B), which further indicated its possible role as a functional promoter or active regulatory region.
P53 bound to miR-492 promoter directly
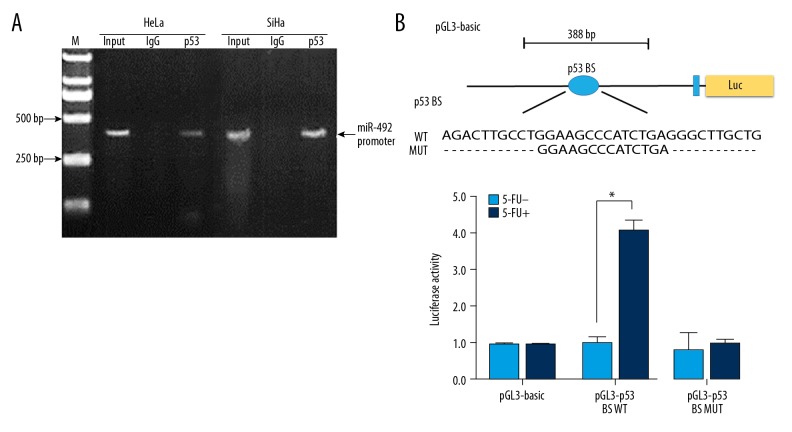
To validate whether p53 mediated transcriptional activation of miR-492, ChIP analysis was performed. HeLa and SiHa cells were cross-linked with formaldehyde and their chromatin was sheared by sonication. Then the cross-linked protein-DNA complex was immunoprecipitated by utilizing a full-length anti-p53 antibody. The harvested DNA fragments were subsequently amplified by semi-quantitative PCR using primers flanking the predicted p53 BS in the miR-492 promoter. As shown in Figure 4A, an enrichment of miR-492 promoter sequence interacting with p53 could be observed in HeLa and SiHa cells, indicating miR-492 could be transcriptionally activated by p53 directly.
Figure 4.
MiR-492 expression was directly regulated by p53. (A) ChIP analysis of p53 enrichment at the promoter region of miR-492 was performed with SiHa and HeLa cells. The chromatin segments bound to p53 or control IgG were purified and further analyzed by semi-quantitative PCR with primers surrounding the p53 BS. (B) p53 BS transcriptional activity with or without 5-FU induction was validated by dual-luciferase reporter assays. pGL3-basic construct harboring wild-type or mutant p53 BS or the control vector was transfected into SiHa cells together with pRL-SV40 as an internal control with or without 300 μM 5-FU for 20 hours. Data were represented as means ±SD from 3 independent experiments, * P<0.05. ChIP – chromatin immunoprecipitation; 5-FU – 5-fluorouracil.
We next performed a dual-luciferase reporter assay to validate the direct interaction between p53 and miR-492 promoter. pGL3-basic constructs harboring either wide type (pGL3-BS WT) or mutant p53 BS (pGL3-BS MUT) as well as the control vector pRL-SV40 were transiently delivered into SiHa cells respectively with or without the presence of 5-FU. Administration of 5-FU could stimulate p53 expression (Figure 2E), thus led to remarkably elevated luciferase activity of pGL3-BS WT construct. While this effect was abolished when 2 repeats of consensus p53 binding motifs were deleted as shown in pGL3-BS MUT group, suggesting miR-492 was a transcriptional effector of p53 (Figure 4B). In addition, no obvious change of luciferase activity could be detected between pGL3-BS WT and pGL3-BS MUT vectors without 5-FU treatment, which may be due to the relatively lower level of wild-type p53 in SiHa cells (Figure 4B).
Discussion
Numerous studies have proven that dysregulated miRNAs participate in each essential process of cancer progression. Due to their smaller size, miRNAs are stably existing in tissues, blood, and different biologic fluids such as urine [45]. They can be reliably extracted or detected as well. Such characteristics make miRNAs as promising molecules to be used as diagnostic, prognostic and predictive biomarkers in cancer treatment. For instance, overexpression of miR-21 not only indicates aggressive progression of HPV-associated cervical cancer, but also suggests increased resistance to radiotherapy [20,46]. Decreased miR-143 is significantly associated with lymph node metastasis and HPV16 infection [22,47]. Since miRNAs could serve as viable targets, chemically engineered antisense oligonucleotides, such as antagomirs and LNA-anti-miR, are developed to reduce excessive miRNA expression, and miRNA replacement therapies are proposed to reintroduce miRNAs either lost or downregulated in vivo. Although most of the miRNA-based therapeutics are in preclinical status and very costly, the prospect is encouraging and promising [45].
Our previous study illustrated that miR-492 was significantly upregulated in cervical cancer specimens which were sensitive to concurrent chemoradiation therapy compared with their resistant counterparts, overexpressed miR-492 further induced cell proliferation which made cervical cancer cells more vulnerable to irradiation [33]. Although miR-492 has been found overexpressed in several malignancies including hepatoblastoma [48], pancreatic adenocarcinoma [49], and prostate cancer [50], the regulation of miR-492 is still poorly understood. A previous study suggested that a coding sequence KRT19 was a novel precursor of hsa-miR-492 in hepatoblastoma [48]. While KRT19 positive colonic cancer-initiating stem cells were resistant to radiotherapy [51]. Since cervical cancer patients with upregulated miR-492 were highly sensitive to chemoradiotherapy, we speculated that there must exist another mechanism to regulate miR-492.
According to the previous report that miR-492 was noticeably upregulated upon p53 activation in human non-small lung cancer cells [34], we examined the expression of p53 and miR-492 in cervical cancer cell lines first. As expected, miR-492 was increased in p53 wild-type SiHa and HeLa cells, while decreased in p53 mutant C-33A cells. The p53 mutants interfered with transactivation of MDM2 which could rapidly degrade p53 through ubiquitination, thus resulted in an accumulation of p53 mutant proteins in C-33A cells [52,53]. Transfection of wild-type p53 plasmid could induce miR-492 upregulation both in SiHa and HeLa cells. Moreover, expression of miR-492 was augmented effectively by p53 in response to genotoxic stress, including x-ray irradiation and 5-FU treatment, which indicated miR-492 was regulated by p53.
We went on to investigate whether miR-492 was a direct effector of p53 through bioinformatics tools. miR-492 is encoded by the positive strand of human chromosome 12. PROMO was employed to search for putative p53 BS 2 kb upstream and 1 kb downstream of the pre-miR-492 locus. A putative p53 BS with 2 tandem repeats of consensus p53 binding motifs separated by a 14 bp spacer was found within 1 kb upstream of the pre-miR-492 sequence. Although a widely accepted canonical p53 response element was composed of 2 copies of decametric half-sites separated by up to 13 bp spacers [54], recent studies suggested that in addition to the classic full site response elements, p53 could transactivate target genes through non-canonical half sites and 3/4 sites [55,56]. Besides, the transactivation effect of p53 was similar when the spacer length was increased from 4 to 14 bases, which was both inferior to the length of 0 to 3 bp spacers [56,57]. A popular computer algorithm, P53MH, which identified putative p53 BS on a genome-wide scale also set the spacers between 2 consensus decametric half-sites up to 14 bp [42]. Moreover, the sequences of consensus 10 bp motifs as well as the spacers of miR-492 were all highly conserved among primate species. Further analysis of the putative p53 BS including histone posttranslational modifications and DNase hypersensitive regions by using ENCODE consortium all indicated p53 could transactivate miR-492 directly through binding to this response element.
Indeed, experimental results from ChIP analysis demonstrated an enrichment of endogenous p53 binding to the putative p53 response element of miR-492 in SiHa and HeLa cells, while no enrichment of the p53 BS fragments could be obtained with a control mouse IgG antibody. Dual-luciferase reporter assay also confirmed that p53 could transactivate miR-492 expression directly when p53 was stimulated by cytotoxic reagents. Therefore, miR-492 is a direct p53 target gene. Besides, according to our findings, we postulate that the correlation between miR-492 upregulation and good responsiveness to concurrent chemoradiation in cervical cancer patients may be attributed to the expression level of wild-type p53. Functional wild-type p53 could activate miR-492 expression, and it is also responsible for the higher sensitivity of cervical cancer patients to chemoradiotherapy. We will validate our theory in future studies.
There were several limitations in the present study. First, although some experimental procedures have been validated in our previous studies and the cervical cancer cell lines were verified by short tandem repeat (STR) analysis before, to improve the interpretation and credibility of the results, positive control cells should be included in the experiments. Second, the regulation of miR-492 by p53 could be validated when 5-FU and irradiation were used in succession, which could further simulate the concomitant chemoradiotherapy in vitro.
Conclusions
We have shown for the first time that miR-492 is a direct p53 target gene which can be induced by DNA damage reagents. Our findings give an insight into the transcriptional regulation of miR-492, which provide us a clearer picture of the role of miR-492 in cervical cancer.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation (81302279, 81402346), CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-001), National Key Research & Development (R&D) Plan (2016YFC1302103), Beijing Natural Science Foundation (No. 7172042), Science Foundation of Peking University Cancer Hospital (No. 2017-14), Capital’s Funds for Health Improvement and Research (CFH 2018-2-2153), P. R. China
Conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Yang BH, Bray FI, Parkin DM, et al. Cervical cancer as a priority for prevention in different world regions: An evaluation using years of life lost. Int J Cancer. 2004;109:418–24. doi: 10.1002/ijc.11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical Cancer, Version 2.2015 Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2015;13(4):395–404. doi: 10.6004/jnccn.2015.0055. quiz 404. [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Olvera A, Cetina L, Coronel J, Duenas-Gonzalez A. Emerging drugs for the treatment of cervical cancer. Expert Opin Emerg Drugs. 2015;20:165–82. doi: 10.1517/14728214.2015.1002768. [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Wang W, El-Deiry WS. Current strategies to target p53 in cancer. Biochem Pharmacol. 2010;80:724–30. doi: 10.1016/j.bcp.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira CG, Tolis C, Giaccone G. p53 and chemosensitivity. Ann Oncol. 1999;10:1011–21. doi: 10.1023/a:1008361818480. [DOI] [PubMed] [Google Scholar]
- 9.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 10.Mirza A, Wu Q, Wang L, et al. Global transcriptional program of p53 target genes during the process of apoptosis and cell cycle progression. Oncogene. 2003;22:3645–54. doi: 10.1038/sj.onc.1206477. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa H, Mitsuhashi N, Sakurai H, et al. The effects of p53 status and human papillomavirus infection on the clinical outcome of patients with stage IIIB cervical carcinoma treated with radiation therapy alone. Cancer. 2001;91:80–89. doi: 10.1002/1097-0142(20010101)91:1<80::aid-cncr11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.McIlwrath AJ, Vasey PA, Ross GM, Brown R. Cell cycle arrests and radiosensitivity of human tumor cell lines: dependence on wild-type p53 for radiosensitivity. Cancer Res. 1994;54:3718–22. [PubMed] [Google Scholar]
- 13.Koivusalo R, Krausz E, Helenius H, Hietanen S. Chemotherapy compounds in cervical cancer cells primed by reconstitution of p53 function after short interfering RNA-mediated degradation of human papillomavirus 18 E6 mRNA: opposite effect of siRNA in combination with different drugs. Mol Pharmacol. 2005;68:372–82. doi: 10.1124/mol.105.011189. [DOI] [PubMed] [Google Scholar]
- 14.Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 15.Crook T, Wrede D, Tidy JA, et al. Clonal p53 mutation in primary cervical cancer: Association with human-papillomavirus-negative tumours. Lancet. 1992;339:1070–73. doi: 10.1016/0140-6736(92)90662-m. [DOI] [PubMed] [Google Scholar]
- 16.Paquette RL, Lee YY, Wilczynski SP, et al. Mutations of p53 and human papillomavirus infection in cervical carcinoma. Cancer. 1993;72:1272–80. doi: 10.1002/1097-0142(19930815)72:4<1272::aid-cncr2820720420>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Cordoba SL, Salido-Guadarrama I, Rodriguez-Dorantes M, Hidalgo-Miranda A. miRNA biogenesis: Biological impact in the development of cancer. Cancer Biol Ther. 2014;15:1444–55. doi: 10.4161/15384047.2014.955442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuanyin L, Xiaona W, Zhiling Y, et al. The association between polymorphisms in microRNA genes and cervical cancer in a Chinese Han population. Oncotarget. 2017;8:87914–27. doi: 10.18632/oncotarget.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granados-Lopez AJ, Ruiz-Carrillo JL, Servin-Gonzalez LS, et al. Use of mature miRNA strand selection in miRNAs families in cervical cancer development. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020407. pii: E407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MY, Hu XX. Meta-analysis of microRNA expression profiling studies in human cervical cancer. Med Oncol. 2015;32:510. doi: 10.1007/s12032-015-0510-5. [DOI] [PubMed] [Google Scholar]
- 21.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: The implications for cancer research. Nat Rev Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma G, Dua P, Agarwal SM. A comprehensive review of dysregulated miRNAs involved in cervical cancer. Curr Genomics. 2014;15:310–23. doi: 10.2174/1389202915666140528003249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Song L, Zhang L, et al. miR-21 modulates resistance of HR-HPV positive cervical cancer cells to radiation through targeting LATS1. Biochem Biophys Res Commun. 2015;459:679–85. doi: 10.1016/j.bbrc.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Yu X, Guo X, et al. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol Med Rep. 2012;5:753–60. doi: 10.3892/mmr.2011.696. [DOI] [PubMed] [Google Scholar]
- 25.Huang TH, Chu TY. Repression of miR-126 and upregulation of adrenomedullin in the stromal endothelium by cancer-stromal cross talks confers angiogenesis of cervical cancer. Oncogene. 2014;33:3636–47. doi: 10.1038/onc.2013.335. [DOI] [PubMed] [Google Scholar]
- 26.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–30. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 27.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corney DC, Flesken-Nikitin A, Godwin AK, et al. MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–38. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 29.Kojima K, Fujita Y, Nozawa Y, et al. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70:1501–12. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- 30.Vinall RL, Ripoll AZ, Wang S, et al. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int J Cancer. 2012;130:2526–38. doi: 10.1002/ijc.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita Y, Kojima K, Hamada N, et al. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem Biophys Res Commun. 2008;377:114–19. doi: 10.1016/j.bbrc.2008.09.086. [DOI] [PubMed] [Google Scholar]
- 32.Duan W, Xu Y, Dong Y, et al. Ectopic expression of miR-34a enhances radiosensitivity of non-small cell lung cancer cells, partly by suppressing the LyGDI signaling pathway. J Radiat Res. 2013;54:611–19. doi: 10.1093/jrr/rrs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, An J, Huang M, et al. MicroRNA-492 overexpression involves in cell proliferation, migration, and radiotherapy response of cervical squamous cell carcinomas. Mol Carcinog. 2018;57:32–43. doi: 10.1002/mc.22717. [DOI] [PubMed] [Google Scholar]
- 34.Raver-Shapira N, Marciano E, Meiri E, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–43. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Baker SJ, Markowitz S, Fearon ER, et al. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–15. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Ridzon DA, Broomer AJ, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Yaginuma Y, Westphal H. Analysis of the p53 gene in human uterine carcinoma cell lines. Cancer Res. 1991;51:6506–9. [PubMed] [Google Scholar]
- 39.Wrede D, Tidy JA, Crook T, Lane D, Vousden KH. Expression of RB and p53 proteins in HPV-positive and HPV-negative cervical carcinoma cell lines. Mol Carcinog. 1991;4:171–75. doi: 10.1002/mc.2940040302. [DOI] [PubMed] [Google Scholar]
- 40.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–38. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 41.Messeguer X, Escudero R, Farre D, et al. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–34. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 42.Hoh J, Jin S, Parrado T, et al. The p53MH algorithm and its application in detecting p53-responsive genes. Proc Natl Acad Sci USA. 2002;99:8467–72. doi: 10.1073/pnas.132268899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbloom KR, Dreszer TR, Long JC, et al. ENCODE whole-genome data in the UCSC Genome Browser: update 2012. Nucleic Acids Res. 2012;40:D912–17. doi: 10.1093/nar/gkr1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Zhang MQ. Histone modification profiles are predictive for tissue/cell-type specific expression of both protein-coding and microRNA genes. BMC Bioinformatics. 2011;12:155. doi: 10.1186/1471-2105-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6:851–64. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Han Y, Tian T, et al. MiR-21-5p, miR-34a, and human telomerase RNA component as surrogate markers for cervical cancer progression. Pathol Res Pract. 2018;214:374–79. doi: 10.1016/j.prp.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Ma C, Zhang W, et al. Down regulation of miR-143 is related with tumor size, lymph node metastasis and HPV16 infection in cervical squamous cancer. Diagn Pathol. 2014;9:88. doi: 10.1186/1746-1596-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Frowein J, Pagel P, Kappler R, et al. MicroRNA-492 is processed from the keratin 19 gene and up-regulated in metastatic hepatoblastoma. Hepatology. 2011;53:833–42. doi: 10.1002/hep.24125. [DOI] [PubMed] [Google Scholar]
- 49.Schultz NA, Werner J, Willenbrock H, et al. MicroRNA expression profiles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod Pathol. 2012;25:1609–22. doi: 10.1038/modpathol.2012.122. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Zhang Z, Yang K, et al. Myeloid zinc-finger 1 (MZF-1) suppresses prostate tumor growth through enforcing ferroportin-conducted iron egress. Oncogene. 2015;34:3839–47. doi: 10.1038/onc.2014.310. [DOI] [PubMed] [Google Scholar]
- 51.Asfaha S, Hayakawa Y, Muley A, et al. Krt19(+)/Lgr5(−) cells are radioresistant cancer-initiating stem cells in the colon and intestine. Cell Stem Cell. 2015;16:627–38. doi: 10.1016/j.stem.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 53.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–99. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 54.el-Deiry WS, Kern SE, Pietenpol JA, et al. Definition of a consensus binding site for p53. Nat Genet. 1992;1:45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- 55.Tebaldi T, Zaccara S, Alessandrini F, et al. Whole-genome cartography of p53 response elements ranked on transactivation potential. BMC Genomics. 2015;16:464. doi: 10.1186/s12864-015-1643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan JJ, Menendez D, Inga A, et al. Noncanonical DNA motifs as transactivation targets by wild type and mutant p53. PLoS Genet. 2008;4:e1000104. doi: 10.1371/journal.pgen.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tokino T, Thiagalingam S, el-Deiry WS, et al. p53 tagged sites from human genomic DNA. Hum Mol Genet. 1994;3:1537–42. doi: 10.1093/hmg/3.9.1537. [DOI] [PubMed] [Google Scholar]